化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3455-3465.DOI: 10.16085/j.issn.1000-6613.2020-1352
赤泥吸附重金属离子性能及其机理研究进展
刘钦1( ), 周新涛1(
), 周新涛1( ), 黄静2, 罗中秋1(
), 黄静2, 罗中秋1( ), 邵周军1, 王路星1, 韦宇1, 雒云龙1
), 邵周军1, 王路星1, 韦宇1, 雒云龙1
- 1.昆明理工大学化学工程学院,云南 昆明 650500
2.西昌学院机械与电气工程学院,四川 西昌 615000
-
收稿日期:2020-07-14修回日期:2020-08-19出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:周新涛,罗中秋 -
作者简介:刘钦(1996—),男,硕士研究生,研究方向为固体物资源化利用与安全化处理。E-mail:15827824086@163.com 。 -
基金资助:国家自然科学基金地区基金(21866018);昆明理工大学分析测试基金(2016T20160009);云南科技厅青年基金(2017FD093);云南教育厅资助性项目(2017ZZX147);云南省万人计划“青年拔尖人才”
Research statue on adsorption properties and mechanism of heavy metal ions using red mud
LIU Qin1( ), ZHOU Xintao1(
), ZHOU Xintao1( ), HUANG Jing2, LUO Zhongqiu1(
), HUANG Jing2, LUO Zhongqiu1( ), SHAO Zhoujun1, WANG Luxing1, WEI Yu1, LUO Yunlong1
), SHAO Zhoujun1, WANG Luxing1, WEI Yu1, LUO Yunlong1
- 1.Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
2.School of Mechanical and Electrical Engineering, Xichang University, Xichang 615000, Sichuan, China
-
Received:2020-07-14Revised:2020-08-19Online:2021-06-06Published:2021-06-22 -
Contact:ZHOU Xintao,LUO Zhongqiu
摘要:
赤泥(RM)是铝土矿提取氧化铝时排放的工业废渣,对其进行改性后可作为一种低成本吸附剂有效吸附废水中的重金属离子。本文从赤泥的性质和组成进行讨论,分析赤泥吸附重金属离子的优势,总结酸改性、焙烧改性和复合改性对赤泥结构及重金属吸附性能的影响。在此基础上,阐述了赤泥吸附重金属离子的机理,列举了吸附热力学及吸附动力学模型。指出赤泥作为一种大宗工业废弃物,用作吸附剂吸附废水中的重金属离子,具有成本低、来源广泛等优点,同时可达到以废治废的目的,具有良好的应用前景。
中图分类号:
引用本文
刘钦, 周新涛, 黄静, 罗中秋, 邵周军, 王路星, 韦宇, 雒云龙. 赤泥吸附重金属离子性能及其机理研究进展[J]. 化工进展, 2021, 40(6): 3455-3465.
LIU Qin, ZHOU Xintao, HUANG Jing, LUO Zhongqiu, SHAO Zhoujun, WANG Luxing, WEI Yu, LUO Yunlong. Research statue on adsorption properties and mechanism of heavy metal ions using red mud[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3455-3465.
| 赤泥类型 | SiO2 | CaO | Al2O3 | Fe2O3 | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|
| 拜耳法赤泥 | 3~20 | 2~8 | 10~20 | 30~60 | 2~10 | — | 0.1~10 |
| 烧结法赤泥 | 20~23 | 46~49 | 5~7 | 7~10 | 2~2.5 | 0.2~0.4 | 2.5~3 |
| 联合法赤泥 | 20~20.5 | 43~48 | 4.4~7.5 | 6.1~7.5 | 2.8~3 | 0.5~5.7 | 6.1~7.7 |
表1 赤泥主要化学成分(质量分数)[13-14] (%)
| 赤泥类型 | SiO2 | CaO | Al2O3 | Fe2O3 | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|
| 拜耳法赤泥 | 3~20 | 2~8 | 10~20 | 30~60 | 2~10 | — | 0.1~10 |
| 烧结法赤泥 | 20~23 | 46~49 | 5~7 | 7~10 | 2~2.5 | 0.2~0.4 | 2.5~3 |
| 联合法赤泥 | 20~20.5 | 43~48 | 4.4~7.5 | 6.1~7.5 | 2.8~3 | 0.5~5.7 | 6.1~7.7 |
| 元素 | 矿物 | 化学结构 | 作用离子 |
|---|---|---|---|
| Fe | 赤铁矿 | α-Fe2O3 | Cu2+、Zn2+、Co2+ |
| 针铁矿 | α-FeOOH | — | |
| 磁铁矿 | Fe3O4 | As3+ | |
| 钛铁矿 | FeO-TiO2 | — | |
| 水滑石 | Mg6Al2CO3(OH)16·4H2O | Ni2+ | |
| 磁赤铁矿 | γ-Fe2O3 | — | |
| Al | 铝矾土 | α-Al2O3·3H2O | — |
| 勃姆石 | γ-AlOOH | — | |
| 三水铝石 | Al(OH)3 | Cu2+、Zn2+ | |
| Ti | 锐钛矿 | TiO2 | — |
| 钙钛矿 | CaTiO3 | Co2+ | |
| 钛铁矿 | FeO-TiO2 | — | |
| Si、Al、Na、Ca | 石英 | SiO2 | — |
| 高岭石 | Al2Si2O5(OH)4 | — | |
| 埃洛石 | Al2Si2O5(OH)4·H2O | — | |
| 方解石 | CaCO3 | Cu2+、Zn2+、Cr2+ | |
| 方钠石 | Na4(Al3Si3O12)Cl | Co2+、Cr3+ | |
| 霞石 | Na8(Al6Si6O24)CO3 | — |
表2 赤泥矿物形式以及矿物作用离子情况[15-16]
| 元素 | 矿物 | 化学结构 | 作用离子 |
|---|---|---|---|
| Fe | 赤铁矿 | α-Fe2O3 | Cu2+、Zn2+、Co2+ |
| 针铁矿 | α-FeOOH | — | |
| 磁铁矿 | Fe3O4 | As3+ | |
| 钛铁矿 | FeO-TiO2 | — | |
| 水滑石 | Mg6Al2CO3(OH)16·4H2O | Ni2+ | |
| 磁赤铁矿 | γ-Fe2O3 | — | |
| Al | 铝矾土 | α-Al2O3·3H2O | — |
| 勃姆石 | γ-AlOOH | — | |
| 三水铝石 | Al(OH)3 | Cu2+、Zn2+ | |
| Ti | 锐钛矿 | TiO2 | — |
| 钙钛矿 | CaTiO3 | Co2+ | |
| 钛铁矿 | FeO-TiO2 | — | |
| Si、Al、Na、Ca | 石英 | SiO2 | — |
| 高岭石 | Al2Si2O5(OH)4 | — | |
| 埃洛石 | Al2Si2O5(OH)4·H2O | — | |
| 方解石 | CaCO3 | Cu2+、Zn2+、Cr2+ | |
| 方钠石 | Na4(Al3Si3O12)Cl | Co2+、Cr3+ | |
| 霞石 | Na8(Al6Si6O24)CO3 | — |
| 吸附剂 | 重金属离子 | 吸附量/mg·g-1 | 主要吸附方式 | 参考文献 |
|---|---|---|---|---|
| 赤泥 | Cd2+ | 83.03 | 物理吸附、化学吸附 | [ |
| Cu2+ | 21.56 | [ | ||
| Pb2+ | 389.16 | [ | ||
| 粉煤灰 | Cd2+ | 0.089 | 化学吸附 | [ |
| Cu2+ | 7 | [ | ||
| Pb2+ | 18 | [ | ||
| 黏土 | Cd2+ | 39.5 | 物理吸附、化学吸附 | [ |
| Pb2+ | 19.5 | [ |
表3 赤泥和其他吸附剂对重金属离子吸附量的比较[18-22]
| 吸附剂 | 重金属离子 | 吸附量/mg·g-1 | 主要吸附方式 | 参考文献 |
|---|---|---|---|---|
| 赤泥 | Cd2+ | 83.03 | 物理吸附、化学吸附 | [ |
| Cu2+ | 21.56 | [ | ||
| Pb2+ | 389.16 | [ | ||
| 粉煤灰 | Cd2+ | 0.089 | 化学吸附 | [ |
| Cu2+ | 7 | [ | ||
| Pb2+ | 18 | [ | ||
| 黏土 | Cd2+ | 39.5 | 物理吸附、化学吸附 | [ |
| Pb2+ | 19.5 | [ |
| 金属离子 | 初始浓度 /mg?L-1 | 吸附剂 | 吸附剂用量 /g?L-1 | 吸附pH | 吸附时间 /h | 吸附温度 /℃ | 吸附量 /mg?g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Cu2+ | 100 | RM | 50 | 5.5 | 8 | 30 | 2.28 | [ |
| Cu2+ | 180 | HCl-RM | 4 | 5.5 | 1 | 23 | 21.56 | [ |
| Pb2+ | 500 | HCl-RM | 4 | 5.5 | 1 | 23 | 123.28 | [ |
| Pb2+ | 25 | RM | 0.5 | 5.5 | 2 | 25 | 46.68 | [ |
| Cd2+ | 50 | RM | 10 | 5 | 24 | 25 | 12.58 | [ |
| Cd2+ | 10 | GRM | 1 | 6.5 | 24 | 50 | 9 | [ |
| Cd2+ | 10 | ARM | 5 | 6 | 1 | 30 | 12.55 | [ |
| Cd2+ | 100 | 改性RM | 1 | 4 | 1 | 30 | 83.03 | [ |
| Cd2+ | 200 | RM | 2 | 6 | 24 | 25 | 68 | [ |
| Zn2+ | 50 | RM | 10 | 5 | 24 | 25 | 12.05 | [ |
| Zn2+ | 200 | RM | 2 | 7 | 24 | 25 | 133 | [ |
| Ni2+ | 50 | RM | 10 | 5 | 24 | 25 | 11.06 | [ |
| Ni2+ | 50 | HCl-RM | 10 | 5 | 24 | 21 | 11.1 | [ |
| Co2+ | 280 | RBRM | 5 | 5 | 48 | 21 | 29.47 | [ |
| Mn2+ | 2 | RM | 10 | 6.5 | 1 | 25 | 0.191 | [ |
表4 不同影响因素下赤泥对重金属离子的吸附效果[18-19, 23-31]
| 金属离子 | 初始浓度 /mg?L-1 | 吸附剂 | 吸附剂用量 /g?L-1 | 吸附pH | 吸附时间 /h | 吸附温度 /℃ | 吸附量 /mg?g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
| Cu2+ | 100 | RM | 50 | 5.5 | 8 | 30 | 2.28 | [ |
| Cu2+ | 180 | HCl-RM | 4 | 5.5 | 1 | 23 | 21.56 | [ |
| Pb2+ | 500 | HCl-RM | 4 | 5.5 | 1 | 23 | 123.28 | [ |
| Pb2+ | 25 | RM | 0.5 | 5.5 | 2 | 25 | 46.68 | [ |
| Cd2+ | 50 | RM | 10 | 5 | 24 | 25 | 12.58 | [ |
| Cd2+ | 10 | GRM | 1 | 6.5 | 24 | 50 | 9 | [ |
| Cd2+ | 10 | ARM | 5 | 6 | 1 | 30 | 12.55 | [ |
| Cd2+ | 100 | 改性RM | 1 | 4 | 1 | 30 | 83.03 | [ |
| Cd2+ | 200 | RM | 2 | 6 | 24 | 25 | 68 | [ |
| Zn2+ | 50 | RM | 10 | 5 | 24 | 25 | 12.05 | [ |
| Zn2+ | 200 | RM | 2 | 7 | 24 | 25 | 133 | [ |
| Ni2+ | 50 | RM | 10 | 5 | 24 | 25 | 11.06 | [ |
| Ni2+ | 50 | HCl-RM | 10 | 5 | 24 | 21 | 11.1 | [ |
| Co2+ | 280 | RBRM | 5 | 5 | 48 | 21 | 29.47 | [ |
| Mn2+ | 2 | RM | 10 | 6.5 | 1 | 25 | 0.191 | [ |
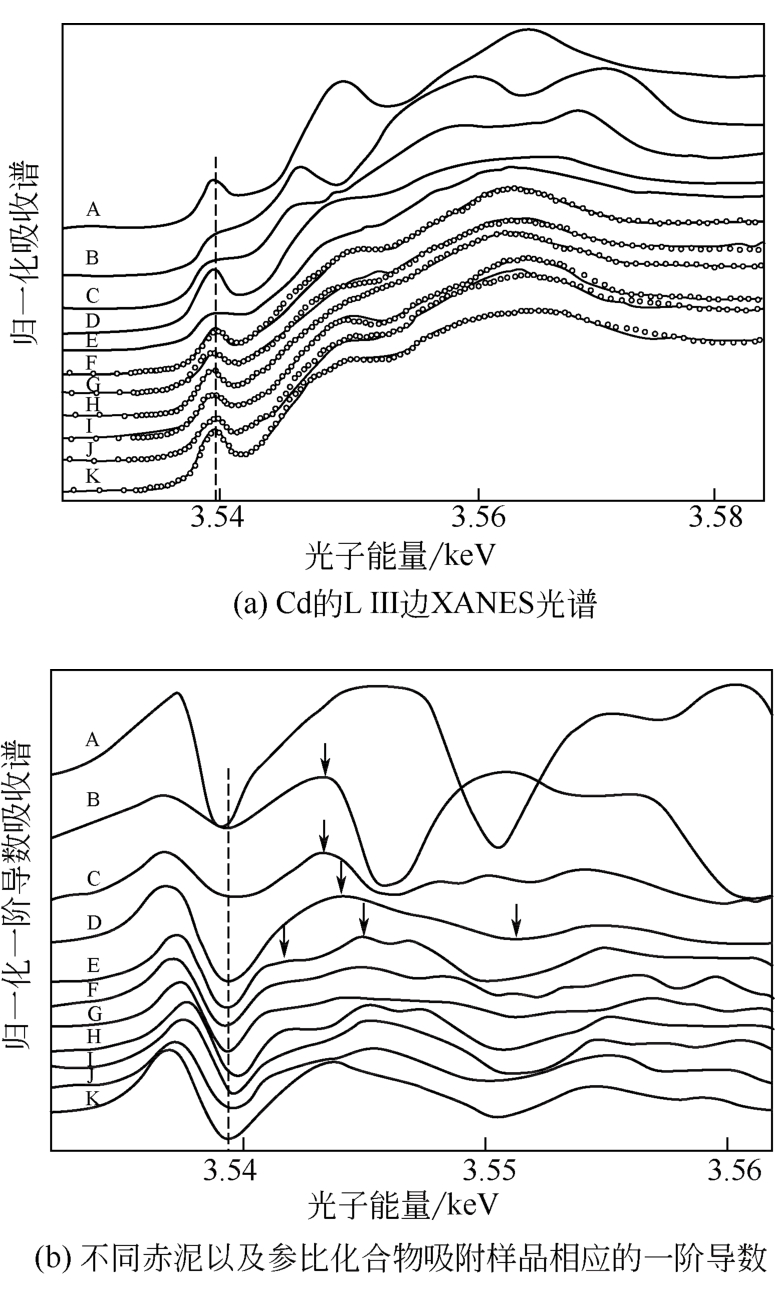
图6 赤泥吸附Cd2+的XANES光谱[44]A—CdCO3;B—CdO;C—Cd(OH)2;D—Cd(NO3)2;E—Cd(OH)Cl;F—RMnano+1mmol/L Cd;G—RMnano+6mmol/L Cd;H—RMa+1mmol/L Cd;I—RMa+6mmol/L Cd;J—RMo+1mmol/L Cd;K—RMo+6mmol/L Cd;其中:RMnano—纳米级赤泥;RMa—酸处理赤泥;RMo—初始赤泥
| 重金属离子 | 吸附剂 | Langmuir等温式 | Freundlich等温式 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| qm/mg?g-1 | KL/L?mg-1 | KF/L?g-1 | ||||||
| Mn2+ | RM | 0.3348 | 00.007 | 0.5352 | 0.9545 | 57.33 | 0.7576 | [ |
| Mn2+ | ARM700 | 88.36 | 0.071 | 0.945 | 5.56 | 29.77 | 0.820 | [ |
| As3+ | RM | 0.5263 | 0.037 | 0.9781 | 1.0710 | 20.48 | 0.9467 | [ |
| As3+ | RMA | 27.8 | 38.2 | 7.11 | 21.6 | [ | ||
| Pb2+ | RM | 6.027 | 2.043 | 0.9938 | 2.3469 | 0.1160 | 0.2123 | [ |
| Zn2+ | ANRM | 14.92 | 0.138 | 0.986 | 1.322 | 3.174 | 0.948 | [ |
| Zn2+ | RM | 12.048 | 0.517 | 0.998 | 4.5 | 4.4 | 0.886 | [ |
| Cd2+ | IOARM | 0.1163 | 11.3 | 0.957 | 1.464 | 3.681 | 0.994 | [ |
| Cd2+ | RM | 12.579 | 0.411 | 0.996 | 6.69 | 3.79 | 0.833 | [ |
| Ni2+ | BRM0.05 | 11.11 | 0.182 | 0.99 | [ | |||
| Ni2+ | BRM0.1 | 9.34 | 0.169 | 0.996 | [ | |||
| Ni2+ | RM | 11.062 | 0.167 | 0.997 | 2.76 | 2.08 | 0.878 | [ |
| Co2+ | RM | 9.0252 | 0.0184 | 0.9702 | 2.9481 | 0.0624 | 0.9335 | [ |
表5 赤泥吸附重金属离子热力学模型拟合情况[26, 30, 32, 53-57]
| 重金属离子 | 吸附剂 | Langmuir等温式 | Freundlich等温式 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| qm/mg?g-1 | KL/L?mg-1 | KF/L?g-1 | ||||||
| Mn2+ | RM | 0.3348 | 00.007 | 0.5352 | 0.9545 | 57.33 | 0.7576 | [ |
| Mn2+ | ARM700 | 88.36 | 0.071 | 0.945 | 5.56 | 29.77 | 0.820 | [ |
| As3+ | RM | 0.5263 | 0.037 | 0.9781 | 1.0710 | 20.48 | 0.9467 | [ |
| As3+ | RMA | 27.8 | 38.2 | 7.11 | 21.6 | [ | ||
| Pb2+ | RM | 6.027 | 2.043 | 0.9938 | 2.3469 | 0.1160 | 0.2123 | [ |
| Zn2+ | ANRM | 14.92 | 0.138 | 0.986 | 1.322 | 3.174 | 0.948 | [ |
| Zn2+ | RM | 12.048 | 0.517 | 0.998 | 4.5 | 4.4 | 0.886 | [ |
| Cd2+ | IOARM | 0.1163 | 11.3 | 0.957 | 1.464 | 3.681 | 0.994 | [ |
| Cd2+ | RM | 12.579 | 0.411 | 0.996 | 6.69 | 3.79 | 0.833 | [ |
| Ni2+ | BRM0.05 | 11.11 | 0.182 | 0.99 | [ | |||
| Ni2+ | BRM0.1 | 9.34 | 0.169 | 0.996 | [ | |||
| Ni2+ | RM | 11.062 | 0.167 | 0.997 | 2.76 | 2.08 | 0.878 | [ |
| Co2+ | RM | 9.0252 | 0.0184 | 0.9702 | 2.9481 | 0.0624 | 0.9335 | [ |
| 重金属离子 | 吸附剂 | 准一级动力学方程 | 准二级动力学方程 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| qe/mg?g-1 | k1/min-1 | R2 | qe/mg?g-1 | k2/g·mg-1·min-1 | R2 | |||
| Pb2+ | RM | 0.0822 | 0.882 | 1.9054 | 0.6437 | 0.999 | [ | |
| Cd2+ | RM500 | 16.62 | 0.061 | 0.56 | 18.05 | 0.0044 | 1 | [ |
| Cd2+ | IOARM | 0.078 | 0.039 | 0.994 | 0.071 | 5.99×10-5 | 0.991 | [ |
| Cd2+ | ARM | 0.966 | 0.0795 | 0.905 | 20.6232 | 0.0052 | 0.992 | [ |
| Zn2+ | ANRM | 30 | 0.0068 | 0.888 | 25.859 | 3.677×10-3 | 0.997 | [ |
| Mn2+ | RM | 17.28 | 0.0093 | 0.93 | 17.96 | 6×10-4 | 0.993 | [ |
| Mn2+ | RM700 | 78.07 | 0.016 | 0.977 | 87.04 | 2.4×10-4 | 0.992 | [ |
| Ni2+ | BRM0.1 | 6.48 | 0.0029 | 0.999 | [ | |||
| Co2+ | RBRM | 20.47 | 0.708 | 0.99 | [ | |||
| Sr2+ | RBRM | 18.22 | 6.044 | 0.99 | [ | |||
表6 赤泥吸附重金属离子动力学模型拟合情况[21, 29-30, 33, 35, 52, 54-55, 59]
| 重金属离子 | 吸附剂 | 准一级动力学方程 | 准二级动力学方程 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| qe/mg?g-1 | k1/min-1 | R2 | qe/mg?g-1 | k2/g·mg-1·min-1 | R2 | |||
| Pb2+ | RM | 0.0822 | 0.882 | 1.9054 | 0.6437 | 0.999 | [ | |
| Cd2+ | RM500 | 16.62 | 0.061 | 0.56 | 18.05 | 0.0044 | 1 | [ |
| Cd2+ | IOARM | 0.078 | 0.039 | 0.994 | 0.071 | 5.99×10-5 | 0.991 | [ |
| Cd2+ | ARM | 0.966 | 0.0795 | 0.905 | 20.6232 | 0.0052 | 0.992 | [ |
| Zn2+ | ANRM | 30 | 0.0068 | 0.888 | 25.859 | 3.677×10-3 | 0.997 | [ |
| Mn2+ | RM | 17.28 | 0.0093 | 0.93 | 17.96 | 6×10-4 | 0.993 | [ |
| Mn2+ | RM700 | 78.07 | 0.016 | 0.977 | 87.04 | 2.4×10-4 | 0.992 | [ |
| Ni2+ | BRM0.1 | 6.48 | 0.0029 | 0.999 | [ | |||
| Co2+ | RBRM | 20.47 | 0.708 | 0.99 | [ | |||
| Sr2+ | RBRM | 18.22 | 6.044 | 0.99 | [ | |||
| 1 | KUMAR S, KUMAR R, BANDOPADHYAY A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries[J]. Resources, Conservation and Recycling, 2006, 48(4): 301-314. |
| 2 | MUKIZA E, ZHANG L L, LIU X M, et al. Utilization of red mud in road base and subgrade materials: a review[J]. Resources Conservation and Recycling, 2019, 141: 187-199. |
| 3 | SGLAVO V M, CAMPOSTRINI R, MAURINA S, et al. Bauxite ‘red mud’ in the ceramic industry. Part 1: Thermal behaviour[J]. Journal of the European Ceramic Society, 2000, 20(3): 235-244. |
| 4 | DI CARLO E, BOULLEMANT A, COURTNEY R. A field assessment of bauxite residue rehabilitation strategies[J]. Science of the Total Environment, 2019, 663: 915-926. |
| 5 | ZHU F, LIAO J X, XUE S G, et al. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography[J]. Science of the Total Environment, 2016, 573: 155-163. |
| 6 | LI L Y. A study of iron mineral transformation to reduce red mud tailings[J]. Waste Management, 2001, 21(6): 525-534. |
| 7 | AHMED M J K, AHMARUZZAMAN M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions[J]. Journal of Water Process Engineering, 2016, 10: 39-47. |
| 8 | MUSHTAQ F, ZAHID M, BHATTI I A, et al. Possible applications of coal fly ash in wastewater treatment[J]. Journal of Environmental Management, 2019, 240: 27-46. |
| 9 | AHMED M J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: review[J]. Environmental Toxicology and Pharmacology, 2017, 50: 1-10. |
| 10 | 张俊杰, 邵敬爱, 黄河洵, 等. 利用污泥制备活性炭及其吸附特性的研究进展[J]. 化工进展, 2017, 36(10): 3876-3886. |
| ZHANG Junjie, SHAO Jingai, HUANG Hexun, et al. Review on the preparation of activated carbon from sludge and its adsorption characteristics[J]. Chemical Industry and Engineering Progress, 2017, 36(10): 3876-3886. | |
| 11 | GUPTA V K, SUHAS, ALI I, et al. Removal of rhodamine B, fast green, and methylene blue from wastewater using red mud, an aluminum industry waste[J]. Industrial & Engineering Chemistry Research, 2004, 43(7): 1740-1747. |
| 12 | KHAIRUL M A, ZANGANE J, MOGHTADER B. The composition, recycling and utilisation of Bayer red mud[J]. Resources Conservation and Recycling, 2019, 141: 483-498. |
| 13 | SANTONA L, CASTALDI P, MELIS P. Evaluation of the interaction mechanisms between red muds and heavy metals[J]. Journal of Hazardous Materials, 2006, 136(2): 324-329. |
| 14 | HUA Y, HEAL K V, FRIESL HAN W. The use of red mud as an immobiliser for metal/metalloid contaminated soil: a review[J]. Journal of Hazardous Materials, 2017, 325: 17-30. |
| 15 | COLLINS R N, CLARK M W, PAYNE T E. Solid phases responsible for Mn-Ⅱ, Cr-Ⅲ, Co-Ⅱ, Ni, Cu-Ⅱand Zn immobilization by a modified bauxite refinery residue (red mud) at pH 7.5[J]. Chemical Engineering Journal, 2014, 236: 419-429. |
| 16 |
MILENKOVIC A, SMICIKLAS I, BUNDALESKI N, et al. The role of different minerals from red mud assemblage in Co( ) sorption mechanism[J]. Colloids and Surfaces Physicochemical and Engineering Aspects, 2016, 508: 8-20. ) sorption mechanism[J]. Colloids and Surfaces Physicochemical and Engineering Aspects, 2016, 508: 8-20.
|
| 17 | WANG S B, ANG H M, TADE M O. Novel applications of red mud as coagulant,adsorbent and catalyst for environmentally benign processes[J]. Chemosphere, 2008, 72(11): 1621-1635. |
| 18 | KALKAN E, NADAROGLU H, DIKBAS N, et al. Bacteria-modified red mud for adsorption of cadmium ions from aqueous solutions[J]. Polish Journal of Environmental Studies, 2013, 22(2): 417-429. |
| 19 | GRUDIC V V, PERIC D, BLAGOJEVIC N Z, et al. Pb(Ⅱ) and Cu(Ⅱ) sorption from aqueous solutions using activated red mud-evaluation of kinetic, equilibrium, and thermodynamic models[J]. Polish Journal of Environmental Studies, 2013, 22(2): 377-385. |
| 20 | MOHAN S, GANDHIMATHI R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent[J]. Journal of Hazardous Materials, 2009, 169: 351-359. |
| 21 | WANG S, TERDKIABURANA T, TADE M. Single and co-adsorption of heavy metals and humic acid on fly ash[J]. Separation and Purification Technology, 2008, 58: 353-358. |
| 22 | BOONAMNUAYVITAYA V, CHAIYA C, TANTHAPANICHAKOON W, et al. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay[J]. Separation and Purification Technology, 2004, 35: 11-22. |
| 23 | AGRAWAL A, SAHU K K, PANDEY B D. A comparative adsorption study of copper on various industrial solid wastes[J]. AIChE Journal, 2004, 50(10): 2430-2438. |
| 24 |
KAZAK O, TOR A. In situ preparation of magnetic hydrochar by co-hydrothermal treatment of waste vinasse with red mud and its adsorption property for Pb( ) in aqueous solution[J]. Journal of Hazardous Materials, 2020, 393: 122391. ) in aqueous solution[J]. Journal of Hazardous Materials, 2020, 393: 122391.
|
| 25 | AYALA J, FERNANDEZ B. Treatment from abandoned mine landfill leachates. Adsorption technology[J]. Journal of Materials Research and Technology, 2019, 8(3): 2732-2740. |
| 26 | GUPTA V K, SHARMA S. Removal of cadmium and zinc from aqueous solutions using red mud[J]. Environmental Science & Technology, 2002, 36(16): 3612-3617. |
| 27 | SAHU M K, MANDA S, YADAV L S, et al. Equilibrium and kinetic studies of Cd(Ⅱ) ion adsorption from aqueous solution by activated red mud[J]. Desalination and Water Treatment, 2016, 57(30): 14251-14265. |
| 28 | VACLAVIKOVA M, MISAELIDES P, GALLIOS G, et al. Removal of cadmium, zinc, copper and lead by red mud, an iron oxides containing hydro metallurgical waste[J]. Oxide Based Materials: New Sources, Novel Phases, New Applications, 2005, 155: 517- 525. |
| 29 | SMICIKLAS I, SMILJANIC S, PERIC GRUJIC A, et al. Effect of acid treatment on red mud properties with implications on Ni(Ⅱ) sorption and stability[J]. Chemical Engineering Journal, 2014, 242: 27-35. |
| 30 | MILENKOVIĆ A S, SMIČIKLASI D, ŠLJIVIĆl IVANOVIĆ M Z, et al. Effect of experimental variables onto Co2+ and Sr2+ sorption behavior in red mud-water suspensions[J]. Journal of Environmental Science and Health Part A: Toxic/Hazardous Substances & Environmental Engineering, 2016, 51(8): 679-690. |
| 31 | PIETRELLI L, IPPOLITO N M, FERRO S, et al. Removal of Mn and As from drinking water by red mud and pyrolusite[J]. Journal of Environmental Management, 2019, 237: 526-533. |
| 32 | SMILJANIC S, SMICIKLAS I, PERIC GRUJIC A, et al. Study of factors affecting Ni2+ immobilization efficiency by temperature activated red mud[J]. Chemical Engineering Journal, 2011, 168(2): 610-619. |
| 33 | SAHU M K, MANDAL S, DASH S S, et al. Removal of Pb(Ⅱ) from aqueous solution by acid activated red mud[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 1315-1324. |
| 34 | GURUDIC V V, BRASANAC S, VESNA L, et al. Sorption of cadmium from water using neutralized red mud and activated neutralized red mud[J]. Journal of Engineeringand Applied Sciences, 2013, 8(11): 933–943. |
| 35 | YANG T X, WANG Y F, SHENG L X, et al. Enhancing Cd(Ⅱ) sorption by red mud with heat treatment: performance and mechanisms of sorption[J]. Journal of Environmental Management, 2020, 255: 109866. |
| 36 | PULFORD I D, HARGREAVES J S J, DERISOVA J, et al. Carbonised red mud—A new water treatment product made from a waste material[J]. Journal of Environmental Management, 2012, 100: 59-64. |
| 37 | LIANG W T, COUPERTHWAITE S J, KAUR G, et al. Effect of strong acids on red mud structural and fluoride adsorption properties[J]. Journal of Colloid and Interface Science, 2014, 423: 158-165. |
| 38 | ANTUNES M L P, COUERTHWAITE S J, CONCEICAO F T DA, et al. Red mud from Brazil: thermal behavior and physical properties[J]. Industrial & Engineering Chemistry Research, 2012, 51(2): 775-779. |
| 39 | SMILJAVIC S, SMUCIKLAS I, PERIN GRUJIC A, et al. Rinsed and thermally treated red mud sorbents for aqueous Ni2+ ions[J]. Chemical Engineering Journal, 2010, 162(1): 75-83. |
| 40 | 王艳秋, 霍维周. 颗粒赤泥吸附剂对重金属离子的吸附性能研究[J]. 工业用水与废水, 2008, 39(6): 82-85. |
| WANG Yanqiu, HUO Weizhou. Adsorption property of granular red mud adsorbent on heavy metal ions[J]. Industrial Water & Wastewater, 2008, 39(6): 82-85. | |
| 41 | 刘江龙, 郭焱, 何小山, 等. 硅烷化赤泥的制备及其对水中铅离子吸附性能分析[J]. 环境工程学报, 2019, 37(11): 36-44. |
| LIU Jianglong, GUO Yan, HE Xiaoshan, et al. Preparation of silanized red mud and its adsorption properties for lead ions in water[J]. Chinese Journal of Environmental Engineering, 2019, 37(11): 36-44. | |
| 42 | 刘江龙, 郭焱, 席艺慧. FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理[J]. 化工进展, 2020, 39(2): 776-789. |
| LIU Jianglong, GUO Yan, XI Yihui. Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 776-789. | |
| 43 | MESGARI ABBASI S, RASHIDI A, GHORBANI A, et al. Synthesis, processing, characterization, and applications of red mud/carbon nanotube composites[J]. Ceramics International, 2016, 42(15): 16738-16743. |
| 44 | LUO L, MA C Y, MA Y B, et al. New insights into the sorption mechanism of cadmium on red mud[J]. Environmental Pollution, 2011, 159(5): 1108-1113. |
| 45 | SERRANO S, ODAY P A, VLASSOPULOS D, et al. A surface complexation and ion exchange model of Pb and Cd competitive sorption on natural soils[J]. Geochimica Et Cosmochimica Acta, 2009, 73(3): 543-558. |
| 46 | KAUR G, COUPERTHWAITE S J, MILLAR G J. Enhanced removal of Mn(Ⅱ) from solution by thermally activated Bayer precipitates[J]. Minerals Engineering, 2019, 134: 166-175. |
| 47 |
THOMPSON H A, PAORKS G A, BROWN G E. Formation and release of cobalt( ) sorption and precipitation products in aging kaolinite-water slurries[J]. Journal of Colloid and Interface Science, 2000, 222(2): 241-253. ) sorption and precipitation products in aging kaolinite-water slurries[J]. Journal of Colloid and Interface Science, 2000, 222(2): 241-253.
|
| 48 | QI X J, WANG H T, ZHANG L, et al. Removal of Cr(Ⅲ) from aqueous solution by using bauxite residue (red mud): identification of active components and column tests[J]. Chemosphere, 2020, 245: 125560. |
| 49 | CASTALdI P, SILVETTI M, ENZO S, et al. Study of sorption processes and FT-IR analysis of arsenate sorbed onto red muds (a bauxite ore processing waste)[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 172-178. |
| 50 | FOO K Y, HAMEED B H. Insights into the modeling of adsorption isotherm systems[J]. Chemical Engineering Journal, 2010, 156(1): 2-10. |
| 51 |
ARAUJO C S T, ALMEIDA I L S, REZENDE H C, et al. Elucidation of mechanism involved in adsorption of Pb( ) onto lobeira fruit (Solanum lycocarpum) using Langmuir,Freundlich and Temkin isotherms[J]. Microchemical Journal, 2018, 137: 348-354. ) onto lobeira fruit (Solanum lycocarpum) using Langmuir,Freundlich and Temkin isotherms[J]. Microchemical Journal, 2018, 137: 348-354.
|
| 52 | CHEN H L, ZHENG J, ZHANG Z Q, et al. Application of annealed red mud to Mn2+ ion adsorption from aqueous solution[J]. Water Science and Technology, 2016, 73(11): 2761-2771. |
| 53 | PEPPER R A, COUERTHWAITE S J, MILLAR G J. A novel akaganeite sorbent synthesised from waste red mud: application for treatment of arsenate in aqueous solutions[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 6308-6316. |
| 54 | SAHU R C, PATEL R, RAY B C. Adsorption of Zn(Ⅱ) on activated red mud: neutralized by CO2[J]. Desalination, 2011, 266(1/2/3): 93-97. |
| 55 | KHAN T A, CHAUDHRY S A, ALI I. Equilibrium uptake, isotherm and kinetic studies of Cd(Ⅱ) adsorption onto iron oxide activated red mud from aqueous solution[J]. Journal of Molecular Liquids, 2015, 202: 165-175. |
| 56 | RIFAAI R A, MOKHEMER S A, SABER E A, et al. Neuroprotective effect of quercetin nanoparticles: a possible prophylactic and therapeutic role in alzheimer’s disease[J]. Journal of Chemical Neuroanatomy, 2020, 107: 101795. |
| 57 | DEIHIMI N, IRANNAJAD M, REZAI B. Equilibrium and kinetic studies of ferricyanide adsorption from aqueous solution by activated red mud[J]. Journal of Environmental Management, 2018, 227: 277-285. |
| 58 | TAN K L, HAMEED B H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 74: 25-48. |
| 59 | LI Y C, HUANG H, XU Z, et al. Mechanism study on manganese(Ⅱ) removal from acid mine wastewater using red mud and its application to a lab-scale column[J]. Journal of Cleaner Production, 2020, 253: 119955. |
| [1] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [9] | 朱杰, 金晶, 丁正浩, 杨会盼, 侯封校. 化学链气化中准东煤灰对CaSO4载氧体改性及其作用机理[J]. 化工进展, 2023, 42(9): 4628-4635. |
| [10] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [13] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [14] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [15] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||