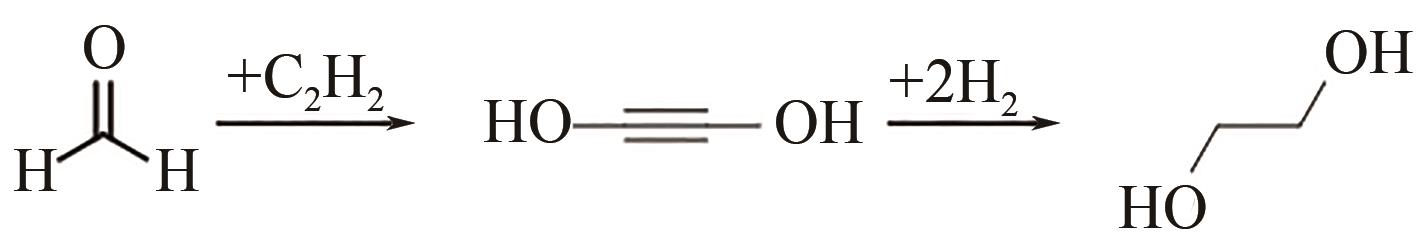化工进展 ›› 2022, Vol. 41 ›› Issue (11): 5771-5782.DOI: 10.16085/j.issn.1000-6613.2022-0102
PBS前体1,4-丁二醇合成的反应工艺和催化剂研究进展
李庆远( ), 王超, 许世佩, 张雪琴, 邱明建, 刘梦瑶, 丛梦晓
), 王超, 许世佩, 张雪琴, 邱明建, 刘梦瑶, 丛梦晓
- 中节能工程技术研究院有限公司,北京 100082
-
收稿日期:2022-01-13修回日期:2022-03-14出版日期:2022-11-25发布日期:2022-11-28 -
通讯作者:李庆远 -
作者简介:李庆远(1984—),男,博士,工程师,研究方向催化反应工程。E-mail:liqy0629@163.com。
Research progress on reaction process and catalysts for PBS precursor of 1,4-butanediol synthesis
LI Qingyuan( ), WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao
), WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao
- CECEP Engineering Technology Research Institute, Beijing 100082, China
-
Received:2022-01-13Revised:2022-03-14Online:2022-11-25Published:2022-11-28 -
Contact:LI Qingyuan
摘要:
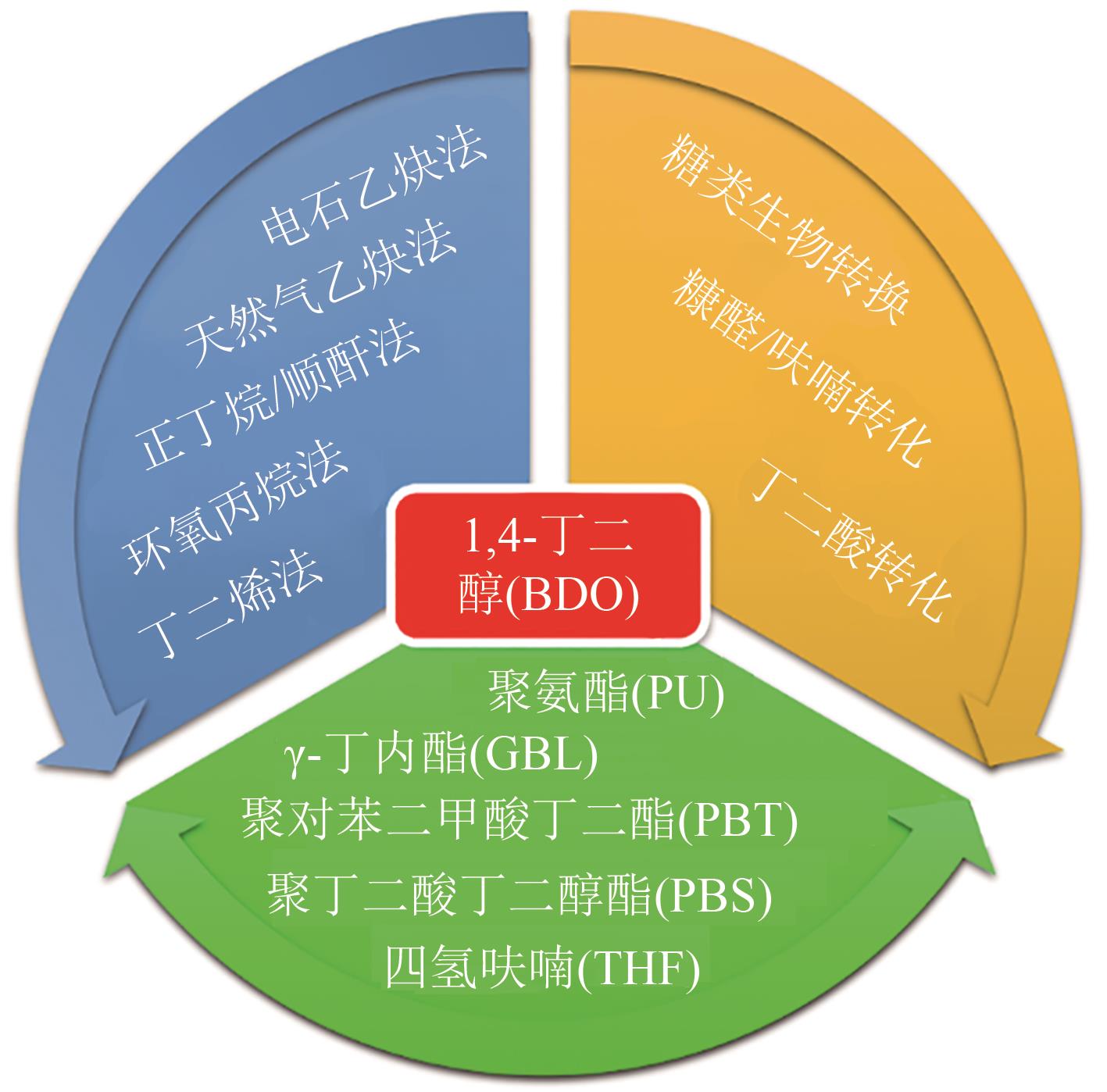
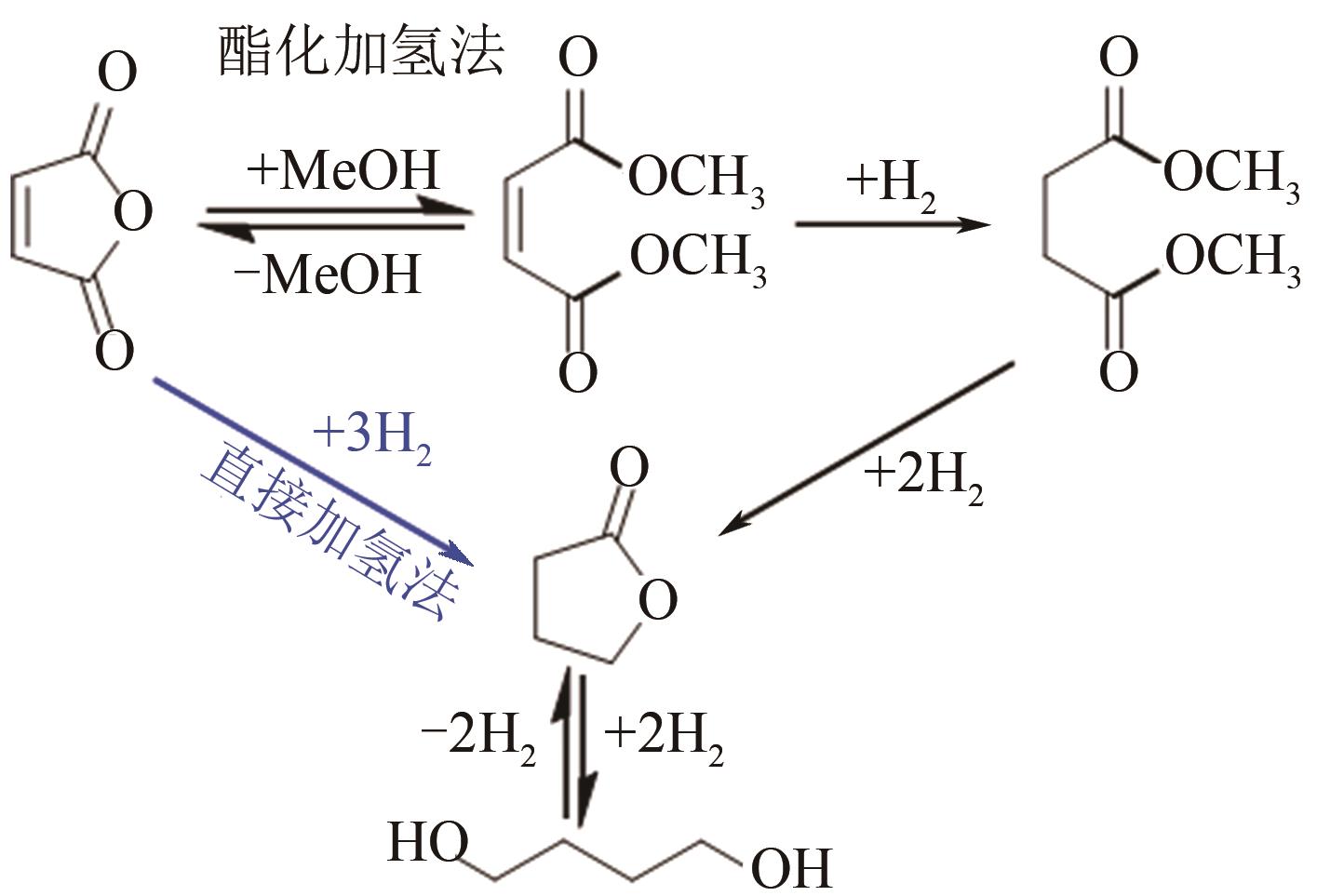
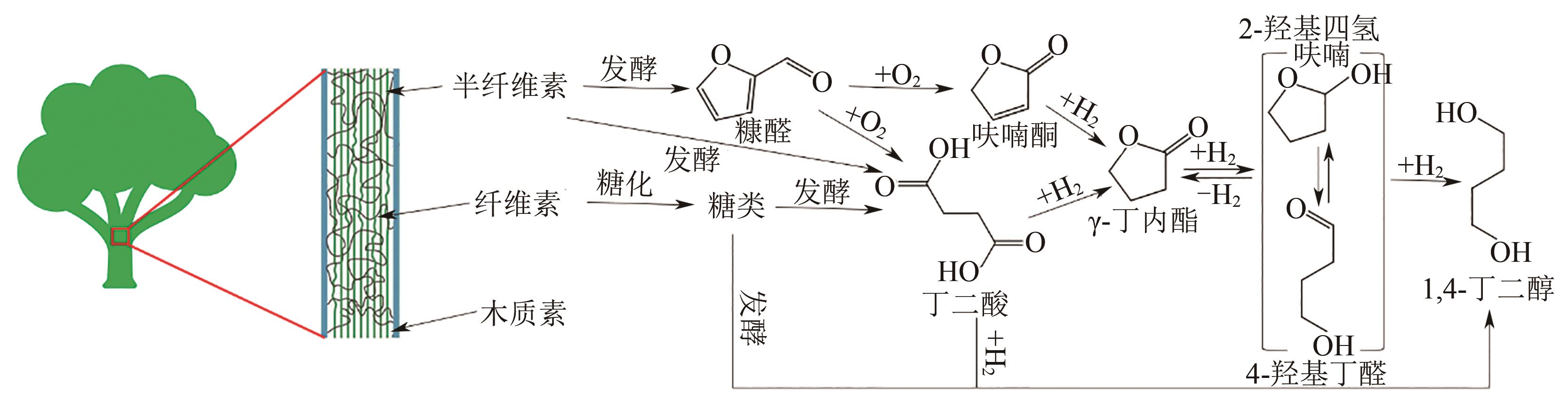
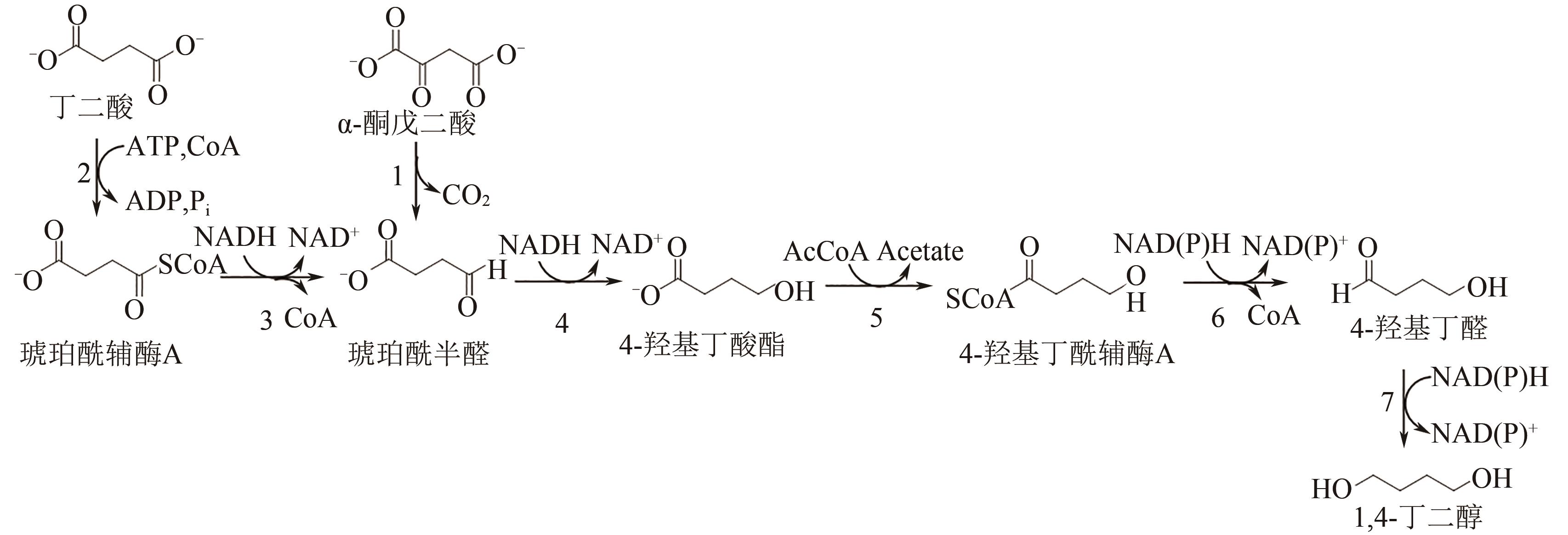
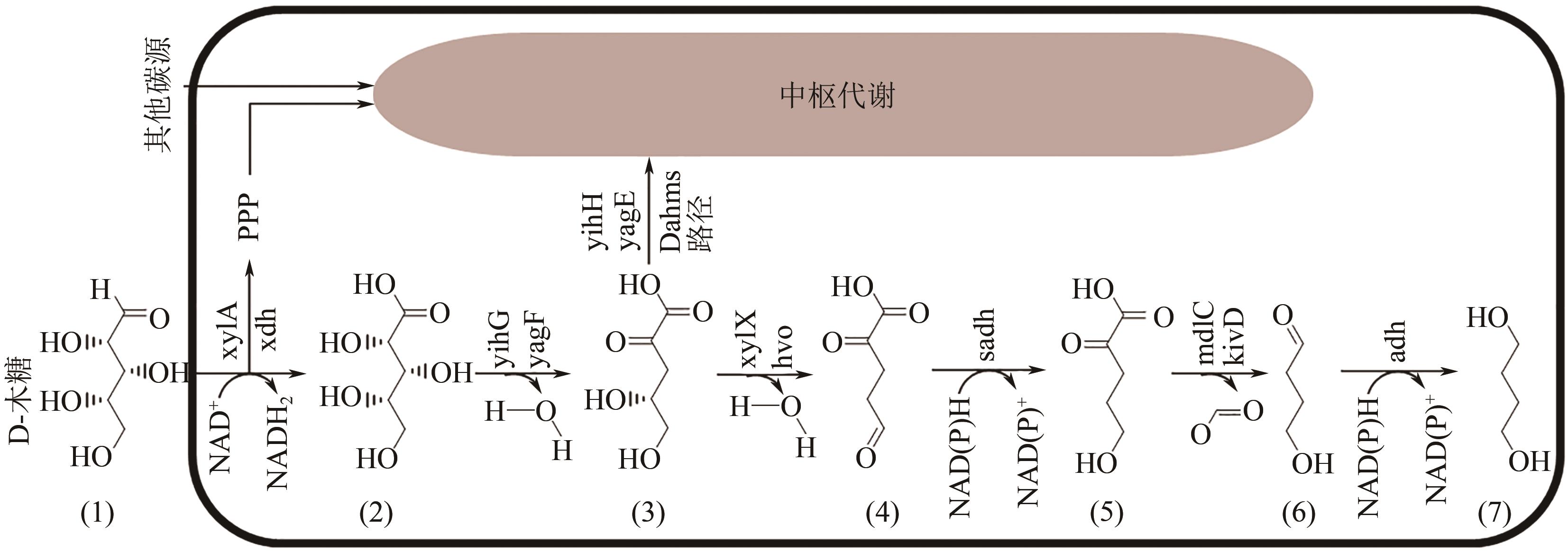
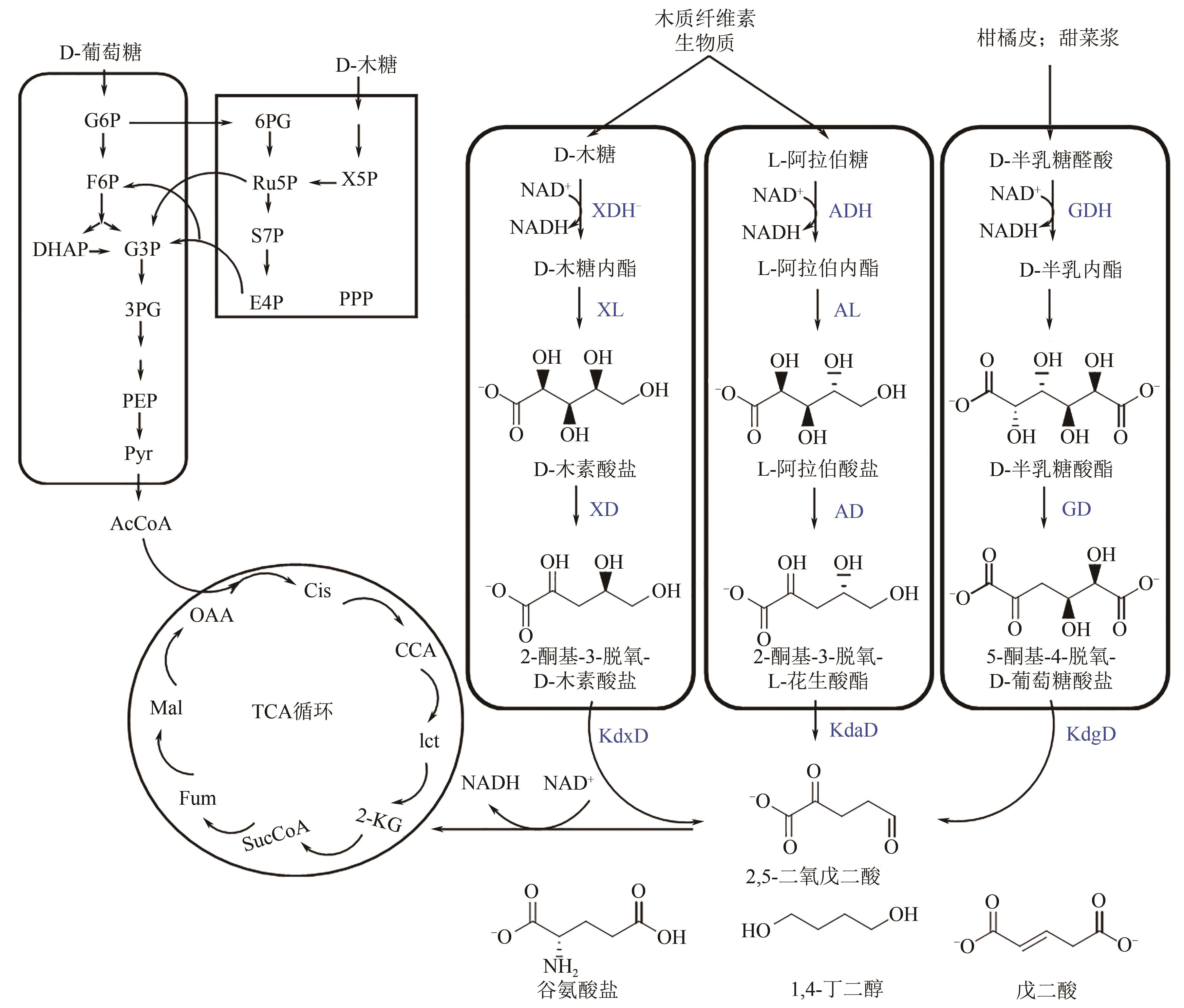
聚丁二酸丁二醇酯(PBS)被公认为是可完全生物降解的可降解塑料,在应用方面具有替代现有普通难降解塑料的潜力,但近年其合成原料,尤其是1,4-丁二醇(BDO)价格不断上升,导致PBS成本不断增加。为了更好地了解和掌握BDO合成的工艺过程、现状、存在问题和最新的发展方向,本文首先简单总结了过往基于化石基方法合成BDO的不同工艺路线,并详细阐述了Cu、Ni、Pd、Pt和Rh基催化剂化石路线合成BDO过程的进展;然后介绍了基于生物路线合成BDO的最新进展,主要着眼于不同生物质基原料(如丁二酸、糠醛/呋喃、1,4-脱水赤藓糖醇和其他糖类等)合成BDO的方法;随后简单地描述了生物质合成BDO生命周期评估以及资本和运营成本,并对化石基和生物基合成BDO做了简要的对比,最后总结和展望了BDO合成中存在的问题和发展方向,即如何开发低能耗和高效催化剂将分别成为化石基和生物基合成BDO的重点所在。
中图分类号:
引用本文
李庆远, 王超, 许世佩, 张雪琴, 邱明建, 刘梦瑶, 丛梦晓. PBS前体1,4-丁二醇合成的反应工艺和催化剂研究进展[J]. 化工进展, 2022, 41(11): 5771-5782.
LI Qingyuan, WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao. Research progress on reaction process and catalysts for PBS precursor of 1,4-butanediol synthesis[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5771-5782.
| 项目 | 化石基路线 | 生物基路线 |
|---|---|---|
| 反应原料 | 来源于煤、石油、天然气等不可再生资源,部分原料在运输和储存方面具有一定的危险(如乙炔等) | 来源于生物质可再生资源,原料相对较为安全 |
| 工艺过程 | 反应条件较为苛刻,通常需要较高的温度和压力,反应能耗较高,工艺较为成熟,工业化装置运行以此为主 | 反应条件较为温和,处于起步期,大多数工艺都处于研究阶段,工业化运行装置较少 |
| 设备装置 | 需耐高温高压,部分反应过程对设备腐蚀较大,设备造价相对较高 | 大都为常压设备,无腐蚀现象,造价相对较低 |
| 反应产物 | 反应的转化率和产物的选择性相对较高 | 反应的转化率和产物的选择性相对较低 |
表1 化石基和生物基路线的特点
| 项目 | 化石基路线 | 生物基路线 |
|---|---|---|
| 反应原料 | 来源于煤、石油、天然气等不可再生资源,部分原料在运输和储存方面具有一定的危险(如乙炔等) | 来源于生物质可再生资源,原料相对较为安全 |
| 工艺过程 | 反应条件较为苛刻,通常需要较高的温度和压力,反应能耗较高,工艺较为成熟,工业化装置运行以此为主 | 反应条件较为温和,处于起步期,大多数工艺都处于研究阶段,工业化运行装置较少 |
| 设备装置 | 需耐高温高压,部分反应过程对设备腐蚀较大,设备造价相对较高 | 大都为常压设备,无腐蚀现象,造价相对较低 |
| 反应产物 | 反应的转化率和产物的选择性相对较高 | 反应的转化率和产物的选择性相对较低 |
| 1 | NAZRIN A, SAPUAN S M, ZUHRI M Y M, et al. Nanocellulose reinforced thermoplastic starch (TPS), polylactic acid (PLA), and polybutylene succinate (PBS) for food packaging applications[J]. Frontiers in Chemistry, 2020, 8: 213. |
| 2 | GIGLI M, FABBRI M, LOTTI N, et al. Poly(butylene succinate)-based polyesters for biomedical applications: a review[J]. European Polymer Journal, 2016, 75: 431-460. |
| 3 | SIRACUSA V, LOTTI N, MUNARI A, et al. Poly(butylene succinate) and poly(butylene succinate-co-adipate) for food packaging applications: gas barrier properties after stressed treatments[J]. Polymer Degradation and Stability, 2015, 119: 35-45. |
| 4 | CHENG J, LI J, ZHENG L G. Achievements and perspectives in 1, 4-butanediol production from engineered microorganisms[J]. Journal of Agricultural and Food Chemistry, 2021, 69(36): 10480-10485. |
| 5 | BURGARD A, BURK M J, OSTERHOUT R, et al. Development of a commercial scale process for production of 1,4-butanediol from sugar[J]. Current Opinion in Biotechnology, 2016, 42: 118-125. |
| 6 | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 7 | LIU H L, JIANG Y Y, ZHAO H Y, et al. Preparation of highly dispersed Cu catalysts from hydrotalcite precursor for the dehydrogenation of 1,4-butanediol[J]. Journal of Industrial and Engineering Chemistry, 2021, 102: 251-259. |
| 8 | 刘响, 廖启江, 张敏卿. 1,4-丁炔二醇加氢过程研究进展[J]. 化工进展, 2017, 36(8): 2787-2797. |
| LIU Xiang, LIAO Qijiang, ZHANG Minqing. Research progress of 1,4-butynediol hydrogenation process[J]. Chemical Industry and Engineering Progress, 2017, 36(8): 2787-2797. | |
| 9 | TAMURA M, NAKAGAWA Y, TOMISHIGE K. Recent developments of heterogeneous catalysts for hydrogenation of caKrboxylic acids to their corresponding alcohols[J]. Asian Journal of Organic Chemistry, 2020, 9(2): 126-143. |
| 10 | MIKLÓSSY I, BODOR Z, SINKLER R, et al. In silico and in vivo stability analysis of a heterologous biosynthetic pathway for 1,4-butanediol production in metabolically engineered E. coli [J]. Journal of Biomolecular Structure and Dynamics, 2017, 35(9): 1874-1889. |
| 11 | VAN DIEN S. From the first drop to the first truckload: commercialization of microbial processes for renewable chemicals[J]. Current Opinion in Biotechnology, 2013, 24(6): 1061-1068. |
| 12 | HAAS T, JAEGER B, WEBER R, et al. New diol processes: 1,3-propanediol and 1,4-butanediol[J]. Applied Catalysis A: General, 2005, 280(1): 83-88. |
| 13 | LUO P, LI X D. Application and market of 1,4-butanediol production of reppe method in China[J]. American Journal of Chemical Engineering, 2021, 9(2): 34-38. |
| 14 | 杨桂花, 王吉德, 徐世美, 等. 炔醛法合成1,4-丁炔二醇催化剂研究进展[J]. 材料导报, 2014, 28(19): 68-74. |
| YANG Guihua, WANG Jide, XU Shimei, et al. Development in catalysts for synthesis of 1,4-butynediol via ethynylation reaction of formaldehyde[J]. Materials Review, 2014, 28(19): 68-74. | |
| 15 | 窦和瑞, 吕荣, 徐晓航, 等. 一种制备生物可降解塑料PBS的方法: CN112694602A[P]. 2021-04-23. |
| DOU Herui, Rong LYU, XU Xiaohang, et al. Method for preparing biodegradable plastic PBS: CN112694602A[P]. 2021-04-23. | |
| 16 | 陈梁峰, 沈伟, 乔明华, 等. 一种用于马来酸二甲酯加氢制备 1, 4-丁二醇的催化剂的制备方法: CN1935375A[P]. 2007-03-28. |
| CHEN Liangfeng, SHEN Wei, QIAO Minghua, et al. A method for preparing a catalyst for hydrogenation of 1,4-butanediol with dimethyl maleate: CN1935375A[P]. 2007-03-28. | |
| 17 | 王春梅, 苏杰, 范丹丹, 等. 一种用于制备 1,4-丁二醇的催化剂及制备方法: CN103801321A[P]. 2014-05-21. |
| WANG Chunmei, SU Jie, FAN Dandan, et al. A catalyst for the preparation of 1,4-butanediol and a preparation method thereof: CN103801321A[P]. 2014-05-21. | |
| 18 | 郭平均, 杨菊群, 刘文艳, 等. 一种生产 1,4-丁二醇的高效铜锰铝催化剂的制备方法: CN103566945A[P]. 2014-02-12. |
| GUO Pingjun, YANG Juqun, LIU Wenyan, et al. A preparation method for high efficiency copper manganese aluminum catalyst for producing 1,4-butanadiol: CN103566945A[P]. 2014-02-12. | |
| 19 | HONG U G, KIM J K, LEE J,et al. Conversion of succinic acid to 1,4-butanediol via dimethyl succinate over rhenium nano-catalyst supported on copper-containing mesoporous carbon[J]. Journal of Nanoscience and Nanotechnology, 2014, 14(11): 8867-8872.. |
| 20 | CHEN L F, GUO P J, ZHU L J, et al. Preparation of Cu/SBA-15 catalysts by different methods for the hydrogenolysis of dimethyl maleate to 1,4-butanediol[J]. Applied Catalysis A: General, 2009, 356(2): 129-136. |
| 21 | HUANG Z W, BARNETT K J, CHADA J P, et al. Hydrogenation of γ-butyrolactone to 1,4-butanediol over CuCo/TiO2 bimetallic catalysts[J]. ACS Catalysis, 2017, 7(12): 8429-8440. |
| 22 | BECKER R, BROCKER F J, KAIBEL G, et al. Process and catalysts for preparing 1,4-butanediol by the hydrogenation of 1,4 -butynediol: WO9815513A1[P].1998-04-16. |
| 23 | 王志钢,代俊桥,史振宇, 等. 一种马来酸二甲酯加氢催化剂的制备方法: CN110368947A[P]. 2019-10-25. |
| 24 | WANG C Z, TIAN Y N, WU R F, et al. Bimetallic synergy effects of phyllosilicate-derived NiCu@SiO2 catalysts for 1,4-butynediol direct hydrogenation to 1,4-butanediol[J]. ChemCatChem, 2019, 11(19): 4777-4787. |
| 25 | FANG Jie, ZHUANG Changjian, MENG Jipeng, et al. Selective hydrogenation of butyne-1,4-diol to butane-1,4-diol over Ni/Al2O3-SiO2 catalysts[J]. China Petroleum Processing & Petrochemical Technology, 2018, 20(4): 20-28. |
| 26 | WANG C Z, JIANG C Y, BAI J, et al. Effect of pore structures on 1,4-butynediol hydrogenation over mesoporous Ni/Al2O3-SiO2 catalysts[J]. Industrial & Engineering Chemistry Research, 2021, 60(49): 17840-17849. |
| 27 | 赵芳, 王长真, 田亚妮, 等. Ni-M/SiO2催化1,4-丁炔二醇加氢的金属助剂效应[J]. 分子催化, 2019, 33(1): 83-89. |
| ZHAO Fang, WANG Changzhen, TIAN Yani, et al. Metal promoter effect of Ni-M/SiO2 in hydrogenation of 1,4-butynediol[J]. Journal of Molecular Catalysis (China), 2019, 33(1): 83-89. | |
| 28 | FRANCOVÁ D, TANCHOUX N, GÉRARDIN C, et al. Hydrogenation of 2-butyne-1,4-diol on supported Pd catalysts obtained from LDH precursors[J]. Microporous and Mesoporous Materials, 2007, 99(1/2): 118-125. |
| 29 | 郭家威, 张蕾, 南军, 等. 1,4-丁炔二醇温和条件下Pd-Ni基加氢催化剂的研究[J]. 厦门大学学报(自然科学版), 2019, 58(5): 661-668. |
| GUO Jiawei, ZHANG Lei, Jun NAN, et al. Investigation on the Pd-Ni based catalysts for 1,4-butynediol hydrogenation under mild condition[J]. Journal of Xiamen University (Natural Science), 2019, 58(5): 661-668. | |
| 30 | YIN D D, LI C, REN H X, et al. Efficient Pd@MIL-101(Cr) hetero-catalysts for 2-butyne-1,4-diol hydrogenation exhibiting high selectivity[J]. RSC Advances, 2017, 7(3): 1626-1633. |
| 31 | CALCIO GAUDINO E, MANZOLI M, CARNAROGLIO D, et al. Sonochemical preparation of alumina-spheres loaded with Pd nanoparticles for 2-butyne-1,4-diol semi-hydrogenation in a continuous flow microwave reactor[J]. RSC Advances, 2018, 8(13): 7029-7039. |
| 32 | 石闯, 蒙龙伟, 陈霄, 等. 强静电吸附法制备PdZn x /Al2O3催化1,4-丁炔二醇选择加氢[J]. 精细化工, 2021, 38(10): 2072-2080. |
| SHI Chuang, MENG Longwei, CHEN Xiao, et al. PdZnx/Al2O3 catalysts prepared by strong electrostatic adsorption for selective hydrogenation of 1,4-butynediol[J]. Fine Chemicals, 2021, 38(10): 2072-2080. | |
| 33 | 张瑞玉, 莫文龙. 1,4-丁炔二醇加氢制1,4-丁烯二醇工艺及催化剂研究进展[J]. 当代化工, 2021, 50(7): 1705-1710. |
| ZHANG Ruiyu, MO Wenlong. Research progress of catalysts for hydrogenation of 1,4-butynediol to 1,4-butenediol[J]. Contemporary Chemical Industry, 2021, 50(7): 1705-1710. | |
| 34 | ZHANG M M, YANG Y B, LI C, et al. PVP-Pd@ZIF-8 as highly efficient and stable catalysts for selective hydrogenation of 1,4-butynediol[J]. Catalysis Science & Technology, 2014, 4(2): 329-332. |
| 35 | 任勇, 袁涛, 刘德蓉, 等. Pd-Cu/Fe3O4@C催化1,4-丁炔二醇选择性加氢的研究[J]. 化学研究与应用, 2017, 29(11): 1686-1692. |
| REN Yong, YUAN Tao, LIU Derong, et al. Study on selective hydrogenation of 1,4-butynediol by Pd-Cu/Fe3O4@C catalyst[J]. Chemical Research and Application, 2017, 29(11): 1686-1692. | |
| 36 | RODE C V, TAYADE P R, NADGERI J M, et al. Continuous hydrogenation of 2-butyne-1,4-diol to 2-butene- and butane-1,4-diols[J]. Organic Process Research & Development, 2006, 10(2): 278-284. |
| 37 | LI C, ZHANG M M, DI X, et al. One-step synthesis of Pt@ZIF-8 catalyst for the selective hydrogenation of 1,4-butynediol to 1,4-butenediol[J]. Chinese Journal of Catalysis, 2016, 37(9): 1555-1561. |
| 38 | 任勇, 潘越, 刘德蓉, 等. Rh/UiO-66-NH2催化1,4-丁炔二醇加氢性能研究[J]. 应用化工, 2019, 48(1): 136-139, 144. |
| REN Yong, PAN Yue, LIU Derong, et al. Study on the hydrogenation properties of 2-butyne-1,4-diol by Rh/UiO-66-NH2 catalyst[J]. Applied Chemical Industry, 2019, 48(1): 136-139, 144. | |
| 39 | GALLEZOT D. Conversion of biomass to selected chemical products[J]. Chemical Society Reviews, 2012, 41(4): 1538-1558. |
| 40 | BECHTHOLD I, BRETZ K, KABASCI S, et al. Succinic acid: a new platform chemical for biobased polymers from renewable resources[J]. Chemical Engineering & Technology, 2008, 31(5): 647-654. |
| 41 | WANG J J, ZENG A P, YUAN W Q. Succinic acid fermentation from agricultural wastes: the producing microorganisms and their engineering strategies[J]. Current Opinion in Environmental Science & Health, 2022: 100313. |
| 42 | CUKALOVIC A, STEVENS C V. Feasibility of production methods for succinic acid derivatives: a marriage of renewable resources and chemical technology[J]. Biofuels, Bioproducts and Biorefining, 2008, 2(6): 505-529. |
| 43 | COK B, TSIROPOULOS I, ROES A L, et al. Succinic acid production derived from carbohydrates: an energy and greenhouse gas assessment of a platform chemical toward a bio-based economy[J]. Biofuels, Bioproducts and Biorefining, 2014, 8(1): 16-29. |
| 44 | CHOI S, SONG C W, SHIN J H, et al. Biorefineries for the production of top building block chemicals and their derivatives[J]. Metabolic Engineering, 2015, 28: 223-239. |
| 45 | NGHIEM N, KLEFF S, SCHWEGMANN S. Succinic acid: technology development and commercialization[J]. Fermentation, 2017, 3(2): 26. |
| 46 | TAPIN B, EPRON F, ESPECEL C, et al. Study of monometallic Pd/TiO2 catalysts for the hydrogenation of succinic acid in aqueous phase[J]. ACS Catalysis, 2013, 3(10): 2327-2335. |
| 47 | HONG U G, KIM J K, LEE J, et al. Hydrogenation of succinic acid to tetrahydrofuran (THF) over ruthenium-carbon composite (Ru-C) catalyst[J]. Applied Catalysis A: General, 2014, 469: 466-471. |
| 48 | KANG K H, HONG U G, BANG Y J, et al. Hydrogenation of succinic acid to 1,4-butanediol over Re-Ru bimetallic catalysts supported on mesoporous carbon[J]. Applied Catalysis A: General, 2015, 490: 153-162. |
| 49 | SILVA R G C, FERREIRA T F, BORGES É R. Identification of potential technologies for 1,4-butanediol production using prospecting methodology[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(12): 3057-3070. |
| 50 | LI F B, LU T, CHEN B F, et al. Pt nanoparticles over TiO2-ZrO2 mixed oxide as multifunctional catalysts for an integrated conversion of furfural to 1,4-butanediol[J]. Applied Catalysis A: General, 2014, 478: 252-258. |
| 51 | DELHOMME C, WEUSTER-BOTZ D, KÜHN F E. Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media[J]. Green Chemistry, 2009, 11(1): 13-26. |
| 52 | JI J C, XU Y, LIU Y, et al. A nanosheet Ru/WO3 catalyst for efficient conversion of glucose to butanediol[J]. Catalysis Communications, 2020, 144: 106074. |
| 53 | LI K T, LI Y S. Hydrogenolysis of succinic acid over Ru and Pd catalysts encapsulated in porous silica nanoparticles[J]. Clean Technologies and Environmental Policy, 2021, 23(7): 2171-2182. |
| 54 | TAPIN B, KHANH LY B, CANAFF C, et al. Characterization by X-ray absorption spectroscopy of bimetallic Re-Pd/TiO2 catalysts efficient for selective aqueous-phase hydrogenation of succinic acid to 1,4-butanediol[J]. Materials Chemistry and Physics, 2020, 252: 123225. |
| 55 | KANG K H, HAN S J, LEE J W, et al. Effect of boron content on 1,4-butanediol production by hydrogenation of succinic acid over Re-Ru/BMC (boron-modified mesoporous carbon) catalysts[J]. Applied Catalysis A: General, 2016, 524: 206-213. |
| 56 | KANG K H, HONG U G, JUN J O, et al. Hydrogenation of succinic acid to γ-butyrolactone and 1,4-butanediol over mesoporous rhenium-copper-carbon composite catalyst[J]. Journal of Molecular Catalysis A: Chemical, 2014, 395: 234-242. |
| 57 | LANGE J P, WADMAN S H. Furfural to 1,4-butanediol/tetrahydrofuran-A detailed catalyst and process design[J]. ChemSusChem, 2020, 13(19): 5329-5337. |
| 58 | LEE Y, KIM Y T, KWON E E, et al. Biochar as a catalytic material for the production of 1,4-butanediol and tetrahydrofuran from furan[J]. Environmental Research, 2020, 184: 109325. |
| 59 | WANG T M, LIU S B, TAMURA M, et al. One-pot catalytic selective synthesis of 1,4-butanediol from 1,4-anhydroerythritol and hydrogen[J]. Green Chemistry, 2018, 20(11): 2547-2557. |
| 60 | WANG T M, TAMURA M, NAKAGAWA Y, et al. Preparation of highly active monometallic rhenium catalysts for selective synthesis of 1,4-butanediol from 1,4-anhydroerythritol[J]. ChemSusChem, 2019, 12(15): 3615-3626. |
| 61 | WANG T M, NAKAGAWA Y, TAMURA M, et al. Tungsten-zirconia-supported rhenium catalyst combined with a deoxydehydration catalyst for the one-pot synthesis of 1,4-butanediol from 1,4-anhydroerythritol[J]. Reaction Chemistry & Engineering, 2020, 5(7): 1237-1250. |
| 62 | BAIDYA P K, SARKAR U, VILLA R, et al. Liquid-phase hydrogenation of bio-refined succinic acid to 1,4-butanediol using bimetallic catalysts[J]. BMC Chemical Engineering, 2019, 1: 10. |
| 63 | VARDON D R, SETTLE A E, VOROTNIKOV V, et al. Ru-Sn/AC for the aqueous-phase reduction of succinic acid to 1,4-butanediol under continuous process conditions[J]. ACS Catalysis, 2017, 7(9): 6207-6219. |
| 64 | Shell Oil Company. Process for the production of n-butanol and 1,4-butanediol from furan: US20170349515A1[P]. 2017-12-07. |
| 65 | LE S D, NISHIMURA S. Highly selective synthesis of 1,4-butanediol via hydrogenation of succinic acid with supported Cu-Pd alloy nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18483-18492. |
| 66 | LIU H W, LU T. Autonomous production of 1,4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli [J]. Metabolic Engineering, 2015, 29: 135-141. |
| 67 | TAI Y S, XIONG M, JAMBUNATHAN P, et al. Engineering nonphosphorylative metabolism to generate lignocellulose-derived products[J]. Nature Chemical Biology, 2016, 12(4): 247-253. |
| 68 | WANG J, JAIN R, SHEN X L, et al. Rational engineering of diol dehydratase enables 1,4-butanediol biosynthesis from xylose[J]. Metabolic Engineering, 2017, 40: 148-156. |
| 69 | DAI L, TAO F, TANG H Z, et al. Directing enzyme devolution for biosynthesis of alkanols and 1, n-alkanediols from natural polyhydroxy compounds[J]. Metabolic Engineering, 2017, 44: 70-80. |
| 70 | DAI L, TAI C, SHEN Y L, et al. Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis[J]. Biocatalysis and Biotransformation, 2019, 37(2): 92-96. |
| 71 | FORTE A, ZUCARO A, BASOSI R, et al. LCA of 1,4-butanediol produced via direct fermentation of sugars from wheat straw feedstock within a territorial biorefinery[J]. Materials (Basel, Switzerland), 2016, 9(7): 563. |
| 72 | SATAM C C, DAUB M, REALFF M J. Techno-economic analysis of 1,4-butanediol production by a single-step bioconversion process[J]. Biofuels, Bioproducts and Biorefining, 2019, 13(5): 1261-1273. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||