化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2600-2610.DOI: 10.16085/j.issn.1000-6613.2024-0001
• 催化与材料技术 • 上一篇
分子筛中限制效应对其酸性表征及催化性能的影响
汪孟宇1,3( ), 范鸿霞1,3, 梁长海2,3, 李文英1,3(
), 范鸿霞1,3, 梁长海2,3, 李文英1,3( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.大连理工大学化工学院,辽宁 大连 116024
3.怀柔实验室山西研究院,山西 太原 030024
-
收稿日期:2024-01-02修回日期:2024-02-28出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:李文英 -
作者简介:汪孟宇(1998—),男,博士研究生,研究方向为工业催化。E-mail:wangmengyu0198@link.tyut.edu.cn。 -
基金资助:国家自然科学基金重点项目(22038008);中国神华煤制油化工有限公司科技创新项目(MZYHG-2021-01)
Influence of zeolite confinement effect on its acidic characterization and catalytic performance
WANG Mengyu1,3( ), FAN Hongxia1,3, LIANG Changhai2,3, LI Wenying1,3(
), FAN Hongxia1,3, LIANG Changhai2,3, LI Wenying1,3( )
)
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China
3.Shanxi Research Institute of Huairou Laboratory, Taiyuan 030024, Shanxi, China
-
Received:2024-01-02Revised:2024-02-28Online:2024-05-15Published:2024-06-15 -
Contact:LI Wenying
摘要:
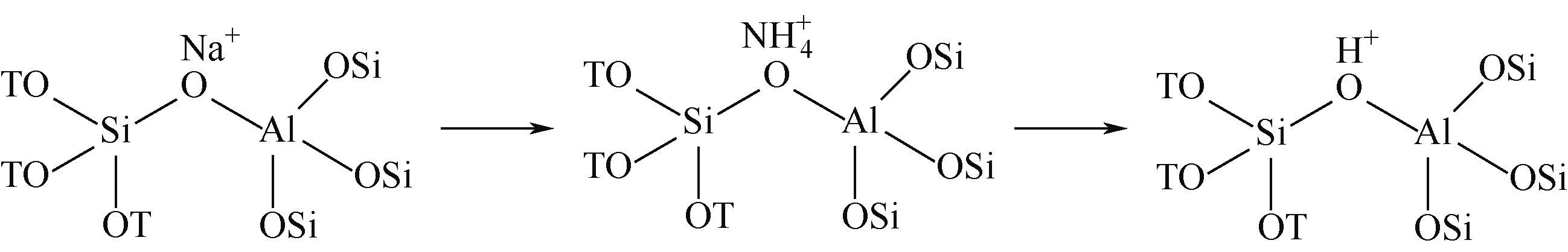
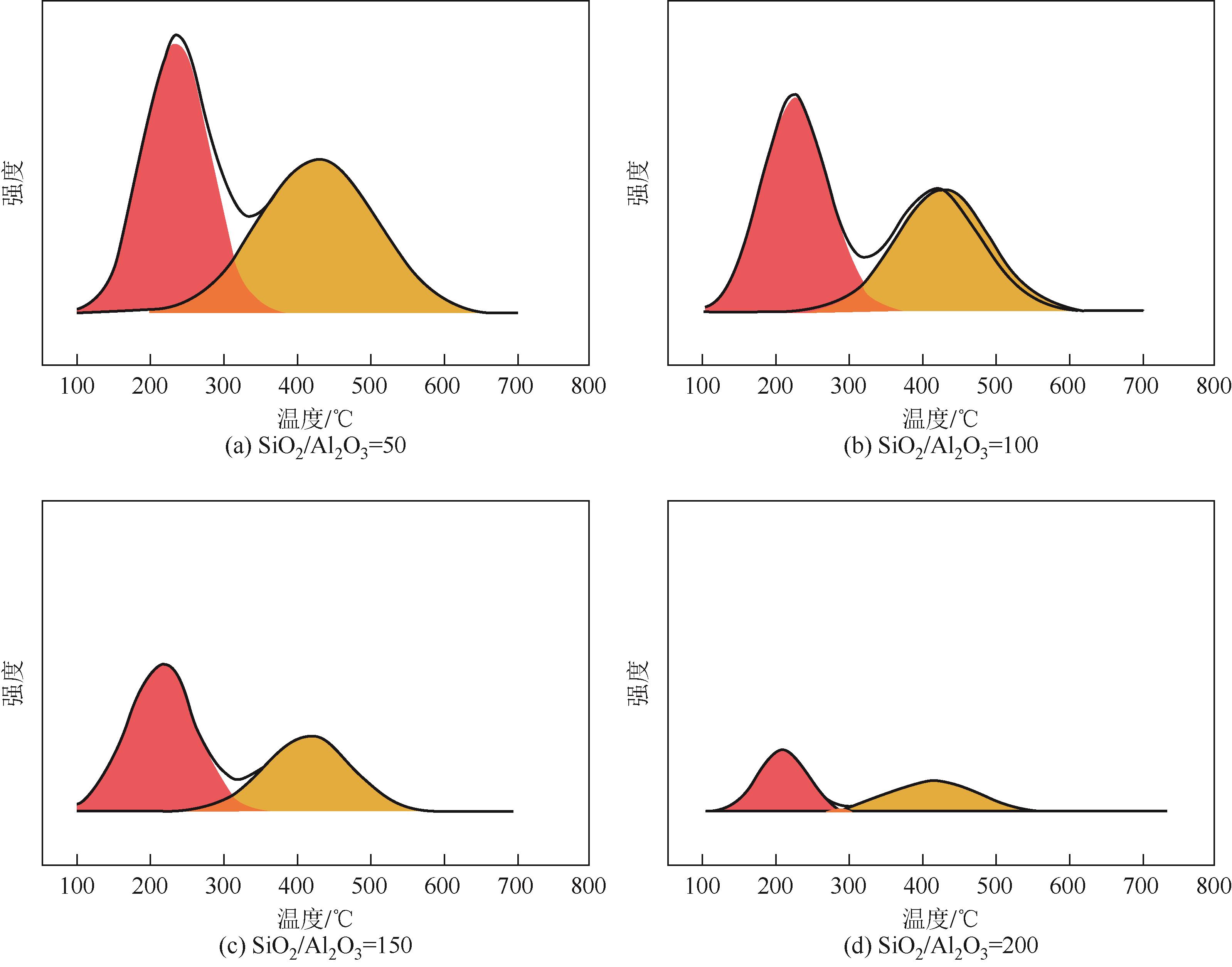
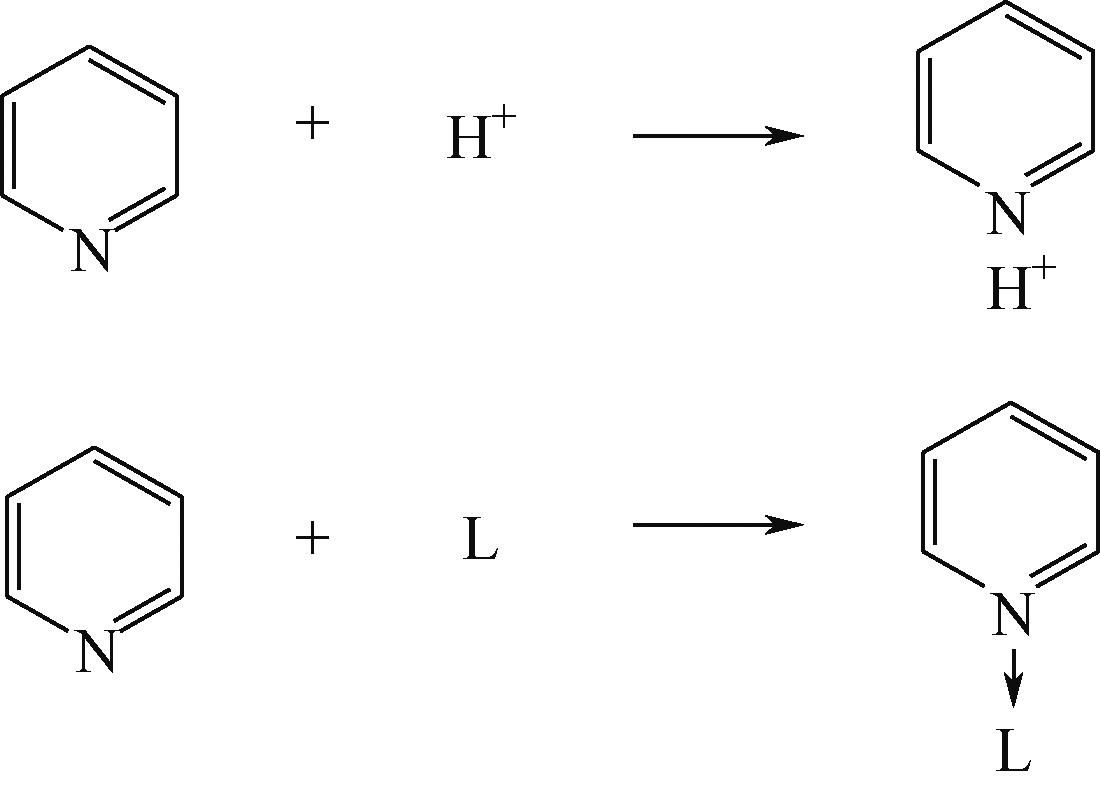
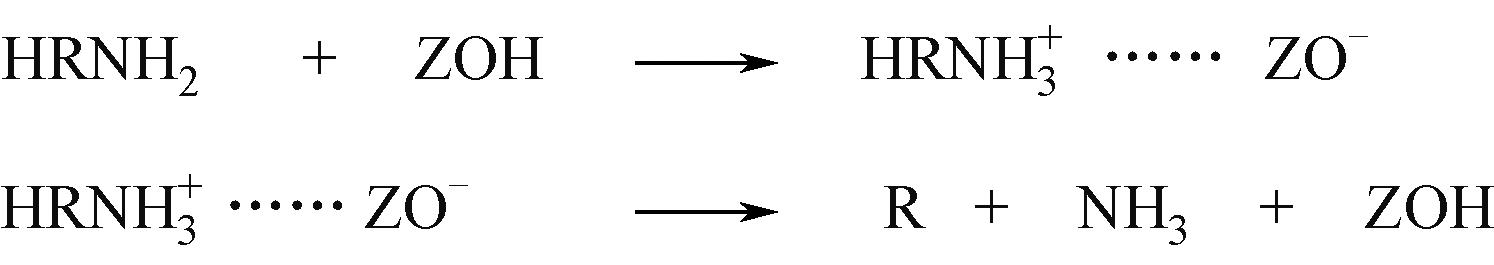
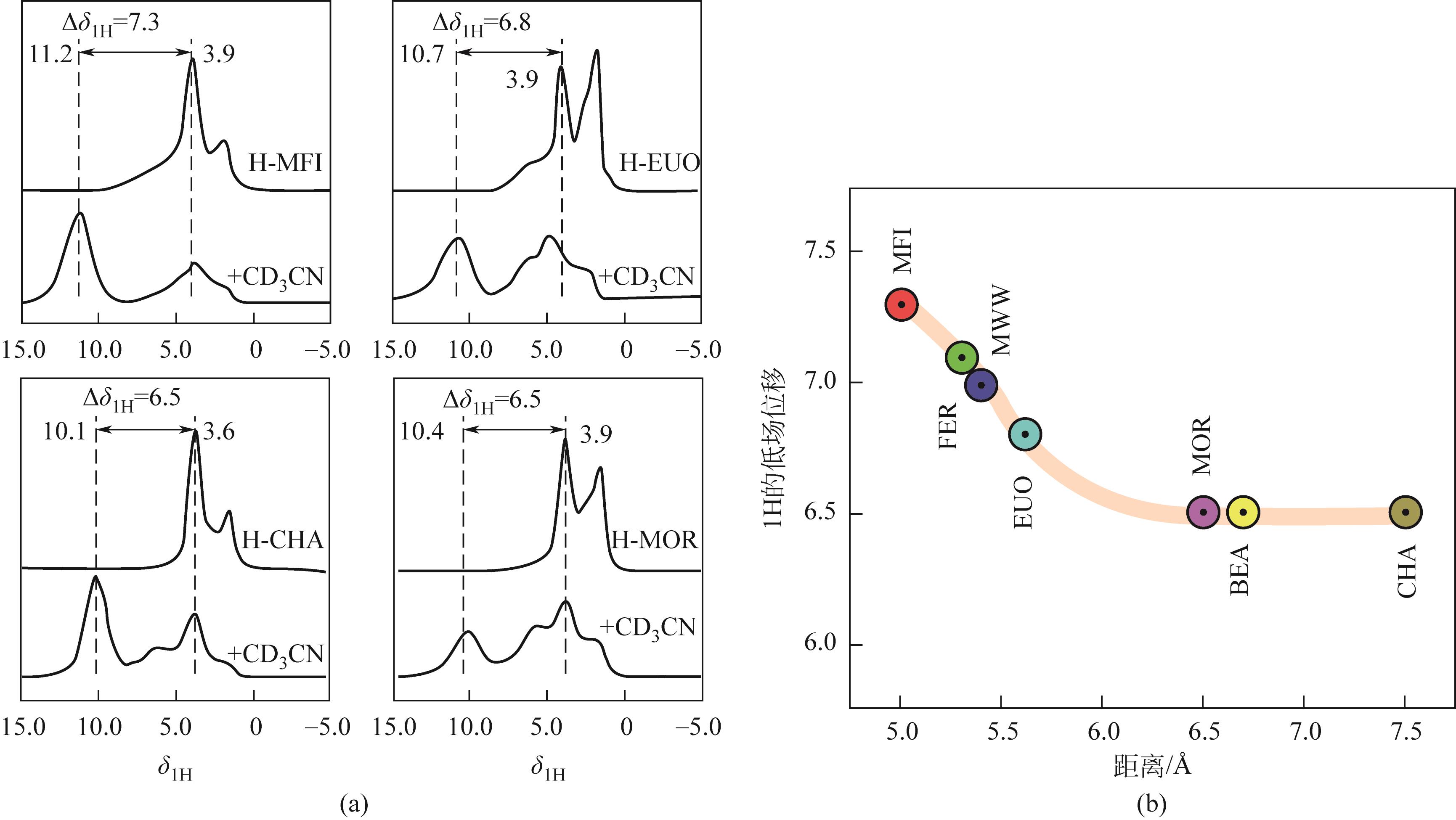
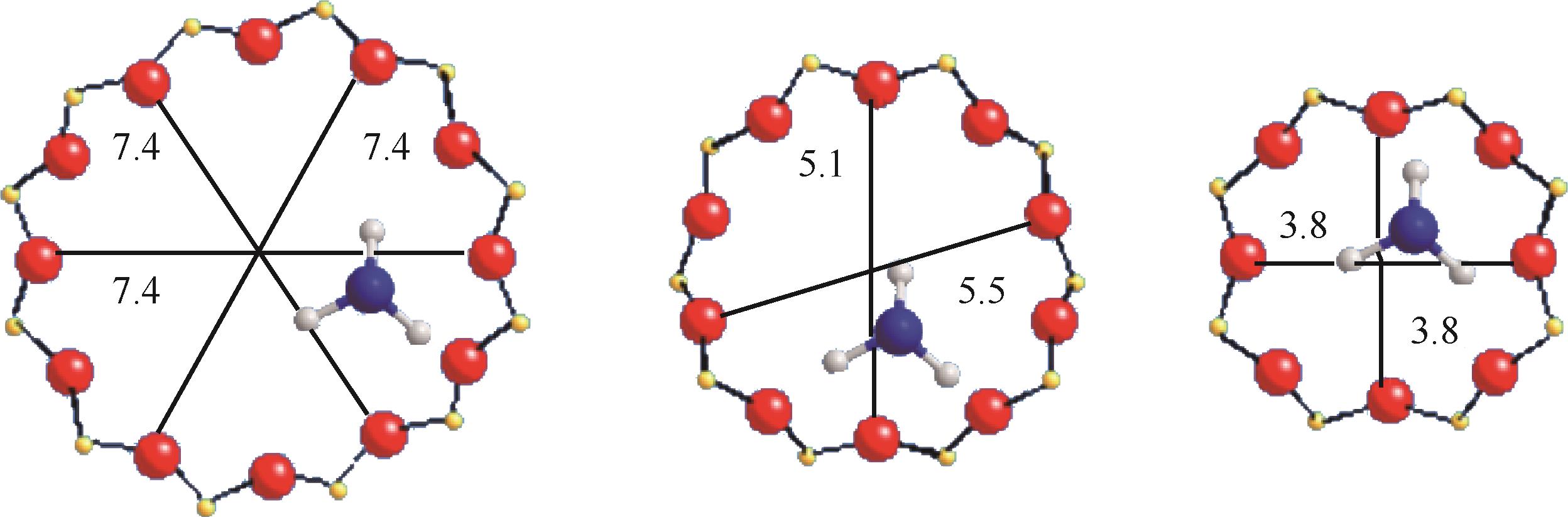
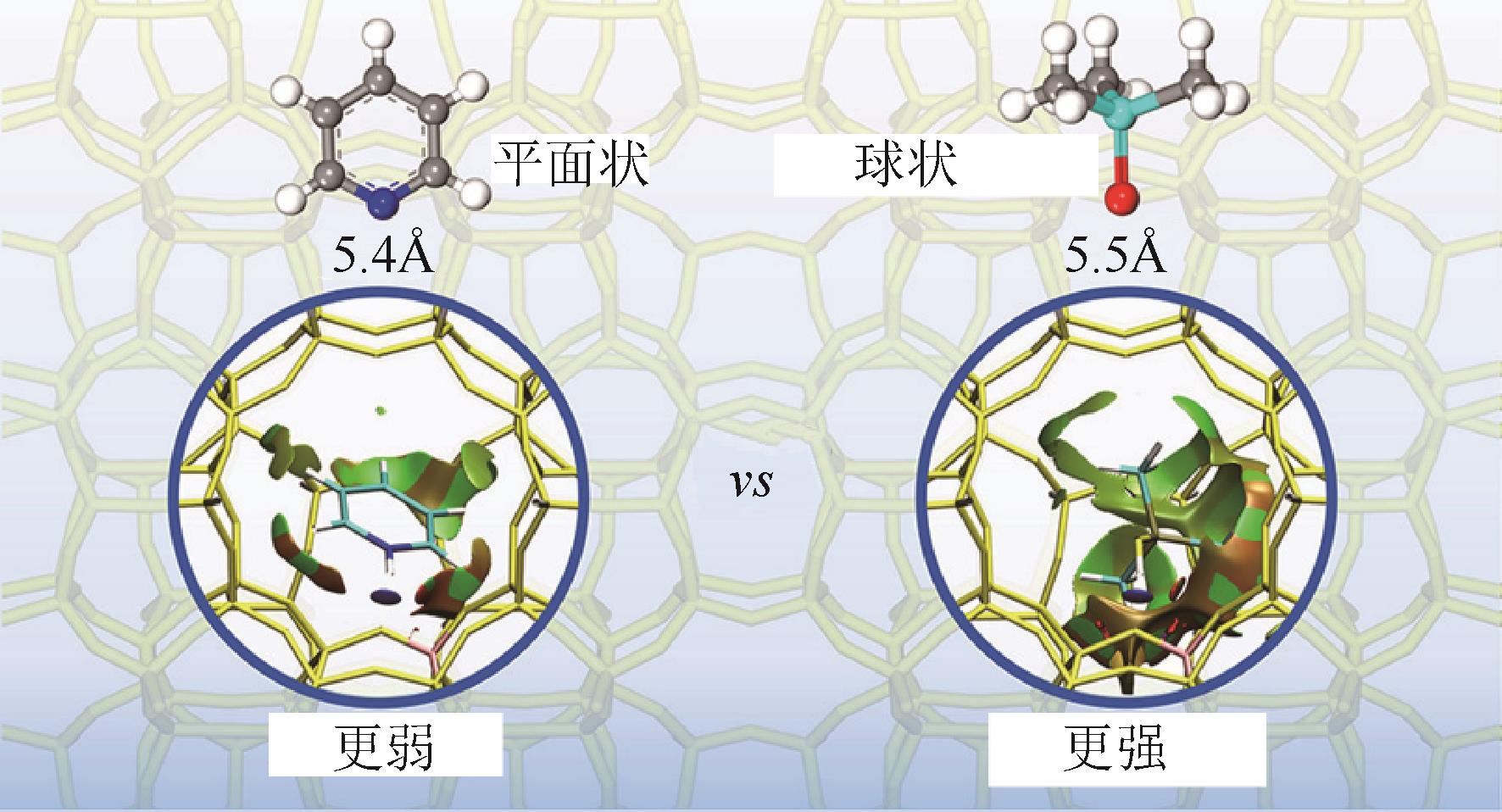
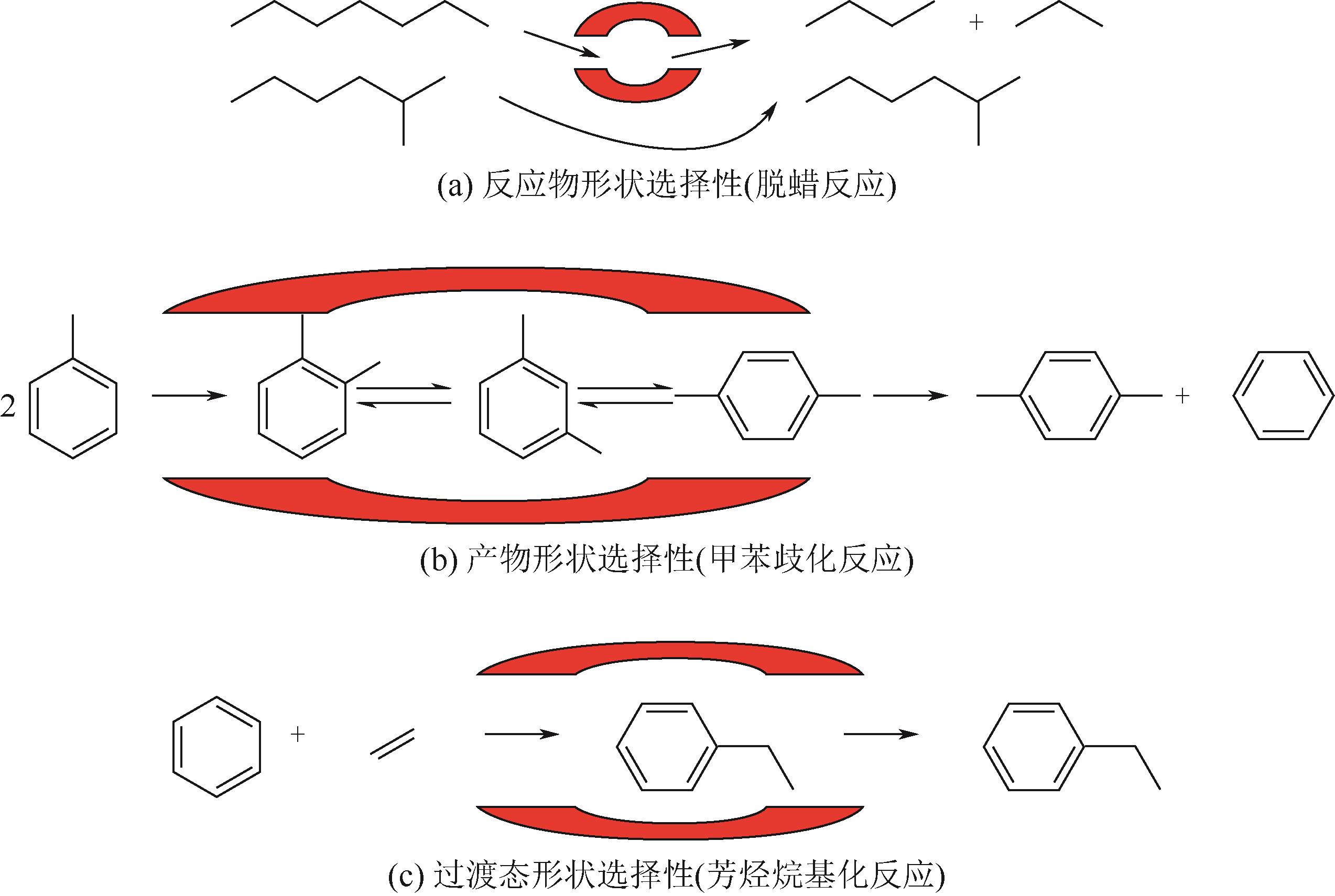
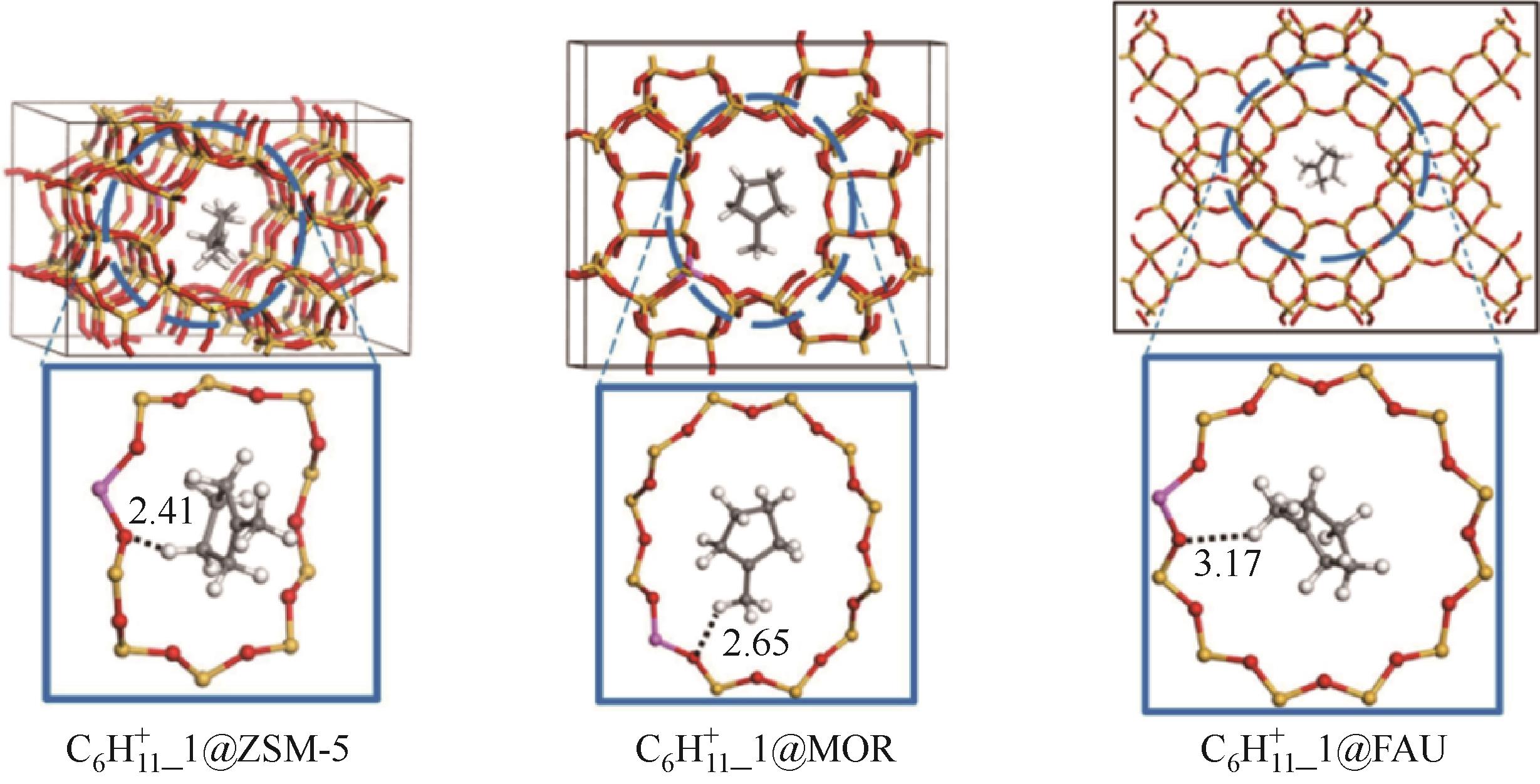
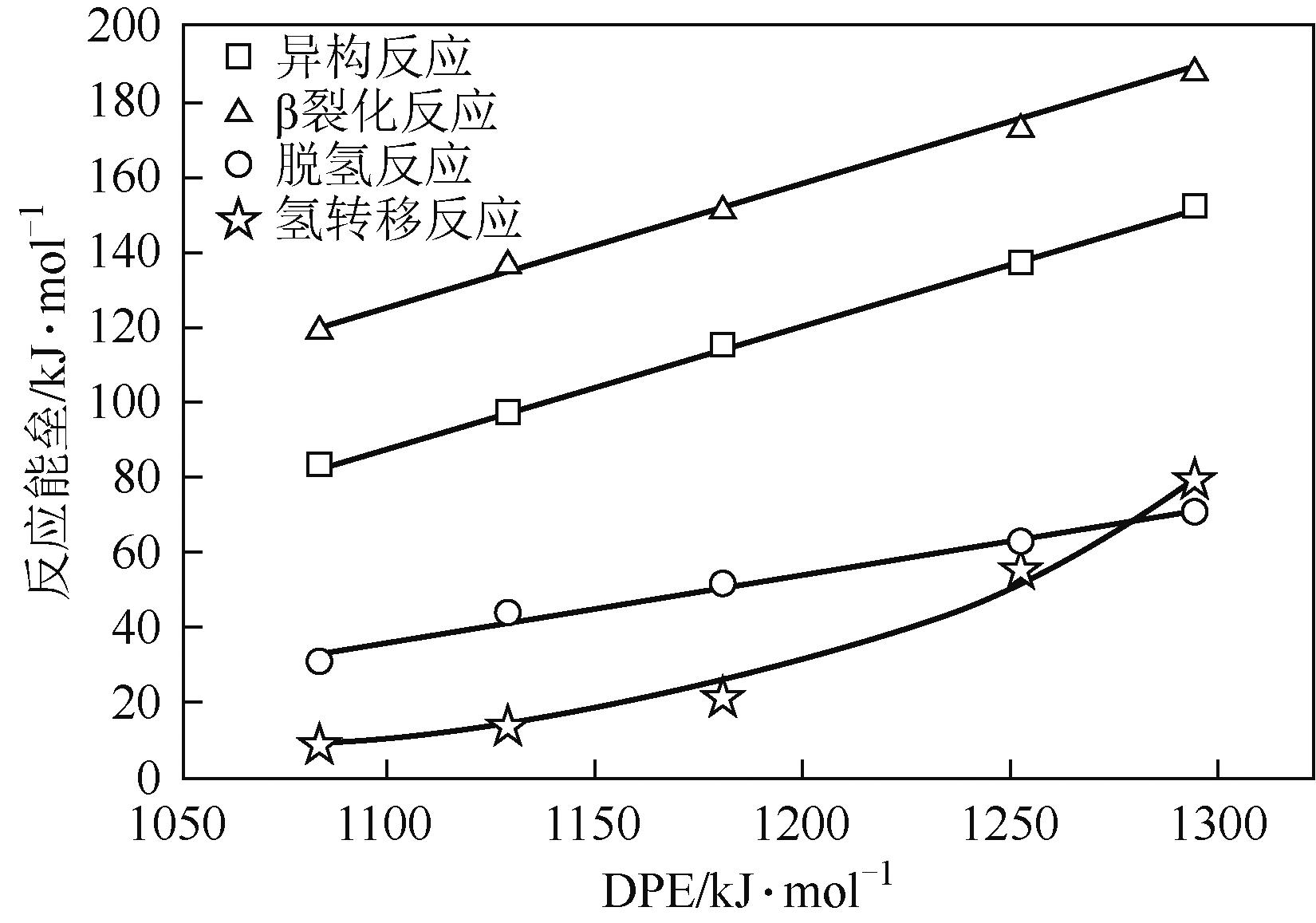
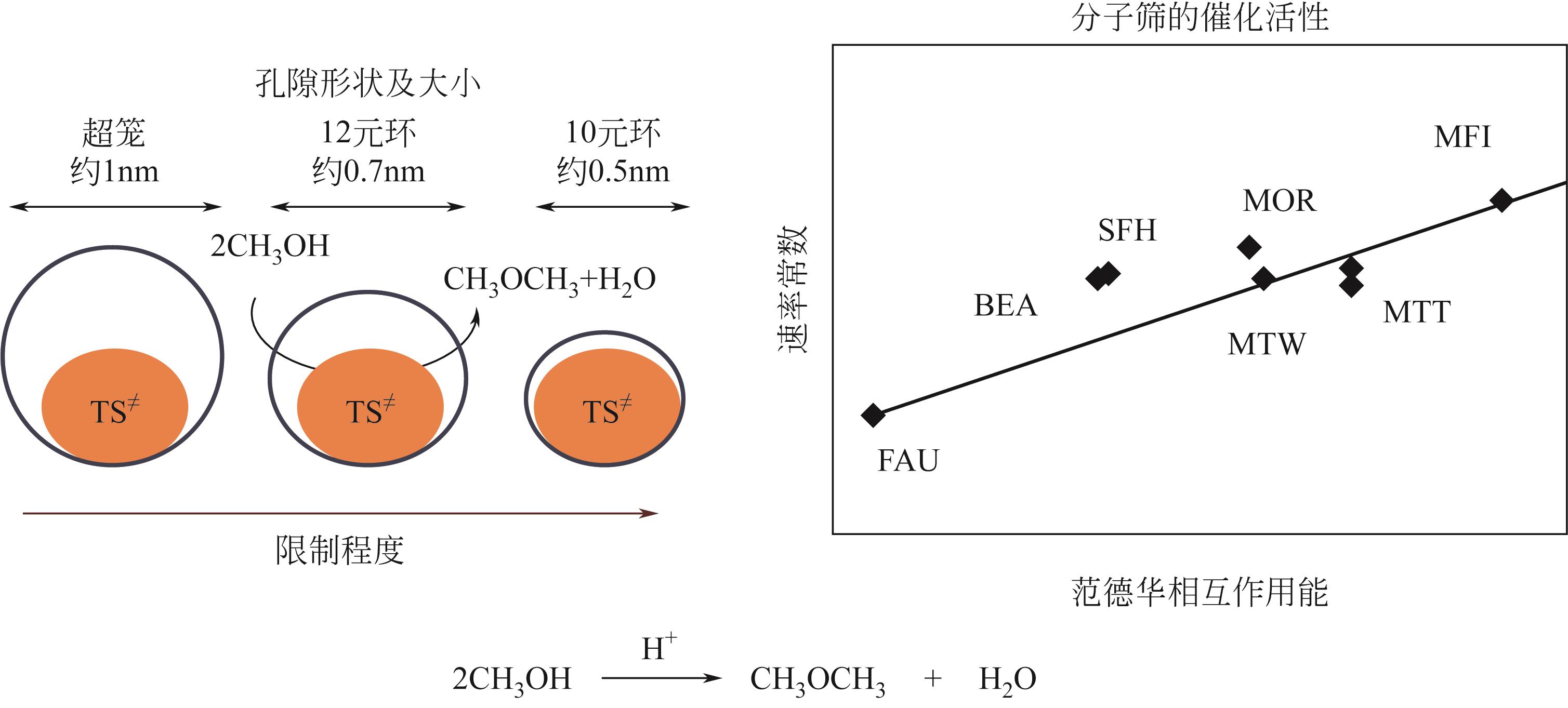
分子筛作为酸催化反应的重要载体,具有酸性可调控、热稳定性强及形状选择性的优点,但特殊刚性孔道结构及内部电荷分布使其具有限制效应会对分子筛的酸性表征及催化反应产生影响。由于分子筛中起催化作用的主要是Brønsted酸位点,因此,本文介绍了Brønsted酸位点及限制效应的形成机理,简述了限制效应对Brønsted酸位点酸强度及酸密度表征的影响,分析了限制效应中的空间约束及局部电场对催化反应的影响。指出在酸性表征中,空间约束限制探针分子对酸位点的可接近性,进一步会影响酸密度的测量。局部电场由于会影响探针分子的吸附与解吸,进而直接影响到酸强度。所以,在分子筛的酸性表征时应该选择与反应物尺寸相近及结构相似的探针分子,才能测量可接近Brønsted酸位点的酸密度与酸强度。在催化主导的热化学反应中,空间约束使分子筛具有形状选择性,通过控制分子筛孔径大小能对热化学反应的反应过程、中间产物过渡态及最终产物分布进行选择。同时,由于局部电场影响表观酸强度,分子筛催化性能与表观酸强度有关。分子筛孔径越小,反应分子所受范德华相互作用越大,通过影响反应过渡态的形成进而改变反应活化能,从而影响催化热化学反应效果。综合分析已有工作表明只有制备一个酸强度适宜、可接近孔径尺寸与反应物分子相近的分子筛才是催化反应的理想酸性载体。
中图分类号:
引用本文
汪孟宇, 范鸿霞, 梁长海, 李文英. 分子筛中限制效应对其酸性表征及催化性能的影响[J]. 化工进展, 2024, 43(5): 2600-2610.
WANG Mengyu, FAN Hongxia, LIANG Changhai, LI Wenying. Influence of zeolite confinement effect on its acidic characterization and catalytic performance[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2600-2610.
| 分子筛 | 反应能/kJ·mol-1 |
|---|---|
| ZSM-5 | -6.78 |
| MOR | -5.69 |
| FAU | 53.93 |
表1 甲基环戊烷正碳离子在三种分子筛上稳定的反应能
| 分子筛 | 反应能/kJ·mol-1 |
|---|---|
| ZSM-5 | -6.78 |
| MOR | -5.69 |
| FAU | 53.93 |
| 1 | TIAN Yijun, ZHANG Fangfang, WANG Jieni, et al. A review on solid acid catalysis for sustainable production of levulinic acid and levulinate esters from biomass derivatives[J]. Bioresource Technology, 2021, 342: 125977. |
| 2 | KERSTENS Dorien, SMEYERS Brent, VAN WAEYENBERG Jonathan, et al. State of the art and perspectives of hierarchical zeolites: Practical overview of synthesis methods and use in catalysis[J]. Advanced Materials, 2020, 32(44): e2004690. |
| 3 | VAN SPEYBROECK Veronique, HEMELSOET Karen, JOOS Lennart, et al. Advances in theory and their application within the field of zeolite chemistry[J]. Chemical Society Reviews, 2015, 44(20): 7044-7111. |
| 4 | ZHAO Ruixue, HALLER Gary L, LERCHER Johannes A. Alkene adsorption and cracking on acidic zeolites—A gradual process of understanding[J]. Microporous and Mesoporous Materials, 2023, 358: 112390. |
| 5 | LIU Peng, LIU Qian, LIU Wei, et al. Mechanistic insights into positional and skeletal isomerization of cyclohexene in the H-BEA zeolite[J]. Physical Chemistry Chemical Physics, 2022, 24(30): 18043-18054. |
| 6 | PARMAR Deependra, Seung Hyeok CHA, HUANG Chenfeng, et al. Impact of medium-pore zeolite topology on para-xylene production from toluene alkylation with methanol[J]. Catalysis Science & Technology, 2023, 13(18): 5227-5236. |
| 7 | XU Guangwen, BAI Dingrong, XU Chunming, et al. Challenges and opportunities for engineering thermochemistry in carbon-neutralization technologies[J]. National Science Review, 2023, 10(9): nwac217. |
| 8 | HAN Z, JIA X, SONG X, et al. Engineering thermochemistry to cope with challenges in carbon neutrality[J]. Journal of Cleaner Production, 2023, 416: 137943. |
| 9 | RAVI Manoj, SUSHKEVICH Vitaly L, VAN BOKHOVEN Jeroen A. Towards a better understanding of Lewis acidic aluminium in zeolites[J]. Nature Materials, 2020, 19: 1047-1056. |
| 10 | LI Shuo, CAO Jianlin, FENG Xiang, et al. Insights into the confinement effect on isobutane alkylation with C4 olefin catalyzed by zeolite catalyst: A combined theoretical and experimental study[J]. Chinese Journal of Chemical Engineering, 2022, 47: 174-184. |
| 11 | ALAITHAN Zainab A, Mallia Giuseppe, HARRISON Nicholas M. Monomolecular cracking of propane: Effect of zeolite confinement and acidity[J]. ACS Omega, 2022, 7(9): 7531-7540. |
| 12 | NIWA Miki, KATADA Naonobu. New method for the temperature- programmed desorption (TPD) of ammonia experiment for characterization of zeolite acidity: A review[J]. The Chemical Record, 2013, 13(5): 432-455. |
| 71 | TIWARI Santosh K, SAHOO Sumanta, WANG Nannan, et al. Graphene research and their outputs: Status and prospect[J]. Journal of Science: Advanced Materials and Devices, 2020, 5(1): 10-29. |
| 72 | SACHSE Alexander, Javier GARCÍA-MARTÍNEZ. Surfactant-templating of zeolites: From design to application[J]. Chemistry of Materials, 2017, 29(9): 3827-3853. |
| 13 | BUSCA Guido. Acidity and basicity of zeolites: A fundamental approach[J]. Microporous and Mesoporous Materials, 2017, 254: 3-16. |
| 14 | TAN Yangchun, HU Wenjing, DU Yanyan, et al. Species and impacts of metal sites over bifunctional catalyst on long chain n-alkane hydroisomerization: A review[J]. Applied Catalysis A: General, 2021, 611: 117916. |
| 15 | WANG Lei, CHEN Yujing, JIN Shaohua, et al. Selective ring-shift isomerization in hydroconversion of fluorene over supported platinum catalysts[J]. Energy & Fuels, 2016, 30(4): 3403-3412. |
| 16 | BRITO Larissa, PIRNGRUBER Gerhard D, GUILLON Emmanuelle, et al. Hydroconversion of perhydrophenanthrene over bifunctional Pt/H-USY zeolite catalyst[J]. ChemCatChem, 2020, 12(13): 3477-3488. |
| 17 | 刘道诚, 王九占, 荆洁颖, 等. 稠环芳烃加氢饱和催化剂研究进展[J]. 化工进展, 2021, 40(2): 835-844. |
| LIU Daocheng, WANG Jiuzhan, JING Jieying, et al. Research progress on the catalysts for saturated hydrogenation of polycyclic aromatic hydrocarbons[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 835-844. | |
| 18 | SCHMIERMUND Torsten. Acid-base theories[M]// The Chemistry Knowledge for Firefighters. Berlin, Heidelberg: Springer, 2023: 205-215. |
| 19 | CHIZALLET C, BOUCHY C, LARMIER K, et al. Molecular views on mechanisms of Brønsted acid-catalyzed reactions in zeolites[J]. Chemical Reviews, 2023, 123(9): 6107-6196. |
| 20 | PALČIĆ A, VALTCHEV V. Analysis and control of acid sites in zeolites [J]. Applied Catalysis A: General, 2020, 606: 117795. |
| 21 | TANG Xiaomin, LIU Zhiqiang, HUANG Ling, et al. Violation or abidance of löwenstein’s rule in zeolites under synthesis conditions?[J]. ACS Catalysis, 2019, 9(12): 10618-10625. |
| 22 | CHAI Yuchao, DAI Weili, WU Guangjun, et al. Confinement in a zeolite and zeolite catalysis[J]. Accounts of Chemical Research, 2021, 54(13): 2894-2904. |
| 23 | DEL CAMPO Pablo, Martínez Cristina, Corma Avelino. Activation and conversion of alkanes in the confined space of zeolite-type materials[J]. Chemical Society Reviews, 2021, 50(15): 8511-8595. |
| 24 | Fernanda ZALAZAR M, CABRAL Néstor Damián, ROMERO OJEDA Gonzalo D, et al. Confinement effects in protonation reactions catalyzed by zeolites with large void structures[J]. The Journal of Physical Chemistry C, 2018, 122(48): 27350-27359. |
| 25 | HIMMEL Daniel, RADTKE Valentin, BUTSCHKE Burkhard, et al. Basic remarks on acidity[J]. Angewandte Chemie (International Ed in English), 2018, 57(16): 4386-4411. |
| 26 | Derouane EG, Védrine JC, Ramos Pinto R, et al. The acidity of zeolites: Concepts, measurements and relation to catalysis: A review on experimental and theoretical methods for the study of zeolite acidity[J]. Catalysis Reviews, 2013, 55(4): 454-515. |
| 27 | Ferenc LÓNYI, József VALYON. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite[J]. Microporous and Mesoporous Materials, 2001, 47(2/3): 293-301. |
| 28 | MIAO Peipei, LI Kang, FAN Jiangtao, et al. Efficient ring-opening reaction of tetralin over nanosized ZSM-5 zeolite: Effect of SiO2/Al2O3 ratio and reaction condition[J]. Energy & Fuels, 2019, 33(10): 9480-9490. |
| 29 | HU Zhongpan, CHEN Lei, CHEN Chong, et al. Fe/ZSM-5 catalysts for ammonia decomposition to CO x -free hydrogen: Effect of SiO2/Al2O3 ratio[J]. Molecular Catalysis, 2018, 455: 14-22. |
| 30 | BEĆ Krzysztof Bernard, GRABSKA Justyna, HUCK Christian Wolfgang. Physical principles of infrared spectroscopy[M]//Comprehensive Analytical Chemistry. Amsterdam: Elsevier, 2022: 1-43. |
| 31 | YUAN P, WU D-Q, HE H-P, et al. The hydroxyl species and acid sites on diatomite surface: A combined IR and Raman study[J]. Applied Surface Science, 2004, 227(1/2/3/4): 30-39. |
| 32 | BORDIGA Silvia, LAMBERTI Carlo, BONINO Francesca, et al. Probing zeolites by vibrational spectroscopies[J]. Chemical Society Reviews, 2015, 44(20): 7262-7341. |
| 33 | GORTE R J. What do we know about the acidity of solid acids?[J]. Catalysis Letters, 1999, 62(1): 1-13. |
| 34 | BANERT Klaus, HAGEDORN Manfred, HECK Manuel, et al. Synthesis of trialkylamines with extreme steric hindrance and their decay by a hofmann-like elimination reaction[J]. The Journal of Organic Chemistry, 2020, 85(21): 13630-13643. |
| 35 | Luís-Ernesto SANDOVAL-DÍAZ, Jhon-Alex GONZÁLEZ-AMAYA, TRUJILLO Carlos-Alexander. General aspects of zeolite acidity characterization[J]. Microporous and Mesoporous Materials, 2015, 215: 229-243. |
| 36 | RAHMAN M, INFANTES-MOLINA A, HOFFMAN A S, et al. Effect of Si/Al ratio of ZSM-5 support on structure and activity of Mo species in methane dehydroaromatization [J]. Fuel, 2020, 278: 118290. |
| 37 | ABDELRAHMAN Omar A, VINTER Katherine P, REN Limin, et al. Simple quantification of zeolite acid site density by reactive gas chromatography[J]. Catalysis Science & Technology, 2017, 7(17): 3831-3841. |
| 38 | 孔维杰, 王焕, 张晓彤, 等. 超稳Y分子筛B酸中心可接近性的研究[J]. 石油炼制与化工, 2020, 51(8): 70-75. |
| KONG Weijie, WANG Huan, ZHANG Xiaotong, et al. Accessibility study of Brønsted acid site in USY zeolite[J]. Petroleum Processing and Petrochemicals, 2020, 51(8): 70-75. | |
| 39 | KIM Kyoungsoo, RYOO Ryong, JANG Hee-Dong, et al. Spatial distribution, strength, and dealumination behavior of acid sites in nanocrystalline MFI zeolites and their catalytic consequences[J]. Journal of Catalysis, 2012, 288: 115-123. |
| 40 | KIM Myoung Yeob, LEE Kyungho, CHOI Minkee. Cooperative effects of secondary mesoporosity and acid site location in Pt/SAPO-11 on n-dodecane hydroisomerization selectivity[J]. Journal of Catalysis, 2014, 319: 232-238. |
| 41 | LOSCH P, JOSHI H R, VOZNIUK O, et al. Proton mobility, intrinsic acid strength, and acid site location in zeolites revealed by varying temperature infrared spectroscopy and density functional theory studies[J]. Journal of the American Chemical Society, 2018, 140(50): 17790-17799. |
| 42 | ZHANG X, LI H, DU Y, et al. Elucidating effect of acid strength on isomerization mechanisms of butene over solid acid catalysts in C4 alkylation [J]. Fuel, 2023, 339: 127397. |
| 43 | PANG Tingting, YANG Xuanyu, YUAN Chenyi, et al. Recent advance in synthesis and application of heteroatom zeolites[J]. Chinese Chemical Letters, 2021, 32(1): 328-338. |
| 44 | YU W, WU X, CHENG B, et al. Synthesis and applications of SAPO-34 zeolite[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2022, 28(11): e202102787. |
| 45 | TANDON Hiteshi, CHAKRABORTY Tanmoy, SUHAG Vandana. A scale of atomic electronegativity in terms of atomic nucleophilicity index[J]. Foundations of Chemistry, 2020, 22(2): 335-346. |
| 46 | LI Li, SHEN Kaixu, HUANG Xin, et al. SAPO-11 with preferential growth along the a-direction as an improved active catalyst in long-alkane isomerization reaction[J]. Microporous and Mesoporous Materials, 2021, 313: 110827. |
| 47 | 叶蔚甄, 任强, 赵毅, 等. Al原子分布对Y型分子筛酸强度的影响[J]. 石油学报(石油加工), 2021, 37(3): 566-571. |
| YE Weizhen, REN Qiang, ZHAO Yi, et al. Effects of Al atom distribution on acid strength of Y zeolite[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(3): 566-571. | |
| 48 | WANG Sen, HE Yue, JIAO Weiyong, et al. Recent experimental and theoretical studies on Al siting/acid site distribution in zeolite framework[J]. Current Opinion in Chemical Engineering, 2019, 23: 146-154. |
| 49 | NYSTROM Steven, HOFFMAN Alexander, HIBBITTS David. Tuning Brønsted acid strength by altering site proximity in CHA framework zeolites[J]. ACS Catalysis, 2018, 8(9): 7842-7860. |
| 50 | BORONAT Mercedes, CORMA Avelino. What is measured when measuring acidity in zeolites with probe molecules?[J]. ACS Catalysis, 2019, 9(2): 1539-1548. |
| 51 | GRIFONI Emanuele, PICCINI Giovanni Maria, LERCHER Johannes A, et al. Confinement effects and acid strength in zeolites[J]. Nature Communications, 2021, 12: 2630. |
| 52 | XIAO Yao, CHEN Wei, YI Xianfeng, et al. Confinement-driven “flexible” acidity properties of porous zeolite catalysts with varied probe-assisted solid-state NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2021, 125(21): 11580-11590. |
| 53 | SHAMZHY Mariya, OPANASENKO Maksym, Patricia CONCEPCIÓN, et al. New trends in tailoring active sites in zeolite-based catalysts[J]. Chemical Society Reviews, 2019, 48(4): 1095-1149. |
| 54 | SMIT Berend, MAESEN Theo L M. Towards a molecular understanding of shape selectivity[J]. Nature, 2008, 451: 671-678. |
| 55 | DEGNAN Thomas F Jr. The implications of the fundamentals of shape selectivity for the development of catalysts for the petroleum and petrochemical industries[J]. Journal of Catalysis, 2003, 216(1/2): 32-46. |
| 56 | FAN Chi, CHEN Zhen, PANG Lei, et al. The influence of Si/Al ratio on the catalytic property and hydrothermal stability of Cu-SSZ-13 catalysts for NH3-SCR[J]. Applied Catalysis A: General, 2018, 550: 256-265. |
| 57 | 向江南, 刘伟, 刘成连, 等. 低硅铝比ZSM-48分子筛合成及其正构十二烷临氢异构催化性能研究[J]. 燃料化学学报, 2020, 48(1): 83-90. |
| XIANG Jiangnan, LIU Wei, LIU Chenglian, et al. Synthesis and hydroisomerization performance of n-C12 over ZSM-48 molecular sieve with low silicon-aluminum ratio[J]. Journal of Fuel Chemistry and Technology, 2020, 48(1): 83-90. | |
| 58 | REN Qinghua, RYBICKI Marcin, SAUER Joachim. Interaction of C3—C5 alkenes with zeolitic Brønsted sites: π-complexes, alkoxides, and carbenium ions in H-FER[J]. The Journal of Physical Chemistry C, 2020, 124(18): 10067-10078. |
| 59 | CHEN Wei, YI Xianfeng, LIU Zhiqiang, et al. Carbocation chemistry confined in zeolites: Spectroscopic and theoretical characterizations[J]. Chemical Society Reviews, 2022, 51(11): 4337-4385. |
| 60 | LIU Jianwen, YIN Yaru, FU Xianzhu, et al. Stability of C3—C6 carbonium ions inside zeolites: A first principles study[J]. Applied Surface Science, 2020, 503: 144148. |
| 61 | 付佳, 冯翔, 刘熠斌, 等. Brønsted酸强度对正碳离子转化方向影响的分子模拟[J]. 化工学报, 2018, 69(2): 725-732. |
| FU Jia, FENG Xiang, LIU Yibin, et al. Influence of Brønsted acid strength on conversion of carbenium ion by molecular simulation[J]. CIESC Journal, 2018, 69(2): 725-732. | |
| 62 | XU B, SIEVERS C, HONG S, et al. Catalytic activity of Brønsted acid sites in zeolites: Intrinsic activity, rate-limiting step, and influence of the local structure of the acid sites[J]. Journal of Catalysis, 2006, 244(2): 163-168. |
| 63 | GOUNDER Rajamani, IGLESIA Enrique. Catalytic consequences of spatial constraints and acid site location for monomolecular alkane activation on zeolites[J]. Journal of the American Chemical Society, 2009, 131(5): 1958-1971. |
| 64 | JONES Andrew J, ZONES Stacey I, IGLESIA Enrique. Implications of transition state confinement within small voids for acid catalysis[J]. The Journal of Physical Chemistry C, 2014, 118(31): 17787-17800. |
| 65 | HERNANDO Héctor, HERNÁNDEZ-GIMÉNEZ Ana M, Cristina OCHOA-HERNÁNDEZ, et al. Engineering the acidity and accessibility of the zeolite ZSM-5 for efficient bio-oil upgrading in catalytic pyrolysis of lignocellulose[J]. Green Chemistry, 2018, 20(15): 3499-3511. |
| 66 | LI Ming, ZHANG Yadong, WANG Hao, et al. Influence of zeolite crystal size on selective conversion of n-alkane: Controlling intermediates’ diffusion distances inside the micropores[J]. Fuel, 2019, 254: 115709. |
| 67 | ZECEVIC Jovana, VANBUTSELE Gina, DE JONG Krijn P, et al. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons[J]. Nature, 2015, 528: 245-248. |
| 68 | BRITO Larissa, PIRNGRUBER Gerhard D, Javier PEREZ-PELLITERO, et al. Shape selectivity effects in the hydroconversion of perhydrophenanthrene over bifunctional catalysts[J]. Catalysis Science & Technology, 2021, 11(23): 7667-7682. |
| 69 | GUO Zhongyuan, LI Xin, HU Shen, et al. Understanding the role of internal diffusion barriers in Pt/beta zeolite catalyzed isomerization of n-heptane[J]. Angewandte Chemie (International Ed in English), 2020, 59(4): 1548-1551. |
| 70 | GUISNET Michel. “Ideal” bifunctional catalysis over Pt-acid zeolites[J]. Catalysis Today, 2013, 218/219: 123-134. |
| [1] | 姚乃瑜, 曹景沛, 庞新博, 赵小燕, 蔡士杰, 徐敏, 赵静平, 冯晓博, 伊凤娇. 低阶煤热解挥发分热催化重整研究进展[J]. 化工进展, 2024, 43(5): 2279-2293. |
| [2] | 张国卿, 宋舒波, 王兴瑞, 巩苗苗, 王旭, 许宇鸿, 冯继越, 张福扬, 陈汇勇. 煤固废基分子筛的制备及其应用进展[J]. 化工进展, 2024, 43(5): 2311-2323. |
| [3] | 吴达, 蒋淑娇, 魏强, 袁胜华, 杨刚, 张成. 能源转型中渣油高效利用技术的研究进展[J]. 化工进展, 2024, 43(5): 2343-2353. |
| [4] | 桂鑫, 陈汇勇, 白柏杨, 贾永梁, 马晓迅. Mo掺杂改性NiC/Al-MCM-41的芘催化加氢性能[J]. 化工进展, 2024, 43(5): 2386-2395. |
| [5] | 丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448. |
| [6] | 刘苗, 焦莹莹, 丁玲, 李城城, 何颖, 孙亮亮, 郝青青, 陈汇勇, 罗群兴. 酸催化己糖脱水合成5-羟甲基糠醛:反应、分离和过程耦合[J]. 化工进展, 2024, 43(5): 2526-2543. |
| [7] | 陈科宇, 徐金鑫, 吴桂波, 杨哲, 陈嘉鸿, 陈永利. 绿氨产业现状及发展展望[J]. 化工进展, 2024, 43(5): 2544-2553. |
| [8] | 段翔, 田野, 董文威, 宋松, 李新刚. 苯酐合成的反应网络及催化反应机制研究现状与展望[J]. 化工进展, 2024, 43(5): 2587-2599. |
| [9] | 王冰, 王磊, 黄欣茹, 袁红鹏, 赖小娟, 李朋. 一种耐酸耐碱高强树脂的合成及性能[J]. 化工进展, 2024, 43(4): 1992-2000. |
| [10] | 薛云娇, 张璇, 刘洋, 陈玉焕, 房静, 杨芳. 伪蛋白生物材料的分类、合成及其应用[J]. 化工进展, 2024, 43(4): 2001-2016. |
| [11] | 刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741. |
| [12] | 王红妍, 马子然, 李歌, 马静, 赵春林, 周佳丽, 王磊, 彭胜攀. 燃煤耦合可再生燃料烟气多污染物协同催化脱除研究进展[J]. 化工进展, 2024, 43(4): 1783-1795. |
| [13] | 陈家一, 高帷韬, 阴亚楠, 王诚, 欧阳鸿武, 毛宗强. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| [14] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [15] | 刘雨蓉, 王兴宝, 李文英. 分子筛负载Pt催化剂酸性位点调控及对蒽深度加氢性能的影响[J]. 化工进展, 2024, 43(4): 1832-1839. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||