化工进展 ›› 2024, Vol. 43 ›› Issue (6): 3061-3079.DOI: 10.16085/j.issn.1000-6613.2023-1068
• 工业催化 • 上一篇
CO/CO2 加氢制低碳醇改性费托合成催化剂研究进展
曾壮1( ), 李柯志1, 苑志伟1, 杜金涛1, 李卓师2(
), 李柯志1, 苑志伟1, 杜金涛1, 李卓师2( ), 王悦2(
), 王悦2( )
)
- 1.中国石化催化剂有限公司工程技术研究院,北京 101111
2.天津大学化工学院,教育部绿色合成与 转化重点实验室,天津 300350
-
收稿日期:2023-06-27修回日期:2023-08-15出版日期:2024-06-15发布日期:2024-07-02 -
通讯作者:李卓师,王悦 -
作者简介:曾壮(1993—),男,博士,工程师,研究方向为催化剂制备技术和清洁生产技术。E-mail:zengzh.chji@sinopec.com。 -
基金资助:国家自然科学基金(U2002212);中国博士后科学基金(2021TQ0239)
Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols
ZENG Zhuang1( ), LI Kezhi1, YUAN Zhiwei1, DU Jintao1, LI Zhuoshi2(
), LI Kezhi1, YUAN Zhiwei1, DU Jintao1, LI Zhuoshi2( ), WANG Yue2(
), WANG Yue2( )
)
- 1.Institute of Engineering Technology, SINOPEC Catalyst Co. , Ltd. , Beijing 101111, China
2.Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
-
Received:2023-06-27Revised:2023-08-15Online:2024-06-15Published:2024-07-02 -
Contact:LI Zhuoshi, WANG Yue
摘要:
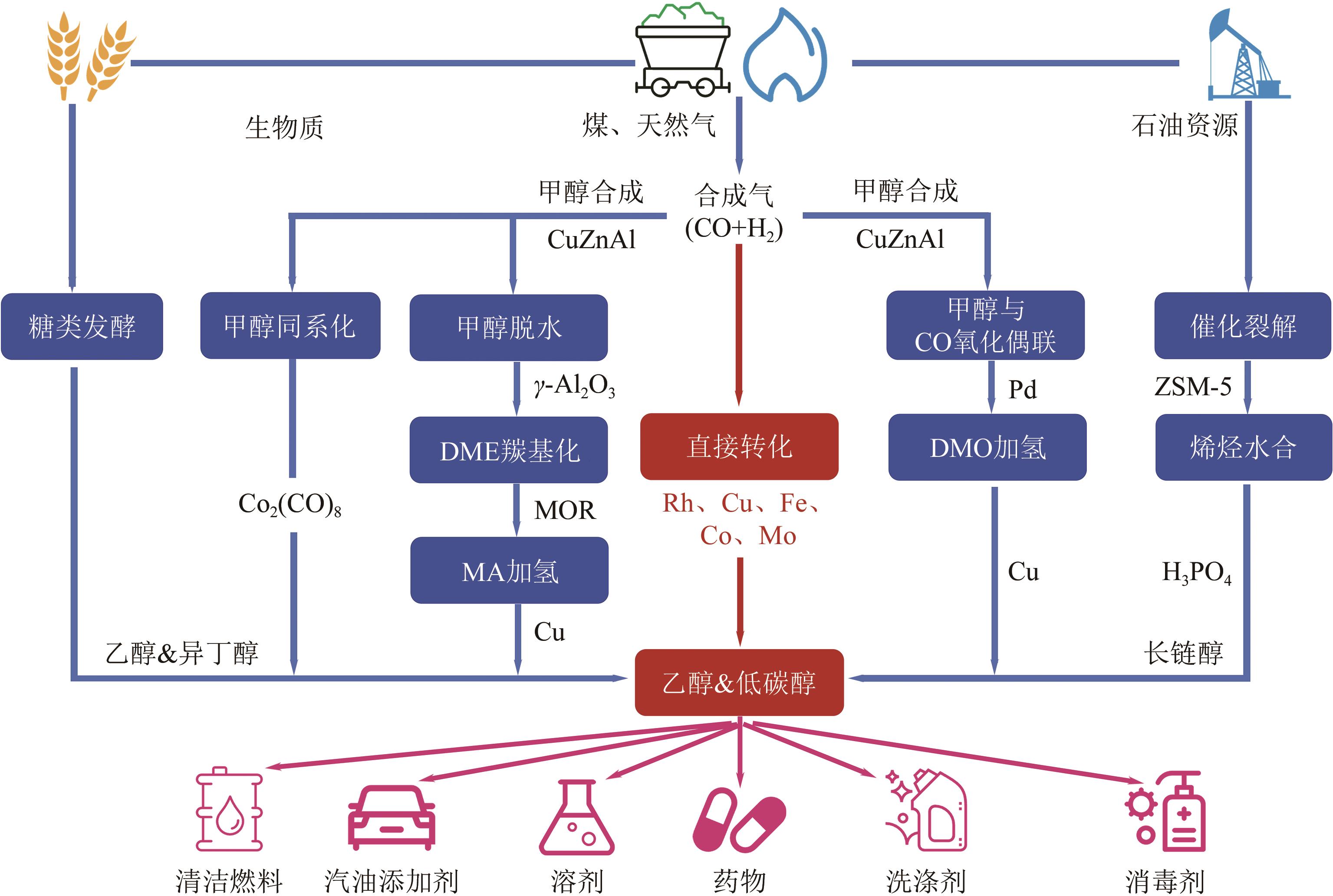
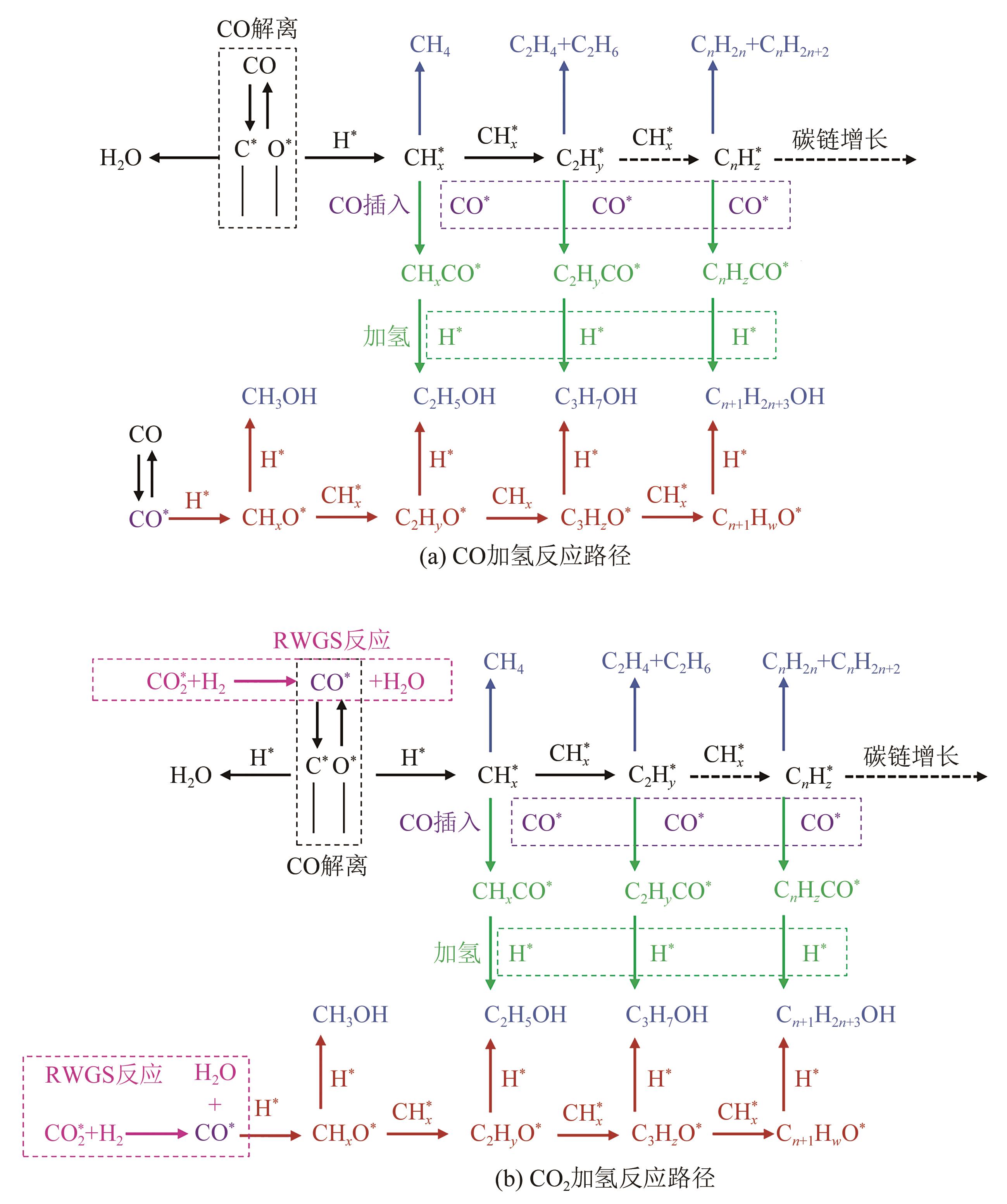
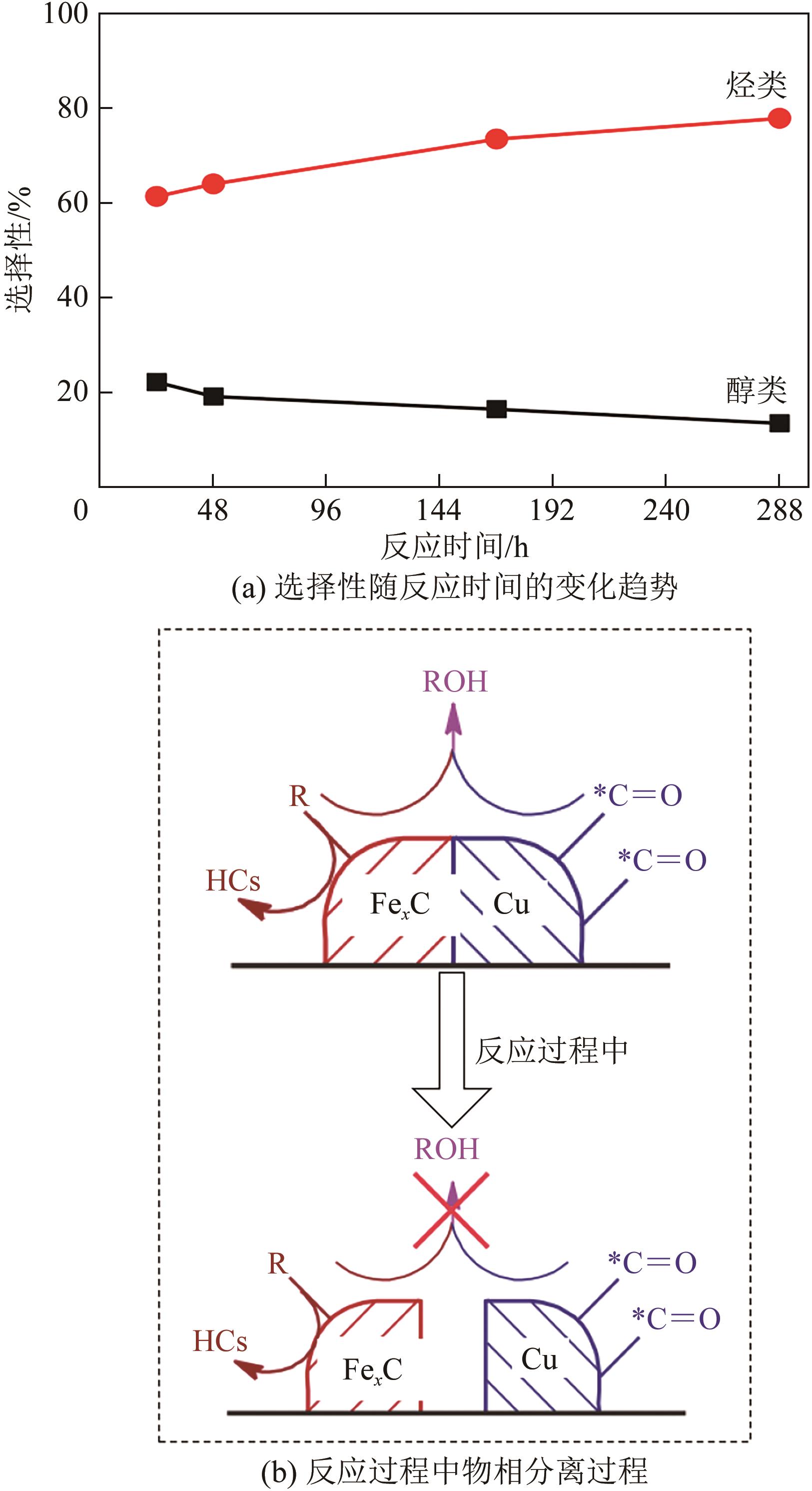
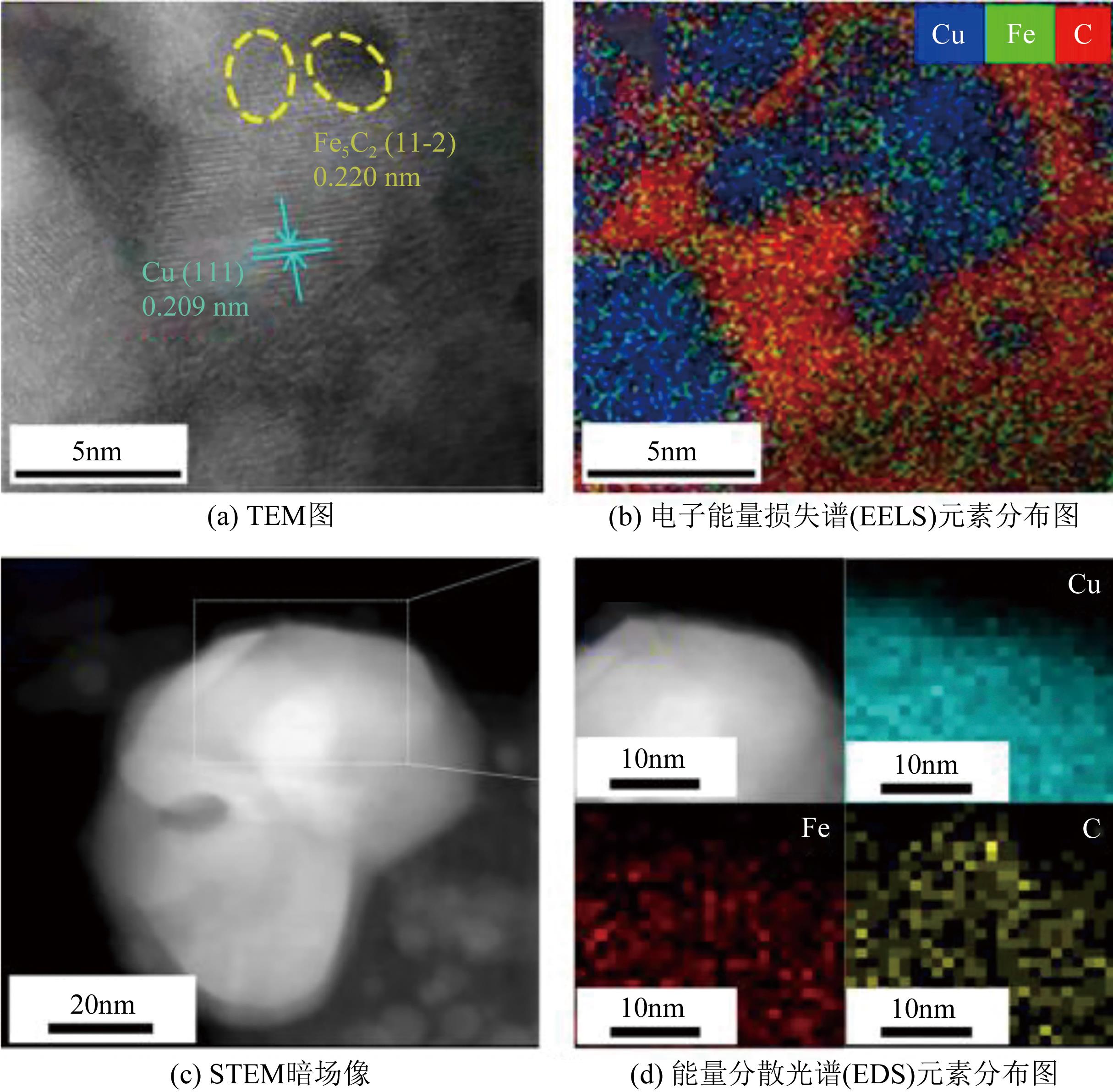
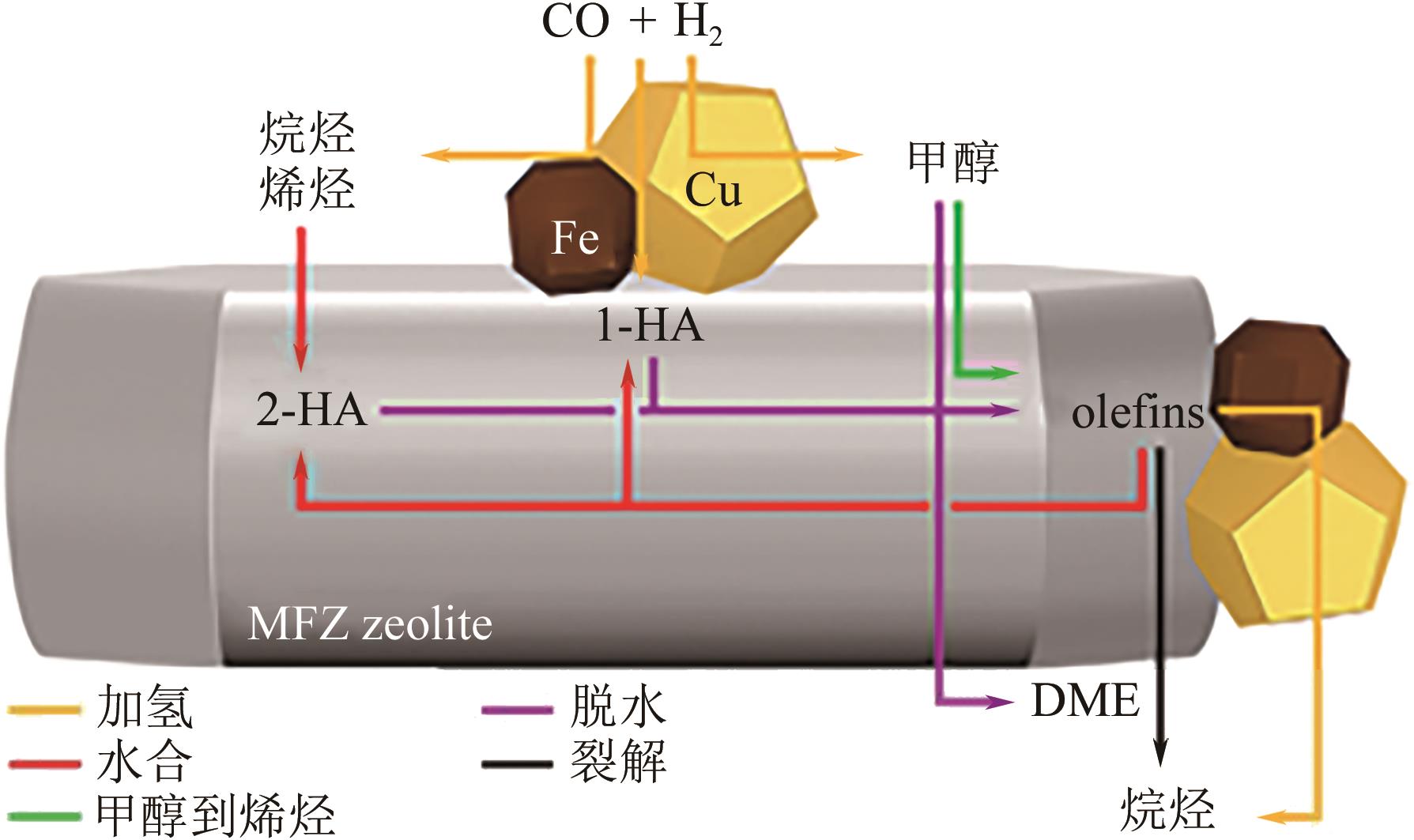
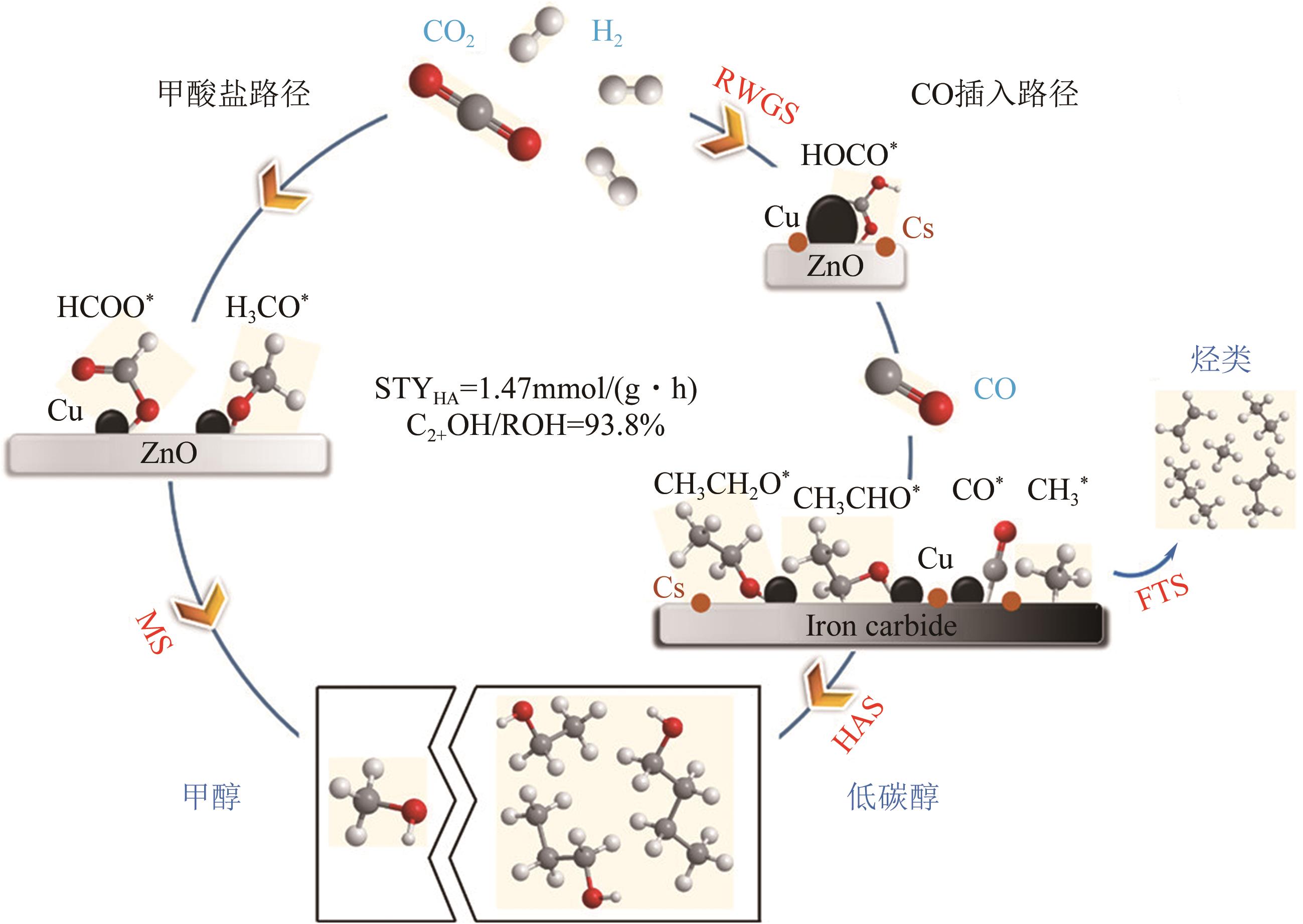
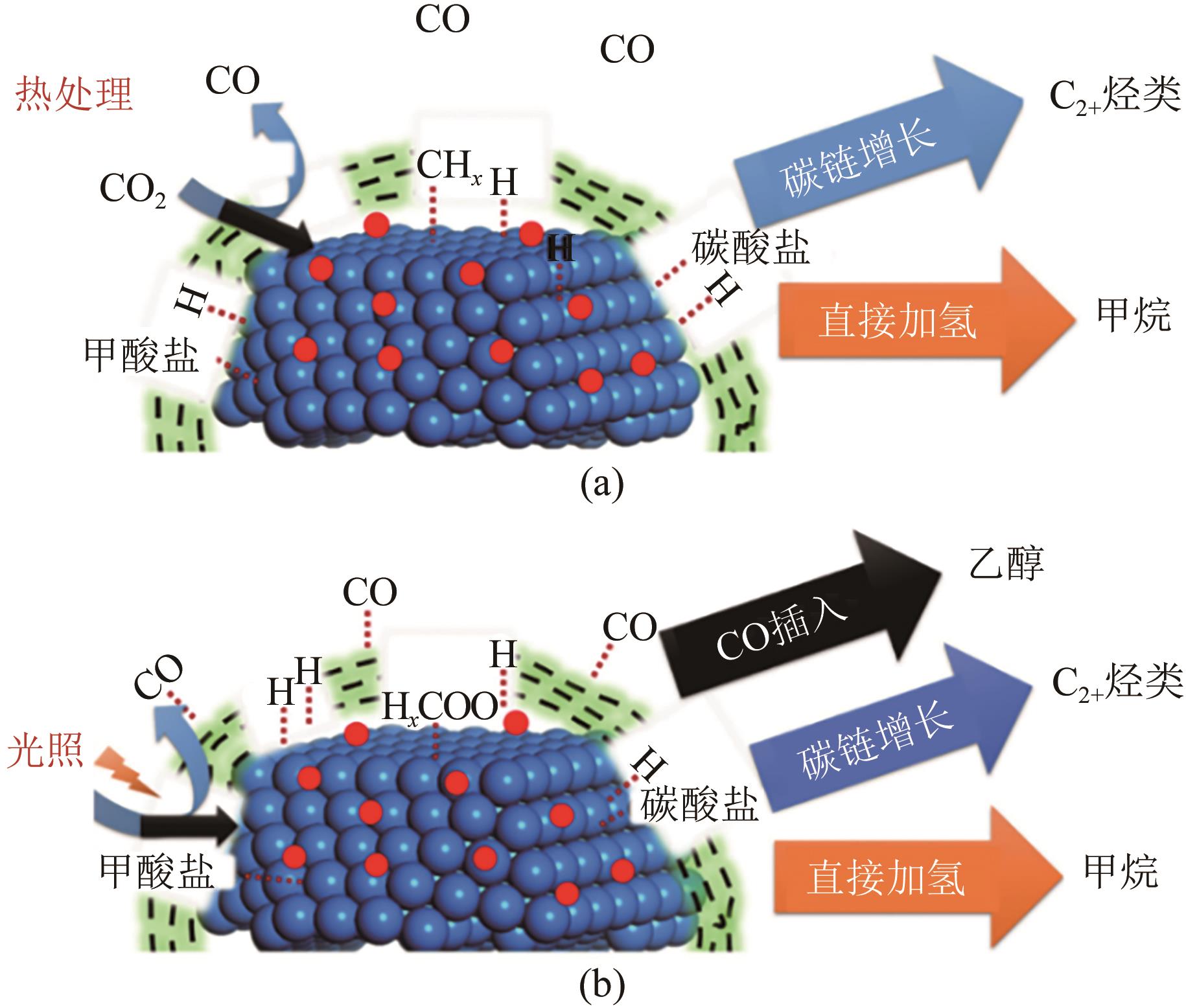
一氧化碳/二氧化碳(CO/CO2)加氢制低碳醇(C2+OH)是碳一化学的重要组成部分,是将煤、天然气、生物质、CO2等非石油资源转化为液体燃料和高附加值化学品的重要途径。该过程具有工艺路线短、原子经济性高、操作可行性强等优点,是颇具前景的合成路线。改性的费托合成催化剂是该体系最具潜力的催化剂之一,但仍存在低碳醇时空收率不理想、反应网络复杂、产物分布难以调控及稳定性差等问题,高效稳定催化剂的开发极具挑战。本文首先从热力学上分析了CO/CO2加氢反应制低碳醇适宜的工艺条件;其次,对改性费托合成催化剂上CO/CO2加氢制低碳醇的反应路径进行了解析,并据此提出催化剂的设计思路。随后,介绍了铁基和钴基催化剂的研究现状,详细阐述了前体结构、助剂、金属-载体相互作用等因素对催化剂性能的影响机制,重点分析了高性能催化剂的构建策略。后续可借助于先进表征技术和理论计算进一步加深对反应机理的认识,并通过催化剂结构的精细调控,获取低碳醇时空收率和催化剂稳定性的提升策略。
中图分类号:
引用本文
曾壮, 李柯志, 苑志伟, 杜金涛, 李卓师, 王悦. CO/CO2 加氢制低碳醇改性费托合成催化剂研究进展[J]. 化工进展, 2024, 43(6): 3061-3079.
ZENG Zhuang, LI Kezhi, YUAN Zhiwei, DU Jintao, LI Zhuoshi, WANG Yue. Advances in modified Fischer-Tropsch synthesis catalysts for CO/CO2 hydrogenation to higher alcohols[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3061-3079.
| 过程 | 反应 | ΔG⊖(25℃)/kJ·mol-1 | ΔH⊖(25℃)/kJ·mol-1 |
|---|---|---|---|
| 甲醇合成 | -25.1 | -90.5 | |
| 3.5 | -49.3 | ||
| 乙醇合成 | -221.1 | -253.6 | |
| -32.4 | -86.7 | ||
| 甲烷化 | -141.9 | -205.8 | |
| -113.5 | -165.0 | ||
| WGS反应 | -28.6 | -41.1 | |
| RWGS反应 | 28.6 | 41.1 |
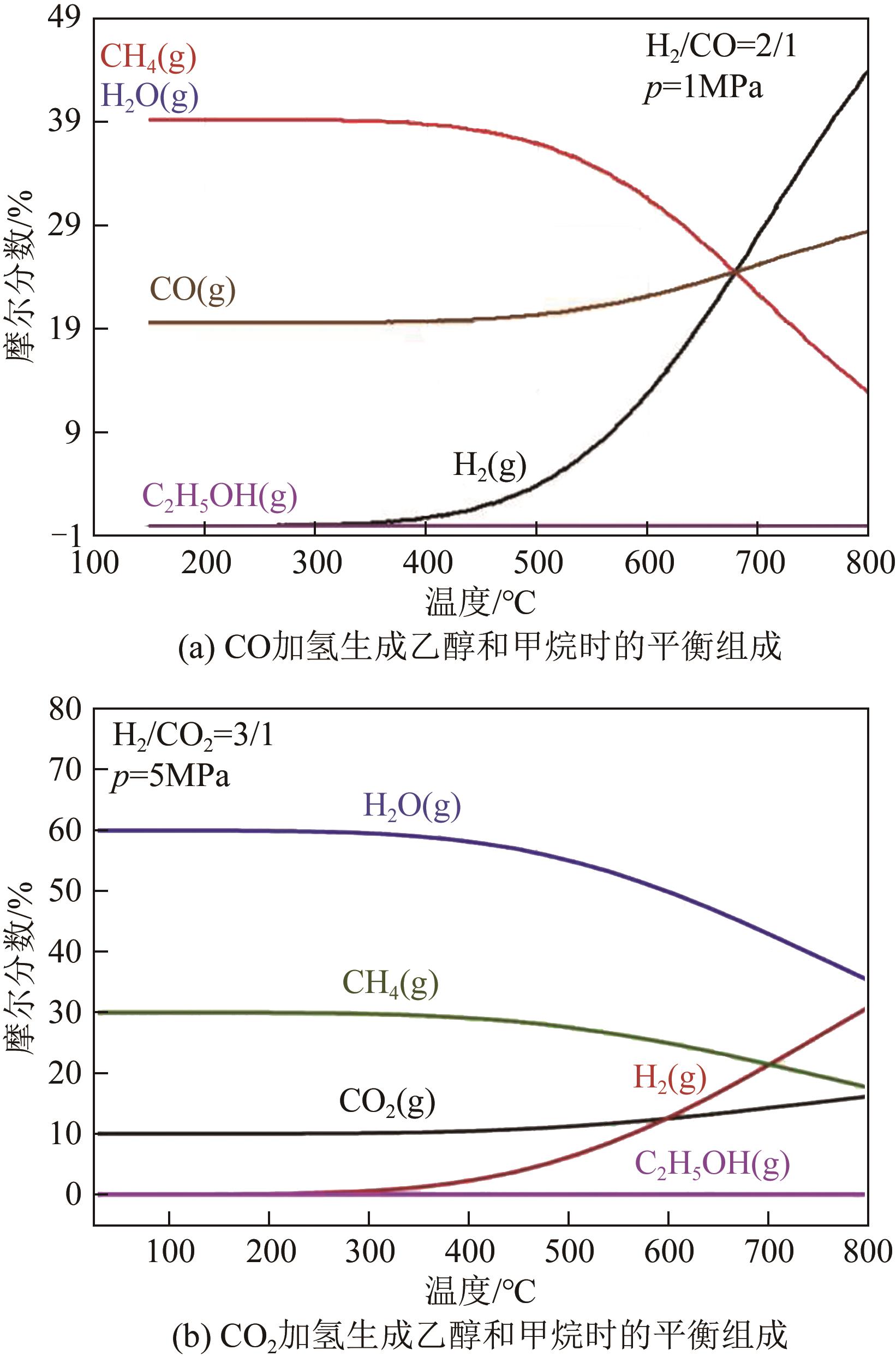
表1 CO/CO2加氢反应过程中重要反应的热力学数据[17-18]
| 过程 | 反应 | ΔG⊖(25℃)/kJ·mol-1 | ΔH⊖(25℃)/kJ·mol-1 |
|---|---|---|---|
| 甲醇合成 | -25.1 | -90.5 | |
| 3.5 | -49.3 | ||
| 乙醇合成 | -221.1 | -253.6 | |
| -32.4 | -86.7 | ||
| 甲烷化 | -141.9 | -205.8 | |
| -113.5 | -165.0 | ||
| WGS反应 | -28.6 | -41.1 | |
| RWGS反应 | 28.6 | 41.1 |
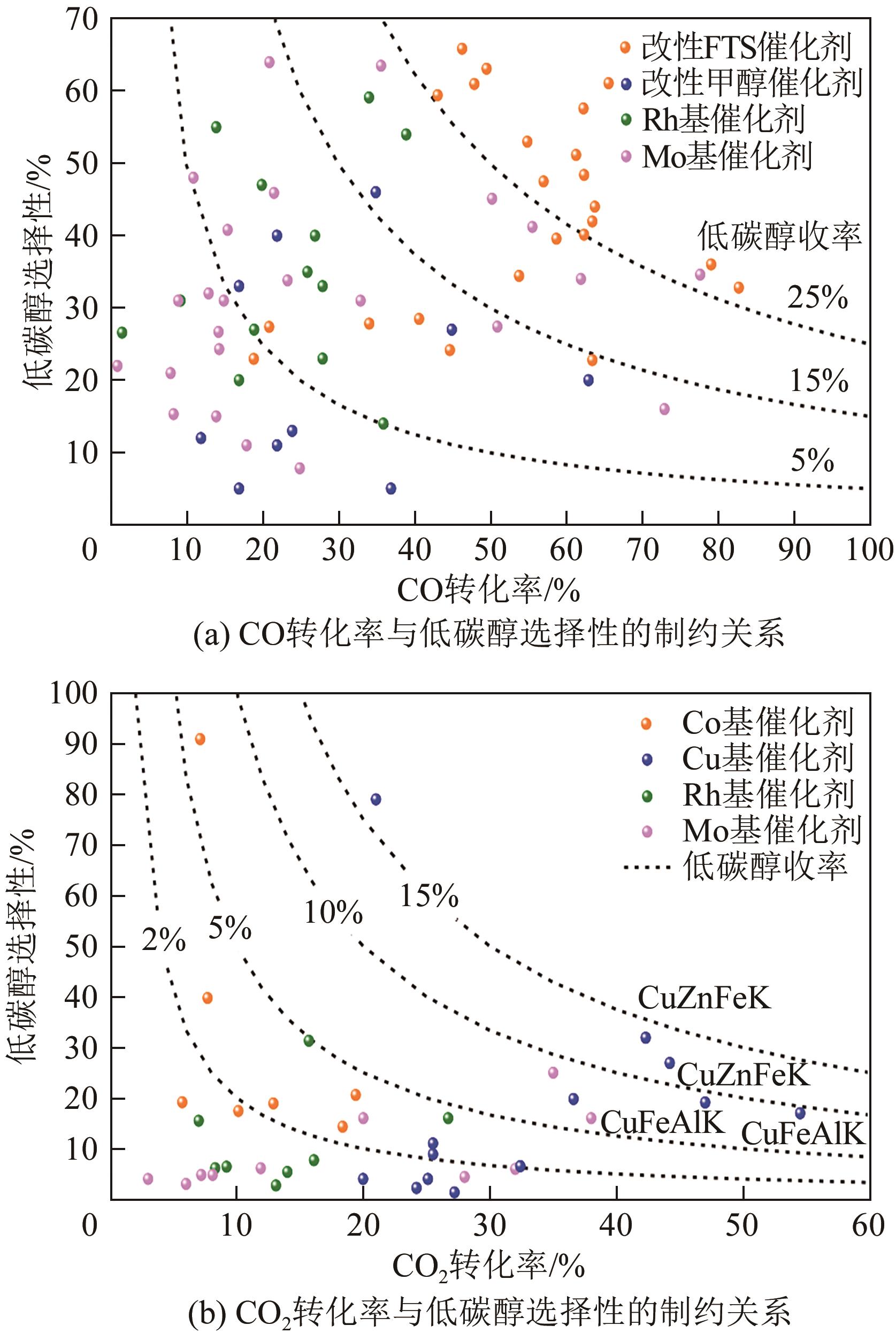
| 催化剂 | 反应条件 | CO转化率 /% | 选择性/% | 收率/% | 时空收率③/g·g-1·h-1 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO比 | 空速/h-1 | 总醇 | 低碳醇 | 低碳醇 | 总醇 | 低碳醇 | |||
| CNF-2-0.005① | 275 | 5 | 1.5 | 32000② | 11 | 47 | 37 | 4.1 | 0.670 | 0.530 | [ |
| Au-Fe-10 | 260 | 3 | 1 | 4800 | 20.0 | 52.5 | 31.7 | 6.3 | 0.268 | 0.195 | [ |
| Co1Fe2 | 260 | 3 | 1 | 9600② | 9.9 | 51.2 | 38.5 | 3.8 | 0.206 | 0.143 | [ |
| CoFe-300-0.25 | 260 | 3 | 2 | 10800② | 24.3 | 34.0 | 31.0 | 7.5 | 0.281 | 0.243 | [ |
| S2-CuFeMg-cat | 300 | 4 | 2 | 2000 | 56.9 | 49.1 | 32.8 | 18.7 | 0.280 | 0.187 | [ |
| CuFe@HHSS① | 300 | 3 | 2 | 5000 | 65.1 | 46.6 | 约27.7 | 18.0 | 0.118 | 0.070 | [ |
| Cu4Fe1 | 260 | 1 | 2 | 2400② | 53.2 | 29.8 | 27.1 | 14.4 | 0.201 | 0.183 | [ |
| 3DOM Cu2Fe1 | 200 | 4.8 | 1 | 2000 | 12.9 | 47.6 | 45.0 | 5.8 | 0.230 | 0.218 | [ |
| Cu5.2Fe4.8 | 350 | 5.5 | 2 | 6000 | 70.5 | 12.4 | 7.2 | 5.1 | 0.177 | 0.102 | [ |
| CF0.5 | 260 | 4 | 2 | 5000 | 18.0 | 20.8 | 10.0 | 1.8 | 0.050 | 0.024 | [ |
| Cu-Fe/SiO2 (WI) | 250 | 3 | 2 | 6000② | 14.0 | 26.8 | 8.47 | 1.2 | 0.104 | 0.033 | [ |
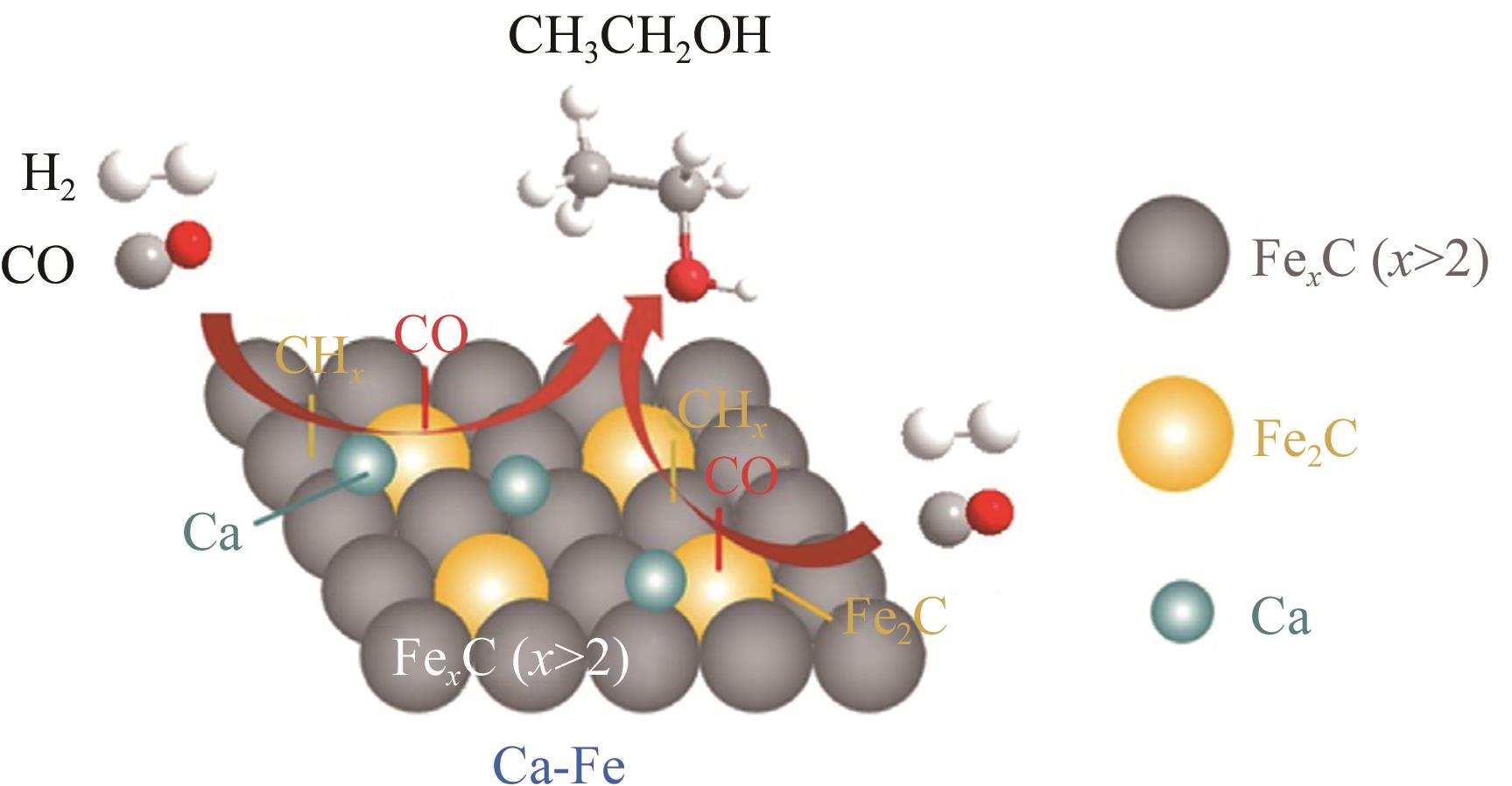
| 2Ca-Fe | 190 | 4 | 2 | 1800② | 22.2 | 37.8 | — | — | 0.039 | — | [ |
表2 CO加氢制低碳醇Fe基催化剂的性能汇总
| 催化剂 | 反应条件 | CO转化率 /% | 选择性/% | 收率/% | 时空收率③/g·g-1·h-1 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO比 | 空速/h-1 | 总醇 | 低碳醇 | 低碳醇 | 总醇 | 低碳醇 | |||
| CNF-2-0.005① | 275 | 5 | 1.5 | 32000② | 11 | 47 | 37 | 4.1 | 0.670 | 0.530 | [ |
| Au-Fe-10 | 260 | 3 | 1 | 4800 | 20.0 | 52.5 | 31.7 | 6.3 | 0.268 | 0.195 | [ |
| Co1Fe2 | 260 | 3 | 1 | 9600② | 9.9 | 51.2 | 38.5 | 3.8 | 0.206 | 0.143 | [ |
| CoFe-300-0.25 | 260 | 3 | 2 | 10800② | 24.3 | 34.0 | 31.0 | 7.5 | 0.281 | 0.243 | [ |
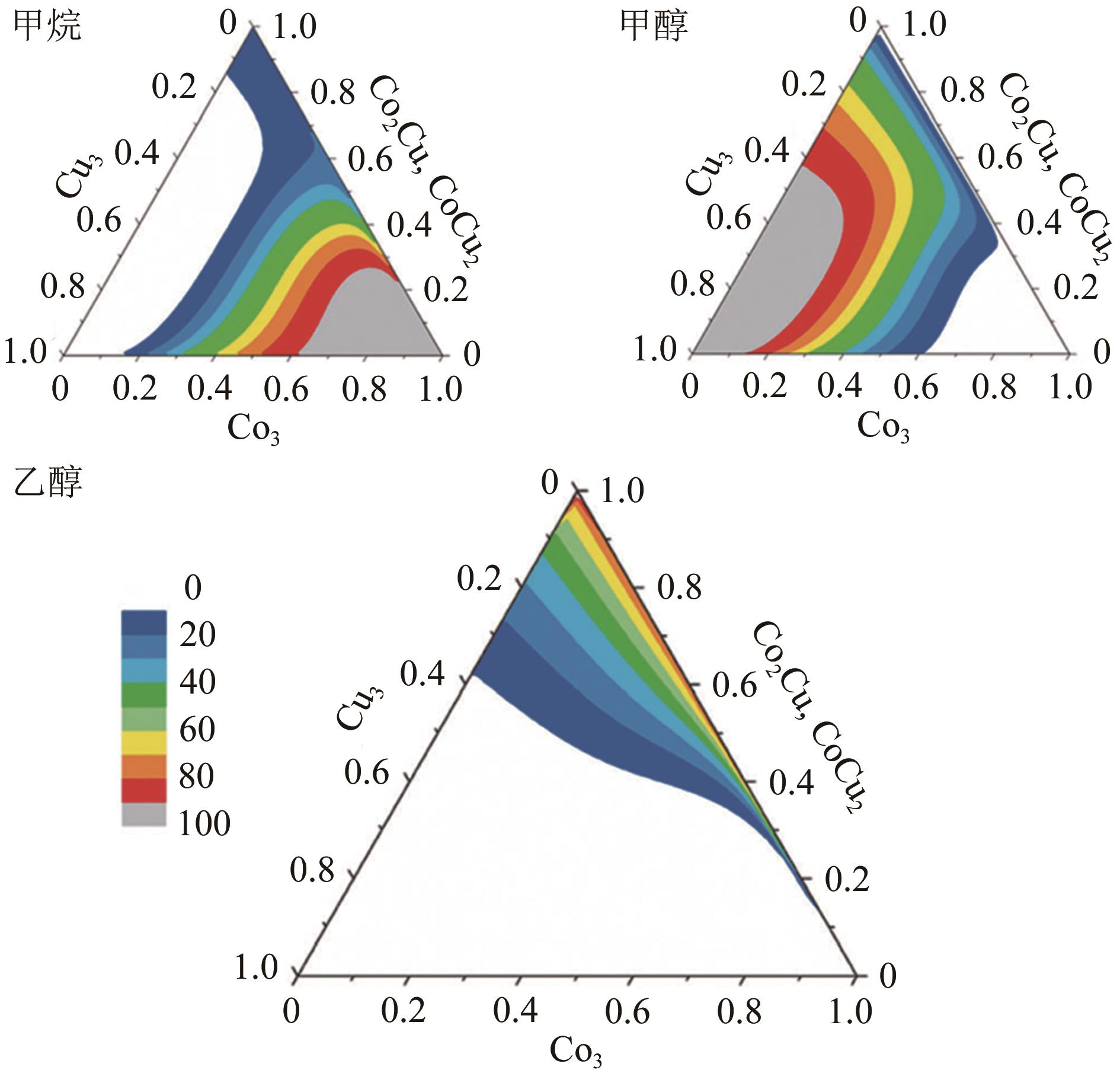
| S2-CuFeMg-cat | 300 | 4 | 2 | 2000 | 56.9 | 49.1 | 32.8 | 18.7 | 0.280 | 0.187 | [ |
| CuFe@HHSS① | 300 | 3 | 2 | 5000 | 65.1 | 46.6 | 约27.7 | 18.0 | 0.118 | 0.070 | [ |
| Cu4Fe1 | 260 | 1 | 2 | 2400② | 53.2 | 29.8 | 27.1 | 14.4 | 0.201 | 0.183 | [ |
| 3DOM Cu2Fe1 | 200 | 4.8 | 1 | 2000 | 12.9 | 47.6 | 45.0 | 5.8 | 0.230 | 0.218 | [ |
| Cu5.2Fe4.8 | 350 | 5.5 | 2 | 6000 | 70.5 | 12.4 | 7.2 | 5.1 | 0.177 | 0.102 | [ |
| CF0.5 | 260 | 4 | 2 | 5000 | 18.0 | 20.8 | 10.0 | 1.8 | 0.050 | 0.024 | [ |
| Cu-Fe/SiO2 (WI) | 250 | 3 | 2 | 6000② | 14.0 | 26.8 | 8.47 | 1.2 | 0.104 | 0.033 | [ |
| 2Ca-Fe | 190 | 4 | 2 | 1800② | 22.2 | 37.8 | — | — | 0.039 | — | [ |
| 催化剂 | 反应条件 | CO转化率 /% | 选择性/% | 收率/% | 时空收率 /g·g-1·h-1 | 参考文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO比 | 空速/h-1 | 甲醇 | 低碳醇 | 烃类 | CO2 | 低碳醇 | 总醇 | 低碳醇 | |||
| Co1Ga0.6-ZnAl-LDO/γ-Al2O3 | 260 | 3 | 2 | 2000 | 43.5 | 4.2 | 54.8 | 39.9 | 0.61 | 23.8 | — | — | [ |
| 0.6Na-Co/AC | 220 | 3 | 2 | 2000② | 12.8 | 3.8 | 27.0 | 56.6 | 12.6 | 3.5 | — | — | [ |
| Co1Mn/AC | 220 | 3 | 2 | 2000 | 29.1 | 1.6 | 19.8 | 76.2 | 2.4 | 5.8 | 0.047 | 0.043 | [ |
| Co/MnOx@quasi-MOF-74 | 230 | 3 | 2 | 15000② | 10.5 | 3.8 | 33.9 | 62.4 | 0.0 | 3.6 | 0.206③ | 0.185 | [ |
| Co4.7Mo@C | 275 | 3 | 2 | 15000② | 48 | 2.3 | 9.6 | 67.0 | 21.1 | 4.6 | 0.123 | 0.099 | [ |
| (Cu1Co2)2Al/CNT | 230 | 3 | 2 | 3900② | 45.4 | 2.1 | 60.8 | 35.3 | 1.8 | 27.6 | 0.149 | 0.137 | [ |
| Co3Cu-11%CNT(h型) | 300 | 5 | 1 | 7200② | 38.5 | 5.0 | 57.9 | 20.7 | 5.0 | 22.3 | 0.683 | 0.611 | [ |
| CoCu/5%GE-LFO① | 300 | 3 | 2 | 3900② | 49.7 | 9.7 | 47.2 | 35.2 | 7.9 | 23.5 | — | — | [ |
| (Cu1Co2)2Al-Red① | 250 | 3 | 2 | 3900② | 51.8 | 1.7 | 44.1 | 51.3 | 2.9 | 22.8 | — | — | [ |
| Co3Cu1/KIT-6 | 270 | 3 | 2 | 14400② | 62.9 | 15.6 | 30.6 | 53.1 | 0.8 | 19.2 | — | — | [ |
| Co2.5Cu1-773 | 270 | 3 | 1 | 4800② | 45.3 | 9.8 | 31.8 | 55.5 | 2.9 | 14.4 | — | — | [ |
| CuCoMn | 270 | 2.5 | 2 | 7500 | 29.7 | 13.7 | 32.5 | 51.9 | 0.4 | 9.7 | — | — | [ |
| 15Cu5Co@MCN① | 220 | 2 | 1 | 4000② | 20.4 | 20.2 | 29.8 | 38.3 | 11.7 | 6.1 | 0.080 | 0.048 | [ |
| Cu0.25Co0.75 | 250 | 3 | 2 | 3900 | 71.3 | 2.6 | 11.2 | 86.2 | — | 8.0 | 0.148 | 0.121 | [ |
表3 CO加氢制低碳醇Co基催化剂的性能汇总
| 催化剂 | 反应条件 | CO转化率 /% | 选择性/% | 收率/% | 时空收率 /g·g-1·h-1 | 参考文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO比 | 空速/h-1 | 甲醇 | 低碳醇 | 烃类 | CO2 | 低碳醇 | 总醇 | 低碳醇 | |||
| Co1Ga0.6-ZnAl-LDO/γ-Al2O3 | 260 | 3 | 2 | 2000 | 43.5 | 4.2 | 54.8 | 39.9 | 0.61 | 23.8 | — | — | [ |
| 0.6Na-Co/AC | 220 | 3 | 2 | 2000② | 12.8 | 3.8 | 27.0 | 56.6 | 12.6 | 3.5 | — | — | [ |
| Co1Mn/AC | 220 | 3 | 2 | 2000 | 29.1 | 1.6 | 19.8 | 76.2 | 2.4 | 5.8 | 0.047 | 0.043 | [ |
| Co/MnOx@quasi-MOF-74 | 230 | 3 | 2 | 15000② | 10.5 | 3.8 | 33.9 | 62.4 | 0.0 | 3.6 | 0.206③ | 0.185 | [ |
| Co4.7Mo@C | 275 | 3 | 2 | 15000② | 48 | 2.3 | 9.6 | 67.0 | 21.1 | 4.6 | 0.123 | 0.099 | [ |
| (Cu1Co2)2Al/CNT | 230 | 3 | 2 | 3900② | 45.4 | 2.1 | 60.8 | 35.3 | 1.8 | 27.6 | 0.149 | 0.137 | [ |
| Co3Cu-11%CNT(h型) | 300 | 5 | 1 | 7200② | 38.5 | 5.0 | 57.9 | 20.7 | 5.0 | 22.3 | 0.683 | 0.611 | [ |
| CoCu/5%GE-LFO① | 300 | 3 | 2 | 3900② | 49.7 | 9.7 | 47.2 | 35.2 | 7.9 | 23.5 | — | — | [ |
| (Cu1Co2)2Al-Red① | 250 | 3 | 2 | 3900② | 51.8 | 1.7 | 44.1 | 51.3 | 2.9 | 22.8 | — | — | [ |
| Co3Cu1/KIT-6 | 270 | 3 | 2 | 14400② | 62.9 | 15.6 | 30.6 | 53.1 | 0.8 | 19.2 | — | — | [ |
| Co2.5Cu1-773 | 270 | 3 | 1 | 4800② | 45.3 | 9.8 | 31.8 | 55.5 | 2.9 | 14.4 | — | — | [ |
| CuCoMn | 270 | 2.5 | 2 | 7500 | 29.7 | 13.7 | 32.5 | 51.9 | 0.4 | 9.7 | — | — | [ |
| 15Cu5Co@MCN① | 220 | 2 | 1 | 4000② | 20.4 | 20.2 | 29.8 | 38.3 | 11.7 | 6.1 | 0.080 | 0.048 | [ |
| Cu0.25Co0.75 | 250 | 3 | 2 | 3900 | 71.3 | 2.6 | 11.2 | 86.2 | — | 8.0 | 0.148 | 0.121 | [ |
| 催化剂 | 反应条件 | CO2转化率 /% | 选择性/% | 收率/% | 参考文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO2比 | 空速/h-1 | 甲醇 | 低碳醇 | 甲烷 | C2+烃 | CO | 低碳醇 | |||
| (K2O)5%CuZnFeZrO2 | 320 | 3 | 3 | 3600 | 25.5 | 12.3 | 11.0 | 23.6(所有烃) | 53.1 | 2.81 | [ | |
| FeNaS-0.6① | 320 | 3 | 3 | 8000② | 32.0 | 0.2 | 12.6 | 10.3 | 56.2 | 20.7 | 4.03 | [ |
| 4.6K-CuMgZnFe | 320 | 5 | 3 | 6000② | 30.4 | 1.3 | 15.9 | 9.7 | 42.7 | 30.6 | 4.83 | [ |
| Cs-Cu0.8Fe1.0Zn1.0 | 330 | 5 | 3 | 4500② | 36.6 | 0.9 | 19.8 | 11.8 | 46.9 | 20.6 | 7.25 | [ |
| K/Cu-Fe-Zn | 300 | 7 | 3 | 5000③ | 44.2 | 2.0 | 26.9 | 46.1(所有烃) | 5.9 | 11.9 | [ | |
| RhFeLi/TiO2NRs-500℃① | 250 | 3 | 3 | 6000 | 15.7 | 2.2 | 31.4 | 53.9 | 0 | 12.5 | 4.93 | [ |
| CuZnFe0.5K0.15 | 300 | 6 | 3 | 5000 | 42.3 | 4.7 | 32.0 | 56.4(所有烃) | 6.9 | 13.5 | [ | |
| Cr(1%)-CuFe | 320 | 4 | 3 | 6000② | 38.4 | — | 29.2 | — | 14.8 | 11.2 | [ | |
| CZA(1)/K-CMZF(1) | 320 | 5 | 3 | 6000② | 42.3 | 1.3 | 17.4 | 67.6(所有烃) | 13.8 | 7.4 | [ | |
| Co@Co3O4/C-N | 220 | 2 | 3 | 6000② | 18.6 | 18.3 | 1.2 | 79.5 | 0.7 | 0 | 0.22 | [ |
| Ir/Co(A)-Na2O/SiO2 | 220 | 2.1 | 3 | 2000 | 7.6 | 7.8 | 7.9 | 35.7 | 9.6 | 38.5 | 0.60 | [ |
| Na-Co/SiO2 | 250 | 5 | 3 | 4000 | 18.8 | ~1.2 | ~8.3 | 38.7 | 22.7 | 29.1 | 1.45 | [ |
| Co3O4-m① | 200 | 2 | 3 | 6000② | 28.9 | 10.2 | 9.0 | 53.6 | 27.2 | 0 | 2.60 | [ |
| Cu/Co3O4-2h | 250 | 3 | 3 | 36000② | 13.9 | 2.4 | 15.2 | 37.9 | 19.9 | 6.5 | 2.11 | [ |
| Pt/Co3O4-m① | 200 | 2 | 3 | 6000② | 10.7 | 23.6 | 23.6 | 14.9 | 9.6 | 28.3 | 2.53 | [ |
表4 CO2加氢制低碳醇改性FTS催化剂的性能汇总
| 催化剂 | 反应条件 | CO2转化率 /% | 选择性/% | 收率/% | 参考文献 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 压力/MPa | H2/CO2比 | 空速/h-1 | 甲醇 | 低碳醇 | 甲烷 | C2+烃 | CO | 低碳醇 | |||
| (K2O)5%CuZnFeZrO2 | 320 | 3 | 3 | 3600 | 25.5 | 12.3 | 11.0 | 23.6(所有烃) | 53.1 | 2.81 | [ | |
| FeNaS-0.6① | 320 | 3 | 3 | 8000② | 32.0 | 0.2 | 12.6 | 10.3 | 56.2 | 20.7 | 4.03 | [ |
| 4.6K-CuMgZnFe | 320 | 5 | 3 | 6000② | 30.4 | 1.3 | 15.9 | 9.7 | 42.7 | 30.6 | 4.83 | [ |
| Cs-Cu0.8Fe1.0Zn1.0 | 330 | 5 | 3 | 4500② | 36.6 | 0.9 | 19.8 | 11.8 | 46.9 | 20.6 | 7.25 | [ |
| K/Cu-Fe-Zn | 300 | 7 | 3 | 5000③ | 44.2 | 2.0 | 26.9 | 46.1(所有烃) | 5.9 | 11.9 | [ | |
| RhFeLi/TiO2NRs-500℃① | 250 | 3 | 3 | 6000 | 15.7 | 2.2 | 31.4 | 53.9 | 0 | 12.5 | 4.93 | [ |
| CuZnFe0.5K0.15 | 300 | 6 | 3 | 5000 | 42.3 | 4.7 | 32.0 | 56.4(所有烃) | 6.9 | 13.5 | [ | |
| Cr(1%)-CuFe | 320 | 4 | 3 | 6000② | 38.4 | — | 29.2 | — | 14.8 | 11.2 | [ | |
| CZA(1)/K-CMZF(1) | 320 | 5 | 3 | 6000② | 42.3 | 1.3 | 17.4 | 67.6(所有烃) | 13.8 | 7.4 | [ | |
| Co@Co3O4/C-N | 220 | 2 | 3 | 6000② | 18.6 | 18.3 | 1.2 | 79.5 | 0.7 | 0 | 0.22 | [ |
| Ir/Co(A)-Na2O/SiO2 | 220 | 2.1 | 3 | 2000 | 7.6 | 7.8 | 7.9 | 35.7 | 9.6 | 38.5 | 0.60 | [ |
| Na-Co/SiO2 | 250 | 5 | 3 | 4000 | 18.8 | ~1.2 | ~8.3 | 38.7 | 22.7 | 29.1 | 1.45 | [ |
| Co3O4-m① | 200 | 2 | 3 | 6000② | 28.9 | 10.2 | 9.0 | 53.6 | 27.2 | 0 | 2.60 | [ |
| Cu/Co3O4-2h | 250 | 3 | 3 | 36000② | 13.9 | 2.4 | 15.2 | 37.9 | 19.9 | 6.5 | 2.11 | [ |
| Pt/Co3O4-m① | 200 | 2 | 3 | 6000② | 10.7 | 23.6 | 23.6 | 14.9 | 9.6 | 28.3 | 2.53 | [ |
| 1 | SUBRAMANI Velu, GANGWAL Santosh K. A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol[J]. Energy & Fuels, 2008, 22(2): 814-839. |
| 2 | SPIVEY James J, EGBEBI Adefemi. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas[J]. Chemical Society Reviews, 2007, 36(9): 1514-1528. |
| 3 | LU Yao, KANG Li, GUO Dan, et al. Double-site doping of a V promoter on Ni x -V-MgAl catalysts for the DRM reaction: Simultaneous effect on CH4 and CO2 activation[J]. ACS Catalysis, 2021, 11(14): 8749-8765. |
| 4 | GUO Dan, LI Maoshuai, LU Yao, et al. Enhanced thermocatalytic stability by coupling nickel step sites with nitrogen heteroatoms for dry reforming of methane[J]. ACS Catalysis, 2022, 12(1): 316-330. |
| 5 | Ho Ting LUK, MONDELLI Cecilia, FERRÉ Daniel Curulla, et al. Status and prospects in higher alcohols synthesis from syngas[J]. Chemical Society Reviews, 2017, 46(5): 1358-1426. |
| 6 | Alex MILLS G. Status and future opportunities for conversion of synthesis gas to liquid fuels[J]. Fuel, 1994, 73(8): 1243-1279. |
| 7 | AO Min, PHAM Gia Hung, SUNARSO Jaka, et al. Active centers of catalysts for higher alcohol synthesis from syngas: A review[J]. ACS Catalysis, 2018, 8(8): 7025-7050. |
| 8 | MOREIRA Marcelo M R, SEABRA Joaquim E A, LYND Lee R, et al. Socio-environmental and land-use impacts of double-cropped maize ethanol in Brazil[J]. Nature Sustainability, 2020, 3(3): 209-216. |
| 9 | LI Zhuoshi, HU Zhiwei, ZENG Zhuang, et al. Lamellar-structured silicate derived highly dispersed CoCu catalyst for higher alcohol synthesis from syngas[J]. Industrial & Engineering Chemistry Research, 2022, 61(20): 6859-6871. |
| 10 | XU Di, DING Mingyue, HONG Xinlin, et al. Mechanistic aspects of the role of K promotion on Cu-Fe-based catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. ACS Catalysis, 2020, 10(24): 14516-14526. |
| 11 | LI Yinwen, GAO Wa, PENG Mi, et al. Interfacial Fe5C2-Cu catalysts toward low-pressure syngas conversion to long-chain alcohols[J]. Nature Communications, 2020, 11(1): 1-8. |
| 12 | SUN Jie, CAI Qiuxia, WAN Yan, et al. Promotional effects of cesium promoter on higher alcohol synthesis from syngas over cesium-promoted Cu/ZnO/Al2O3 catalysts[J]. ACS Catalysis, 2016, 6(9): 5771-5785. |
| 13 | BAI Hui, MA Mengmeng, BAI Bing, et al. The active site of syngas conversion into ethanol over Cu/ZnO/Al2O3 ternary catalysts in slurry bed[J]. Journal of Catalysis, 2019, 380: 68-82. |
| 14 | WANG Chengtao, ZHANG Jian, QIN Gangqiang, et al. Direct conversion of syngas to ethanol within zeolite crystals[J]. Chem, 2020, 6(3): 646-657. |
| 15 | ZENG Feng, XI Xiaoying, CAO Huatang, et al. Synthesis of mixed alcohols with enhanced C3+ alcohol production by CO hydrogenation over potassium promoted molybdenum sulfide[J]. Applied Catalysis B: Environmental, 2019, 246: 232-241. |
| 16 | LI Jianli, HU Ruijue, QU Hao, et al. Radio-frequency thermal plasma-induced novel chainmail-like core-shell MoO2 as highly stable catalyst for converting syngas to higher alcohols[J]. Applied Catalysis B: Environmental, 2019, 249: 63-71. |
| 17 | ZENG Feng, MEBRAHTU Chalachew, XI Xiaoying, et al. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives[J]. Applied Catalysis B: Environmental, 2021, 291: 120073. |
| 18 | GUPTA Mayank, SMITH Miranda L, SPIVEY James J. Heterogeneous catalytic conversion of dry syngas to ethanol and higher alcohols on Cu-based catalysts[J]. ACS Catalysis, 2011, 1(6): 641-656. |
| 19 | XU Di, WANG Yanqiu, DING Mingyue, et al. Advances in higher alcohol synthesis from CO2 hydrogenation[J]. Chem, 2021, 7(4): 849-881. |
| 20 | LIN Tiejun, AN Yunlei, YU Fei, et al. Advances in selectivity control for Fischer-Tropsch synthesis to fuels and chemicals with high carbon efficiency[J]. ACS Catalysis, 2022, 12(19): 12092-12112. |
| 21 | XU Xiaoding, DOESBURG E B M, SCHOLTEN J J F. Synthesis of higher alcohols from syngas-Recently patented catalysts and tentative ideas on the mechanism[J]. Catalysis Today, 1987, 2(1): 125-170. |
| 22 | Ho Ting LUK, MONDELLI Cecilia, MITCHELL Sharon, et al. Role of carbonaceous supports and potassium promoter on higher alcohols synthesis over copper-iron catalysts[J]. ACS Catalysis, 2018, 8(10): 9604-9618. |
| 23 | ZENG Zhuang, LI Zhuoshi, GUO Shaoxia, et al. Janus Au-Fe2.2C catalyst for direct conversion of syngas to higher alcohols[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(33): 11258-11268. |
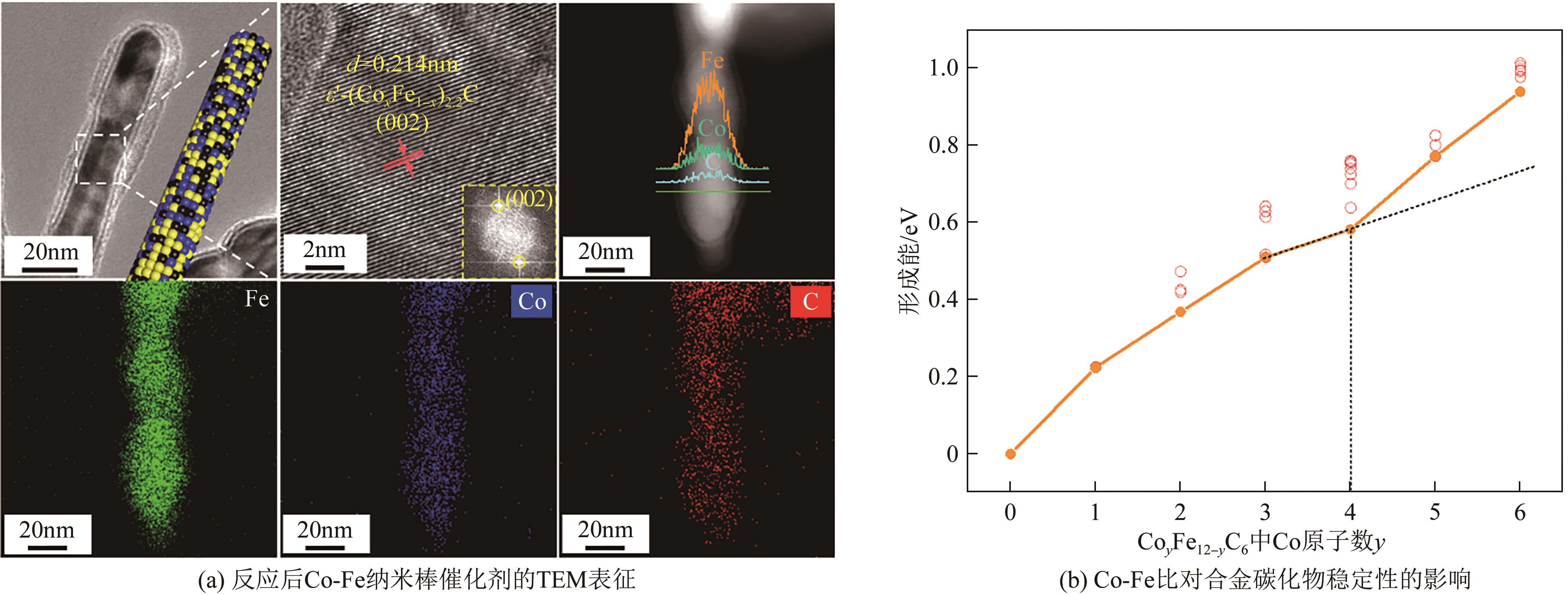
| 24 | ZENG Zhuang, LI Zhuoshi, KANG Li, et al. A monodisperse ε′-(Co x Fe1- x )2.2C bimetallic carbide catalyst for direct conversion of syngas to higher alcohols[J]. ACS Catalysis, 2022, 12(10): 6016-6028. |
| 25 | ZENG Zhuang, LI Zhuoshi, GUAN Tong, et al. CoFe alloy carbide catalysts for higher alcohols synthesis from syngas: Evolution of active sites and Na promoting effect[J]. Journal of Catalysis, 2022, 405: 430-444. |
| 26 | GAO Wa, ZHAO Yufei, LIU Junmin, et al. Catalytic conversion of syngas to mixed alcohols over CuFe-based catalysts derived from layered double hydroxides[J]. Catalysis Science & Technology, 2013, 3(5): 1324-1332. |
| 27 | YANG Wanliang, CHEN Meng, ZHOU Jiayuan, et al. Preparation and evaluation of highly dispersed HHSS supported Cu-Fe bimetallic catalysts for higher alcohols synthesis from syngas[J]. Applied Catalysis A: General, 2020, 608: 117868. |
| 28 | LU Yongwu, CAO Baobao, YU Fei, et al. High selectivity higher alcohols synthesis from syngas over three-dimensionally ordered macroporous Cu-Fe catalysts[J]. ChemCatChem, 2014, 6(2): 473-478. |
| 29 | GUO Haijun, ZHANG Hairong, PENG Fen, et al. Effects of Cu/Fe ratio on structure and performance of attapulgite supported CuFeCo-based catalyst for mixed alcohols synthesis from syngas[J]. Applied Catalysis A: General, 2015, 503: 51-61. |
| 30 | HAN Xinyou, FANG Kegong, ZHOU Juan, et al. Synthesis of higher alcohols over highly dispersed Cu-Fe based catalysts derived from layered double hydroxides[J]. Journal of Colloid and Interface Science, 2016, 470: 162-171. |
| 31 | SUN Chao, MAO Dongsen, HAN Lupeng, et al. Effect of preparation method on performance of Cu-Fe/SiO2 catalysts for higher alcohols synthesis from syngas[J]. RSC Advances, 2016, 6(60): 55233-55239. |
| 32 | XU Jing, WEI Jian, ZHANG Jixin, et al. Precisely synergistic synthesis of higher alcohols from syngas over iron carbides[J]. Chem Catalysis, 2023, 3(4): 100584. |
| 33 | PIJOLAT M, PERRICHON V. Synthesis of alcohols from CO and H2 on a Fe/Al2O3 catalyst at 8~30 bars pressure[J]. Applied Catalysis, 1985, 13(2): 321-333. |
| 34 | ZHANG Xiaobing, LI Zhong, GUO Qihai, et al. Selective synthesis of mixed alcohols from syngas over catalyst Fe2O3/Al2O3 in slurry reactor[J]. Fuel Processing Technology, 2010, 91(4): 379-382. |
| 35 | XU Jing, WEI Jian, ZHANG Jixin, et al. Highly selective production of long-chain aldehydes, ketones or alcohols via syngas at a mild condition[J]. Applied Catalysis B: Environmental, 2022, 307: 121155. |
| 36 | XIAO Kang, BAO Zhenghong, QI Xingzhen, et al. Advances in bifunctional catalysis for higher alcohol synthesis from syngas[J]. Chinese Journal of Catalysis, 2013, 34(1): 116-129. |
| 37 | HOU Bin, HAN Xinyou, LIN Minggui, et al. Preparation of SiO2-coated CuFe catalysts for synthesis of higher alcohols from CO hydrogenation[J]. Journal of Fuel Chemistry and Technology, 2016, 44(2): 217-224. |
| 38 | XIAO Kang, BAO Zhenghong, QI Xingzhen, et al. Unsupported CuFe bimetallic nanoparticles for higher alcohol synthesis via syngas[J]. Catalysis Communications, 2013, 40: 154-157. |
| 39 | XIAO Kang, BAO Zhenghong, QI Xingzhen, et al. Structural evolution of CuFe bimetallic nanoparticles for higher alcohol synthesis[J]. Journal of Molecular Catalysis A: Chemical, 2013, 378: 319-325. |
| 40 | CAO Ang, YANG Qilei, WEI Ying, et al. Synthesis of higher alcohols from syngas over CuFeMg-LDHs/CFs composites[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17425-17434. |
| 41 | DING Mingyue, TU Junling, QIU Minghuang, et al. Impact of potassium promoter on Cu-Fe based mixed alcohols synthesis catalyst[J]. Applied Energy, 2015, 138: 584-589. |
| 42 | LU Yongwu, YU Fei, HU Jin, et al. Catalytic conversion of syngas to mixed alcohols over Zn-Mn promoted Cu-Fe based catalyst[J]. Applied Catalysis A: General, 2012, 429/430: 48-58. |
| 43 | LUK H T, MONDELLI C, MITCHELL S, et al. Impact of carrier acidity on the conversion of syngas to higher alcohols over zeolite-supported copper-iron catalysts[J]. Journal of Catalysis, 2019, 371: 116-125. |
| 44 | AN Zhe, NING Xun, HE Jing. Ga-promoted CO insertion and C-C coupling on Co catalysts for the synthesis of ethanol and higher alcohols from syngas[J]. Journal of Catalysis, 2017, 356: 157-164. |
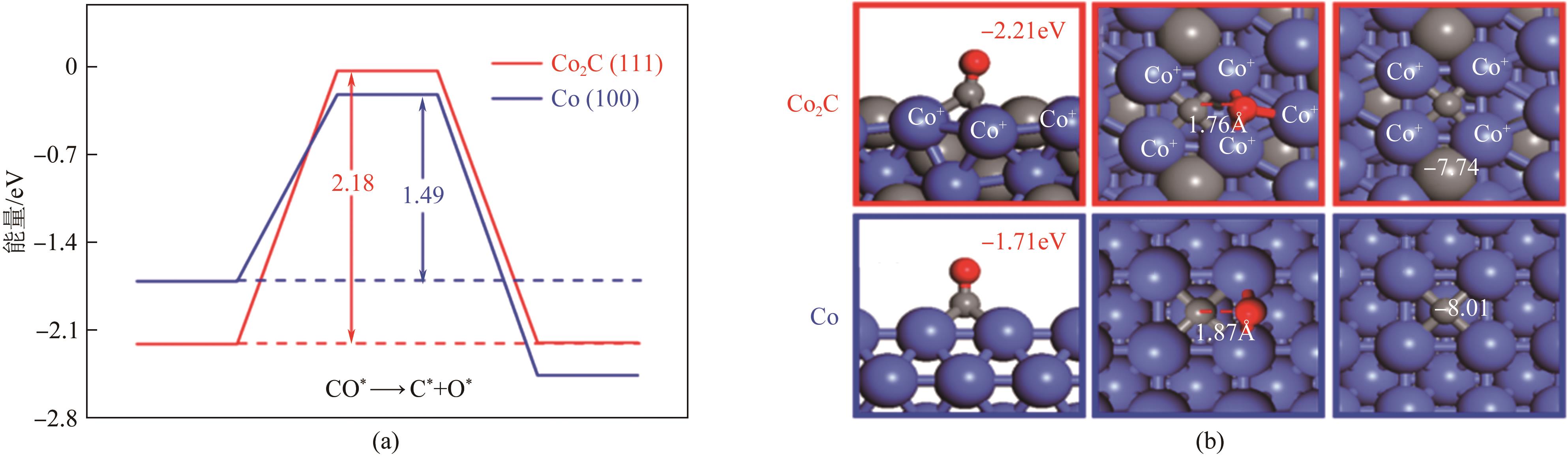
| 45 | LI Liusha, LIN Tiejun, LI Xiao, et al. Control of Co0/Co2C dual active sites for higher alcohols synthesis from syngas[J]. Applied Catalysis A: General, 2020, 602: 117704. |
| 46 | ZHAO Ziang, LU Wei, YANG Ruoou, et al. Insight into the formation of Co@Co2C catalysts for direct synthesis of higher alcohols and olefins from syngas[J]. ACS Catalysis, 2018, 8(1): 228-241. |
| 47 | CUI Wen-Gang, LI Yan-Ting, ZHANG Hongbo, et al. In situ encapsulated Co/MnO x nanoparticles inside quasi-MOF-74 for the higher alcohols synthesis from syngas[J]. Applied Catalysis B: Environmental, 2020, 278: 119262. |
| 48 | LI Fuping, LI Jiaquan, WANG Kai, et al. Co/Co6Mo6C@C nanoreactors derived from ZIF-67 composite for higher alcohols synthesis[J]. Composites Part B: Engineering, 2021, 209: 108608. |
| 49 | CAO Ang, LIU Guilong, WANG Lianfang, et al. Growing layered double hydroxides on CNTs and their catalytic performance for higher alcohol synthesis from syngas[J]. Journal of Materials Science, 2016, 51(11): 5216-5231. |
| 50 | DONG Xin, LIANG Xuelian, LI Haiyan, et al. Preparation and characterization of carbon nanotube-promoted Co-Cu catalyst for higher alcohol synthesis from syngas[J]. Catalysis Today, 2009, 147(2): 158-165. |
| 51 | NIU T, LIU G L, CHEN Y, et al. Hydrothermal synthesis of graphene-LaFeO3 composite supported with Cu-Co nanocatalyst for higher alcohol synthesis from syngas[J]. Applied Surface Science, 2016, 364: 388-399. |
| 52 | CAO Ang, LIU Guilong, YUE Yizhi, et al. Nanoparticles of Cu-Co alloy derived from layered double hydroxides and their catalytic performance for higher alcohol synthesis from syngas[J]. RSC Advances, 2015, 5(72): 58804-58812. |
| 53 | LI Zhuoshi, ZENG Zhuang, YAO Dawei, et al. High-performance CoCu catalyst encapsulated in KIT-6 for higher alcohol synthesis from syngas[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(1): 200-209. |
| 54 | SUN Kai, TAN Minghui, BAI Yunxing, et al. Design and synthesis of spherical-platelike ternary copper-cobalt-manganese catalysts for direct conversion of syngas to ethanol and higher alcohols[J]. Journal of Catalysis, 2019, 378: 1-16. |
| 55 | Subhasis DAS, SENGUPTA Manideepa, LIPPI Renata, et al. Insights into mesoporous nitrogen-rich carbon induced synergy for the selective synthesis of ethanol[J]. Carbon, 2020, 168: 337-353. |
| 56 | SHUI Meiling, HUANG Chao, MA Peiyu, et al. Accelerating C2+ alcohols synthesis from syngas by simultaneous optimizations of CO dissociation and chain growth over CuCo alloy catalyst[J]. Chinese Chemical Letters, 2021, 32(7): 2203-2206. |
| 57 | CLAEYS M, DRY M E, VAN STEEN E, et al. In situ magnetometer study on the formation and stability of cobalt carbide in Fischer-Tropsch synthesis[J]. Journal of Catalysis, 2014, 318: 193-202. |
| 58 | XIONG Jianmin, DING Yunjie, WANG Tao, et al. The formation of Co2C species in activated carbon supported cobalt-based catalysts and its impact on Fischer-Tropsch reaction[J]. Catalysis Letters, 2005, 102(3): 265-269. |
| 59 | PEI Yanpeng, DING Yunjie, ZHU Hejun, et al. Study on the effect of alkali promoters on the formation of cobalt carbide (Co2C) and on the performance of Co2C via CO hydrogenation reaction[J]. Reaction Kinetics, Mechanisms and Catalysis, 2014, 111(2): 505-520. |
| 60 | PEI Yanpeng, LIU Jinxun, ZHAO Yonghui, et al. High alcohols synthesis via Fischer-Tropsch reaction at cobalt metal/carbide interface[J]. ACS Catalysis, 2015, 5(6): 3620-3624. |
| 61 | ZHANG Riguang, WEN Guangxiang, ADIDHARMA Hertanto, et al. C2 oxygenate synthesis via Fischer-Tropsch synthesis on Co2C and Co/Co2C interface catalysts: How to control the catalyst crystal facet for optimal selectivity[J]. ACS Catalysis, 2017, 7(12): 8285-8295. |
| 62 | WANG Zi, KUMAR Nitin, SPIVEY James J. Preparation and characterization of lanthanum-promoted cobalt-copper catalysts for the conversion of syngas to higher oxygenates: Formation of cobalt carbide[J]. Journal of Catalysis, 2016, 339: 1-8. |
| 63 | XIANG Yizhi, KRUSE Norbert. Tuning the catalytic CO hydrogenation to straight- and long-chain aldehydes/alcohols and olefins/paraffins[J]. Nature Communications, 2016, 7(1): 1-6. |
| 64 | FAN Siqi, WANG Yue, LI Zhuoshi, et al. Graphene oxide-ordered mesoporous silica composite supported Co-based catalysts for CO hydrogenation to higher alcohols[J]. Applied Catalysis A: General, 2019, 583: 117123. |
| 65 | NING Xun, AN Zhe, HE Jing. Remarkably efficient CoGa catalyst with uniformly dispersed and trapped structure for ethanol and higher alcohol synthesis from syngas[J]. Journal of Catalysis, 2016, 340: 236-247. |
| 66 | CHEN Tianyuan, SU Junjie, ZHANG Zhengpai, et al. Structure evolution of Co-CoO x interface for higher alcohol synthesis from syngas over Co/CeO2 catalysts[J]. ACS Catalysis, 2018, 8(9): 8606-8617. |
| 67 | Nguyen TIEN-THAO, Hassan ZAHEDI-NIAKI M, ALAMDARI Houshang, et al. Conversion of syngas to higher alcohols over nanosized LaCo0.7Cu0.3O3 perovskite precursors[J]. Applied Catalysis A: General, 2007, 326(2): 152-163. |
| 68 | PRIETO Gonzalo, BEIJER Steven, SMITH Miranda L, et al. Design and synthesis of copper-cobalt catalysts for the selective conversion of synthesis gas to ethanol and higher alcohols[J]. Angewandte Chemie International Edition, 2014, 53(25): 6397-6401. |
| 69 | NISHIZAWA T, ISHIDA K. The Co-Cu (cobalt-copper) system[J]. Bulletin of Alloy Phase Diagrams, 1984, 5(2): 161-165. |
| 70 | YANG Qilei, CAO Ang, KANG Na, et al. Bimetallic nano Cu-Co based catalyst for direct ethanol synthesis from syngas and its structure variation with reaction time in slurry reactor[J]. Industrial & Engineering Chemistry Research, 2017, 56(11): 2889-2898. |
| 71 | GAO Wa, ZHAO Yufei, CHEN Haoran, et al. Core-shell Cu@(CuCo-alloy)/Al2O3 catalysts for the synthesis of higher alcohols from syngas[J]. Green Chemistry, 2015, 17(3): 1525-1534. |
| 72 | LIU Guilong, PAN Dongming, NIU Ting, et al. Nanoparticles of Cu-Co alloy supported on high surface area LaFeO3—Preparation and catalytic performance for higher alcohol synthesis from syngas[J]. RSC Advances, 2015, 5(40): 31637-31647. |
| 73 | LIU Guilong, GENG Yuxia, PAN Dongming, et al. Bi-metal Cu-Co from LaCo1- x Cu x O3 perovskite supported on zirconia for the synthesis of higher alcohols[J]. Fuel Processing Technology, 2014, 128: 289-296. |
| 74 | LI Zhuoshi, LUO Guangyuan, CHEN Tao, et al. Bimetallic CoCu catalyst derived from in situ grown Cu-ZIF-67 encapsulated inside KIT-6 for higher alcohol synthesis from syngas[J]. Fuel, 2020, 278: 118292. |
| 75 | LIN Tiejun, QI Xingzhen, WANG Xinxing, et al. Direct production of higher oxygenates by syngas conversion over a multifunctional catalyst[J]. Angewandte Chemie International Edition, 2019, 58(14): 4627-4631. |
| 76 | HUANG Chao, ZHU Can, ZHANG Mingwei, et al. Design of efficient ZnO/ZrO2 modified CuCoAl catalysts for boosting higher alcohol synthesis in syngas conversion[J]. Applied Catalysis B: Environmental, 2022, 300: 120739. |
| 77 | GUO Wei, GAO Wengui, WANG Hua, et al. Higher alcohols synthesis from CO2 hydrogenation over K2O-modified CuZnFeZrO2 catalysts[J]. Advanced Materials Research, 2013, 827: 20-24. |
| 78 | YAO Ruwei, WEI Jian, GE Qingjie, et al. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation[J]. Applied Catalysis B: Environmental, 2021, 298: 120556. |
| 79 | XU Di, DING Mingyue, HONG Xinlin, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catalysis, 2020, 10(9): 5250-5260. |
| 80 | TAKAGAWA Makoto, OKAMOTO Atsushi, FUJIMURA Hiromitsu, et al. Ethanol synthesis from carbon dioxide and hydrogen[J]. Studies in Surface Science and Catalysis, 1998, 114: 525-528. |
| 81 | YANG Chengsheng, MU Rentao, WANG Guishuo, et al. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation[J]. Chemical Science, 2019, 10(11): 3161-3167. |
| 82 | LI Shanggui, GUO Haijun, LUO Cairong, et al. Effect of iron promoter on structure and performance of K/Cu-Zn catalyst for higher alcohols synthesis from CO2 hydrogenation[J]. Catalysis Letters, 2013, 143(4): 345-355. |
| 83 | ZHANG Qian, WANG Sen, GENG Rui, et al. Hydrogenation of CO2 to higher alcohols on an efficient Cr-modified CuFe catalyst[J]. Applied Catalysis B: Environmental, 2023, 337: 123013. |
| 84 | XU Di, YANG Hengquan, HONG Xinlin, et al. Tandem catalysis of direct CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2021, 11(15): 8978-8984. |
| 85 | LIAN Yun, FANG Tingfeng, ZHANG Yuhua, et al. Hydrogenation of CO2 to alcohol species over Co@Co3O4/C-N catalysts[J]. Journal of Catalysis, 2019, 379: 46-51. |
| 86 | OKABE Kiyomi, YAMADA Hidekazu, HANAOKA Takaaki, et al. CO2 hydrogenation to alcohols over highly dispersed Co/SiO2 catalysts derived from acetate[J]. Chemistry Letters, 2001, 30(9): 904-905. |
| 87 | ZHANG Shunan, LIU Xiaofang, SHAO Zilong, et al. Direct CO2 hydrogenation to ethanol over supported Co2C catalysts: Studies on support effects and mechanism[J]. Journal of Catalysis, 2020, 382: 86-96. |
| 88 | LIU Bing, OUYANG Bi, ZHANG Yuhua, et al. Effects of mesoporous structure and Pt promoter on the activity of Co-based catalysts in low-temperature CO2 hydrogenation for higher alcohol synthesis[J]. Journal of Catalysis, 2018, 366: 91-97. |
| 89 | YANG Chengsheng, LIU Sihang, WANG Yanan, et al. The interplay between structure and product selectivity of CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2019, 58(33): 11242-11247. |
| 90 | HUANG Jie, JIANG Suyu, WANG Meng, et al. Dynamic evolution of Fe and carbon species over different ZrO2 supports during CO prereduction and their effects on CO2 hydrogenation to light olefins[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(23): 7891-7903. |
| 91 | GUO Haijun, LI Shanggui, PENG Fen, et al. Roles investigation of promoters in K/Cu-Zn catalyst and higher alcohols synthesis from CO2 hydrogenation over a novel two-stage bed catalyst combination system[J]. Catalysis Letters, 2015, 145(2): 620-630. |
| 92 | GNANAMANI Muthu Kumaran, JACOBS Gary, KEOGH Robert A, et al. Fischer-Tropsch synthesis: Effect of pretreatment conditions of cobalt on activity and selectivity for hydrogenation of carbon dioxide[J]. Applied Catalysis A: General, 2015, 499: 39-46. |
| 93 | LIU Lichen, PUGA Alberto V, CORED Jorge, et al. Sunlight-assisted hydrogenation of CO2 into ethanol and C2+ hydrocarbons by sodium-promoted Co@C nanocomposites[J]. Applied Catalysis B: Environmental, 2018, 235: 186-196. |
| 94 | GNANAMANI Muthu Kumaran, HAMDEH Hussein H, JACOBS Gary, et al. Hydrogenation of carbon dioxide over K-promoted FeCo bimetallic catalysts prepared from mixed metal oxalates[J]. ChemCatChem, 2017, 9(7): 1303-1312. |
| 95 | WANG Lingxiang, HE Shenxian, WANG Liang, et al. Cobalt-nickel catalysts for selective hydrogenation of carbon dioxide into ethanol[J]. ACS Catalysis, 2019, 9(12): 11335-11340. |
| 96 | DING Liping, SHI Taotao, GU Jing, et al. CO2 hydrogenation to ethanol over Cu@Na-beta[J]. Chem, 2020, 6(10): 2673-2689. |
| [1] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [2] | 周爱国, 郑家乐, 杨川箬, 杨小艺, 赵俊德, 李兴春. 直接空气二氧化碳捕集技术工业化进展[J]. 化工进展, 2024, 43(6): 2928-2939. |
| [3] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [4] | 张真, 张凡, 云祉婷. 绿氢在石化和化工行业的减碳经济性分析[J]. 化工进展, 2024, 43(6): 3021-3028. |
| [5] | 何世坤, 张文豪, 冯君锋, 潘晖. 负载金属型固体酸催化木质纤维生物质定向转化为乙酰丙酸甲酯[J]. 化工进展, 2024, 43(6): 3042-3050. |
| [6] | 陈富强, 仲兆平, 戚仁志. 铜基催化剂电还原二氧化碳为甲酸研究进展[J]. 化工进展, 2024, 43(6): 3051-3060. |
| [7] | 冯勇强, 王洁茹, 王超娴, 李芳, 苏婉婷, 孙宇, 赵彬然. γ-Al2O3 负载的Ni、Fe、Cu对介质阻挡放电等离子体转化CO2/CH4的影响[J]. 化工进展, 2024, 43(5): 2705-2713. |
| [8] | 解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722. |
| [9] | 周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738. |
| [10] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| [11] | 黄澎, 邹颖, 王宝焕, 王逍妍, 赵勇, 梁鑫, 胡迪. 二氧化碳电催化还原反应制合成气催化剂研究进展[J]. 化工进展, 2024, 43(5): 2760-2775. |
| [12] | 周秋明, 牛丛丛, 吕帅帅, 李红伟, 文富利, 徐润, 李明丰. 通过产物转化分离推动CO2加氢制甲醇过程的研究进展[J]. 化工进展, 2024, 43(5): 2776-2785. |
| [13] | 卢欣欣, 蔡东仁, 詹国武. 基于固体前体构建集成催化剂及CO2加氢研究进展[J]. 化工进展, 2024, 43(5): 2786-2802. |
| [14] | 武西宁, 张宁, 秦佳敏, 徐龙, 魏朝阳, 马晓迅. 低冷量下强化CO2吸收的甲醇基纳米流体性能[J]. 化工进展, 2024, 43(5): 2811-2822. |
| [15] | 李海鹏, 吴桐, 王琪, 郜时旺, 王晓龙, 李旭, 高新华, 年佩, 魏逸彬. 透水NaA分子筛膜强化的CO2加氢高效制甲醇[J]. 化工进展, 2024, 43(5): 2834-2842. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||