化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4230-4245.DOI: 10.16085/j.issn.1000-6613.2023-1210
• 化工过程与装备 • 上一篇
盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺
何海霞1( ), 万亚萌1, 李帆帆1, 牛心雨1, 张静雯1, 李涛2, 任保增2(
), 万亚萌1, 李帆帆1, 牛心雨1, 张静雯1, 李涛2, 任保增2( )
)
- 1.河南工程学院化工与印染工程学院,河南 郑州 450007
2.郑州大学化工学院,河南 郑州 450001
-
收稿日期:2023-07-16修回日期:2023-10-03出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:任保增 -
作者简介:何海霞(1991—),女,博士,研究方向为相平衡与工业结晶。E-mail:xiaxia3502@163.com。 -
基金资助:河南省重大科技专项(201300310900);河南省高等学校重点科研项目(19A530004);2022年度河南工程学院一流本科课程项目(2022YLKCX02)
Kinetics and crystallization process of naphazoline hydrochloride in methanol-ethyl acetate system
HE Haixia1( ), WAN Yameng1, LI Fanfan1, NIU Xinyu1, ZHANG Jingwen1, LI Tao2, REN Baozeng2(
), WAN Yameng1, LI Fanfan1, NIU Xinyu1, ZHANG Jingwen1, LI Tao2, REN Baozeng2( )
)
- 1.School of Chemical and Printing-Dyeing Engineering, Henan University of Engineering, Zhengzhou 450007, Henan, China
2.School of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, Henan, China
-
Received:2023-07-16Revised:2023-10-03Online:2024-08-15Published:2024-09-02 -
Contact:REN Baozeng
摘要:
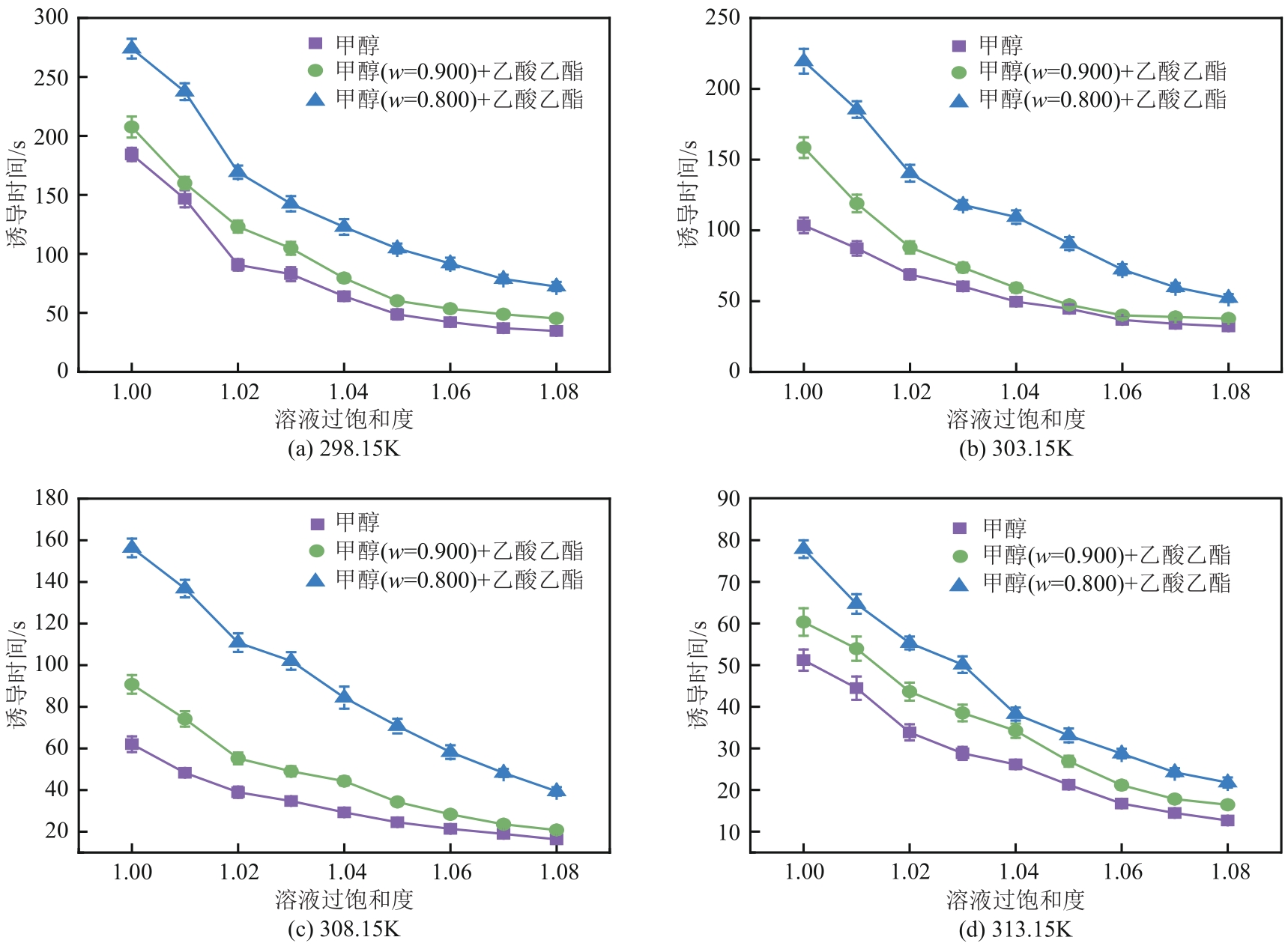
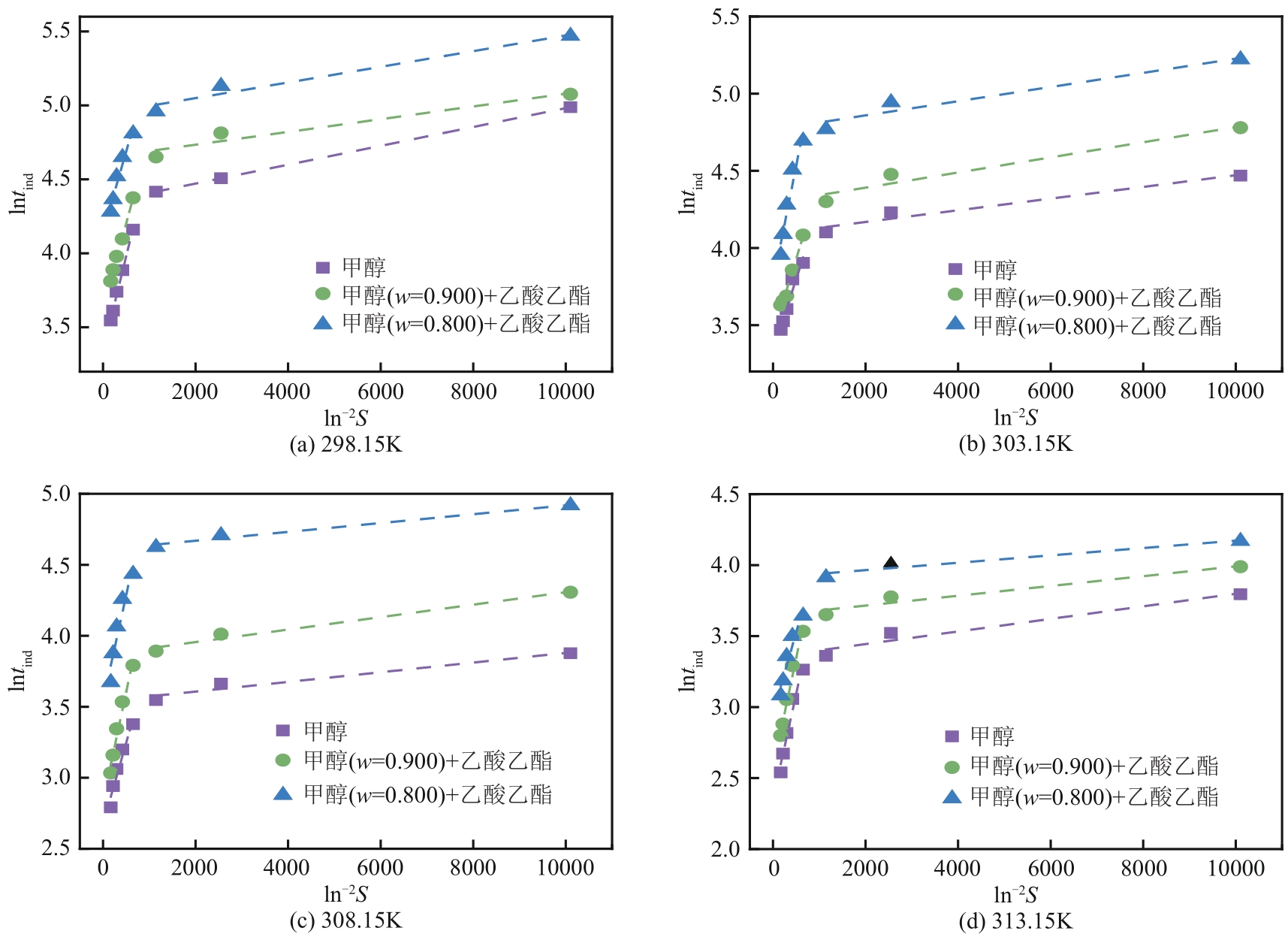
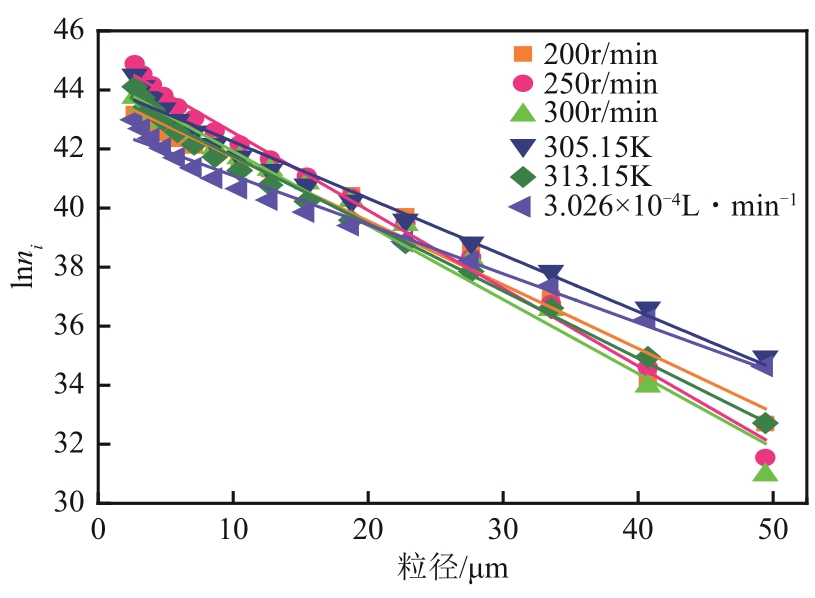
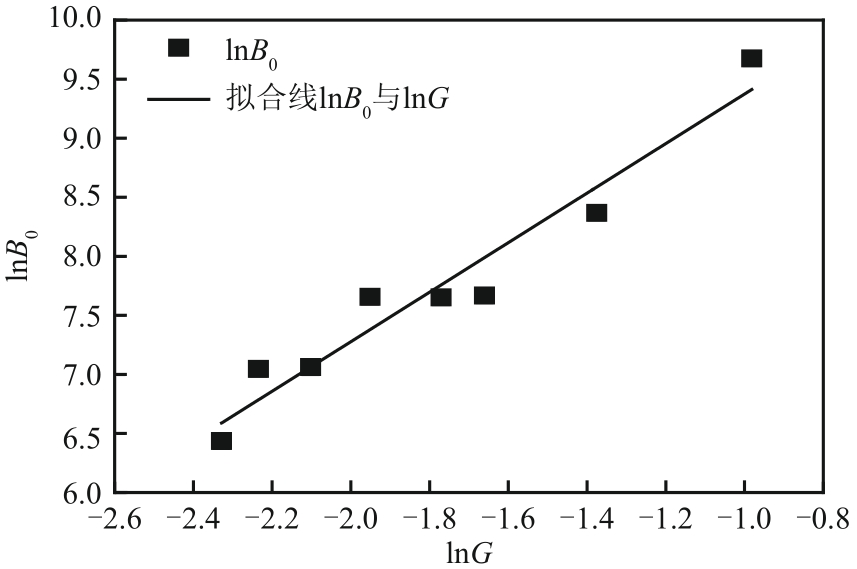
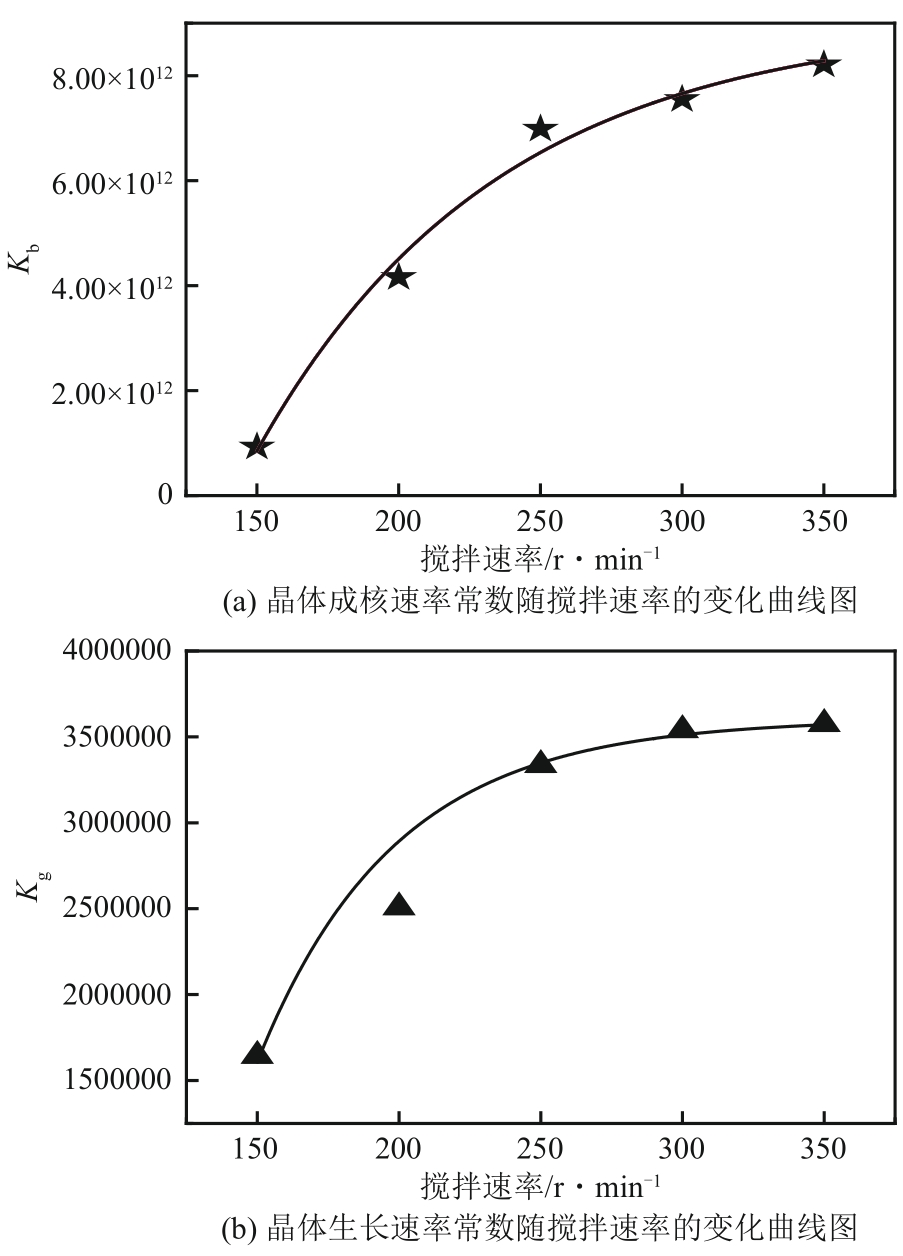
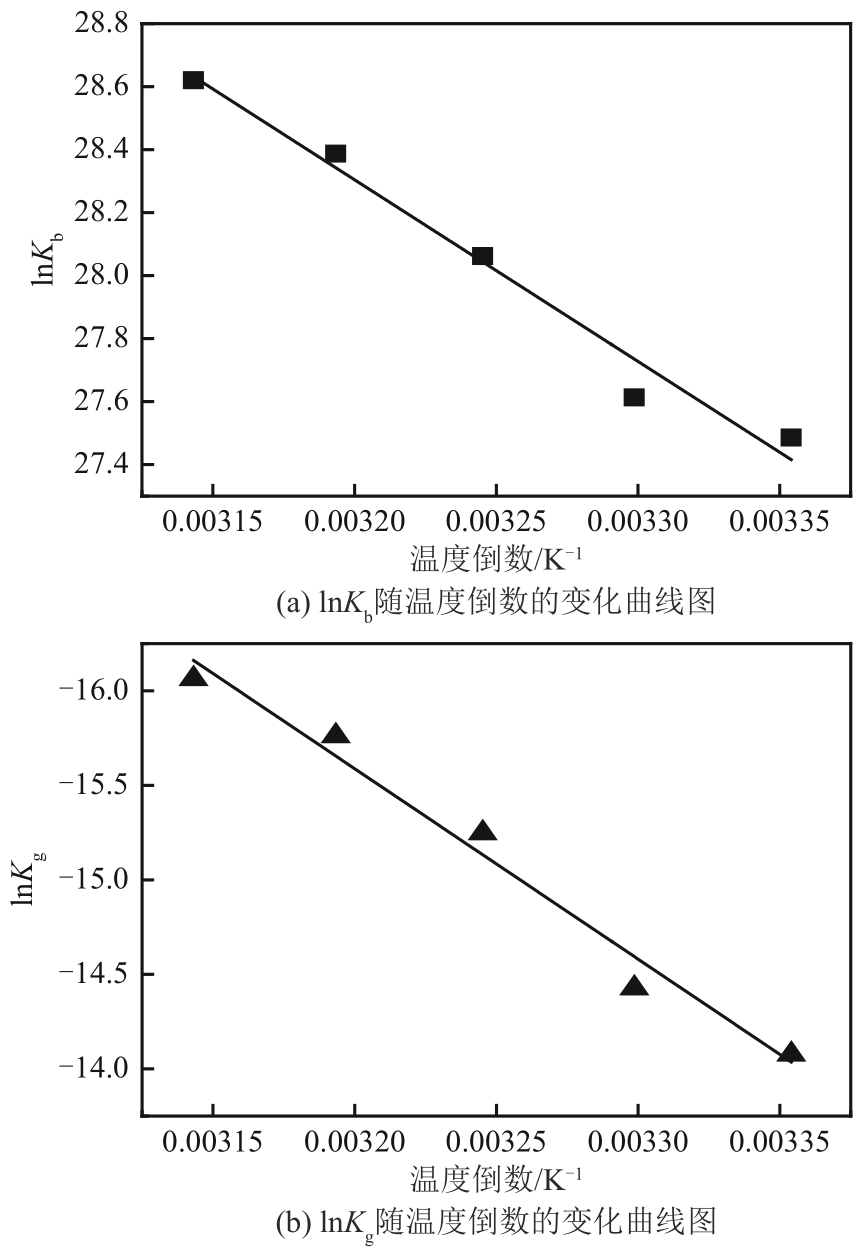
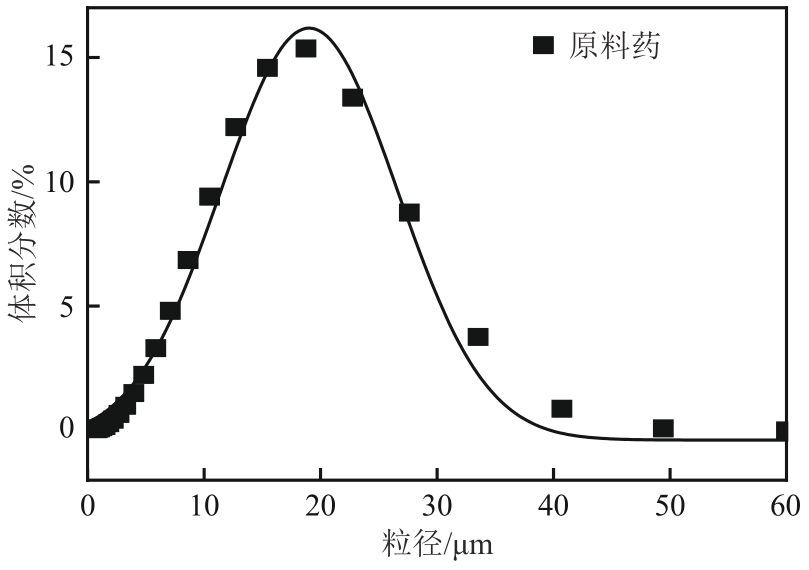
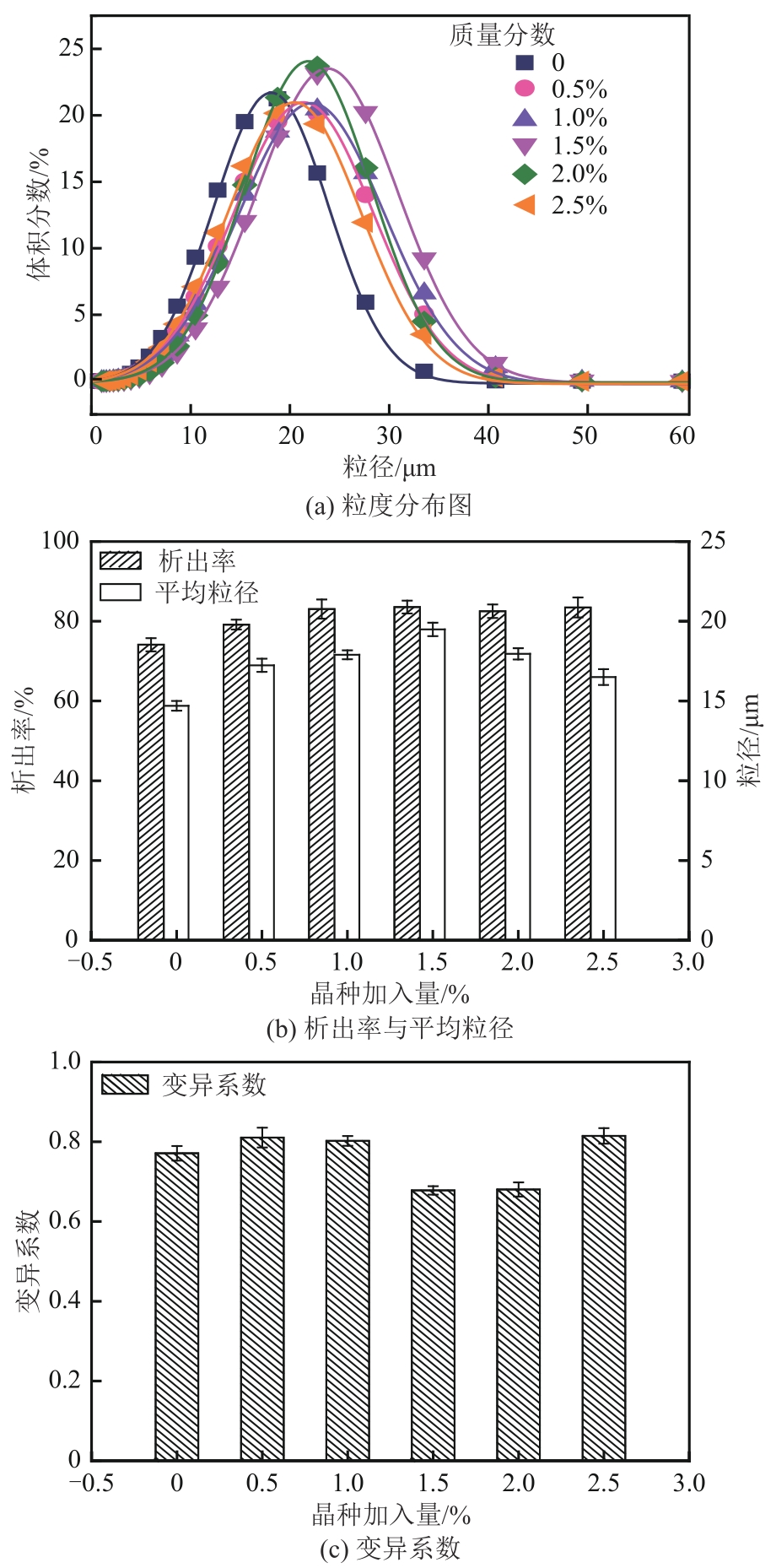
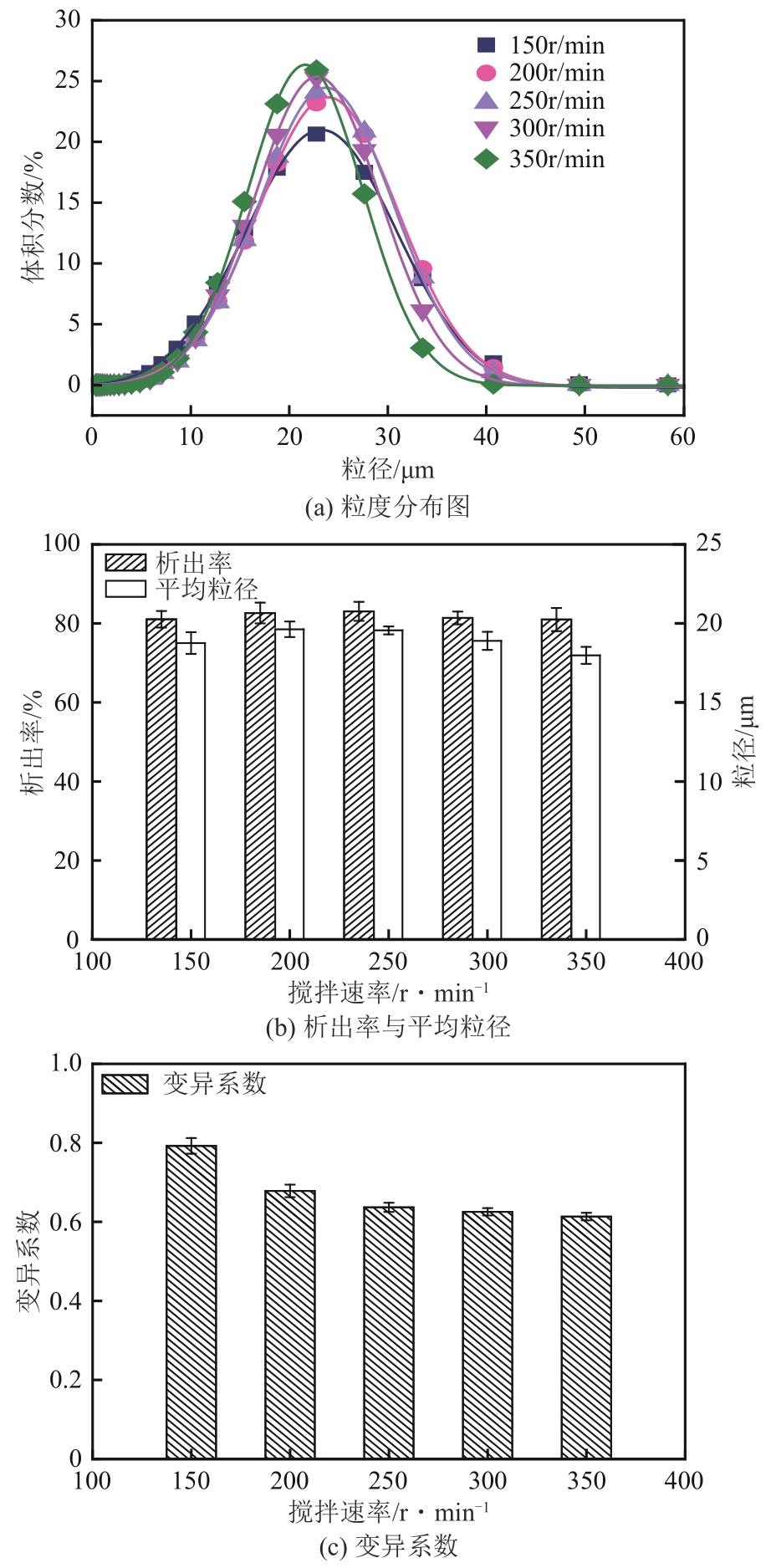
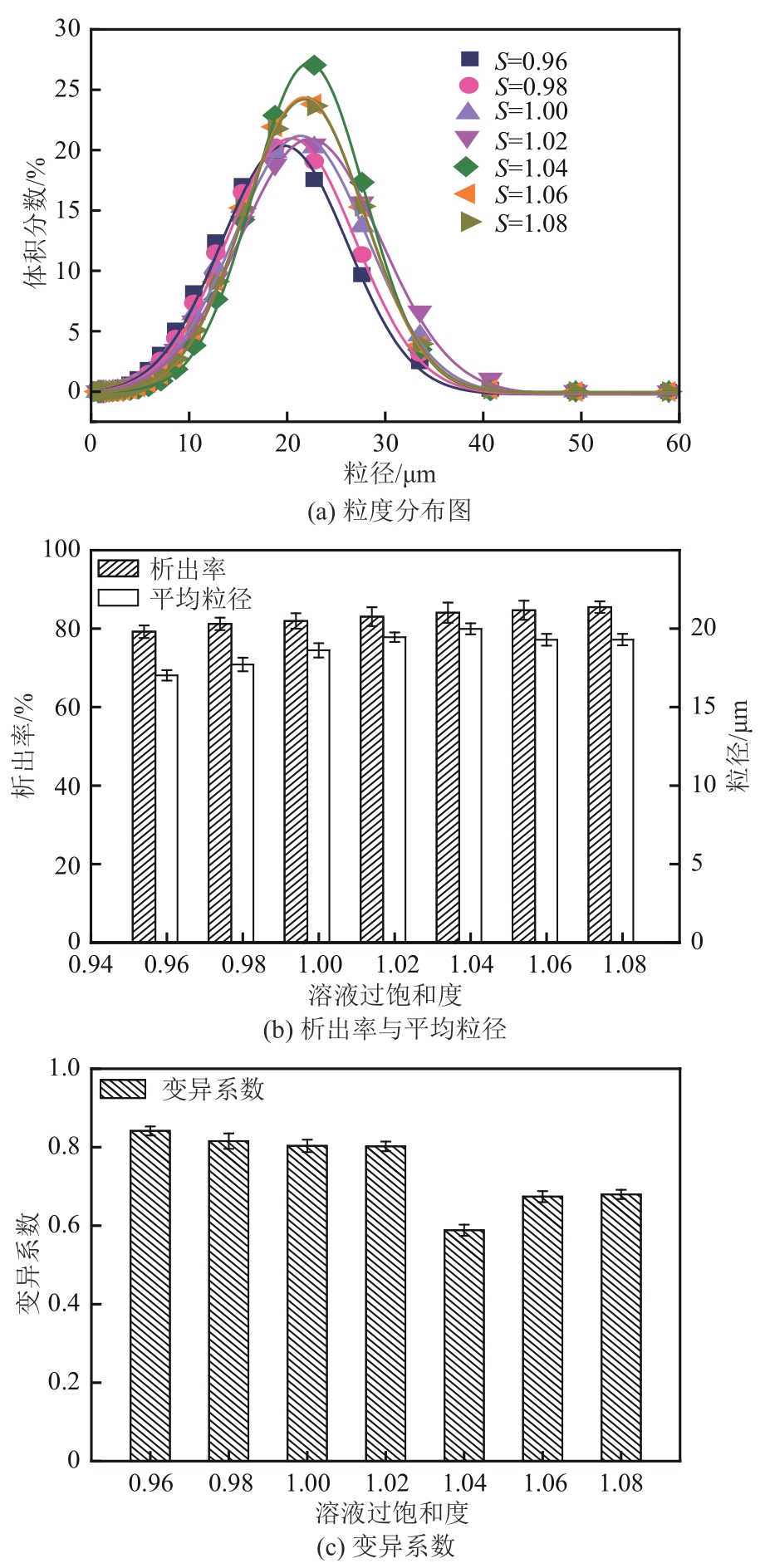
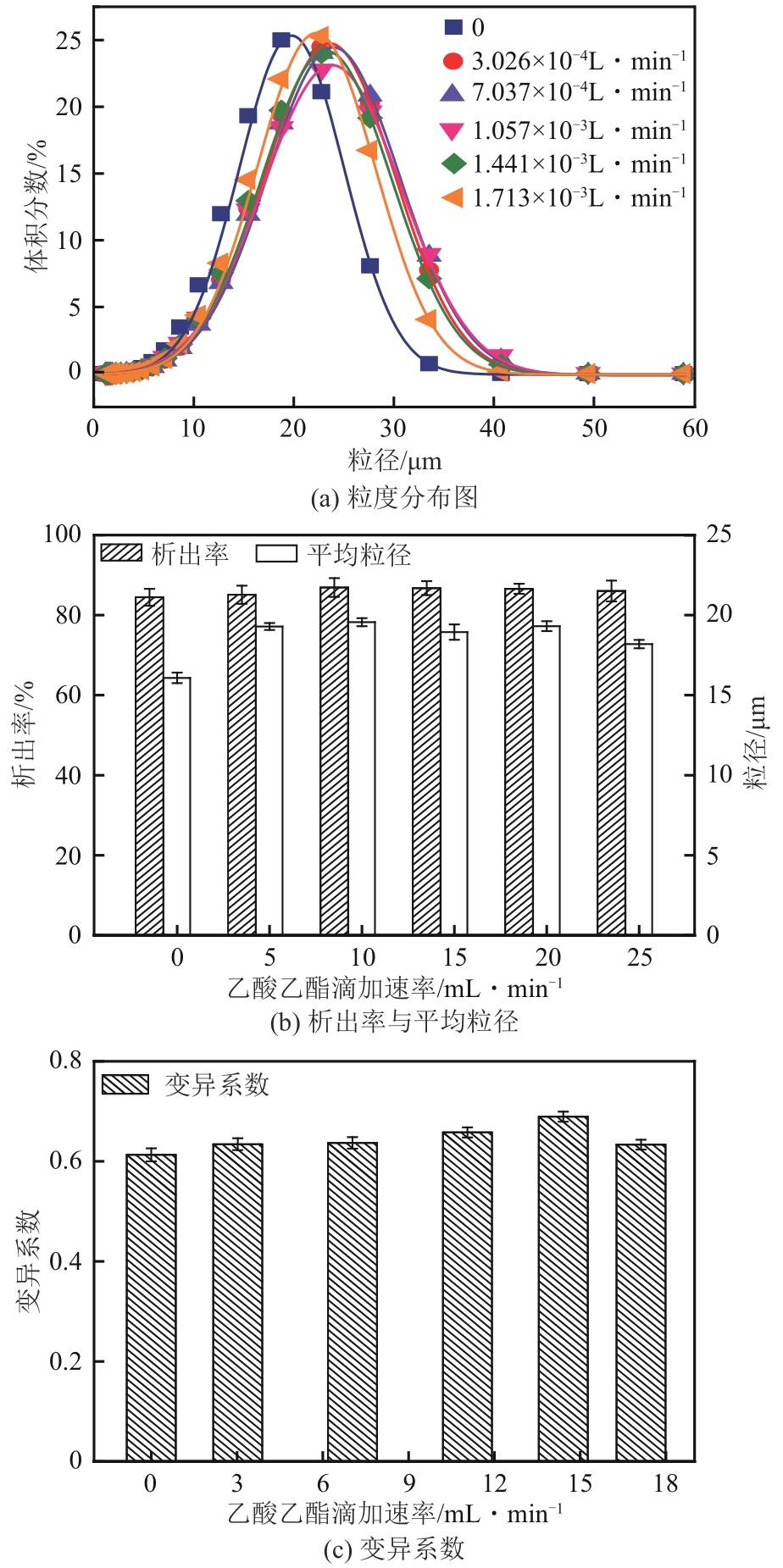
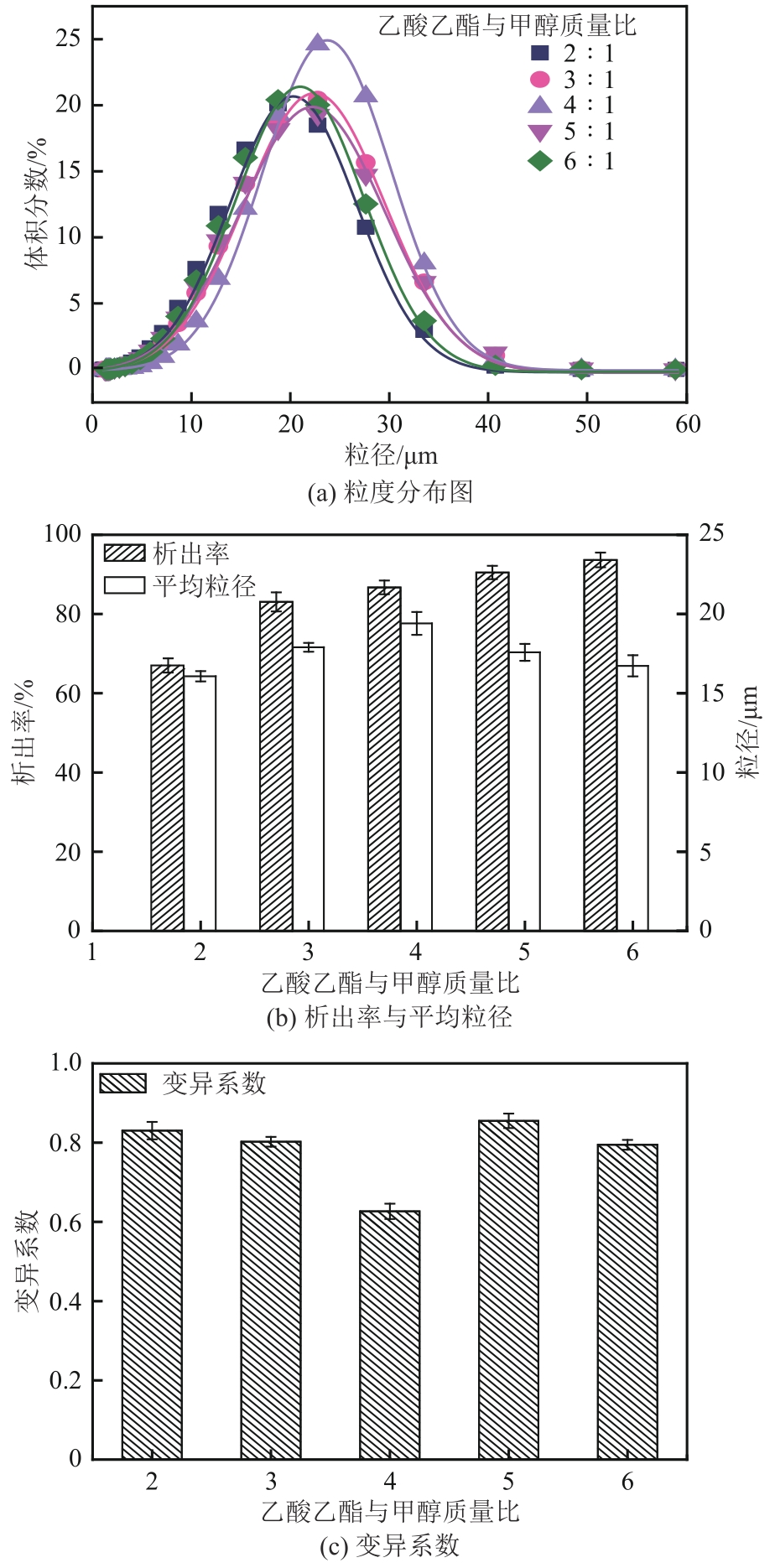
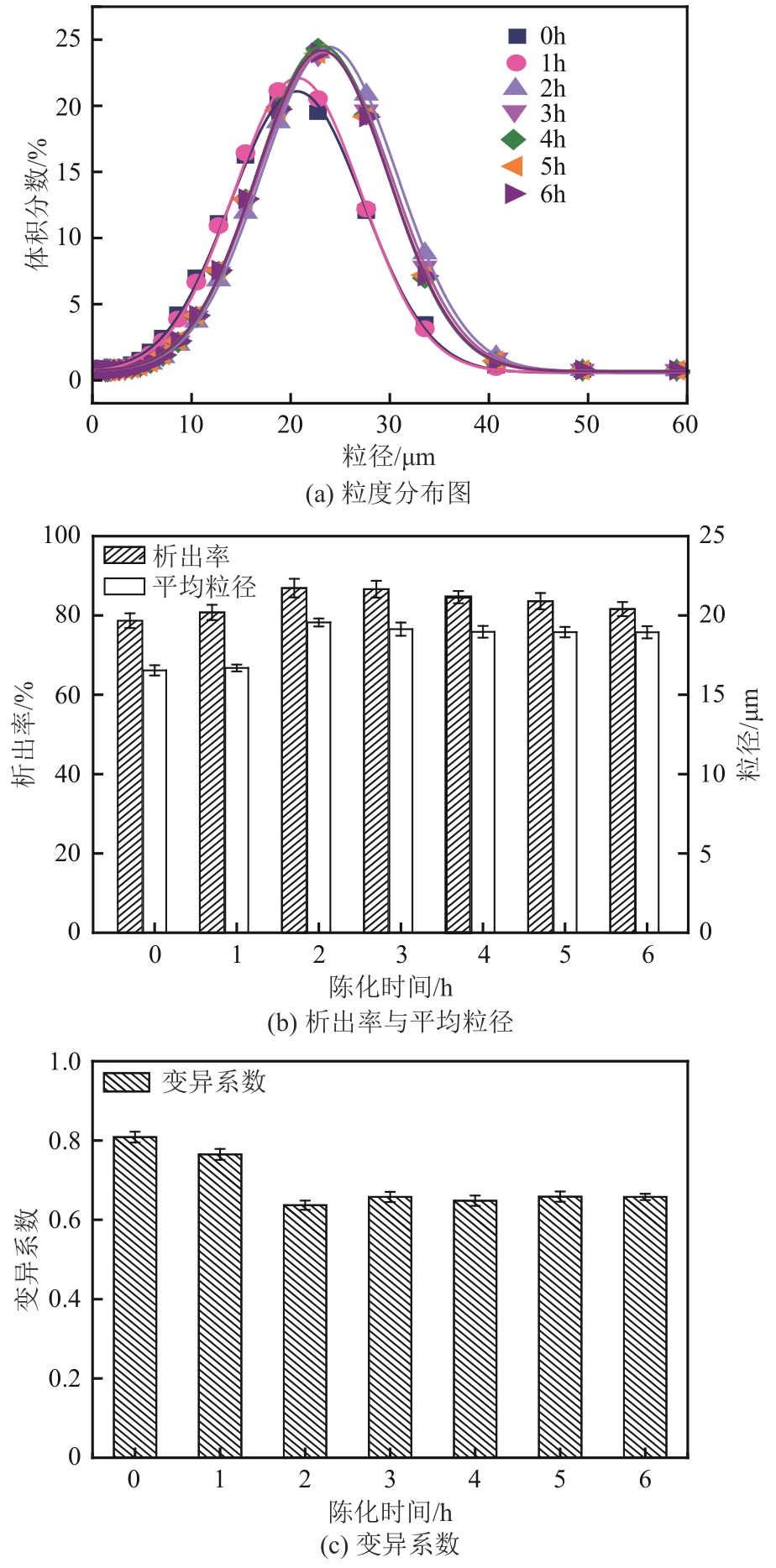
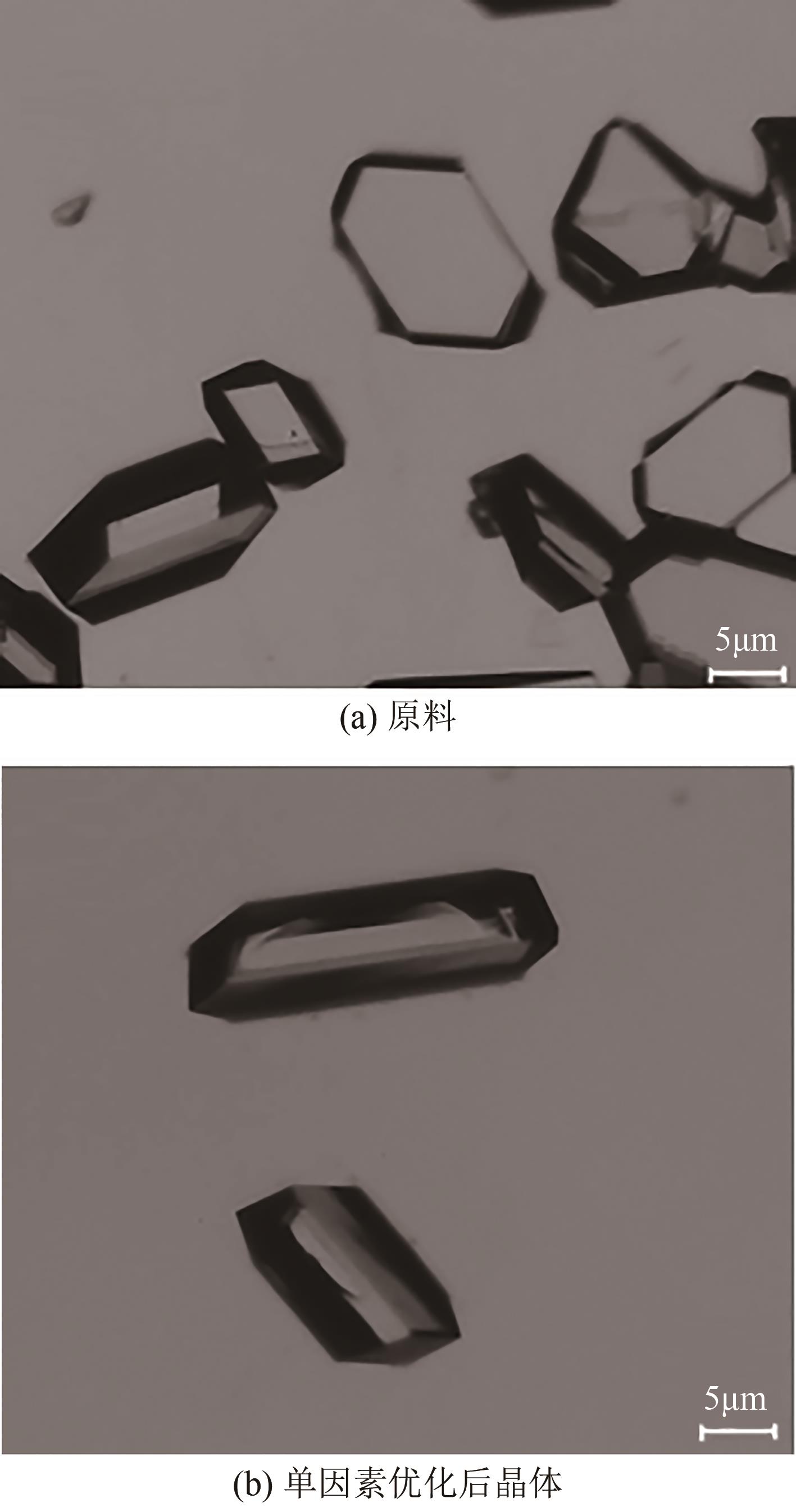
盐酸萘甲唑啉(NPZ)是一种作用于循环系统的血管收缩类药物。针对原料药平均粒径偏小及粒度分布不均的问题,本文提出运用间歇动态法对NPZ在甲醇-乙酸乙酯体系中的结晶动力学进行研究,并系统考察了不同结晶工艺条件对晶体析出率、粒径以及变异系数的影响规律。结果表明:随着溶液过饱和度的增大,NPZ晶体成核机制由非均相成核转为均相成核,晶体表面生长符合连续生长模式;NPZ生长动力学模型、成核速率与生长速率方程分别为
中图分类号:
引用本文
何海霞, 万亚萌, 李帆帆, 牛心雨, 张静雯, 李涛, 任保增. 盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺[J]. 化工进展, 2024, 43(8): 4230-4245.
HE Haixia, WAN Yameng, LI Fanfan, NIU Xinyu, ZHANG Jingwen, LI Tao, REN Baozeng. Kinetics and crystallization process of naphazoline hydrochloride in methanol-ethyl acetate system[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4230-4245.
| 甲醇质量分数 | 过饱和度 | 初级成核速率/N·m-3·s-1 | |||
|---|---|---|---|---|---|
| 298.15K | 303.15K | 308.15K | 313.15K | ||
| 1.000 | 1.01 | 574.8 | 896.7 | 1716 | 1669 |
| 1.02 | 992.1 | 1156 | 2149 | 2215 | |
| 1.03 | 1096 | 1327 | 2421 | 2615 | |
| 1.04 | 1450 | 1634 | 2884 | 2887 | |
| 1.05 | 1945 | 1827 | 3466 | 3568 | |
| 1.06 | 2268 | 2231 | 3987 | 4556 | |
| 1.07 | 2594 | 2420 | 4513 | 5277 | |
| 1.08 | 2783 | 2561 | 5251 | 6034 | |
| 0.900 | 1.01 | 472.1 | 579.6 | 1038 | 1277 |
| 1.02 | 644.3 | 805.0 | 1420 | 1593 | |
| 1.03 | 764.6 | 970.8 | 1610 | 1813 | |
| 1.04 | 1035 | 1220 | 1789 | 2046 | |
| 1.05 | 1396 | 1545 | 2334 | 2622 | |
| 1.06 | 1583 | 1839 | 2840 | 3347 | |
| 1.07 | 1744 | 1899 | 3434 | 3991 | |
| 1.08 | 1889 | 1955 | 3907 | 4330 | |
| 0.800 | 1.01 | 270.7 | 393.3 | 480.7 | 1185 |
| 1.02 | 403.6 | 539.7 | 605.9 | 1399 | |
| 1.03 | 491.5 | 655.5 | 662.5 | 1552 | |
| 1.04 | 581.0 | 711.4 | 812.8 | 1573 | |
| 1.05 | 694.4 | 873.3 | 981.2 | 2388 | |
| 1.06 | 800.7 | 1113 | 1203 | 2764 | |
| 1.07 | 946.8 | 1363 | 1465 | 3293 | |
| 1.08 | 1036 | 1570 | 1810 | 3671 | |
表1 初级成核速率计算结果
| 甲醇质量分数 | 过饱和度 | 初级成核速率/N·m-3·s-1 | |||
|---|---|---|---|---|---|
| 298.15K | 303.15K | 308.15K | 313.15K | ||
| 1.000 | 1.01 | 574.8 | 896.7 | 1716 | 1669 |
| 1.02 | 992.1 | 1156 | 2149 | 2215 | |
| 1.03 | 1096 | 1327 | 2421 | 2615 | |
| 1.04 | 1450 | 1634 | 2884 | 2887 | |
| 1.05 | 1945 | 1827 | 3466 | 3568 | |
| 1.06 | 2268 | 2231 | 3987 | 4556 | |
| 1.07 | 2594 | 2420 | 4513 | 5277 | |
| 1.08 | 2783 | 2561 | 5251 | 6034 | |
| 0.900 | 1.01 | 472.1 | 579.6 | 1038 | 1277 |
| 1.02 | 644.3 | 805.0 | 1420 | 1593 | |
| 1.03 | 764.6 | 970.8 | 1610 | 1813 | |
| 1.04 | 1035 | 1220 | 1789 | 2046 | |
| 1.05 | 1396 | 1545 | 2334 | 2622 | |
| 1.06 | 1583 | 1839 | 2840 | 3347 | |
| 1.07 | 1744 | 1899 | 3434 | 3991 | |
| 1.08 | 1889 | 1955 | 3907 | 4330 | |
| 0.800 | 1.01 | 270.7 | 393.3 | 480.7 | 1185 |
| 1.02 | 403.6 | 539.7 | 605.9 | 1399 | |
| 1.03 | 491.5 | 655.5 | 662.5 | 1552 | |
| 1.04 | 581.0 | 711.4 | 812.8 | 1573 | |
| 1.05 | 694.4 | 873.3 | 981.2 | 2388 | |
| 1.06 | 800.7 | 1113 | 1203 | 2764 | |
| 1.07 | 946.8 | 1363 | 1465 | 3293 | |
| 1.08 | 1036 | 1570 | 1810 | 3671 | |
| 温度/K | 甲醇质量分数 | 界面张力/×10-5J·m-2 | 表面熵因子 | 特征因子 | ||
|---|---|---|---|---|---|---|
| 均相成核 | 非均相成核 | 均相成核 | 非均相成核 | |||
| 298.15 | 1.000 | 5.801 | 2.140 | 0.02534 | 9.350×10-3 | 0.05019 |
| 303.15 | 1.000 | 5.287 | 1.819 | 0.02272 | 7.810×10-3 | 0.04069 |
| 308.15 | 1.000 | 5.720 | 1.774 | 0.02418 | 7.500×10-3 | 0.02986 |
| 313.15 | 1.000 | 6.321 | 1.959 | 0.02630 | 8.150×10-3 | 0.02976 |
| 298.15 | 0.900 | 5.595 | 1.878 | 0.02445 | 8.210×10-3 | 0.03782 |
| 303.15 | 0.900 | 5.372 | 1.982 | 0.02309 | 8.520×10-3 | 0.05023 |
| 308.15 | 0.900 | 6.323 | 1.932 | 0.02673 | 8.170×10-3 | 0.02851 |
| 313.15 | 0.900 | 6.405 | 1.802 | 0.02664 | 7.490×10-3 | 0.02225 |
| 298.15 | 0.800 | 5.496 | 2.012 | 0.02401 | 8.790×10-3 | 0.04907 |
| 303.15 | 0.800 | 6.214 | 1.940 | 0.02670 | 8.340×10-3 | 0.03044 |
| 308.15 | 0.800 | 6.254 | 1.722 | 0.02644 | 7.280×10-3 | 0.02089 |
| 313.15 | 0.800 | 5.765 | 1.638 | 0.02398 | 6.810×10-3 | 0.02294 |
表2 NPZ在甲醇-乙酸乙酯体系中的界面张力与表面熵因子
| 温度/K | 甲醇质量分数 | 界面张力/×10-5J·m-2 | 表面熵因子 | 特征因子 | ||
|---|---|---|---|---|---|---|
| 均相成核 | 非均相成核 | 均相成核 | 非均相成核 | |||
| 298.15 | 1.000 | 5.801 | 2.140 | 0.02534 | 9.350×10-3 | 0.05019 |
| 303.15 | 1.000 | 5.287 | 1.819 | 0.02272 | 7.810×10-3 | 0.04069 |
| 308.15 | 1.000 | 5.720 | 1.774 | 0.02418 | 7.500×10-3 | 0.02986 |
| 313.15 | 1.000 | 6.321 | 1.959 | 0.02630 | 8.150×10-3 | 0.02976 |
| 298.15 | 0.900 | 5.595 | 1.878 | 0.02445 | 8.210×10-3 | 0.03782 |
| 303.15 | 0.900 | 5.372 | 1.982 | 0.02309 | 8.520×10-3 | 0.05023 |
| 308.15 | 0.900 | 6.323 | 1.932 | 0.02673 | 8.170×10-3 | 0.02851 |
| 313.15 | 0.900 | 6.405 | 1.802 | 0.02664 | 7.490×10-3 | 0.02225 |
| 298.15 | 0.800 | 5.496 | 2.012 | 0.02401 | 8.790×10-3 | 0.04907 |
| 303.15 | 0.800 | 6.214 | 1.940 | 0.02670 | 8.340×10-3 | 0.03044 |
| 308.15 | 0.800 | 6.254 | 1.722 | 0.02644 | 7.280×10-3 | 0.02089 |
| 313.15 | 0.800 | 5.765 | 1.638 | 0.02398 | 6.810×10-3 | 0.02294 |
| 停留时间/min | 悬浮密度/kg·m-3 | 斜率 | 生长速率/μm·min-1 | 初始粒度密度对数值 | 初始粒度密度/1019μm-4 | 初始成核速率/×104L·min-1 |
|---|---|---|---|---|---|---|
| 10 | 5.64 | -0.26692 | 0.37464 | 45.195 | 4.2483 | 15.916 |
| 15 | 6.46 | -0.26378 | 0.25274 | 44.281 | 1.7034 | 4.3051 |
| 20 | 6.98 | -0.63040 | 0.19009 | 43.867 | 1.1251 | 2.1387 |
| 25 | 7.64 | -0.23503 | 0.17019 | 43.961 | 1.2370 | 2.1053 |
| 30 | 8.06 | -0.23454 | 0.14212 | 44.145 | 1.4870 | 2.1134 |
| 35 | 8.38 | -0.23379 | 0.12221 | 43.702 | 9.5488 | 1.1669 |
| 40 | 8.84 | -0.23354 | 0.10705 | 43.818 | 1.0723 | 1.1449 |
| 45 | 9.38 | -0.22802 | 0.097460 | 43.302 | 0.64000 | 0.62372 |
表3 不同结晶时间下的晶桨悬浮密度、成核速率和生长速率
| 停留时间/min | 悬浮密度/kg·m-3 | 斜率 | 生长速率/μm·min-1 | 初始粒度密度对数值 | 初始粒度密度/1019μm-4 | 初始成核速率/×104L·min-1 |
|---|---|---|---|---|---|---|
| 10 | 5.64 | -0.26692 | 0.37464 | 45.195 | 4.2483 | 15.916 |
| 15 | 6.46 | -0.26378 | 0.25274 | 44.281 | 1.7034 | 4.3051 |
| 20 | 6.98 | -0.63040 | 0.19009 | 43.867 | 1.1251 | 2.1387 |
| 25 | 7.64 | -0.23503 | 0.17019 | 43.961 | 1.2370 | 2.1053 |
| 30 | 8.06 | -0.23454 | 0.14212 | 44.145 | 1.4870 | 2.1134 |
| 35 | 8.38 | -0.23379 | 0.12221 | 43.702 | 9.5488 | 1.1669 |
| 40 | 8.84 | -0.23354 | 0.10705 | 43.818 | 1.0723 | 1.1449 |
| 45 | 9.38 | -0.22802 | 0.097460 | 43.302 | 0.64000 | 0.62372 |
| 13 | LI Xiang, YANG Ke, LI Bing, et al. Crystallization kinetics of Hf28Be18Ti17Zr17Cu7.5Ni12.5 high-entropy bulk metallic glass[J]. Thermochimica Acta, 2023, 724: 179497. |
| 14 | CAPUTO Maria Rosaria, OLMOS Asier, LI Bo, et al. Synthesis, morphology, and crystallization kinetics of polyheptalactone (PHL)[J]. Biomacromolecules, 2023, 24(7): 3256-3267. |
| 15 | WU Wenya, LEONG Fong Yew, TONG Shi Wun, et al. Time-resolved dynamic crystallization at liquid/vapor interface[J]. The Journal of Physical Chemistry C, 2022, 126(46): 19926-19933. |
| 16 | 范嘉昊, 张洋, 范兵强, 等. (NH4)2SO4和Na2SO4混合溶液中(NH4)2SO4结晶动力学及铁/铝/锰/铬等离子对(NH4)2SO4结晶的影响规律[J]. 化工进展, 2023, 42(1): 488-496. |
| FAN Jiahao, ZHANG Yang, FAN Bingqiang, et al. Crystallization kinetics of (NH4)2SO4 in mixed solution of (NH4)2SO4 and Na2SO4 and the influence of Fe/Al/Mn/Cr ions on crystallization[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 488-496. | |
| 17 | TIERNEY Teresa B, RASMUSON Åke C, HUDSON Sarah P. Size and shape control of micron-sized salicylic acid crystals during antisolvent crystallization[J]. Organic Process Research & Development, 2017, 21(11): 1732-1740. |
| 18 | ZHOU Yanan, WANG Jingkang, WANG Ting, et al. Self-assembly of monodispersed carnosine spherical crystals in a reverse antisolvent crystallization process[J]. Crystal Growth & Design, 2019, 19(5): 2695-2705. |
| 19 | OSTERGAARD Iben, DE DIEGO Heidi Lopez, QU Haiyan, et al. Risk-based operation of a continuous mixed-suspension-mixed-product-removal antisolvent crystallization process for polymorphic control[J]. Organic Process Research & Development, 2020, 24(12): 2840-2852. |
| 20 | 万亚萌. 盐酸去氧肾上腺素溶析结晶过程研究[D]. 郑州: 郑州大学, 2021. |
| WAN Yameng. Study on the crystallization process of phenylephrine hydrochloride dissolution[D]. Zhengzhou: Zhengzhou University, 2021. | |
| 21 | 陈建新. 氢化可的松结晶过程研究[D]. 天津: 天津大学, 2005. |
| CHEN Jianxin. A study on crystallization process of hydrocortisone[D]. Tianjin: Tianjin University, 2005. | |
| 22 | 国家市场监督管理总局, 国家标准化管理委员会. 颗粒材料物理性能测试 第3部分:流动性指数的测量: [S]. 北京: 中国标准出版社, 2019. |
| State Administration for Market Regulation, Standardization Administration of the People's Republic of China. Granular materials-Physical properties: Part 3: Fluidity index: [S]. Beijing: Standards Press of China, 2019. | |
| 23 | 吴灏. 酰胺类药物晶体生长过程强化研究[D]. 天津: 天津大学, 2021. |
| WU Hao. Study on strengthening the crystal growth process of amide drugs[D]. Tianjin: Tianjin University, 2021. | |
| 24 | 鲍颖. 盐酸大观霉素溶析结晶过程研究[D]. 天津: 天津大学, 2003. |
| BAO Ying. Study on dissolution and crystallization process of spectinomycin hydrochloride[D]. Tianjin: Tianjin University, 2003. | |
| 25 | HEFFERNAN Claire, UKRAINCZYK Marko, ZEGLINSKI Jacek, et al. Influence of structurally related impurities on the crystal nucleation of curcumin[J]. Crystal Growth & Design, 2018, 18(8): 4715-4723. |
| 26 | DAVEY Roger J, SCHROEDER Sven L M, HORST Joop H TER. Nucleation of organic crystals-a molecular perspective[J]. Angewandte Chemie International Edition, 2013, 52(8): 2166-2179. |
| 27 | ZOU Fengxia, ZHUANG Wei, WU Jinglan, et al. Determination of metastable zone widths and the primary nucleation and growth mechanisms for the crystallization of disodium guanosine 5'-monophosphate from a water-ethanol system[J]. Industrial & Engineering Chemistry Research, 2015, 54(1): 137-145. |
| 28 | MAHADEVAN C, JEGATHEESAN C S. Nucleation studies in supersaturated aqueous solutions of chromate doped potassium dihydrogen phosphate[J]. Journal of the Indian Chemical Society, 1999, 76(1): 47-48. |
| 29 | 时钧. 化学工程手册[M]. 2版. 北京: 化学工业出版社, 1996. |
| SHI Jun. Chemical engineering handbook[M]. 2nd ed. Beijing: Chemical Industry Press, 1996. | |
| 1 | QUAN Lin, HE Hua. Treatment with olopatadine and naphazoline hydrochloride reduces allergic conjunctivitis in mice through alterations in inflammation, NGF and VEGF[J]. Molecular Medicine Reports, 2016, 13(4): 3319-3325. |
| 2 | 李苗, 童颖, 乔戈, 等. 盐酸萘甲唑啉滴鼻液质量评价[J]. 医药导报, 2021, 40(3): 369-373. |
| LI Miao, TONG Ying, QIAO Ge, et al. Quality evaluation of naphazoline hydrochloride nasal drops[J]. Herald of Medicine, 2021, 40(3): 369-373. | |
| 3 | 卜晔, 杜建勇, 郑丽霞, 等. 盐酸萘甲唑啉的药物新用途: CN114652717A[P]. 2022-06-24. |
| BU Ye, DU Jianyong, ZHENG Lixia, et al. New medicinal application of naphazoline hydrochloride: CN114652717A[P]. 2022-06-24. | |
| 4 | 李明乐, 李盼欣, 张志光, 等. 盐酸萘甲唑啉的合成工艺研究[J]. 河北科技大学学报, 2021, 42(3): 265-270. |
| LI Mingle, LI Panxin, ZHANG Zhiguang, et al. Study on the synthesis process of naphazoline hydrochloride[J]. Journal of Hebei University of Science and Technology, 2021, 42(3): 265-270. | |
| 5 | PODDER A, MUKHOPADHYAY B P, DATTAGUPTA J K, et al. 2-(1-Naphthylmethyl)-2-imidazoline hydrochloride (naphazoline hydrochloride), C14H15N2+.Cl-, an α-adrenergic agonist[J]. Acta Crystallographica Section C Crystal Structure Communications, 1983, 39(4): 495-497. |
| 6 | CRAVER B, CHASE H, YONKMAN F F. Pharmacologic studies of a new vasoconstrictor: 2-naphthyl-(1')-methyl-imidazoline hydrochloride (privine or naphthazoline) Ⅱ. vascular and respiratory reactions in the anesthetized dog[J]. The Journal of Pharmacology and Experimental Therapeutics, 1944, 82(3): 275-287. |
| 7 | 邢科, 燕禹辛, 刘学艳. 高效液相色谱法和电位滴定法测定盐酸萘甲唑啉原料药含量[J]. 山东化工, 2021, 50(7): 101-102, 105. |
| XING Ke, YAN Yuxin, LIU Xueyan. Determination of naphazoline hydrochloride by HPLC and potentiometry titration[J]. Shandong Chemical Industry, 2021, 50(7): 101-102, 105. | |
| 8 | 龚俊波, 孙杰, 王静康. 面向智能制造的工业结晶研究进展[J]. 化工学报, 2018, 69(11): 4505-4517. |
| GONG Junbo, SUN Jie, WANG Jingkang. Research progress of industrial crystallization towards intelligent manufacturing[J]. CIESC Journal, 2018, 69(11): 4505-4517. | |
| 9 | Marko TRAMPUŽ, Dušan TESLIĆ, LIKOZAR Blaž. Crystallization of fesoterodine fumarate active pharmaceutical ingredient: Modelling of thermodynamic equilibrium, nucleation, growth, agglomeration and dissolution kinetics and temperature cycling[J]. Chemical Engineering Science, 2019, 201: 97-111. |
| 10 | 黄炎, 孙海龙, 孟子超, 等. 溶析结晶在医药领域的研究进展[J]. 化工进展, 2019, 38(5): 2380-2388. |
| HUANG Yan, SUN Hailong, MENG Zichao, et al. Progress in antisolvent crystallization in pharmaceutical field[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2380-2388. | |
| 11 | 张宇. 基于溶析法强化结晶的氨法捕碳过程研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| ZHANG Yu. Study on carbon capture process by ammonia method based on enhanced crystallization by dissolution method[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 12 | TRIFKOVIC M, SHEIKHZADEH M, ROHANI S. Kinetics estimation and single and multi-objective optimization of a seeded, anti-solvent, isothermal batch crystallizer[J]. Industrial & Engineering Chemistry Research, 2008, 47(5): 1586-1595. |
| 30 | LI Yao, XIN Haoran, ZONG Yukai, et al. A novel nucleation-induced crystallization process towards simultaneous removal of hardness and organics[J]. Separation and Purification Technology, 2023, 307: 122785. |
| 31 | 陈葵. 红霉素结晶过程研究[D]. 上海: 华东理工大学, 2011. |
| CHEN Kui. Study on the erythromycin crystallization processes[D]. Shanghai: East China University of Science and Technology, 2011. | |
| 32 | 丁绪淮, 谈遒. 工业结晶[M]. 北京: 化学工业出版社, 1985. |
| DING Xuhuai, TAN Qiu. Industrial crystallization[M]. Beijing: Chemical Industry Press, 1985. |
| [1] | 焦文磊, 刘震, 陈俊先, 张天钰, 姬忠礼. 叶片式分离元件结构及性能影响因素研究进展[J]. 化工进展, 2024, 43(8): 4187-4202. |
| [2] | 顾颂琦, 孙凡飞, 韦尧, 宋兴飞, 南兵, 李丽娜, 黄宇营. 时间分辨热化学原位XAFS方法[J]. 化工进展, 2024, 43(7): 3747-3755. |
| [3] | 曹景沛, 姚乃瑜, 庞新博, 赵小燕, 赵静平, 蔡士杰, 徐敏, 冯晓博, 伊凤娇. 煤热解研究进展及其发展历程[J]. 化工进展, 2024, 43(7): 3620-3636. |
| [4] | 丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612. |
| [5] | 张昊, 陆小明. 纳米钛酸钡前体热分解反应动力学及颗粒演化机理[J]. 化工进展, 2024, 43(7): 3987-3995. |
| [6] | 马栋, 解桂林, 田治华, 王勤辉, 张建国, 宋慧林, 钟晋. 流化床中煤气化细渣高温还原磷石膏过程[J]. 化工进展, 2024, 43(6): 3479-3491. |
| [7] | 熊远帆, 李华山, 龚宇烈. 非共沸工质蒸发式冷凝器多目标优化设计[J]. 化工进展, 2024, 43(6): 2950-2960. |
| [8] | 王东亮, 李婧玮, 孟文亮, 杨勇, 周怀荣, 范宗良. 二氧化碳加氢制甲醇过程碳氢利用率的影响因素与工艺优化分析[J]. 化工进展, 2024, 43(5): 2843-2850. |
| [9] | 江安迪, 丁雪兴, 王世鹏, 丁俊华, 力宁. 超临界CO2干气密封热动力学性能研究进展[J]. 化工进展, 2024, 43(5): 2354-2369. |
| [10] | 黄淄博, 周文静, 魏进家. 基于ReaxFF MD模拟的低阶煤热解产物演化规律及反应机理[J]. 化工进展, 2024, 43(5): 2409-2419. |
| [11] | 石鎏, 胡振中, 李显, 孙一鸣, 童珊, 刘显哲, 郭丽, 刘豪, 彭冰, 李硕, 罗光前, 姚洪. 生物质气压烘焙技术研究进展[J]. 化工进展, 2024, 43(5): 2494-2511. |
| [12] | 张宝, 王鹏, 安勇攀, 吕平, 蒋建良. 船舶应用燃料电池系统的设计与试验[J]. 化工进展, 2024, 43(5): 2554-2567. |
| [13] | 刘思宇, 杨卷, 陈培, 陈祖田, 闫斌, 刘育红, 邱介山. 富氮多孔碳纳米片的氮掺杂构型调控及其储锌性能[J]. 化工进展, 2024, 43(5): 2673-2683. |
| [14] | 王德斌, 林梦雨, 杨雪, 董殿权. 锌掺杂型钛系铯离子筛的制备及其吸附性能[J]. 化工进展, 2024, 43(4): 1953-1961. |
| [15] | 张鹏飞, 陈伟鹏, 肖卓楠, 吕青岗, 张顺风, 张子峰. 红砖掺杂改性白云鄂博铁精矿载氧体性能[J]. 化工进展, 2024, 43(4): 2226-2234. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||