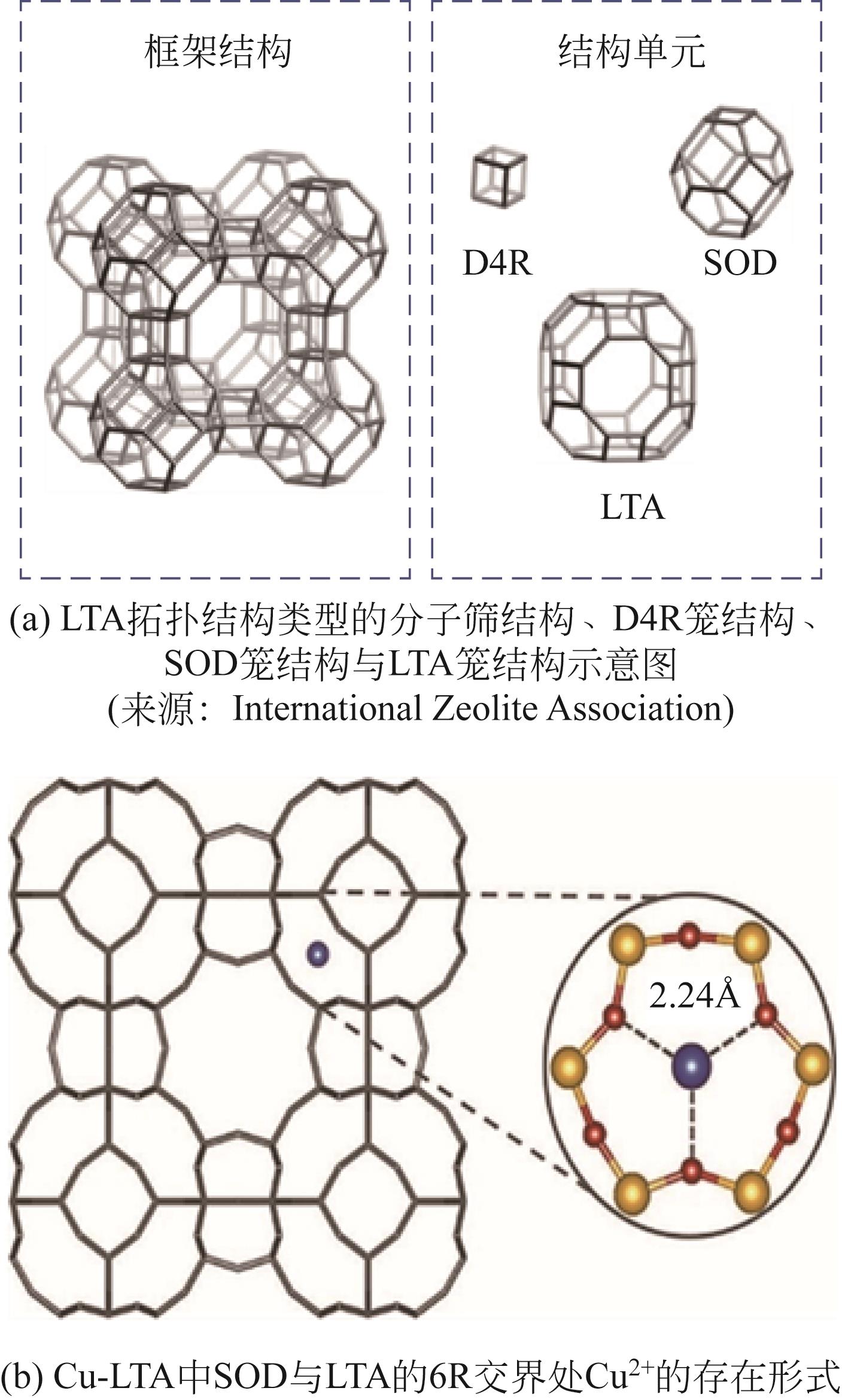
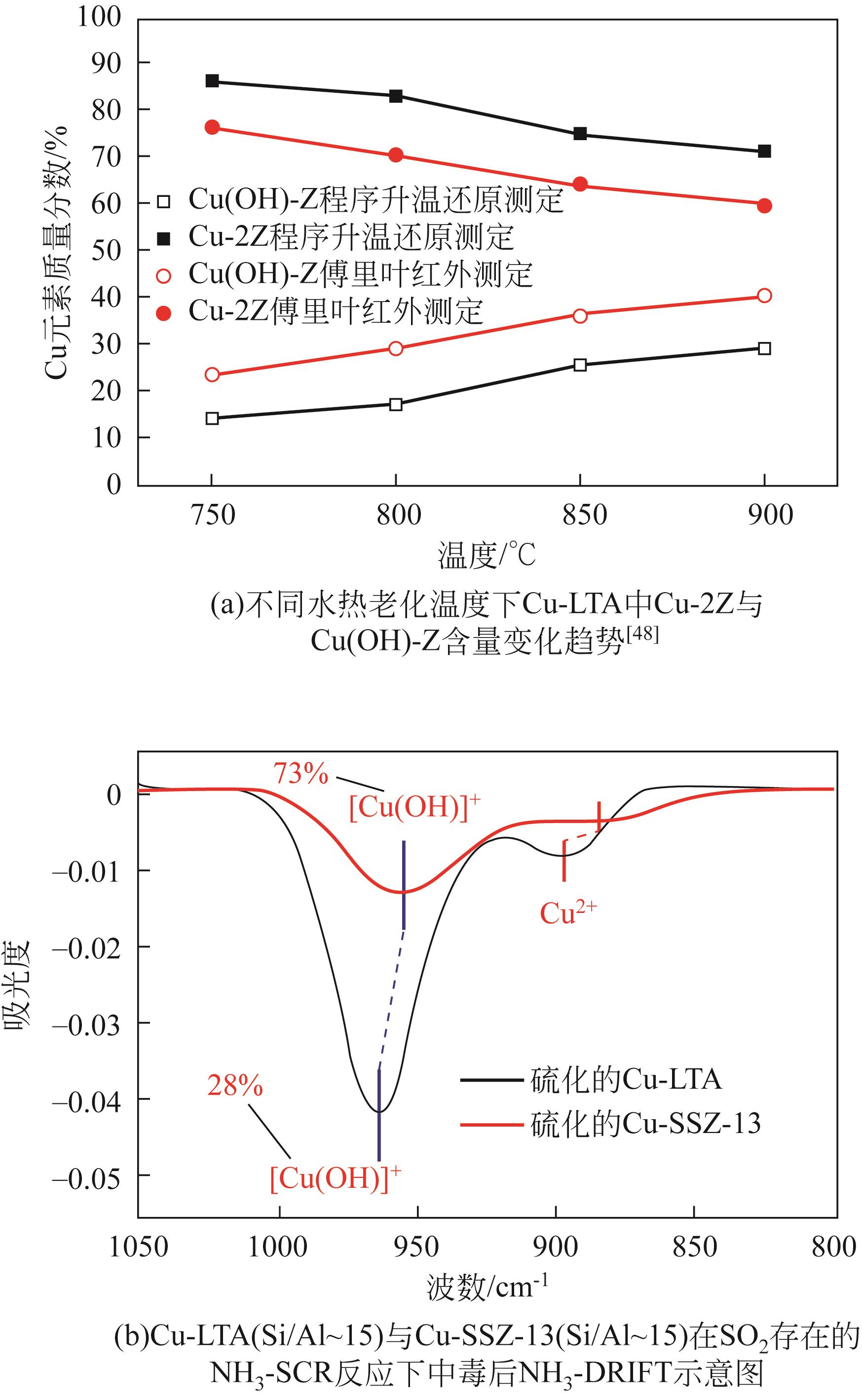
化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1321-1331.DOI: 10.16085/j.issn.1000-6613.2022-1006
柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展
张晨光1( ), 封硕1, 邢玉烨2, 沈伯雄1,2(
), 封硕1, 邢玉烨2, 沈伯雄1,2( ), 苏立超3
), 苏立超3
- 1.河北工业大学能源与环境工程学院,天津市清洁能源利用与污染物控制重点实验室,天津 300401
2.河北工业大学化工学院,天津 300401
3.邻筑(河北)工程产业技术研究有限公司,河北 保定 054001
-
收稿日期:2022-05-30修回日期:2022-10-24出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:沈伯雄 -
作者简介:张晨光(1999—),男,硕士研究生,研究方向为烟气中污染物控制。E-mail:2287588410@qq.com。 -
基金资助:国家自然科学基金联合资助项目(U20A20302);天津市重点研发项目(19ZXZSN00050);河北省重点研发项目(20373701D);河北省重大科技攻关项目(22183701Z)
Research progress of isolated Cu2+ in copper based zeolite NH3-SCR catalyst for diesel vehicles
ZHANG Chenguang1( ), FENG Shuo1, XING Yuye2, SHEN Boxiong1,2(
), FENG Shuo1, XING Yuye2, SHEN Boxiong1,2( ), SU Lichao3
), SU Lichao3
- 1.Key Laboratory of Clean Energy Utilization and Pollution Control in Tianjin, College of Energy and Environmental Engineering, Hebei University of Technology, Tianjin 300401, China
2.College of Chemical Engineering, Hebei University of Technology, Tianjin 300401, China
3.Linzhu(Hebei) Engineering Industry Technology Research Corporation Limited, Baoding 054001, Hebei, China
-
Received:2022-05-30Revised:2022-10-24Online:2023-03-15Published:2023-04-10 -
Contact:SHEN Boxiong
摘要:
NH3选择催化还原技术(NH3-SCR)作为一种高效去除NO x 的手段,已广泛地应用到柴油车尾气脱硝过程当中。车用NH3-SCR技术常采用铜基分子筛作为催化剂,尾气中的氮氧化物可在催化剂的作用下,与NH3反应转化为N2;但在实际应用过程中,N2O的生成、催化剂的水热老化与尾气中的SO2会影响铜基分子筛催化剂的脱硝性能。因此,本文以Cu-SSZ-13分子筛催化剂中孤立态Cu2+的研究进展为基础,总结了Cu-SSZ-13中两种孤立态Cu2+对NH3-SCR反应与N2O生成的影响;综述了两种孤立态Cu2+对水热老化与SO2响应的差异;归纳了诱导Cu-2Z生成的手段。同时,本文以Cu-LTA分子筛催化剂的研究现状为例,简要回顾了Cu-LTA中孤立态Cu2+的研究进展,对Cu-LTA的研究趋势与应用前景作出了展望。本文可为其他类型的铜基分子筛催化剂在柴油车尾气脱硝领域的研究提供思路,即明确分子筛中两种孤立态Cu2+相关性质,通过调控孤立态Cu2+对催化剂进行合理设计,在保持高催化活性的同时,进一步提升催化剂的水热稳定性与SO2抗性。
中图分类号:
引用本文
张晨光, 封硕, 邢玉烨, 沈伯雄, 苏立超. 柴油车用NH3-SCR铜基分子筛催化剂孤立态Cu2+研究进展[J]. 化工进展, 2023, 42(3): 1321-1331.
ZHANG Chenguang, FENG Shuo, XING Yuye, SHEN Boxiong, SU Lichao. Research progress of isolated Cu2+ in copper based zeolite NH3-SCR catalyst for diesel vehicles[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1321-1331.
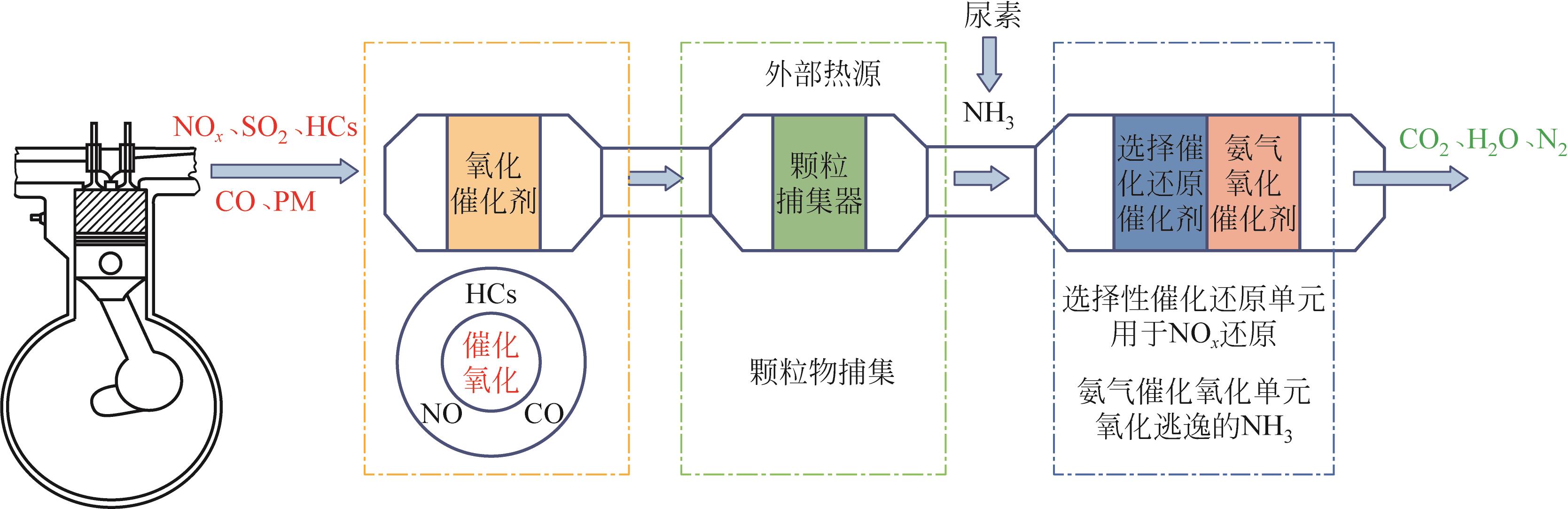
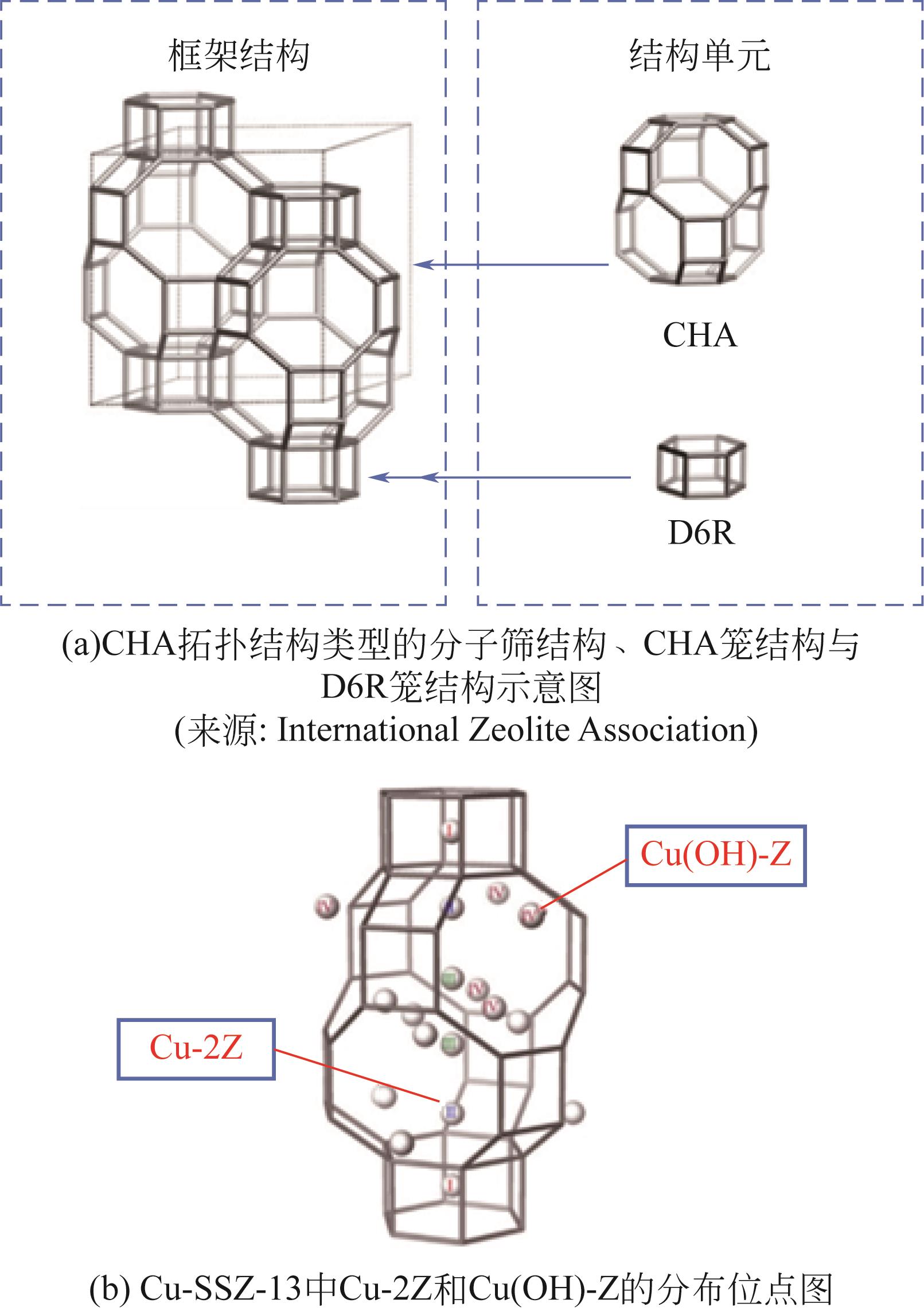
图1 柴油机尾气处理系统布局示意图氧化催化剂(DOC):将碳氢化合物(HCs)、CO等物质氧化为H2O和CO2,部分NO氧化为NO2;颗粒捕集器(DPF):用于捕集颗粒物(PM),外部热源(external heat source)用于去除DPF单元工作过程中产生的积炭;选择性催化还原催化剂(SCR):用于还原NO x,此过程需要的NH3源于尿素分解;氨氧化催化剂(AOC):用于将SCR单元逃逸的NH3氧化为N2
| Si/Al | 制备方式 | 测试条件 | 脱硝效率 | 参考文献 |
|---|---|---|---|---|
| 6 | 液相离子交换 | [NO]=[NH3]=500mg/kg,[O2]=5%,体积空速=25000h-1 | >90%(175~550℃) | [ |
| 6.5 | 液相离子交换 | [NO]=[NH3]=200mg/kg,[O2]=5%,体积空速=60000h-1 | >90%(250~500℃) | [ |
| 11 | 液相离子交换 | [NO]=[NH3]=500mg/kg,[O2]=5%,体积空速=120000h-1 | >90%(200~500℃) | [ |
| 24 | 液相离子交换 | [NO]=[NH3]=350mg/kg,[O2]=14%,体积空速=30000h-1 | >90%(200~450℃) | [ |
表1 Cu-SSZ-13催化下作用下NO高效转化的温度区间
| Si/Al | 制备方式 | 测试条件 | 脱硝效率 | 参考文献 |
|---|---|---|---|---|
| 6 | 液相离子交换 | [NO]=[NH3]=500mg/kg,[O2]=5%,体积空速=25000h-1 | >90%(175~550℃) | [ |
| 6.5 | 液相离子交换 | [NO]=[NH3]=200mg/kg,[O2]=5%,体积空速=60000h-1 | >90%(250~500℃) | [ |
| 11 | 液相离子交换 | [NO]=[NH3]=500mg/kg,[O2]=5%,体积空速=120000h-1 | >90%(200~500℃) | [ |
| 24 | 液相离子交换 | [NO]=[NH3]=350mg/kg,[O2]=14%,体积空速=30000h-1 | >90%(200~450℃) | [ |
| 催化剂 | 拓扑结构 | 水热老化条件 | 测试条件 | 脱硝效率 | 参考文献 |
|---|---|---|---|---|---|
| Cu-LTA | LTA | [温度]=900℃,[H2O]=10%,[时间]=12h | [NH3]=[NO]=0.05%,体积空速=100000h-1,[O2]=5%,[H2O]=10% | >90%(250~600℃)-新鲜,>80%(250~550℃)-老化 | [ |
| Cu-SSZ-39 | AFI | [温度]=850℃,[H2O]=10%,[时间]=16h | [NH3]=[NO]=0.05%,体积空速=25000h-1,[O2]=5%,[H2O]=5% | 约100%(225~450℃)-新鲜,>80%(250~500℃)-老化 | [ |
| Cu-AFX | AFX | [温度]=800℃,[H2O]=5%,[时间]=16h | [NH3]=[NO]=0.03%,体积空速=144000h-1,[O2]=5%,[H2O]=3% | 约100%(250~400℃)-新鲜,约100%(250~350℃)-老化 | [ |
| Cu-ERI | ERI | [温度]=850℃,[H2O]=10%,[时间]=5h | [NH3]=[NO]=0.03%,体积空速=50000h-1,[O2]=5%,[H2O]=3% | >90%(250~600℃)-新鲜,>70%(250~500℃)-老化 | [ |
| Cu-ZJM-7 | KFI | [温度]=850℃,[H2O]=10%,[时间]=12h | [NH3]=[NO]=0.05%,体积空速=80000h-1,[O2]=5%,[H2O]=5% | 约100%(200~400℃)-新鲜,>80%(250~400℃)-老化 | [ |
| Cu-UZM-35 | MSE | [温度]=800℃,[H2O]=10%,[时间]=24h | [NH3]=[NO]=0.05%,体积空速=100000h-1,[O2]=5%,[H2O]=10% | 约100%(200~450℃)-新鲜,>80%(250~500℃)-老化 | [ |
表2 具有不同拓扑结构的铜基催化剂在水热老化前后的脱硝性能
| 催化剂 | 拓扑结构 | 水热老化条件 | 测试条件 | 脱硝效率 | 参考文献 |
|---|---|---|---|---|---|
| Cu-LTA | LTA | [温度]=900℃,[H2O]=10%,[时间]=12h | [NH3]=[NO]=0.05%,体积空速=100000h-1,[O2]=5%,[H2O]=10% | >90%(250~600℃)-新鲜,>80%(250~550℃)-老化 | [ |
| Cu-SSZ-39 | AFI | [温度]=850℃,[H2O]=10%,[时间]=16h | [NH3]=[NO]=0.05%,体积空速=25000h-1,[O2]=5%,[H2O]=5% | 约100%(225~450℃)-新鲜,>80%(250~500℃)-老化 | [ |
| Cu-AFX | AFX | [温度]=800℃,[H2O]=5%,[时间]=16h | [NH3]=[NO]=0.03%,体积空速=144000h-1,[O2]=5%,[H2O]=3% | 约100%(250~400℃)-新鲜,约100%(250~350℃)-老化 | [ |
| Cu-ERI | ERI | [温度]=850℃,[H2O]=10%,[时间]=5h | [NH3]=[NO]=0.03%,体积空速=50000h-1,[O2]=5%,[H2O]=3% | >90%(250~600℃)-新鲜,>70%(250~500℃)-老化 | [ |
| Cu-ZJM-7 | KFI | [温度]=850℃,[H2O]=10%,[时间]=12h | [NH3]=[NO]=0.05%,体积空速=80000h-1,[O2]=5%,[H2O]=5% | 约100%(200~400℃)-新鲜,>80%(250~400℃)-老化 | [ |
| Cu-UZM-35 | MSE | [温度]=800℃,[H2O]=10%,[时间]=24h | [NH3]=[NO]=0.05%,体积空速=100000h-1,[O2]=5%,[H2O]=10% | 约100%(200~450℃)-新鲜,>80%(250~500℃)-老化 | [ |
| 1 | ZHANG Wenbo, CHEN Jialing, GUO Li, et al. Research progress on NH3-SCR mechanism of metal-supported zeolite catalysts[J]. Journal of Fuel Chemistry and Technology, 2021, 49(9): 1294-1315. |
| 2 | 陶汉国, 徐富强, 汪利峰, 等. 柴油机超低排放后处理系统及催化剂的研发探讨[J]. 中国环保产业, 2021(11): 52-57, 62. |
| TAO Hanguo, XU Fuqiang, WANG Lifeng, et al. Research and development of after-treatment system and catalyst for ultra-low emission of diesel engine[J]. China Environmental Protection Industry, 2021(11): 52-57, 62. | |
| 3 | Beñat PEREDA-AYO, DE LA TORRE Unai, ILLÁN-GÓMEZ María José, et al. Role of the different copper species on the activity of Cu/zeolite catalysts for SCR of NO x with NH3 [J]. Applied Catalysis B: Environmental, 2014, 147: 420-428. |
| 4 | 钟秋月, 高延新, 胡帅. 柴油机Cu基分子筛催化剂的特性研究[J]. 汽车实用技术, 2018(23): 257-258. |
| ZHONG Qiuyue, GAO Yanxin, HU Shuai. The research on the characteristics of Cu-based zeolite for diesel engine[J]. Automobile Applied Technology, 2018(23): 257-258. | |
| 5 | YASHNIK Svetlana, ISMAGILOV Zinfer. Cu-substituted ZSM-5 catalyst: Controlling of DeNO x reactivity via ion-exchange mode with copper-ammonia solution[J]. Applied Catalysis B: Environmental, 2015, 170: 241-254. |
| 6 | 张冉冉, 李永红. Cu基分子筛NH3-SCR脱硝催化剂的研究进展[J]. 现代化工, 2015, 35(8): 67-71. |
| ZHANG Ranran, LI Yonghong. Progress of Cu-zeolites catalysts for removal of NO with NH3 selective catalytic reduction technology[J]. Modern Chemical Industry, 2015, 35(8): 67-71. | |
| 7 | SHAN Yulong, DU Jinpeng, ZHANG Yan, et al. Selective catalytic reduction of NO x with NH3: Opportunities and challenges of Cu-based small-pore zeolites[J]. National Science Review, 2021, 8(10): nwab010. |
| 8 | SHAN Wenpo, YU Yunbo, ZHANG Yan, et al. Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NO x with NH3 [J]. Catalysis Today, 2021, 376: 292-301. |
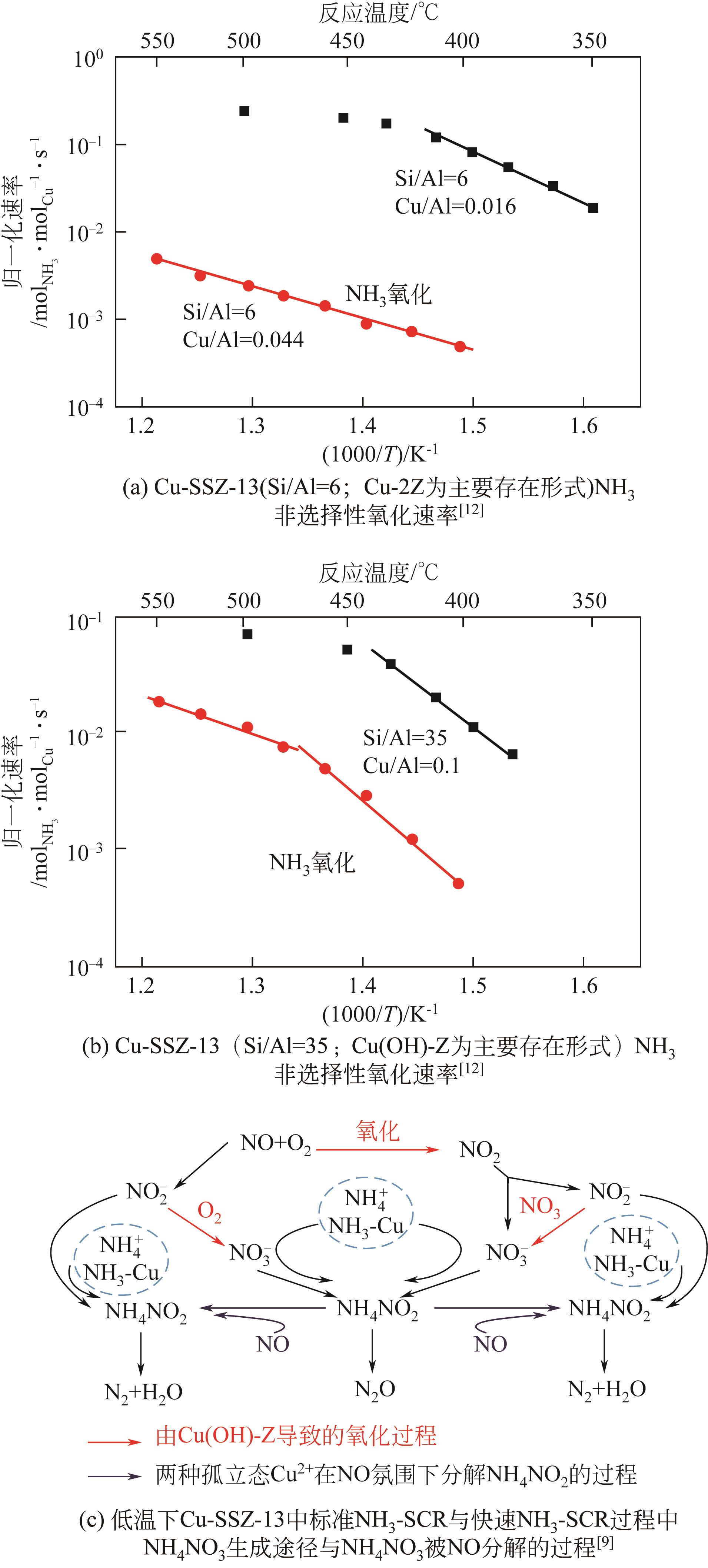
| 9 | YAO Dongwei, LIU Biao, WU Feng, et al. N2O formation mechanism during low-temperature NH3-SCR over Cu-SSZ-13 catalysts with different Cu loadings[J]. Industrial & Engineering Chemistry Research, 2021, 60(28): 10083-10093. |
| 10 | JOHNSON Timothy, JOSHI Ameya. Review of vehicle engine efficiency and emissions[J]. SAE International Journal of Engines, 2018, 11(6): 1307-1330. |
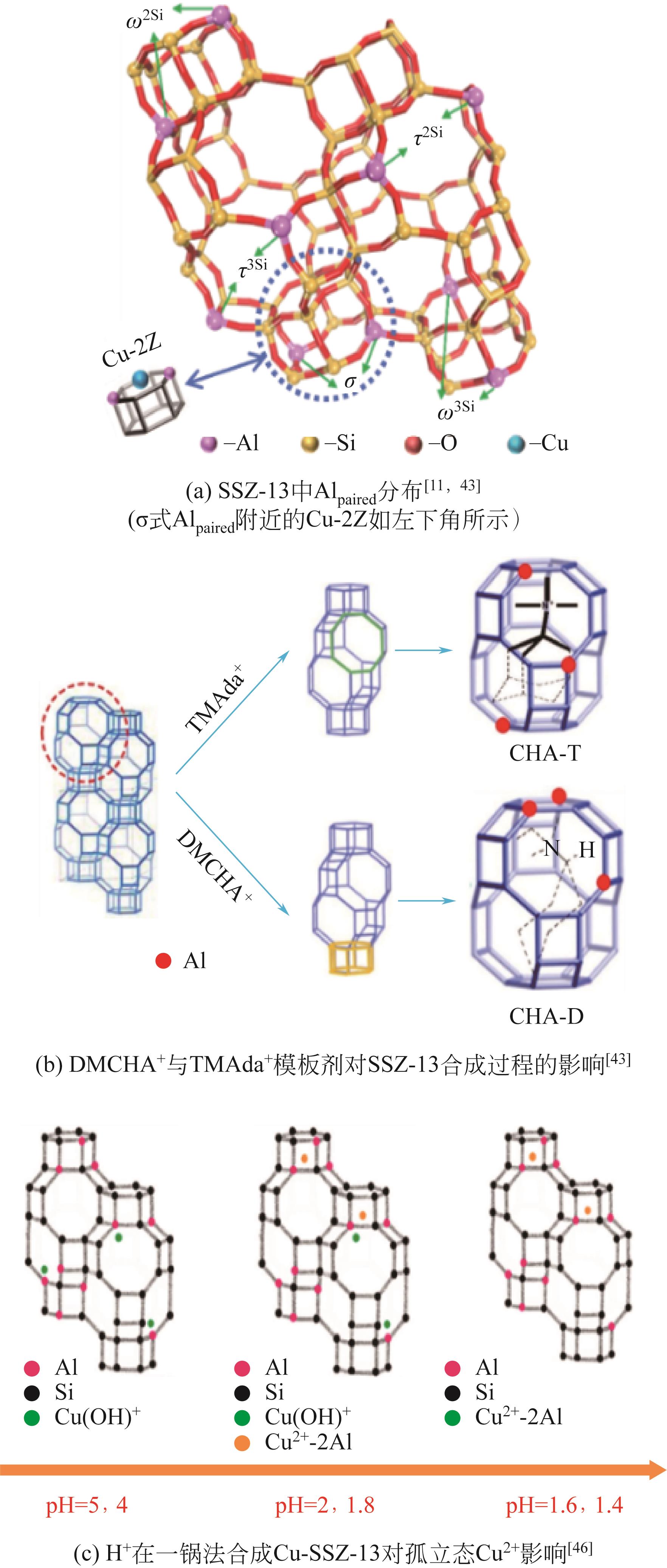
| 11 | CHEN Zhiqiang, YE Tianle, QU Hongxia, et al. Progressive regulation of Al sites and Cu distribution to increase hydrothermal stability of hierarchical SSZ-13 for the selective catalytic reduction reaction[J]. Applied Catalysis B: Environmental, 2022, 303: 120867. |
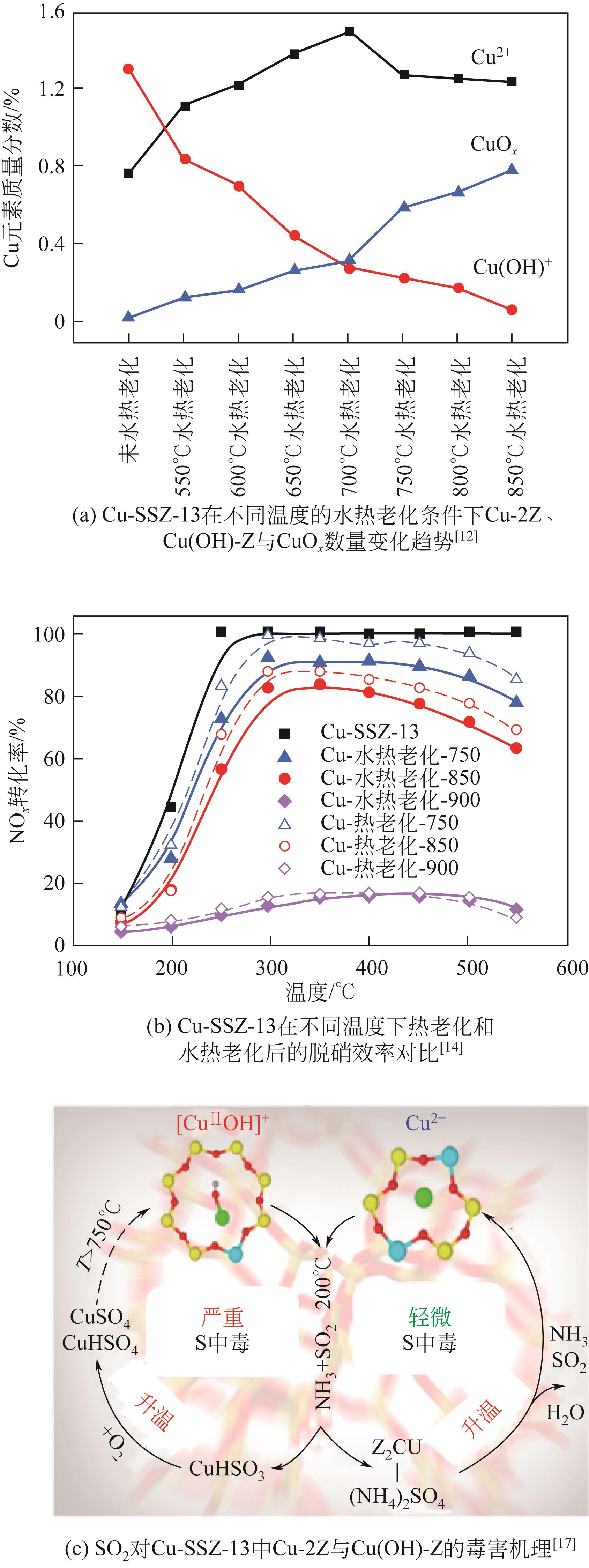
| 12 | SONG James, WANG Yilin, WALTER Eric D, et al. Toward rational design of Cu/SSZ-13 selective catalytic reduction catalysts: Implications from atomic-level understanding of hydrothermal stability[J]. ACS Catalysis, 2017, 7(12): 8214-8227. |
| 13 | MA Yue, CHENG Songqi, WU Xiaodong, et al. Improved hydrothermal durability of Cu-SSZ-13 NH3-SCR catalyst by surface Al modification: Affinity and passivation[J]. Journal of Catalysis, 2022, 405: 199-211. |
| 14 | ZHAO Huawang, WU Xiaomin, HUANG Zhiwei, et al. A comparative study of the thermal and hydrothermal aging effect on Cu-SSZ-13 for the selective catalytic reduction of NO x with NH3 [J]. Chinese Journal of Chemical Engineering, 2022, 45: 68-77. |
| 15 | ZHANG Li, WANG Di, LIU Yong, et al. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst[J]. Applied Catalysis B: Environmental, 2014, 156/157: 371-377. |
| 16 | LIANG Jian, TAO Jinxiong, MI Yangyang, et al. Unraveling the boosting low-temperature performance of ordered mesoporous Cu-SSZ-13 catalyst for NO x reduction[J]. Chemical Engineering Journal, 2021, 409: 128238. |
| 17 | JANGJOU Yasser, Quan DO, GU Yuntao, et al. Nature of Cu active centers in Cu-SSZ-13 and their responses to SO2 exposure[J]. ACS Catalysis, 2018, 8(2): 1325-1337. |
| 18 | MESILOV Vitaly V, BERGMAN Susanna L, DAHLIN Sandra, et al. Differences in oxidation-reduction kinetics and mobility of Cu species in fresh and SO 2 - poisoned Cu-SSZ-13 catalysts[J]. Applied Catalysis B: Environmental, 2021, 284: 119756. |
| 19 | ZHANG Yani, ZHU Hongchang, ZHANG Tao, et al. Revealing the synergistic deactivation mechanism of hydrothermal aging and SO2 poisoning on Cu/SSZ-13 under SCR condition[J]. Environmental Science & Technology, 2022, 56(3): 1917-1926. |
| 20 | YONG Xin, ZHANG Cuijuan, WEI Miao, et al. Promotion of the performance of Cu-SSZ-13 for selective catalytic reduction of NO x by ammonia in the presence of SO2 during high temperature hydrothermal aging[J]. Journal of Catalysis, 2021, 394: 228-235. |
| 21 | KWAK Ja Hun, TRAN Diana, BURTON Sarah D, et al. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites[J]. Journal of Catalysis, 2012, 287: 203-209. |
| 22 | GAO Feng, János SZANYI. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts[J]. Applied Catalysis A: General, 2018, 560: 185-194. |
| 23 | FICKEL Dustin W, LOBO Raul F. Copper coordination in Cu-SSZ-13 and Cu-SSZ-16 investigated by variable-temperature XRD[J]. The Journal of Physical Chemistry C, 2010, 114(3): 1633-1640. |
| 24 | KWAK Ja Hun, ZHU Haiyang, LEE Jong H, et al. Two different cationic positions in Cu-SSZ-13?[J]. Chemical Communications, 2012, 48(39): 4758-4760. |
| 25 | ANDERSEN Casper Welzel, BREMHOLM Martin, VENNESTRØM Peter Nicolai Ravnborg, et al. Location of Cu2+ in CHA zeolite investigated by X-ray diffraction using the Rietveld/maximum entropy method[J]. International Union of Crystallography, 2014, 1(6): 382-386. |
| 26 | SHAN Yulong, SHAN Wenpo, SHI Xiaoyan, et al. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13[J]. Applied Catalysis B: Environmental, 2020, 264: 118511. |
| 27 | USUI Toyohiro, LIU Zhendong, Sayoko IBE, et al. Improve the hydrothermal stability of Cu-SSZ-13 zeolite catalyst by loading a small amount of Ce[J]. ACS Catalysis, 2018, 8(10): 9165-9173. |
| 28 | BEALE A M, LEZCANO-GONZALEZ I, SLAWINSKI W A, et al. Correlation between Cu ion migration behaviour and deNO x activity in Cu-SSZ-13 for the standard NH3-SCR reaction[J]. Chemical Communications, 2016, 52(36): 6170-6173. |
| 29 | HU Wenshuo, Selleri Tommaso, Gramigni Federica, et al. On the redox mechanism of low-temperature NH3-SCR over Cu-CHA: A combined experimental and theoretical study of the reduction half cycle[J]. Angewandte Chemie International Edition, 2021, 60(13): 7197-7204. |
| 30 | HU Wenshuo, GRAMIGNI Federica, NASELLO Nicole Daniela, et al. Dynamic binuclear CuII sites in the reduction half-cycle of low-temperature NH3–SCR over Cu-CHA catalysts[J]. ACS Catalysis, 2022, 12(9): 5263-5274. |
| 31 | ZHANG Dong, YANG Ralph T. N2O formation pathways over zeolite-supported Cu and Fe catalysts in NH3-SCR[J]. Energy & Fuels, 2018, 32(2): 2170-2182. |
| 32 | SHIH Arthur J, GONZÁLEZ Juan M, KHURANA Ishant, et al. Influence of ZCuOH, Z2Cu, and extraframework Cu x O y species in Cu-SSZ-13 on N2O formation during the selective catalytic reduction of NO x with NH3 [J]. ACS Catalysis, 2021, 11(16): 10362-10376. |
| 33 | Magdalena JABŁOŃSKA, Kinga GÓRA-MAREK, GRILC Miha, et al. Effect of textural properties and presence of Co-cation on NH3-SCR activity of Cu-exchanged ZSM-5[J]. Catalysts, 2021, 11(7): 843. |
| 34 | WANG Hao, XU Ruinian, JIN Yi, et al. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts[J]. Catalysis Today, 2019, 327: 295-307. |
| 35 | ZHANG Yani, ZHANG Jun, WANG Houlin, et al. Selective catalytic reduction of NO x with NH3 over Cu/SSZ-13: Elucidating dynamics of Cu active sites with in situ UV-Vis spectroscopy and DFT calculations[J]. The Journal of Physical Chemistry C, 2022, 126(20): 8720-8733. |
| 36 | DAYA Rohil, TRANDAL Dylan, MENON Unmesh, et al. Kinetic model for the reduction of CuII sites by NO + NH3 and reoxidation of NH3-solvated CuI sites by O2 and NO in Cu-SSZ-13[J]. ACS Catalysis, 2022, 12(11): 6418-6433. |
| 37 | LEE Hwangho, SONG Inhak, JEON Se Won, et al. Mobility of Cu ions in Cu-SSZ-13 determines the reactivity of selective catalytic reduction of NO x with NH3 [J]. The Journal of Physical Chemistry Letters, 2021, 12(12): 3210-3216. |
| 38 | WANG Xiaofeng, XU Yang, QIN Mengyue, et al. Insight into the effects of Cu2+ ions and CuO species in Cu-SSZ-13 catalysts for selective catalytic reduction of NO by NH3 [J]. Journal of Colloid and Interface Science, 2022, 622: 1-10. |
| 39 | WIJAYANTI Kurnia, LEISTNER Kirsten, CHAND Shilpa, et al. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions[J]. Catalysis Science & Technology, 2016, 6(8): 2565-2579. |
| 40 | WEI Lingang, GUO Ruitang, ZHOU Jue, et al. Chemical deactivation and resistance of Mn-based SCR catalysts for NOx removal from stationary sources[J]. Fuel, 2022, 316: 123438. |
| 41 | ZHAO Ling, ZHANG Yu, KANG Mengdi. Recent advances in heighten sulfur resistance of SCR catalysts: A review[J]. Environmental Engineering Research, 2022, 27(1): 200642. |
| 42 | SHIH Arthur J, KHURANA Ishant, LI Hui, et al. Spectroscopic and kinetic responses of Cu-SSZ-13 to SO2 exposure and implications for NO x selective catalytic reduction[J]. Applied Catalysis A: General, 2019, 574: 122-131. |
| 43 | ZHANG Juan, SHAN Yulong, ZHANG Ling, et al. Importance of controllable Al sites in CHA framework by crystallization pathways for NH3-SCR reaction[J]. Applied Catalysis B: Environmental, 2020, 277: 119193. |
| 44 | Wenting LYU, WANG Sen, WANG Pengfei, et al. Regulation of Al distributions and Cu2+ locations in SSZ-13 zeolites for NH3-SCR of NO by different alkali metal cations[J]. Journal of Catalysis, 2021, 393: 190-201. |
| 45 | JIANG Han, GUAN Bin, PENG Xuesong, et al. Influence of synthesis method on catalytic properties and hydrothermal stability of Cu/SSZ-13 for NH3-SCR reaction[J]. Chemical Engineering Journal, 2020, 379: 122358. |
| 46 | ZHAO Huawang, YANG Guangpeng, HILL Alexander J, et al. One-step ion-exchange from Na-SSZ-13 to Cu-SSZ-13 for NH3-SCR by adjusting the pH value of Cu-exchange solution: The effect of H+ ions on activity and hydrothermal stability[J]. Microporous and Mesoporous Materials, 2021, 324: 111271. |
| 47 | Taekyung RYU, Nak Ho AHN, SEO Seungwan, et al. Fully copper-exchanged high-silica LTA zeolites as unrivaled hydrothermally stable NH3-SCR catalysts[J]. Angewandte Chemie International Edition, 2017, 56(12): 3256-3260. |
| 48 | WANG Aiyong, ARORA Prakhar, BERNIN Diana, et al. Investigation of the robust hydrothermal stability of Cu/LTA for NH3-SCR reaction[J]. Applied Catalysis B: Environmental, 2019, 246: 242-253. |
| 49 | OGURA Masaru, SHIMADA Yumiko, OHNISHI Takeshi, et al. AFX zeolite for use as a support of NH3-SCR catalyst mining through AICE joint research project of industries-academia-academia[J]. Catalysts, 2021, 11(2): 163. |
| 50 | ZHU Jie, LIU Zhendong, XU Le, et al. Understanding the high hydrothermal stability and NH3-SCR activity of the fast-synthesized ERI zeolite[J]. Journal of Catalysis, 2020, 391: 346-356. |
| 51 | HAN Shichao, TANG Xiaomin, WANG Lijin, et al. Potassium-directed sustainable synthesis of new high silica small-pore zeolite with KFI structure (ZJM-7) as an efficient catalyst for NH3-SCR reaction[J]. Applied Catalysis B: Environmental, 2021, 281: 119480. |
| 52 | LEE Jeong Hwan, KIM Young Jin, Taekyung RYU, et al. Synthesis of zeolite UZM-35 and catalytic properties of copper-exchanged UZM-35 for ammonia selective catalytic reduction[J]. Applied Catalysis B: Environmental, 2017, 200: 428-438. |
| 53 | Taekyung RYU, KIM Hyojun, HONG Suk Bong. Nature of active sites in Cu-LTA NH3-SCR catalysts: A comparative study with Cu-SSZ-13[J]. Applied Catalysis B: Environmental, 2019, 245: 513-521. |
| 54 | WANG Aiyong, OLSSON Louise. Insight into the SO2 poisoning mechanism for NO x removal by NH3-SCR over Cu/LTA and Cu/SSZ-13[J]. Chemical Engineering Journal, 2020, 395: 125048. |
| 55 | 谭丕强, 段立爽, 楼狄明, 等. 柴油机选择性催化还原捕集技术(SDPF)的研究现状与发展趋势[J]. 中国环境科学, 2021, 41(12): 5495-5511. |
| TAN Piqiang, DUAN Lishuang, LOU Diming, et al. Research status and development trend of selective catalytic reduction filter(SDPF) technology of diesel engines[J]. China Environmental Science, 2021, 41(12): 5495-5511. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [6] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [7] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [8] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [14] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [15] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||