化工进展 ›› 2024, Vol. 43 ›› Issue (11): 6397-6411.DOI: 10.16085/j.issn.1000-6613.2023-1765
• 资源与环境化工 • 上一篇
胺吸收剂逃逸引发的大气气相反应进展
陆诗建1,2( ), 祝文举1,2(
), 祝文举1,2( ), 刘玲1,2, 康国俊1,2, 陈思铭1
), 刘玲1,2, 康国俊1,2, 陈思铭1
- 1.中国矿业大学碳中和研究院,江苏 徐州 221116
2.中国矿业大学化工学院,江苏 徐州 221116
-
收稿日期:2023-10-10修回日期:2024-03-04出版日期:2024-11-15发布日期:2024-12-07 -
通讯作者:陆诗建 -
作者简介:陆诗建(1984—),男,博士,研究员,研究方向为CCUS与废气治理技术。E-mail:lushijian@cumt.edu.cn
祝文举(2000—),男,硕士研究生,研究方向为CCUS与废气治理技术。E-mail:TS22040155P31@cumt.edu.cn。 -
基金资助:中央高校基本科研业务费专项(XJ2022000501);江苏省科技厅科技项目-碳达峰碳中和科技创新专项(BE2022613)
Advances in atmospheric gas-phase reactions initiated by amine absorbent escape
LU Shijian1,2( ), ZHU Wenju1,2(
), ZHU Wenju1,2( ), LIU Ling1,2, KANG Guojun1,2, CHEN Siming1
), LIU Ling1,2, KANG Guojun1,2, CHEN Siming1
- 1.Carbon Neutral Research Institute, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
2.School of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2023-10-10Revised:2024-03-04Online:2024-11-15Published:2024-12-07 -
Contact:LU Shijian
摘要:
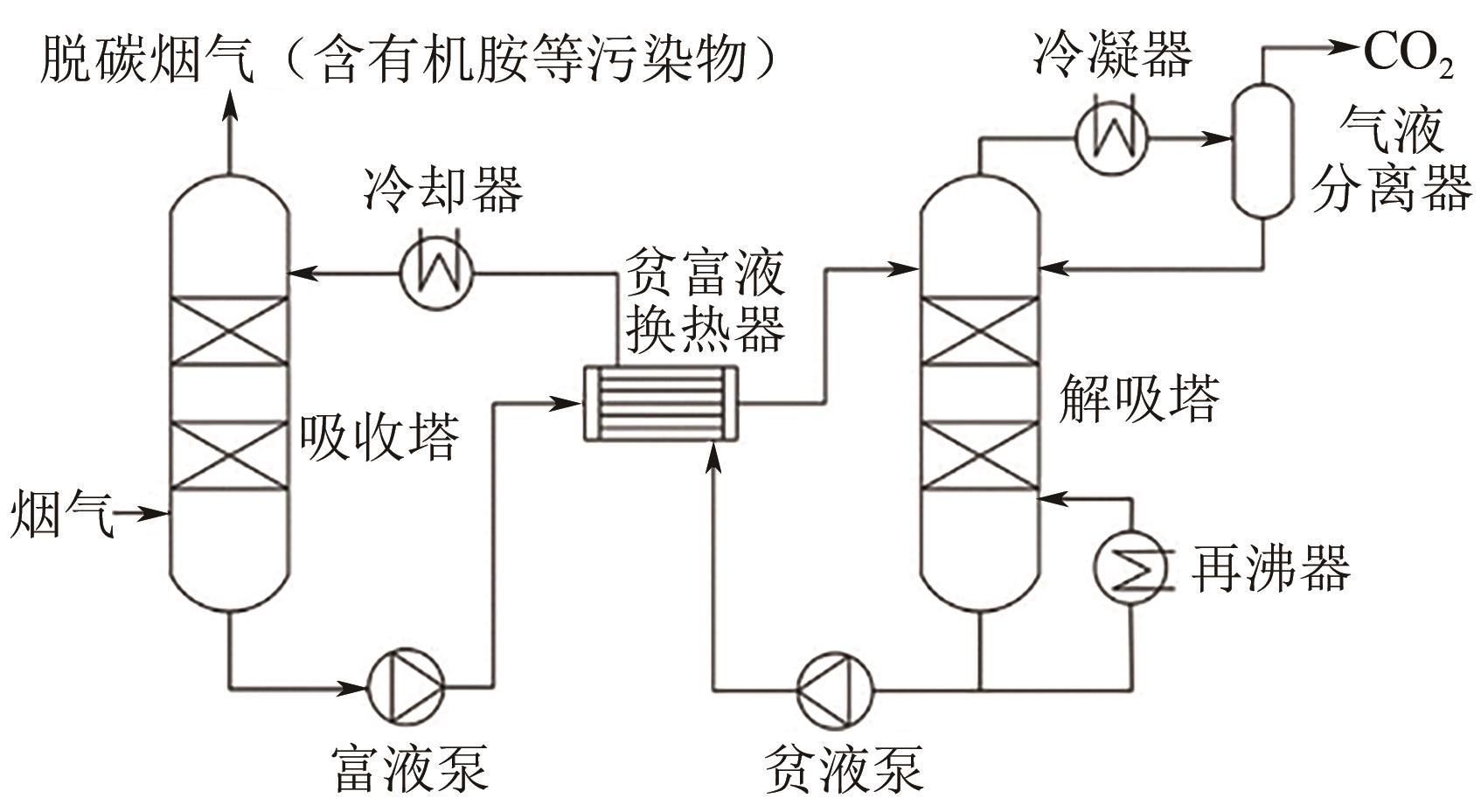
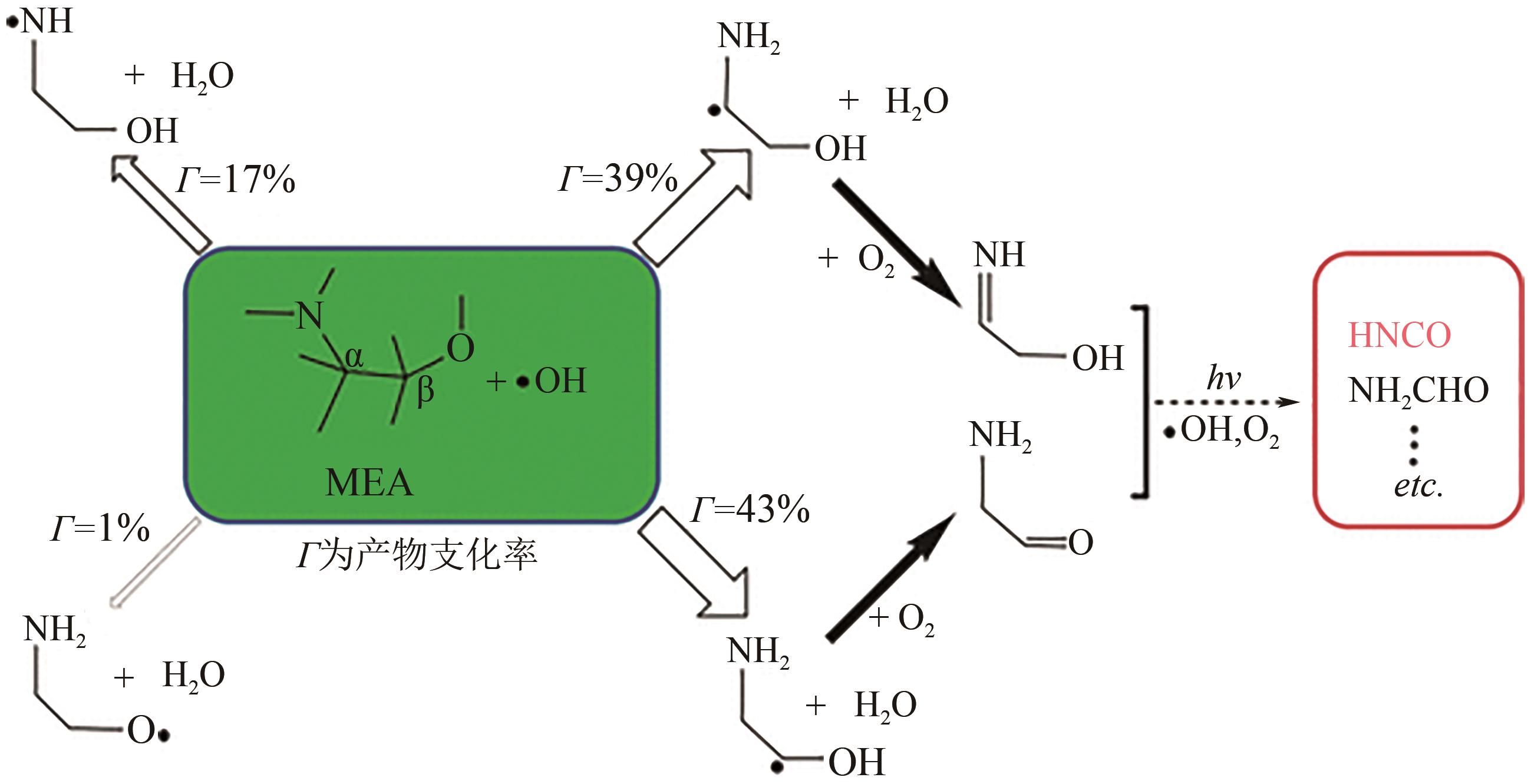
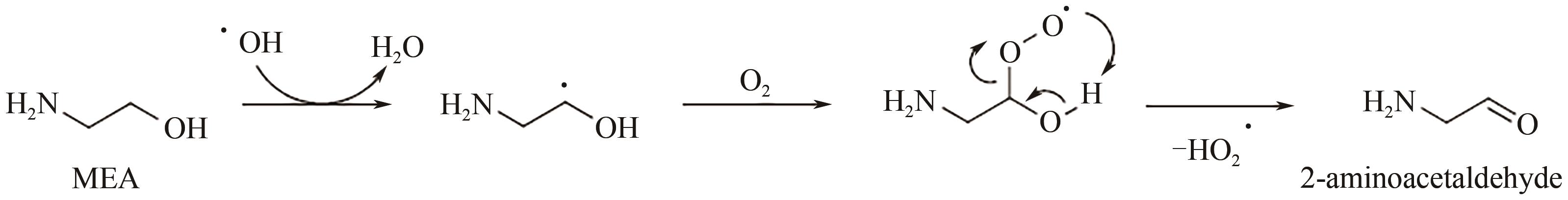
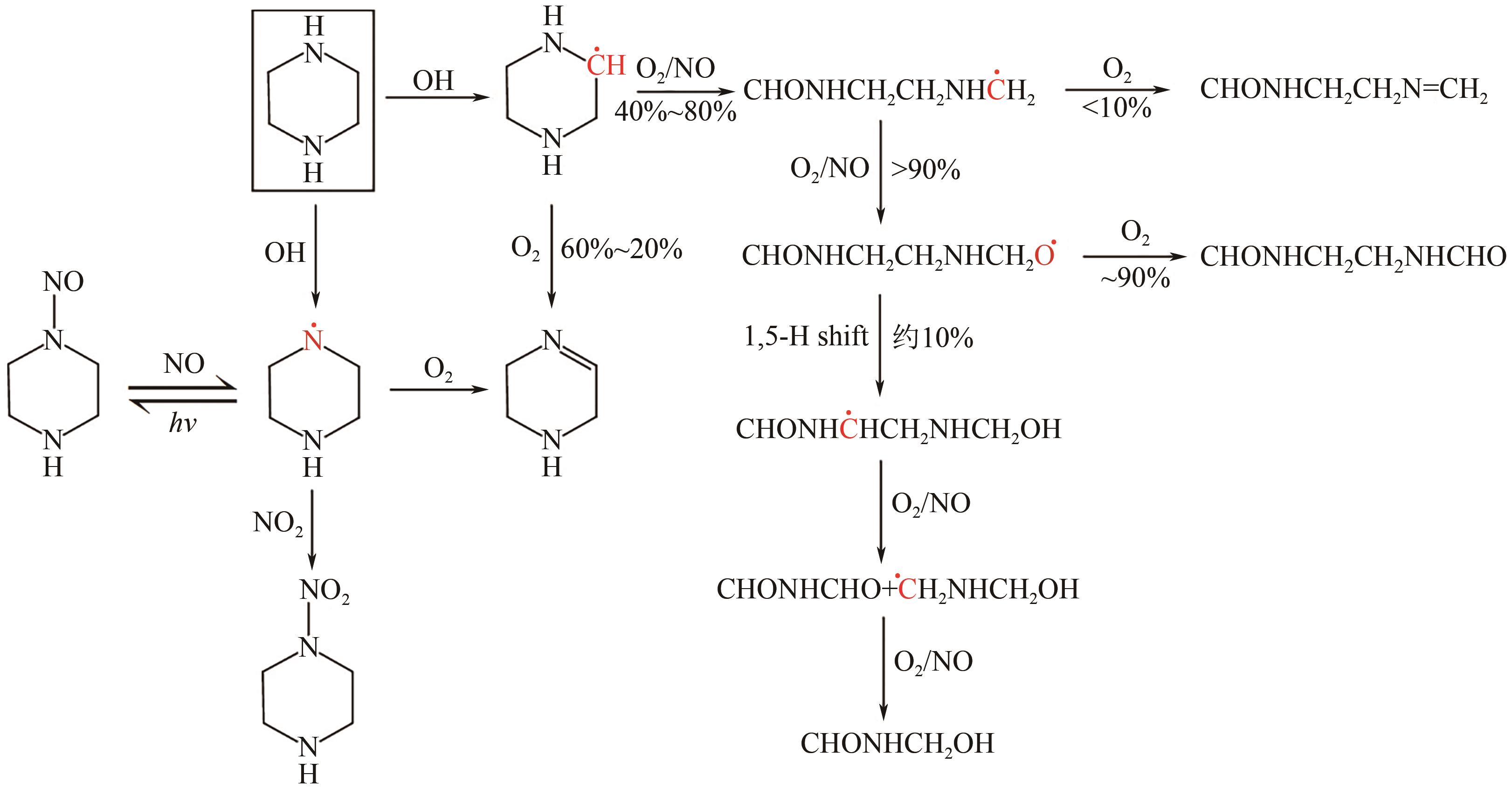
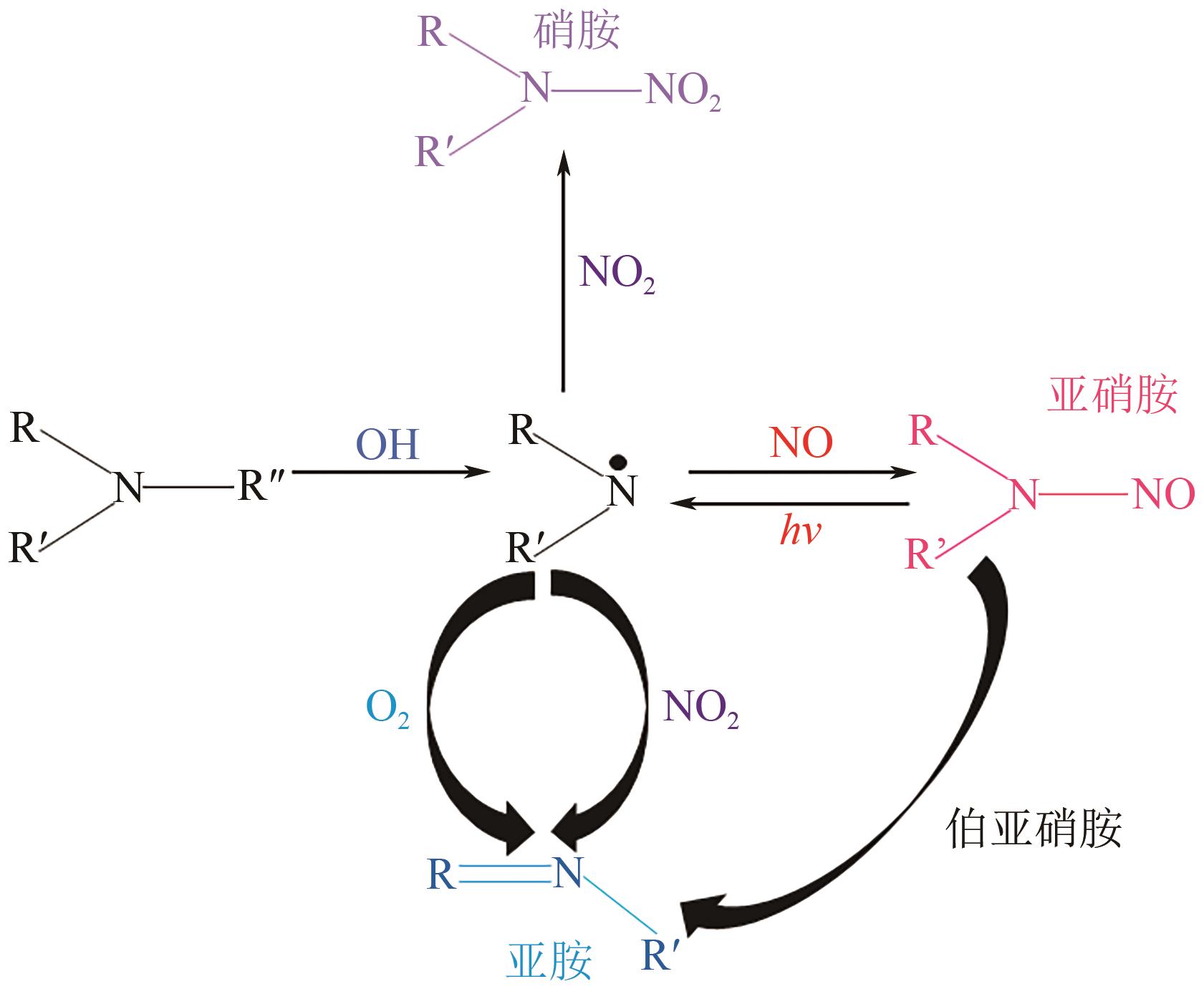
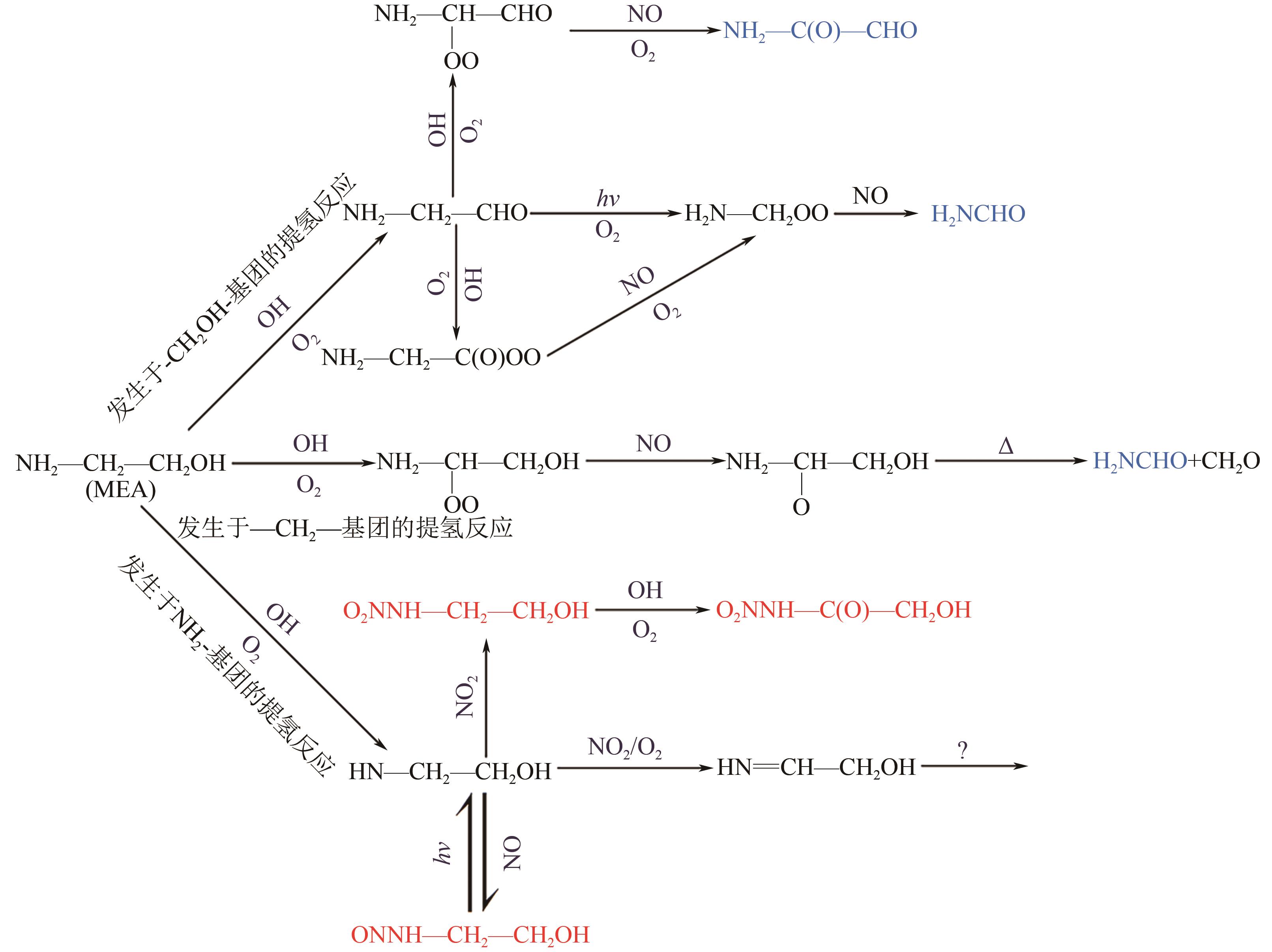
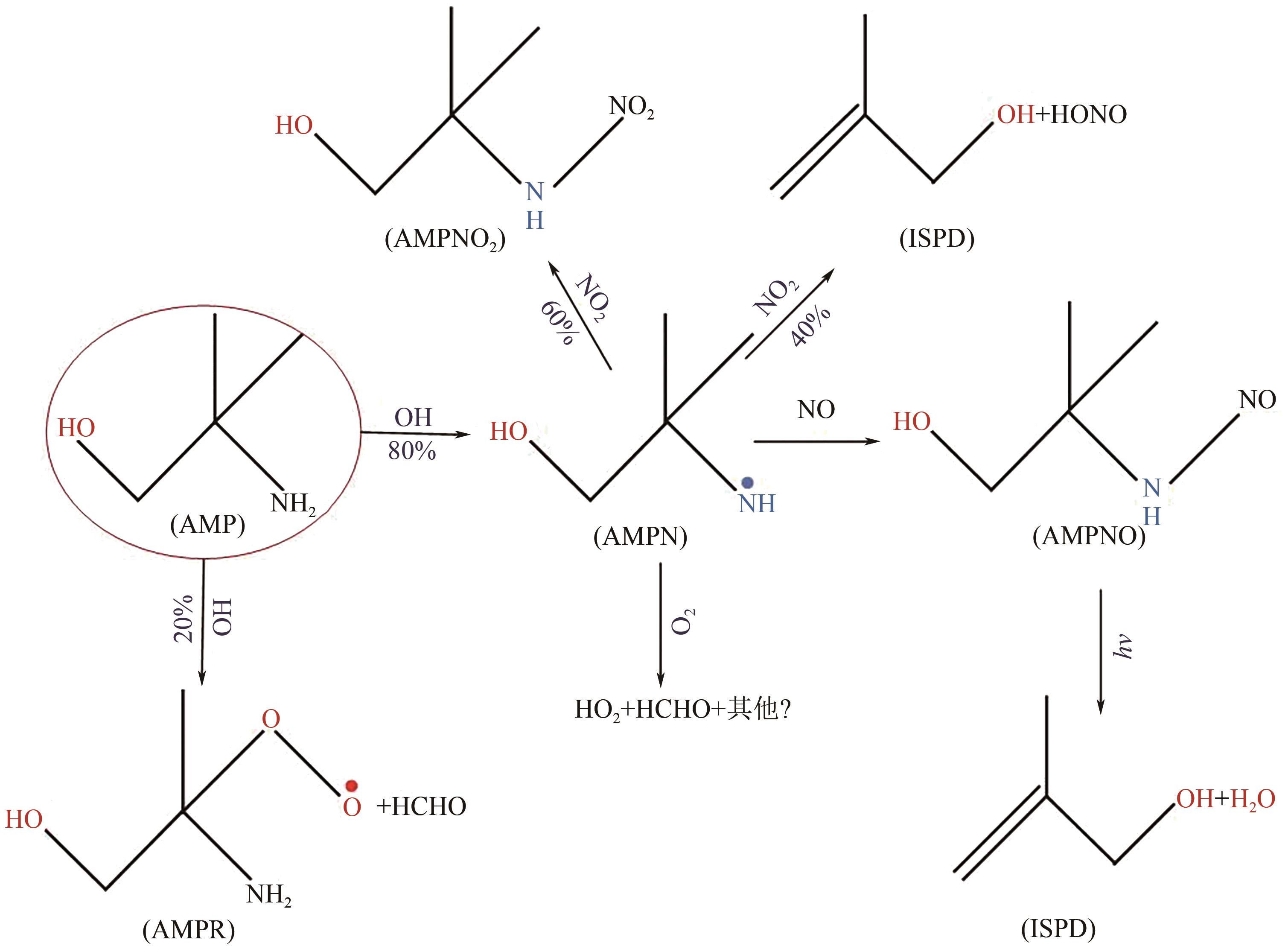
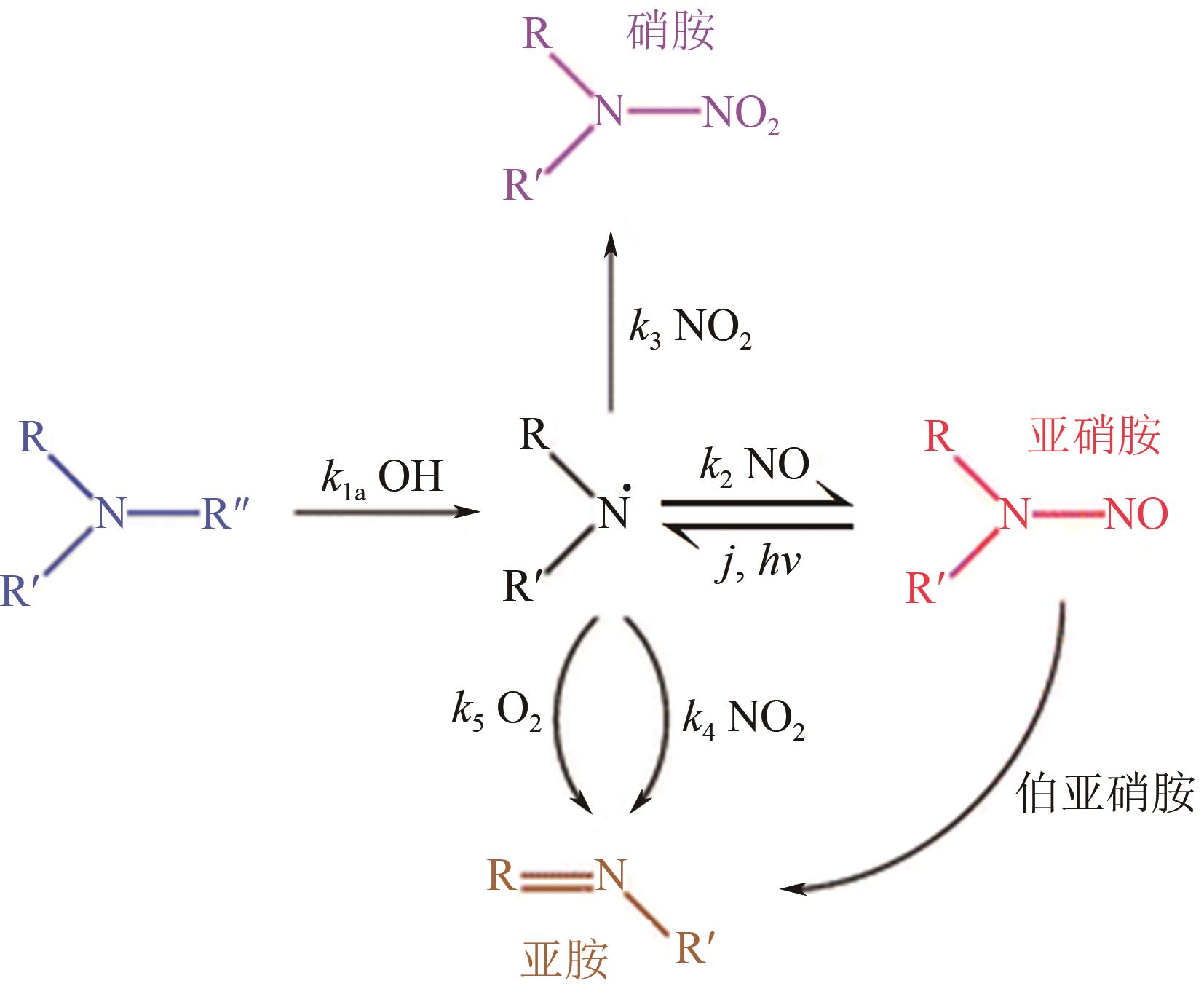
碳捕集化学吸收技术是目前最具潜力的减少发电厂及其他使用化石燃料行业CO2排放的技术,但碳捕集化学吸收系统带来的胺吸收剂逃逸排放会引发一系列大气化学反应。碳捕集化学系统运行中胺吸收剂会以液滴、气态、气溶胶颗粒形式排向大气进而引发大气气相反应。胺吸收剂与OH自由基反应主要涉及—CH2基团、C—H键之间的H-抽提反应,少量在N—H键之间发生,极少部分在—OH基团之间发生,生成亚胺、醛类等;与Cl自由基反应涉及—CH2基团、—NH2基团、—OH基团,生成氮氧化物、HCl等;与NO x 反应包含氨基或烷基的抽氢反应,与不饱和化合物的加成反应生成硝酸、硝胺、亚硝胺等。胺与臭氧的反应主要产生酰胺、异氰酸、亚硝基化合物等。这些化合物对大气环境和人体健康都具有潜在的危害,可能对空气质量和生态系统造成严重影响,甚至引发癌症等健康问题。国内外对碳捕集排放限值皆有规定,但对总胺排放限定缺乏标准。尽管胺排放控制取得一些进展,但小颗粒气溶胶的脱除效率低、适用局限、工艺复杂、能耗高等问题亟待解决。
中图分类号:
引用本文
陆诗建, 祝文举, 刘玲, 康国俊, 陈思铭. 胺吸收剂逃逸引发的大气气相反应进展[J]. 化工进展, 2024, 43(11): 6397-6411.
LU Shijian, ZHU Wenju, LIU Ling, KANG Guojun, CHEN Siming. Advances in atmospheric gas-phase reactions initiated by amine absorbent escape[J]. Chemical Industry and Engineering Progress, 2024, 43(11): 6397-6411.
| 排放类型 | 形态 | 产生原因 | 吸收塔后胺排放数量级/μL·L-1 |
|---|---|---|---|
| 物理夹带 | 液滴D≥100μm | 气液接触时部分吸收剂被气流携带 | <1 |
| 气体 | 气态 | 吸收剂及其降解产物挥发 | 0~102 |
| 气溶胶 | 气溶胶颗粒100μm>D>1nm | 吸收塔内非均相成核、均相成核、气泡破裂等 | 0~103 |
表1 CO2化学吸收系统胺排放类型[9]
| 排放类型 | 形态 | 产生原因 | 吸收塔后胺排放数量级/μL·L-1 |
|---|---|---|---|
| 物理夹带 | 液滴D≥100μm | 气液接触时部分吸收剂被气流携带 | <1 |
| 气体 | 气态 | 吸收剂及其降解产物挥发 | 0~102 |
| 气溶胶 | 气溶胶颗粒100μm>D>1nm | 吸收塔内非均相成核、均相成核、气泡破裂等 | 0~103 |
| 来源 | 报道的排放浓度/mg·m-3 | 组分 |
|---|---|---|
| IEAGHG技术报告试验厂[ | 0.5~3 | MEA |
| Niederaussem中试工厂[ | 0.02~0.03 | MEA |
| CESAR中试工厂[ | <0.3 | MEA |
| ENEL中试工厂[ | 1.2~1.5 | MEA(WESP运行与否) |
| Maasvlakte火电站CO2捕集中试厂,1500m3/h[ | 0.97~4 | MEA(除雾器后) |
| 206 | MEA(水洗塔后) | |
| 336~460 | MEA(吸收塔后) | |
| Loy Yang中试工厂[ | 2.4 | MEA |
| KIT中试火电厂CO2捕集系统,180m3/h[ | 3000 | MEA |
| TNO微型移动捕集试验厂,4m3/h[ | 100~200 | MEA(进口烟气含有烟尘) |
| 600~1100 | MEA(进口烟气含有SO3) | |
| TNO微型移动捕集试验厂,4m3/h[ | 1000~1900 | MEA(贫液温度40~80℃)) |
| 100~2940 | AMP(贫液pH=9.4~11.0,硫酸气溶胶浓度变化) | |
| 0~416 | PZ(CO2体积分数0.7%~13%,硫酸气溶胶浓度变化) | |
| TNO微型移动捕集试验厂,4m3/h[ | 383 1051 | MEA(进口烟气无SO3) MEA(进口烟气SO3浓度5.25μL/L) |
表2 胺吸收剂排放状况表
| 来源 | 报道的排放浓度/mg·m-3 | 组分 |
|---|---|---|
| IEAGHG技术报告试验厂[ | 0.5~3 | MEA |
| Niederaussem中试工厂[ | 0.02~0.03 | MEA |
| CESAR中试工厂[ | <0.3 | MEA |
| ENEL中试工厂[ | 1.2~1.5 | MEA(WESP运行与否) |
| Maasvlakte火电站CO2捕集中试厂,1500m3/h[ | 0.97~4 | MEA(除雾器后) |
| 206 | MEA(水洗塔后) | |
| 336~460 | MEA(吸收塔后) | |
| Loy Yang中试工厂[ | 2.4 | MEA |
| KIT中试火电厂CO2捕集系统,180m3/h[ | 3000 | MEA |
| TNO微型移动捕集试验厂,4m3/h[ | 100~200 | MEA(进口烟气含有烟尘) |
| 600~1100 | MEA(进口烟气含有SO3) | |
| TNO微型移动捕集试验厂,4m3/h[ | 1000~1900 | MEA(贫液温度40~80℃)) |
| 100~2940 | AMP(贫液pH=9.4~11.0,硫酸气溶胶浓度变化) | |
| 0~416 | PZ(CO2体积分数0.7%~13%,硫酸气溶胶浓度变化) | |
| TNO微型移动捕集试验厂,4m3/h[ | 383 1051 | MEA(进口烟气无SO3) MEA(进口烟气SO3浓度5.25μL/L) |
| 胺吸收剂种类 | 结构 | kOH/cm3·mol-1·s-1 |
|---|---|---|
| MEA | 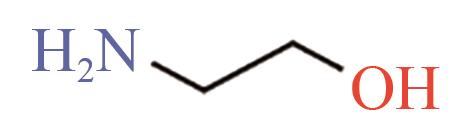 | (7.61±0.76)×10-11[ (9.2±1.1)×10-11[ (7.02±0.46)×10-11[ |
| DMEA | 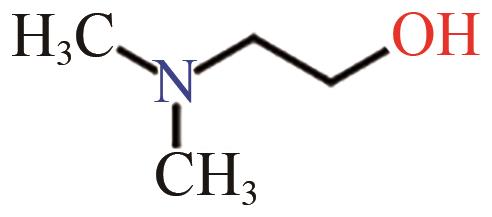 | (9.0±2.0)×10-11[ (4.7±1.2)×10-11[ (7.29±0.72)×10-11[ |
| AMP | 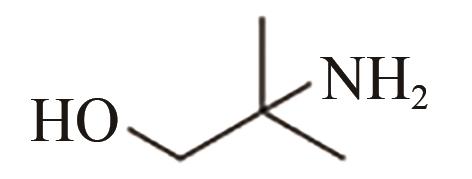 | (2.8±0.5)×10-11[ |
| PZ | 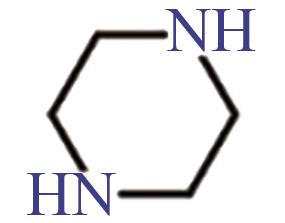 | (2.8±0.6)×10-10[ |
| MMEA | 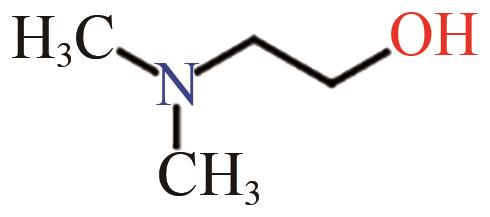 | (8.26±0.82)×10-11[ |
表3 常温下OH自由基与胺吸收剂的实验速率常数
| 胺吸收剂种类 | 结构 | kOH/cm3·mol-1·s-1 |
|---|---|---|
| MEA |  | (7.61±0.76)×10-11[ (9.2±1.1)×10-11[ (7.02±0.46)×10-11[ |
| DMEA |  | (9.0±2.0)×10-11[ (4.7±1.2)×10-11[ (7.29±0.72)×10-11[ |
| AMP |  | (2.8±0.5)×10-11[ |
| PZ |  | (2.8±0.6)×10-10[ |
| MMEA |  | (8.26±0.82)×10-11[ |

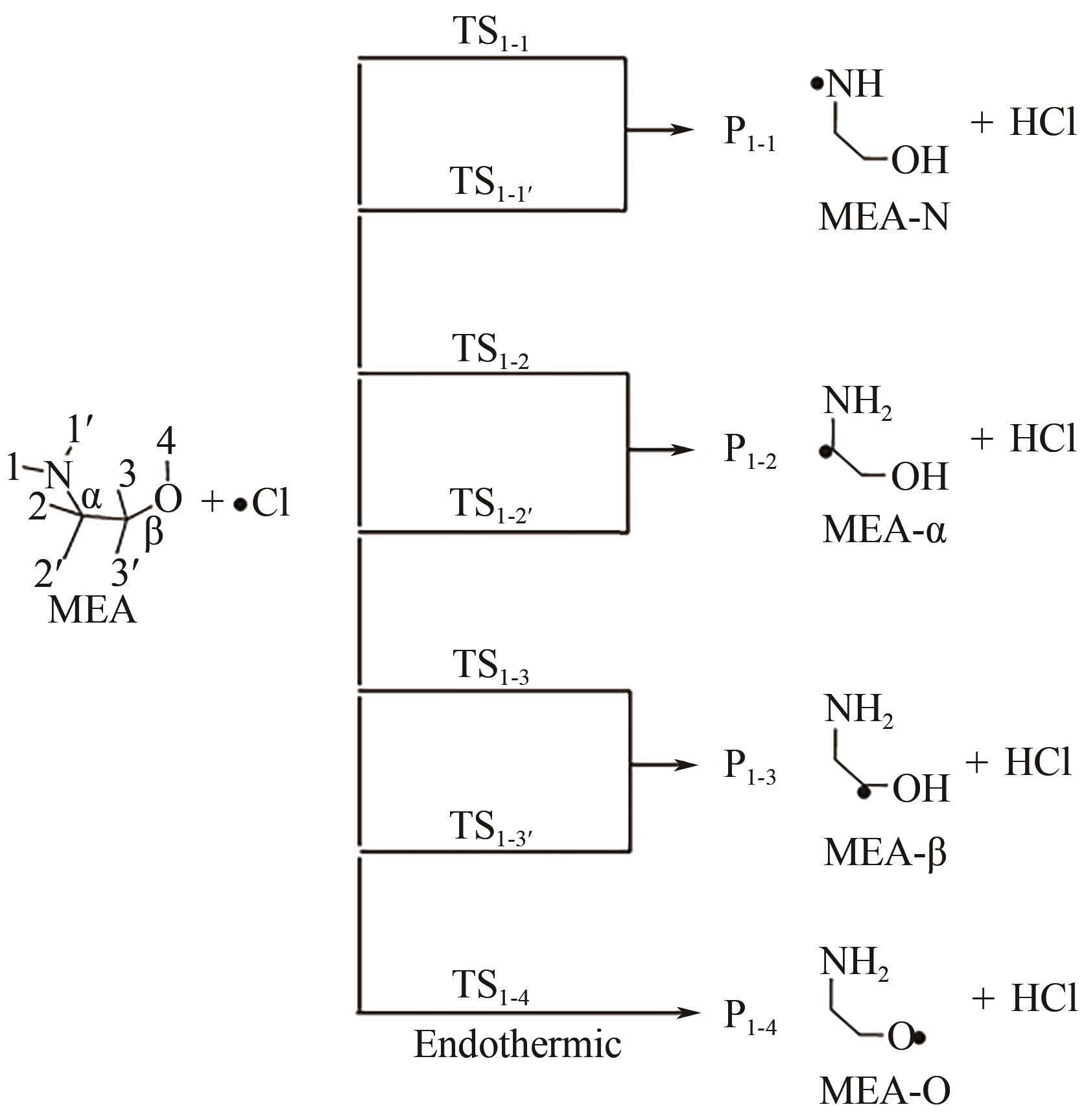
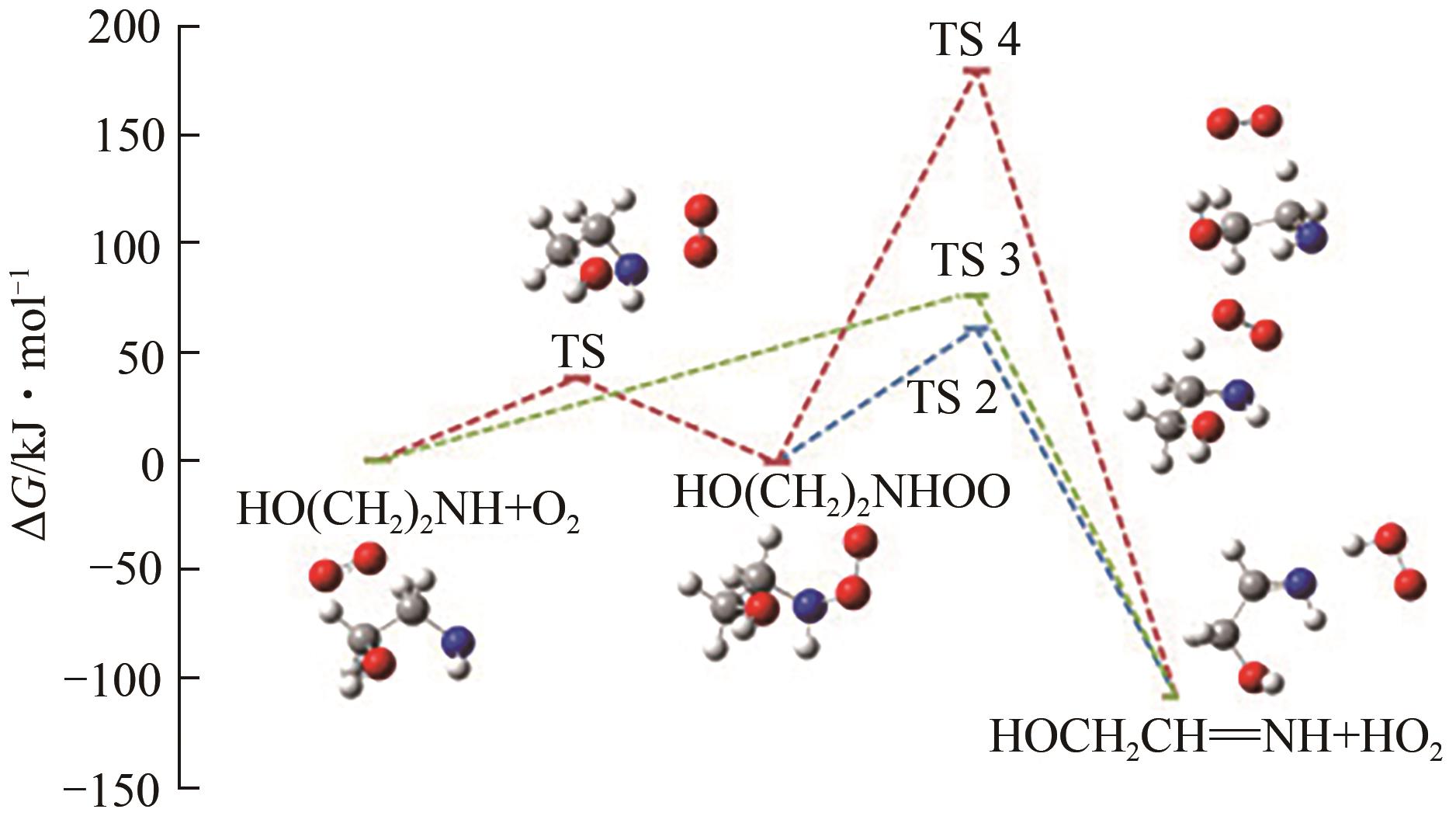
图8 CCSD(T)/Aug-cc-pVTZ//MP2/6-31+G(3df,2p)水平上计算的PZ+Cl反应势能面[34][反应物PZ+Cl的总能量设置为零(参考态)]R1、Rc1-1、Pc1-m 、Ts1-m 和P1-m —反应物、反应前络合物、反应后络合物、过渡态和参与反应的产物;m—不同数字
| 胺吸收剂种类 | 结构 | k |
|---|---|---|
| MEA | 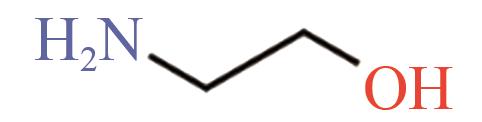 | (1.09±0.05)×10-18[ |
| AMP | 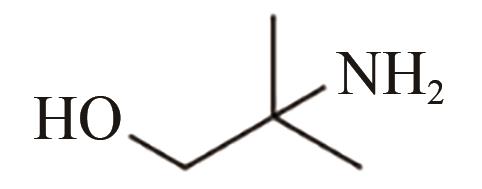 | 1.9×10-19[ |
| DMAE | 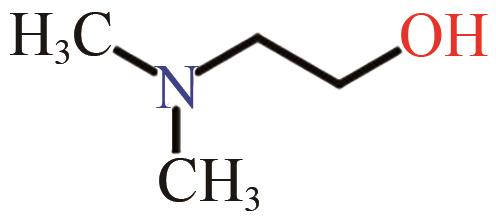 | (6.76±0.83)×10-18[ |
表4 常温下O3与胺吸收剂的实验速率常数
| 胺吸收剂种类 | 结构 | k |
|---|---|---|
| MEA |  | (1.09±0.05)×10-18[ |
| AMP |  | 1.9×10-19[ |
| DMAE |  | (6.76±0.83)×10-18[ |
| 化学名称/种类 | 缩写名称 | 是否鉴定 |
|---|---|---|
| 2-氨基-2-甲基-1-丙醇(碱胺) | AMP | 是 |
| AMP的氨基 | AMPN | 否(未观察到自由基) |
| AMP的过氧基团 | AMPR | 否(未观察到自由基) |
| AMP硝酸气溶胶 | AMPNTR | 通过SMPS测量间接观察到 |
| AMP亚硝胺 | AMPNO | 否(亚硝胺很难观察到) |
| AMP硝胺 | AMPNO2 | 通过SIFT-MS观察到, 但很难量化 |
| 甲烯丙醇等产品 | ISPD | 否 |
表5 AMP反应产物种类汇总
| 化学名称/种类 | 缩写名称 | 是否鉴定 |
|---|---|---|
| 2-氨基-2-甲基-1-丙醇(碱胺) | AMP | 是 |
| AMP的氨基 | AMPN | 否(未观察到自由基) |
| AMP的过氧基团 | AMPR | 否(未观察到自由基) |
| AMP硝酸气溶胶 | AMPNTR | 通过SMPS测量间接观察到 |
| AMP亚硝胺 | AMPNO | 否(亚硝胺很难观察到) |
| AMP硝胺 | AMPNO2 | 通过SIFT-MS观察到, 但很难量化 |
| 甲烯丙醇等产品 | ISPD | 否 |
| 污染物 | 最高允许排放浓度/mg·m-3 | 最高允许排放速率/kg·h-1 | 无组织排放监控浓度限值 | |||
|---|---|---|---|---|---|---|
| 排放气筒高度/m | 二级 | 三级 | 监控点 | 浓度/mg·m-3 | ||
| 苯胺类 | 20 | 15 | 0.52 | 0.78 | 周界外最高浓度点 | 0.40 |
| 20 | 0.87 | 7.3 | ||||
| 30 | 2.9 | 4.4 | ||||
| 40 | 5.0 | 7.6 | ||||
| 50 | 7.7 | 12 | ||||
| 60 | 11 | 17 | ||||
| 氮氧化物 | 240(硝酸使用和其他) | 15 | 0.77 | 1.2 | 周界外最高浓度点 | 0.12 |
| 20 | 1.3 | 2.0 | ||||
| 30 | 4.4 | 6.6 | ||||
| 40 | 7.5 | 11 | ||||
| 50 | 12 | 18 | ||||
| 60 | 16 | 25 | ||||
| 70 | 23 | 35 | ||||
| 80 | 31 | 47 | ||||
| 90 | 40 | 61 | ||||
| 100 | 52 | 78 | ||||
| 甲醛 | 25 | 15 | 0.26 | 0.39 | 周界外最高浓度点 | 0.20 |
| 20 | 0.43 | 0.65 | ||||
| 30 | 1.4 | 2.2 | ||||
| 40 | 2.6 | 3.8 | ||||
| 50 | 3.8 | 5.9 | ||||
| 60 | 5.4 | 8.3 | ||||
表6 涉及碳捕集工业大气排放污染物排放标准[40]
| 污染物 | 最高允许排放浓度/mg·m-3 | 最高允许排放速率/kg·h-1 | 无组织排放监控浓度限值 | |||
|---|---|---|---|---|---|---|
| 排放气筒高度/m | 二级 | 三级 | 监控点 | 浓度/mg·m-3 | ||
| 苯胺类 | 20 | 15 | 0.52 | 0.78 | 周界外最高浓度点 | 0.40 |
| 20 | 0.87 | 7.3 | ||||
| 30 | 2.9 | 4.4 | ||||
| 40 | 5.0 | 7.6 | ||||
| 50 | 7.7 | 12 | ||||
| 60 | 11 | 17 | ||||
| 氮氧化物 | 240(硝酸使用和其他) | 15 | 0.77 | 1.2 | 周界外最高浓度点 | 0.12 |
| 20 | 1.3 | 2.0 | ||||
| 30 | 4.4 | 6.6 | ||||
| 40 | 7.5 | 11 | ||||
| 50 | 12 | 18 | ||||
| 60 | 16 | 25 | ||||
| 70 | 23 | 35 | ||||
| 80 | 31 | 47 | ||||
| 90 | 40 | 61 | ||||
| 100 | 52 | 78 | ||||
| 甲醛 | 25 | 15 | 0.26 | 0.39 | 周界外最高浓度点 | 0.20 |
| 20 | 0.43 | 0.65 | ||||
| 30 | 1.4 | 2.2 | ||||
| 40 | 2.6 | 3.8 | ||||
| 50 | 3.8 | 5.9 | ||||
| 60 | 5.4 | 8.3 | ||||
| 1 | NABAT Mohammad Hossein, ZEYNALIAN Mirhadi, RAZMI Amir Reza, et al. Energy, exergy, and economic analyses of an innovative energy storage system; liquid air energy storage (LAES) combined with high-temperature thermal energy storage (HTES)[J]. Energy Conversion and Management, 2020, 226: 113486. |
| 2 | AGENCY International Energy. Energy technology perspectives 2020 - special report on carbon capture utilisation and storage: CCUS in clean energy transitions[M]. OECD, 2020. |
| 3 | GCCSI. Global status of CCS 2022[R]. Melbourne: Global CCS Institute, 2022. |
| 4 | AFKHAMIPOUR Morteza, MOFARAHI Masoud. A modeling-optimization framework for assessment of CO2 absorption capacity by novel amine solutions: 1DMA2P, 1DEA2P, DEEA, and DEAB[J]. Journal of Cleaner Production, 2018, 171: 234-249. |
| 5 | REYNOLDS Alicia J, Vincent VERHEYEN T, ADELOJU Samuel B, et al. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts[J]. Environmental Science & Technology, 2012, 46(7): 3643-3654. |
| 6 | LEPAUMIER Hélène, SILVA Eirik F DA, EINBU Aslak, et al. Comparison of MEA degradation in pilot-scale with lab-scale experiments[J]. Energy Procedia, 2011, 4: 1652-1659. |
| 7 | SHAO R, STANGELAND A. Amines used in CO2 capture-health and environmental impacts[J]. Bellona report, 2009, 49: 1-49. |
| 8 | NIELSEN C, D’ANNA B, KARL M, et al. Atmospheric degradation of amines (ADA) summary report: Photo-oxidation of methylamine, dimethylamine and trimethylamine CLIMIT project no. 201604[M]. NILU, 2011. |
| 9 | KHAKHARIA Purvil, BRACHERT Leonie, MERTENS Jan, et al. Understanding aerosol based emissions in a post combustion CO2 capture process: Parameter testing and mechanisms[J]. International Journal of Greenhouse Gas Control, 2015, 34: 63-74. |
| 10 | SILVA Eirik F DA, KOLDERUP Herman, GOETHEER Earl, et al. Emission studies from a CO2 capture pilot plant[J]. Energy Procedia, 2013, 37: 778-783. |
| 11 | NGUYEN Thu, HILLIARD Marcus, ROCHELLE Gary. Volatility of aqueous amines in CO2 capture[J]. Energy Procedia, 2011, 4: 1624-1630. |
| 12 | NGUYEN Thu, HILLIARD Marcus, ROCHELLE Gary T. Amine volatility in CO2 capture[J]. International Journal of Greenhouse Gas Control, 2010, 4(5): 707-715. |
| 13 | IEA GHG. Environmental impact of solvent scrubbing of CO2 [R/OL]. (2006-10)[2023-07-18]. . |
| 14 | MOSER Peter, SCHMIDT Sandra, STAHL Knut. Investigation of trace elements in the inlet and outlet streams of a MEA-based post-combustion capture process results from the test programme at the Niederaussem pilot plant[J]. Energy Procedia, 2011, 4: 473-479. |
| 15 | Project CESAR EU. SINTEF materials and chemistry. emission measurements at Dong’s pilot plant for CO2 capture in esbjerg[EB/OL]. [2023-7-18]. . |
| 16 | OCTAVIUS Evénements. Workshop emissions[C/OL]// Projets IFPEN. OCTAVIUS FP 7. Allemagne: EnBW Heilbronn, 2014: 2.13-2.14. . |
| 17 | HALLIBURTON B, DAY S J, LAVRENCIC S, et al. Project A-Establishing sampling and analytical procedures for potentially harmful components from post-combustion amine based CO2 capture[C]. 2010. |
| 18 | MERTENS Jan, BRACHERT L, DESAGHER D, et al. ELPI+ measurements of aerosol growth in an amine absorption column[J]. International Journal of Greenhouse Gas Control, 2014, 23: 44-50. |
| 19 | KHAKHARIA Purvil, BRACHERT Leonie, MERTENS Jan, et al. Investigation of aerosol based emission of MEA due to sulphuric acid aerosol and soot in a Post Combustion CO2 Capture process[J]. International Journal of Greenhouse Gas Control, 2013, 19: 138-144. |
| 20 | HARSHA Shreyas, KHAKHARIA Purvil, HUIZINGA Arjen, et al. In-situ experimental investigation on the growth of aerosols along the absorption column in post combustion carbon capture[J]. International Journal of Greenhouse Gas Control, 2019, 85: 86-99. |
| 21 | NIELSEN Claus J, HERRMANN Hartmut, WELLER Christian. Atmospheric chemistry and environmental impact of the use of amines in carbon capture and storage (CCS)[J]. Chemical Society Reviews, 2012, 41(19): 6684-6704. |
| 22 | ATKINSON Roger. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions[J]. Chemical Reviews, 1986, 86(1): 69-201. |
| 23 | ATKINSON Roger, CARTER William P L. Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions[J]. Chemical Reviews, 1984, 84(5): 437-470. |
| 24 | ONEL L, BLITZ M A, SEAKINS P W. Direct determination of the rate coefficient for the reaction of OH radicals with monoethanol amine (MEA) from 296 to 510 K[J]. The Journal of Physical Chemistry Letters, 2012, 3(7): 853-856. |
| 25 | KARL M, DYE C, SCHMIDBAUER N, et al. Study of OH-initiated degradation of 2-aminoethanol[J]. Atmospheric Chemistry & Physics, 2012, 12(4): 1881-1901. |
| 26 | BORDUAS Nadine, ABBATT Jonathan P D, MURPHY Jennifer G. Gas phase oxidation of monoethanolamine (MEA) with OH radical and ozone: Kinetics, products, and particles[J]. Environmental Science & Technology, 2013, 47(12): 6377-6383. |
| 27 | ANDERSON Larry G, STEPHENS Robert D. Kinetics of the reaction of hydroxyl radicals with 2-(dimethylamino)ethanol from 234–364 K[J]. International Journal of Chemical Kinetics, 1988, 20(2): 103-110. |
| 28 | HARRIS Geoffrey W, PITTS James N. Notes. Rates of reaction of hydroxyl radicals with 2-(dimethylamino)ethanol and 2-amino-2-methyl-1-propanol in the gas phase at 300±2K[J]. Environmental Science & Technology, 1983, 17(1): 50-51. |
| 29 | ONEL L, BLITZ M A, BREEN J, et al. Branching ratios for the reactions of OH with ethanol amines used in carbon capture and the potential impact on carcinogen formation in the emission plume from a carbon capture plant[J]. Physical Chemistry Chemical Physics, 2015, 17(38): 25342-25353. |
| 30 | TAN Wen, ZHU Liang, MIKOVINY Tomas, et al. Experimental and theoretical study of the OH-initiated degradation of piperazine under simulated atmospheric conditions[J]. The Journal of Physical Chemistry A, 2021, 125(1): 411-422. |
| 31 | XIE Hongbin, LI Chao, HE Ning, et al. Atmospheric chemical reactions of monoethanolamine initiated by OH radical: Mechanistic and kinetic study[J]. Environmental Science & Technology, 2014, 48(3): 1700-1706. |
| 32 | TAN Wen, ZHU Liang, MIKOVINY Tomáš, et al. Atmospheric chemistry of 2-amino-2-methyl-1-propanol: A theoretical and experimental study of the OH-initiated degradation under simulated atmospheric conditions[J]. The Journal of Physical Chemistry A, 2021, 125(34): 7502-7519. |
| 33 | XIE Hongbin, MA Fangfang, WANG Yuanfang, et al. Quantum chemical study on ·Cl-initiated atmospheric degradation of monoethanolamine[J]. Environmental Science & Technology, 2015, 49(22): 13246-13255. |
| 34 | MA Fangfang, DING Zhezheng, Jonas ELM, et al. Atmospheric oxidation of piperazine initiated by ·Cl: Unexpected high nitrosamine yield[J]. Environmental Science & Technology, 2018, 52(17): 9801-9809. |
| 35 | MANZOOR Saba, SIMPERLER Alexandra, KORRE Anna. A theoretical study of the reaction kinetics of amines released into the atmosphere from CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 41: 219-228. |
| 36 | LI Kangwei, WHITE Stephen, ZHAO Bin, et al. Evaluation of a new chemical mechanism for 2-amino-2-methyl-1-propanol in a reactive environment from CSIRO smog chamber experiments[J]. Environmental Science & Technology, 2020, 54(16): 9844-9853. |
| 37 | TUAZON E C, ATKINSON R, ASCHMANN S M, et al. Kinetics and products of the gas-phase reactions of O3 with amines and related compounds[J]. Research on Chemical Intermediates, 1994, 20(3): 303-320. |
| 38 | NIELSEN Claus Jørgen, Barbara D’ANNA, Christian DYE, et al. Atmospheric chemistry of 2-aminoethanol (MEA)[J]. Energy Procedia, 2011, 4: 2245-2252. |
| 39 | DAG T. Update and improvement of dispersion calculations for emissions to air from TCM’s amine plant. Part II-Likely case nitrosamines, nitramines and formaldehyde[R/OL]. (2011)[2023-8-14]. . |
| 40 | 国家环境保护局. 大气污染物综合排放标准: [S]. 北京: 中国标准出版社, 1997. |
| State Bureau of Environmental Protection of the People’s Republic of China. Comprehensive emission standard of air pollutants: [S]. Beijing: Standards Press of China, 1997. | |
| 41 | HSE. EH40/2005 Workplace exposure limits [S/OL]. 2007. [2023-8-15]. . |
| 42 | LAG M P, LINDEMAN B, INSTANES C, et al. Health effects of amines and derivatives associated with CO2 capture[C].2011. |
| 43 | Review of Amine Emissions from Carbon Capture Systems[C].2015. |
| 44 | BERGLEN T, TØNNESEN D, DYE C, et al. CO2 Technology Centre Mongstad-updated air dispersion calculations[C]. 2010. |
| 45 | 中华人民共和国环境保护部. 环境空气和废气 酰胺类化合物的测定 液相色谱法: [S]. 北京: 中国环境科学出版社, 2016. |
| Ministry of Environmental Protection of the People’s Republic of China. Ambient air and waste gas-Determination of amide compounds-Liquid chromatography: [S]. Beijing: China Environmental Science Press, 2016. | |
| 46 | 环境空气. 挥发性有机物的测定 吸附管采样-热脱附/气相色谱-质谱法: [S]. |
| Determination of volatile organic compounds in ambient air Adsorbent tube sampling-thermal desorption/gas chromatography-mass spectrometry: [S]. | |
| 47 | ALAWODE A O. Oxidative degradation of piperazine in the absorption of carbon dioxide[D]. The University of Texas at Austin, 2005. |
| 48 | EINBU Aslak, DASILVA Eirik, HAUGEN Geir, et al. A new test rig for studies of degradation of CO2 absorption solvents at process conditions; comparison of test rig results and pilot plant data for degradation of MEA[J]. Energy Procedia, 2013, 37: 717-726. |
| 49 | FINE Nathan A, ROCHELLE Gary T. Thermal decomposition of N-nitrosopiperazine[J]. Energy Procedia, 2013, 37: 1678-1686. |
| 50 | FINE Nathan A, GOLDMAN Mark J, NIELSEN Paul T, et al. Managing N-nitrosopiperazine and dinitrosopiperazine[J]. Energy Procedia, 2013, 37: 273-284. |
| 51 | FINE Nathan A, NIELSEN Paul T, ROCHELLE Gary T. Decomposition of nitrosamines in CO2 capture by aqueous piperazine or monoethanolamine[J]. Environmental Science and Technology, 2014, 48(10): 5996-6002. |
| 52 | GOLDMAN Mark J, FINE Nathan A, ROCHELLE Gary T. Kinetics of N-nitrosopiperazine formation from nitrite and piperazine in CO2 capture[J]. Environmental Science & Technology, 2013, 47(7): 3528-3534. |
| 53 | CHEN Yifei, LIN Qinhao, LI Guiying, et al. A new method of simultaneous determination of atmospheric amines in gaseous and particulate phases by gas chromatography-mass spectrometry[J]. Journal of Environmental Sciences, 2022, 114: 401-411. |
| 54 | Berit FOSTÅS, GANGSTAD Audun, NENSETER Bjarne, et al. Effects of NO x in the flue gas degradation of MEA[J]. Energy Procedia, 2011, 4: 1566-1573. |
| 55 | 杨正大, 贤振楠, 邵凌宇, 等. 胺法碳捕集过程胺气溶胶形成与排放研究现状[J]. 能源环境保护, 2023, 37(3): 195-203. |
| YANG Zhengda, XIAN Zhennan, SHAO Lingyu, et al. Research status of amine aerosol formation and emission in amine carbon capture process[J]. Energy Environmental Protection, 2023, 37(3): 195-203. | |
| 56 | 方梦祥, 狄闻韬, 易宁彤, 等. CO2化学吸收系统污染物排放与控制研究进展[J]. 洁净煤技术, 2021, 27(2): 8-16. |
| FANG Mengxiang, DI Wentao, YI Ningtong, et al. Research progress on pollutant emission and control from CO2 chemical absorption system[J]. Clean Coal Technology, 2021, 27(2): 8-16. | |
| 57 | MERTENS Jan, ANDERLOHR C, ROGIERS P, et al. A wet electrostatic precipitator (WESP) as countermeasure to mist formation in amine based carbon capture[J]. International Journal of Greenhouse Gas Control, 2014, 31: 175-181. |
| 58 | FUJITA Koshito, MURAOKA Daigo, OGAWA Takashi, et al. Evaluation of amine emissions from the post-combustion CO2 capture pilot plant[J]. Energy Procedia, 2013, 37: 727-734. |
| 59 | MERTENS Jan, KHAKHARIA P, ROGIERS Pieter, et al. Prevention of mist formation in amine based carbon capture: Field testing using a wet ElectroStatic precipitator (WESP) and a gas-gas heater (GGH)[J]. Energy Procedia, 2017, 114: 987-999. |
| 60 | HUANG Jiayu, ZHANG Fan, SHI Yingjie, et al. Investigation of a pilot-scale wet electrostatic precipitator for the control of sulfuric acid mist from a simulated WFGD system[J]. Journal of Aerosol Science, 2016, 100: 38-52. |
| 61 | KHAKHARIA Purvil, HUIZINGA Arjen, TRAP Henk, et al. Lab scale investigation on the formation of aerosol nuclei by a wet electrostatic precipitator in the presence of SO2 in a gas stream[J]. International Journal of Greenhouse Gas Control, 2019, 86: 22-33. |
| 62 | LI Chao, YI Ningtong, FANG Mengxiang, et al. Solvent emissions control in large scale chemical absorption CO2 capture plant[J]. International Journal of Greenhouse Gas Control, 2021, 111: 103444. |
| 63 | MOSER Peter, SCHMIDT Sandra, STAHL Knut, et al. Demonstrating emission reduction-results from the post-combustion capture pilot plant at niederaussem[J]. Energy Procedia, 2014, 63: 902-910. |
| 64 | LU Shijian, YANG Fei, ZHANG Juanjuan, et al. Research and design experience of a 150kt/a CO2 capture and purification project in the Shaanxi Guohua Jinjie power plant[J]. Separation and Purification Technology, 2023, 320: 124089. |
| [1] | 于梦洁, 吴语童, 罗发祥, 豆义波. 低浓度二氧化碳还原光催化剂结构设计的研究进展[J]. 化工进展, 2024, 43(S1): 335-350. |
| [2] | 王博葳, 郑明朕, 王乐萌, 付东, 王珊, 朱生俊, 赵昆, 张盼. Na2SO4电解制备NaOH捕集CO2[J]. 化工进展, 2024, 43(S1): 604-614. |
| [3] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
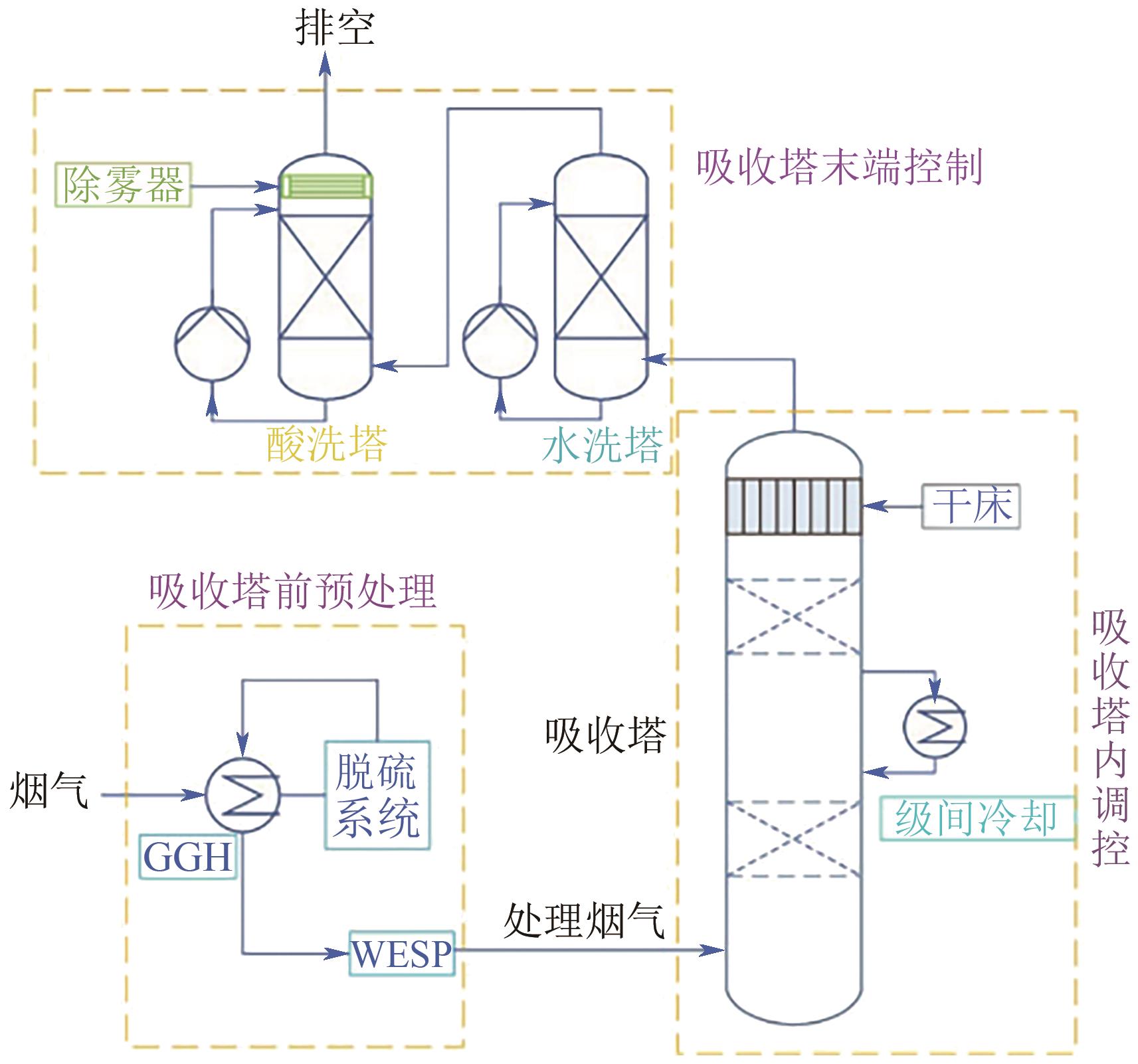
| [4] | 陆诗建, 张娟娟, 杨菲, 刘玲, 陈思铭, 康国俊, 房芹芹. 化学吸收法胺液逃逸控制技术研究进展[J]. 化工进展, 2024, 43(8): 4562-4570. |
| [5] | 罗丛佳, 豆义波, 卫敏. 水滑石光催化剂结构调控用于二氧化碳还原的研究进展[J]. 化工进展, 2024, 43(7): 3891-3909. |
| [6] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [7] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| [8] | 苗诒贺, 王耀祖, 刘雨杭, 朱炫灿, 李佳, 于立军. 添加剂改性固态胺吸附剂用于碳捕集的研究进展[J]. 化工进展, 2024, 43(5): 2739-2759. |
| [9] | 武西宁, 张宁, 秦佳敏, 徐龙, 魏朝阳, 马晓迅. 低冷量下强化CO2吸收的甲醇基纳米流体性能[J]. 化工进展, 2024, 43(5): 2811-2822. |
| [10] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [11] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [12] | 孙伟吉, 刘浪, 方治余, 朱梦博, 解耿, 何伟, 高宇恒. 改性镁渣的湿法碳酸化工艺[J]. 化工进展, 2024, 43(4): 2161-2173. |
| [13] | 徐泽文, 王明, 王强, 侯影飞. 胺基材料在二氧化碳分离膜领域研究进展[J]. 化工进展, 2024, 43(3): 1374-1386. |
| [14] | 张瑞凯, 张会书, 郑龙云, 曾爱武. CO2吸收过程中气相分压对Rayleigh对流传质特性的影响[J]. 化工进展, 2024, 43(2): 913-924. |
| [15] | 焦竞, 刘琳琳, 都健. 基于代理模型的碳捕集与电厂集成调度优化[J]. 化工进展, 2024, 43(11): 6059-6067. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||