化工进展 ›› 2024, Vol. 43 ›› Issue (12): 6883-6895.DOI: 10.16085/j.issn.1000-6613.2023-2057
• 生物与医药化工 • 上一篇
高产琥珀酸工程菌株E.coli SUC37的代谢途径优化及分析
黄超1( ), 任晓洁1, 裴疆森2, 赵新河1(
), 任晓洁1, 裴疆森2, 赵新河1( ), 赵玉斌3, 王灵云3, 荆宇航1
), 赵玉斌3, 王灵云3, 荆宇航1
- 1.山东理工大学农业工程与食品科学学院,山东 淄博 255000
2.中国食品发酵工业研究院有限公司,北京 100125
3.鲁洲生物科技有限公司,山东 临沂 276400
-
收稿日期:2023-11-24修回日期:2024-01-29出版日期:2024-12-15发布日期:2025-01-11 -
通讯作者:赵新河 -
作者简介:黄超(1996—),男,硕士研究生,研究方向为发酵工程。E-mail:1833829611@qq.com。 -
基金资助:山东省自然科学基金博士项目(ZR2023MC194);促进与加拿大、澳大利亚、新西兰及拉美地区科研合作与高层次人才培养项目(留金美2022-1007)
Optimization and analysis of the metabolic pathway of succinic acid-producing viaE.coli SUC37
HUANG Chao1( ), REN Xiaojie1, PEI Jiangsen2, ZHAO Xinhe1(
), REN Xiaojie1, PEI Jiangsen2, ZHAO Xinhe1( ), ZHAO Yubin3, WANG Lingyun3, JING Yuhang1
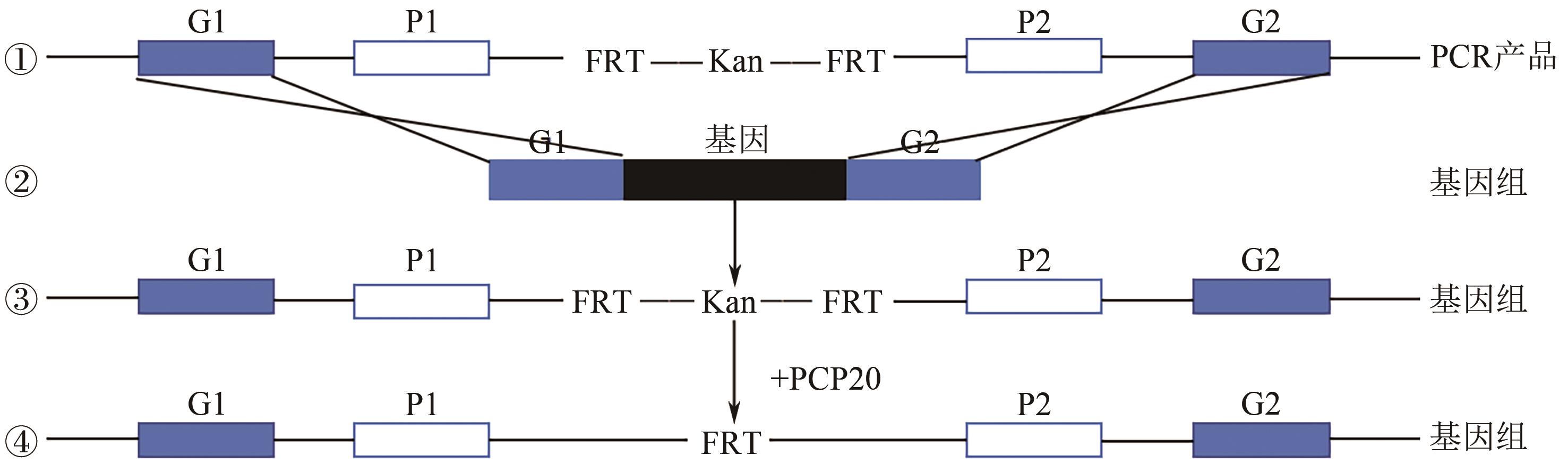
), ZHAO Yubin3, WANG Lingyun3, JING Yuhang1
- 1.School of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo 255000, Shandong, China
2.China National Research Institute of Food and Fermentation Industry Co. , Ltd. , Beijing 100125, China
3.Luzhou Bio-Chem Technology Limited, Linyi 276400, Shandong, China
-
Received:2023-11-24Revised:2024-01-29Online:2024-12-15Published:2025-01-11 -
Contact:ZHAO Xinhe
摘要:
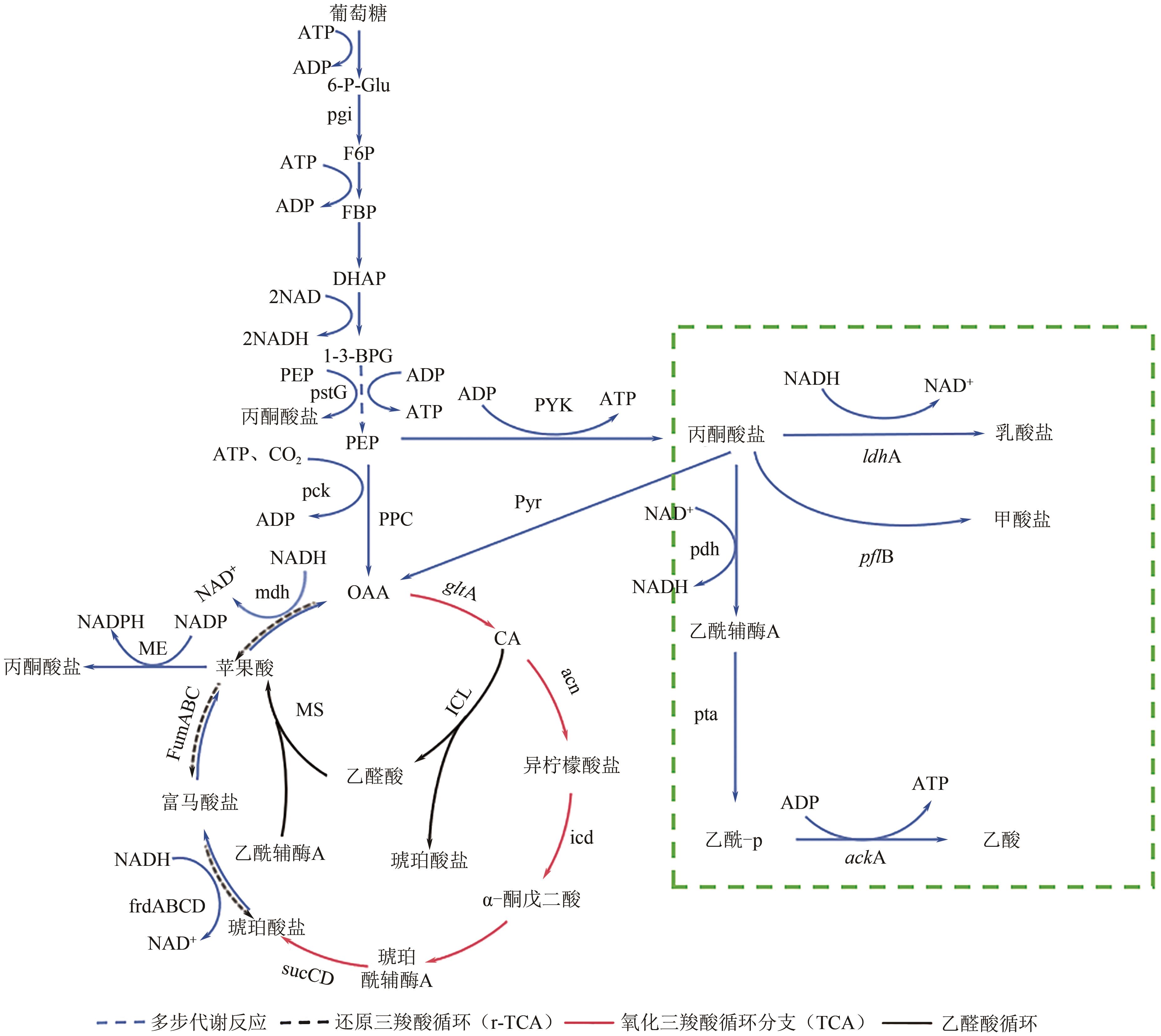
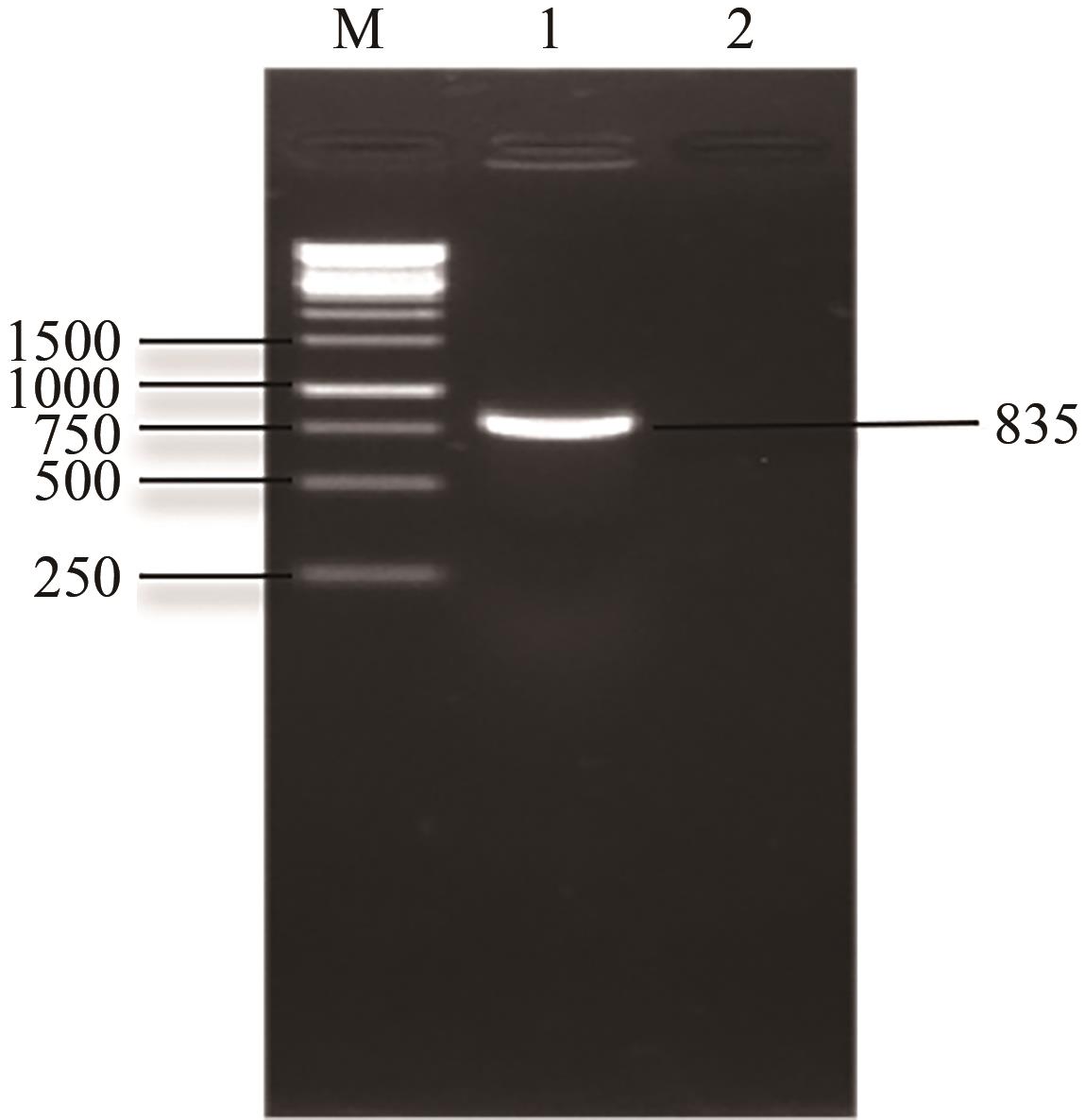
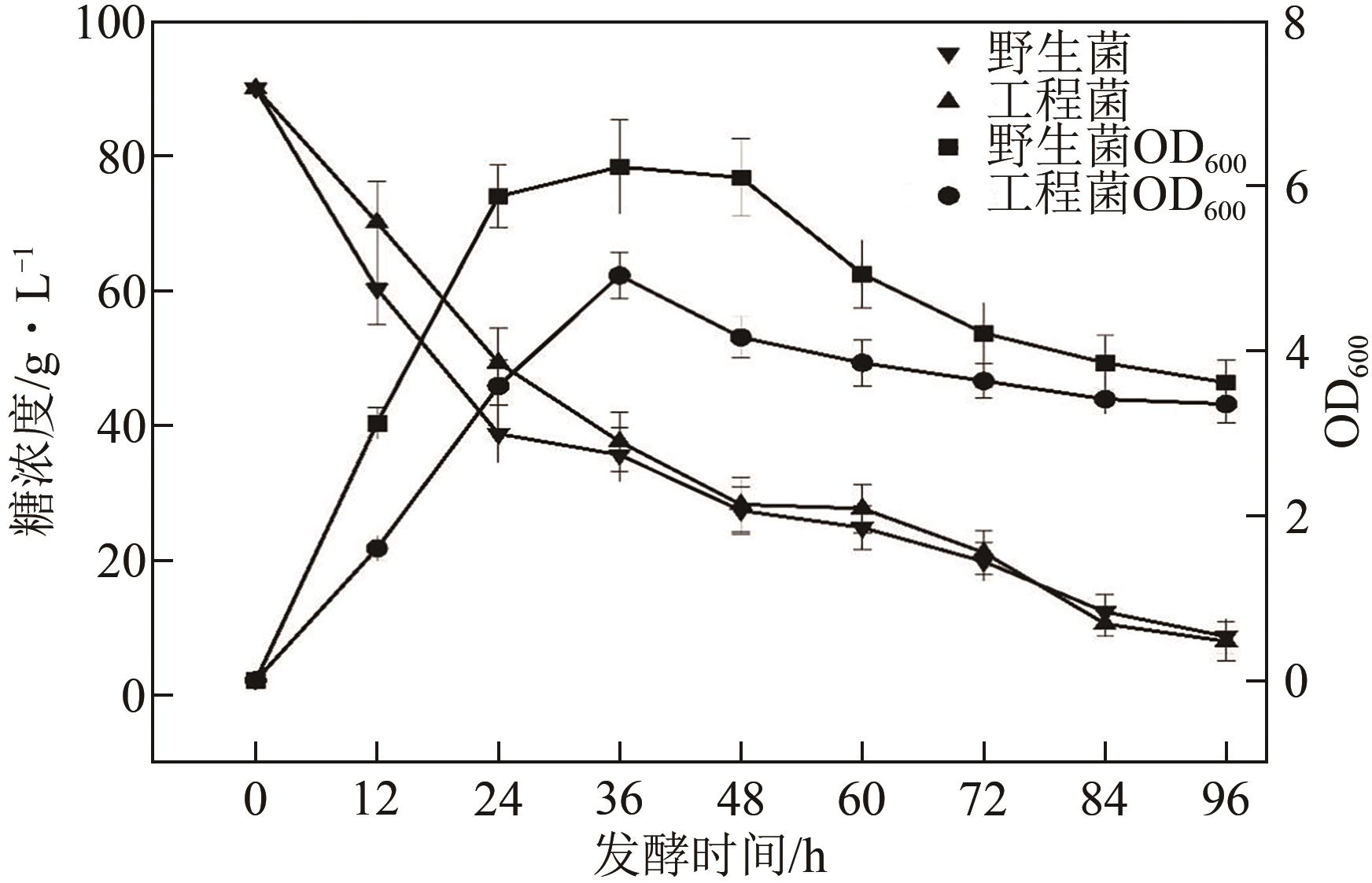
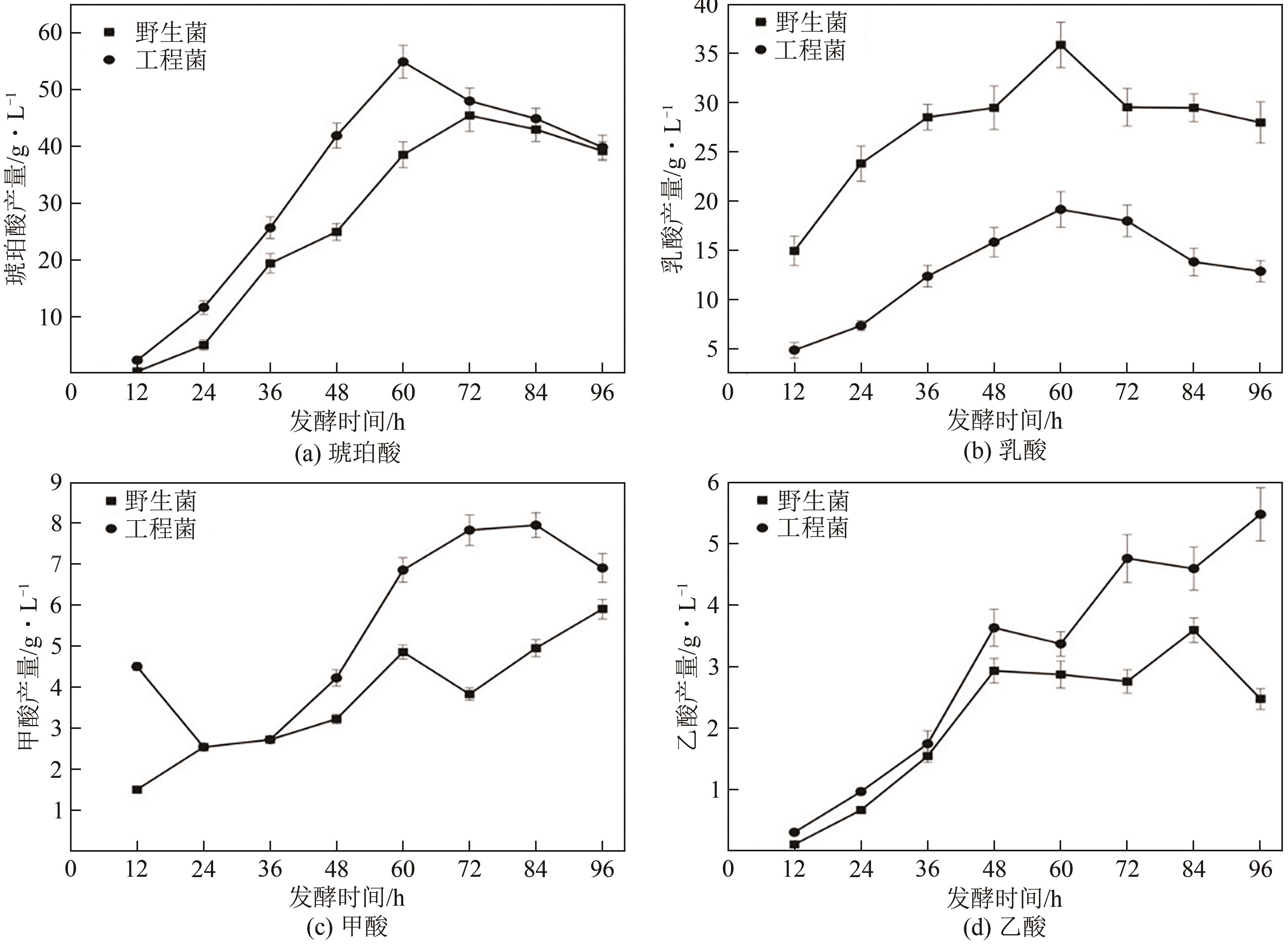
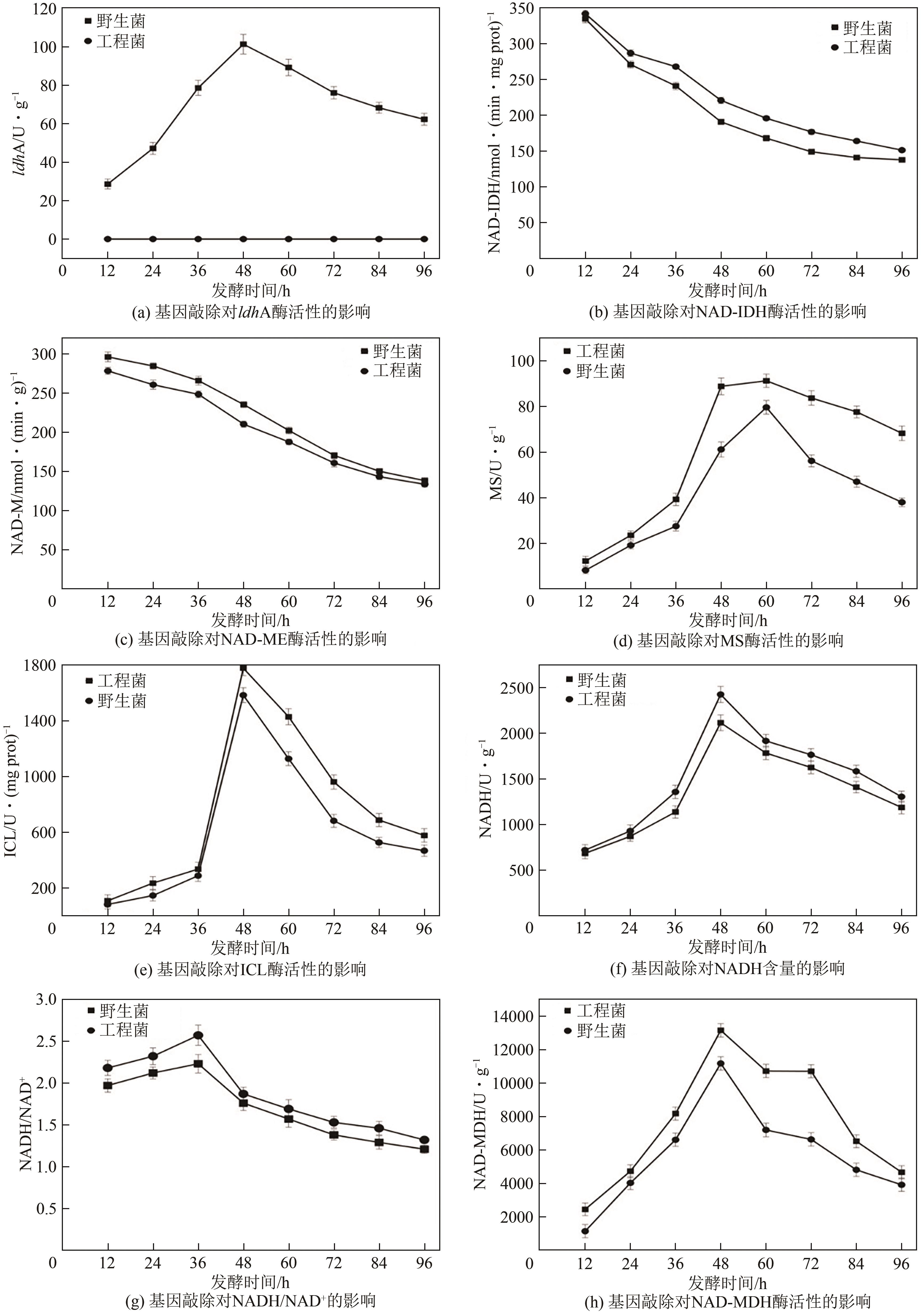
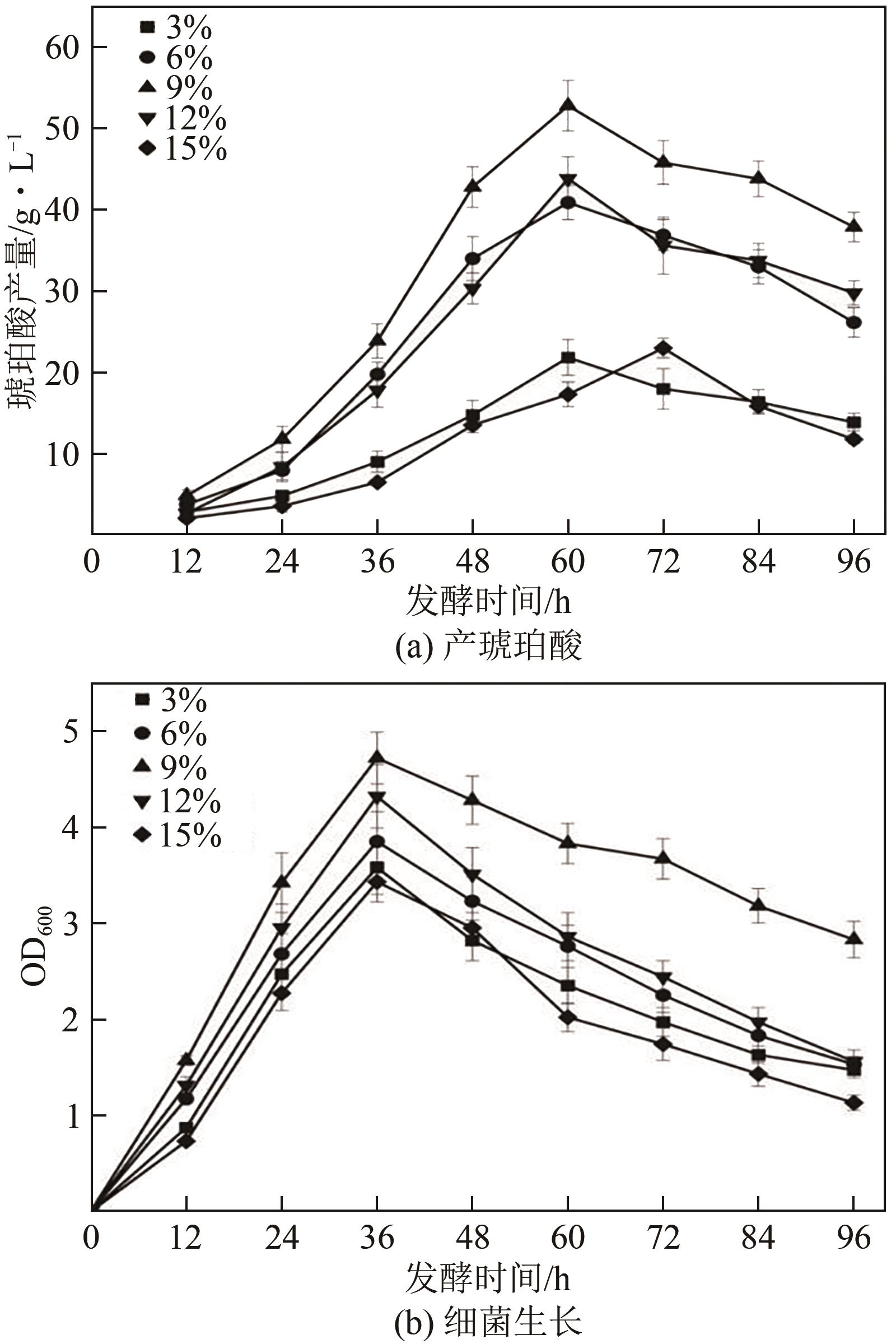
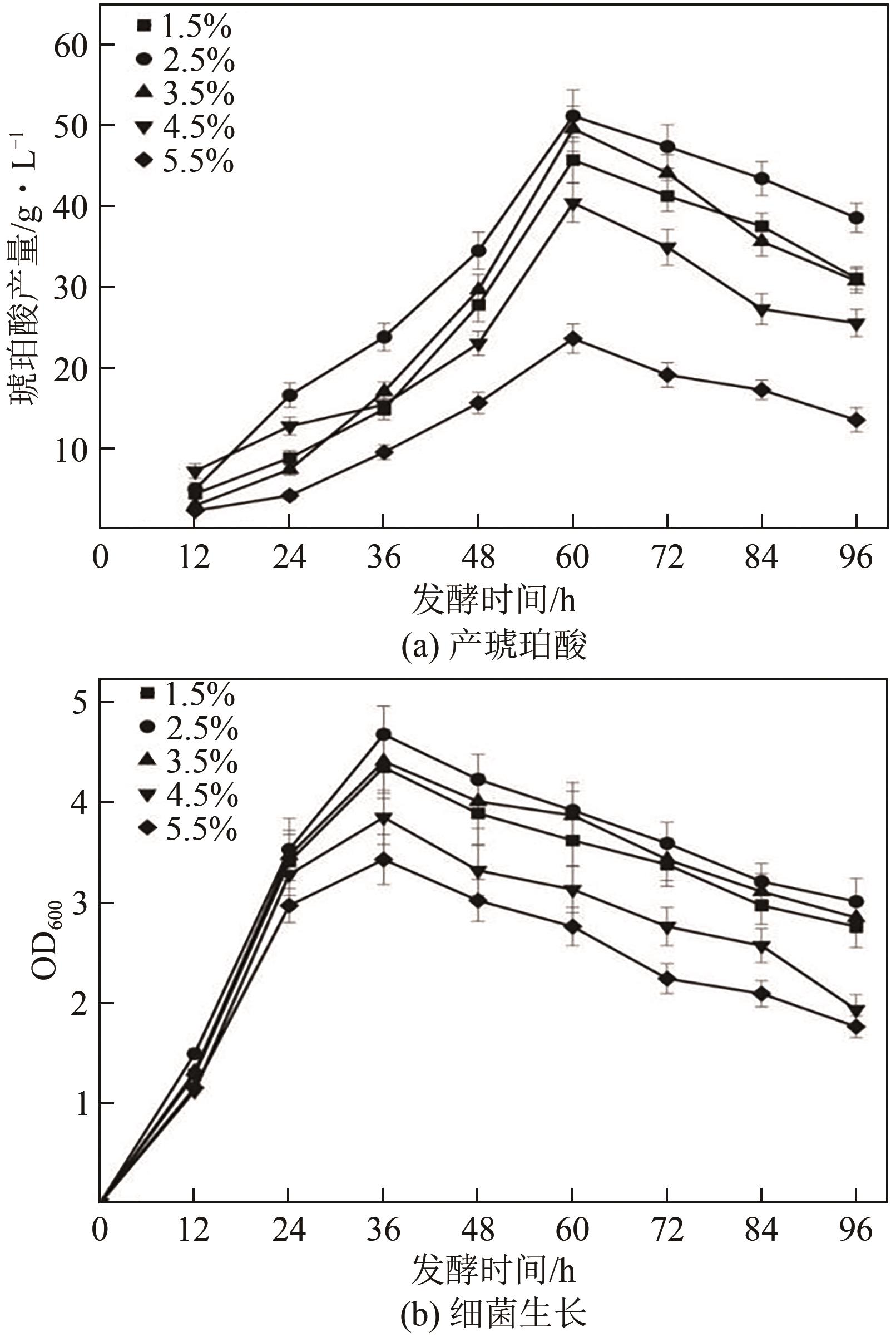
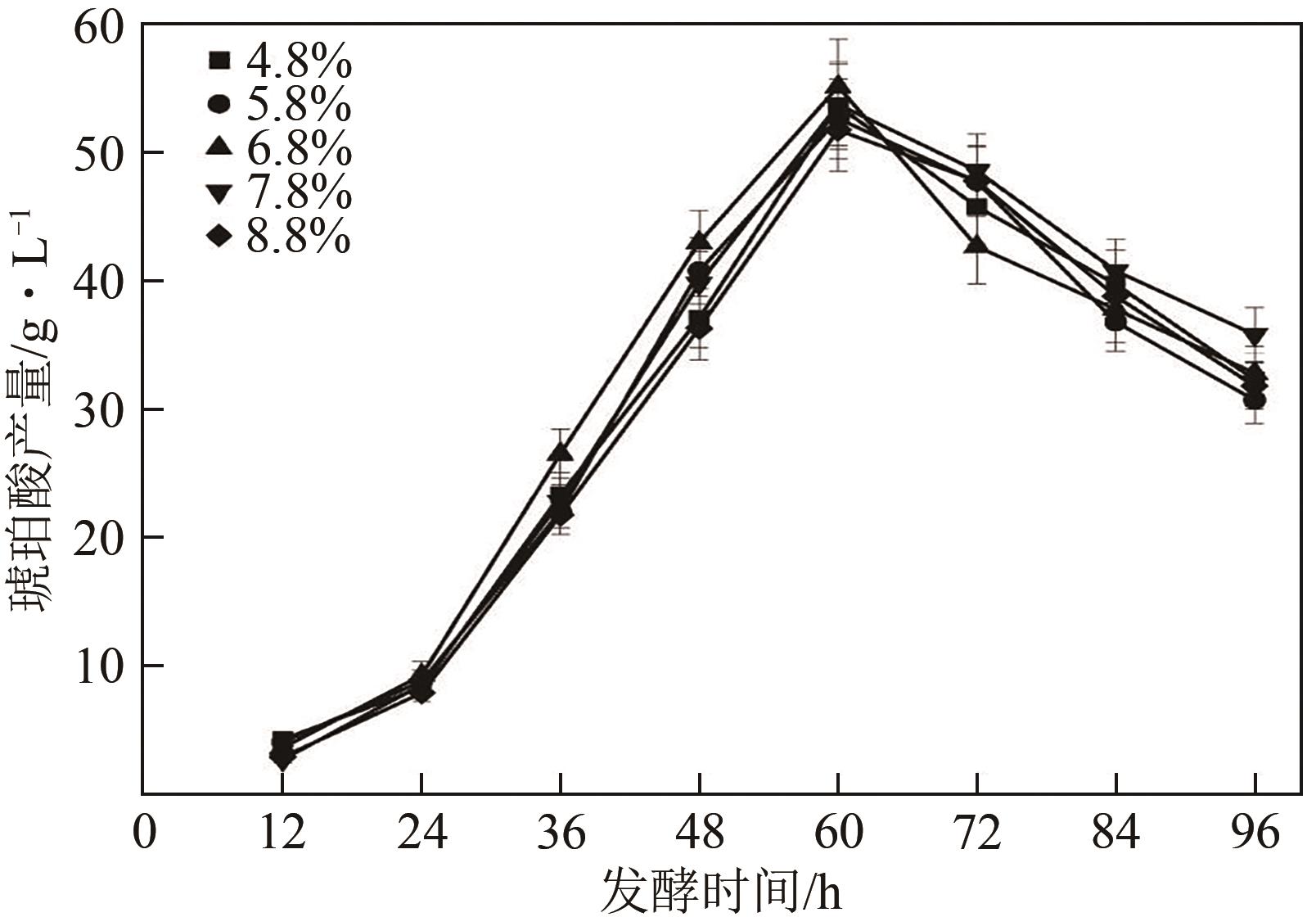
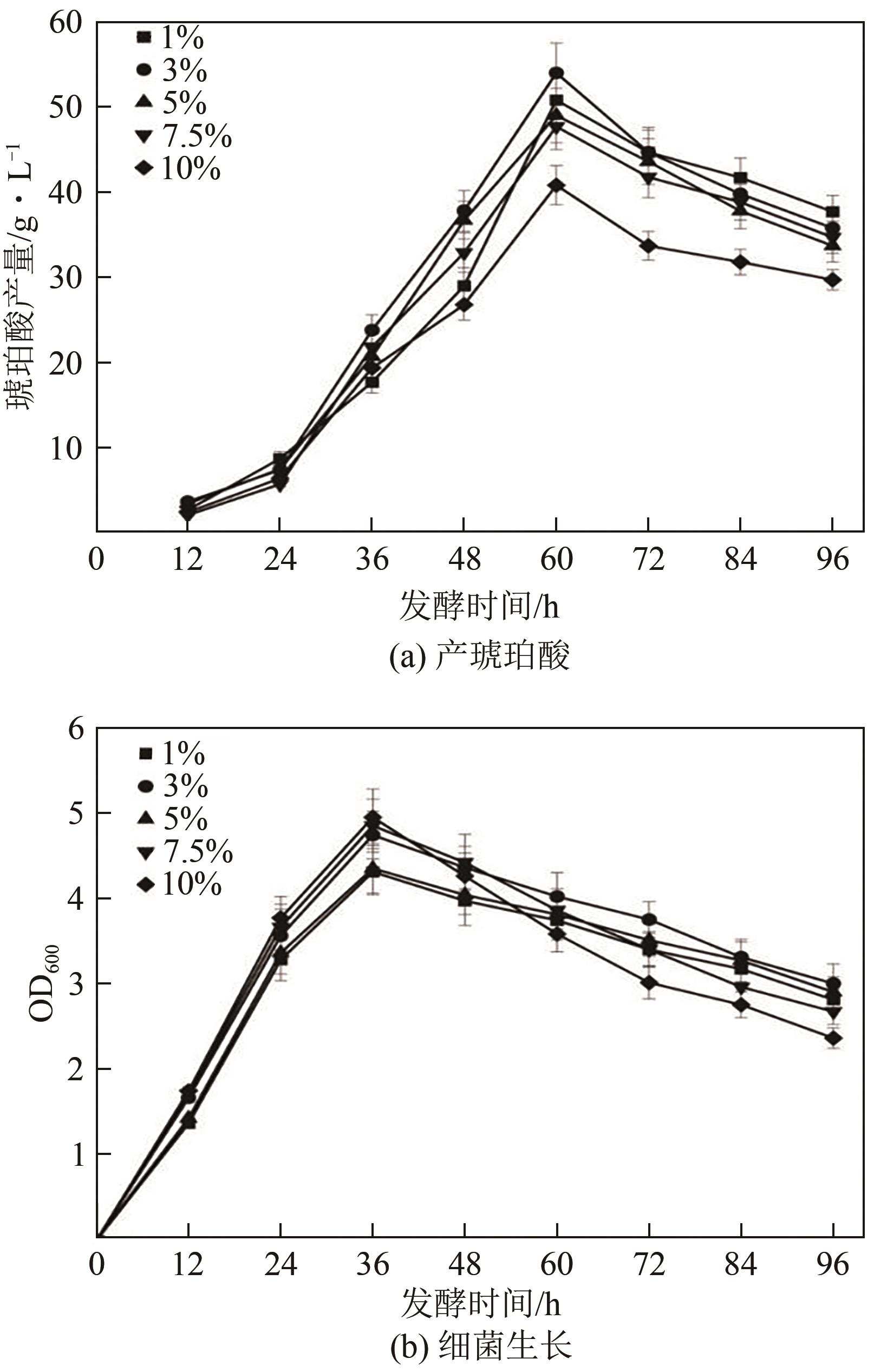
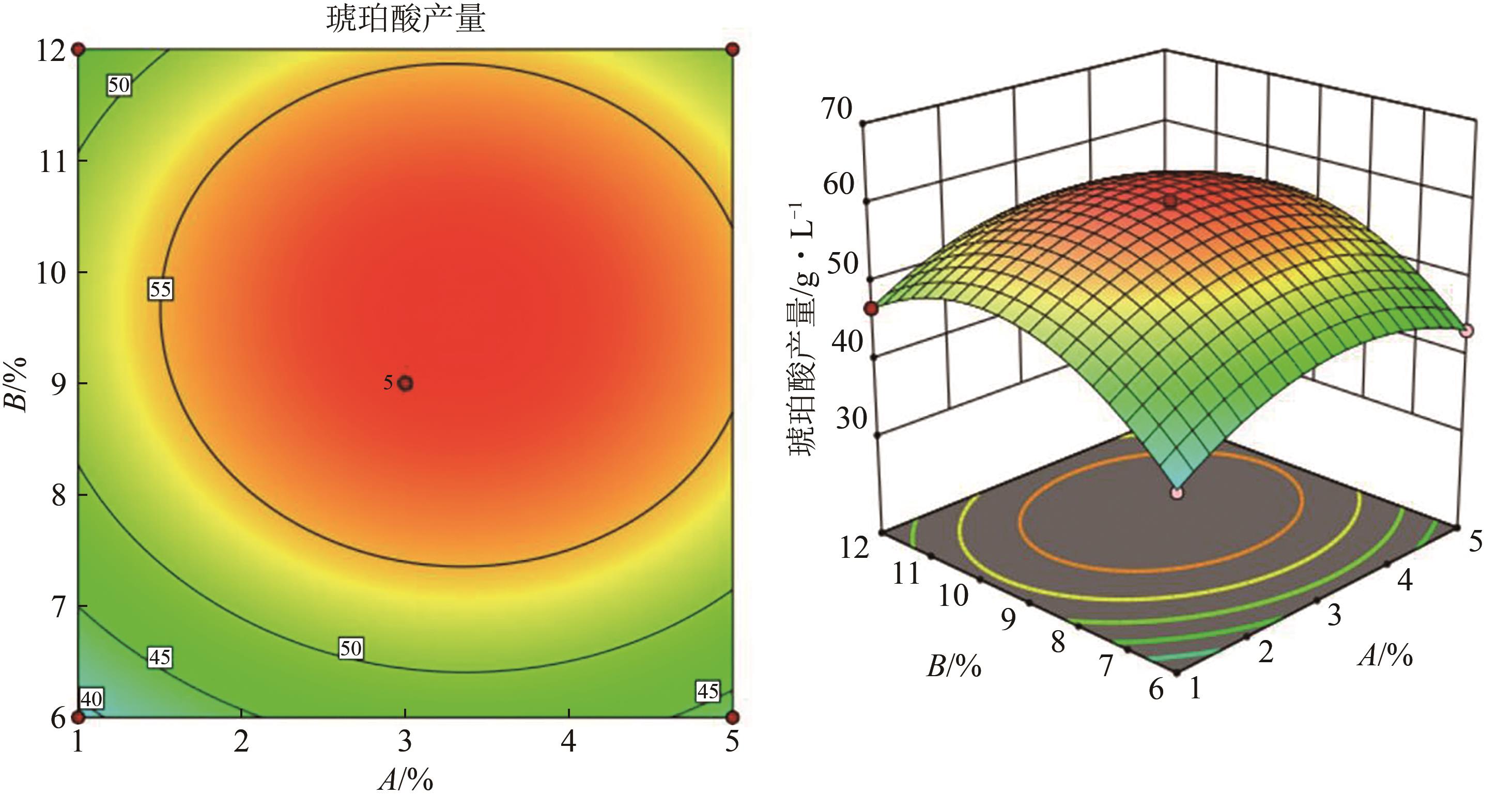
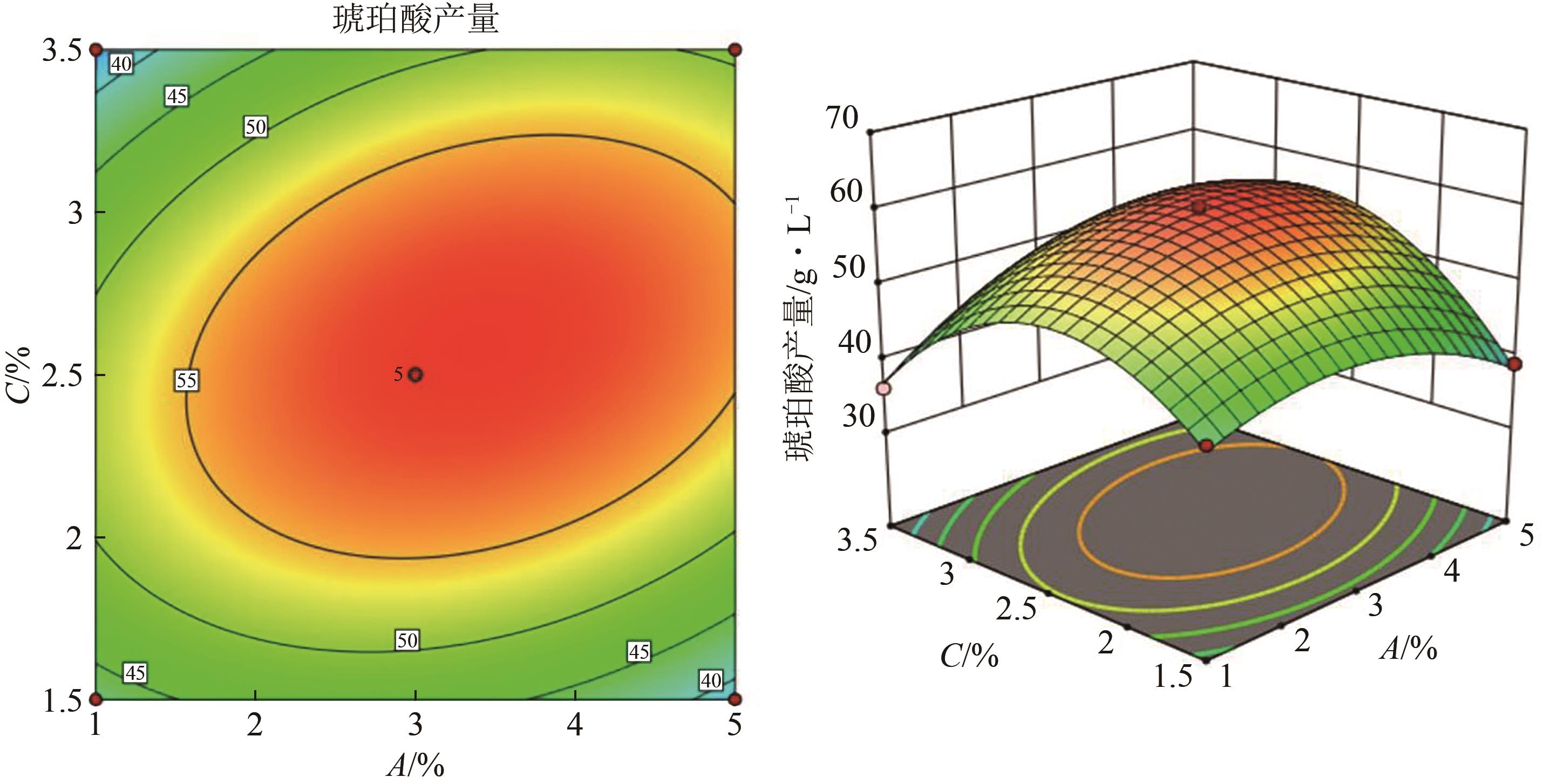
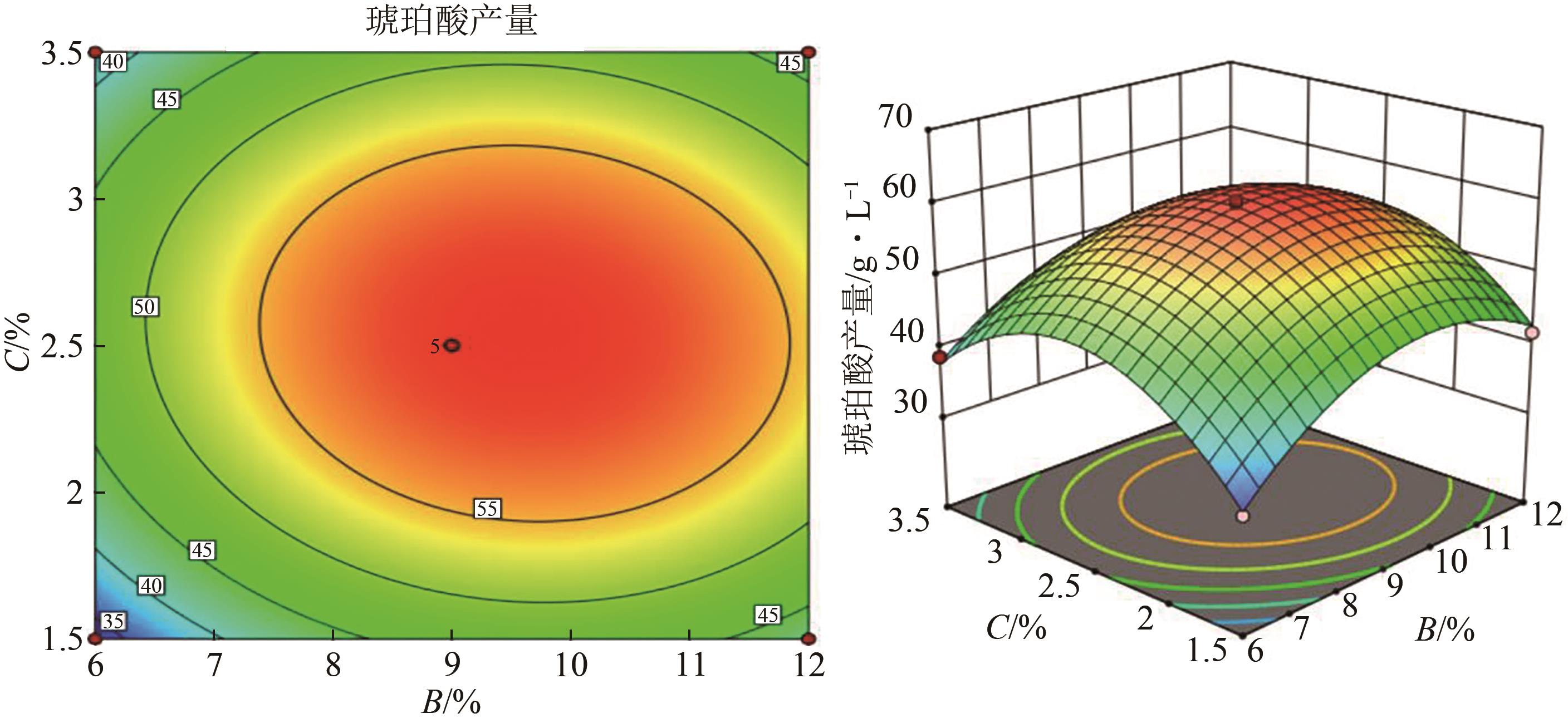
琥珀酸作为最具有前途的化学中间体之一,受到社会广泛的关注。利用微生物法生产琥珀酸具有环境友好、成本低廉等优点,但是也存在发酵得率低、副产物复杂等问题。本文通过对大肠杆菌(E. coli SUC37)的工程改造,有效提高了琥珀酸的产量。首先以E. coli SUC37为出发菌株,通过Red同源重组技术,构建乳酸脱氢酶基因(ldhA)失活突变菌株,阻断主要冗余代谢支路,减少副产物的积累进而增加琥珀酸的产量。对照分析了发酵过程中出发菌株和工程菌株中线粒体异柠檬酸脱氢酶(NAD-IDH)、NAD-苹果酸酶(NAD-ME)、苹果酸合酶(MS)、异柠檬酸裂合酶(ICL)、辅酶ⅠNAD(H)、NAD-苹果酸脱氢酶(NAD-MDH)的活性,进一步探索高产菌株发酵过程中关键酶的代谢差异,发现ldhA的敲除主要通过增强TCA循环的还原支路以及乙醛酸途径,从而促进琥珀酸的积累。优化以玉米淀粉工业废弃物玉米浆为氮源产琥珀酸的发酵条件得到,初始葡萄糖含量为9%、初始玉米浆含量为2.5%、初始碳酸镁含量为6.8%、接种量为3%、发酵温度为37℃的条件下,发酵60h琥珀酸的转化率为0.658g/g葡萄糖,提高了30.2%;产量达到54.9g/L,提高了20.7%;副产物乳酸积累了19.16g/L,降低了46.5%,为琥珀酸的工业化生产奠定了基础。
中图分类号:
引用本文
黄超, 任晓洁, 裴疆森, 赵新河, 赵玉斌, 王灵云, 荆宇航. 高产琥珀酸工程菌株E.coli SUC37的代谢途径优化及分析[J]. 化工进展, 2024, 43(12): 6883-6895.
HUANG Chao, REN Xiaojie, PEI Jiangsen, ZHAO Xinhe, ZHAO Yubin, WANG Lingyun, JING Yuhang. Optimization and analysis of the metabolic pathway of succinic acid-producing viaE.coli SUC37[J]. Chemical Industry and Engineering Progress, 2024, 43(12): 6883-6895.
| 菌株/质粒 | 相关特性 | 来源 |
|---|---|---|
| pKD4 | oriR6Kγ, Kan, rgnB (Ter) 3267bp | 武汉转导生物 |
| pKD46 | pSC101ts ori, bla, PBAD, gam, bet, exo 6329bp | 武汉转导生物 |
| pCP20 | pSC101ts ori, Cm, Flp 9332bp | 武汉转导生物 |
表1 本研究所用的质粒
| 菌株/质粒 | 相关特性 | 来源 |
|---|---|---|
| pKD4 | oriR6Kγ, Kan, rgnB (Ter) 3267bp | 武汉转导生物 |
| pKD46 | pSC101ts ori, bla, PBAD, gam, bet, exo 6329bp | 武汉转导生物 |
| pCP20 | pSC101ts ori, Cm, Flp 9332bp | 武汉转导生物 |
| 反应体系(50μL) | 扩增条件 |
|---|---|
| 超纯水(22μL) | 预变形95℃,3min |
| 底物(1μL) | 扩增95℃,30s |
| IdhF(1μL) | 退火55℃,30s |
| IdhR(1μL) | 延伸72℃,10min |
| PCRMM(25μL) | 共30个循环 |
表2 PCR扩增条件及配置反应体系
| 反应体系(50μL) | 扩增条件 |
|---|---|
| 超纯水(22μL) | 预变形95℃,3min |
| 底物(1μL) | 扩增95℃,30s |
| IdhF(1μL) | 退火55℃,30s |
| IdhR(1μL) | 延伸72℃,10min |
| PCRMM(25μL) | 共30个循环 |
| 引物 | 序列(5'→3') |
|---|---|
| IdhF | ATGAAACTC |
| IdhR | TTAAACCAGTTCGTTCGGGCA |
| VerifyldhF | TGCAACAGGTGAACGAGTC |
| VerifyldhR | TACTGGTCAGTGCTTCTGC |
表3 引物序列
| 引物 | 序列(5'→3') |
|---|---|
| IdhF | ATGAAACTC |
| IdhR | TTAAACCAGTTCGTTCGGGCA |
| VerifyldhF | TGCAACAGGTGAACGAGTC |
| VerifyldhR | TACTGGTCAGTGCTTCTGC |
| 水平 | 因素 | ||
|---|---|---|---|
| 接种量(A)/% | G添加量(B)/% | 玉米浆浓度(C)/% | |
| -1 | 1 | 6 | 1.5 |
| 0 | 3 | 9 | 2.5 |
| 1 | 5 | 12 | 3.5 |
表4 实验因素水平
| 水平 | 因素 | ||
|---|---|---|---|
| 接种量(A)/% | G添加量(B)/% | 玉米浆浓度(C)/% | |
| -1 | 1 | 6 | 1.5 |
| 0 | 3 | 9 | 2.5 |
| 1 | 5 | 12 | 3.5 |
| 序号 | 接种量(A)/% | 葡萄糖(B)/% | 玉米浆浓度(C)/% | 琥珀酸产量/g·L-1 |
|---|---|---|---|---|
| 1 | 1 | 6 | 2.5 | 38.605 |
| 2 | 5 | 6 | 2.5 | 43.302 |
| 3 | 1 | 12 | 2.5 | 46.742 |
| 4 | 5 | 12 | 2.5 | 50.265 |
| 5 | 1 | 9 | 1.5 | 43.763 |
| 6 | 5 | 9 | 1.5 | 38.852 |
| 7 | 1 | 9 | 3.5 | 36.049 |
| 8 | 5 | 9 | 3.5 | 48.287 |
| 9 | 3 | 6 | 1.5 | 32.632 |
| 10 | 3 | 12 | 1.5 | 41.578 |
| 11 | 3 | 6 | 3.5 | 38.653 |
| 12 | 3 | 12 | 3.5 | 43.34 |
| 13 | 3 | 9 | 2.5 | 58.864 |
| 14 | 3 | 9 | 2.5 | 59.01 |
| 15 | 3 | 9 | 2.5 | 59.963 |
| 16 | 3 | 9 | 2.5 | 58.873 |
| 17 | 3 | 9 | 2.5 | 60.26 |
表5 琥珀酸生产的RSM实验设计及结果
| 序号 | 接种量(A)/% | 葡萄糖(B)/% | 玉米浆浓度(C)/% | 琥珀酸产量/g·L-1 |
|---|---|---|---|---|
| 1 | 1 | 6 | 2.5 | 38.605 |
| 2 | 5 | 6 | 2.5 | 43.302 |
| 3 | 1 | 12 | 2.5 | 46.742 |
| 4 | 5 | 12 | 2.5 | 50.265 |
| 5 | 1 | 9 | 1.5 | 43.763 |
| 6 | 5 | 9 | 1.5 | 38.852 |
| 7 | 1 | 9 | 3.5 | 36.049 |
| 8 | 5 | 9 | 3.5 | 48.287 |
| 9 | 3 | 6 | 1.5 | 32.632 |
| 10 | 3 | 12 | 1.5 | 41.578 |
| 11 | 3 | 6 | 3.5 | 38.653 |
| 12 | 3 | 12 | 3.5 | 43.34 |
| 13 | 3 | 9 | 2.5 | 58.864 |
| 14 | 3 | 9 | 2.5 | 59.01 |
| 15 | 3 | 9 | 2.5 | 59.963 |
| 16 | 3 | 9 | 2.5 | 58.873 |
| 17 | 3 | 9 | 2.5 | 60.26 |
| 源项 | 平方差 | DF | 均方 | E | F* |
|---|---|---|---|---|---|
| A | 30.21 | 1 | 30.21 | 31.40 | 0.0008 |
| B | 103.20 | 1 | 103.30 | 107.25 | <0.0001 |
| C | 11.29 | 1 | 11.29 | 11.73 | 0.0111 |
| A2 | 151.04 | 1 | 151.04 | 156.96 | <0.0001 |
| B2 | 316.96 | 1 | 316.96 | 329.39 | <0.0001 |
| C2 | 573.13 | 1 | 573.13 | 595.62 | <0.0001 |
| AB | 0.34 | 1 | 0.34 | 0.36 | 0.5684 |
| AC | 73.52 | 1 | 73.52 | 76.41 | <0.0001 |
| BC | 4.53 | 1 | 4.53 | 4.71 | 0.0665 |
| 残差 | 6.74 | 7 | 60.96 | ||
| 失拟项 | 4.96 | 3 | 1.65 | 3.73 | 0.1371 |
| 纯误差 | 1.77 | 4 | 0.44 | ||
| 总和 | 1382.06 | 16 |
表6 琥珀酸生产二次模型方差分析
| 源项 | 平方差 | DF | 均方 | E | F* |
|---|---|---|---|---|---|
| A | 30.21 | 1 | 30.21 | 31.40 | 0.0008 |
| B | 103.20 | 1 | 103.30 | 107.25 | <0.0001 |
| C | 11.29 | 1 | 11.29 | 11.73 | 0.0111 |
| A2 | 151.04 | 1 | 151.04 | 156.96 | <0.0001 |
| B2 | 316.96 | 1 | 316.96 | 329.39 | <0.0001 |
| C2 | 573.13 | 1 | 573.13 | 595.62 | <0.0001 |
| AB | 0.34 | 1 | 0.34 | 0.36 | 0.5684 |
| AC | 73.52 | 1 | 73.52 | 76.41 | <0.0001 |
| BC | 4.53 | 1 | 4.53 | 4.71 | 0.0665 |
| 残差 | 6.74 | 7 | 60.96 | ||
| 失拟项 | 4.96 | 3 | 1.65 | 3.73 | 0.1371 |
| 纯误差 | 1.77 | 4 | 0.44 | ||
| 总和 | 1382.06 | 16 |
| 1 | NGHIEM Nhuan, KLEFF Susanne, SCHWEGMANN Stefan. Succinic acid: Technology development and commercialization[J]. Fermentation, 2017, 3(2): 26. |
| 2 | BEAUPREZ Joeri J, DE MEY Marjan, SOETAERT Wim K. Microbial succinic acid production: Natural versus metabolic engineered producers[J]. Process Biochemistry, 2010, 45(7): 1103-1114. |
| 3 | Benjamin COK, TSIROPOULOS Ioannis, ROES Alexander L, et al. Succinic acid production derived from carbohydrates: An energy and greenhouse gas assessment of a platform chemical toward a bio-based economy[J]. Biofuels, Bioproducts and Biorefining, 2014, 8(1): 16-29. |
| 4 | LU Jiasheng, LI Jiawen, GAO Hao, et al. Recent progress on bio-succinic acid production from lignocellulosic biomass[J]. World Journal of Microbiology and Biotechnology, 2021, 37(1): 16. |
| 5 | LU Jiasheng, Yang LYU, JIANG Yujia, et al. Consolidated bioprocessing of hemicellulose-enriched lignocellulose to succinic acid through a microbial cocultivation system[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(24): 9035-9045. |
| 6 | VERMA Minal, MANDYAL Parteek, SINGH Dilbag, et al. Recent developments in heterogeneous catalytic routes for the sustainable production of succinic acid from biomass resources[J]. ChemSusChem, 2020, 13(16): 4026-4034. |
| 7 | Enlin LO, Luiza BRABO-CATALA, DOGARIS Ioannis, et al. Biochemical conversion of sweet sorghum bagasse to succinic acid[J]. Journal of Bioscience and Bioengineering, 2020, 129(1): 104-109. |
| 8 | SU Hsiang-Yen, LI Huaying, XIE Caiyun, et al. Co-production of acetoin and succinic acid by metabolically engineered Enterobacter cloacae[J]. Biotechnology for Biofuels, 2021, 14(1): 26. |
| 9 | SHEN Naikun, ZHANG Hongyan, QIN Yan, et al. Efficient production of succinic acid from duckweed (Landoltia punctata) hydrolysate by Actinobacillus succinogenes GXAS137[J]. Bioresource Technology, 2018, 250: 35-42. |
| 10 | CHOI Sol, SONG Hyohak, Sung Won LIM, et al. Highly selective production of succinic acid by metabolically engineered Mannheimia succiniciproducens and its efficient purification[J]. Biotechnology and Bioengineering, 2016, 113(10): 2168-2177. |
| 11 | YU Yong, ZHU Xinna, XU Hongtao, et al. Construction of an energy-conserving glycerol utilization pathways for improving anaerobic succinate production in Escherichia coli [J]. Metabolic Engineering, 2019, 56: 181-189. |
| 12 | ZHU Liwen, TANG Yajie. Current advances of succinate biosynthesis in metabolically engineered Escherichia coli [J]. Biotechnology Advances, 2017, 35(8): 1040-1048. |
| 13 | FERONE Mariateresa, RAGANATI Francesca, OLIVIERI Giuseppe, et al. Bioreactors for succinic acid production processes[J]. Critical Reviews in Biotechnology, 2019, 39(4): 571-586. |
| 14 | JANTAMA Kaemwich, HAUPT M J, SVORONOS Spyros A, et al. Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate[J]. Biotechnology and Bioengineering, 2008, 99(5): 1140-1153. |
| 15 | CHEN Xiaoju, ZHOU Yaojie, ZHANG Di. Engineering Corynebacterium crenatum for enhancing succinic acid production[J]. Journal of Food Biochemistry, 2018, 42(6): e12645. |
| 16 | Jung Ho AHN, SEO Hogyun, PARK Woojin, et al. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase[J]. Nature Communications, 2020, 11(1): 1970. |
| 17 | DATSENKO K A, WANNER B L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(12): 6640-6645. |
| 18 | ZHAO Busi, SUN Liankun, JIANG Xianrui, et al. Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway[J]. European Journal of Pharmacology, 2019, 845: 56-64. |
| 19 | JIANG Yongshun, CAO Sai, ZHOU Bin, et al. Hemocytes in blue mussel Mytilus edulis adopt different energy supply modes to cope with different BDE-47 exposures[J]. Science of the Total Environment, 2023, 885: 163766. |
| 20 | ROUCOURT Bart, MINNEBO Nikki, AUGUSTIJNS Patrick, et al. Biochemical characterization of malate synthase G of P. aeruginosa [J]. BMC Biochemistry, 2009, 10: 20. |
| 21 | SUN Jikang, JIA Hao, WANG Ping, et al. Exogenous gibberellin weakens lipid breakdown by increasing soluble sugars levels in early germination of zanthoxylum seeds[J]. Plant Science, 2019, 280: 155-163. |
| 22 | YANG Kui, YIN Qin, MAO Qingcheng, et al. Metabolomics analysis reveals therapeutic effects of α-mangostin on collagen-induced arthritis in rats by down-regulating nicotinamide phosphoribosyltransferase[J]. Inflammation, 2019, 42(2): 741-753. |
| 23 | YAO Yuxin, LI Ming, ZHAI Heng, et al. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis[J]. Journal of Plant Physiology, 2011, 168(5): 474-480. |
| 24 | SUN Wenhui, JIANG Bo, ZHANG Yue, et al. Enabling the biosynthesis of malic acid in Lactococcus lactis by establishing the reductive TCA pathway and promoter engineering[J]. Biochemical Engineering Journal, 2020, 161: 107645. |
| 25 | LU Ping, GAO Ting, BAI Ruoxuan, et al. Regulation of carbon flux and NADH/NAD+ supply to enhance 2,3-butanediol production in Enterobacter aerogenes [J]. Journal of Biotechnology, 2022, 358: 67-75. |
| 26 | KUIT Wouter, MINTON Nigel P, LÓPEZ-CONTRERAS Ana M, et al. Disruption of the acetate kinase (ack) gene of Clostridium acetobutylicum results in delayed acetate production[J]. Applied Microbiology and Biotechnology, 2012, 94(3): 729-741. |
| 27 | GE Jingping, WANG Jiawang, YE Guangbin, et al. Disruption of the lactate dehydrogenase and acetate kinase genes in Klebsiella pneumoniae HD79 to enhance 2, 3-butanediol production, and related transcriptomics analysis[J]. Biotechnology Letters, 2020, 42(4): 537-549. |
| 28 | KYNSHI Balakyntiewshisha Lyngdoh, SACHU Meguovilie, SYIEM Mayashree B. Modulation in isocitrate dehydrogenase activity under citrate enrichment affects carbon and nitrogen fixations in the cyanobacterium Nostoc muscorum Meg 1[J]. Biochimie, 2021, 186: 94-104. |
| 29 | AFZAL Aqeel Rana, JEON Jinyoung, JUNG Che-Hun. Fumarase activity in NAD-dependent malic enzyme, MaeA, from Escherichia coli [J]. Biochemical and Biophysical Research Communications, 2023, 678: 144-147. |
| 30 | Céline BROCHIER-ARMANET, MADERN Dominique. Phylogenetics and biochemistry elucidate the evolutionary link between l-malate and l-lactate dehydrogenases and disclose an intermediate group of sequences with mix functional properties[J]. Biochimie, 2021, 191: 140-153. |
| 31 | ZHOU Shenghu, DING Nana, HAN Runhua, et al. Metabolic engineering and fermentation optimization strategies for producing organic acids of the tricarboxylic acid cycle by microbial cell factories[J]. Bioresource Technology, 2023, 379: 128986. |
| 32 | NGUYEN Diep Thi Ngoc, LEE Ok Kyung, HADIYATI Susila, et al. Metabolic engineering of the type Ⅰ methanotroph Methylomonas sp. DH-1 for production of succinate from methane[J]. Metabolic Engineering, 2019, 54: 170-179. |
| 33 | DENG Yu, MA Ning, ZHU Kangjia, et al. Balancing the carbon flux distributions between the TCA cycle and glyoxylate shunt to produce glycolate at high yield and titer in Escherichia coli [J]. Metabolic Engineering, 2018, 46: 28-34. |
| 34 | ZHU Fayin, WANG Chengqiang, Ka-Yiu SAN, et al. Metabolic engineering of Escherichia coli to produce succinate from woody hydrolysate under anaerobic conditions[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(2): 223-232. |
| 35 | SHEN Naikun, LIAO Siming, WANG Qingyan, et al. Economical succinic acid production from sugarcane juice by Actinobacillus succinogenes supplemented with corn steep liquor and peanut meal as nitrogen sources[J]. Sugar Tech, 2016, 18(3): 292-298. |
| 36 | Lisbeth VALLECILLA-YEPEZ, RAMCHANDRAN Divya, LONG Dianna, et al. Corn fiber as a biomass feedstock for production of succinic acid[J]. Bioresource Technology Reports, 2021, 16: 100868. |
| 37 | XI Yonglan, CHEN Kequan, DAI Wenyu, et al. Succinic acid production by Actinobacillus succinogenes NJ113 using corn steep liquor powder as nitrogen source[J]. Bioresource Technology, 2013, 136: 775-779. |
| 38 | XI Yonglan, DAI Wenyu, XU Rong, et al. Ultrasonic pretreatment and acid hydrolysis of sugarcane bagasse for succinic acid production using Actinobacillus succinogenes [J]. Bioprocess and Biosystems Engineering, 2013, 36(11): 1779-1785. |
| 39 | CARVALHO Margarida, MATOS Mariana, ROCA Christophe, et al. Succinic acid production from glycerol by Actinobacillus succinogenes using dimethylsulfoxide as electron acceptor[J]. New Biotechnology, 2014, 31(1): 133-139. |
| 40 | NARISETTY Vivek, OKIBE Maureen Chiebonam, AMULYA K, et al. Technological advancements in valorization of second generation (2G) feedstocks for bio-based succinic acid production[J]. Bioresource Technology, 2022, 360: 127513. |
| 41 | JIANG Min, CHEN Kequan, LIU Zhongmin, et al. Succinic acid production by Actinobacillus succinogenes using spent brewer’s yeast hydrolysate as a nitrogen source[J]. Applied Biochemistry and Biotechnology, 2010, 160(1): 244-254. |
| 42 | NGHIEM Nhuan P, DAVISON Brian H, THOMPSON James E, et al. The effect of biotin on the production of succinic acid by Anaerobiospirillum succiniciproducens [J]. Applied Biochemistry and Biotechnology, 1996, 57(1): 633-638. |
| 43 | CHENG Keke, ZHAO Xuebing, ZENG Jing, et al. Biotechnological production of succinic acid: Current state and perspectives[J]. Biofuels, Bioproducts and Biorefining, 2012, 6(3): 302-318. |
| 44 | LIU Rongming, LIANG Liya, WU Mingke, et al. CO2 fixation for succinic acid production by engineered Escherichia coli co-expressing pyruvate carboxylase and nicotinic acid phosphoribosyltransferase[J]. Biochemical Engineering Journal, 2013, 79: 77-83. |
| 45 | SONG Hyohak, LEE Jeong Wook, CHOI Sol, et al. Effects of dissolved CO2 levels on the growth of Mannheimia succiniciproducens and succinic acid production[J]. Biotechnology and Bioengineering, 2007, 98(6): 1296-1304. |
| 46 | PIWOWAREK Kamil, Edyta LIPIŃSKA, Elżbieta HAĆ-SZYMAŃCZUK, et al. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry[J]. Applied Microbiology and Biotechnology, 2018, 102(2): 515-538. |
| 47 | MELLEFONT L A, MCMEEKIN T A, ROSS T. Effect of relative inoculum concentration on Listeria monocytogenes growth in co-culture[J]. International Journal of Food Microbiology, 2008, 121(2): 157-168. |
| [1] | 佘丹, 王舒婷, 陆信曜, 宗红, 诸葛斌. 基因工程改造促进大肠杆菌发酵纤维素水解液合成1,2,4-丁三醇[J]. 化工进展, 2024, 43(2): 1063-1068. |
| [2] | 唐文秀, 王学明, 郭亮, 季立豪, 高聪, 陈修来, 刘立明. 代谢工程改造大肠杆菌生产琥珀酸[J]. 化工进展, 2022, 41(2): 938-950. |
| [3] | 郭超, 冯奥, 陆信曜, 宗红, 诸葛斌. 重组大肠杆菌1,2,4-丁三醇合成途径的平衡优化[J]. 化工进展, 2022, 41(12): 6531-6539. |
| [4] | 陶雨萱, 张尚杰, 景艺文, 信丰学, 董维亮, 周杰, 蒋羽佳, 章文明, 姜岷. 甲基营养型大肠杆菌构建策略的研究进展[J]. 化工进展, 2021, 40(7): 3932-3941. |
| [5] | 李阳, 朱晨辉, 范代娣. 重组胶原蛋白的绿色生物制造及其应用[J]. 化工进展, 2021, 40(3): 1262-1275. |
| [6] | 周末,刘彦君,佟欣瑞,董延甲,吴欣瑜,王迎香,应明. E. coli K-12琥珀酸脱氢酶sdhC基因纳米锥的设计及自组装[J]. 化工进展, 2020, 39(2): 679-685. |
| [7] | 陈露,刘丁玉,汪保卫,赵玉姣,贾广韬,陈涛,王智文. 大肠杆菌乙酰辅酶A代谢调控及其应用研究进展[J]. 化工进展, 2019, 38(9): 4218-4226. |
| [8] | 陈丹,张小里,李冰麟,卫龙辉,赵彬侠,张耀中. 正己烷/丙酮混合溶剂中非均相催化合成α-生育酚琥珀酸酯[J]. 化工进展, 2019, 38(9): 4095-4101. |
| [9] | 张玲, 宋祖坤, 林荣, 王男, 杨海麟. 乳糖诱导E. coli发酵生产FAD为辅基的葡萄糖脱氢酶[J]. 化工进展, 2019, 38(04): 1879-1886. |
| [10] | 富敏霞, 祝铃钰, 贠军贤. 乳酸脱氢酶的分离纯化及其催化合成苯乳酸的研究进展[J]. 化工进展, 2018, 37(12): 4814-4820. |
| [11] | 宋鑫, 张双虹, 陈涛, 刘训理, 王智文. 大肠杆菌调控因子工程及其菌株耐受性研究进展[J]. 化工进展, 2018, 37(07): 2780-2789. |
| [12] | 李亿, 张红岩, 朱婧, 秦艳, 王青艳, 申乃坤. 响应面优化木糖母液发酵产丁二酸[J]. 化工进展, 2018, 37(01): 252-259. |
| [13] | 孙明荣, 刘晓欣, 谢文华, 宗保宁, 郜亮. 生物炼制经济的发展思路和展望[J]. 化工进展, 2017, 36(09): 3250-3256. |
| [14] | 李海花, 高美玲, 张利辉, 高玉华, 刘振法. 含磺酸基团的环氧琥珀酸类共聚物的合成及性能比较[J]. 化工进展, 2017, 36(04): 1491-1498. |
| [15] | 刘长高, 何忠平, 张玉苍, 席小勇. 香蕉假茎中产碱性果胶酶细菌的筛选发酵优化与酶学性质[J]. 化工进展, 2015, 34(09): 3415-3420. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||