化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2489-2504.DOI: 10.16085/j.issn.1000-6613.2024-1598
• 合成生物制造 • 上一篇
微生物细胞活力调控生产丁二酸
- 江南大学生物工程学院,工业生物技术教育部重点实验室,江苏 无锡 214122
-
收稿日期:2024-10-08修回日期:2025-01-04出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:陈修来 -
作者简介:唐永圣(2000—),男,硕士研究生,研究方向为微生物代谢工程。E-mail:6230209063@stu.jiangnan.edu.cn。 -
基金资助:国家重点研发计划(2019YFA0904900);中央高校基本科研业务费专项资金(JUSRP622001)
Regulation of microbial cell viability for succinic acid production
TANG Yongsheng( ), GU Ziyun, CHEN Xiulai(
), GU Ziyun, CHEN Xiulai( )
)
- Key Laboratory of Industrial Biotechnology of Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2024-10-08Revised:2025-01-04Online:2025-05-25Published:2025-05-20 -
Contact:CHEN Xiulai
摘要:
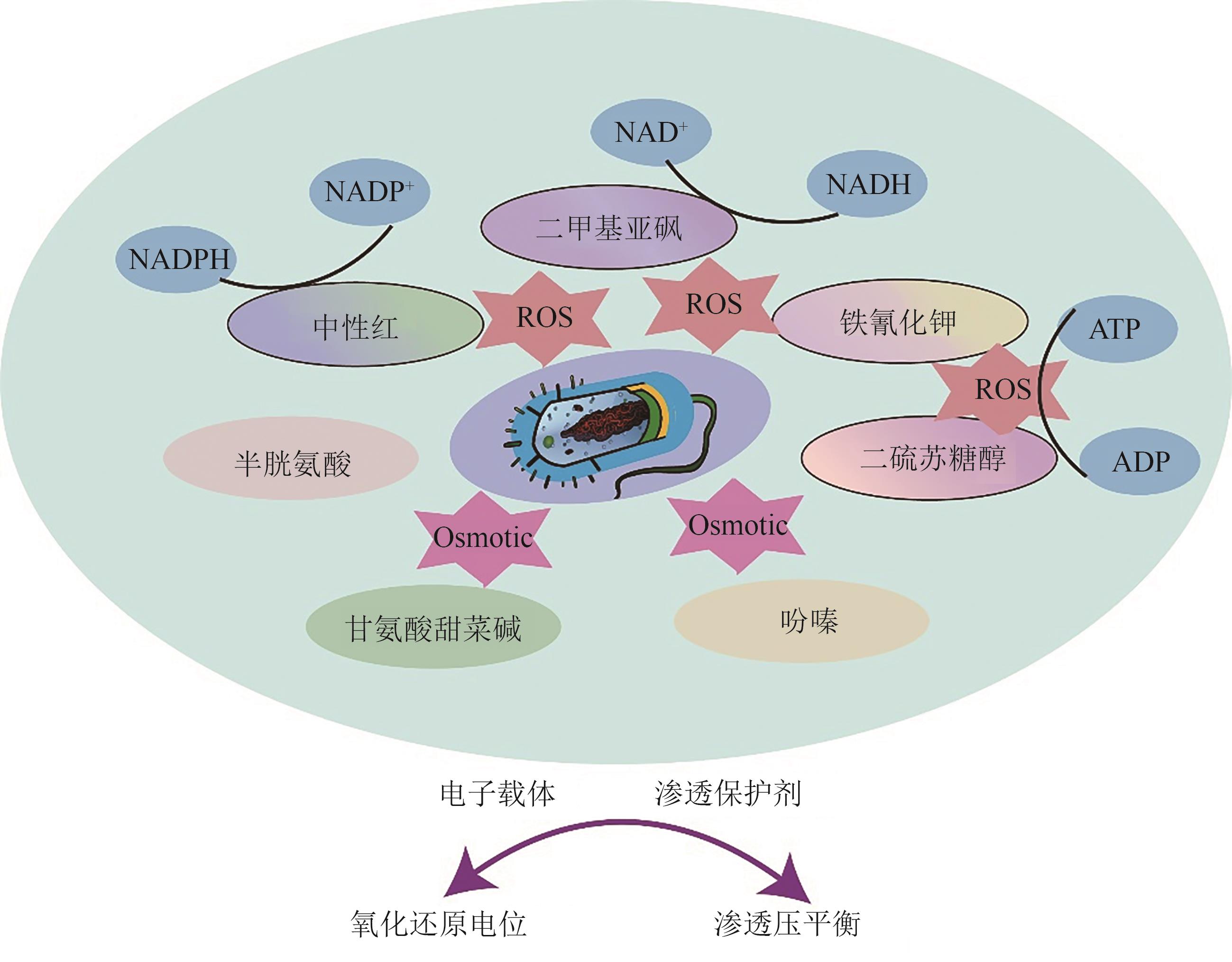
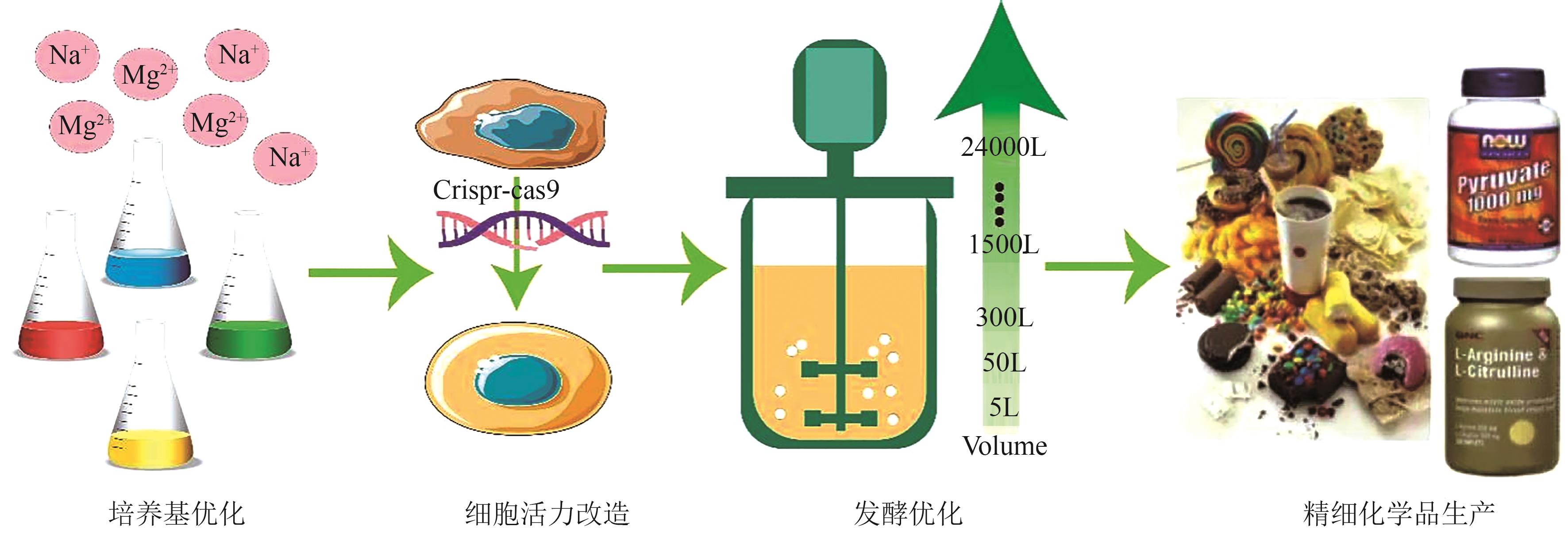
随着代谢工程技术的发展,微生物发酵法生产丁二酸受到广泛关注。目前,研究人员已经开发了多种高产丁二酸的工程菌株,包括大肠杆菌、产琥珀酸曼氏杆菌、产琥珀酸放线杆菌、谷氨酸棒状杆菌、解脂耶氏酵母等。然而,由于丁二酸发酵后期微生物细胞活力的不足,导致丁二酸生产效率严重下降,从而制约了丁二酸的高效生产与工业化应用。本文围绕微生物发酵生产丁二酸,探讨了提升微生物细胞活力的关键策略与方法,主要包括:基于内外源特定化合物供给的化学工程方法、基于改良细胞生长性能与环境适应性的代谢工程策略和基于发酵工程的发酵工艺优化方案。最后,对微生物发酵法生产丁二酸的产业化应用进行了展望。
中图分类号:
引用本文
唐永圣, 顾子蕴, 陈修来. 微生物细胞活力调控生产丁二酸[J]. 化工进展, 2025, 44(5): 2489-2504.
TANG Yongsheng, GU Ziyun, CHEN Xiulai. Regulation of microbial cell viability for succinic acid production[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2489-2504.
| 菌株 | 改造策略 | 发酵条件 | 时间 | 产量 | 产率 | 参考文献 |
|---|---|---|---|---|---|---|
| E. coli FY-65 | ΔpflAB,ΔldhA,ΔackA-pta,ΔpoxB 过表达ribF | 葡萄糖;两阶段分批补料 | 72h | 153.4g/L | 0.90 | [ |
| M.succiniciproducens PALFK | ΔldhA,ΔfruA,Δpta-ackA | 蔗糖、甘油;厌氧分批补料 | 14h | 78.4g/L | 1.07 | [ |
| E. coli W1485 | ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料 | 80h | 79.8g/L | 0.78 | [ |
| E. coli DY329 | ΔackA,Δpta,ΔldhA,ΔpstG,过表达mdh | 葡萄糖;两阶段分批发酵 | 80h | 32.2g/L | [ | |
| Y. lipolytica Y-3314 | 过表达pcK,scS2,ΔacH | 甘油;好氧分批补料发酵 | 138h | 110.7g/L | 0.53 | [ |
| E. coli SD121 | 过表达ppc,ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料发 | 75h | 116.2g/L | 1.13 | [ |
| E. coli AFP111 | ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段发酵 | 54h | 101.2g/L | 1.07 | [ |
| E. coli MG1655 | ΔadhE,ΔldhA | 葡萄糖;厌氧分批补料 | 24h | 15.6g/L | 0.85 | [ |
| E. coli AFP184 | ΔpflB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料发酵 | 72h | 77.0g/L | 0.75 | [ |
| Y. lipolytica PGC01003 | ΔsdH5 | 甘油;好氧分批补料发酵 | — | 198.3g/L | — | [ |
| E. coli W1485 | ΔackA-pta,ΔiclR,ΔpoxB,ΔmgsA,ΔsdhA | 葡萄糖;好氧分批补料 | 52h | 36.1g/L | 0.37 | [ |
| E. coli AS1600a | ΔldhA,ΔadhE,ΔackA,ΔfocA-pflB,ΔmgsA, ΔpoxB,ΔtdcDE,ΔcitF,ΔaspC,ΔsfcA | 木糖;厌氧分批补料 | — | 84.3g/L | 0.90 | [ |
| C. glutamicum ATCC 13032 | ΔldhA,Δpta-ackA,ΔactA,ΔpoxB | 葡萄糖;两阶段分批补料发酵 | 160h | 152.2g/L | 1.1 | [ |
| E. coli HX024 | ΔptsI,ΔldhA,ΔpflB,Ppck*-galP,Ppck*-pck, ΔackA-pta,Ppck*-aceBA,Ppck*-dcuC, ΔmgsA | 葡萄糖;两阶段分批补料发酵 | — | 96.0g/L | 0.89 | [ |
| A. succinogene ΔldhA-pCRA71 | ΔldhA | 葡萄糖;微有氧分批补料发酵 | 48h | 146.0g/L | 0.92 | [ |
| E. coli NZN11 | ΔpflB, ΔldhA | 醋酸盐、液化木薯淀粉;两阶段分批补料发酵 | 72h | 127.1g/L | 0.71 | [ |
| E. coli SD121 | ΔpflB, ΔldhA, ΔptsG | 葡萄糖;两阶段分批补料发酵 | 75h | 116.2g/L | 1.13 | [ |
| C. glutamicum BOL | 过表达pyC,fdH,gapA,ΔcaT,ΔpqO,ΔldhA,Δpta-ackA | 葡萄糖、甲酸;两阶段分批补料发酵 | 54h | 133.8g/L | 1.09 | [ |
| E. coli YL106/pSCsfcA | ΔptsG, ΔpoxB, Δpta, ΔiclR, ΔsdhA, ΔarcA, ΔadhE, ΔldhA, pckA* | 葡萄糖;两阶段分批补料发酵 | 40h | 85.3g/L | 0.65 | [ |
表1 不同改造策略下的微生物生产性能对比
| 菌株 | 改造策略 | 发酵条件 | 时间 | 产量 | 产率 | 参考文献 |
|---|---|---|---|---|---|---|
| E. coli FY-65 | ΔpflAB,ΔldhA,ΔackA-pta,ΔpoxB 过表达ribF | 葡萄糖;两阶段分批补料 | 72h | 153.4g/L | 0.90 | [ |
| M.succiniciproducens PALFK | ΔldhA,ΔfruA,Δpta-ackA | 蔗糖、甘油;厌氧分批补料 | 14h | 78.4g/L | 1.07 | [ |
| E. coli W1485 | ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料 | 80h | 79.8g/L | 0.78 | [ |
| E. coli DY329 | ΔackA,Δpta,ΔldhA,ΔpstG,过表达mdh | 葡萄糖;两阶段分批发酵 | 80h | 32.2g/L | [ | |
| Y. lipolytica Y-3314 | 过表达pcK,scS2,ΔacH | 甘油;好氧分批补料发酵 | 138h | 110.7g/L | 0.53 | [ |
| E. coli SD121 | 过表达ppc,ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料发 | 75h | 116.2g/L | 1.13 | [ |
| E. coli AFP111 | ΔpflAB,ΔldhA,ΔptsG | 葡萄糖;两阶段发酵 | 54h | 101.2g/L | 1.07 | [ |
| E. coli MG1655 | ΔadhE,ΔldhA | 葡萄糖;厌氧分批补料 | 24h | 15.6g/L | 0.85 | [ |
| E. coli AFP184 | ΔpflB,ΔldhA,ΔptsG | 葡萄糖;两阶段分批补料发酵 | 72h | 77.0g/L | 0.75 | [ |
| Y. lipolytica PGC01003 | ΔsdH5 | 甘油;好氧分批补料发酵 | — | 198.3g/L | — | [ |
| E. coli W1485 | ΔackA-pta,ΔiclR,ΔpoxB,ΔmgsA,ΔsdhA | 葡萄糖;好氧分批补料 | 52h | 36.1g/L | 0.37 | [ |
| E. coli AS1600a | ΔldhA,ΔadhE,ΔackA,ΔfocA-pflB,ΔmgsA, ΔpoxB,ΔtdcDE,ΔcitF,ΔaspC,ΔsfcA | 木糖;厌氧分批补料 | — | 84.3g/L | 0.90 | [ |
| C. glutamicum ATCC 13032 | ΔldhA,Δpta-ackA,ΔactA,ΔpoxB | 葡萄糖;两阶段分批补料发酵 | 160h | 152.2g/L | 1.1 | [ |
| E. coli HX024 | ΔptsI,ΔldhA,ΔpflB,Ppck*-galP,Ppck*-pck, ΔackA-pta,Ppck*-aceBA,Ppck*-dcuC, ΔmgsA | 葡萄糖;两阶段分批补料发酵 | — | 96.0g/L | 0.89 | [ |
| A. succinogene ΔldhA-pCRA71 | ΔldhA | 葡萄糖;微有氧分批补料发酵 | 48h | 146.0g/L | 0.92 | [ |
| E. coli NZN11 | ΔpflB, ΔldhA | 醋酸盐、液化木薯淀粉;两阶段分批补料发酵 | 72h | 127.1g/L | 0.71 | [ |
| E. coli SD121 | ΔpflB, ΔldhA, ΔptsG | 葡萄糖;两阶段分批补料发酵 | 75h | 116.2g/L | 1.13 | [ |
| C. glutamicum BOL | 过表达pyC,fdH,gapA,ΔcaT,ΔpqO,ΔldhA,Δpta-ackA | 葡萄糖、甲酸;两阶段分批补料发酵 | 54h | 133.8g/L | 1.09 | [ |
| E. coli YL106/pSCsfcA | ΔptsG, ΔpoxB, Δpta, ΔiclR, ΔsdhA, ΔarcA, ΔadhE, ΔldhA, pckA* | 葡萄糖;两阶段分批补料发酵 | 40h | 85.3g/L | 0.65 | [ |
| 83 | LI Feng, MA Jiangfeng, WU Mingke, et al. Succinic acid production from sucrose and sugarcane molasses by metabolically engineered Escherichia coli [J]. Chinese Journal of Biotechnology, 2015, 31(4): 534-541. |
| 84 | 吴明科, 刘嵘明, 梁丽亚, 等. 大肠杆菌AFP111菌体回收连续转化生产丁二酸[J]. 生物工程学报, 2013, 29(12): 1875-1879. |
| WU Mingke, LIU Rongming, LIANG Liya, et al. Succinic acid production with Escherichia coli AFP111 recovered from fermentation[J]. Chinese Journal of Biotechnology, 2013, 29(12): 1875-1879. | |
| 85 | ANDERSSON Christian, HELMERIUS Jonas, HODGE David, et al. Inhibition of succinic acid production in metabolically engineered Escherichia coli by neutralizing agent, organic acids, and osmolarity[J]. Biotechnology Progress, 2009, 25(1): 116-123. |
| 86 | ANDERSSON Christian, HODGE David, BERGLUND Kris A, et al. Effect of different carbon sources on the production of succinic acid using metabolically engineered Escherichia coli [J]. Biotechnology Progress, 2007, 23(2): 381-388. |
| 87 | BOADA Yadira, VIGNONI Alejandro, Jesús PICÓ, et al. Extended metabolic biosensor design for dynamic pathway regulation of cell factories[J]. iScience, 2020, 23(7): 101305. |
| 88 | 夏建业, 刘晶, 庄英萍. 人工智能时代发酵优化与放大技术的机遇与挑战[J]. 生物工程学报, 2022, 38(11): 4180-4199. |
| XIA Jianye, LIU Jing, ZHUANG Yingping. Opportunities and challenges for fermentation optimization and scale-up technology in the artificial intelligence era[J]. Chinese Journal of Biotechnology, 2022, 38(11): 4180-4199. | |
| 89 | PURDY Hugh M, REED Jennifer L. Evaluating the capabilities of microbial chemical production using genome-scale metabolic models[J]. Current Opinion in Systems Biology, 2017, 2: 91-97. |
| 90 | NIELSEN Alec A K, DER Bryan S, SHIN Jonghyeon, et al. Genetic circuit design automation[J]. Science, 2016, 352(6281): aac7341. |
| 91 | CHAE Tong Un, CHOI So Young, KIM Je Woong, et al. Recent advances in systems metabolic engineering tools and strategies[J]. Current Opinion in Biotechnology, 2017, 47: 67-82. |
| 92 | WEHRS Maren, TANJORE Deepti, Thomas ENG, et al. Engineering robust production microbes for large-scale cultivation[J]. Trends in Microbiology, 2019, 27(6): 524-537. |
| 93 | FENG Yao, TIAN Xiwei, CHEN Yang, et al. Real-time and on-line monitoring of ethanol fermentation process by viable cell sensor and electronic nose[J]. Bioresources and Bioprocessing, 2021, 8(1): 37. |
| 94 | ZOU Yejun, WANG Aoxue, HUANG Li, et al. Illuminating NAD+ metabolism in live cells and in vivo using a genetically encoded fluorescent sensor[J]. Developmental Cell, 2020, 53(2): 240-252.e247. |
| 95 | ZHAO Yuzheng, HU Qingxun, CHENG Feixiong, et al. SoNar, a highly responsive NAD+/NADH sensor, allows high-throughput metabolic screening of anti-tumor agents[J]. Cell Metabolism, 2015, 21(5): 777-789. |
| 96 | ZHAO Yuzheng, JIN Jing, HU Qingxun, et al. Genetically encoded fluorescent sensors for intracellular NADH detection[J]. Cell Metabolism, 2011, 14(4): 555-566. |
| 97 | WANG Baowei, WANG Zhiwen, CHEN Tao, et al. Development of novel bioreactor control systems based on smart sensors and actuators[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8:7. |
| 98 | FERONE Mariateresa, RAGANATI Francesca, OLIVIERI Giuseppe, et al. Bioreactors for succinic acid production processes[J]. Critical Reviews in Biotechnology, 2019, 39(4): 571-586. |
| 99 | 夏建业, 田锡炜, 刘娟, 等. 人工智能时代的智能生物制造[J]. 生物加工过程, 2020, 18(1): 13-20. |
| XIA Jianye, TIAN Xiwei, LIU Juan, et al. Intelligent biological manufacturing in the age of artificial intelligence[J]. Chinese Journal of Bioprocess Engineering, 2020, 18(1): 13-20. | |
| 100 | 刘嵘明, 梁丽亚, 吴明科, 等. 微生物发酵生产丁二酸研究进展[J]. 生物工程学报, 2013, 29(10): 1386-1397. |
| LIU Rongming, LIANG Liya, WU Mingke, et al. Progress in microbial production of succinic Acid[J]. Chinese Journal of Biotechnology, 2013, 29(10):1386-1397. | |
| 101 | CHENG Keke, ZHAO Xuebing, ZENG Jing, et al. Biotechnological production of succinic acid: Current state and perspectives[J]. Biofuels, Bioproducts and Biorefining, 2012, 6(3): 302-318. |
| 102 | KWON Ohsuk, GEORGELLIS Dimitris, LYNCHA Simon, et al. The ArcB sensor kinase of Escherichia coli: Genetic exploration of the transmembrane region[J]. Journal of Bacteriology, 2000, 182(10): 2960-2966. |
| 1 | 王学明. 代谢工程改造大肠杆菌生产琥珀酸[D]. 无锡: 江南大学, 2023. |
| WANG Xueming. Metabolic engineering of Escherichia coli for the production of succinic acid[D]. Wuxi: Jiangnan University, 2023. | |
| 2 | 张耀, 邱晓曼, 陈程鹏, 等. 生物法制造丁二酸研究进展[J]. 化工学报, 2020, 71(5): 1964-1975. |
| ZHANG Yao, QIU Xiaoman, CHEN Chengpeng, et al. Recent progress in microbial production of succinic acid[J]. CIESC Journal, 2020, 71(5): 1964-1975. | |
| 3 | 万屹东, 高有军, 马江锋. 生物法制备丁二酸的研究及产业化进展[J]. 生物加工过程, 2020, 18(5): 583-591, 630. |
| WAN Yidong, GAO Youjun, MA Jiangfeng. Progress in industrialization on succinic acid production by fermentation[J]. Chinese Journal of Bioprocess Engineering, 2020, 18(5): 583-591, 630. | |
| 4 | 王学明, 潘静宇, 吴静, 等. 调控大肠杆菌胞内ATP和NADH水平促进琥珀酸生产[J]. 生物工程学报, 2023, 39(8): 3236-3252. |
| WANG Xueming, PAN Jingyu, WU Jing, et al. Regulation of intracellular level of ATP and NADH in Escherichia coli to promote succinic acid production[J]. Chinese Journal of Biotechnology, 2023, 39(8): 3236-3252. | |
| 5 | Thomas KLASSON K, STURM Matthew P, COLE Marsha R. Acid hydrolysis of sucrose in sweet sorghum syrup followed by succinic acid production using a genetically engineered Escherichia coli [J]. Biocatalysis and Agricultural Biotechnology, 2022, 39: 102231. |
| 6 | 孙涛, 孙美莉, 陆然, 等. 合成生物学改造酵母驱动丁二酸绿色生物制造[J]. 化工学报, 2024, 75(4): 1382-1393. |
| SUN Tao, SUN Meili, LU Ran, et al. Synthetic biology of yeasts drives green biomanufacturing of succinic acid[J]. CIESC Journal, 2024, 75(4): 1382-1393. | |
| 7 | 钟驭涛, 尚长宇, 王言东, 等. 利用酵母细胞工厂合成丁二酸的研究进展[J]. 生物工程学报, 2024, 40(8): 2644-2665. |
| ZHONG Yutao, SHANG Changyu, WANG Yandong, et al. Advances in synthesis of succinic acid using yeast cell factories[J]. Chinese Journal of Biotechnology, 2024, 40(8): 2644-2665. | |
| 8 | VEMURI G N, EITEMAN M A, ALTMAN E. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli [J]. Applied and Environmental Microbiology, 2002, 68(4): 1715-1727. |
| 9 | SKOROKHODOVA Allexandra Yu, MORZHAKOVA Anastasiya A, GULEVICH Andrey Yu, et al. Manipulating pyruvate to acetyl-CoA conversion in Escherichia coli for anaerobic succinate biosynthesis from glucose with the yield close to the stoichiometric maximum[J]. Journal of Biotechnology, 2015, 214: 33-42. |
| 10 | BAGRAMYAN Karine, GALSTYAN Anna, TRCHOUNIAN Armen. Redox potential is a determinant in the Escherichia coli anaerobic fermentative growth and survival: Effects of impermeable oxidant[J]. Bioelectrochemistry, 2000, 51(2): 151-156. |
| 11 | KAMPERS Linde F C, VAN HECK Ruben G A, DONATI Stefano, et al. In silico-guided engineering of Pseudomonas putida towards growth under micro-oxic conditions[J]. Microbial Cell Factories, 2019, 18(1): 179. |
| 12 | LEE Jong An, Jung Ho AHN, KIM Gi Bae, et al. Metabolic engineering of Mannheimia succiniciproducens for malic acid production using dimethylsulfoxide as an electron acceptor[J]. Biotechnology and Bioengineering, 2023, 120(1): 203-215. |
| 13 | MARKARIAN S A, POLADYAN A A, KIRAKOSYAN G R, et al. Effect of diethylsulphoxide on growth, survival and ion exchange of Escherichia coli [J]. Letters in Applied Microbiology, 2002, 34(6): 417-421. |
| 14 | LI Jian, JIANG Min, CHEN Kequan, et al. Effect of redox potential regulation on succinic acid production by Actinobacillus succinogenes [J]. Bioprocess and Biosystems Engineering, 2010, 33(8): 911-920. |
| 15 | CHEN Xiaoju, JIANG Shaotong, ZHENG Zhi, et al. Effects of culture redox potential on succinic acid production by Corynebacterium crenatum under anaerobic conditions[J]. Process Biochemistry, 2012, 47(8): 1250-1255. |
| 16 | KAHRU Anne, PAALME Toomas, VILU Raivo. Effect of temperature on the ATP pool and adenylate energy charge in Escherichia coli [J]. FEMS Microbiology Letters, 1987, 41(3): 305-308. |
| 17 | PHUE Je-Nie, SHILOACH Joseph. Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21 evaluating transcription levels of key genes at different dissolved oxygen conditions[J]. Metabolic Engineering, 2005, 7(5/6): 353-363. |
| 18 | LARA Alvaro R, JAÉN Karim E, FOLARIN Olusegun, et al. Effect of the oxygen transfer rate on oxygen-limited production of plasmid DNA by Escherichia coli [J]. Biochemical Engineering Journal, 2019, 150: 107303. |
| 19 | ARENSE Paula, BERNAL Vicente, IBORRA José L, et al. Metabolic adaptation of Escherichia coli to long-term exposure to salt stress[J]. Process Biochemistry, 2010, 45(9): 1459-1467. |
| 20 | XIAO Mengyong, ZHU Xinna, FAN Feiyu, et al. Osmotolerance in Escherichia coli is improved by activation of copper efflux genes or supplementation with sulfur-containing amino acids[J]. Applied and Environmental Microbiology, 2017, 83(7): e03050-16. |
| 103 | LOPEZ Pau Cabaneros, UDUGAMA Isuru A, THOMSEN Sune T, et al. Towards a digital twin: A hybrid data-driven and mechanistic digital shadow to forecast the evolution of lignocellulosic fermentation[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(5): 1046-1060. |
| 21 | MUKHTAR Salma, AHMAD Samia, BASHIR Aftab, et al. Identification of plasmid encoded osmoregulatory genes from halophilic bacteria isolated from the rhizosphere of halophytes[J]. Microbiological Research, 2019, 228: 126307. |
| 22 | KANESHIRO Kiyomi R., STROME Susan. Inheritance of protection from osmotic stress[J]. Nature Cell Biology, 2017, 19(3): 151-152. |
| 23 | FANG Xiaojiang, LI Jian, ZHENG Xiaoyu, et al. Influence of osmotic stress on fermentative production of succinic acid by Actinobacillus succinogenes [J]. Applied Biochemistry and Biotechnology, 2011, 165(1): 138-147. |
| 24 | CIEMNIECKI John A, NEWMAN Dianne K. The potential for redox-active metabolites to enhance or unlock anaerobic survival metabolisms in aerobes[J]. Journal of Bacteriology, 2020, 202(11): 10.1128/jb.00797-19. |
| 25 | WANG Jun, GUO Xin, LI Heng, et al. Hydrogen sulfide from cysteine desulfurase, not 3-mercaptopyruvate sulfurtransferase, contributes to sustaining cell growth and bioenergetics in E. coli under anaerobic conditions[J]. Frontiers in Microbiology, 2019, 10:2357. |
| 26 | 张岩, 梁萌, 刘德华. 克鲁氏假丝酵母在高渗环境中海藻糖和胞内甘油积累的研究[J]. 生物工程学报, 2001,17(3): 328-331. |
| ZHANG Yan, LIANG Meng, LIU Dehua. The metabolism of trehalose and intracellular glycerol in Candida krusei responsing to high osmosis[J]. Chinese Journal of Biotechnology, 2001,17(3): 328-331. | |
| 27 | ZHANG Wenming, ZHU Junru, ZHU Xinggui, et al. Expression of global regulator IrrE for improved succinate production under high salt stress by Escherichia coli [J]. Bioresource Technology, 2018, 254: 151-156. |
| 28 | VON WEYMARN N, NYYSSÖLÄ A, REINIKAINEN T, et al. Improved osmotolerance of recombinant Escherichia coli by de novo glycine betaine biosynthesis[J]. Applied Microbiology and Biotechnology, 2001, 55(2): 214-218. |
| 29 | VAZULKA Sophie, SCHIAVINATO Matteo, WAGENKNECHT Martin, et al. Interaction of periplasmic fab production and intracellular redox balance in Escherichia coli affects product yield[J]. ACS Synthetic Biology, 2022, 11(2): 820-834. |
| 30 | KAILA Ville R I, Mårten WIKSTRÖM. Architecture of bacterial respiratory chains[J]. Nature Reviews Microbiology, 2021, 19(5): 319-330. |
| 31 | XU Hongtao, ZHOU Zhihui, WANG Chen, et al. Enhanced succinic acid production in Corynebacterium glutamicum with increasing the available NADH supply and glucose consumption rate by decreasing H+-ATPase activity[J]. Biotechnology Letters, 2016, 38(7): 1181-1186. |
| 32 | JUNG Hwi-Min, HAN Jae-Ho, Min-Kyu OH. Improved production of 2,3-butanediol and isobutanol by engineering electron transport chain in Escherichia coli [J]. Microbial Biotechnology, 2021, 14(1): 213-226. |
| 33 | ZHOU Hang, ZHANG Yiwen, LONG Christopher P, et al. A citric acid cycle-deficient Escherichia coli as an efficient chassis for aerobic fermentations[J]. Nature Communications, 2024, 15(1): 2372. |
| 34 | WU Zaiqiang, WANG Junsong, ZHANG Xueli, et al. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate by increasing the intracellular FAD pool[J]. Biochemical Engineering Journal, 2019, 146: 132-142. |
| 35 | Julian TIX, GOTTHARDT Leon, BODE Joshua, et al. Enhancement of succinic acid production by Actinobacillus succinogenes in an electro-bioreactor[J]. Fermentation, 2024, 10(10): 504. |
| 36 | BUDIN Itay, DE ROND Tristan, CHEN Yan, et al. Viscous control of cellular respiration by membrane lipid composition[J]. Science, 2018, 362(6419): 1186-1189. |
| 37 | LIU Qiaojie, LIN Zhenquan, ZHANG Yan, et al. Improved poly(3-hydroxybutyrate) production in Escherichia coli by inactivation of cytochrome bd-Ⅱ oxidase or/and NDH-Ⅱ dehydrogenase in low efficient respiratory chains[J]. Journal of Biotechnology, 2014, 192: 170-176. |
| 38 | LIU Jianming, WANG Zhihao, KANDASAMY Vijayalakshmi, et al. Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis[J]. Metabolic Engineering, 2017, 44: 22-29. |
| 39 | PAN Jingyu, TANG Yongsheng, LIU Jia, et al. Reprogramming protein stability in Escherichia coli to improve four-carbon dicarboxylic acids production[J]. Chemical Engineering Journal, 2024, 493: 152893. |
| 40 | LEE Jee Whu, Tee Gee ONG, SAMIAN Mohammed Razip, et al. Screening of selected ageing-related proteins that extend chronological life span in yeast Saccharomyces cerevisiae [J]. Scientific Reports, 2021, 11(1): 24148. |
| 41 | GUO Liang, DIAO Wenwen, GAO Cong, et al. Engineering Escherichia coli lifespan for enhancing chemical production[J]. Nature Catalysis, 2020, 3(3): 307-318. |
| 42 | 郭亮. 时空代谢调控大肠杆菌生产精细化学品[D]. 无锡: 江南大学, 2020. |
| GUO Liang. Temporo-spatial metabolic regulation of Escherichia coli for poduction of fine chemicals[D]. Wuxi: Jiangnan University, 2020. | |
| 43 | GUO Liang, QI Mengya, GAO Cong, et al. Engineering microbial cell viability for enhancing chemical production by second codon engineering[J]. Metabolic Engineering, 2022, 73: 235-246. |
| 44 | NIELSEN Jens, KEASLING Jay D. Engineering cellular metabolism[J]. Cell, 2016, 164(6): 1185-1197. |
| 45 | FONTAINE Fanette, STEWART Eric J, LINDNER Ariel B, et al. Mutations in two global regulators lower individual mortality in Escherichia coli [J]. Molecular Microbiology, 2008, 67(1): 2-14. |
| 46 | KARGETI Manika, VENKATESH K V. Effect of global transcriptional regulators on kinetic behavior of Escherichia coli under anaerobic fermentation conditions[J]. Archives of Microbiology, 2018, 200(6): 979-987. |
| 47 | ZHU Liwen, XIA Shitao, WEI Lina, et al. Enhancing succinic acid biosynthesis in Escherichia coli by engineering its global transcription factor, catabolite repressor/activator (Cra)[J]. Scientific Reports, 2016, 6(1): 36526. |
| 48 | ZENG Duwen, YANG Yongqiang, WANG Qi, et al. Transcriptome analysis of Kluyveromyces marxianus under succinic acid stress and development of robust strains[J]. Applied Microbiology and Biotechnology, 2024, 108(1): 293. |
| 49 | Jaana MÄNNIK, KAR Prathitha, AMARASINGHE Chathuddasie, et al. Determining the rate-limiting processes for cell division in Escherichia coli [J]. Nature Communications, 2024, 15(1): 9948. |
| 50 | MICALI Gabriele, GRILLI Jacopo, MARCHI Jacopo, et al. Dissecting the control mechanisms for DNA replication and cell division in E. coli [J]. Cell Reports, 2018, 25(3): 761-771. |
| 51 | KARIMOVA Gouzel, ROBICHON Carine, LADANT Daniel. Characterization of YmgF, a 72-residue inner membrane protein that associates with the Escherichia coli cell division machinery[J]. Journal of Bacteriology, 2009, 191(1): 333-346. |
| 52 | JEONG Ki Jun, LEE Sang Yup. Enhanced production of recombinant proteins in Escherichia coli by filamentation suppression[J]. Applied and Environmental Microbiology, 2003, 69(2): 1295-1298. |
| 53 | BISICCHIA Paola, ARUMUGAM Senthil, SCHWILLE Petra, et al. MinC, MinD, and MinE drive counter-oscillation of early-cell-division proteins prior to Escherichia coli septum formation[J]. mBio, 2013, 4(6): e00856-13. |
| 54 | AUER George K, OLIVER Piercen M, RAJENDRAM Manohary, et al. Bacterial swarming reduces proteus mirabilis and vibrio parahaemolyticus cell stiffness and increases β-lactam susceptibility[J]. mBio, 2019, 10(5):e00210-219. |
| 55 | DING Qiang, MA Danlei, LIU Gaoqiang, et al. Light-powered Escherichia coli cell division for chemical production[J]. Nature Communications, 2020, 11(1): 2262. |
| 56 | WALLDEN Mats, FANGE David, LUNDIUS Ebba Gregorsson, et al. The synchronization of replication and division cycles in individual E. coli cells[J]. Cell, 2016, 166(3): 729-739. |
| 57 | URSINUS Astrid, DEN ENT Fusinita VAN, BRECHTEL Sonja, et al. Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli [J]. Journal of Bacteriology, 2004, 186(20): 6728-6737. |
| 58 | GERDING Matthew A, LIU Bing, BENDEZÚ Felipe O, et al. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction[J]. Journal of Bacteriology, 2009, 191(24): 7383-7401. |
| 59 | CHEN Xiulai, ZHOU Jie, DING Qiang, et al. Morphology engineering of Aspergillus oryzae for l-malate production[J]. Biotechnology and Bioengineering, 2019, 116(10): 2662-2673. |
| 60 | ZHENG Hai, Po-Yi HO, JIANG Meiling, et al. Interrogating the Escherichia coli cell cycle by cell dimension perturbations[J]. Proceedings of the National Academy of Sciences, 2016, 113(52): 15000-15005. |
| 61 | MOLINARI Sara, SHIS David L, BHAKTA Shyam P, et al. A synthetic system for asymmetric cell division in Escherichia coli [J]. Nature Chemical Biology, 2019, 15(9): 917-924. |
| 62 | LEE Jeong Wook, YI Jongho, KIM Tae Yong, et al. Homo-succinic acid production by metabolically engineered Mannheimia succiniciproducens [J]. Metabolic Engineering, 2016, 38: 409-417. |
| 63 | ZHU Xinna, TAN Zaigao, XU Hongtao, et al. Metabolic evolution of two reducing equivalent-conserving pathways for high-yield succinate production in Escherichia coli [J]. Metabolic Engineering, 2014, 24: 87-96. |
| 64 | YUZBASHEV Tigran V, YUZBASHEVA Evgeniya Y, SOBOLEVSKAYA Tatiana I, et al. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica [J]. Biotechnology and Bioengineering, 2010, 107(4): 673-682. |
| 65 | WANG Dan, LI Qiang, SONG Ziyu, et al. High cell density fermentation via a metabolically engineered Escherichia coli for the enhanced production of succinic acid[J]. Journal of Chemical Technology & Biotechnology, 2011, 86(4): 512-518. |
| 66 | JIANG Min, LIU Shuwen, MA Jiangfeng, et al. Effect of growth phase feeding strategies on succinate production by metabolically engineered Escherichia coli [J]. Applied and Environmental Microbiology, 2010, 76(4): 1298-1300. |
| 67 | SÁNCHEZ Ailen M, BENNETT George N, Ka-Yiu SAN. Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant[J]. Biotechnology Progress, 2005, 21(2): 358-365. |
| 68 | HODGE David B, ANDERSSON Christian, BERGLUND Kris A, et al. Detoxification requirements for bioconversion of softwood dilute acid hydrolyzates to succinic acid[J]. Enzyme and Microbial Technology, 2009, 44(5): 309-316. |
| 69 | LI Jiaojiao, LI Yikui, CUI Zhiyong, et al. Enhancement of succinate yield by manipulating NADH/NAD+ ratio and ATP generation[J]. Applied Microbiology and Biotechnology, 2017, 101(8): 3153-3161. |
| 70 | SAWISIT Apichai, JANTAMA Kaemwich, ZHENG Huabao, et al. Mutation in galP improved fermentation of mixed sugars to succinate using engineered Escherichia coli AS1600a and AM1 mineral salts medium[J]. Bioresource Technology, 2015, 193: 433-441. |
| 71 | CHUNG Soon-Chun, PARK Joon-Song, YUN Jiae, et al. Improvement of succinate production by release of end-product inhibition in Corynebacterium glutamicum [J]. Metabolic Engineering, 2017, 40: 157-164. |
| 72 | OKINO Shohei, NOBURYU Ryoji, SUDA Masako, et al. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain[J]. Applied Microbiology and Biotechnology, 2008, 81(3): 459-464. |
| 73 | CHEN Tao, ZHU Nianqing, XIA Huihua. Aerobic production of succinate from arabinose by metabolically engineered Corynebacterium glutamicum [J]. Bioresource Technology, 2014, 151: 411-414. |
| 74 | LITSANOV Boris, BROCKER Melanie, BOTT Michael. Toward homosuccinate fermentation: Metabolic engineering of Corynebacterium glutamicum for anaerobic production of succinate from glucose and formate[J]. Applied and Environmental Microbiology, 2012, 78(9): 3325-3337. |
| 75 | LI Yikui, LI Mingji, ZHANG Xu, et al. A novel whole-phase succinate fermentation strategy with high volumetric productivity in engineered Escherichia coli [J]. Bioresource Technology, 2013, 149: 333-340. |
| 76 | LIU Yuan, WU Hui, LI Qing, et al. Process development of succinic acid production by Escherichia coli NZN111 using acetate as an aerobic carbon source[J]. Enzyme and Microbial Technology, 2011, 49(5): 459-464. |
| 77 | ZHANG Wenming, ZHANG Ting, SONG Meng, et al. Metabolic engineering of Escherichia coli for high yield production of succinic acid driven by methanol[J]. ACS Synthetic Biology, 2018, 7(12): 2803-2811. |
| 78 | GUO Feng, QIAO Yangyi, XIN Fengxue, et al. Bioconversion of C1 feedstocks for chemical production using Pichia pastoris [J]. Trends in Biotechnology, 2023, 41(8): 1066-1079. |
| 79 | WU Jing, WANG Chen, LIU Fuqiang, et al. Optimization of pretreatment and fermentation processes to enhance the production of succinic acid from corn straw by Actinobacillus succinogenes [J]. Industrial Crops and Products, 2024, 222: 119673. |
| 80 | MIKOYAN G, KARAPETYAN L, VASSILIAN A, et al. External succinate and potassium ions influence Dcu dependent F0F1-ATPase activity and H+ flux of Escherichia coli at different pHs[J]. Journal of Bioenergetics and Biomembranes, 2020, 52(5): 377-382. |
| 81 | KIM Ji Yeon, LEE Jong An, Jung Ho AHN, et al. High-level succinic acid production by overexpressing a magnesium transporter in Mannheimia succiniciproducens [J]. Proceedings of the National Academy of Sciences, 2024, 121(37): e2407455121. |
| 82 | 王洪辉. 大肠杆菌发酵木糖母液产丁二酸的研究[D]. 重庆: 重庆大学, 2015. |
| WANG Honghui. Succinic acid production by engineered Escherichia coli using xylose mother liquor[D]. Chongqing: Chongqing University, 2015. | |
| 83 | 李凤, 马江锋, 吴明科, 等. 重组大肠杆菌利用蔗糖及糖蜜发酵生产丁二酸[J]. 生物工程学报, 2015, 31: 534-541. |
| [1] | 倪新, 高教琪, 周雍进. 酵母细胞工厂用于木质纤维素生物转化研究进展[J]. 化工进展, 2025, 44(5): 2475-2488. |
| [2] | 盛华康, 张博, 申晓林, 孙新晓, 王佳, 袁其朋. 微生物合成白藜芦醇及其衍生物[J]. 化工进展, 2025, 44(5): 2463-2474. |
| [3] | 王媛媛, 张翀, 韩双艳, 邢新会. 毕赤酵母利用甲醇生产重组蛋白技术的研究进展[J]. 化工进展, 2025, 44(5): 2441-2450. |
| [4] | 王婉莹, 周颖喆, 刘华娟, 蒋国强. 我国生物制造领域竞争优劣势分析[J]. 化工进展, 2024, 43(S1): 662-666. |
| [5] | 张锐, 江静, 徐鸿飞, 杨盛凯, 李亚红, 周靖原, 曾坚贤, 黄小平, 刘鹏飞, 张明明, 李志强. 陶瓷膜分离技术及其在生物制造领域的应用进展[J]. 化工进展, 2024, 43(8): 4550-4561. |
| [6] | 佘丹, 王舒婷, 陆信曜, 宗红, 诸葛斌. 基因工程改造促进大肠杆菌发酵纤维素水解液合成1,2,4-丁三醇[J]. 化工进展, 2024, 43(2): 1063-1068. |
| [7] | 黄超, 任晓洁, 裴疆森, 赵新河, 赵玉斌, 王灵云, 荆宇航. 高产琥珀酸工程菌株E.coli SUC37的代谢途径优化及分析[J]. 化工进展, 2024, 43(12): 6883-6895. |
| [8] | 徐涛, 王勇军, 林启松, 戴钧明, 查全亮, 吕汪洋, 陈文兴. 聚丁二酸丁二醇酯熔融缩聚增黏特性[J]. 化工进展, 2024, 43(10): 5663-5670. |
| [9] | 吕学东, 罗发亮, 林海涛, 宋丹青, 刘义, 牛瑞雪, 郑柳春. 聚丁二酸丁二醇酯的合成工艺及气体阻隔性最新进展[J]. 化工进展, 2023, 42(5): 2546-2554. |
| [10] | 陶雨萱, 郭亮, 高聪, 宋伟, 陈修来. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52. |
| [11] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [12] | 姚伦, 周雍进. 一碳化合物生物利用和转化研究进展[J]. 化工进展, 2023, 42(1): 16-29. |
| [13] | 唐文秀, 王学明, 郭亮, 季立豪, 高聪, 陈修来, 刘立明. 代谢工程改造大肠杆菌生产琥珀酸[J]. 化工进展, 2022, 41(2): 938-950. |
| [14] | 郭超, 冯奥, 陆信曜, 宗红, 诸葛斌. 重组大肠杆菌1,2,4-丁三醇合成途径的平衡优化[J]. 化工进展, 2022, 41(12): 6531-6539. |
| [15] | 李庆远, 王超, 许世佩, 张雪琴, 邱明建, 刘梦瑶, 丛梦晓. PBS前体1,4-丁二醇合成的反应工艺和催化剂研究进展[J]. 化工进展, 2022, 41(11): 5771-5782. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||