化工进展 ›› 2021, Vol. 40 ›› Issue (1): 401-414.DOI: 10.16085/j.issn.1000-6613.2020-0129
相变吸收酸性气体的发展现状
赵文波1( ), 李广振1, 许胜超1, 李雪霏1, 王志友2
), 李广振1, 许胜超1, 李雪霏1, 王志友2
- 1.昆明理工大学化学工程学院,云南 昆明 650500
2.中国电建集团山东电力建设第一工程有限公司河北分公司,河北 石家庄 050040
-
收稿日期:2020-01-20出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:赵文波 -
作者简介:赵文波(1982—),男,教授,博士生导师,研究方向为酸性气体吸收。E-mail:wenshuixing@126.com 。 -
基金资助:国家自然科学基金(21666011);云南省万人计划
Recent developments of acid gas absorption by phase-change
Wenbo ZHAO1( ), Guangzhen LI1, Shengchao XU1, Xuefei LI1, Zhiyou WANG2
), Guangzhen LI1, Shengchao XU1, Xuefei LI1, Zhiyou WANG2
- 1.Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
2.China Electric Power Construction Group Shandong Electric Power Construction First Engineering Co. , Ltd. , Hebei Branch, Shijiazhuang 050040, Hebei, China
-
Received:2020-01-20Online:2021-01-05Published:2021-01-12 -
Contact:Wenbo ZHAO
摘要:
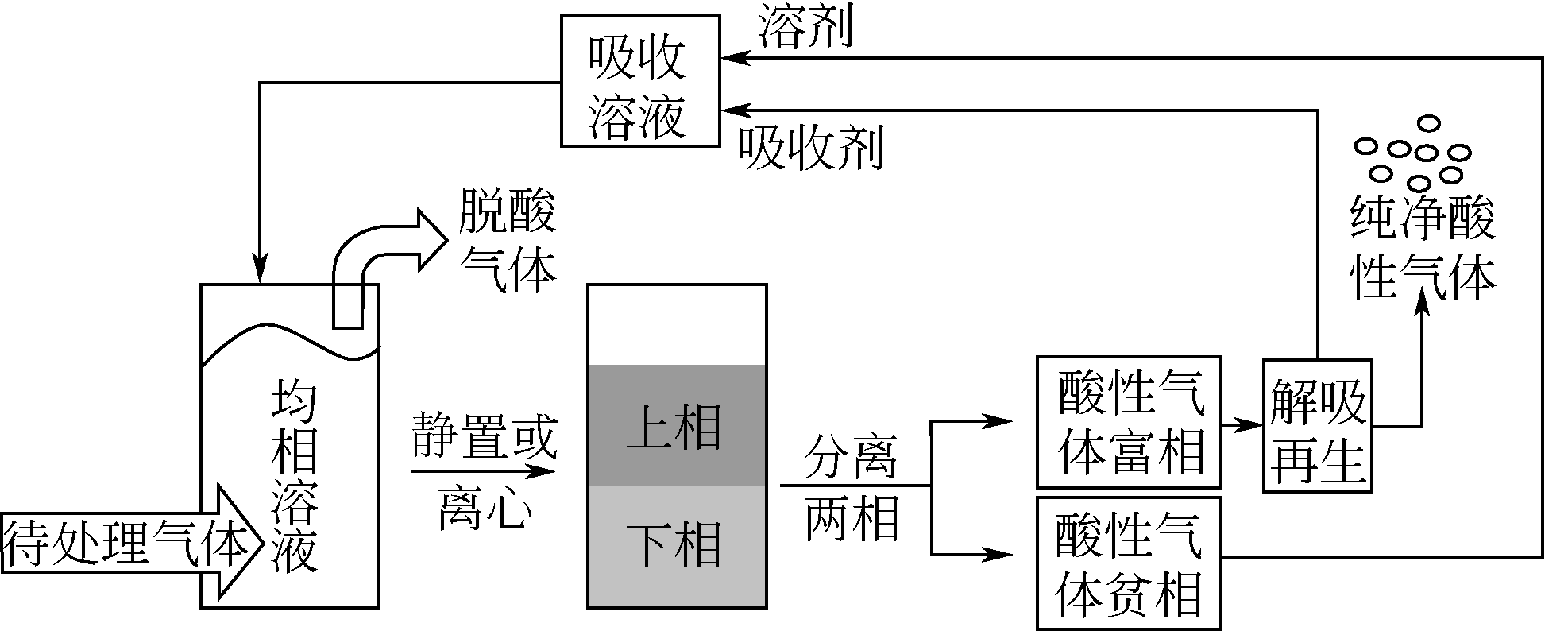
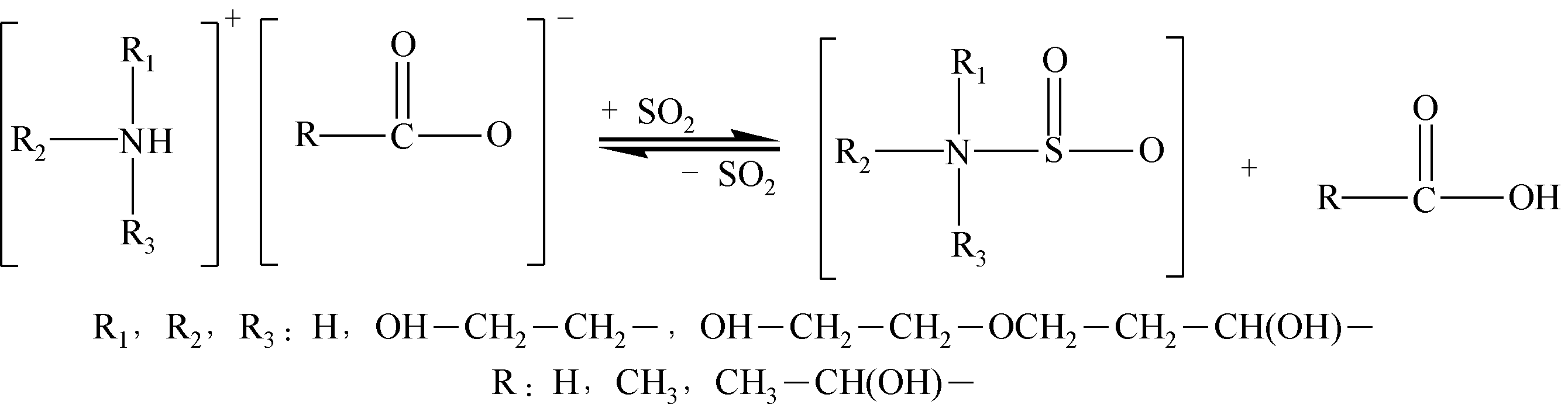
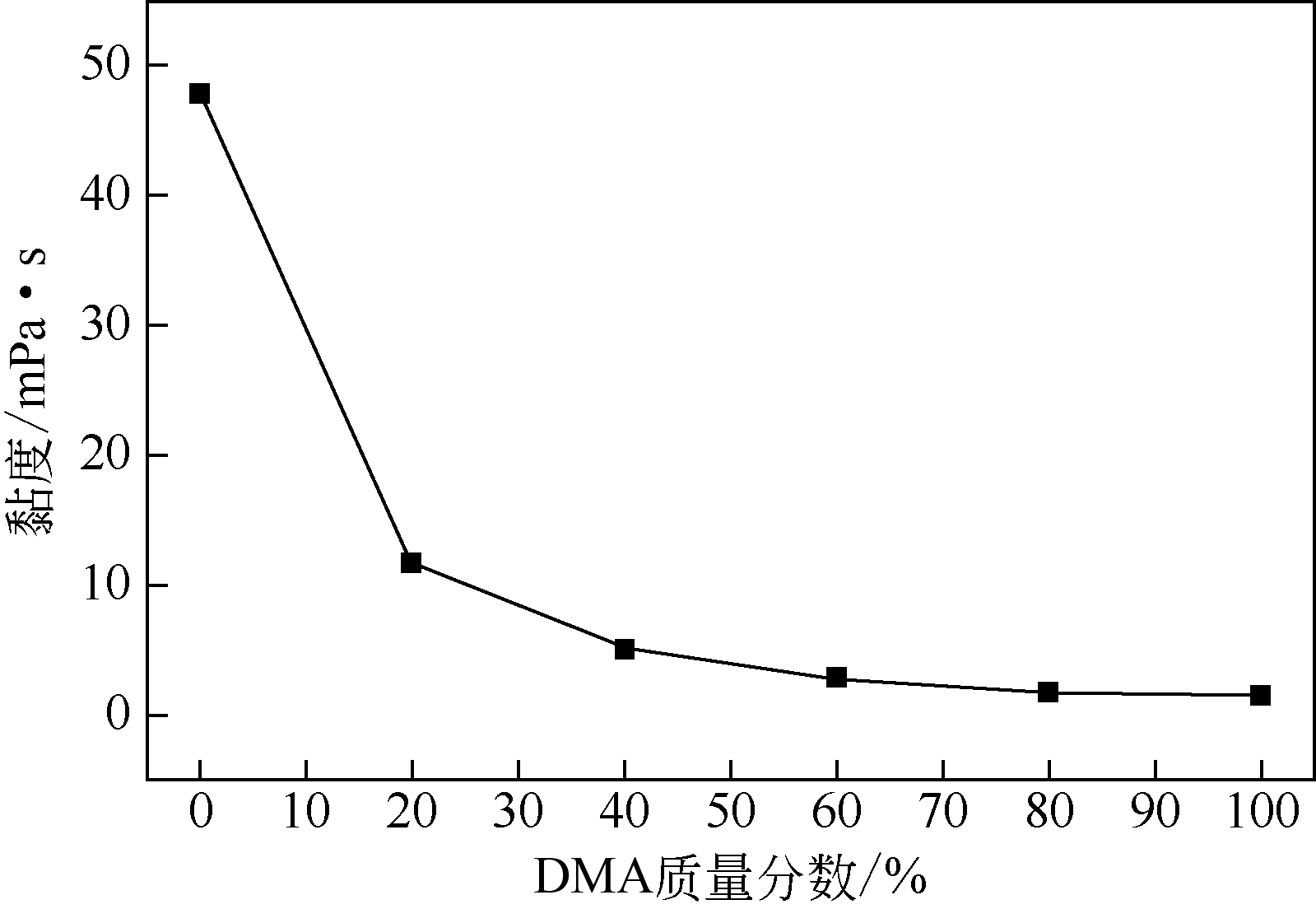
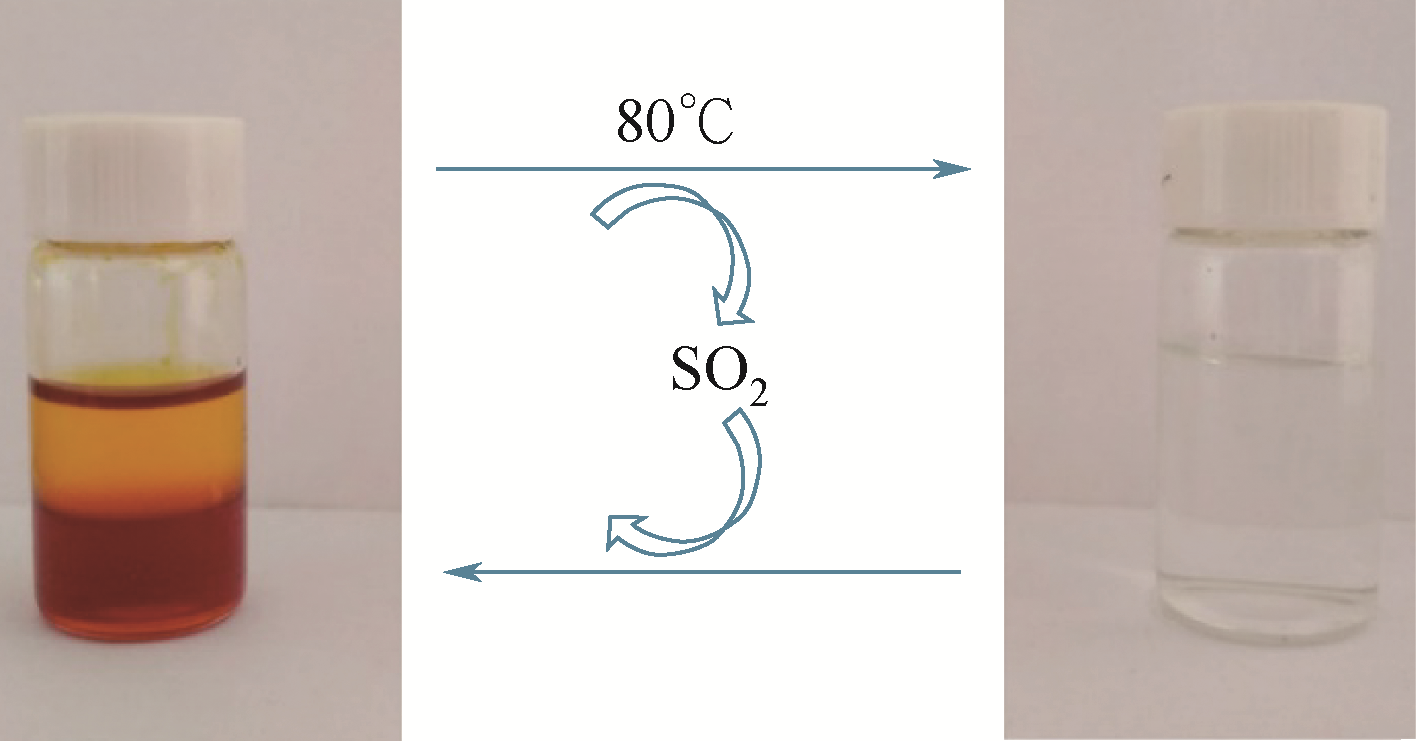
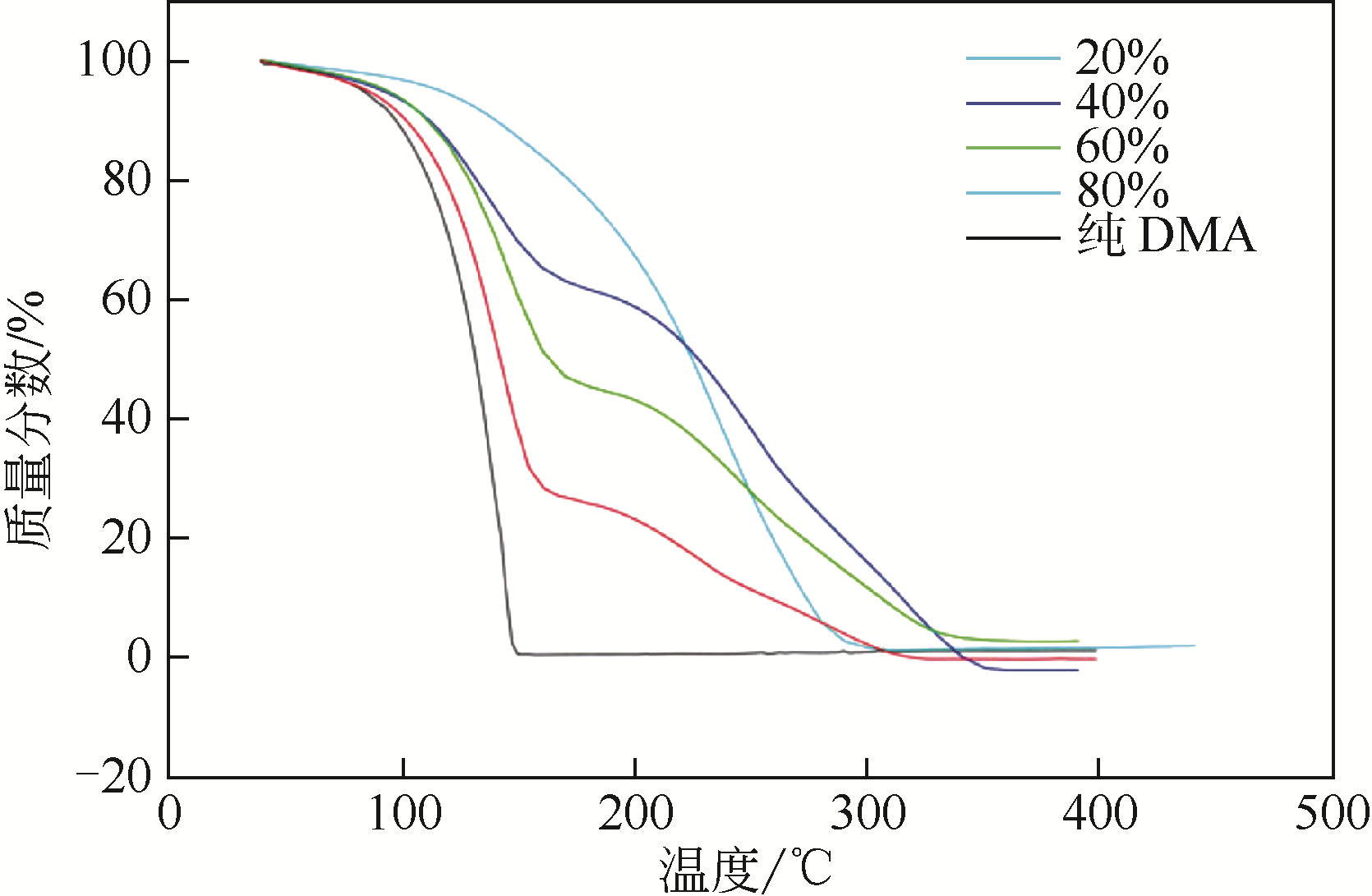
酸性气体,如CO2、SO2和H2S的过量排放已经造成温室效应或酸雨等一系列环境危害。传统的溶剂吸收法由于技术成熟、成本低廉而得到了广泛应用,但它依然存在吸收剂再生能耗高、产物附加值低的缺点。近年来,一种新的吸收方式,相变吸收(即均相体系在吸收酸性气体后分为两相,酸性气体主要富集于其中一相),引起了人们的广泛关注。该法在吸收剂再生时只需要对其中的富相进行处理,从而减少了处理量,降低了解吸能耗,且富相中的酸性气体作为一种已经被改性的原料可以直接用于合成含硫或含碳化学品,从根本上避免再生过程。本文阐述了相变吸收的研究现状,依次对CO2、SO2以及H2S体系作了详细的介绍。将相变吸收分为液-液相变、液-固相变两类,介绍了两种相变吸收的研究进展,并分析比较了它们优缺点。在实际使用中,不同领域应综合考虑。目前,相变吸收实现了吸收剂对SO2气体的质量吸收量为1.56g SO2 /g DMEA,对H2S的为0.205g H2S/ g DBN,对CO2的摩尔吸收量为1.87mol CO2/mol (TETA/PEG200),与其他方法相比,相变吸收的吸收量非常高。这些结果表明相变吸收是一种极具潜力的酸性气体捕集方式。
中图分类号:
引用本文
赵文波, 李广振, 许胜超, 李雪霏, 王志友. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414.
Wenbo ZHAO, Guangzhen LI, Shengchao XU, Xuefei LI, Zhiyou WANG. Recent developments of acid gas absorption by phase-change[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 401-414.
| 吸收方法 | 吸收剂 | 吸收量 | 反应条件 |
|---|---|---|---|
| 钙法脱硫[ | Ca(OH)2 | 0.12g/g | 60min |
| 海水法脱硫[ | 海水 | 7.463mmol/L | 25℃ |
| 离子液体[ | GT [P4442][BTFA] | 0.44g/g 0.61g/g | 20℃,1.0atm |
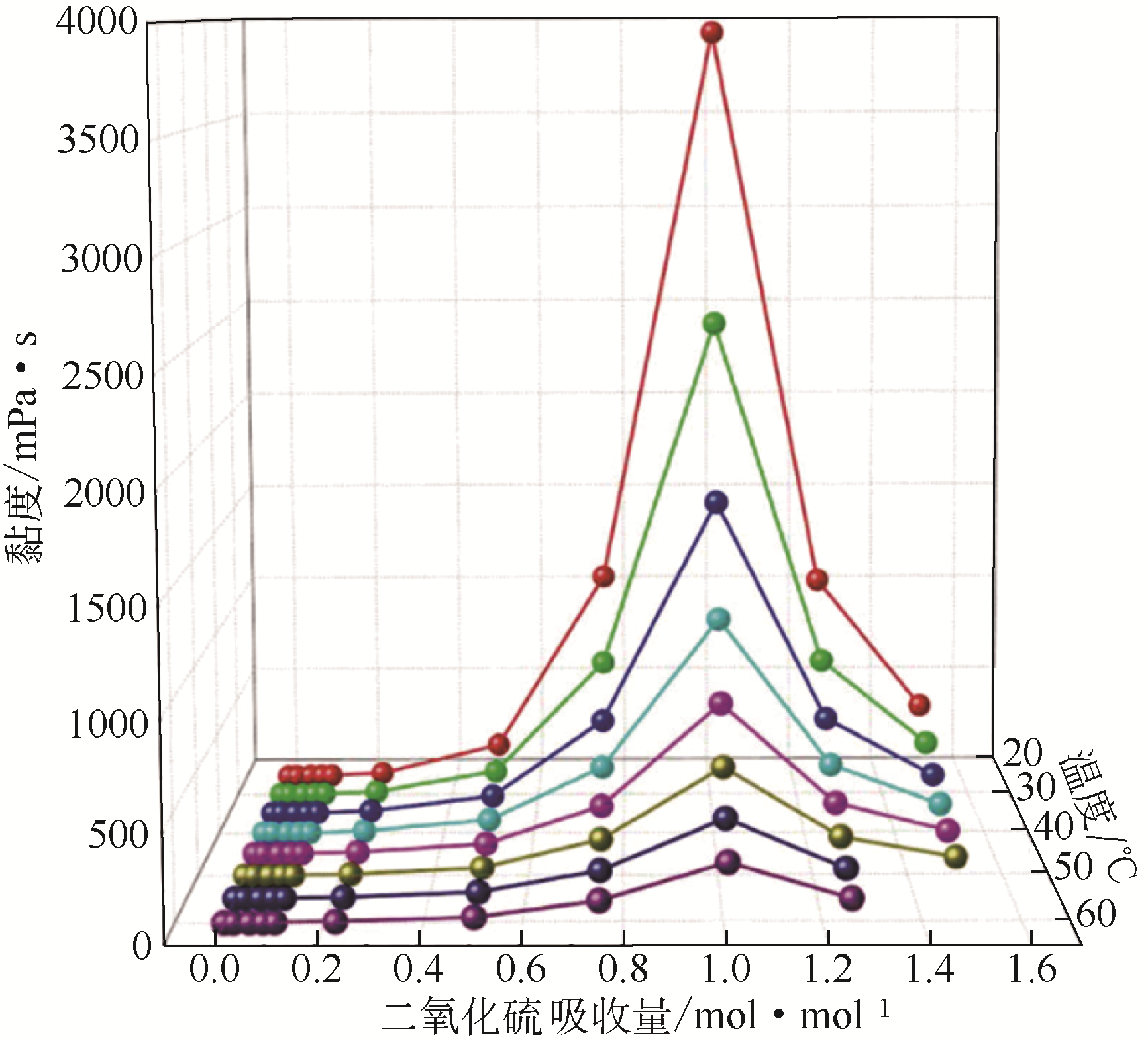
| 低共熔法[ | Emim-EG(2∶1) 己内酰胺/乙酰胺 | 1.15g/g 0.497g/g | 30℃,0.1MPa |
相变吸收[ | DMCHA/LP DMEA/正十六烷 Emim-AA | 1.19g/g 1.56g/g 1.39g/g | 20℃, 1atm |
表1 不同方法吸收SO2的吸收量
| 吸收方法 | 吸收剂 | 吸收量 | 反应条件 |
|---|---|---|---|
| 钙法脱硫[ | Ca(OH)2 | 0.12g/g | 60min |
| 海水法脱硫[ | 海水 | 7.463mmol/L | 25℃ |
| 离子液体[ | GT [P4442][BTFA] | 0.44g/g 0.61g/g | 20℃,1.0atm |
| 低共熔法[ | Emim-EG(2∶1) 己内酰胺/乙酰胺 | 1.15g/g 0.497g/g | 30℃,0.1MPa |
相变吸收[ | DMCHA/LP DMEA/正十六烷 Emim-AA | 1.19g/g 1.56g/g 1.39g/g | 20℃, 1atm |
| 名称 | 相对分子 质量 | 结构式 | 理论吸收能力 /mol·mol-1 |
|---|---|---|---|
| 乙醇胺(MEA) | 61.01 |  | 0.5 |
| 二乙醇胺(DEA) | 105.14 |  | 0.5 |
| 三乙醇胺(TEA) | 149.19 |  | 1.0 |
| N-甲基二乙醇胺(MDEA) | 119.16 |  | 1.0 |
表2 胺类吸收剂的吸收能力
| 名称 | 相对分子 质量 | 结构式 | 理论吸收能力 /mol·mol-1 |
|---|---|---|---|
| 乙醇胺(MEA) | 61.01 |  | 0.5 |
| 二乙醇胺(DEA) | 105.14 |  | 0.5 |
| 三乙醇胺(TEA) | 149.19 |  | 1.0 |
| N-甲基二乙醇胺(MDEA) | 119.16 |  | 1.0 |
| 吸收剂类别 | 典型吸收剂 | 优势 | 存在问题 |
|---|---|---|---|
| 热钾碱法 | K2CO3 | 低成本、低毒性、低挥发性、无降解 | 吸收速率低,不适合电厂烟气等低分压的情况,高温下设备易腐蚀 |
| 醇胺类 | MEA,DEA,MDEA,EDA,PZ | 伯胺和仲胺吸收速率高;叔胺再生能耗低 | 不能同时满足高吸收率和低再生能耗;易氧化 |
| 氨水 | NH3·H2O | 成本低;脱除效率和吸收负荷高,再生能耗低 | 吸收再生过程中挥发性大 |
| 混合胺[ | MEA/PZ,MDEA/PZ,空间位阻胺类 | 同时具有高吸收负荷、吸收速率和低再生能耗 | 受限于胺类自身的性质,再生性提升有限 |
| 离子液体[ | 氨基功能化离子液体 | CO2具有高选择性;高吸收负荷和低再生能耗 | 成本高,黏度大,吸收速率慢 |
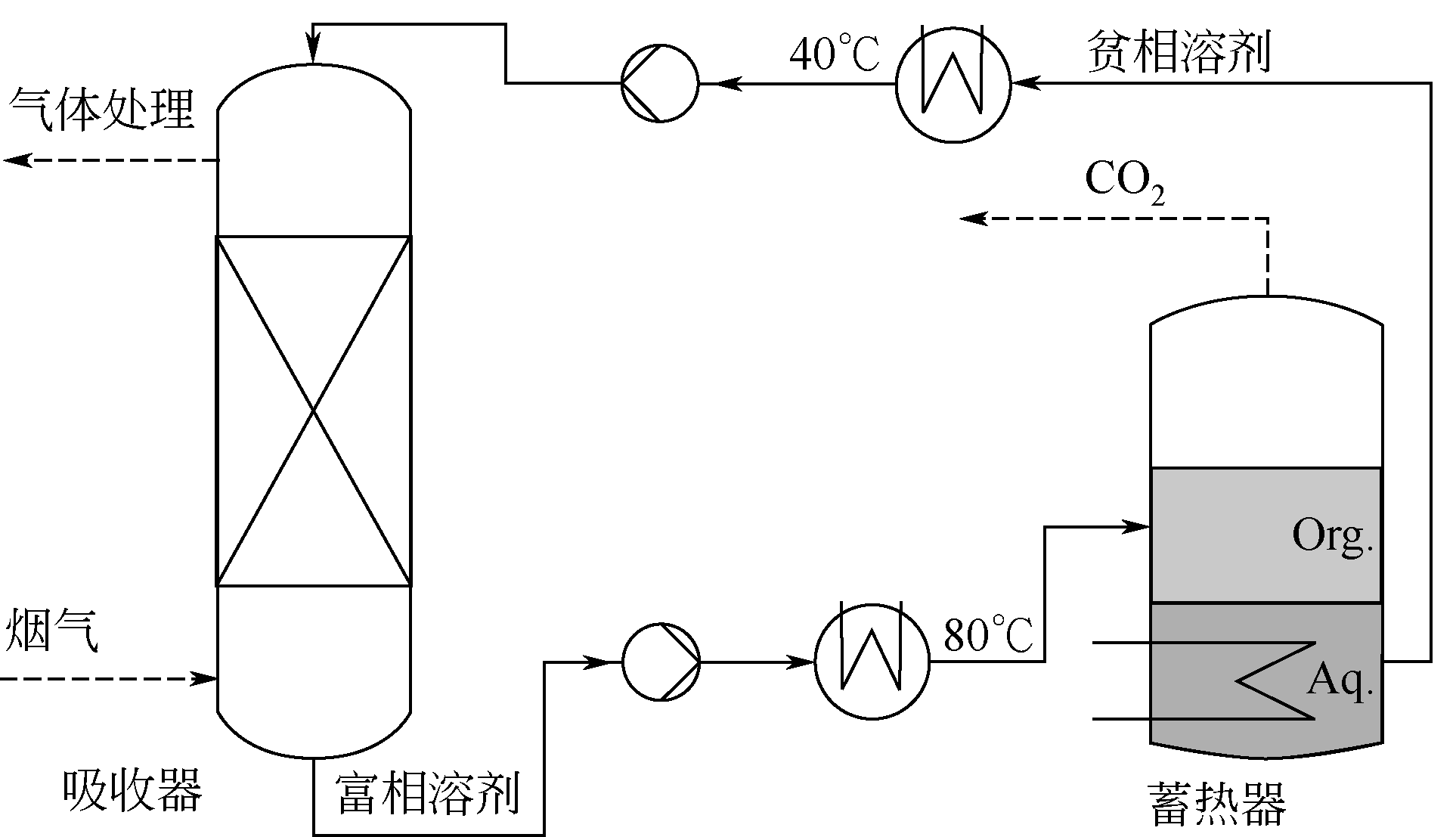
| 相变吸收剂[ | DPA/DMCA等液液相变;EDA/PEG300等液固相变 | CO2富相体积小,大大降低再生能耗;相变过程可促进反应向右移,增大吸收负荷 | 富相黏度较大 |
表3 不同的CO2吸收方法比较
| 吸收剂类别 | 典型吸收剂 | 优势 | 存在问题 |
|---|---|---|---|
| 热钾碱法 | K2CO3 | 低成本、低毒性、低挥发性、无降解 | 吸收速率低,不适合电厂烟气等低分压的情况,高温下设备易腐蚀 |
| 醇胺类 | MEA,DEA,MDEA,EDA,PZ | 伯胺和仲胺吸收速率高;叔胺再生能耗低 | 不能同时满足高吸收率和低再生能耗;易氧化 |
| 氨水 | NH3·H2O | 成本低;脱除效率和吸收负荷高,再生能耗低 | 吸收再生过程中挥发性大 |
| 混合胺[ | MEA/PZ,MDEA/PZ,空间位阻胺类 | 同时具有高吸收负荷、吸收速率和低再生能耗 | 受限于胺类自身的性质,再生性提升有限 |
| 离子液体[ | 氨基功能化离子液体 | CO2具有高选择性;高吸收负荷和低再生能耗 | 成本高,黏度大,吸收速率慢 |
| 相变吸收剂[ | DPA/DMCA等液液相变;EDA/PEG300等液固相变 | CO2富相体积小,大大降低再生能耗;相变过程可促进反应向右移,增大吸收负荷 | 富相黏度较大 |
| 1 | BARZAGLI F, LAI S, MANI F, et al. Novel non-aqueous amine solvents for biogas upgrading[J]. Energy & Fuels, 2014, 28(8): 5252-5258. |
| 2 | CASTRILLON M C, MOURA K O, ALVES C A, et al. CO2 and H2S removal from CH4-rich streams by adsorption on activated carbons modified with K2CO3, NaOH, or Fe2O3[J]. Energy & Fuels, 2016, 30(11): 9596-9604. |
| 3 | CHEN Xiaoyuan, VINH-THANG H, RAMIREZ A A, et al. Membrane gas separation technologies for biogas upgrading[J]. RSC Advances, 2015, 5(31): 24399-24448. |
| 4 | DIMARIA P C, DUTTA A, MAHMUD S. Syngas purification in cryogenic packed beds using a one-dimensional pseudo-homogenous model[J]. Energy & Fuels, 2015, 29(8): 5028-5035. |
| 5 | YAN Rong, LIANG D T, TSEN L, et al. Kinetics and mechanisms of H2S adsorption by alkaline activated carbon[J]. Environmental Science & Technology, 2002, 36(20): 4460-4466. |
| 6 | AHMED I, JHUNG Sung Hwa. Adsorptive desulfurization and denitrogenation using metal-organic frameworks[J]. Journal of Hazardous Materials, 2016, 301: 259-276. |
| 7 | WANG Xianfeng, AKHMEDOV N G, HOPKINSON D, et al. Phase change amino acid salt separates into CO2-rich and CO2-lean phases upon interacting with CO2[J]. Applied Energy, 2016, 161: 41-47. |
| 8 | SRIVASTAVA R K, JOZEWICZ W, SINGER C. SO2 scrubbing technologies: a review[J]. Environmental Progress, 2001, 20(4): 219-228. |
| 9 | 周理明, 史永永, 李海洋, 等. 氨法烟气脱硫过程的工艺优化[J]. 化学工程, 2014, 42(4): 7-12. |
| ZHOU Liming, SHI Yongyong, LI Haiyang, et al. Process optimization of ammonia flue gas desulfurization process[J]. Chemical Engineering, 2014, 42(4): 7-12. | |
| 10 | OIKAWA K, YONGSIRI C, TAKEDA K, et al. Seawater flue gas desulfurization: its technical implications and performance results[J]. Environmental Progress & Sustainable Energy, 2003, 22(1): 67-73. |
| 11 | 魏巍. 烟气氧化镁法脱硫技术研究[J]. 山西能源与节能, 2004(3): 20-21. |
| WEI Wei. Study on desulphurization technology of flue gas magnesium oxide[J]. Shanxi Energy and Energy Conservation, 2004(3): 20-21. | |
| 12 | 王智友, 陈雯, 耿家锐. 有机胺烟气脱硫现状[J]. 云南冶金, 2009, 38(1): 40-43. |
| WANG Zhiyou, CHEN Wen, GENG Jiarui. Status of organic amine flue gas desulfurization[J]. Yunnan Metallurgy, 2009, 38(1): 40-43. | |
| 13 | WALKER R J, WILDMAN D J, GASIOR S J. Evaluation of some regenerate sulfur dioxide absorbents for flue gas desulfurization[J]. Journal of the Air Pollution Control Association, 1983, 33(11): 1061-1067. |
| 14 | 汤志刚, 周长城, 陈成. 乙二胺/磷酸溶液化学吸收法烟气脱硫的研究[J]. 高校化学工程学报, 2005, 19(3): 285-291. |
| TANG Zhigang, ZHOU Changcheng, CHEN Cheng. Chemical absorption of SO2 by using ethylene diamine/phosphoric acid solution[J]. Journal of Chemical Engineering of Chinese Universities, 2005, 19(3): 285-291. | |
| 15 | WU Weize, HAN Buxing, GAO Haixiang, et al. Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angewandte Chemie International Edition, 2004, 43(18): 2415-2417. |
| 16 | HUANG Jun, RIISAGER A, WASSERSCHEID P, et al. Reversible physical absorption of SO2 by ionic liquids[J]. Chemical Communications, 2006, 38: 4027-4029. |
| 17 | 崔国凯, 张峰涛, 黄艳杰, 等. 酰胺基离子液体在酸性气体捕集中的应用[C]//中国化学会第30届学术年会摘要集-第三十三分会: 绿色化学, 2016. |
| CUI Guokai, ZHANG Fengtao, HUANG Yanjie,et al. Acid gas capture by acylamido-functionalized ionic liquids[C]// Abstract of the 30th annual meeting of the Chinese Chemical Society, Chapter33: Green Chemistry, 2016. | |
| 18 | WANG Congmin, ZHENG Junjie, CUI Guokai, et al. Highly efficient SO2 capture through tuning the interaction between anion-functionalized ionic liquids and SO2[J]. Chemical Communications, 2013, 49(12): 1166-1168. |
| 19 | CUI Guokai, LIN Wenjun, DING Fang, et al. Highly efficient SO2 capture by phenyl-containing azole-based ionic liquids through multiple-site interactions[J]. Green Chemistry, 2014, 16(3): 1211-1216. |
| 20 | DING Fang, ZHENG Junjie, CHEN Yaqian, et al. Highly efficient and reversible SO2 capture by surfactant-derived dual functionalized ionic liquids with metal chelate cations[J]. Industrial & Engineering Chemistry Research, 2014, 53(48): 18568-18574. |
| 21 | CHEN Kaihong, LIN Wenjun, YU Xini, et al. Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas[J]. AIChE Journal, 2015, 61(6): 2028-2034. |
| 22 | YUAN Xiaoliang, ZHANG Suojiang, LU Xingmei. Hydroxyl ammonium ionic liquids: synthesis, properties, and solubility of SO2[J]. Journal of Chemical & Engineering Data, 2007, 52(2): 596-599. |
| 23 | YANG Dezhong, HOU Minqiang, NING Hui, et al. Efficient SO2 absorption by renewable choline chloride-glycerol deep eutectic solvents[J]. Green Chemistry, 2013, 15(8): 2261-2265. |
| 24 | LI Hongping, CHANG Yonghui, ZHU Wenshuai, et al. Theoretical evidence of charge transfer interaction between SO2 and deep eutectic solvents formed by choline chloride and glycerol[J]. Physical Chemistry Chemical Physics, 2015, 17(43): 28729-28742. |
| 25 | ZHANG Kai, REN Shuhang, HOU Yucui, et al. Efficient absorption of SO2 with low-partial pressures by environmentally benign functional deep eutectic solvents[J]. Journal of Hazardous Materials, 2017, 324(B): 457-463. |
| 26 | DUAN Erhong, GUO Bin, ZHANG Miaomiao, et al. Efficient capture of SO2 by a binary mixture of caprolactam tetrabutyl ammonium bromide ionic liquid and water[J]. Journal of Hazardous Materials, 2011, 194: 48-52. |
| 27 | HELDEBRANT D J, KOECH P K, YONKER C R. A reversible zwitterionic SO2-binding organic liquid[J]. Energy & Environmental Science, 2010, 3(1): 111-113. |
| 28 | CHAI Muyuan, ZHAO Wenbo, LI Genming, et al. Novel SO2 phase-change absorbent: mixture of N,N-dimethylaniline and liquid paraffin[J]. Industrial & Engineering Chemistry Research, 2018, 57(37): 12502-12510. |
| 29 | LI Genming, ZHAO Wenbo, CHAI Muyuan, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethylcyclohexylamine as absorbent and liquid paraffin as solvent[J]. Journal of Hazardous Materials, 2018, 360: 89-96. |
| 30 | XU Shengchao, ZHAO Wenbo, CHAI Muyuan, et al. Development of SO2 phase change absorption: viscosity change and component distribution rules[J]. Energy & Fuels, 2019, 33(10): 10029-10038. |
| 31 | SHANNON M S, IRVIN A C, LIU Haining, et al. Chemical and physical absorption of SO2 by N-functionalized imidazoles: experimental results and molecular-level insight[J]. Industrial & Engineering Chemistry Research, 2014, 54(1): 462-471. |
| 32 | BARA J E. Versatile and scalable method for producing N-functionalized imidazoles[J]. Industrial & Engineering Chemistry Research, 2011, 50(24): 13614-13619. |
| 33 | 汪洋. 有机体系相变捕集酸性气体研究[D]. 昆明: 昆明理工大学, 2018. |
| WANG Yang. Study on phase change trapping of acidic gases in organic systems[D]. Kunming: Kunming University of Science and Technology, 2018. | |
| 34 | WANG Yang, ZHAO Wenbo, CHAI Muyuan, et al. Phase-change absorption of SO2 by imidazole in organic solvents and conversion of the absorption product in the presence of water and oxygen[J]. Energy & Fuels, 2017, 31(12): 13999-14006. |
| 35 | ZHAO Qian, ZHAO Wenbo, CHAI Muyuan, et al. Phase-change absorption of SO2 by N,N,Nʹ,Nʹ-tetramethyl-p-phenylenediamine in organic solvents and utilization of absorption product[J]. Energy & Fuels, 2018, 32(2): 2073-2080. |
| 36 | 何柯佳, 郑娜, 宋蔷, 等. 钙基吸收剂脱除SOx过程中SO3吸收量的测量方法[J]. 中国电机工程学报, 2019, 39(20): 5973-5978. |
| HE Kejia, ZHENG Na, SONG Qiang, et al. A novel method for the measurement of SO3 absorption amount by calcium based absorbent during SOx removal process[J]. Proceedings of the CSEE, 2019, 39(20): 5973-5978. | |
| 37 | 张清凤. 基于喷射鼓泡塔的船用海水法脱硫特性研究[D]. 南京: 东南大学, 2016. |
| ZHANG Qingfeng. Study on desulfurization characteristics of marine seawater process based on injection bubble tower[D]. Nanjing: Southeast University, 2016. | |
| 38 | DENG Dongshun, LIU Xiaobang, CUI Yanhong, et al. Investigation of SO2 solubilities in some biobased solvents and their thermodynamic properties[J]. Journal of Chemical Thermodynamics, 2018, 119: 84-91. |
| 39 | YANG Dezhong, HAN Yanling, QI Hongbin, et al. Efficient absorption of SO2 by EmimCl-EG deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6382-6386. |
| 40 | CUI Guokai, ZHAO Ning, LI Yanan, et al. Limited number of active sites strategy for improving SO2 capture by ionic liquids with fluorinated acetylacetonate anion[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7985-7992. |
| 41 | LIU Baoyou, ZHAO Jingjing, WEI Fuxiang. Characterization of caprolactam based eutectic ionic liquids and their application in SO2 absorption[J]. Journal of Molecular Liquids, 2013, 180: 19-25. |
| 42 | EMMETT E J, RICHARDS-TAYLOR C S, NGUYEN B, et al. Palladium-catalysed aminosulfonylation of aryl-, alkenyl- and heteroaryl halides: scope of the three-component synthesis of N-aminosulfonamides[J]. Organic & Biomolecular Chemistry, 2012, 10(20): 4007-4014. |
| 43 | NGUYEN B, EMMETT E J, WILLIS M C. Palladium-catalyzed aminosulfonylation of aryl halides[J]. Journal of the American Chemical Society, 2010, 132(46): 16372-16373. |
| 44 | EMMETT E J, HAYTER B R, WILLIS M C. Palladium-catalyzed synthesis of ammonium sulfinates from aryl halides and a sulfur dioxide surrogate: a gas- and reductant-free process[J]. Angewandte Chemie International Edition, 2014, 53(38): 10204-10208. |
| 45 | ROCKE B N, BAHNCK K B, HERR M, et al. Synthesis of sulfones from organozinc reagents, DABSO, and alkyl halides[J]. Organic Letters, 2013, 16(1): 154-157. |
| 46 | MALET-SANZ L, MADRZAK J, LEY S V, et al. Preparation of arylsulfonyl chlorides by chlorosulfonylation of in situ generated diazonium salts using a continuous flow reactor[J]. Organic & Biomolecular Chemistry, 2010, 8(23): 5324-5332. |
| 47 | YANG Zhenzhen, HE Liangnian, ZHAO Yanan, et al. Highly efficient SO2 absorption and its subsequent utilization by weak base/polyethylene glycol binary system[J]. Environmental Science & Technology, 2013, 47(3): 1598-1605. |
| 48 | WU Jiangping, EMEIGH J, SU Xiping. Alkylation of magnesium sulfinates: a direct transformation of functionalized aromatic/heteroaromatic halides into sulfones[J]. Organic Letters, 2005, 7(7): 1223-1225. |
| 49 | MUMFORD K A, SMITH K H, ANDERSON C J, et al. Post-combustion capture of CO2: results from the solvent absorption capture plantat hazelwood power station using potassium carbonate solvent[J]. Energy Fuels, 2012, 26(1): 138-146. |
| 50 | ZHANG Minkai, GUO Yincheng. Process simulations of NH3 abatement system for large-scale CO2 capture using aqueous ammonia solution[J]. International Journal of Greenhouse Gas Control, 2013, 18: 114-127. |
| 51 | PUXTY G, ROWLAND R, ALLPORT A, et al. Carbon dioxide postcombustion capture: a novel screening study of the carbon dioxide absorption performance of 76 amines[J]. Environmental Science & Technology, 2009, 43(16): 6427-6433. |
| 52 | MANDAL B P, BANDYOPADHYAY S S. Simultaneous absorption of carbon dioxide and hydrogen sulfide into aqueous blends of 2-amino-2-methyl-1-propanol and diethanolamine[J]. Chemical Engineering Science, 2005, 60(22): 6438-6451. |
| 53 | 王金莲, 方梦祥, 晏水平, 等. 吸收CO2新型混合化学吸收剂的研究[J]. 环境科学, 2007, 28(11): 2630-2636. |
| WANG Jinlian, FANG Mengxiang, YAN Shuiping, et al. Study of new blended chemical absorbents to absorb CO2[J]. Environmental Science, 2007, 28(11): 2630-2636. | |
| 54 | SAKWATTANAPONG R, AROONWILAS A, VEAWAB A. Behavior of reboiler heat duty for CO2 capture plants using regenerable single and blended alkanolamines[J]. Industrial & Engineering Chemistry Research, 2005, 44(12): 4465-4473. |
| 55 | MANDAL B P, BANDYOPADHYAY S S. Absorption of carbon dioxide into aqueous blends of 2-amino-2-methyl-1-propanol and monoethanolamine[J]. Chemical Engineering Science, 2006, 61(16): 5440-5547. |
| 56 | MANDAL B P, BISWAS A K, BANDYOPADHYAY S S. Absorption of carbon dioxide into aqueous blends of 2-amino-2-methyl-1-propanol and diethanolamine[J]. 2003, 58(18): 4137-4144. |
| 57 | MANDAL B P, GUHA M, BISWAS A K, et al. Removal of carbon dioxide by absorption in mixed amines: modelling of absorption in aqueous MDEA/MEA and AMP/MEA solutions[J]. Chemical Engineering Science, 2001, 56(21): 6217-6224. |
| 58 | SISTLA Y S, JAIN L, KHANNA A. Validation and prediction of solubility parameters of ionic liquids for CO2 capture[J]. Separation & Purification Technology, 2012, 97: 51-64. |
| 59 | MORTAZAVI-MANESH S, SATYRO M, MARRIOTT R A. A semi-empirical Henry’s law expression for carbon dioxide dissolution in ionic liquids[J]. Fluid Phase Equilibria, 2011, 307(2): 208-215. |
| 60 | BATES E D, MAYTON R D, NTAI I, et al. CO2 capture by a task-specific ionic liquid[J]. Journal of the American Chemical Society, 2002, 124(6): 926-927. |
| 61 | ZHANG Yanqiang, ZHANG Suojiang, LU Xingmei, et al. Dual amino-functionalised phosphonium ionic liquids for CO2 capture[J]. Chemistry: A European Journal, 2009, 15(12): 3003-3011. |
| 62 | GURKAN B E, FUENTE J C D L, MINDRUP E M, et al. Equimolar CO2 absorption by anion-functionalized ionic liquids[J]. Journal of the American Chemical Society, 2010, 132(7): 2116-2117. |
| 63 | LI Xiaoyong, HOU Minqiang, HAN Buxing, et al. Solubility of CO2 in a choline chloride + urea eutectic mixture[J]. Journal of Chemical & Engineering Data, 2008, 53(2): 548-550. |
| 64 | LERON R B, CAPARANGA A, LI Menghui. Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T=303.15-343.15K and moderate pressures[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(6): 879-885. |
| 65 | LERON R B, LI Menghui. Solubility of carbon dioxide in a eutectic mixture of choline chloride and glycerol at moderate pressures[J]. Journal of Chemical Thermodynamics, 2013, 57: 131-136. |
| 66 | ZHANG Jiafei, AGAR D W, ZHANG Xiaohui, et al. CO2 absorption in biphasic solvents with enhanced low temperature solvent regeneration[J]. Energy Procedia, 2011, 4: 67-74. |
| 67 | ZHANG Jiafei. Study on CO2 capture using thermomorphic biphasic solvents with energy efficient regeneration[D]. Dortmund: TU-Dortmund University, 2014. |
| 68 | ZHANG Jiafei, QIAO Yu, WANG Wanzhong, et al. Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents[J]. Energy Procedia, 2013, 37: 1254-1261. |
| 69 | ZHANG Jiafei, QIAO Yu, AGAR D W. Intensification of low temperature thermomorphic biphasic amine solvent regeneration for CO2 capture[J]. Chemical Engineering Research and Design, 2012, 90(6): 743-749. |
| 70 | 徐志成, 王淑娟, 陈昌和. MAPA、DEEA、BDA及BDA/DEEA混合物吸收CO2的试验研究[J]. 燃烧科学与技术, 2013, 19(2): 103-108. |
| XU Zhicheng, WANG Shujuan, CHEN Changhe. CO2 solvent by MAPA, DEEA, BDA and BDA/DEEA mixtures[J]. Combustion Science and Technology, 2013, 19(2): 103-108. | |
| 71 | XU Zhicheng, WANG Shujuan, CHEN Changhe. CO2 absorption by biphasic solvents: mixtures of 1,4-butanediamine and 2-(diethylamino)-ethanol[J]. International Journal of Greenhouse Gas Control, 2013, 16: 107-115. |
| 72 | XU Zhicheng, WANG Shujuan, QI Guojie, et al. CO2 absorption by biphasic solvents: comparison with lower phase alone[J]. Oil & Gas Science and Technology, 2014, 69(5): 851-864. |
| 73 | 徐志成, 王淑娟, 陈昌和. 液液两相吸收剂吸收CO2的试验研究[J]. 清华大学学报(自然科学版), 2013, 53(3): 336-341. |
| XU Zhicheng, WANG Shujuan, CHEN Changhe. Experimental study of CO2 absorption by liquid-liquid biphasic solvents[J]. Journal of Tsinghua University (Science and Technology), 2013, 53(3): 336-341. | |
| 74 | 徐志成, 王淑娟, 陈昌和. BDA/DEEA两相吸收剂吸收CO2的研究[J]. 工程热物理学报, 2013, 34(5): 993-997. |
| XU Zhicheng, WANG Shujuan, CHEN Changhe. CO2 absorption by BDA/DEEA biphasic solvents[J]. Journal of Engineering Thermophysics, 2013, 34(5): 993-997. | |
| 75 | 安山龙. 相变溶剂吸收二氧化碳反应动力学研究[D]. 保定: 华北电力大学, 2017. |
| AN Shanlong. Kinetics of carbon dioxide absorption by phase-change absorbents[D]. Baoding: North China Electric Power University, 2017. | |
| 76 | ZHOU Haicheng, XU Xin, CHEN Xiaochun, et al. Novel ionic liquids phase change solvents for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2020, 98: 103068. |
| 77 | 张政. 有机胺非水体系相变吸收CO2研究[D]. 昆明: 昆明理工大学, 2016. |
| ZHANG Zheng. CO2 phase change absorption in non-aqueous organic amine systems[D]. Kunming: Kunming University of Science and Technology, 2016. | |
| 78 | ZHANG Zheng, ZHAO Wenbo, NONG Jiejing, et al. Liquid-solid phase-change behavior of diethylenetriamine in nonaqueous systems for carbon dioxide absorption[J]. Energy Technology, 2017, 5(3): 461-468. |
| 79 | ZHANG Zheng, LIU Biao, QIN Xianye, et al. Relationship between the property of cloud point and CO2 absorption and desorption of amines[C]// Proceedings of the Advanced Materials Research, 2014. |
| 80 | DARDE V C A, THOMSEN K, WELL W J M VAN, et al. Chilled ammonia process for CO capture[J]. International Journal of Greenhouse Gas Control, 2010, 4(2): 131-136. |
| 81 | MA’MUN S, KIM Inna. Selection and characterization of phase-change solvent for carbon dioxide capture: precipitating system[J]. Energy Procedia, 2013, 37: 331-339. |
| 82 | ZHANG Shihan, YE Xinhuai, LU Yongqi. Development of a potassium carbonate-based absorption process with crystallization-enabled high-pressure stripping for CO2 capture: vapor-liquid equilibrium behavior and CO2 stripping performance of carbonate/bicarbonate aqueous systems[J]. Energy Procedia, 2014, 63: 665-675. |
| 83 | MIMURA T, SIMAYOSHI H, SUDA T, et al. Development of energy saving technology for flue gas carbon dioxide recovery in power plant by chemical absorption method and steam system[J]. Energy Conversion & Management, 1997, 38(S1): S57-S62. |
| 84 | PAULECHKA Y U. Heat capacity of room-temperature ionic liquids: a critical review[J]. Journal of Physical & Chemical Reference Data, 2010, 39(3): 033108. |
| 85 | KAR S, SEN R, GOEPPERT A, et al. Integrative CO2 capture and hydrogenation to methanol with reusable catalyst and amine: toward a carbon neutral methanol economy[J]. Journal of the American Chemical Society, 2018, 140(5): 1580-1583. |
| 86 | KÖNST P, MIRELES I H, STEL R VAN DER, et al. Integrated system for capturing CO2 as feedstock for algae production[J]. Energy Procedia, 2017, 114: 7126-7132. |
| 87 | ZHAO Yi, WANG Tianhao, WANG Yongbin, et al. Simultaneous absorption and hydrogenation of CO2 from flue gas by KBH4 catalyzed by nickel nanoparticles supported on TiO2[J]. Chemical Engineering Journal, 2020, 380: 122523. |
| 88 | Fan-Yuan JOU, MATHER A E. Solubility of hydrogen sulfide in[bmim][PF6][J]. International Journal of Thermophysics, 2007, 28(2): 490. |
| 89 | HUANG Kuan, CAI Daniu, CHEN Yongle, et al. Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption[J]. AIChE Journal, 2013, 59(6): 2227-2235. |
| 90 | ZHENG Wentao, WU Dongsheng, FENG Xi, et al. Low viscous protic ionic liquids functionalized with multiple Lewis base for highly efficient capture of H2S[J]. Journal of Molecular Liquids, 2018, 263: 209-217. |
| 91 | LIU Fujian, CHEN Wei, MI Jinxing, et al. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures[J]. AIChE Journal, 2019, 65(5): e16574. |
| 92 | XU Zhiyong, ZHAO Wenbo, XIE Xuhao, et al. Phase-change reversible absorption of hydrogen sulfide by the superbase 1,5-diazabicyclo[4.3.0] non-5-ene in organic solvents[J]. Industrial & Engineering Chemistry Research, 2019, 58(4): 1701-1710. |
| 93 | 董群, 武显春, 单希林, 等. 化学吸收-催化氧化法脱除酸性气中硫化氢的试验研究[J]. 石油炼制与化工, 2000, 31(9): 17-19. |
| DONG Qun, WU Xianchun, SHAN Xilin. et al. Experimental study on removal of hydrogen sulfide from acidic gas by chemical absorption and catalytic oxidation[J]. Petroleum Processing and Petrochemicals, 2000, 31(9): 17-19. | |
| 94 | 朱菊华, 黄妍, 童志权. Fe2(SO4)3溶液吸收H2S废气工艺研究[J]. 化工进展, 2004, 23(3): 277-281. |
| ZHU Juhua, HUANG Yan, TONG Zhiquan. Study on absorption process of H2S waste gas by Fe2(SO4)3 solution[J]. Chemical Industry and Engineering Progress, 2004, 23(3): 277-281. | |
| 95 | 严召, 童志权, 张俊丰. Zn/Fe体系湿法催化氧化高效脱除沼气中H2S的研究[J]. 环境工程学报, 2008, 2(1): 137-141. |
| YAN Zhao, TONG Zhiquan, ZHANG Junfeng. Efficient removal of H2S from biogas by catalytic wet oxidation with zinc/iron catalyst[J]. Chinese Journal of Environmental Engineering, 2008, 2(1): 137-141. | |
| 96 | 袁响玲. 铁基湿式氧化净化药厂硫化氢的研究[D]. 石家庄: 河北科技大学, 2013. |
| YUAN Xiangling. Study on Iron-based wet-oxidation purification of hydrogen sulfide from Pharmaceutical factory[D]. Shijiazhuang: Hebei University of Science & Technology, 2013. | |
| 97 | 纪罗军. 硫化氢气体制备甲硫醇的现状与前景[J]. 精细石油化工进展, 2003, 4(3): 13-15. |
| JI Luojun. The present status and prospects of producing methanethi ol from hydrogen sulfide[J]. Advance in Fine Petrochemicals, 2003, 4(3): 13-15. | |
| 98 | 耿胜楚. 利用煤化工装置含硫化氢酸性气生产甲硫醇钠的工艺研究[J]. 氮肥与合成气, 2019, 47(7): 16-18. |
| GENG Shengchu. Study on the production of sodium methanethiol by hydrogen sulphide acid gas in coal chemical plant[J]. Nitrogen Fertilizer and Syngas, 2019, 47(7): 16-18. | |
| 99 | SINGH G, NAKADE P G, CHETIA D, et al. Kinetics and mechanism of phase transfer catalyzed synthesis of aromatic thioethers by H2S-rich methyldiethanolamine[J]. Journal of Industrial & Engineering Chemistry, 2016, 37: 190-197. |
| [1] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [2] | 桑伟, 唐建峰, 花亦怀, 陈洁, 孙培源, 许义飞. 物理溶剂及有机胺的性质对相变吸收性能的影响[J]. 化工进展, 2023, 42(4): 2151-2159. |
| [3] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [4] | 王子宗, 刘罡, 王振维. 乙烯丙烯生产过程强化技术进展及思考[J]. 化工进展, 2023, 42(4): 1669-1676. |
| [5] | 毛停停, 李双福, 黄李茗铭, 周川玲, 韩凯. 面向水处理与有机溶剂回收的太阳能界面蒸发系统与材料[J]. 化工进展, 2023, 42(1): 178-193. |
| [6] | 肖周荣, 李国柱, 王涖, 张香文, 谷建民, 王德松. 液体碳氢燃料蒸汽重整制氢催化剂研究进展[J]. 化工进展, 2022, 41(S1): 97-107. |
| [7] | 闫鹏, 程易. 用于分布式制氢的甲烷蒸汽重整膜反应器的数值模拟[J]. 化工进展, 2022, 41(7): 3446-3454. |
| [8] | 石一慈, 潘艳秋, 王成宇, 范嘉禾, 俞路. 焦耳效应强化气隙式膜蒸馏脱盐过程的实验研究[J]. 化工进展, 2022, 41(5): 2285-2291. |
| [9] | 孙逊, 赵越, 玄晓旭, 赵珊, YOON Joon Yong, 陈颂英. 基于水力空化的化工过程强化研究进展[J]. 化工进展, 2022, 41(5): 2243-2255. |
| [10] | 宋飞, 王君妍, 何林, 隋红, 李鑫钢. 表面活性剂强化重质油矿溶剂萃取残渣中残留溶剂鼓泡分离[J]. 化工进展, 2022, 41(4): 2007-2014. |
| [11] | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| [12] | 张春, 王学瑞, 刘华, 高雪超, 张玉亭, 顾学红. 面向工业过程碳减排的分子筛膜技术研究进展[J]. 化工进展, 2022, 41(3): 1376-1390. |
| [13] | 唐思扬, 李星宇, 鲁厚芳, 钟山, 梁斌. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| [14] | 孟祥伟, 吴晓莉, 高展鹏, 李文鹏, 王景涛. 蛭石层状膜的制备及有机溶剂纳滤性能[J]. 化工进展, 2022, 41(11): 5986-5995. |
| [15] | 刘庆丰, 廖亚龙, 吴越, 郗家俊, 嵇广雄. 黄铜矿强化浸出研究进展[J]. 化工进展, 2022, 41(11): 6099-6110. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||