化工进展 ›› 2021, Vol. 40 ›› Issue (5): 2873-2881.DOI: 10.16085/j.issn.1000-6613.2020-2071
四乙烯五胺接枝膨润土对酸性大红GR的吸附性能
- 1.榆林学院能源工程学院,陕西 榆林 719000
2.榆林学院化学与化工学院,陕西 榆林 719000
-
收稿日期:2020-10-14出版日期:2021-05-06发布日期:2021-05-24 -
通讯作者:孙志勇 -
作者简介:孙志勇(1981—),男,副教授,研究方向为环境功能材料。E-mail:sunzy11@126.com 。 -
基金资助:国家自然科学基金(52064048);陕西省科技厅项目(2019SF-271);陕西省科技资源开发共享平台项目(2019PT-18);榆林市科技局项目(CXY-2020-005-007)
Adsorption properties of acid scarlet GR by tetraethylenepentamine grafted bentonite
SUN Zhiyong1( ), SHANG Tielin1, WANG Jindong1, MA Xiangrong2
), SHANG Tielin1, WANG Jindong1, MA Xiangrong2
- 1.School of Energy Engineering, Yulin College, Yulin 719000, Shaanxi, China
2.School of Chemistry and Chemical Engineering, Yulin College, Yulin 719000, Shaanxi, China
-
Received:2020-10-14Online:2021-05-06Published:2021-05-24 -
Contact:SUN Zhiyong
摘要:
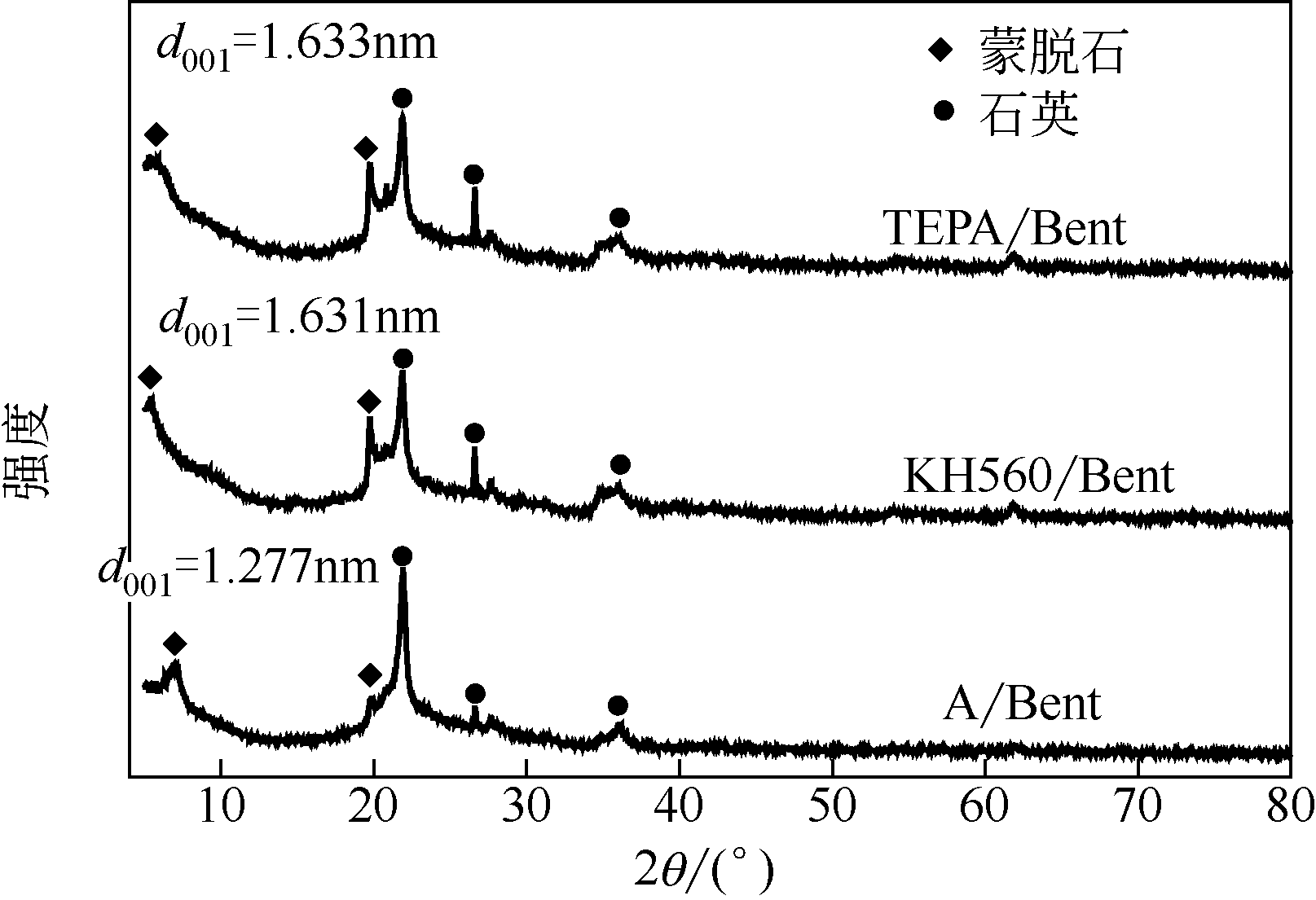
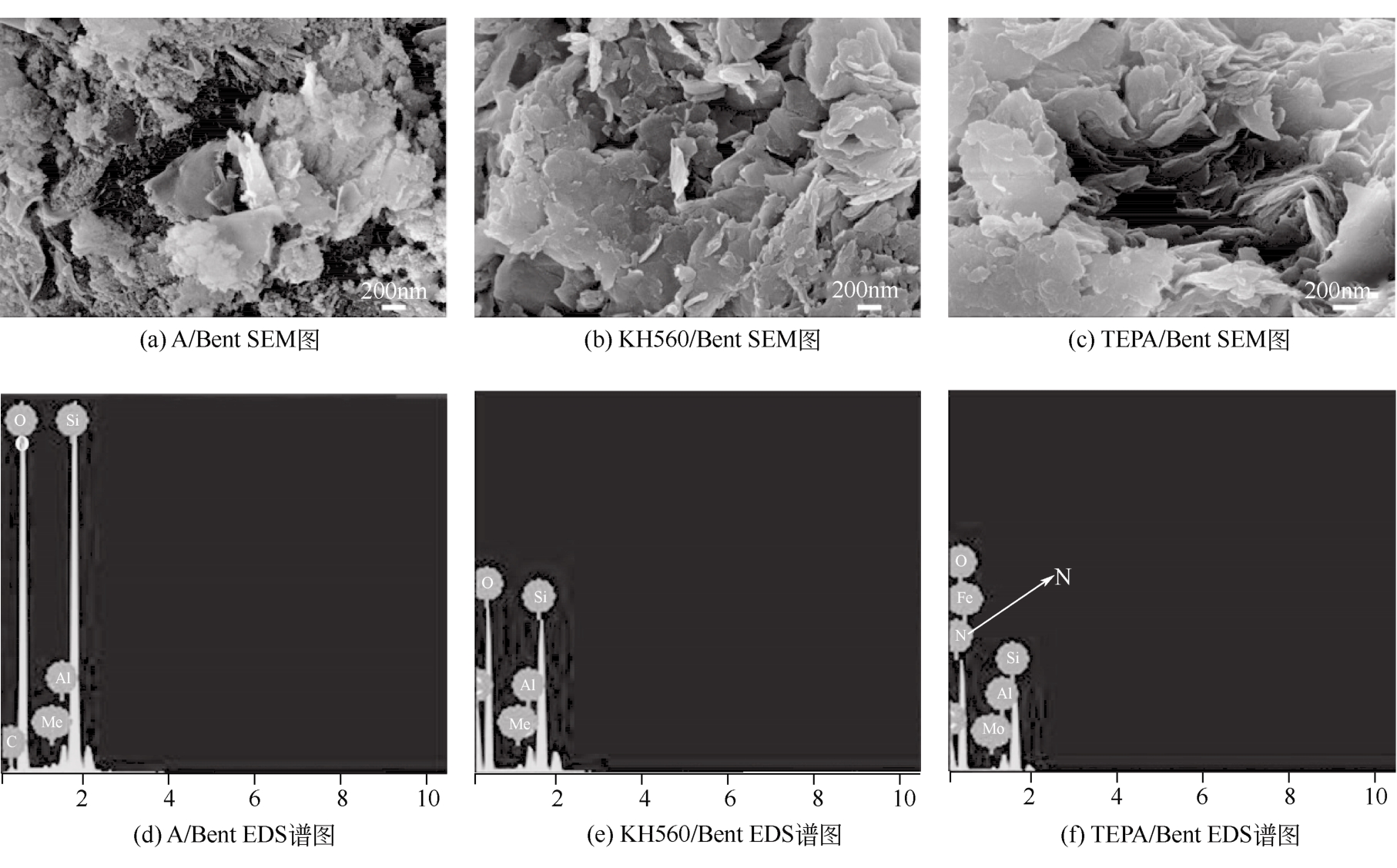
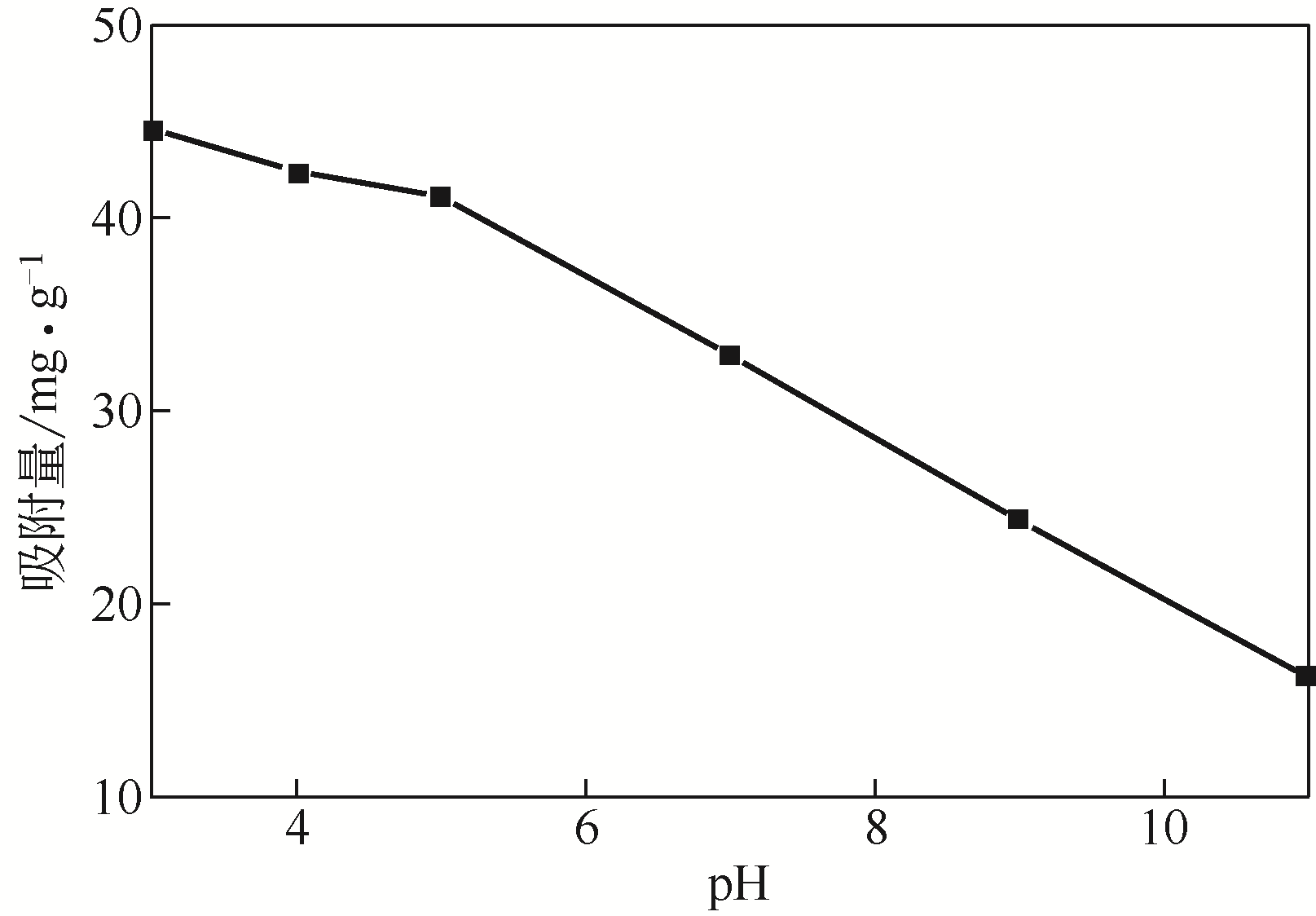
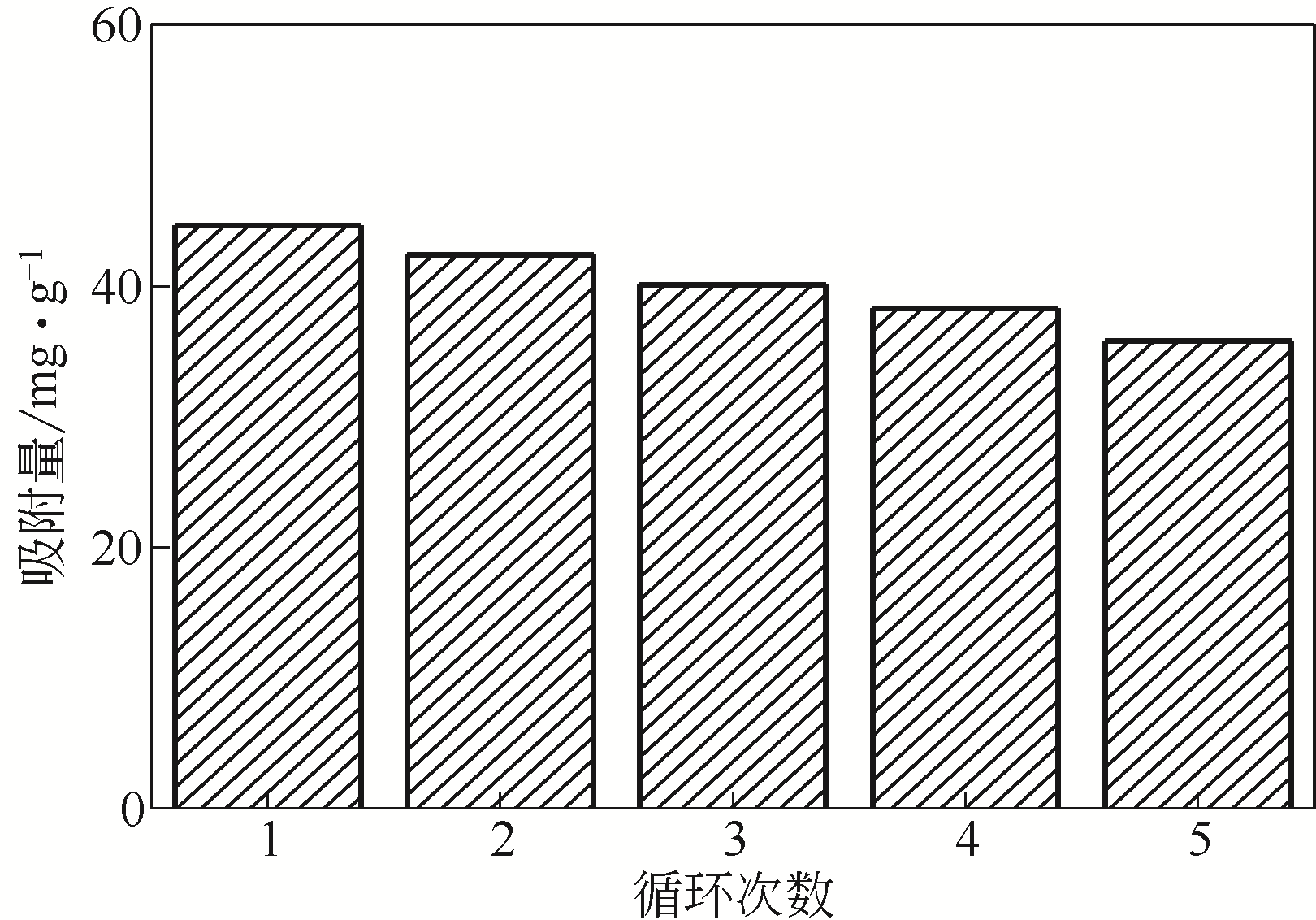
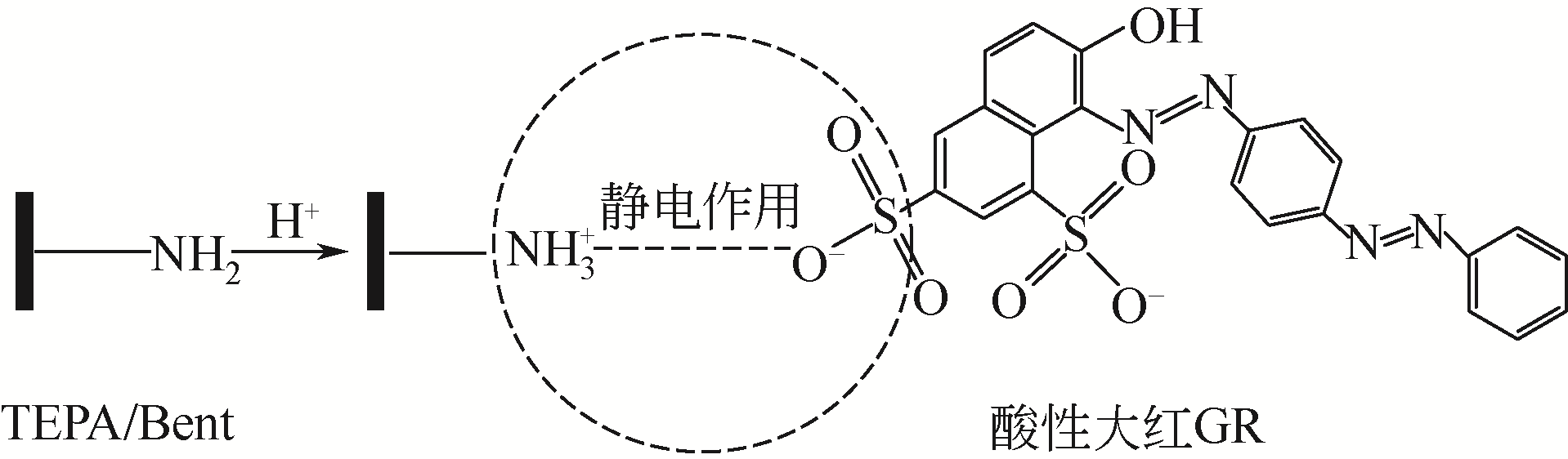
在膨润土(Bent)表面接枝四乙烯五胺(TEPA)制备四乙烯五胺改性膨润土(TEPA/Bent),利用FTIR(红外光谱仪)、XRD(X射线衍射仪)、EA(元素分析)、SEM(扫描电镜)和EDS(能谱仪)对其进行表征分析,并考察对水体中阴离子染料酸性大红GR的吸附性能。结果表明:TEPA成功接枝于膨润土表面,提高了膨润土对酸性大红GR的吸附量;pH对TEPA/Bent表面电位和吸附量影响较大;随着初始pH的增大,TEPA/Bent的zeta电位由正变为负,对酸性大红GR吸附量减少;在pH=3,染料初始质量浓度为100mg/L条件下,TEPA/Bent对酸性大红GR的吸附量可达44.63mg/g;TEPA/Bent对酸性大红GR的吸附动力学符合准二级动力学模型;吸附等温线符合Langmuir吸附等温模型,为单分子层吸附;吸附热力学表明该吸附为自发吸热过程。吸附剂经过5次再生后,吸附量仍保持为初始80%以上。研究表明,TEPA/Bent是从水溶液中去除阴离子染料的潜在有效吸附剂。
中图分类号:
引用本文
孙志勇, 商铁林, 王金东, 马向荣. 四乙烯五胺接枝膨润土对酸性大红GR的吸附性能[J]. 化工进展, 2021, 40(5): 2873-2881.
SUN Zhiyong, SHANG Tielin, WANG Jindong, MA Xiangrong. Adsorption properties of acid scarlet GR by tetraethylenepentamine grafted bentonite[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2873-2881.
| 样品 | 元素质量分数/% | ||
|---|---|---|---|
| N | C | H | |
| A/Bent | 0 | 0.97 | 0.51 |
| KH560/Bent | 0 | 12.73 | 2.41 |
| TEPA/Bent | 2.07 | 15.26 | 3.06 |
表1 吸附材料元素分析
| 样品 | 元素质量分数/% | ||
|---|---|---|---|
| N | C | H | |
| A/Bent | 0 | 0.97 | 0.51 |
| KH560/Bent | 0 | 12.73 | 2.41 |
| TEPA/Bent | 2.07 | 15.26 | 3.06 |
| C0/mg·L-1 | 准一级吸附速率方程 | 准二级吸附速率方程 | 颗粒内扩散模型 | |||||
|---|---|---|---|---|---|---|---|---|
| qe/mg·g-1 | K1/min-1 | R2 | qe/mg·g-1 | K2/g·mg-1·min-1 | R2 | Kp/mg·g-1·min-0.5 | R2 | |
| 100 | 54.20 | 0.092 | 0.9555 | 46.71 | 1.963×10-3 | 0.9904 | 3.47 | 0.7591 |
| 200 | 59.10 | 0.076 | 0.9334 | 54.61 | 1.383×10-3 | 0.9928 | 4.38 | 0.8227 |
表2 TEPA/Bent吸附酸性大红GR的动力学模型参数
| C0/mg·L-1 | 准一级吸附速率方程 | 准二级吸附速率方程 | 颗粒内扩散模型 | |||||
|---|---|---|---|---|---|---|---|---|
| qe/mg·g-1 | K1/min-1 | R2 | qe/mg·g-1 | K2/g·mg-1·min-1 | R2 | Kp/mg·g-1·min-0.5 | R2 | |
| 100 | 54.20 | 0.092 | 0.9555 | 46.71 | 1.963×10-3 | 0.9904 | 3.47 | 0.7591 |
| 200 | 59.10 | 0.076 | 0.9334 | 54.61 | 1.383×10-3 | 0.9928 | 4.38 | 0.8227 |
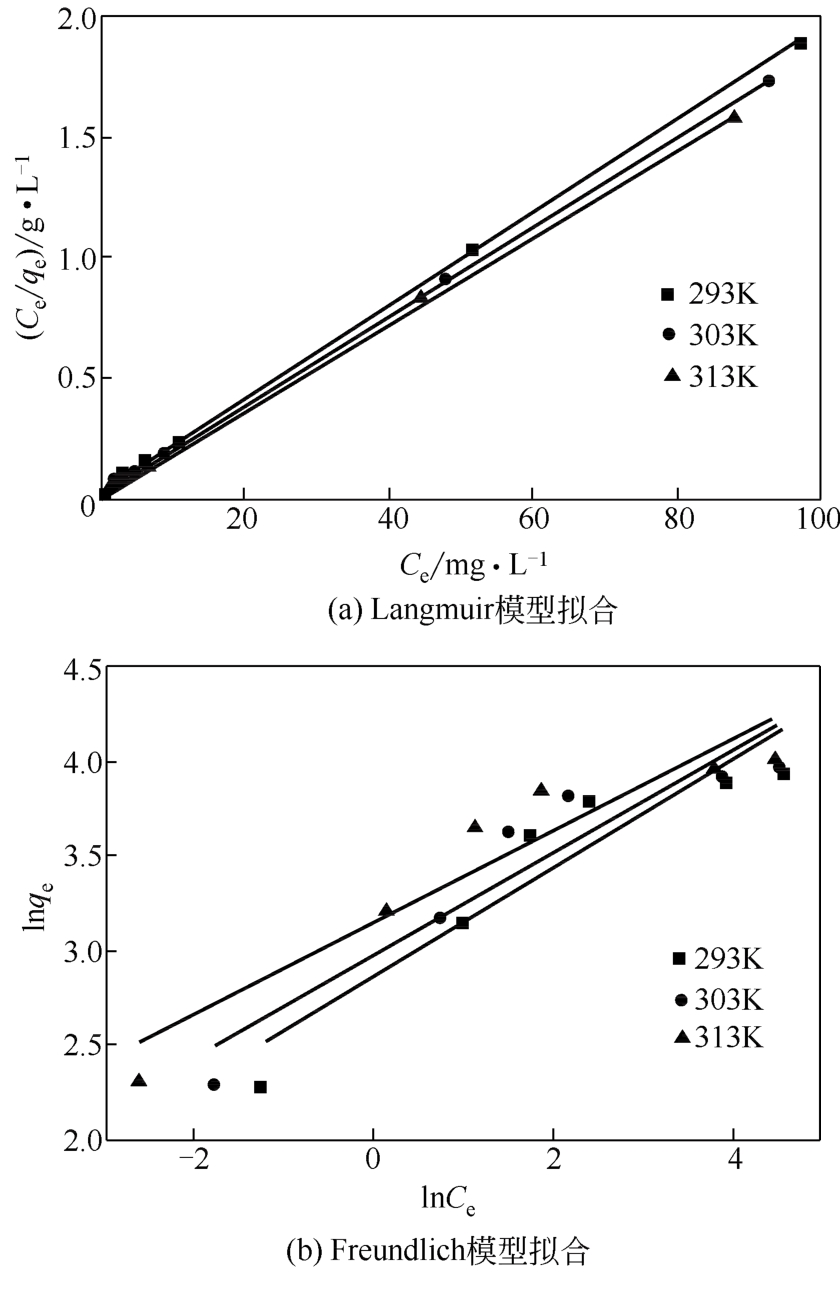
| T/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
qm /mg·g-1 | KL /L·mg-1 | R2 | KF /L·mg-1 | 1/n | R2 | |
| 293 | 52.41 | 0.4395 | 0.9995 | 17.62 | 0.2828 | 0.8545 |
| 303 | 54.29 | 0.5384 | 0.9995 | 19.70 | 0.2686 | 0.8668 |
| 313 | 56.05 | 0.7683 | 0.9994 | 23.14 | 0.2420 | 0.8768 |
表3 不同温度下的Langmuir和Freundlich模型参数
| T/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
qm /mg·g-1 | KL /L·mg-1 | R2 | KF /L·mg-1 | 1/n | R2 | |
| 293 | 52.41 | 0.4395 | 0.9995 | 17.62 | 0.2828 | 0.8545 |
| 303 | 54.29 | 0.5384 | 0.9995 | 19.70 | 0.2686 | 0.8668 |
| 313 | 56.05 | 0.7683 | 0.9994 | 23.14 | 0.2420 | 0.8768 |
| 吸附剂 | 染料 | 浓度 /mg·L-1 | 吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| CTAB改性磁性膨润土 | 酸性大红 | 600 | 48.00 | [ |
| 酸化膨润土 | 酸性大红 | 400 | 17.00 | [ |
| 聚环氧氯丙烷二甲胺改性膨润土 | 酸性大红GR | — | 51.79 | [ |
| 四乙烯五胺接枝膨润土 | 酸性大红GR | 200 | 55.66 | 本研究 |
表4 本研究与其他改性膨润土吸附性能比较
| 吸附剂 | 染料 | 浓度 /mg·L-1 | 吸附量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| CTAB改性磁性膨润土 | 酸性大红 | 600 | 48.00 | [ |
| 酸化膨润土 | 酸性大红 | 400 | 17.00 | [ |
| 聚环氧氯丙烷二甲胺改性膨润土 | 酸性大红GR | — | 51.79 | [ |
| 四乙烯五胺接枝膨润土 | 酸性大红GR | 200 | 55.66 | 本研究 |
| T/K | ΔG0/kJ·mol-1 | ΔH0/kJ·mol-1 | ΔS0/J·mol-1 |
|---|---|---|---|
| 293 | -3.47 | ||
| 303 | -4.18 | 21.12 | 83.78 |
| 313 | -5.15 |
表5 吸附热力学参数
| T/K | ΔG0/kJ·mol-1 | ΔH0/kJ·mol-1 | ΔS0/J·mol-1 |
|---|---|---|---|
| 293 | -3.47 | ||
| 303 | -4.18 | 21.12 | 83.78 |
| 313 | -5.15 |
| 1 | VAKILI M, RAFATULLAH M, SALAMATINIA B, et al. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: a review[J]. Carbohydrate Polymers, 2014, 113: 115-130. |
| 2 | ZHU L F, WANG L H, XU Y. Chitosan and surfactant co-modified montmorillonite: a multifunctional adsorbent for contaminant removal[J]. Applied Clay Science, 2017, 146: 35-42. |
| 3 | PRIYADARSHINI A, TAY S W, ONG P J, et al. Zeolite Y-carbonaceous composite membrane with a pseudo solid foam structure assessed by nanofiltration of aqueous dye solutions[J]. Journal of Membrane Science, 2018, 567: 146-156. |
| 4 | 王会丽, 赵越, 马乐宽, 等. 复合改性膨胀石墨的制备及对酸性艳蓝染料的吸附[J]. 高等学校化学学报, 2016, 37(2): 335-341. |
| WANG Huili, ZHAO Yue, MA Lekuan, et al. Preparation of composite modified expanded graphite and its adsorption on acid brilliant blue dye[J]. Chemical Journal of Chinese Universities, 2016, 37(2): 335-341. | |
| 5 | NEKOUEI MARNANI N, SHAHBAZI A. A novel environmental-friendly nanobiocomposite synthesis by EDTA and chitosan functionalized magnetic grapheme oxide for high removal of Rhodamine B: adsorption mechanism and separation property[J]. Chemosphere, 2019, 218: 715-725. |
| 6 | ZHANG C, LI Y Y, LI Y J, et al. Synthesis and Zn(Ⅱ) modification of hierarchical porous carbon materials from petroleum pitch for effective adsorption of organic dyes[J]. Chemosphere, 2019, 216: 379-386. |
| 7 | CERRÓN-CALLE G A, ARANDA-AGUIRRE A J, LUYO C, et al. Photoelectrocatalytic decolorization of azo dyes with nano-composite oxide layers of ZnO nanorods decorated with Ag nanoparticles[J]. Chemosphere, 2019, 219: 296-304. |
| 8 | KARIMIFARD S, ALAVI MOGHADDAM M R. Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review[J]. Science of the Total Environment, 2018, 640/641: 772-797. |
| 9 | RATOVA M, REDFERN J, VERRAN J, et al. Highly efficient photocatalytic bismuth oxide coatings and their antimicrobial properties under visible light irradiation[J]. Applied Catalysis B: Environmental, 2018, 239: 223-232. |
| 10 | KURTAN U, AMIR M, YILDIZ A, et al. Synthesis of magnetically recyclable MnFe2O4@SiO2@Ag nanocatalyst: its high catalytic performances for azo dyes and nitro compounds reduction[J]. Applied Surface Science, 2016, 376: 16-25. |
| 11 | MOHEBALI S, BASTANI D, SHAYESTEH H. Equilibrium, kinetic and thermodynamic studies of a low-cost biosorbent for the removal of Congo red dye: acid and CTAB-acid modified celery (Apium graveolens)[J]. Journal of Molecular Structure, 2019, 1176: 181-193. |
| 12 | LI Y, ZHOU K, HE M, et al. Synthesis of ZIF-8 and ZIF-67 using mixed-base and their dye adsorption[J]. Microporous and Mesoporous Materials, 2016, 234: 287-292. |
| 13 | 李芳郴, 罗岳平, 陈镇, 等. 镉和磷酸根离子在铝柱撑膨润土上的协同吸附[J]. 环境工程学报, 2015, 9(11): 5482-5486. |
| LI Fangchen, LUO Yueping, CHEN Zhen, et al. Collaborative sorption of cadmium and phosphate ions to hydroxyaluminum pillared bentonites[J]. Chinese Journal of Environmental Engineering, 2015, 9(11): 5482-5486. | |
| 14 | 张连科, 刘心宇, 王维大, 等. 镧柱撑膨润土对铅的吸附特性[J]. 环境污染与防治, 2018, 40(4): 435-439, 444. |
| ZHANG Lianke, LIU Xinyu, WANG Weida, et al. Adsorption properties of lead on lanthanum pillared bentonite[J]. Environmental Pollution & Prevention, 2018, 40(4): 435-439, 444. | |
| 15 | 汤睿, 张寒冰, 施华珍, 等. CTAB改性磁性膨润土对刚果红和酸性大红的吸附[J]. 高校化学工程学报, 2019, 33(3):748-757. |
| TANG Rui, ZHANG Hanbing, SHI Huazhen, et al. Adsorption of Congo Red and Acidic Red by magnetic bentonite modified with CTAB[J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33 (3): 748-757. | |
| 16 | 肖讴, 陈兵, 杨志泉. CTAC改性膨润土吸附去除水体中高氯酸盐的离子交换性能研究[J]. 环境科学学报, 2013, 33(2): 415-423. |
| XIAO Ou, CHEN Bing, YANG Zhiquan. Bentonite modified with cetyltrimethylammonium chloride as highly efficient anion exchanger for perchlorate ion[J]. Acta Scientiae Circumstantiae, 2013, 33(2): 415-423. | |
| 17 | JING P, HOU M F, ZHAO P, et al. Adsorption of 2-mercaptobenzothiazole from aqueous solution by organo-bentonite[J]. Journal of Environmental Sciences, 2013, 25(6): 1139-1144. |
| 18 | 陈平, 曹晓强, 张燕. 有机膨润土对阴离子和非离子染料的吸附研究[J]. 水处理技术, 2016, 42(8): 74-78. |
| CHEN Ping, CAO Xiaoqiang, ZHANG Yan. Adsorption of anionic and nonionic dyes onto organo-modified bentonite[J]. Technology of Water Treatment, 2016, 42(8): 74-78. | |
| 19 | ZHANG L J, HU P, WANG J, et al. Adsorption of Amido Black 10B from aqueous solutions onto Zr(Ⅳ) surface-immobilized cross-linked chitosan/bentonite composite[J]. Applied Surface Science, 2016, 369: 558-566. |
| 20 | 邵红, 程慧, 李佳琳. 负载壳聚糖膨润土的制备及其吸附性能的影响[J]. 环境工程学报, 2009, 3(9): 1597-1601. |
| SHAO Hong, CHENG Hui, LI Jialin. Preparation of bentonite coated chitosan and its adsorptive properties[J]. Chinese Journal of Environmental Engineering, 2009, 3(9): 1597-1601. | |
| 21 | CALAGUI M J C, SENORO D B, KAN C C, et al. Adsorption of indium(Ⅲ) ions from aqueous solution using chitosan-coated bentonite beads[J]. Journal of Hazardous Materials, 2014, 277(30): 120-126. |
| 22 | 周娟, 张寒冰, 童张法, 等. 亚甲基蓝在有机酸膨润土上的吸附行为[J]. 环境工程学报, 2015, 9(3): 1057-1061. |
| ZHOU Juan, ZHANG Hanbing, TONG Zhangfa, et al. Adsorption behavior of methylene blue on citric acid bentonite[J]. Chinese Journal of Environmental Engineering, 2015, 9(3): 1057-1061. | |
| 23 | 覃岳隆, 张寒冰, 陈宁华, 等. 乙酸膨润土对孔雀石绿的吸附去除[J]. 化工进展, 2016, 35(3): 944-949. |
| QIN Yuelong, ZHANG Hanbing, CHEN Ninghua, et al. Malachite green adsorption on acetic acid bentonite[J]. Chemical Industry and Engineering Progress, 2016, 35(3): 944-949. | |
| 24 | 陈焕利, 邢宝林, 谌伦建, 等. 有机膨润土对模拟废水中苯酚的吸附特性[J]. 化工进展, 2017, 36(2): 735-741. |
| CHEN Huanli, XING Baolin, CHEN Lunjian, et al. Adsorption of phenol from simulated wastewater by organic bentonite[J]. Chemical Industry and Engineering Progress, 2017, 36(2): 735-741. | |
| 25 | 施华珍, 刘坤, 汤睿, 等. 有机改性磁性碱性钙基膨润土的制备及对Cu(Ⅱ)和Mn(Ⅱ)的吸附[J]. 化工进展, 2018, 37(11): 4509-4521. |
| SHI Huazhen, LIU Kun, TANG Rui, et al. Organically modification of magnetic alkaline Ca-bentonite and its adsorption for Cu(Ⅱ) and Mn(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4509-4521. | |
| 26 | ZAWRAH M F, KHATTAB R M, SAAD E M, et al. Effect of surfactant types and their concentration on the structural characteristics of nanoclay[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 122: 616-623. |
| 27 | 姚培, 李树白, 刘媛, 等. HTMAC/KH550/膨润土吸附剂的制备、表征及其在甲基橙废水的吸附行为[J]. 化工进展, 2017, 36(4): 1374-1380. |
| YAO Pei, LI Shubai, LIU Yuan, et al. Preparation, characterization of HTMAC/KH550/bentonite adsorbent and its adsorption behavior on methyl orange wastewater[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1374-1380. | |
| 28 | PARK Y, AYOKO G A, KURDI R, et al. Adsorption of phenolic compounds by organoclays: implications for the removal of organic pollutants from aqueous media[J]. Journal of Colloid and Interface Science, 2013, 406: 196-208. |
| 29 | GAPUSAN R B, BALELA M D L. Adsorption of anionic methyl orange dye and lead(Ⅱ) heavy metal ion by polyaniline-kapok fiber nanocomposite[J]. Materials Chemistry and Physics, 2020, 243: 122682. |
| 30 | LIU C, JIN R N, OUYANG X K, et al. Adsorption behavior of carboxylated cellulose nanocrystal-polyethyleneimine composite for removal of Cr(Ⅵ) ions[J]. Applied Surface Science, 2017, 408: 77-87. |
| 31 | CHEN Q Z, ZHU R L, ZHU Y P, et al. Adsorption of polyhydroxy fullerene on polyethylenimine-modified montmorillonite[J]. Applied Clay Science, 2016, 132/133: 412-418. |
| 32 | HORRI N, SANZ-PEREZ E S, ARENCIBIA A, et al. Amine grafting of acid-activated bentonite for carbon dioxide capture[J]. Applied Clay Science, 2019, 180: 105195. |
| 33 | 王姝凡, 徐卫华, 刘云国, 等. 壳聚糖/改性平菇复合吸附剂对Cr(Ⅵ)的吸附特性[J]. 中国环境科学, 2019, 39(8): 3264-3270. |
| WANG Shufan, XU Weihua, LIU Yunguo, et al. Characteristics of Cr(Ⅵ) removal by cross-linked chitosan/tartaric acid modified Pleurotus ostreatus composite adsorbent[J]. China Environmental Science, 2019, 39(8): 3264-3270. | |
| 34 | SHAN R R, YAN L G, YANG Y K, et al. Adsorption of Cd(Ⅱ) by Mg-Al-CO3- and magnetic Fe3O4/Mg-Al-CO3-layered double hydroxides: kinetic, isothermal, thermodynamic and mechanistic studies[J]. Journal of Hazardous Materials, 2015, 299: 42-49. |
| 35 | SALEH T A, MUSA A M, ALI S A. Synthesis of hydrophobic cross-linked polyzwitterionic acid for simultaneous sorption of Eriochrome Black T and chromium ions from binary hazardous waters[J]. Journal of Colloid and Interface Science, 2016, 468: 324-333. |
| 36 | MU'AZU N D, JARRAH N, KAZEEM T S, et al. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T[J]. Applied Clay Science, 2018, 161: 23-34. |
| 37 | LIU Y, GAO Q, LI C, et al. Effective coating of crosslinked polyethyleneimine on elastic spongy monolith for highly efficient batch and continuous flow adsorption of Pb(Ⅱ) and Acidic Red 18[J]. Chemical Engineering Journal, 2020, 391: 123610. |
| 38 | BASKARALINGAM P, PULIKESI M, ELANGO D, et al. Adsorption of acid dye onto organobentonite[J]. Journal of Hazardous Materials, 2006, 128(2/3): 138-144. |
| 39 | 王喜全, 王玲玲, 张秋霞, 等. 改性膨润土处理酸性大红的实验研究[J]. 环境科学与管理, 2012, 37(7): 85-87, 105. |
| WANG Xiquan, WANG Lingling, ZHANG Qiuxia, et al. Study on treatment of acid brilliant scarlet GR with modified bentonite[J]. Environmental Science and Management, 2012, 37(7): 85-87, 105. | |
| 40 | LI Q, YUE Q Y, SU Y, et al. Equilibrium, thermodynamics and process design to minimize adsorbent amount for the adsorption of acid dyes onto cationic polymer-loaded bentonite[J]. Chemical Engineering Journal, 2010, 158: 489-497. |
| 41 | TANG Y L, LI M H, MU C H, et al. Ultrafast and efficient removal of anionic dyes from wastewater by polyethyleneimine-modified silica nanoparticles[J]. Chemosphere, 2019, 229: 570-579. |
| 42 | SU J Z, HE S, ZHAO Z G, et al. Efficient preparation of cetyltrimethylammonium bromide-graphene oxide composite and its adsorption of congo red from aqueous solutions[J]. Colloids and Surfaces A, 2018, 554: 227-236. |
| 43 | JAVADIAN H, ANGAJI M T, NAUSHAD M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: a comparative study for the adsorption of three different anionic dyes[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3890-3900. |
| 44 | LIN Q W, GAO M F, CHANG J L, et al. Adsorption properties of crosslinking carboxymethyl cellulose grafting dimethyldiallylammonium chloride for cationic and anionic dyes[J]. Carbohydrate Polymers, 2016, 151: 283-294. |
| 45 | SONG W, GAO B Y, XU X, et al. Adsorption-desorption behavior of magnetic amine/Fe3O4 functionalized biopolymer resin towards anionic dyes from wastewater[J]. Bioresource Technology, 2016, 210: 123-130. |
| 46 | HUO Y X, WU H, WANG Z L, et al. Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the efficient adsorption of anionic dyes[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 549: 174-183. |
| 47 | WONG S, TUMARI H H, NGADI N, et al. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL) [J]. Journal of Cleaner Production, 2019, 206: 394-406. |
| 48 | FU J W, ZHU J H, WANG Z W, et al. Highly-efficient and selective adsorption of anionic dyes onto hollow polymer microcapsules having a high surface-density of amino groups: Isotherms, kinetics, thermodynamics and mechanism[J]. Journal of Colloid and Interface Science, 2019, 542: 123-135. |
| 49 | XU J, DU P F, BI W D, et al. Graphene oxide aerogels co-functionalized with polydopamine and polyethylenimine for the adsorption of anionic dyes and organic solvents[J]. Chemical Engineering Research and Design, 2020, 154: 192-202. |
| 50 | NORDIN A H, WONG S, NGADI N, et al. Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104639. |
| 51 | LIU Q, YANG B C, ZHANG L J, et al. Adsorption of an anionic azo dye by cross-linked chitosan/bentonite composite[J]. International Journal of Biological Macromolecules, 2015, 72: 1129-1135. |
| 52 | AIN Q U, RASHEED U, YASEEN M, et al. Fabrication of magnetically separable 3-acrylamidopropyltrimethylammonium chloride intercalated bentonite composite for the efficient adsorption of cationic and anionic dyes[J]. Applied Surface Science, 2020, 514: 145929. |
| 53 | CHENG J, SHI L, LU J. Amino ionic liquids-modified magnetic core/shell nanocomposite as an efficient adsorbent for dye removal[J]. Journal of Industrial and Engineering Chemistry, 2016, 36: 206-214. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||