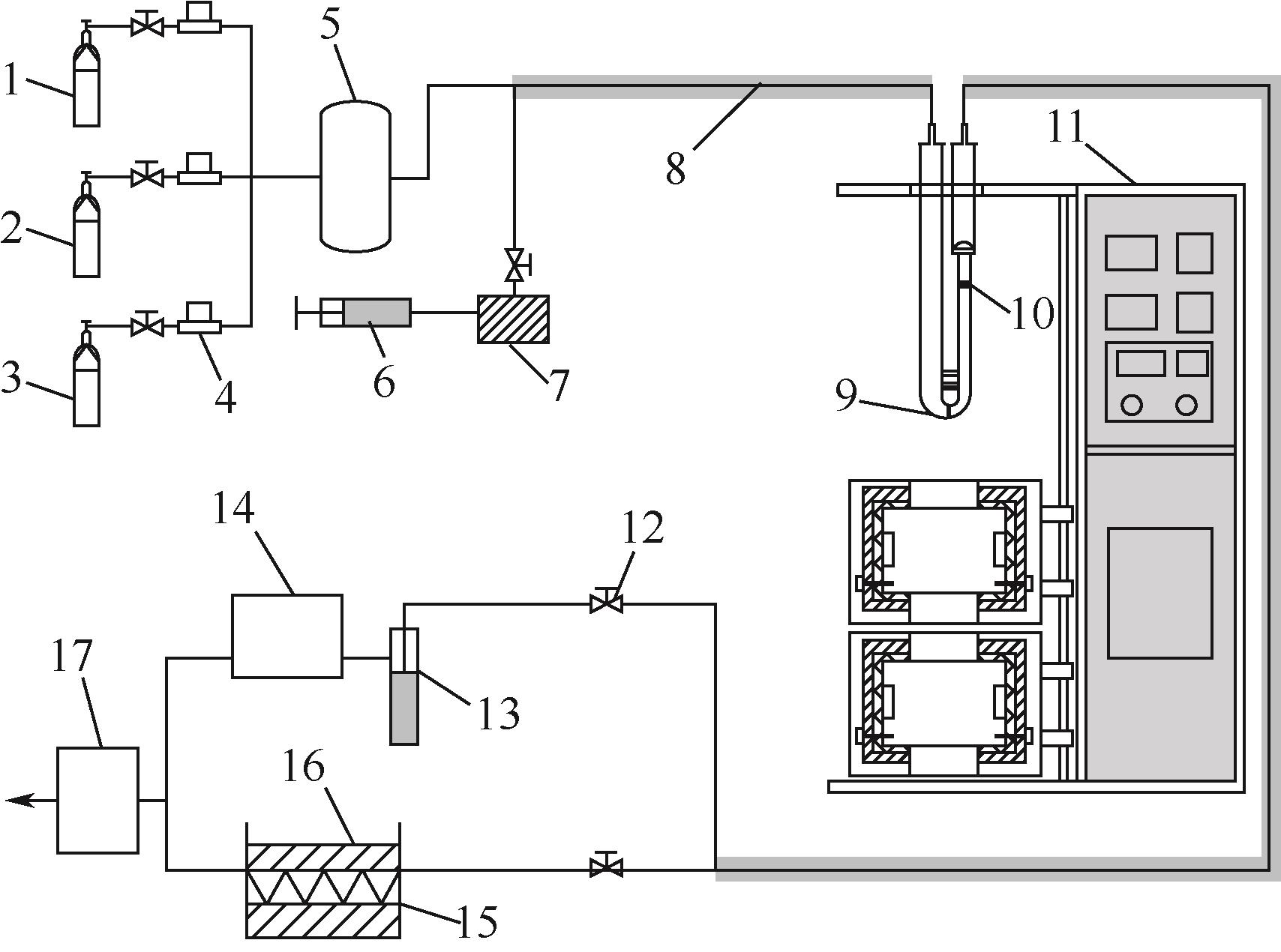
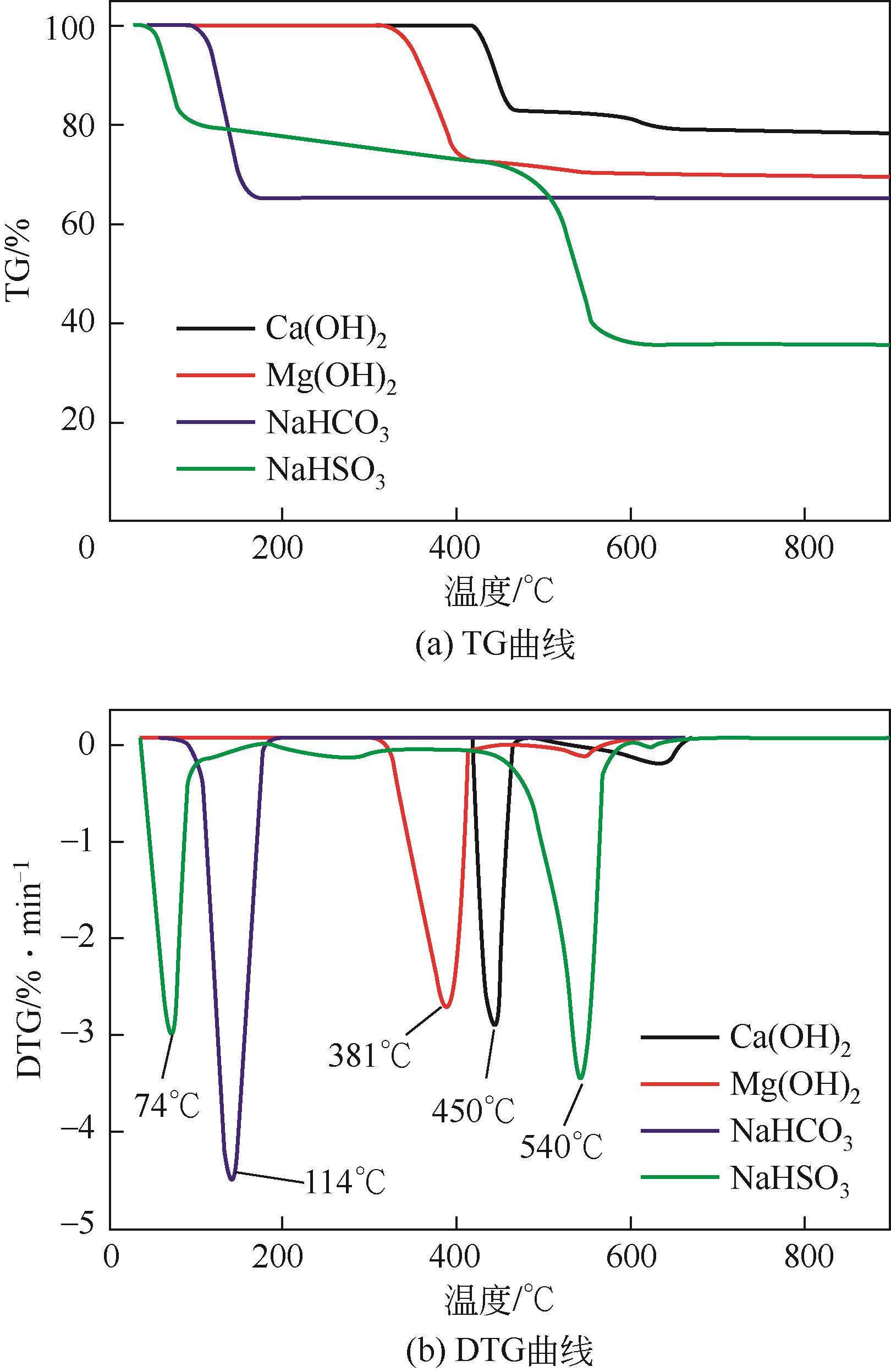
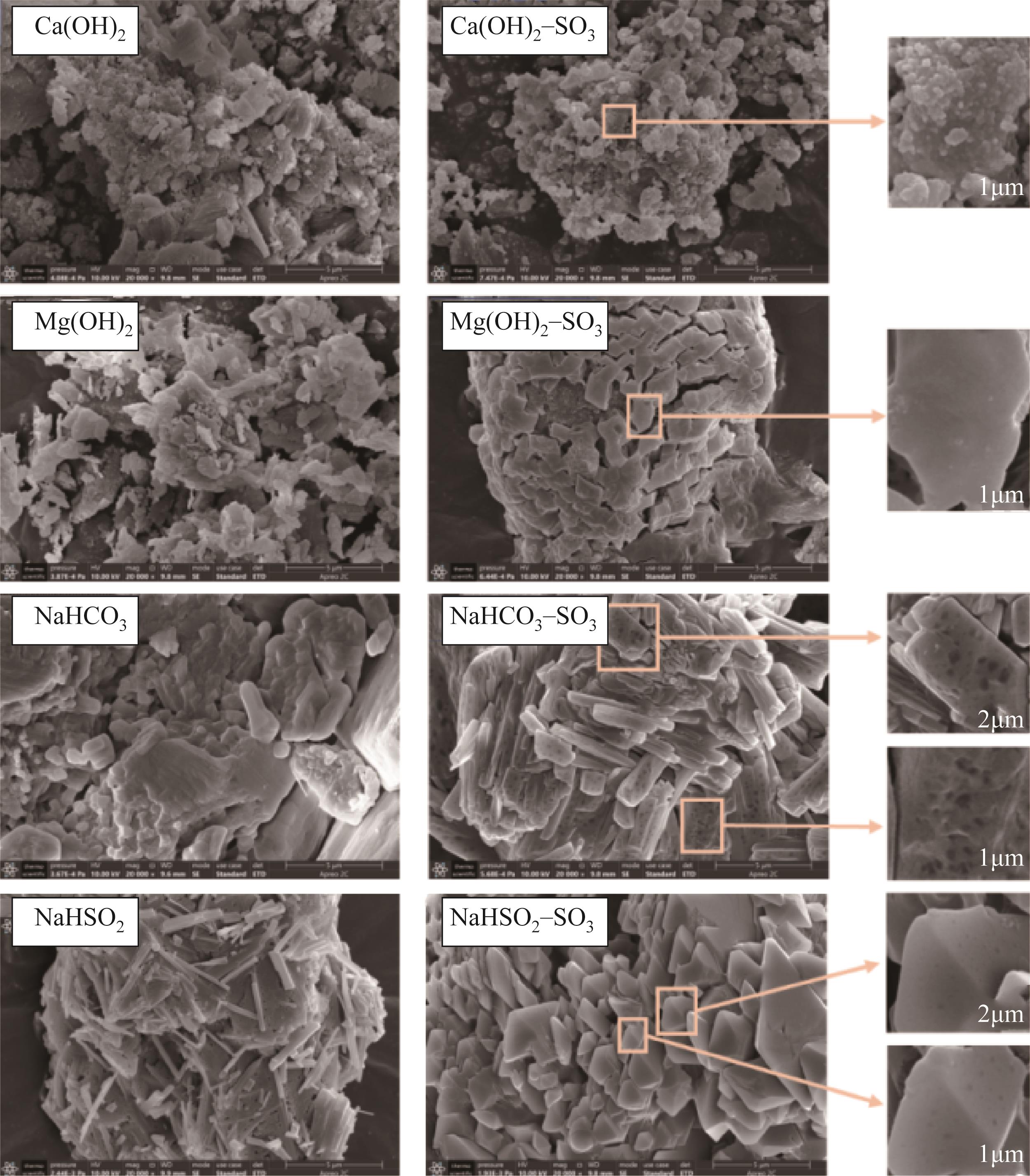
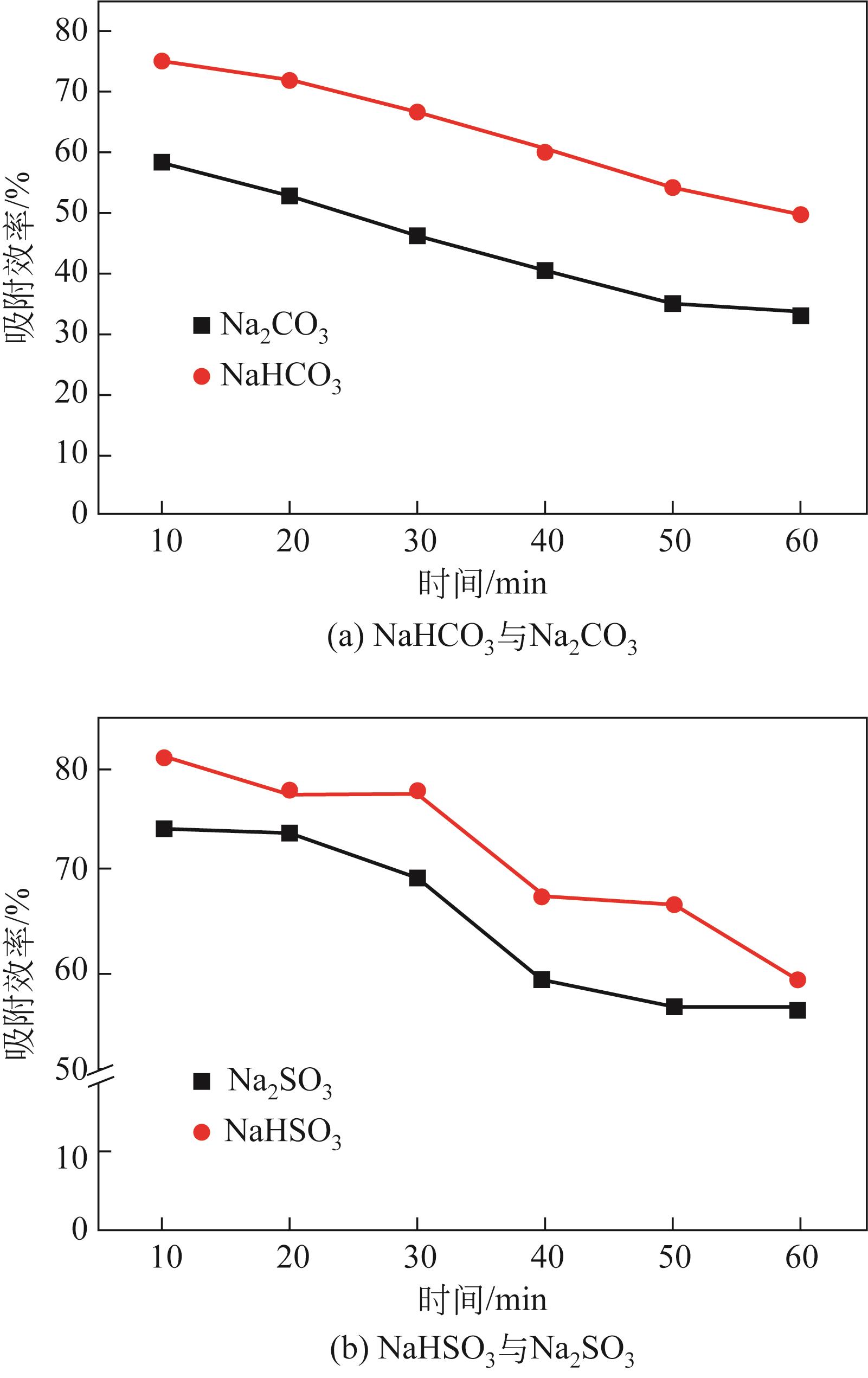
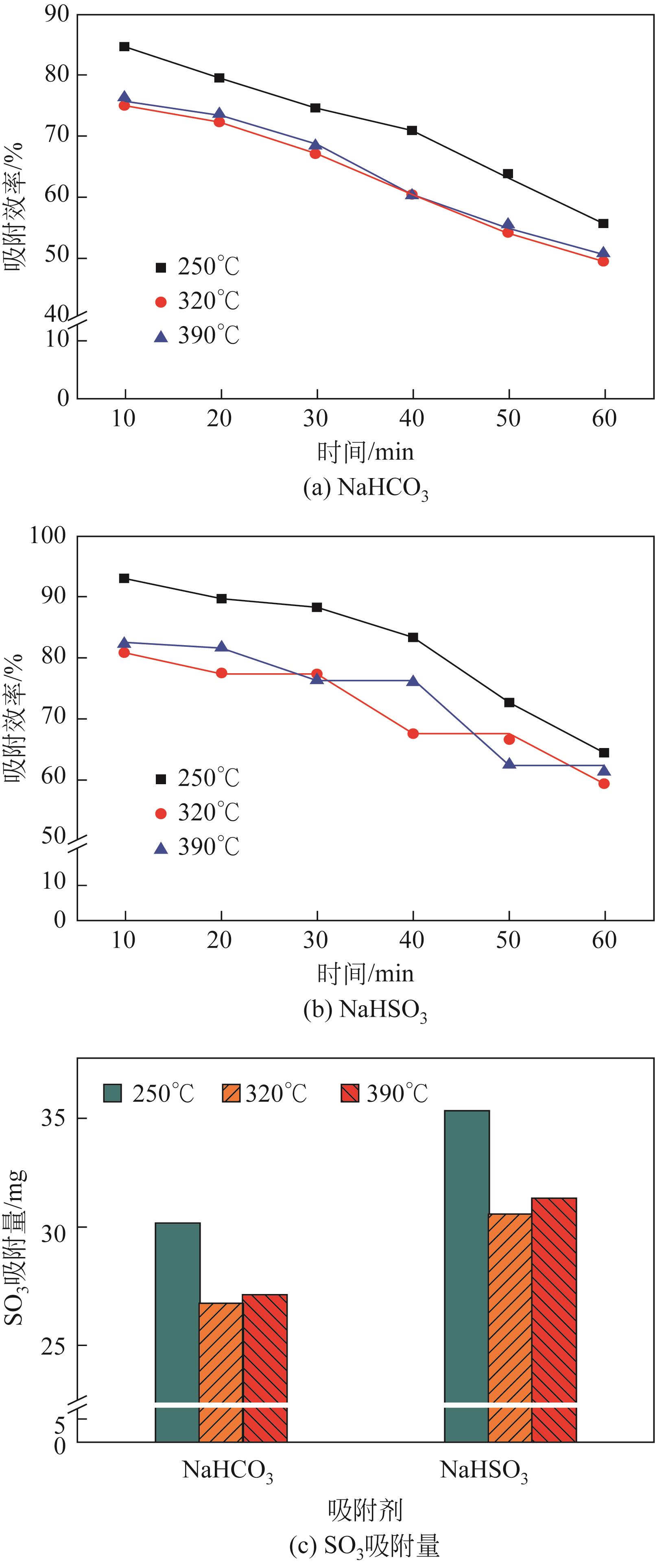
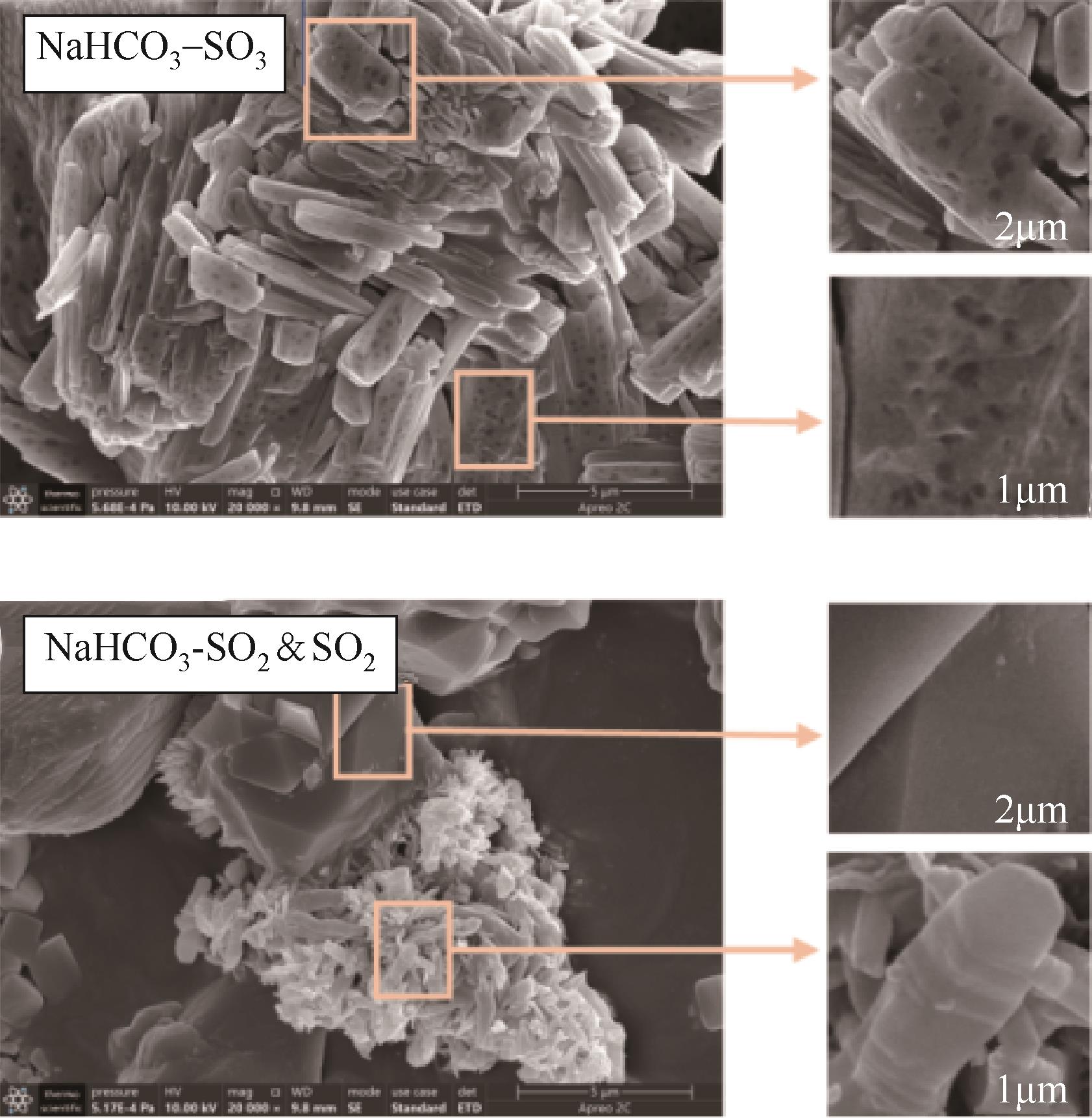
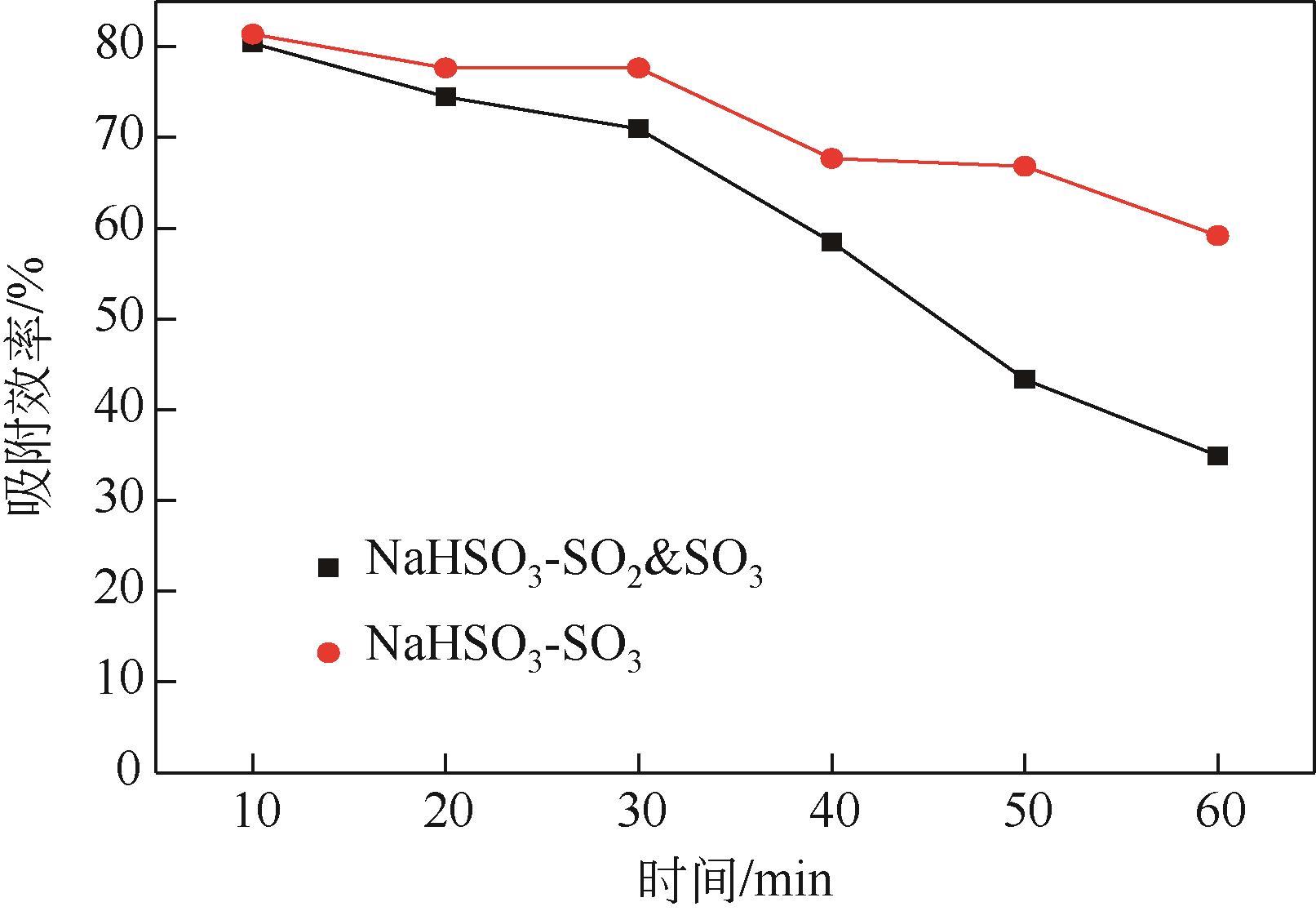
| 1 |
Reinhold SPÖRL, WALKER Johannes, BELO Lawrence, et al. SO3 emissions and removal by ash in coal-fired oxy-fuel combustion[J]. Energy & Fuels, 2014, 28(8): 5296-5306.
|
| 2 |
LU J, ZHOU Z, ZHANG H, et al. Influenced factors study and evaluation for SO2/SO3 conversion rate in SCR process[J]. Fuel, 2019, 245: 528-533.
|
| 3 |
HE K, TANG Z, SONG Q, et al. Process analysis of SO3 removal by Ca(OH)2 particles from flue gas[J]. Chemical Engineering Science, 2022, 247: 117054.
|
| 4 |
李欣怡, 潘丹萍, 胡斌, 等. 燃煤烟气中SO3迁移转化特性及其控制的研究现状及展望[J]. 化工进展, 2018, 37(12): 4887-4896.
|
|
LI Xinyi, PAN Danping, HU Bin, et al. Research status and prospects of migration, transformation and control of SO3 from coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4887-4896.
|
| 5 |
刘亚明, 束航, 徐齐胜, 等. SCR脱硝过程中SO2催化氧化的原位红外研究[J]. 燃料化学学报, 2015, 43(8): 1018-1024.
|
|
LIU Yaming, SHU Hang, XU Qisheng, et al. FT-IR study of the catalytic oxidation of SO2 during the process of selective catalytic reduction of NO with NH3 over commercial catalysts[J]. Journal of Fuel Chemistry and Technology, 2015, 43(8): 1018-1024.
|
| 6 |
YANG Zhengda, ZHENG Chenghang, ZHANG Xuefeng, et al. Highly efficient removal of sulfuric acid aerosol by a combined wet electrostatic precipitator[J]. RSC Advances, 2018, 8(1): 59-66.
|
| 7 |
HU B, ZHANG L, YI Y, et al. PM2.5 and SO3 collaborative removal in electrostatic precipitator[J]. Powder Technology, 2017, 318: 484-490.
|
| 8 |
XIANG Baixiang, ZHANG Man, WU Yuxin, et al. Experimental and modeling studies on sulfur trioxide of flue gas in a coal-fired boiler[J]. Energy & Fuels, 2017, 31(6): 6284-6297.
|
| 9 |
ZHENG C, WANG Y, LIU Y, et al. Formation, transformation, measurement, and control of SO3 in coal-fired power plants[J]. Fuel, 2019, 241: 327-346.
|
| 10 |
MITSUI Y, IMADA N, KIKKAWA H, et al. Study of Hg and SO3 behavior in flue gas of oxy-fuel combustion system[J]. International Journal of Greenhouse Gas Control, 2011, 5: S143-S150.
|
| 11 |
张道军, 马子然, 孙琦, 等. 硫酸氢铵在钒基选择性催化还原催化剂表面的生成、作用及防治[J]. 化工进展, 2018, 37(7): 2635-2643.
|
|
ZHANG Daojun, MA Ziran, SUN Qi, et al. Formation mechanism, effects and prevention of NH4HSO4 formed on the surface of V2O5 based catalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2635-2643.
|
| 12 |
FLEIG Daniel, ANDERSSON Klas, JOHNSSON Filip. Influence of operating conditions on SO3 formation during air and oxy-fuel combustion[J]. Industrial & Engineering Chemistry Research, 2012, 51(28): 9483-9491.
|
| 13 |
PAN Danping, GU Conghui, ZHANG Dongping, et al. Removal characteristics of sulfuric acid aerosols in the wet electrostatic precipitator system[J]. Energy & Fuels, 2019, 33(8): 7813-7818.
|
| 14 |
ZHENG Chenghang, HONG Yipan, XU Zhewei, et al. Experimental study on removal characteristics of SO3 by wet flue gas desulfurization absorber[J]. Energy & Fuels, 2018, 32(5): 6031-6038.
|
| 15 |
何柯佳, 郑娜, 宋蔷, 等. 钙基吸收剂脱除SO x 过程中SO3吸收量的测量方法[J]. 中国电机工程学报, 2019, 39(20): 5973-5978, 6177.
|
|
HE Kejia, ZHENG Na, SONG Qiang, et al. A novel method for the measurement of SO3 absorption amount by calcium based absorbent during SO x removal process[J]. Proceedings of the CSEE, 2019, 39(20): 5973-5978, 6177.
|
| 16 |
Dean THIBAULT J, STEWARD Frank R, RUTHVEN Douglas M. The kinetics of absorption of SO3 in calcium and magnesium oxides[J]. The Canadian Journal of Chemical Engineering, 1982, 60(6): 796-801.
|
| 17 |
GALLOWAY B D, SASMAZ E, PADAK B. Binding of SO3 to fly ash components: CaO, MgO, Na2O and K2O[J]. Fuel, 2015, 145: 79-83.
|
| 18 |
WANG Z, HU Y, CHENG X, et al. Study of adsorption characteristics of calcium-based sorbents with SO3 [J]. Energy Procedia, 2018, 144: 43-49.
|
| 19 |
何川, 宋玉宝, 马云龙, 等. 钙镁氧化物及氢氧化物脱除SO3协同防治催化剂低温失活[J]. 化工进展, 2020, 39(11): 4619-4624.
|
|
HE Chuan, SONG Yubao, MA Yunlong, et al. Synergetic control catalyst deactivation at low temperature through SO3 removal by calcium magnesium oxide and hydroxide[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4619-4624.
|
| 20 |
QI L Q, YUAN Y T. Influence of SO3 in flue gas on electrostatic precipitability of high-alumina coal fly ash from a power plant in China[J]. Powder Technology, 2013, 245: 163-167.
|
| 21 |
CAO Jun, ZHONG Wenqi, JIN Baosheng, et al. Treatment of hydrochloric acid in flue gas from municipal solid waste incineration with Ca-Mg-Al mixed oxides at medium-high temperatures[J]. Energy & Fuels, 2014, 28(6): 4112-4117.
|
| 22 |
HE Kejia, SONG Qiang, YAN Zhennan, et al. SO3 removal from flue gas by using Na2SO3 [J]. Energy & Fuels, 2020, 34(6): 7232-7241.
|
| 23 |
陈朋. 钙基吸收剂脱除燃煤烟气中SO3的研究[D]. 济南: 山东大学, 2011.
|
|
CHEN Peng. Research of SO3 removal from flue-gas by calcium-based absorbents[D]. Jinan: Shandong University, 2011.
|
| 24 |
ZHENG C, LUO C, LIU Y, et al. Experimental study on the removal of SO3 from coal-fired flue gas by alkaline sorbent[J]. Fuel, 2020, 259: 116306.
|
| 25 |
HE K, SONG Q, YAN Z, et al. Study on competitive absorption of SO3 and SO2 by calcium hydroxide[J]. Fuel, 2019, 242: 355-361.
|
| 26 |
RASMUSSEN M H, WEDEL S, PEDERSEN K H, et al. Initial reaction between CaO and SO2 under carbonating and non-carbonating conditions[J]. Chemical Engineering Science, 2015, 134: 169-177.
|
| 27 |
ZUO W, ZHANG X, LI Y, et al. Evaluation of the controlled condensation method for flue gas SO3/H2SO4 measurement[J]. Fuel Processing Technology, 2020, 206: 106461.
|
| 28 |
FANG F, LI Z S, CAI N S, et al. AFM investigation of solid product layers of MgSO4 generated on MgO surfaces for the reaction of MgO with SO2 and O2 [J]. Chemical Engineering Science, 2011, 66(6): 1142-1149.
|
| 29 |
SUN Z, LUO S, QI P, et al. Ionic diffusion through calcite (CaCO3) layer during the reaction of CaO and CO2 [J]. Chemical Engineering Science, 2012, 81: 164-168.
|
| 30 |
KEENER T C, KHANG S J. Kinetics of the sodium bicarbonate—Sulfur dioxide reaction[J]. Chemical Engineering Science, 1993, 48(16): 2859-2865.
|
 ), 黄亚继1(
), 黄亚继1( ), 许月阳2, 程好强1, 朱志成1, 李金壘1, 丁雪宇1, 王圣2, 张荣初3
), 许月阳2, 程好强1, 朱志成1, 李金壘1, 丁雪宇1, 王圣2, 张荣初3
 ), HUANG Yaji1(
), HUANG Yaji1( ), XU Yueyang2, CHENG Haoqiang1, ZHU Zhicheng1, LI Jinlei1, DING Xueyu1, WANG Sheng2, ZHANG Rongchu3
), XU Yueyang2, CHENG Haoqiang1, ZHU Zhicheng1, LI Jinlei1, DING Xueyu1, WANG Sheng2, ZHANG Rongchu3