化工进展 ›› 2021, Vol. 40 ›› Issue (1): 415-426.DOI: 10.16085/j.issn.1000-6613.2020-0387
MOF及其复合材料吸附去除VOCs应用研究进展
李孟1( ), 李炜1, 张帅1, 李雨薇1, 刘芳1,2(
), 李炜1, 张帅1, 李雨薇1, 刘芳1,2( ), 赵朝成1,2, 王永强1,2
), 赵朝成1,2, 王永强1,2
- 1.中国石油大学(华东)化工学院,山东 青岛 266580
2.石油石化污染物控制与处理国家重点实验室,北京 102206
-
收稿日期:2020-03-16出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:刘芳 -
作者简介:李孟(1993—),男,硕士研究生,研究方向环境污染控制技术。E-mail:1115018012@qq.com 。 -
基金资助:石油石化污染物控制与处理国家重点实验室自主课题(PPCIP2017005)
Research progress on adsorption of VOCs by MOF and its composite
Meng LI1( ), Wei LI1, Shuai ZHANG1, Yuwei LI1, Fang LIU1,2(
), Wei LI1, Shuai ZHANG1, Yuwei LI1, Fang LIU1,2( ), Chaocheng ZHAO1,2, Yongqiang WANG1,2
), Chaocheng ZHAO1,2, Yongqiang WANG1,2
- 1.College of Chemical Engineering, China University of Petroleum, Qingdao 266580, Shandong, China
2.State Key Laboratory of Petroleum Pollution Control, Beijing 102206, China
-
Received:2020-03-16Online:2021-01-05Published:2021-01-12 -
Contact:Fang LIU
摘要:
金属有机骨架材料(MOF)是一种高比表面积、活性位点丰富、易化学修饰的新型多孔材料,但较差的水稳定性限制其在吸附挥发性有机化合物(VOCs)领域的应用,因此如何提高和巩固MOF的吸附性能已成为研究的热点。本文从单体MOF的合成、MOF复合材料的制备、吸附机理和吸附影响因素等方面综述了MOF及其复合材料吸附去除VOCs的研究进展。针对目前MOF材料在吸附VOCs方面的不足提出研究建议,展望其在VOCs吸附领域的发展方向为制备富含微-介孔结构和活性位点、水热稳定性强、抗水蒸气竞争吸附好和循环利用率高的新型高稳定性材料,以及开发新型MOF材料合成方法。
中图分类号:
引用本文
李孟, 李炜, 张帅, 李雨薇, 刘芳, 赵朝成, 王永强. MOF及其复合材料吸附去除VOCs应用研究进展[J]. 化工进展, 2021, 40(1): 415-426.
Meng LI, Wei LI, Shuai ZHANG, Yuwei LI, Fang LIU, Chaocheng ZHAO, Yongqiang WANG. Research progress on adsorption of VOCs by MOF and its composite[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 415-426.
| 制备方法 | 优点 | 缺点 |
|---|---|---|
| 水热法 | 工艺成熟、反应条件温和 | 耗时长、合成量少、二次污染 |
| 溶剂热法 | 材料结晶度高、反应条件温和 | 耗时长、需大量溶剂、成本高、二次污染 |
| 机械化学法 | 简单快捷、无溶剂、产量高、无污染 | 需高机械能、成本高 |
| 微波法 | 合成快、加热均匀、材料粒径小、产量高 | 需大量溶剂、成本高、不稳定 |
| 电化学合成法 | 合成时间短、易于控制、产量高 | 能耗大 |
| 溶胶-凝胶法 | 易操作控制、均匀性好 | 成本较高、耗时长 |
表1 MOF材料制备方法对比
| 制备方法 | 优点 | 缺点 |
|---|---|---|
| 水热法 | 工艺成熟、反应条件温和 | 耗时长、合成量少、二次污染 |
| 溶剂热法 | 材料结晶度高、反应条件温和 | 耗时长、需大量溶剂、成本高、二次污染 |
| 机械化学法 | 简单快捷、无溶剂、产量高、无污染 | 需高机械能、成本高 |
| 微波法 | 合成快、加热均匀、材料粒径小、产量高 | 需大量溶剂、成本高、不稳定 |
| 电化学合成法 | 合成时间短、易于控制、产量高 | 能耗大 |
| 溶胶-凝胶法 | 易操作控制、均匀性好 | 成本较高、耗时长 |
典型 VOCs | 吸附剂 | 比表面积 /m3?g-1 | 孔径分布 /nm | 孔容 /cm3?g-1 | 吸附 位点 | 亲、 疏水性 | 吸附条件 | 吸附量 /m3?g-1 | 吸附机理 |
|---|---|---|---|---|---|---|---|---|---|
甲苯 | Cu-BTC[ | 793.49 | 3.49 | 0.31 | 微、介孔 | 亲水性 | T=298K,c0=1300mg·m-3, m=0.3g | 62.7 | 孔道填充吸附 |
| Cu-BTC@GO-20%[ | 512.6 | 3.86 | 0.35 | 183.6 | |||||
| Uio-66[ | 1335 | 0.45~0.7 | 0.83 | 微孔 | 疏水性 | T=298K | 151 | 孔道填充吸附 | |
| ZG-4[ | 1112 | — | — | 微孔 | 亲水性 | T=298K,55% RH | 116 | 孔道填充吸附、π-π键作用 | |
Uio-66-NH2[ | 1250 | 1.98 | 0.62 | 微孔 | 疏水性 | T=298K | 147 | 孔道填充吸附、疏水性作用、氢键作用 | |
CZ-5%[ | 1484.46 | 3.27 | 0.60 | 微、介孔 | 疏水性 | T=298K,c0=1300mg·m-3, m=0.3g,30% RH | 158.6 | 孔道填充吸附、π-π键作用、阳离子-π键作用、疏水性作用 | |
| HK@CMC [ | 545 | 0.371 | 微、介孔 | 疏水性 | T=298K,P=0.0379bar | 285.6 | 孔道填充吸附 | ||
苯 | Mg-MOF-74[ | — | 1.6 | 0.88 | 微、介孔 | — | T=300K、P=20Pa | 640.5 | 范德华力、库仑作用、孔道填充吸附 |
| MC-500-6[ | 2320 | — | 1.05 | 微、介孔 | 疏水性 | T=298K | 999.8 | 孔道填充吸附、π-π键作用 | |
甲苯、 二甲苯 | H-MOZs[ | 1570 | — | 0.90 | 微孔、介孔、大孔 | — | T=298K | 296.7(甲苯)、 148.2(二甲苯) | 孔道填充吸附(介孔和大孔的存在促进吸附) |
| Uio-66[ | — | — | — | 微、介孔 | — | T=298K,m=5mg,V=10mL,C=100μL·L-1 | 22.94(甲苯) 38.31(二甲苯) | 孔道填充吸附 | |
| Uio-66-F4[ | — | — | — | 微、介孔 | 疏水性 | 30.49(甲苯) 38.31(二甲苯) | 孔道填充吸附、疏水性作用 | ||
二氯甲烷 | ZG-15[ | 559.3 | 2.2 | 0.32 | 微、介孔 | 亲水性 | 气流速率=20mL·min-1,床层高度=10cm | 240 | 孔道填充吸附、氢键作用、π-π键作用 |
苯酚 | CNT@MOF-68(Al)[ | 1290 | 1.6 | — | 微、介孔 | 疏水性 | c0=2000/μL·L-1、CNT含量=0.75% | 341.1 | 孔道填充吸附、氢键作用和π-π键作用 |
甲醛 | ED-MIL-101-3[ | 382 | 2.3 | — | 微、介孔 | 疏水性 | T=298K、潮湿环境 | 164.86 | 疏水性作用、孔道填充吸附(微孔为主) |
甲醇、 乙醇、 正己烷 | RGO(2%wt)/ Cu-BTC[ | 1549 | 1.54 | — | 微、介孔 | 亲水性 | T=298K、潮湿环境 | 421(甲醇) 474.52(乙9醇) 406(正己烷) | 静电排斥、亲水性作用、孔道填充吸附、π-π键作用 |
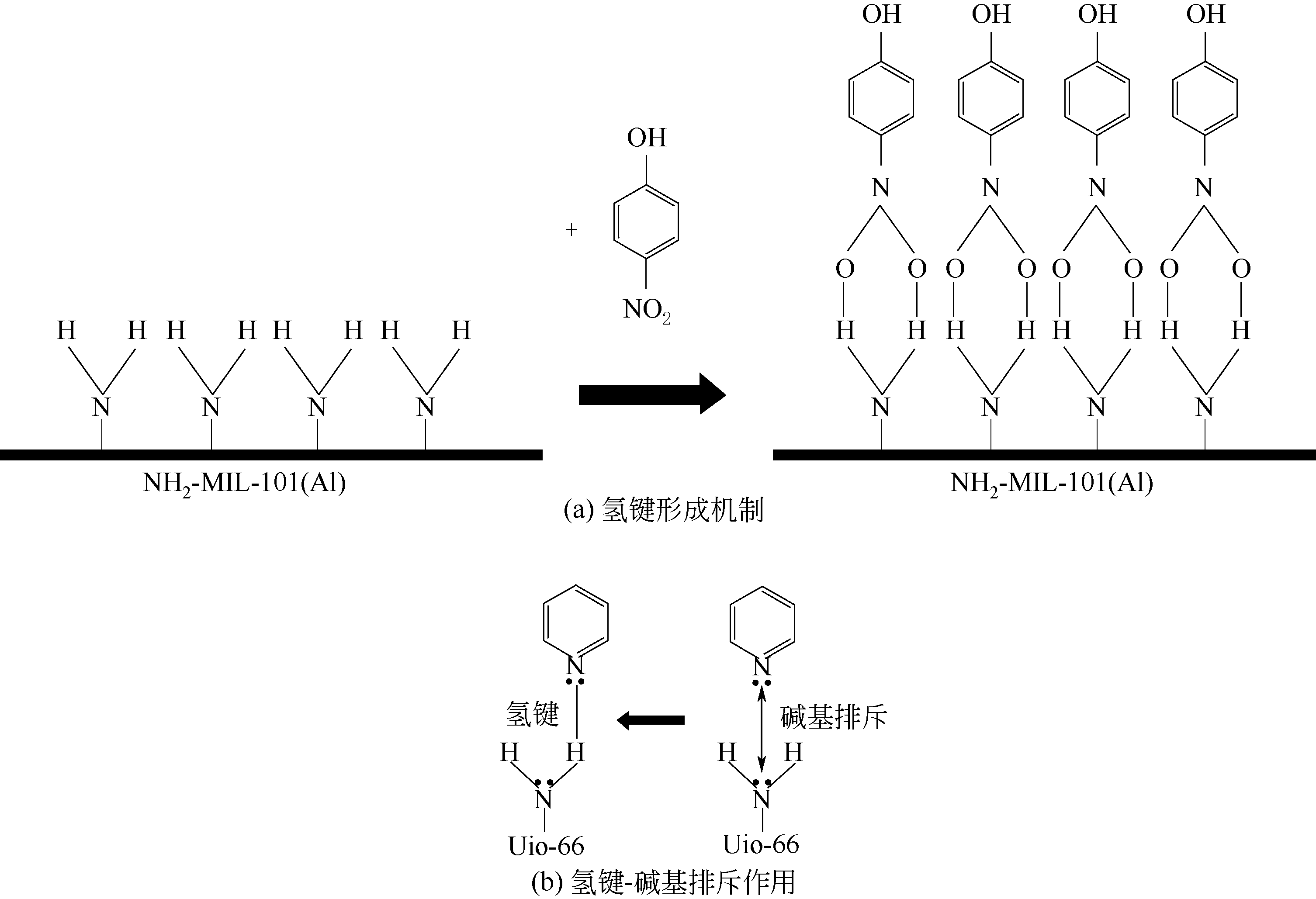
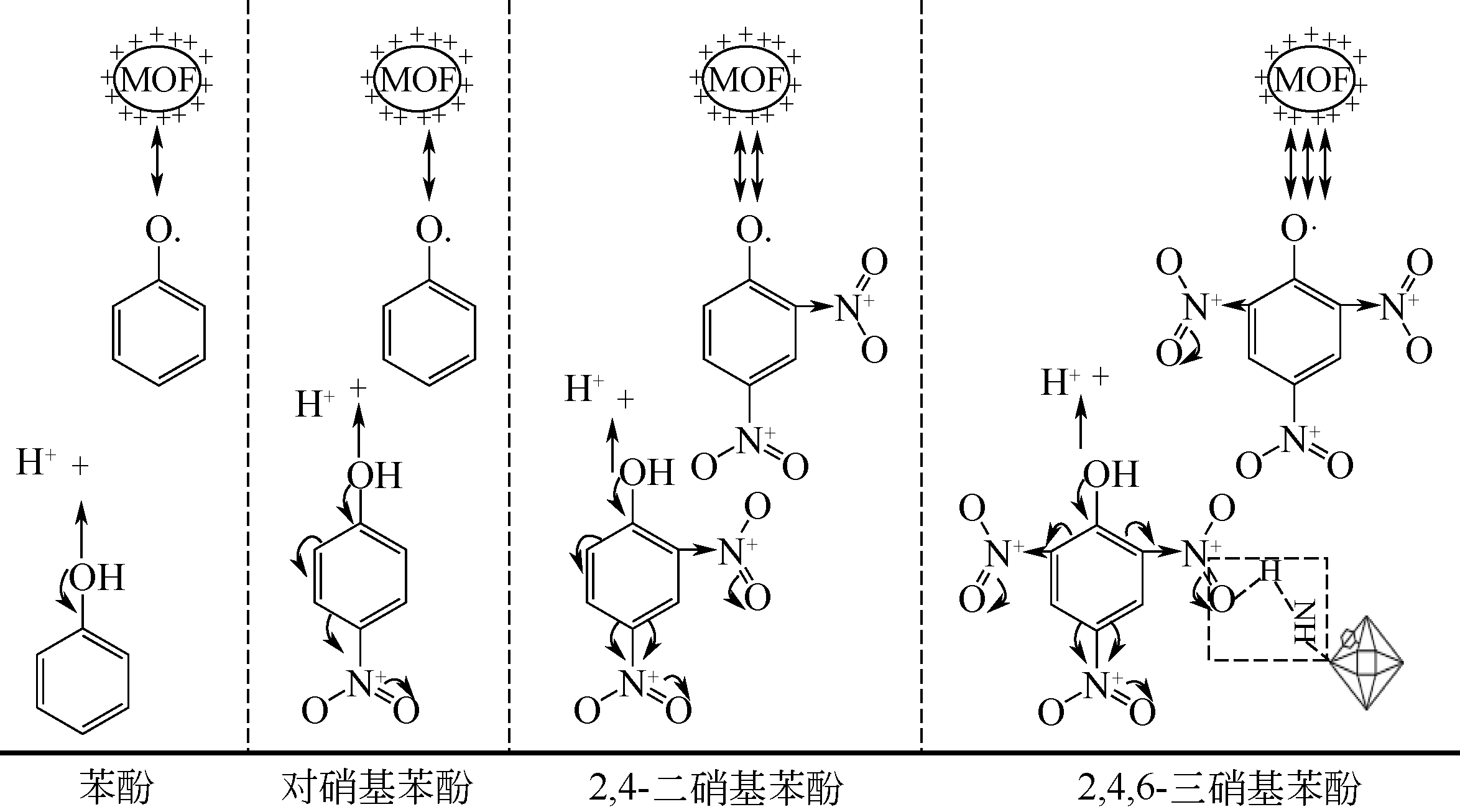
不同 硝基酚 | Uio-66-NH2[ | 1023 | — | 0.45 | 微、介孔 | 疏水性 | pH=4,m=20mg,c0=80mg·L-1,T=288K,V=50mL | 6.05(苯酚) 44.96(4-NP) 144.10(DNP) 141.09(TNP) | 氢键作用、π-π键作用、静电相互作用、孔道填充吸附、配位反应 |
表2 不同MOF材料吸附VOCs的性能对比
典型 VOCs | 吸附剂 | 比表面积 /m3?g-1 | 孔径分布 /nm | 孔容 /cm3?g-1 | 吸附 位点 | 亲、 疏水性 | 吸附条件 | 吸附量 /m3?g-1 | 吸附机理 |
|---|---|---|---|---|---|---|---|---|---|
甲苯 | Cu-BTC[ | 793.49 | 3.49 | 0.31 | 微、介孔 | 亲水性 | T=298K,c0=1300mg·m-3, m=0.3g | 62.7 | 孔道填充吸附 |
| Cu-BTC@GO-20%[ | 512.6 | 3.86 | 0.35 | 183.6 | |||||
| Uio-66[ | 1335 | 0.45~0.7 | 0.83 | 微孔 | 疏水性 | T=298K | 151 | 孔道填充吸附 | |
| ZG-4[ | 1112 | — | — | 微孔 | 亲水性 | T=298K,55% RH | 116 | 孔道填充吸附、π-π键作用 | |
Uio-66-NH2[ | 1250 | 1.98 | 0.62 | 微孔 | 疏水性 | T=298K | 147 | 孔道填充吸附、疏水性作用、氢键作用 | |
CZ-5%[ | 1484.46 | 3.27 | 0.60 | 微、介孔 | 疏水性 | T=298K,c0=1300mg·m-3, m=0.3g,30% RH | 158.6 | 孔道填充吸附、π-π键作用、阳离子-π键作用、疏水性作用 | |
| HK@CMC [ | 545 | 0.371 | 微、介孔 | 疏水性 | T=298K,P=0.0379bar | 285.6 | 孔道填充吸附 | ||
苯 | Mg-MOF-74[ | — | 1.6 | 0.88 | 微、介孔 | — | T=300K、P=20Pa | 640.5 | 范德华力、库仑作用、孔道填充吸附 |
| MC-500-6[ | 2320 | — | 1.05 | 微、介孔 | 疏水性 | T=298K | 999.8 | 孔道填充吸附、π-π键作用 | |
甲苯、 二甲苯 | H-MOZs[ | 1570 | — | 0.90 | 微孔、介孔、大孔 | — | T=298K | 296.7(甲苯)、 148.2(二甲苯) | 孔道填充吸附(介孔和大孔的存在促进吸附) |
| Uio-66[ | — | — | — | 微、介孔 | — | T=298K,m=5mg,V=10mL,C=100μL·L-1 | 22.94(甲苯) 38.31(二甲苯) | 孔道填充吸附 | |
| Uio-66-F4[ | — | — | — | 微、介孔 | 疏水性 | 30.49(甲苯) 38.31(二甲苯) | 孔道填充吸附、疏水性作用 | ||
二氯甲烷 | ZG-15[ | 559.3 | 2.2 | 0.32 | 微、介孔 | 亲水性 | 气流速率=20mL·min-1,床层高度=10cm | 240 | 孔道填充吸附、氢键作用、π-π键作用 |
苯酚 | CNT@MOF-68(Al)[ | 1290 | 1.6 | — | 微、介孔 | 疏水性 | c0=2000/μL·L-1、CNT含量=0.75% | 341.1 | 孔道填充吸附、氢键作用和π-π键作用 |
甲醛 | ED-MIL-101-3[ | 382 | 2.3 | — | 微、介孔 | 疏水性 | T=298K、潮湿环境 | 164.86 | 疏水性作用、孔道填充吸附(微孔为主) |
甲醇、 乙醇、 正己烷 | RGO(2%wt)/ Cu-BTC[ | 1549 | 1.54 | — | 微、介孔 | 亲水性 | T=298K、潮湿环境 | 421(甲醇) 474.52(乙9醇) 406(正己烷) | 静电排斥、亲水性作用、孔道填充吸附、π-π键作用 |
不同 硝基酚 | Uio-66-NH2[ | 1023 | — | 0.45 | 微、介孔 | 疏水性 | pH=4,m=20mg,c0=80mg·L-1,T=288K,V=50mL | 6.05(苯酚) 44.96(4-NP) 144.10(DNP) 141.09(TNP) | 氢键作用、π-π键作用、静电相互作用、孔道填充吸附、配位反应 |
| 1 | 赵朝成, 吴光锐. MOFs复合材料催化降解水中有机污染物的应用研究进展[J]. 化工进展, 2019, 38(4): 1775-1784. |
| ZHAO Chaocheng, WU Guangrui. Research progress on the mechanism and applications of MOFs composite materials for catalytic degradation of organic pollutants in the solution[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1775-1784. | |
| 2 | 周玲玲, 汤立红, 宁平, 等.金属有机骨架材料在气体吸附与分离中的应用研究进展[J]. 材料导报, 2017, 31(19): 112-121. |
| ZHOU Lingling, TANG Lihong, NING Ping, et al. Progress in gas adsorption and separation application of metal-organic framework materials[J]. Materials Reports, 2017, 31(19): 112-121. | |
| 3 | YANG Q, WANG Y, WANG J, et al. High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOF (UiO-67)[J]. Food Chemistry, 2018, 254: 241-248. |
| 4 | 于吉行, 俞俊, 薛晓雅, 等.金属有机骨架UiO-66在催化领域的应用[J]. 化工进展, 2019, 38(S1): 144-151. |
| YU Jixing, YU Jun, XUE Xiaoya, et al. Applications in the field of catalysis of metal organic framework UiO-66[J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 144-151. | |
| 5 | FAIG R W, POPP T M O, FRACAROLI A M, et al. The chemistry of CO2 capture in an amine-functionalized metal-organic framework under dry and humid conditions[J]. Journal of the American Chemical Society, 2017, 139(35): 12125-12128. |
| 6 | 李小娟, 何长发, 黄斌, 等.金属有机骨架材料吸附去除环境污染物的进展[J]. 化工进展, 2016, 35(2): 586-594. |
| LI Xiaojuan, HE Changfa, HUANG Bin, et al. Progress in the applications of metal-organic frameworks in adsorption removal of hazardous materials [J]. Chemical Industry and Engineering Progress, 2016, 35(2): 586-594. | |
| 7 | SHEBERLA D, BACHMAN J C, ELIAS J S, et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance[J]. Nature Materials, 2017, 16(2): 220-224. |
| 8 | JOYARAMULU K, GEYER F, SCHNEEMANN A, et al. Hydrophobic metal-organic frameworks[J]. Advanced Materials, 2019, 31(32): 1900820. |
| 9 | ZHANG X, LV X, SHI X, et al. Enhanced hydrophobic UiO-66 (university of oslo 66) metal-organic framework with high capacity and selectivity for toluene capture from high humid air[J]. Journal of Colloid and Interface Science, 2019, 539: 152-160. |
| 10 | ZHANG Z, NGUYEN H T H, MILLER S A, et al. Polymer-metal-organic frameworks (polyMOF) water tolerant materials for selective carbon dioxide separations[J]. Journal of the American Chemical Society, 2016, 138(3): 920-925. |
| 11 | LI Y, MIAO J, SUN X, et al. Mechanochemical synthesis of Cu-BTC@GO with enhanced water stability and toluene adsorption capacity[J]. Chemical Engineering Journal, 2016, 298: 191-197. |
| 12 | JABBARI V, VELETA J M, ZAREI-CHALESHTORI M, et al. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants[J]. Chemical Engineering Journal, 2016, 304: 774-783. |
| 13 | ZHU J, USOV P M, XU W, et al. A new class of metal-cyclam based zirconium metal-organic frameworks for CO2 adsorption and chemical fixation[J]. Journal of the American Chemical Society, 2017, 140(3): 993-1003. |
| 14 | WANG D F, WU G P, ZHAO Y F, et al. Study on the copper(Ⅱ)-doped MIL-101(Cr) and its performance in VOCs adsorption[J]. Environmental Science and Pollution Research, 2018, 25(28): 28109-28119. |
| 15 | WANG Z, WANG W, JIANG D, et al. Diamine-appended metal-organic frameworks: enhanced formaldehyde-vapor adsorption capacity, superior recyclability and water resistibility [J]. Dalton Transactions, 2016, 45(28): 11306-11311. |
| 16 | BIBI R, WEI L, SHEN Q, et al. Effect of amino functionality on the uptake of cationic dye by titanium-based metal organic frameworks[J]. Journal of Chemical & Engineering Data, 2017, 62(5): 1615-1622. |
| 17 | FAN Y, ZHANG S, QIN S, et al. An enhanced adsorption of organic dyes onto NH2 functionalization titanium-based metal-organic frameworks and the mechanism investigation[J]. Microporous and Mesoporous Materials, 2018, 263: 120-127. |
| 18 | SEO Y S, KHAN N A, JHUNG S H. Adsorptive removal of methylchlorophenoxypropionic acid from water with a metal-organic framework[J]. Chemical Engineering Journal, 2015, 270: 22-27. |
| 19 | QIU J, FENG Y, ZHANG X, et al. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: adsorption performance and mechanisms[J]. Journal of Colloid and Interface Science, 2017, 499: 151-158. |
| 20 | KHAN N A, JUN J W, JEONG J H, et al. Remarkable adsorptive performance of a metal-organic framework, vanadium-benzenedicarboxylate (MIL-47), for benzothiophene[J]. Chemical Communications, 2011, 47(4): 1306-1308. |
| 21 | AHMED I, HASAN Z, KHAN N A, et al. Adsorptive denitrogenation of model fuels with porous metal-organic frameworks (MOF): effect of acidity and basicity of MOF[J]. Applied Catalysis B: Environmental, 2013, 129(2): 123-129. |
| 22 | HASAN Z, CHOI E J, JHUNG S H. Adsorption of naproxen and clofibric acid over a metal-organic framework MIL-101 functionalized with acidic and basic groups[J]. Chemical Engineering Journal, 2013, 219: 537-544. |
| 23 | KHAN N A, JUNG B K, HASAN Z, et al. Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal-organic frameworks[J]. Journal of Hazardous Materials, 2015, 282: 194-200. |
| 24 | LIU B J, YANG F, ZOU Y X, et al. Adsorption of phenol and p-nitrophenol from aqueous solutions on metal-organic frameworks: effect of hydrogen bonding[J]. Journal of Chemical and Engineering Data, 2014, 59(5): 1476-1482. |
| 25 | HASAN Z, TONG M, JUNG B K, et al. Adsorption of pyridine over amino-functionalized metal-organic frameworks: attraction via hydrogen bonding versus base-base repulsion[J]. The Journal of Physical Chemistry C, 2014, 118(36): 21049-21056. |
| 26 | AHMED I, JHUNG S H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks[J]. Journal of Hazardous Materials, 2016, 301: 259-276. |
| 27 | WU Y, CHEN H, LIU D, et al. Effective ligand functionalization of zirconium-based metal-organic frameworks for the adsorption and separation of benzene and toluene: a multiscale computational study[J]. ACS Applied Materials and Interfaces, 2015, 7(10): 5775-5787. |
| 28 | LAHOZ-MARTIN F D, MARTÍN-CALVO A, CALERO S. Selective separation of BTEX mixtures using metal-organic frameworks[J]. Journal of Physical Chemistry C, 2014, 118(24): 13126-13136. |
| 29 | BOZBIYIK B, LANNOEYE J, DE-VOS D E, et al. Shape selective properties of the Al-fumarate metal-organic framework in the adsorption and separation of n-alkanes, iso-alkanes, cyclo-alkanes and aromatic hydrocarbons[J]. Physical Chemistry Chemical Physics, 2016, 18(4): 3294-3301. |
| 30 | LI M, LI Y W, LI W, et al. Synthesis and application of Cu-BTC@ZSM-5 composites as effective adsorbents for removal of toluene gas under moist ambience: kinetics, thermodynamics and mechanism studies[J]. Environmental Science and Pollution Research, 2020, 27(6): 6052-6065. |
| 31 | LI Y, YANG Z, WANG Y, et al. A mesoporous cationic thorium-organic framework that rapidly traps anionic persistent organic pollutants[J]. Nature Communications, 2017, 8(1): 1354. |
| 32 | PI Y, LI X, XIA Q, et al. Adsorptive and photocatalytic removal of persistent organic pollutants (POPs) in water by metal-organic frameworks (MOF)[J]. Chemical Engineering Journal, 2018, 337: 351-371. |
| 33 | DAI Y X, LI M, LIU F, et al. Graphene oxide wrapped copper-benzene-1,3,5-tricarbxylate metal organic framework as efficient absorbent for gaseous toluene under ambient conditions[J]. Environmental Science and Pollution Research, 2019, 26(3): 2477-2491. |
| 34 | SUN X J, XIA Q B, ZHAO Z X, et al. Synthesis and adsorption performance of MIL-101(Cr)/graphite oxide composites with high capacities of n-hexane[J]. Chemical Engineering Journal, 2014, 239: 226-232. |
| 35 | YANG Q, REN S, ZHAO Q, et al. Selective separation of methyl orange from water using magnetic ZIF-67 composites[J]. Chemical Engineering Journal, 2018, 333: 49-57. |
| 36 | HUANG C Y, SONG M, GU Z Y, et al. Probing the adsorption characteristic of metal-organic framework MIL-101 for volatile organic compounds by quartz crystal microbalance[J]. Environmental Science & Technology, 2011, 45(10): 4490-4496. |
| 37 | DUAN C, YANG M, LI F, et al. Soft-templating synthesis of mesoporous metal-organic frameworks with enhanced toluene adsorption capacity[J]. Chemistryselect, 2018, 3(45): 12888-12893. |
| 38 | YANG J, ZHANG Y, LIU Q, et al. Principles of designing extra-large pore openings and cages in zeolitic imidazolate frameworks[J]. Journal of the American Chemical Society, 2017, 139(18): 6448-645. |
| 39 | AL-JANABI N, HILL P, TORRENTE-MURCIANO L, et al. Mapping the Cu-BTC metal-organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases[J]. Chemical Engineering Journal, 2015, 281: 669-677. |
| 40 | ZHAO Z, WANG S, YANG Y, et al. Competitive adsorption and selectivity of benzene and water vapor on the microporous metal organic frameworks (HKUST-1)[J]. Chemical Engineering Journal, 2015, 259: 79-89. |
| 41 | VELLINGIRI K, KUMAR P, DEEP A, et al. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure[J]. Chemical Engineering Journal, 2017, 307: 1116-1126. |
| 42 | ZHU M, HU P, TONG Z, et al. Enhanced hydrophobic MIL(Cr) metal-organic framework with high capacity and selectivity for benzene VOCs capture from high humid air[J]. Chemical Engineering Journal, 2017, 313: 1122-1131. |
| 43 | PETIT C, BANDOSZ T J. MOF-graphite oxide composites: combining the uniqueness of graphene layers and metal-organic frameworks[J]. Advanced Materials, 2009, 21(46): 4753-4757. |
| 44 | ZHENG Y, CHU F, ZHANG B, et al. Ultrahigh adsorption capacities of carbon tetrachloride on MIL-101 and MIL-101/graphene oxide composites[J]. Microporous and Mesoporous Materials, 2018, 263: 71-76. |
| 45 | ZHOU Y, ZHOU L, ZHANG X, et al. Preparation of zeolitic imidazolate framework-8/graphene oxide composites with enhanced VOCs adsorption capacity[J]. Microporous and Mesoporous Materials, 2016, 225: 488-493. |
| 46 | HU P, LIANG X, YASEEN M, et al. Preparation of highly-hydrophobic novel N-coordinated UiO-66(Zr) with dopamine via fast mechano-chemical method for (CHO-/Cl-)-VOCs competitive adsorption in humid environment[J]. Chemical Engineering Journal, 2018, 322: 608-618. |
| 47 | KIM B, LEE Y R, KIM H Y, et al. Adsorption of volatile organic compounds over MIL-125-NH2[J]. Polyhedron, 2018, 154: 343-349. |
| 48 | LYU G, LIU J, XIONG Z, et al. Selectivity Adsorptive mechanism of different nitrophenols on UIO-66 and UIO-66-NH2 in aqueous solution[J]. Journal of Chemical and Engineering Data, 2016, 61(11): 3868-3876. |
| 49 | HAN T T, XIAO Y L, TONG M M, et al. Synthesis of CNT@MIL-68(Al) composites with improved adsorption capacity for phenol in aqueous solution[J]. Chemical Engineering Journal, 2015, 275: 134-141. |
| 50 | PLANCHAIS A, DEVAUTOUR-VINOT S, GIRET S, et al. Adsorption of benzene in the cation-containing MOFs MIL-141[J]. Journal of Physical Chemistry C, 2015, 117(38): 19393-19401. |
| 51 | SAINI V K, JOÃO P. Development of metal organic fromwork-199 immobilized zeolite foam for adsorption of common indoor VOCs[J]. Journal of Environmental Sciences, 2016, 55(5): 321-330. |
| 52 | DUAN C X, LI F E, YANG M H, et al. Rapid synthesis of hierarchically structured multifunctional metal-organic zeolites with enhanced volatile organic compounds adsorption capacity[J]. Industrial & Engineering Chemistry Research, 2018, 57(45): 15385-15394. |
| 53 | ZHANG X, YANG Y, LYU X, et al. Adsorption/desorption kinetics and breakthrough of gaseous toluene for modified microporous-mesoporous UiO-66 metal organic framework[J]. Journal of Hazardous Materials, 2019, 366: 140-150. |
| 54 | CHU F, ZHENG Y, WEN B, et al. Adsorption of toluene with water on zeolitic imidazolate framework-8/graphene oxide hybrid nanocomposites in a humid atmosphere[J]. RSC Advances, 2018, 8(5): 2426-2432. |
| 55 | CUI X F, SUN X D, LIU L, et al. In-situ fabrication of cellulose foam HKUST-1 and surface modification with polysaccharides for enhanced selective adsorption of toluene and acidic dipeptides[J]. Chemical Engineering Journal2019, 359: 898-907. |
| 56 | LIU A, PENG X, JIN Q, et al. Adsorption and diffusion of benzene in Mg-MOF-74 with open metal sites[J]. ACS Applied Materials & Interfaces, 2019, 11(4): 4686-4700. |
| 57 | WANG C P, YIN H, TIAN P J, et al. Remarkable adsorption performance of MOF-199 derived porous carbons for benzene vapor[J]. Environmental Research, 2020,184: 109323. |
| 58 | NAVARRO A R, CIRRE L, CARBONI M, et al. BTEX removal from aqueous solution with hydrophobic Zr metal organic frameworks[J]. Journal of Environmental Management, 2018, 214: 17-22. |
| 59 | SUN Y F, MA M, TANG B, et al. Graphene modified Cu-BTC with high stability in water and controllable selective adsorption of various gases[J]. Journal of Alloys and Compounds, 2019, 808: 151721. |
| 60 | GU C, HOSONO N, ZHENG J, et al. Design and control of gas diffusion process in a nanoporous soft crystal[J]. Science, 2019, 363(6425): 387-391. |
| 61 | LIU Q, SONG Y Y, MA Y H, et al. Mesoporous cages in chemically robust MOFs created by a large number of vertices with reduced connectivity[J]. Journal of the American Chemical Society, 2019, 141(1): 488-496. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [3] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [4] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [5] | 许中硕, 周盼盼, 王宇晖, 黄威, 宋新山. 硫铁矿介导的自养反硝化研究进展[J]. 化工进展, 2023, 42(9): 4863-4871. |
| [6] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [7] | 单雪影, 张濛, 张家傅, 李玲玉, 宋艳, 李锦春. 阻燃型环氧树脂的燃烧数值模拟[J]. 化工进展, 2023, 42(7): 3413-3419. |
| [8] | 于志庆, 黄文斌, 王晓晗, 邓开鑫, 魏强, 周亚松, 姜鹏. B掺杂Al2O3@C负载CoMo型加氢脱硫催化剂性能[J]. 化工进展, 2023, 42(7): 3550-3560. |
| [9] | 杨子育, 朱玲, 王文龙, 于超凡, 桑义敏. 阴燃法处理含油污泥的研究及应用进展[J]. 化工进展, 2023, 42(7): 3760-3769. |
| [10] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [11] | 许春树, 姚庆达, 梁永贤, 周华龙. 氧化石墨烯/碳纳米管对几种典型高分子材料的性能影响[J]. 化工进展, 2023, 42(6): 3012-3028. |
| [12] | 朱雅静, 徐岩, 简美鹏, 李海燕, 王崇臣. 金属有机框架材料用于海水提铀的研究进展[J]. 化工进展, 2023, 42(6): 3029-3048. |
| [13] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [14] | 陈飞, 刘成宝, 陈丰, 钱君超, 邱永斌, 孟宪荣, 陈志刚. g-C3N4基超级电容器用电极材料的研究进展[J]. 化工进展, 2023, 42(5): 2566-2576. |
| [15] | 刘念, 陈葵, 武斌, 纪利俊, 吴艳阳, 韩金玲. 蛋黄-壳介孔磁性炭微球的制备及其对红霉素的高效吸附[J]. 化工进展, 2023, 42(5): 2724-2732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||