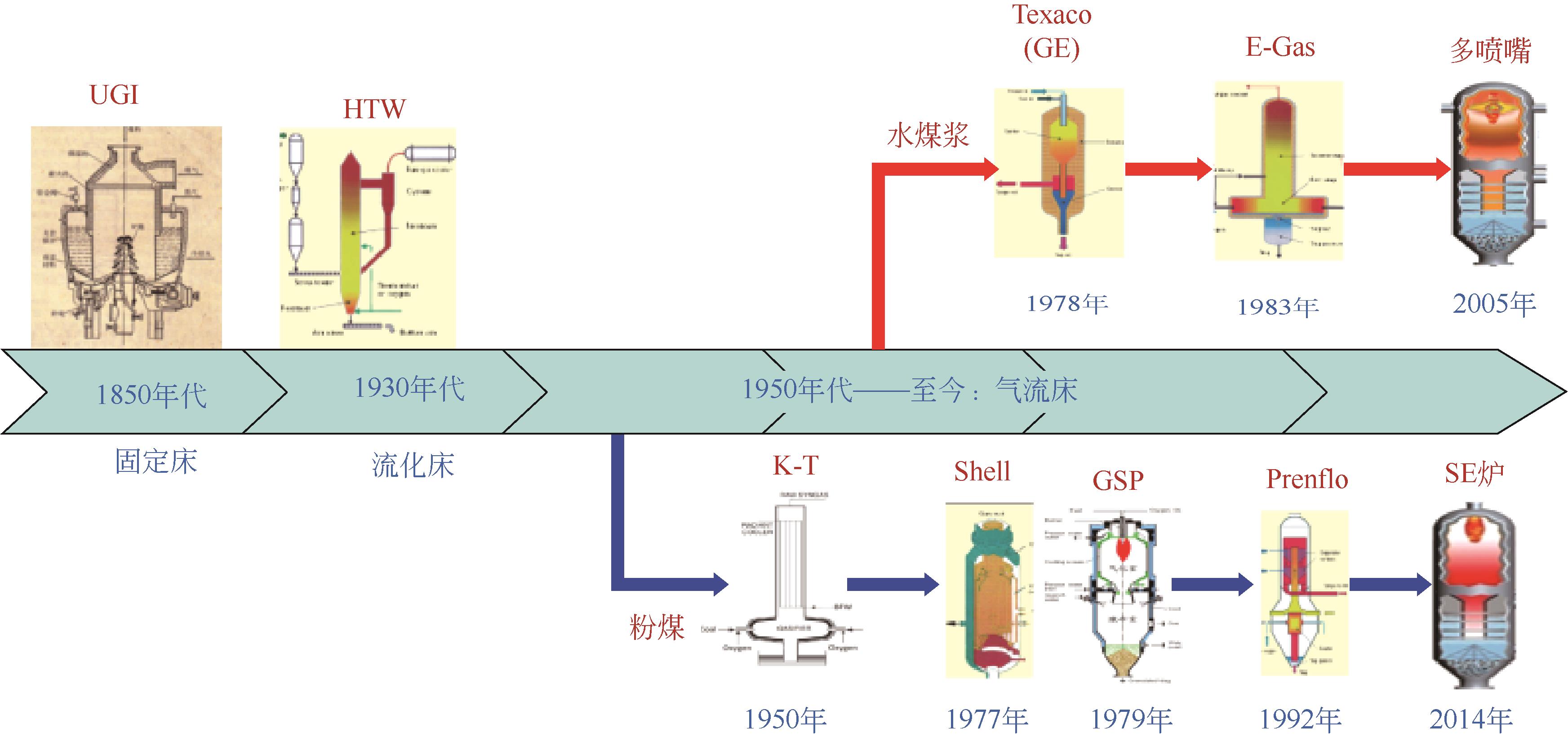
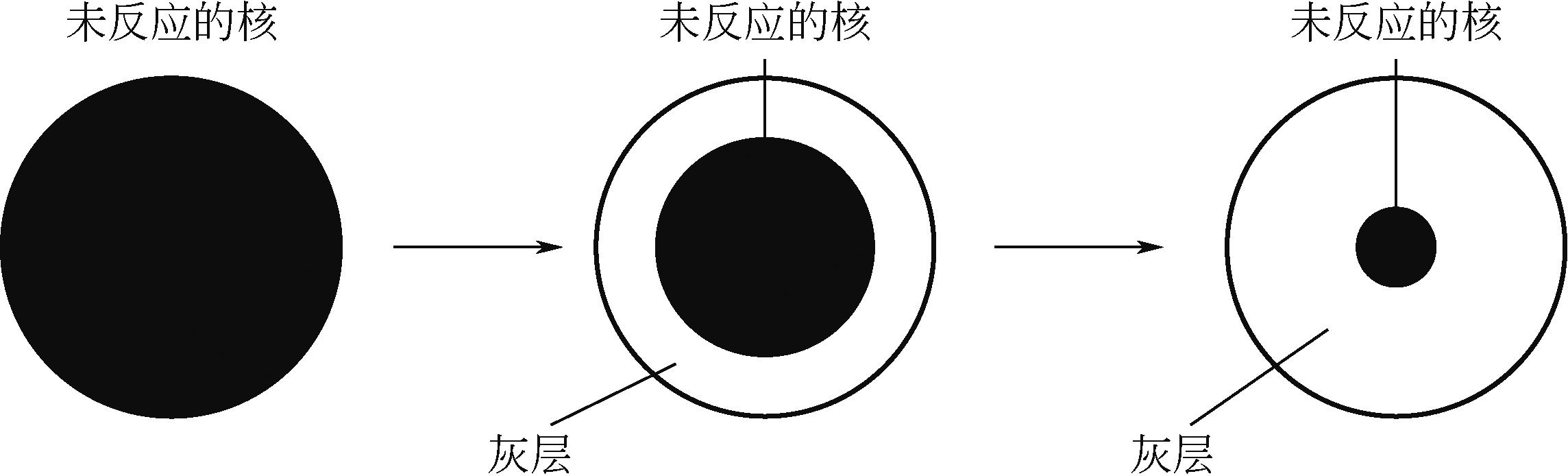
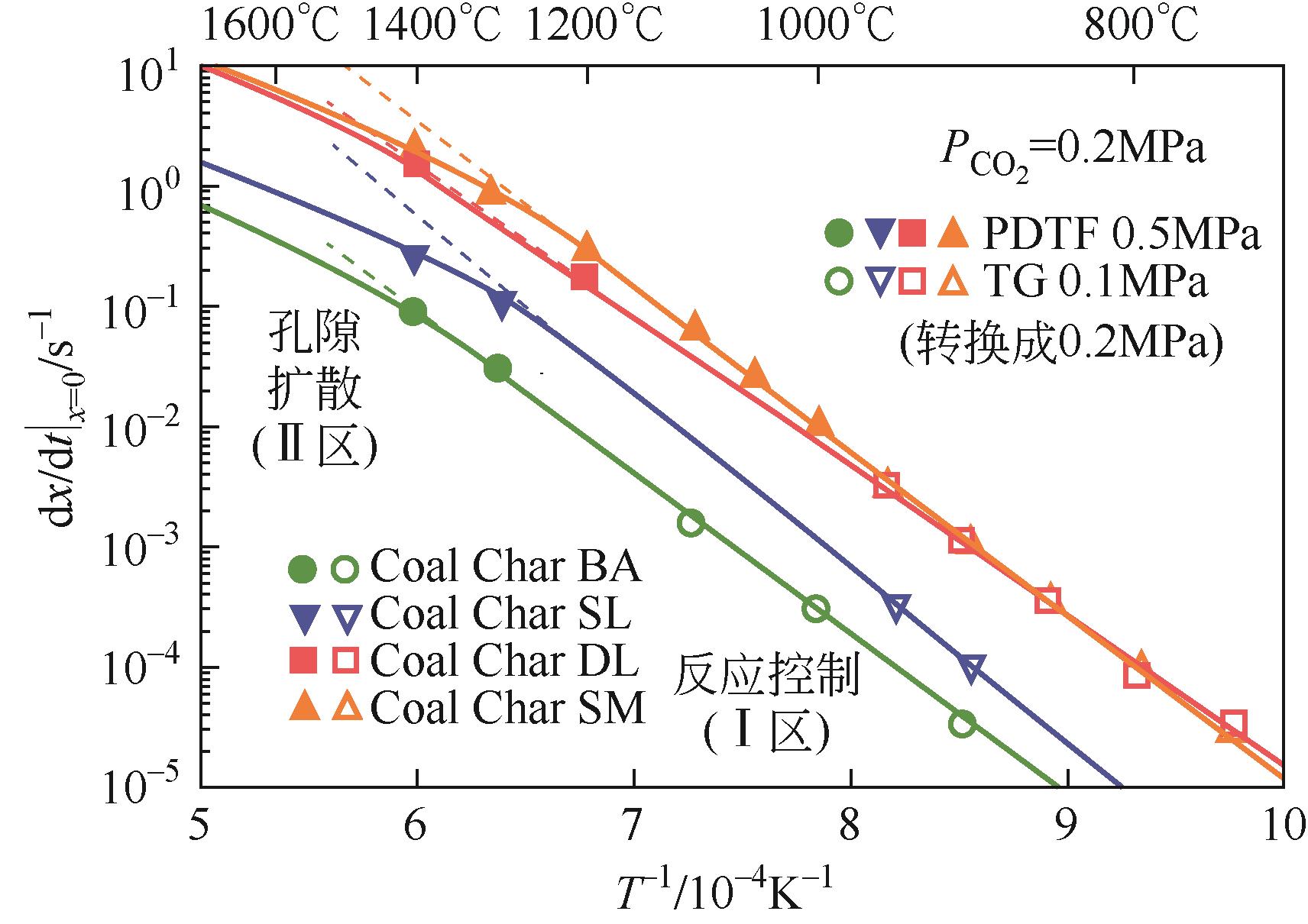
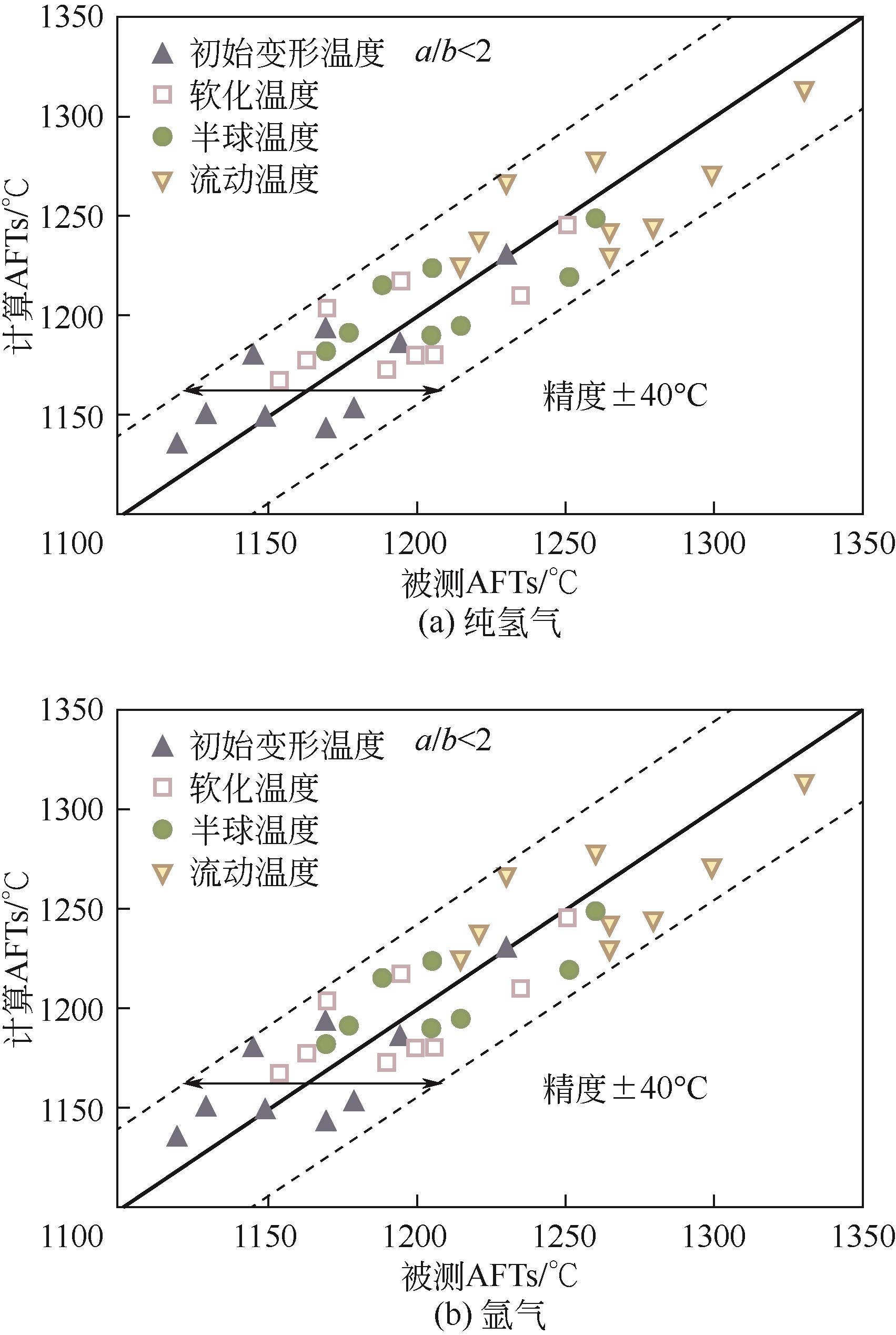
化工进展 ›› 2024, Vol. 43 ›› Issue (7): 3593-3612.DOI: 10.16085/j.issn.1000-6613.2024-0037
煤气化过程反应模型研究进展
丁路1,2( ), 王培尧1,2(
), 王培尧1,2( ), 孔令学3(
), 孔令学3( ), 白进3, 于广锁1,2, 李文3, 王辅臣1,2
), 白进3, 于广锁1,2, 李文3, 王辅臣1,2
- 1.华东理工大学洁净煤技术研究所,上海 200237
2.华东理工大学含碳废弃物资源化零碳利用教育部工程研究 中心,上海 200237
3.中国科学院山西煤炭化学研究所煤炭高效低碳利用全国重点实验室,山西 太原 030001
-
收稿日期:2023-01-05修回日期:2024-04-07出版日期:2024-07-25发布日期:2024-08-14 -
通讯作者:丁路,孔令学 -
作者简介:丁路(1987—),男,教授,博士生导师,研究方向为含碳物料气化。E-mail:dinglu@ecust.edu.cn
王培尧(2001—),男,本科生,研究方向为含碳物料气化。E-mail:20001633@mail.ecust.edu.cn。 -
基金资助:国家自然科学基金(22278142);国家自然科学基金(U23A20129);山西煤化所创新基金(SCJC-DT-2022-01);化学工程联合国家重点实验室开放课题(SKL-ChE-23C05);山西煤化所创新基金(SCJC-DT-2022-01)
Progress on reaction models for coal gasification processes
DING Lu1,2( ), WANG Peiyao1,2(
), WANG Peiyao1,2( ), KONG Lingxue3(
), KONG Lingxue3( ), BAI Jin3, YU Guangsuo1,2, LI Wen3, WANG Fuchen1,2
), BAI Jin3, YU Guangsuo1,2, LI Wen3, WANG Fuchen1,2
- 1.Institute of Clean Coal Technology, East China University of Science and Technology, Shanghai 200237, China
2.Carbon-Containing Waste Resource Zero Carbon Utilization Engineering Center of the Ministry of Education, East China University of Science and Technology, Shanghai 200237, China
3.State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, Shanxi, China
-
Received:2023-01-05Revised:2024-04-07Online:2024-07-25Published:2024-08-14 -
Contact:DING Lu, KONG Lingxue
摘要:
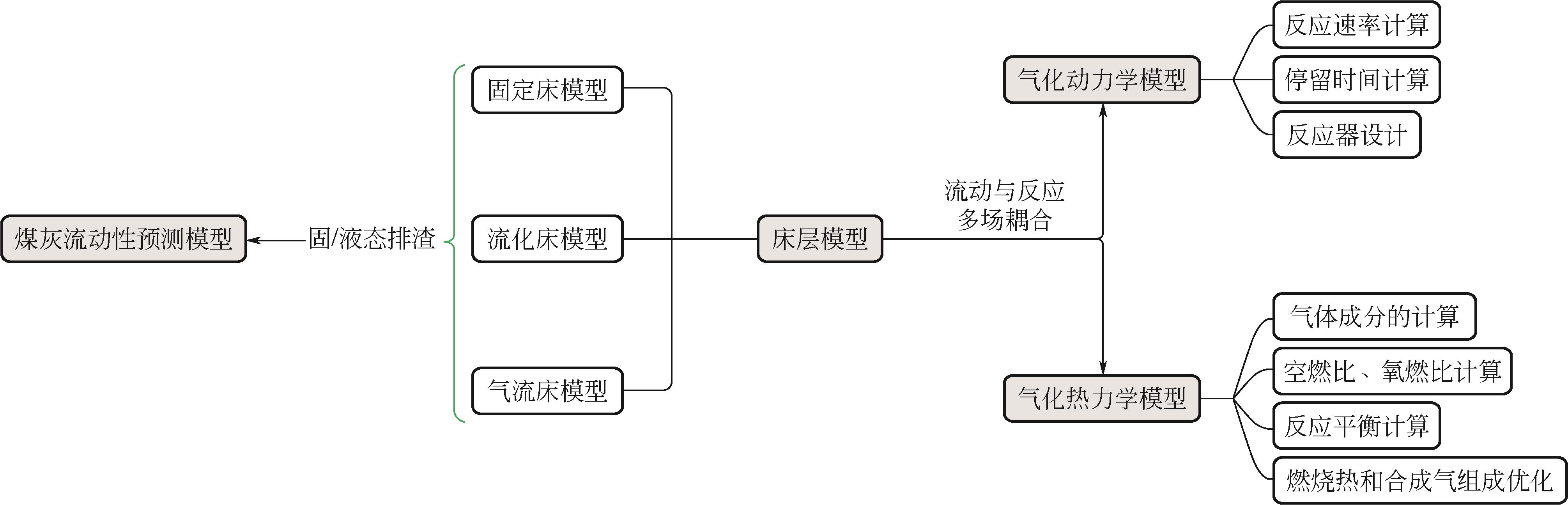
煤炭是我国能源安全的压舱石,煤气化作为煤炭清洁高效利用的核心技术对实现“碳达峰、碳中和”的战略目标具有重要作用。基于动态原位表征揭示煤气化反应机理并建立煤气化模型,对拓展煤炭和生物质等含碳物料作为气化原料的适用性,开发新型高效的气化技术有重要的理论指导价值。同时,煤灰的流动性质是影响气化炉长周期稳定运行的关键指标。本文详细综述了煤气化过程的动力学模型、热力学模型、床层模型以及煤灰流动性的预测模型,对比了各类模型的优缺点、适用的条件以及描述气化过程的性能,指出了不同方法建立的模型存在的问题,为气化过程的总包反应模型建立提供了理论指导,并针对煤气化过程反应模型未来的研究重点进行了展望。
中图分类号:
引用本文
丁路, 王培尧, 孔令学, 白进, 于广锁, 李文, 王辅臣. 煤气化过程反应模型研究进展[J]. 化工进展, 2024, 43(7): 3593-3612.
DING Lu, WANG Peiyao, KONG Lingxue, BAI Jin, YU Guangsuo, LI Wen, WANG Fuchen. Progress on reaction models for coal gasification processes[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3593-3612.
| 优点 | 缺点 |
|---|---|
| 不需考虑气化炉形状 | 无论何种气化炉在低工作温度下都无法获得热力学平衡 |
| 不需要了解转换机制 | 结果不精确 |
| 设置操作条件不受限制 | 难以解释炭化和焦油的形成 |
| 可提供最大的反应物转化率和最大的产物收率 | 忽视了CO2和CH4的产生 |
| 可便捷地进行灵敏度分析 | 高估了H2、CO的产品收率 |
| 相对容易实现 | 气化炉的几何形状和设计未在模型中考虑 |
| 动力学模型与之相较计算更加密集 | 无法准确估计焦油含量 |
| 可以获得最佳条件 | 不考虑出口流中的焦油、焦炭和CH4 |
表1 热力学平衡模型优缺点
| 优点 | 缺点 |
|---|---|
| 不需考虑气化炉形状 | 无论何种气化炉在低工作温度下都无法获得热力学平衡 |
| 不需要了解转换机制 | 结果不精确 |
| 设置操作条件不受限制 | 难以解释炭化和焦油的形成 |
| 可提供最大的反应物转化率和最大的产物收率 | 忽视了CO2和CH4的产生 |
| 可便捷地进行灵敏度分析 | 高估了H2、CO的产品收率 |
| 相对容易实现 | 气化炉的几何形状和设计未在模型中考虑 |
| 动力学模型与之相较计算更加密集 | 无法准确估计焦油含量 |
| 可以获得最佳条件 | 不考虑出口流中的焦油、焦炭和CH4 |
| 作者 | 年份 | 定义 |
|---|---|---|
| Reid和Cohen | 1944 | 降温过程中全液相流体变为塑性流体对应的温度 |
| Corey | 1964 | 全流体与塑性流体的分界温度,也是降温过程中首次出现屈服应力的温度 |
| Watt和Fereday | 1969 | 结晶相与流体开始发生相互作用时的温度 |
| Winegartner | 1974 | 熔渣由牛顿流体变为假塑性流体的温度 |
| Singer(editor) | 1991 | 固相开始结晶和从液相中析出的温度 |
| Nowok和Benson | 1991 | 熔体的流动性由牛顿流体变为非牛顿流体特性的温度 |
| Nowok等 | 1993 | 熔渣由均相变为多相混合时的温度 |
| Mills和Broadbent | 1994 | 黏度快速上升时对应的温度 |
| Seggiani | 1999 | 熔渣由牛顿流体变为假塑性流体的温度 |
| Vargas等 | 2001 | 晶体开始影响熔渣黏度的温度 |
| Stultz和Kitto | 2005 | 黏度的对数与温度不为线性的温度 |
| Spliethoff | 2010 | 黏度达到250P的温度 |
| Massoudi和Wang | 2011 | 熔渣由牛顿流体变为非牛顿流体的温度 |
表2 临界黏度温度的定义
| 作者 | 年份 | 定义 |
|---|---|---|
| Reid和Cohen | 1944 | 降温过程中全液相流体变为塑性流体对应的温度 |
| Corey | 1964 | 全流体与塑性流体的分界温度,也是降温过程中首次出现屈服应力的温度 |
| Watt和Fereday | 1969 | 结晶相与流体开始发生相互作用时的温度 |
| Winegartner | 1974 | 熔渣由牛顿流体变为假塑性流体的温度 |
| Singer(editor) | 1991 | 固相开始结晶和从液相中析出的温度 |
| Nowok和Benson | 1991 | 熔体的流动性由牛顿流体变为非牛顿流体特性的温度 |
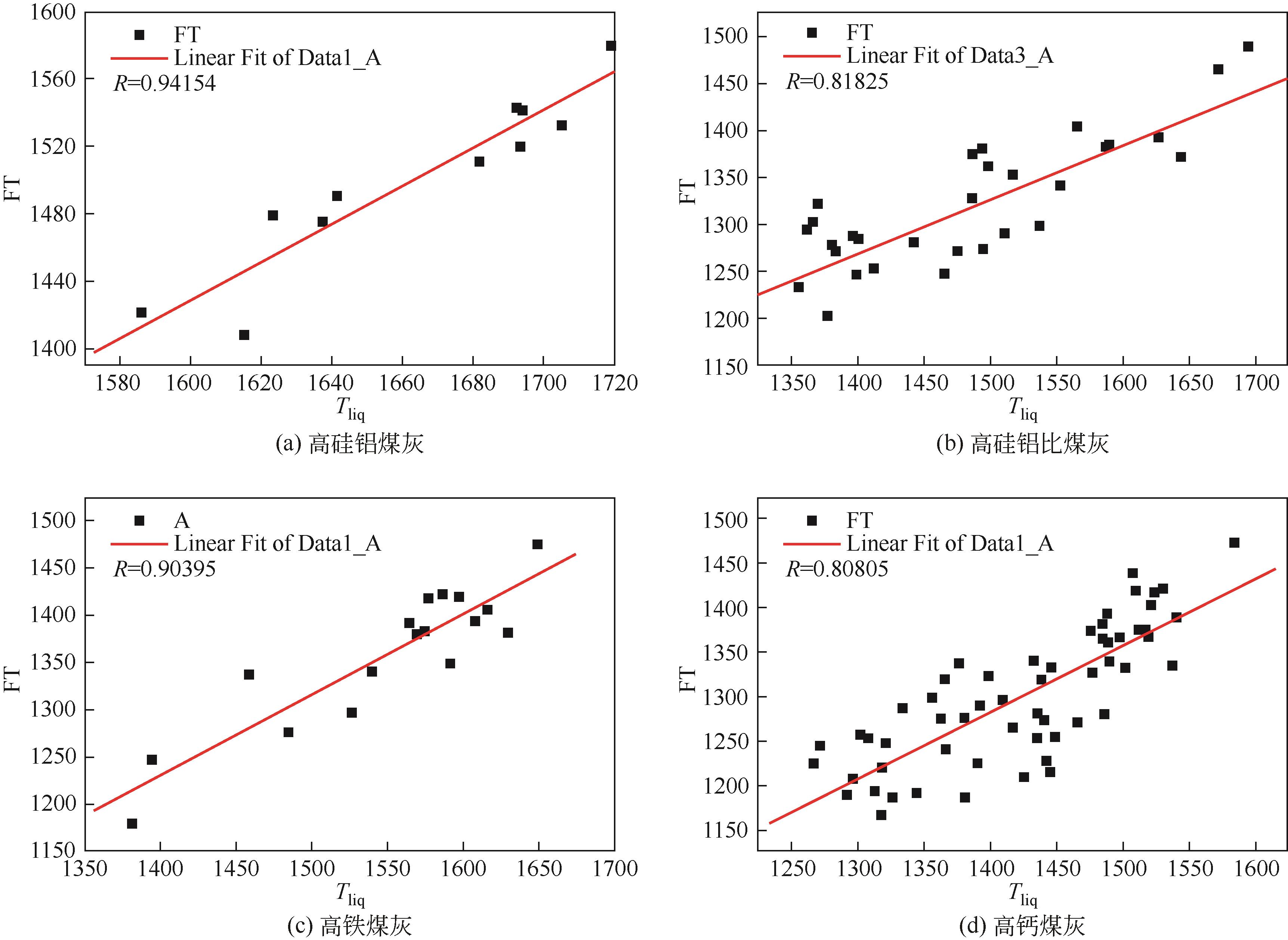
| Nowok等 | 1993 | 熔渣由均相变为多相混合时的温度 |
| Mills和Broadbent | 1994 | 黏度快速上升时对应的温度 |
| Seggiani | 1999 | 熔渣由牛顿流体变为假塑性流体的温度 |
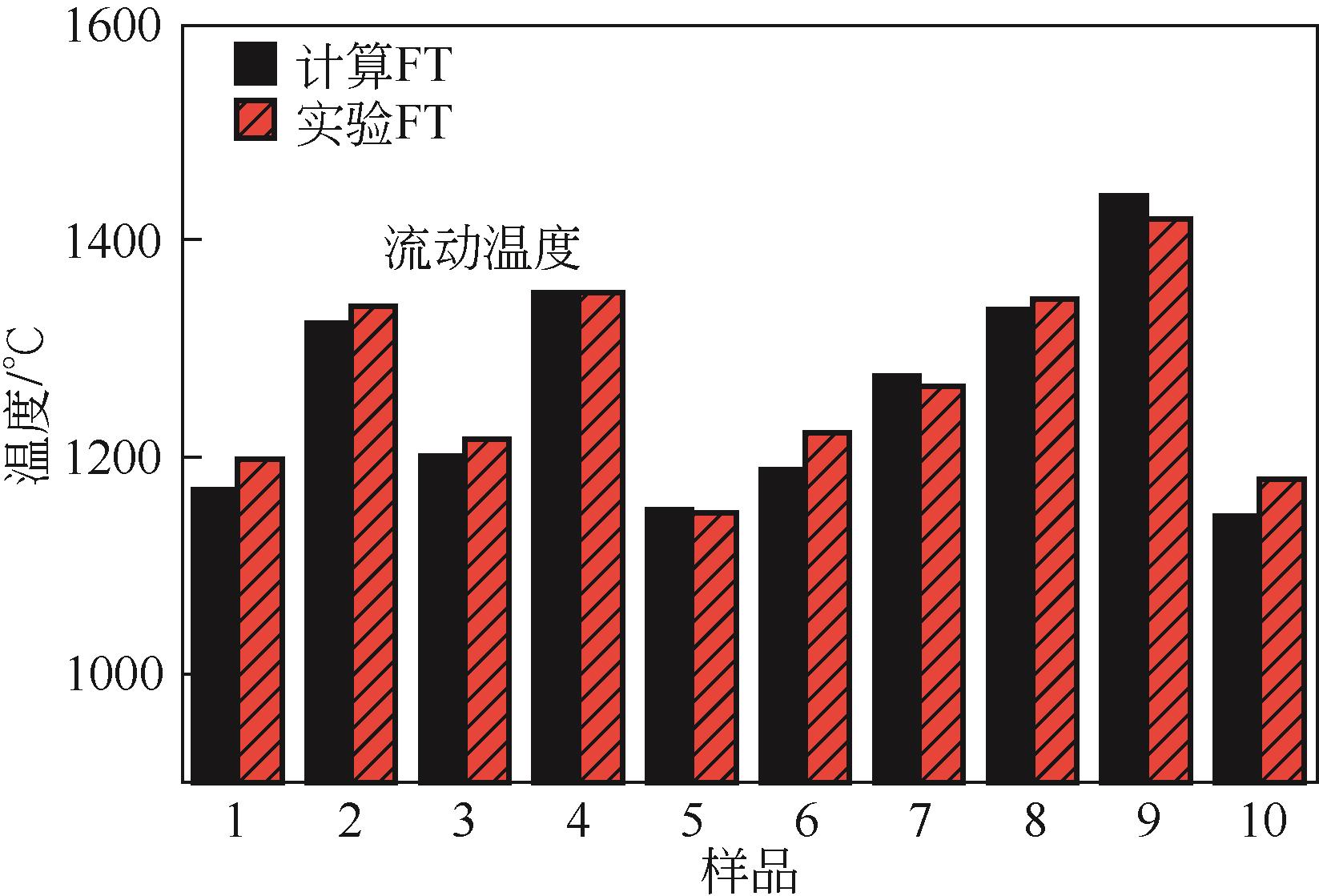
| Vargas等 | 2001 | 晶体开始影响熔渣黏度的温度 |
| Stultz和Kitto | 2005 | 黏度的对数与温度不为线性的温度 |
| Spliethoff | 2010 | 黏度达到250P的温度 |
| Massoudi和Wang | 2011 | 熔渣由牛顿流体变为非牛顿流体的温度 |
| 作者 | 年份 | 公式 |
|---|---|---|
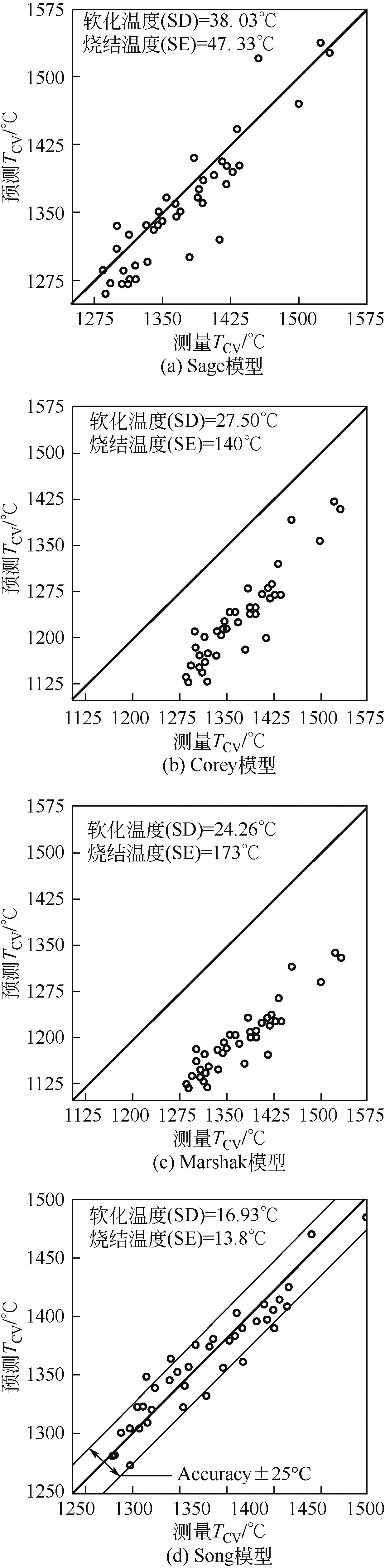
| Reid | 1944 | TCV=TS |
| Sage | 1960 | TCV=HT+111K |
| Watt | 1963 | TCV=3263-1470×(SiO2/Al2O3)+360×(SiO2 /Al2O3)2-14.7×(Fe2O3+CaO+MgO)+0.15×( Fe2O3+CaO+MgO)2 |
| Corey | 1964 | TCV=ST |
| Marshak | 1969 | TCV=0.75×HT+548K |
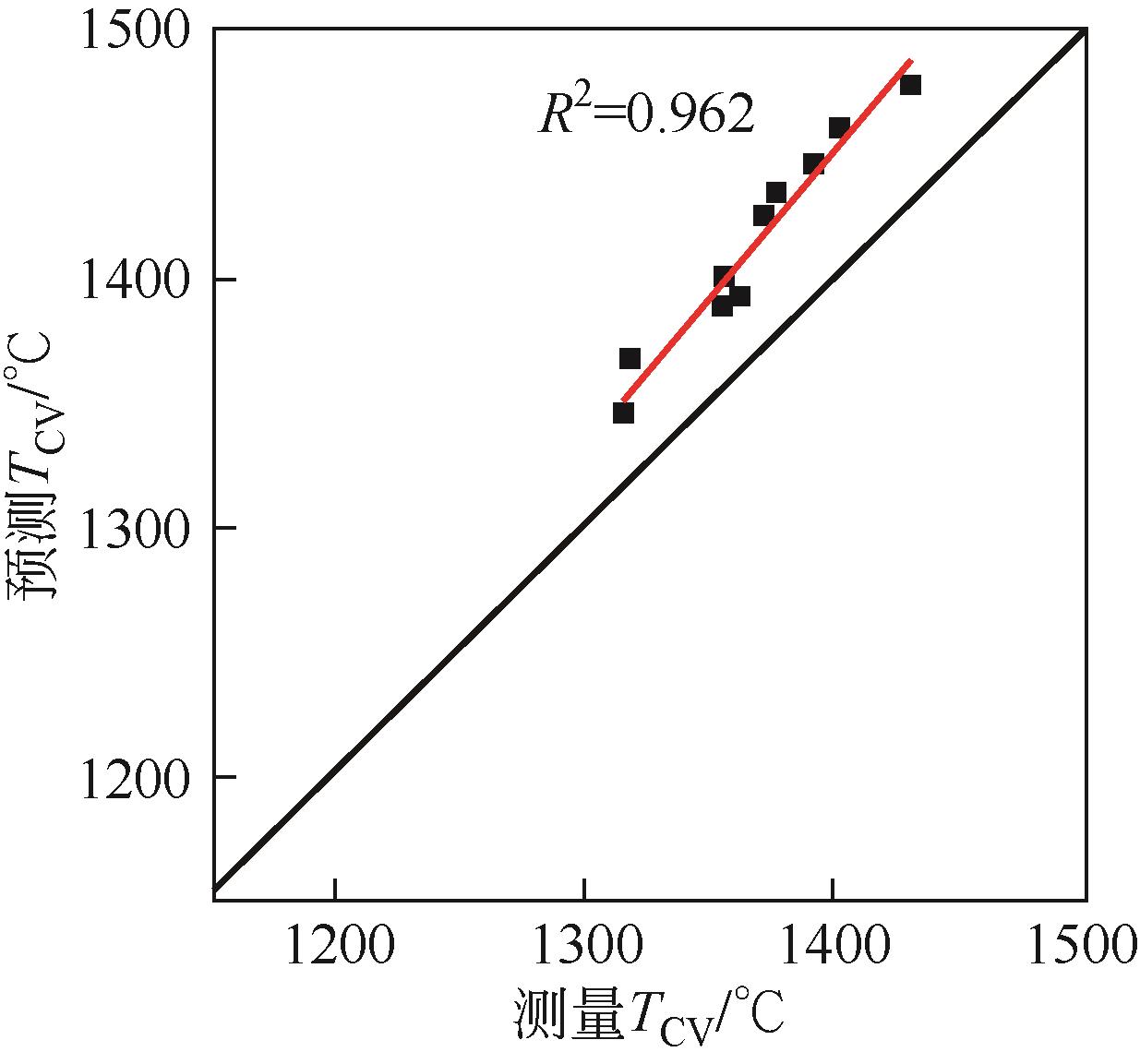
| Song | 2011 | TCV=118+0.894Tliq |
| Kong | 2013 | TCV=0.98Tmax +17.33 |
| Hsieh | 2016 | TCV=FT,TCV=1900-148.3×(SiO2/Al2O3)-8.04×(Fe2O3+1.1FeO+1.43Fe) |
| Ge | 2020 |
表3 临界黏度温度预测公式
| 作者 | 年份 | 公式 |
|---|---|---|
| Reid | 1944 | TCV=TS |
| Sage | 1960 | TCV=HT+111K |
| Watt | 1963 | TCV=3263-1470×(SiO2/Al2O3)+360×(SiO2 /Al2O3)2-14.7×(Fe2O3+CaO+MgO)+0.15×( Fe2O3+CaO+MgO)2 |
| Corey | 1964 | TCV=ST |
| Marshak | 1969 | TCV=0.75×HT+548K |
| Song | 2011 | TCV=118+0.894Tliq |
| Kong | 2013 | TCV=0.98Tmax +17.33 |
| Hsieh | 2016 | TCV=FT,TCV=1900-148.3×(SiO2/Al2O3)-8.04×(Fe2O3+1.1FeO+1.43Fe) |
| Ge | 2020 |
| 1 | XU Guangwen, BAI Dingrong, XU Chunming, et al. Challenges and opportunities for engineering thermochemistry in carbon-neutralization technologies[J]. National Science Review, 2022, 10(9): nwac217. |
| 2 | WU Xiaoxiang, GONG Yan, GUO Qinghua, et al. Experimental study of particle evolution characteristics below the burner plane in an impinging entrained-flow gasifier[J]. Chemical Engineering Science, 2022, 251: 117445. |
| 3 | 李文, 白进. 煤的灰化学[M]. 北京: 科学出版社, 2013. |
| LI Wen, BAI Jin. Chemistry of ash from coal[M]. Beijing: Science Press, 2013. | |
| 4 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 煤灰熔融性的测定方法: [S]. 北京: 中国标准出版社, 2008. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Determination of fusibility of coal ash: [S]. Beijing: Standards Press of China, 2008. | |
| 5 | SONG Wen J, TANG Li H, ZHU Xue D, et al. Effect of coal ash composition on ash fusion temperatures[J]. Energy & Fuels, 2010, 24(1): 182-189. |
| 6 | KONG Lingxue, BAI Jin, LI Wen. Viscosity-temperature property of coal ash slag at the condition of entrained flow gasification: A review[J]. Fuel Processing Technology, 2021, 215: 106751. |
| 7 | RONCANCIO Rathziel, GORE Jay P. CO2 char gasification: A systematic review from 2014 to 2020[J]. Energy Conversion and Management: X, 2021, 10: 100060. |
| 8 | ROBERTS D G, HARRIS D J. Char gasification with O2, CO2, and H2O: Effects of pressure on intrinsic reaction kinetics[J]. Energy and Fuels, 2000, 14(2): 483-489. |
| 9 | LIU Guisu, TATE A G, BRYANT G W, et al. Mathematical modeling of coal char reactivity with CO2 at high pressures and temperatures[J]. Fuel, 2000, 79(10): 1145-1154. |
| 10 | GOMEZ Arturo, VARGAS Marlon, MAHINPEY Nader. A theoretical model to estimate steam and CO2 gasification rates based on feedstock characterization properties[J]. Fuel Processing Technology, 2016, 149: 187-194. |
| 11 | 张凡. 一种堡德河盆地次烟煤催化CO2气化的研究[D]. 北京: 中国矿业大学(北京), 2015. |
| ZHANG Fan. Catalytic CO2 gasification of a sub-bituminous coal from powder river basin[D]. Beijing: China University of Mining & Technology, Beijing, 2015. | |
| 12 | PORADA Stanisław, CZERSKI Grzegorz, GRZYWACZ Przemysław, et al. Comparison of the gasification of coals and their chars with CO2 based on the formation kinetics of gaseous products[J]. Thermochimica Acta, 2017, 653: 97-105. |
| 13 | ZUO Haibin, GENG Weiwei, ZHANG Jianliang, et al. Comparison of kinetic models for isothermal CO2 gasification of coal char-biomass char blended char[J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(4): 363-370. |
| 14 | LIU Zhengjian, WANG Guangwei, ZHANG Jianliang, et al. Study on CO2 gasification reactivity and structure characteristics of carbonaceous materials from the corex furnace[J]. Energy & Fuels, 2018, 32(5): 6155-6166. |
| 15 | LI Rongpeng, ZHANG Jianliang, WANG Guangwei, et al. Study on CO2 gasification reactivity of biomass char derived from high-temperature rapid pyrolysis[J]. Applied Thermal Engineering, 2017, 121: 1022-1031. |
| 16 | KOMAROVA Evgeniia, ABOSTEIF Ziad, GUHL Stefan, et al. Brown coal char CO2-gasification kinetics with respect to the char structure Part Ⅱ: Kinetics and correlations[J]. The Canadian Journal of Chemical Engineering, 2019, 97(1): 226-237. |
| 17 | ZHANG Fan, XU Deping, WANG Yonggang, et al. CO2 gasification of Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst[J]. Applied Energy, 2015, 145: 295-305. |
| 18 | GOMEZ Arturo, SILBERMANN Rico, MAHINPEY Nader. A comprehensive experimental procedure for CO2 coal gasification: Is there really a maximum reaction rate?[J]. Applied Energy, 2014, 124: 73-81. |
| 19 | TANNER Joanne, BHATTACHARYA Sankar. Kinetics of CO2 and steam gasification of Victorian brown coal chars[J]. Chemical Engineering Journal, 2016, 285: 331-340. |
| 20 | ZHANG Jianliang, WANG Guangwei, SHAO Jiugang, et al. A modified random pore model for the kinetics of char gasification[J]. BioResources, 2014, 9(2): 3497-3507. |
| 21 | WANG Guangwei, ZHANG Jianliang, SHAO Jiugang, et al. Experimental and modeling studies on CO2 gasification of biomass chars[J]. Energy, 2016, 114: 143-154. |
| 22 | LIU Hao, LUO Chunhua, KANEKO Masahiro, et al. Unification of gasification kinetics of char in CO2 at elevated temperatures with a modified random pore model[J]. Energy & Fuels, 2003, 17(4): 961-970. |
| 23 | ZHANG Yan, ASHIZAWA Masami, KAJITANI Shiro, et al. Proposal of a semi-empirical kinetic model to reconcile with gasification reactivity profiles of biomass chars[J]. Fuel, 2008, 87(4/5): 475-481. |
| 24 | GUPTA Ankita, THENGANE Sonal K, MAHAJANI Sanjay. CO2 gasification of char from lignocellulosic garden waste: Experimental and kinetic study[J]. Bioresource Technology, 2018, 263: 180-191. |
| 25 | KRAMB Jason, KONTTINEN Jukka, Alberto GÓMEZ-BAREA, et al. Modeling biomass char gasification kinetics for improving prediction of carbon conversion in a fluidized bed gasifier[J]. Fuel, 2014, 132: 107-115. |
| 26 | BETANCUR Mariluz, NATALIA ARENAS Cindy, DANIEL MARTÍNEZ Juan, et al. CO2 gasification of char derived from waste tire pyrolysis: Kinetic models comparison[J]. Fuel, 2020, 273: 117745. |
| 27 | SHA Xingzhong, CHEN Yigong, CAO Jianqin, et al. Effects of operating pressure on coal gasification[J]. Fuel, 1990, 69(5): 656-659. |
| 28 | NOZAKI Takao, ADSCHIRI Tadafumi, FUJIMOTO Kaoru. Coal char gasification under pressurized CO2 atmosphere[J]. Fuel, 1992, 71(3): 349-350. |
| 29 | ROBERTS D G, HARRIS D J. Char gasification in mixtures of CO2 and H2O: Competition and inhibition[J]. Fuel, 2007, 86(17/18): 2672-2678. |
| 30 | HUANG Zhimin, ZHANG Jiansheng, ZHAO Yong, et al. Kinetic studies of char gasification by steam and CO2 in the presence of H2 and CO[J]. Fuel Processing Technology, 2010, 91(8): 843-847. |
| 31 | EVERSON Raymond C, NEOMAGUS Hein W J P, KASAINI Henry, et al. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam[J]. Fuel, 2006, 85(7/8): 1076-1082. |
| 32 | 霍威. 煤等含碳物质热解特性及气化反应特性模型化研究[D]. 上海: 华东理工大学, 2015 |
| HUO Wei. Research on pyrolysis characteristics and gasification kinetics modeling of coal and other carbonaceous materials[D]. Shanghai: East China University of Science and Technology, 2015. | |
| 33 | ZOU Jian hui, ZHOU Zhi jie, WANG Fu chen, et al. Modeling reaction kinetics of petroleum coke gasification with CO2 [J]. Chemical Engineering and Processing: Process Intensification, 2007, 46(7): 630-636. |
| 34 | THIELE E W. Relation between catalytic activity and size of particle[J]. Industrial & Engineering Chemistry, 1939, 31(7): 916-920. |
| 35 | HONG Jianhui, HECKER William C, FLETCHER Thomas H. Improving the accuracy of predicting effectiveness factors for mth order and Langmuir rate equations in spherical coordinates[J]. Energy & Fuels, 2000, 14(3): 663-670. |
| 36 | 陈甘棠, 陈建峰, 陈纪忠. 化学反应工程[M]. 4版. 北京: 化学工业出版社, 2021. |
| CHEN Gantang, CHEN Jianfeng, CHEN Jizhong. Chemical reaction engineering[M]. 4th ed. Beijing: Chemical Industry Press, 2021. | |
| 37 | LIU Guisu, NIKSA Stephen. Coal conversion submodels for design applications at elevated pressures. Part Ⅱ. Char gasification[J]. Progress in Energy and Combustion Science, 2004, 30(6): 679-717. |
| 38 | 柳明. 高温气固反应特性与颗粒行为相互作用研究[D]. 上海: 华东理工大学, 2021. |
| LIU Ming. Investigation on interactions between high-temperature gas solid reaction and particle behavior[D]. Shanghai: East China University of Science and Technology, 2021. | |
| 39 | SEIDER W D, WHITE III C W.Chemical reaction equilibrium analysis: Theory and algorithms[J]. AIChE Journal, 1985, 31(1): 176-176. |
| 40 | LOHA Chanchal, CHATTERJEE Pradip K, CHATTOPADHYAY Himadri. Performance of fluidized bed steam gasification of biomass—Modeling and experiment[J]. Energy Conversion and Management, 2011, 52(3): 1583-1588. |
| 41 | LOHA Chanchal, CHATTOPADHYAY Himadri, CHATTERJEE Pradip K. Thermodynamic analysis of hydrogen rich synthetic gas generation from fluidized bed gasification of rice husk[J]. Energy, 2011, 36(7): 4063-4071. |
| 42 | LA VILLETTA M, COSTA M, MASSAROTTI N. Modelling approaches to biomass gasification: A review with emphasis on the stoichiometric method[J]. Renewable and Sustainable Energy Reviews, 2017, 74: 71-88. |
| 43 | Maria PUIG-ARNAVAT, BRUNO Juan Carlos, CORONAS Alberto. Modified thermodynamic equilibrium model for biomass gasification: A study of the influence of operating conditions[J]. Energy & Fuels, 2012, 26(2): 1385-1394. |
| 44 | RAMOS Ana, MONTEIRO Eliseu, ROUBOA Abel. Numerical approaches and comprehensive models for gasification process: A review[J]. Renewable and Sustainable Energy Reviews, 2019, 110: 188-206. |
| 45 | PATRA Tapas Kumar, SHETH Pratik N. Biomass gasification models for downdraft gasifier: A state-of-the-art review[J]. Renewable and Sustainable Energy Reviews, 2015, 50: 583-593. |
| 46 | Maria PUIG-ARNAVAT, BRUNO Joan Carles, CORONAS Alberto. Review and analysis of biomass gasification models[J]. Renewable and Sustainable Energy Reviews, 2010, 14(9): 2841-2851. |
| 47 | RODRIGUEZ-ALEJANDRO David A, Hyungseok NAM, MAGLINAO Amado L, et al. Development of a modified equilibrium model for biomass pilot-scale fluidized bed gasifier performance predictions[J]. Energy, 2016, 115: 1092-1108. |
| 48 | NÁSNER Albany Milena Lozano, LORA Electo Eduardo Silva, PALACIO José Carlos Escobar, et al. Refuse Derived Fuel (RDF) production and gasification in a pilot plant integrated with an Otto cycle ICE through Aspen plus™ modelling: Thermodynamic and economic viability[J]. Waste Management, 2017, 69: 187-201. |
| 49 | RENKEL Maria F, Norbert LÜMMEN. Supplying hydrogen vehicles and ferries in Western Norway with locally produced hydrogen from municipal solid waste[J]. International Journal of Hydrogen Energy, 2018, 43(5): 2585-2600. |
| 50 | SAHA Pretom, HELAL UDDIN M, TOUFIQ REZA M. A steady-state equilibrium-based carbon dioxide gasification simulation model for hydrothermally carbonized cow manure[J]. Energy Conversion and Management, 2019, 191: 12-22. |
| 51 | RUPESH S, MURALEEDHARAN C, ARUN P. ASPEN plus modelling of air-steam gasification of biomass with sorbent enabled CO2 capture[J]. Resource-Efficient Technologies, 2016, 2(2): 94-103. |
| 52 | SHABBAR Syed, JANAJREH Isam. Thermodynamic equilibrium analysis of coal gasification using Gibbs energy minimization method[J]. Energy Conversion and Management, 2013, 65: 755-763. |
| 53 | ROKNI Masoud. Thermodynamic analyses of municipal solid waste gasification plant integrated with solid oxide fuel cell and Stirling hybrid system[J]. International Journal of Hydrogen Energy, 2015, 40(24): 7855-7869. |
| 54 | SAFARIAN Sahar, Rúnar UNNÞÓRSSON, RICHTER Christiaan. A review of biomass gasification modelling[J]. Renewable and Sustainable Energy Reviews, 2019, 110: 378-391. |
| 55 | BARUAH Dipal, BARUAH D C. Modeling of biomass gasification: A review[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 806-815. |
| 56 | 于遵宏, 王辅臣. 煤炭气化技术[M]. 北京: 化学工业出版社, 2010. |
| YU Zunhong, WANG Fuchen. coal gasification technology[M]. Beijing: Chemical Industry Press, 2010. | |
| 57 | HOBBS Michael L, RADULOVIC Predrag T, Douglas SMOOT L. Modeling fixed-bed coal gasifiers[J]. AIChE Journal, 1992, 38(5): 681-702. |
| 58 | YOON H, WEI J, M-M DENN. A model for moving-bed coal gasification reactors[J]. AIChE Journal, 1978, 24: 885-903. |
| 59 | AMUNDSON Neal R, ARRI Luis Ernesto. Char gasification in a countercurrent reactor[J]. AIChE Journal, 1978, 24(1): 87-101. |
| 60 | MACAK Jiri, MALECHA Jiri. Mathematical model for the gasification of coal under pressure[J]. Industrial & Engineering Chemistry Process Design and Development, 1978, 17(1): 92-98. |
| 61 | EARL W, ISLAM K. Steady state model of a Lurgi type coal gasifier[C]//Chemeca 85: Innovation Process Resour. Ind., 13th Aust. Chem. Eng. Conf. 1985: 289-294. |
| 62 | THORSNESS Charles Bennett, KANG Sang Wook. Further development of a general-purpose, packed-bed model for analysis of underground coal gasification processes[R].Lawrence Livermore National Lab., CA (USA), 1985. |
| 63 | BHATTACHARYA Amitava, SALAM Lyle, DUDUKOVIC M P, et al. Experimental and modeling studies in fixed-bed char gasification[J]. Industrial & Engineering Chemistry Process Design and Development, 1986, 25(4): 988-996. |
| 64 | ADANEZ Juan, Garcia LABIANO F. Modeling of moving-bed coal gasifiers[J]. Industrial & Engineering Chemistry Research, 1990, 29(10): 2079-2088. |
| 65 | SOLOMON P R, HAMBLEN D G, CARANGELO R M, et al. General model of coal devolatilization[J]. Energy & Fuels, 1988, 2(4): 405-422. |
| 66 | KATO K, WEN C Y. Bubble assemblage model for fluidized bed catalytic reactors[J]. Chemical Engineering Science, 1969, 24(8): 1351-1369. |
| 67 | 肖显斌, 杨海瑞, 吕俊复, 等. 循环流化床燃烧数学模型[J]. 煤炭转化, 2002, 25(3): 11-16, 42. |
| XIAO Xianbin, YANG Hairui, Junfu LÜ, et al. Review of mathematical modeling in circulating fluidized bed reacor[J]. Coal Conversion, 2002, 25(3): 11-16, 42. | |
| 68 | KIM Y J, 李大骥. 一带有进风管的内循环流化床煤气化反应炉中的模型(续)[J]. 洁净煤燃烧与发电技术, 2000(3): 46-55. |
| KIM Y J, LI Daji. Model of an internal circulating fluidized bed coal gasification reactor with air inlet pipe (continued) [J]. Clean Coal Combustion and Power Generation Technology, 2000, (3): 46-55. | |
| 69 | 岑可法, 倪明江, 骆仲泱, 等. 循环流化床锅炉理论、设计与运行[M]. 北京: 中国电力出版社, 1998. |
| CEN Kefa, Ni Mingjiang, Luo Zhongyang, et al. Theory, design and operation of circulating fluidized bed boiler[M]. Beijing: China Electric Power Press, 1998. | |
| 70 | 叶正才, 吴韬, 王辅臣, 等. 射流携带床气化炉内混合过程的研究[J]. 华东理工大学学报(自然科学版), 1998, 24(4):385-388. |
| YE Zhengcai, WU Tao, WANG Fuchen, et al. Studies on the mixing process in jet-entrained flow bed gasifier[J]. Journal of East China University of Science and Technology, 1998, 24(4):385-388. | |
| 71 | LOLJA Saimir A, HAXHI Hajri, MARTIN Duncan J. Correlations in the properties of Albanian coals[J]. Fuel, 2002, 81(9): 1095-1100. |
| 72 | 刘新兵, 陈茺. 煤灰熔融性的研究[J]. 煤化工, 1995, 23(2): 48-52, 47. |
| LIU Xinbing, CHEN Chong. Study on the fusibility of coal ash[J]. Coal Chemical Industry, 1995, 23(2): 48-52, 47. | |
| 73 | 张德祥, 龙永华, 高晋生, 等. 煤灰中矿物的化学组成与灰熔融性的关系[J]. 华东理工大学学报, 2003, 29(6): 590-594. |
| ZHANG Dexiang, LONG Yonghua, GAO Jinsheng, et al. Relationship between the coal ash fusibility and its chemical composition[J]. Journal of East China University of Science and Technology, 2003, 29(6): 590-594. | |
| 74 | WINEGARTNER E C, RHODES B T. An empirical study of the relation of chemical properties to ash fusion temperatures[J]. Journal of Engineering for Power, 1975, 97(3): 395-403. |
| 75 | SEGGIANI M. Empirical correlations of the ash fusion temperatures and temperature of critical viscosity for coal and biomass ashes[J]. Fuel, 1999, 78(9): 1121-1125. |
| 76 | 陈文敏, 姜宁. 煤灰成分和煤灰熔融性的关系[J]. 洁净煤技术, 1996, 2(2): 34-37. |
| CHEN Wenmin, JIANG Ning. Relation between the coal ash composition and fusibility[J]. Clean Coal Technology, 1996, 2(2): 34-37. | |
| 77 | XU Jie, LIU Xia, LI Dexia, et al. Prediction model for flow temperature of coal ash [J]. 燃料化学学报 (中英文), 2012, 40(12): 1415-1421. |
| 78 | 徐志明, 郑娇丽, 文孝强. 基于偏最小二乘回归的灰熔点预测[J]. 动力工程学报, 2010, 30(10): 788-792, 803. |
| XU Zhiming, ZHENG Jiaoli, WEN Xiaoqiang. Prediction for ash fusion point based on partial least square regression[J]. Journal of Chinese Society of Power Engineering, 2010, 30(10): 788-792, 803. | |
| 79 | 张玉磊, 尹永志, 王希闯. 煤灰成分预测灰熔点的模型[J]. 中氮肥, 2012(6): 10-12. |
| ZHANG Yulei, YIN Yongzhi, WANG Xichuang. Component analysis based ash fusion point prediction model[J]. M-Sized Nitrogenous Fertilizer Progress, 2012(6): 10-12. | |
| 80 | 刘硕, 周安宁, 杨伏生, 等. 煤灰流动温度的预测研究[J]. 煤炭与化工, 2017, 40(3): 20-24. |
| LIU Shuo, ZHOU Anning, YANG Fusheng, et al. Study on prediction of coal ash flow temperature[J]. Coal and Chemical Industry, 2017, 40(3): 20-24. | |
| 81 | HUGGINS Frank E, KOSMACK Deborah A, HUFFMAN Gerald P. Correlation between ash-fusion temperatures and ternary equilibrium phase diagrams[J]. Fuel, 1981, 60(7): 577-584. |
| 82 | Evgueni JAK. Prediction of coal ash fusion temperatures with the F*A*C*T thermodynamic computer package[J]. Fuel, 2002, 81(13): 1655-1668. |
| 83 | SONG Wen J, TANG Li H, ZHU Xue D, et al. Prediction of Chinese coal ash fusion temperatures in Ar and H2 atmospheres[J]. Energy & Fuels, 2009, 23(4): 1990-1997. |
| 84 | SHI Wenju, LAABS Marcel, Markus REINMÖLLER, et al. The fusion mechanism of complex minerals mixture and prediction model for flow temperature of coal ash for gasification[J]. Fuel, 2021, 305: 121448. |
| 85 | SONG Wenjia, DONG Yanhe, WU Yongqiang, et al. Prediction of temperature of critical viscosity for coal ash slag[J]. AIChE Journal, 2011, 57(10): 2921-2925. |
| 86 | YAN Tinggui, BAI Jin, KONG Lingxue, et al. Improved prediction of critical-viscosity temperature by fusion behavior of coal ash[J]. Fuel, 2019, 253: 1521-1530. |
| [1] | 赵翔宇, 徐东宇, 陈政宇, 徐春明, 张霖宙. 甲醇制烯烃反应-再生过程分子级模型构建及优化[J]. 化工进展, 2025, 44(8): 4785-4794. |
| [2] | 陈嵩嵩, 鲍艾丽, 霍锋, 侯亚慧, 崔改静, 张军平. 人工智能(AI)在复杂化工过程设计中的应用:现状、挑战与展望[J]. 化工进展, 2025, 44(8): 4821-4837. |
| [3] | 冯思瑶, 潘艳秋, 马佳宁, 孙延吉. 柴油分子重构模型及分子水平柴油加氢精制反应动力学模型构建[J]. 化工进展, 2025, 44(8): 4852-4861. |
| [4] | 刘燕燕, 李飞泉, 刘栋, 王俊涛, 罗雪. 纳观尺度下再生沥青-集料界面性质的分子模拟[J]. 化工进展, 2025, 44(8): 4302-4310. |
| [5] | 张嘉政, 毛岩鹏, 魏光朔, 逄栋杰, 徐剑, 董婧祎, 王旭江, 李敬伟, 王文龙. 煤气化渣替代水泥窑燃料的协同处理工艺[J]. 化工进展, 2025, 44(7): 4202-4211. |
| [6] | 马晶, 马玉龙, 朱莉, 乔松, 孙永刚, 吉文欣. 不同方法对煤气化粗渣中硅铝矿物的活化[J]. 化工进展, 2025, 44(7): 4251-4266. |
| [7] | 吴展华, 孔德宝, 田均均, 梁汝军, 卢朋慧, 盛敏. “1+N”模式的反应安全技术体系探讨[J]. 化工进展, 2025, 44(6): 3199-3207. |
| [8] | 袁梦丽, 宋云彩, 李文英, 冯杰. 光热驱动褐煤固定床气化过程热质传递规律[J]. 化工进展, 2025, 44(4): 2008-2019. |
| [9] | 王沛淦, 李乐利, 谢颂专, 宋冰冰, 孔巧平, 刘改革, 麻微微, 施雪卿. 污泥基FeCa-ALE复合材料对磷酸盐的吸附机制[J]. 化工进展, 2025, 44(4): 2365-2373. |
| [10] | 孙雅娟, 段思宇, 张宏, 周冬冬, 路广军, 马志斌. 化学外加剂对固废基胶凝材料性能及水化行为的影响[J]. 化工进展, 2025, 44(3): 1739-1748. |
| [11] | 王奇, 张乾, 杨凯, 高晨明, 孙岳鹏, 黄伟. 煤气化渣提炭分质用于橡胶补强填充料[J]. 化工进展, 2025, 44(3): 1749-1757. |
| [12] | 何丽君, 张昊颖, 马新青, 王常艳, 刘东方. 煤气化粗渣重金属形态及充填浸出行为[J]. 化工进展, 2025, 44(12): 7299-7307. |
| [13] | 张善超, 张德亮, 王露, 梁豪, 杨倩, 杨冠杰, 苏伟, 马晓迅, 朱燕燕. 铁基钙钛矿载氧体化学链制合成气热力学性能分析与实验[J]. 化工进展, 2025, 44(10): 5703-5716. |
| [14] | 张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201. |
| [15] | 曹树扬, 施静波, 董友明, 吕建雄. 不同温度下斜叶桉木材吸湿、解吸等温线与热力学性质[J]. 化工进展, 2024, 43(9): 5095-5105. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||