化工进展 ›› 2022, Vol. 41 ›› Issue (4): 2090-2101.DOI: 10.16085/j.issn.1000-6613.2021-0740
相变吸收捕集烟气中CO2技术的发展现状
- 华东交通大学土木建筑学院,江西 南昌 330013
-
收稿日期:2021-04-08修回日期:2021-06-23出版日期:2022-04-23发布日期:2022-04-25 -
通讯作者:张卫风 -
作者简介:张卫风(1977—),男,博士,副教授,研究方向为大气污染及其控制、温室气体CO2减排。E-mail:wfzhang2002@126.com 。
Recent developments of phase-change absorption technology for CO2 capture from flue gas
ZHANG Weifeng( ), ZHOU Wu, WANG Qiuhua
), ZHOU Wu, WANG Qiuhua
- School of Civil Engineering and Architecture, East China Jiaotong University, Nanchang 330013, Jiangxi, China
-
Received:2021-04-08Revised:2021-06-23Online:2022-04-23Published:2022-04-25 -
Contact:ZHANG Weifeng
摘要:
化学吸收法作为目前最有效的CO2捕集技术,吸收剂常用有机胺,但过高的再生能耗和成本限制了其在工业中的应用。基于传统有机胺溶剂开发出来的相变吸收剂被认为可以大幅减少解吸能耗,成为近几年研究的热点。本文详细介绍了相变吸收剂的常见类型、分相机理,并根据其具体组成进行了种类划分,对比分析了常用相变吸收剂和传统乙醇胺(MEA)吸收液的再生能耗,并指出温度、CO2负荷以及相分离等因素对相变吸收剂的工艺流程长期运行稳定性的影响。在制备相变吸收剂的过程中,可加入活化剂来降低CO2富液黏度,加入助溶剂来提高传质特性。本文阐述了现有相变吸收剂的挥发、降解和腐蚀等特性的研究现状。最后,结合研究现状和烟气捕集需求对相变吸收剂今后的研究方向给出了建议。
中图分类号:
引用本文
张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101.
ZHANG Weifeng, ZHOU Wu, WANG Qiuhua. Recent developments of phase-change absorption technology for CO2 capture from flue gas[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2090-2101.
| 类型 | 体系 | 特点 |
|---|---|---|
| 单元基吸收剂 | MDEA+正丁醇+水 | 最优条件下吸收剂在膜器内停留时间仅为数秒,CO2的解吸率均高于80%,最高可达92%[ |
| MEA+叔丁醇+水 | 相比于质量分数为30%的MEA水溶液,MEA/叔丁醇/水相变吸收剂具有更高的循环负载和更少的解吸液量[ | |
| MEA+1-丙醇+水 | 与传统的质量分数为30%的MEA水溶液相比,初始吸收率更高,循环负载量增加了一半[ | |
| 多元混合吸收剂 | TETA+DEEA | 下层相吸收CO2达到90%以上,合适的吸收和解吸温度分别为30℃和90℃[ |
| DMCA+TETA | 有较高的CO2吸收循环能力,良好的相分离行为和较低的再生热。与5mol/L MEA相比,TETA-DMCA的再生能耗可降低约40%[ | |
| MAPA+DEEA | 具有较低的挥发性,相比于质量分数为30%的MEA水溶液其再生能耗可以进一步降低[ | |
| DMCA+MCA+AMP | 有良好的相变温度,高CO2净负荷以及良好的抗氧化降解和热降解能力,且能耗可降至2.0GJ·(t CO2)-1 以下[ | |
| 无水吸收剂 | MEA+三甘醇 | 醇不参与反应,仅MEA和CO2反应,MEA浓度为5mol/L,吸收剂循环处理量高,能耗接近 2.0GJ·(t CO2)-1,优于MEA+水吸收剂[ |
| MEA+环丁砜 | MEA/环丁砜相变溶剂的总再生能耗为2.67GJ·(t CO2)-1,与传统的5mol/L MEA相比,降低了31%[ |
表1 液-液相变吸收剂常见类型
| 类型 | 体系 | 特点 |
|---|---|---|
| 单元基吸收剂 | MDEA+正丁醇+水 | 最优条件下吸收剂在膜器内停留时间仅为数秒,CO2的解吸率均高于80%,最高可达92%[ |
| MEA+叔丁醇+水 | 相比于质量分数为30%的MEA水溶液,MEA/叔丁醇/水相变吸收剂具有更高的循环负载和更少的解吸液量[ | |
| MEA+1-丙醇+水 | 与传统的质量分数为30%的MEA水溶液相比,初始吸收率更高,循环负载量增加了一半[ | |
| 多元混合吸收剂 | TETA+DEEA | 下层相吸收CO2达到90%以上,合适的吸收和解吸温度分别为30℃和90℃[ |
| DMCA+TETA | 有较高的CO2吸收循环能力,良好的相分离行为和较低的再生热。与5mol/L MEA相比,TETA-DMCA的再生能耗可降低约40%[ | |
| MAPA+DEEA | 具有较低的挥发性,相比于质量分数为30%的MEA水溶液其再生能耗可以进一步降低[ | |
| DMCA+MCA+AMP | 有良好的相变温度,高CO2净负荷以及良好的抗氧化降解和热降解能力,且能耗可降至2.0GJ·(t CO2)-1 以下[ | |
| 无水吸收剂 | MEA+三甘醇 | 醇不参与反应,仅MEA和CO2反应,MEA浓度为5mol/L,吸收剂循环处理量高,能耗接近 2.0GJ·(t CO2)-1,优于MEA+水吸收剂[ |
| MEA+环丁砜 | MEA/环丁砜相变溶剂的总再生能耗为2.67GJ·(t CO2)-1,与传统的5mol/L MEA相比,降低了31%[ |
| 类型 | 体系 | 特点 |
|---|---|---|
| 氨基酸盐溶液 | 牛磺酸钾 | 固液分离温度为40℃,与30% MEA基准相比,可将捕获过程的总能量降低15%[ |
| K2CO3溶液 | 高浓度K2CO3溶液 | 低成本、低毒性、不易挥发,能够吸收多杂质的CO2、SO x 和NO x 以及有价值的副产物的产生[ |
| K2CO3溶液+MEA | 向K2CO3中添加MEA会有效提高吸收速率[ | |
| 冷氨水溶液 | 冷氨水溶液 | 低成本,化学稳定和对氧气的高稳定性,较高的CO2负载能力[ |
| 非水溶液吸收剂 | 脯氨酸钾+乙醇 | 与30% MEA水溶液相比,使用脯氨酸钾+乙醇溶液可以显著提高CO2的溶解度,尤其在低CO2负荷下[ |
| AMP/PZ/DME | 具有高吸收负荷和再生效率,吸收CO2后,富含CO2的下相体积占溶液总体积的43%,而约占总负载的94%[ |
表2 液-固相变吸收剂常见类型
| 类型 | 体系 | 特点 |
|---|---|---|
| 氨基酸盐溶液 | 牛磺酸钾 | 固液分离温度为40℃,与30% MEA基准相比,可将捕获过程的总能量降低15%[ |
| K2CO3溶液 | 高浓度K2CO3溶液 | 低成本、低毒性、不易挥发,能够吸收多杂质的CO2、SO x 和NO x 以及有价值的副产物的产生[ |
| K2CO3溶液+MEA | 向K2CO3中添加MEA会有效提高吸收速率[ | |
| 冷氨水溶液 | 冷氨水溶液 | 低成本,化学稳定和对氧气的高稳定性,较高的CO2负载能力[ |
| 非水溶液吸收剂 | 脯氨酸钾+乙醇 | 与30% MEA水溶液相比,使用脯氨酸钾+乙醇溶液可以显著提高CO2的溶解度,尤其在低CO2负荷下[ |
| AMP/PZ/DME | 具有高吸收负荷和再生效率,吸收CO2后,富含CO2的下相体积占溶液总体积的43%,而约占总负载的94%[ |
| 试剂 | 浓度 | 相变 形式 | 再生能耗 /MJ·(kg CO2)-1 | 参考 文献 |
|---|---|---|---|---|
| MEA | 30% | — | 4.22 | [ |
| MEA/1-丙醇/水 | 30% | 液-液 | 2.40 | [ |
| MEA/环丁砜 | 4mol/L | 液-液 | 2.67 | [ |
| TETA/DEEA | 5mol/L | 液-液 | 2.7 | [ |
| TETA/DMCA | 4mol/L | 液-液 | 2.6 | [ |
| MAPA/DEEA | 7mol/L | 液-液 | 2.2~2.4 | [ |
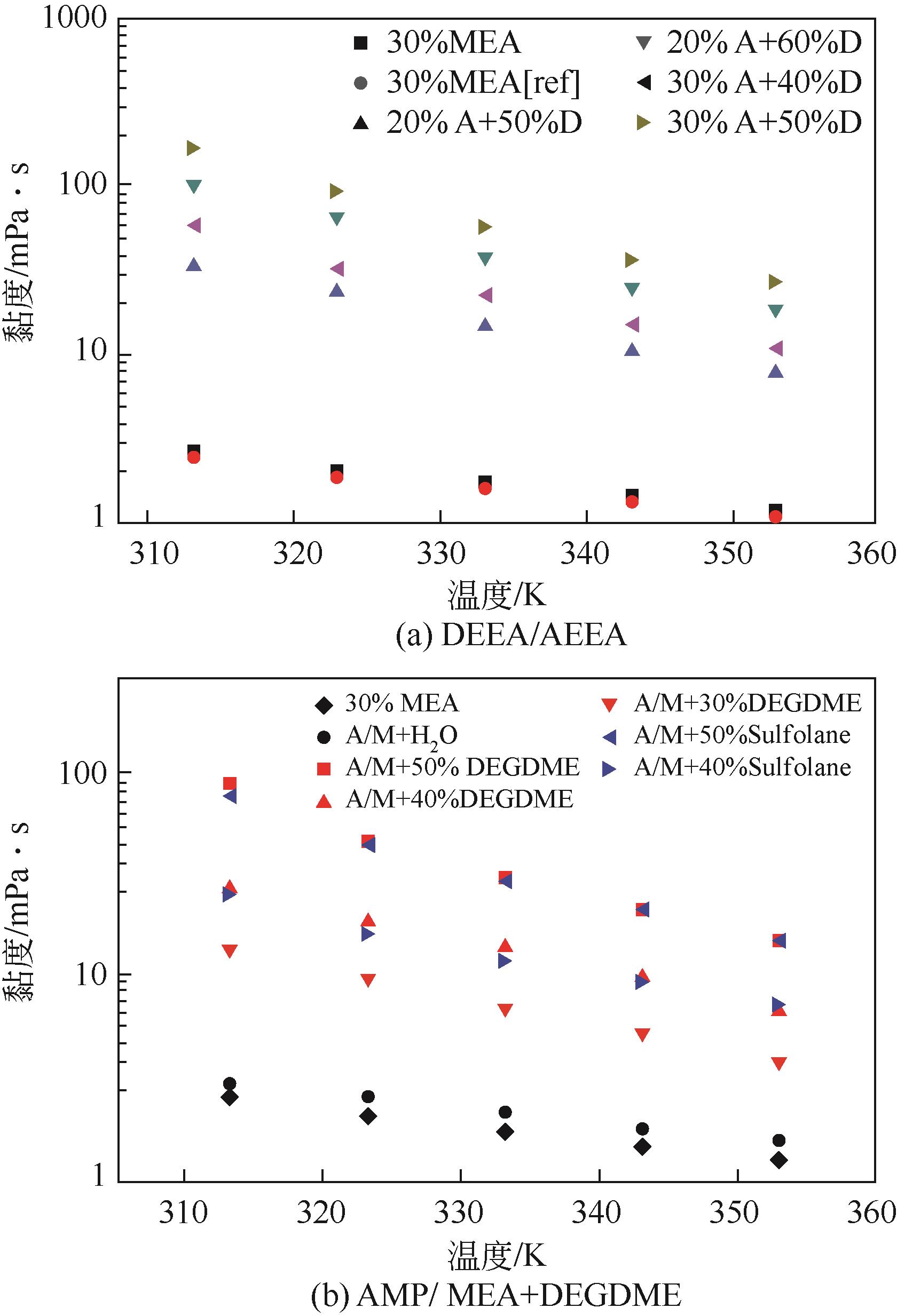
| AEEA/DEEA | 20%+60% | 液-液 | 2.46 | [ |
| DMCA/MCA/AMP | (3+1+1)mol/L | 液-液 | 2.0 | [ |
| 脯氨酸钾/乙醇 | 3.3~5.9mol/kg乙醇 | 液-固 | 2.0~2.5 | [ |
| 牛磺酸钾溶液 | 4mol/L | 液-固 | 3.25 | [ |
| AMP/PZ/DME | 1mol/kg | 液-固 | 1.61 | [ |
| K2CO3溶液 | 20%~40% | 液-固 | 2.0~2.5 | [ |
| 冷氨水溶液 | 30% | 液-固 | 2.5 | [ |
表3 相变吸收剂和常用MEA吸收液再生能耗的比较
| 试剂 | 浓度 | 相变 形式 | 再生能耗 /MJ·(kg CO2)-1 | 参考 文献 |
|---|---|---|---|---|
| MEA | 30% | — | 4.22 | [ |
| MEA/1-丙醇/水 | 30% | 液-液 | 2.40 | [ |
| MEA/环丁砜 | 4mol/L | 液-液 | 2.67 | [ |
| TETA/DEEA | 5mol/L | 液-液 | 2.7 | [ |
| TETA/DMCA | 4mol/L | 液-液 | 2.6 | [ |
| MAPA/DEEA | 7mol/L | 液-液 | 2.2~2.4 | [ |
| AEEA/DEEA | 20%+60% | 液-液 | 2.46 | [ |
| DMCA/MCA/AMP | (3+1+1)mol/L | 液-液 | 2.0 | [ |
| 脯氨酸钾/乙醇 | 3.3~5.9mol/kg乙醇 | 液-固 | 2.0~2.5 | [ |
| 牛磺酸钾溶液 | 4mol/L | 液-固 | 3.25 | [ |
| AMP/PZ/DME | 1mol/kg | 液-固 | 1.61 | [ |
| K2CO3溶液 | 20%~40% | 液-固 | 2.0~2.5 | [ |
| 冷氨水溶液 | 30% | 液-固 | 2.5 | [ |
| 69 | GAO Hongxia, RONGWONG Wichitpan, PENG Chao, et al. Thermal and oxidative degradation of aqueous N,N-diethylethanolamine (DEEA) at stripping conditions for CO2 capture[J]. Energy Procedia, 2014, 63: 1911-1918. |
| 70 | 刘畅, 陈旭, 杨江. CO2腐蚀及其缓蚀剂应用研究进展[J]. 化工进展, 2021, 39(6): 1-13. |
| LIU Chang, CHEN Xu, YANG Jiang. Progress in CO2 corrosion and application of corrosion inhibitors[J]. Chemical Industry and Engineering Progress, 2021, 39(6): 1-13. | |
| 71 | 杨钧晗. 醇胺水溶液吸收CO2过程的腐蚀特性研究[D]. 北京: 华北电力大学, 2018. |
| YANG Junhan. Study on corrosion characteristics of CO2 absorption of amine aqueous solution[D]. Beijing: North China Electric Power University, 2018. | |
| 72 | TZIRAKIS F, TSIVINTZELIS I, PAPADOPOULOS A I, et al. Experimental measurement and assessment of equilibrium behaviour for phase change solvents used in CO2 capture[J]. Chemical Engineering Science, 2019, 199: 20-27. |
| 73 | 方童波, 赵兵涛, 王大淇, 等. 二元复合溶液脱除烟气中CO2的过程模拟与评价[J]. 化工进展, 2019, 38(3): 1561-1566. |
| FANG Tongbo, ZHAO Bingtao, WANG Daqi, et al. Process simulation and evaluation of CO2 removal from flue gas by binary compound solutions[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1561-1566. | |
| 1 | 乔明, 李雪静, 周笑洋. 新形势下石油公司二氧化碳减排策略的变化[J]. 化工进展, 2018, 37(1): 1-6. |
| QIAO Ming, LI Xuejing, ZHOU Xiaoyang. Transformation of carbon emission reduction strategy of oil petroleum companies under the new situation[J]. Chemical Industry and Engineering Progress, 2018, 37(1): 1-6. | |
| 2 | SPECHT E, REDEMANN T, LORENZ N. Simplified mathematical model for calculating global warming through anthropogenic CO2 [J]. International Journal of Thermal Sciences, 2016, 102: 1-8. |
| 3 | 康丽娜, 尚会建, 郑学明. CO2的捕集封存技术进展及在我国的应用前景[J]. 化工进展, 2010, 29(S1): 24-27. |
| KANG Lina, SHANG Huijian, ZHENG Xueming. Progress in CO2 capture and storage technology and its application prospect in China[J]. Chemical Industry and Engineering Progress, 2010, 29(S1): 24-27. | |
| 4 | 白宏山, 赵东亚, 田群宏, 等. CO2捕集、运输、驱油与封存全流程随机优化[J]. 化工进展, 2019, 38(11): 4911-4920. |
| BAI Hongshan, ZHAO Dongya, TIAN Qunhong, et al. Stochastic optimization of the whole process of CO2 capture, transportation, utilization and sequestration[J]. Chemical Industry and Engineering Progress, 2019, 38(11): 4911-4920. | |
| 5 | 彭召静, 赵彦杰, 黄成德, 等. 用于燃烧后CO2捕集系统的胺基固态吸附材料研究进展[J]. 化工进展, 2018, 37(2): 610-620. |
| PENG Zhaojing, ZHAO Yanjie, HUANG Chengde, et al. Recent advances in amine-based solid sorbents for post-combustion CO2 capture system[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 610-620. | |
| 6 | 张卫风, 李娟, 王秋华, 等. 燃煤烟气中CO2膜吸收分离技术的膜浸润特性述评[J]. 化工进展, 2019, 38(8): 3866-3873. |
| ZHANG Weifeng, LI Juan, WANG Qiuhua, et al. Review on membrane wettability of membrane CO2 absorption method from coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3866-3873. | |
| 7 | WANG M H, JOEL A S, RAMSHAW C, et al. Process intensification for post-combustion CO2 capture with chemical absorption:a critical review[J]. Applied Energy, 2015, 158: 275-291. |
| 8 | SHAVALIEVA G, KAZEPIDIS P, PAPADOPOULOS A I, et al. Environmental, health and safety assessment of post-combustion CO2 capture processes with phase-change solvents[J]. Sustainable Production and Consumption, 2021, 25: 60-76. |
| 9 | PINTO D D D, KNUUTILA H, FYTIANOS G, et al. CO2 post combustion capture with a phase change solvent. Pilot plant campaign[J]. International Journal of Greenhouse Gas Control, 2014, 31: 153-164. |
| 10 | 赵文波, 李广振, 许胜超, 等. 相变吸收酸性气体的发展现状[J]. 化工进展, 2021, 40(1): 401-414. |
| ZHAO Wenbo, LI Guangzhen, XU Shengchao, et al. Recent developments of acid gas absorption by phase-change[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 401-414. | |
| 11 | PAPADOPOULOS A I, PERDOMO F A, TZIRAKIS F, et al. Molecular engineering of sustainable phase-change solvents: from digital design to scaling-up for CO2 capture[J]. Chemical Engineering Journal, 2021, 420: 127624. |
| 12 | 张亚萍, 刘建周, 季芹芹, 等. 醇胺法捕集燃煤烟气CO2工艺模拟及优化[J]. 化工进展, 2013, 32(4): 930-935. |
| ZHANG Yaping, LIU Jianzhou, JI Qinqin, et al. Process simulation and optimization of flue gas CO2 capture by the alkanolamine solutions[J]. Chemical Industry and Engineering Progress, 2013, 32(4): 930-935. | |
| 13 | ZHANG J F, NWANI O, TAN Y, et al. Carbon dioxide absorption into biphasic amine solvent with solvent loss reduction[J]. Chemical Engineering Research and Design, 2011, 89(8): 1190-1196. |
| 14 | ZHANG Jiafei, QIAO Yu, WANG Wanzhong, et al. Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents[J]. Energy Procedia, 2013, 37: 1254-1261. |
| 15 | ZHANG Jiafei, QIAO Yu, AGAR D W. Intensification of low temperature thermomorphic biphasic amine solvent regeneration for CO2 capture[J]. Chemical Engineering Research and Design, 2012, 90(6): 743-749. |
| 16 | ZHOU Haicheng, XU Xin, CHEN Xiaochun, et al. Novel ionic liquids phase change solvents for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2020, 98: 103068. |
| 17 | 金显杭. 面向CO2捕集的相变吸收剂开发及应用研究[D]. 北京: 北京化工大学, 2017. |
| JIN Xianhang. The development and application of phase change absorbents for CO2 capture[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 18 | 涂巍巍, 方佳伟, 李竹石, 等. 基于MEA的CO2相变化吸收剂的开发[J]. 中国科学: 化学, 2018, 48(6): 641-647. |
| TU Weiwei, FANG Jiawei, LI Zhushi, et al. Development of CO2 phase change absorbent based on MEA[J]. Scientia Sinica (Chimica), 2018, 48(6): 641-647. | |
| 19 | ZHANG Weidong, JIN Xianhang, TU Weiwei, et al. A novel CO2 phase change absorbent: MEA/1-propanol/H2O[J]. Energy & Fuels, 2017, 31(4): 4273-4279. |
| 20 | LI Yaoyao, LIU Changjun, PARNAS Richard, et al. The CO2 absorption and desorption performance of the triethylenetetramine + N,N-diethylethanolamine + H2O system[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2351-2360. |
| 21 | ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6) : 3660-3668. |
| 22 | KIERZKOWSKA-PAWLAK H, SOABLA K. Heat of absorption of CO2 in aqueous solutions of DEEA and DEEA + MAPA blends—A new approach to measurement methodology[J]. International Journal of Greenhouse Gas Control, 2020, 100: 103102. |
| 23 | TAN J, SHAO H W, XU J H, et al. Mixture absorption system of monoethanolamine-triethylene glycol for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2011, 50(7): 3966-3976. |
| 24 | WANG Lidong, ZHANG Yifeng, WANG Rujie, et al. Advanced monoethanolamine absorption using sulfolane as a phase splitter for CO2 capture[J]. Environmental Science & Technology, 2018, 52(24) : 14556-14563. |
| 25 | YE Qing, WANG Xinlei, LU Yongqi. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| 26 | RAYNAL L, ALIX P, BOUILLON P A, et al. The DMX™ process:an original solution for lowering the cost of post-combustion carbon capture[J]. Energy Procedia, 2011, 4: 779-786. |
| 27 | ALEIXO M, PRIGENT M, GIBERT A, et al. Physical and chemical properties of DMXTM solvents[J]. Energy Procedia, 2011, 4: 148-155. |
| 28 | RAYNAL L, BRIOT P, DREILLARD M, et al. Evaluation of the DMX process for industrial pilot demonstration-methodology and results[J]. Energy Procedia, 2014, 63: 6298-6309. |
| 29 | 安山龙, 汪黎东, 于松华, 等. 相变溶剂捕集CO2技术的研究进展[J]. 化工环保, 2017, 37(1): 31-37. |
| AN Shanlong, WANG Lidong, YU Songhua, et al. Research progresses in CO2 capture technology using phase change solvents[J]. Environmental Protection of Chemical Industry, 2017, 37(1): 31-37. | |
| 30 | CIFTJA A F, HARTONO A, SVENDSEN H F. Experimental study on phase change solvents in CO2 capture by NMR spectroscopy[J]. Chemical Engineering Science, 2013, 102: 378-386. |
| 31 | 王敏. 液-液相变溶剂吸收CO2关键设备设计与数值模拟研究[D]. 青岛: 青岛科技大学, 2019. |
| WANG Min. Design and numerical simulation of key equipment for CO2 absorption by liquid-liquid phase change solvents [D]. Qingdao: Qingdao University of Science and Technology, 2019. | |
| 32 | SANCHEZ-FERNANDEZ E, HEFFERNAN K, VAN DER HAM L, et al. Analysis of process configurations for CO2 capture by precipitating amino acid solvents[J]. Industrial & Engineering Chemistry Research, 2014, 53(6): 2348-2361. |
| 33 | SMITH K, XIAO G K, MUMFORD K, et al. Demonstration of a concentrated potassium carbonate process for CO2 capture[J]. Energy & Fuels, 2014, 28(1): 299-306. |
| 34 | THEE H, SURYAPUTRADINATA Y A, MUMFORD K A, et al. A kinetic and process modeling study of CO2 capture with MEA-promoted potassium carbonate solutions[J]. Chemical Engineering Journal, 2012, 210: 271-279. |
| 35 | GAZZANI M, SUTTER D, MAZZOTTI M. Improving the efficiency of a chilled ammonia CO2 capture plant through solid formation: a thermodynamic analysis[J]. Energy Procedia, 2014, 63: 1084-1090. |
| 36 | BIAN Yangyang, SHEN Shufeng. CO2 absorption into a phase change absorbent: water-lean potassium prolinate/ethanol solution[J].Chinese Journal of Chemical Engineering, 2018, 26(11): 2318-2326. |
| 37 | CHEN Zhibiao, JING Guohua, Bihong LYU, et al. An efficient solid-liquid biphasic solvent for CO2 capture: crystalline powder product and low heat duty[J]. ACS Sustainable Chemistry & Engineering, 2020, 8: 14493-14503. |
| 38 | SANCHEZ-FERNANDEZ E, MERCADER F D M, MISIAK K, et al. New process concepts for CO2 capture based on precipitating amino acids[J]. Energy Procedia, 2013, 37: 1160-1171. |
| 39 | SANCHEZ-FERNANDEZ E, HEFFERNAN K, VAN DER HAM L, et al. Precipitating amino acid solvents for CO2 capture. opportunities to reduce costs in post combustion capture[J]. Energy Procedia, 2014, 63: 727-738. |
| 40 | WANG Xianfeng, AKHMEDOV N G, HOPKINSON D, et al. Phase change amino acid salt separates into CO2-rich and CO2-lean phases upon interacting with CO2 [J]. Applied Energy, 2016, 161: 41-47. |
| 41 | MOIOLI S, HO M T, WILEY D E, et al. Thermodynamic modeling of the system of CO2 and potassium taurate solution for simulation of the carbon dioxide capture process[J]. Chemical Engineering Research and Design, 2018, 136: 834-845. |
| 42 | MOIOLI S, HO M T, WILEY D E, et al. Assessment of carbon dioxide capture by precipitating potassium taurate solvent[J]. International Journal of Greenhouse Gas Control, 2019, 87: 159-169. |
| 43 | HO M T, GARCIA-CALVO CONDE E, MOIOLI S, et al Wiley. The effect of different process configurations on the performance and cost of potassium taurate solvent absorption[J]. International Journal of Greenhouse Gas Control, 2019, 81: 1-10. |
| 44 | DARDE V, THOMSEN K, WELL W J M VAN, et al. Chilled ammonia process for CO2 capture[J]. Energy Procedia, 2009, 1(1): 1035-1042. |
| 45 | PARK J Y, YOON S J, LEE H. Effect of steric hindrance on carbon dioxide absorption into new amine solutions: thermodynamic and spectroscopic verification through solubility and NMR analysis[J]. Environmental Science & Technology, 2003, 37(8): 1670-1675. |
| 46 | 张政. 有机胺非水体系相变吸收CO2研究[D]. 昆明: 昆明理工大学, 2016. |
| ZHANG Zheng. Study on CO2 absorption by phase change in non-aqueous organic amines[D]. Kunming: Kunming University of Science and Technology, 2016. | |
| 47 | ALIVAND M S, MAZAHERI O, WU Y, et al. Data in brief on CO2 absorption-desorption of aqueous-based amino acid solvents with phase change behaviour.[J]. Data in Brief, 2019, 27: 104741. |
| 48 | LI Yannan, CHENG Jun, HU Leiqing, et al. Regulating crystal structures of EDA-carbamates in solid-liquid phase- changing CO2 capture solutions[J]. Fuel, 2019, 252(9): 47-54. |
| 49 | 张克舫, 刘中良, 王远亚, 等. 化学吸收法CO2捕集解吸能耗的分析计算[J]. 化工进展, 2013, 32(12): 3008-3014. |
| ZHANG Kefang, LIU Zhongliang, WANG Yuanya, et al. Analysis and calculation of the desorption energy consumption of CO2 capture process by chemical absorption method[J]. Chemical Industry and Engineering Progress, 2013, 32(12): 3008-3014. | |
| 50 | 柳康, 许世森, 李广宇, 等. 基于整体煤气化联合循环的燃烧前CO2捕集工艺及系统分析[J]. 化工进展, 2018, 37(12): 4897-4907. |
| LIU Kang, XU Shisen, LI Guangyu, et al. Technological process and system analysis of pre-combustion CO2 capture based on IGCC[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4897-4907. | |
| 51 | 陆诗建, 高丽娟, 王家凤, 等. 烟气CO2捕集热能梯级利用节能工艺耦合优化[J]. 化工进展, 2020, 39(2): 728-737. |
| LU Shijian, GAO Lijuan, WANG Jiafeng, et al. Coupling optimization of energy-saving technology for cascade utilization of flue gas CO2 capture system[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 728-737. | |
| 52 | MÄNNISTÖ M, UUSI-KYYNY P, RICHON D, et al. Study of CO2 absorption into phase change solvents MAPA and DEEA[J]. Journal of Chemical & Data, 2017, 62(8): 2261-2271. |
| 53 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Biphasic behaviors and regeneration energy of a 2-(diethylamino)-ethanol and 2-((2-aminoethyl)amino) ethanol blend for CO2 capture[J]. Sustainable Energy & Fuels, 2019, 3(12): 3594-3602. |
| 54 | 彭艳娇, 汤谧琼. 国际领先“相变型”二氧化碳捕集工业装置在华能运行成功[N]. 中国电力报, 2020-11-27. |
| PENG Yanjiao, TANG Miqiong. The world’s leading “phase-variant” carbon dioxide capture industrial plant has been successfully run in China[N]. China Electric Power News, 2020-11-27. | |
| 55 | WANG Lidong, AN Shanlong, YU Songhua, et al. Mass transfer characteristics of CO2 absorption into a phase-change solvent in a wetted-wall column[J]. International Journal of Greenhouse Gas Control, 2017, 64: 276-283. |
| 56 | LEE J, HONG Y K, YOU J K. Phase separation characteristics in biphasic solvents based on mutually miscible amines for energy efficient CO2 capture[J]. Korean Journal of Chemical Engineering, 2017, 34(6) : 1840-1845. |
| 57 | WANG Lidong, LIU Shanshan, WANG Rujie, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science and Technology, 2019, 53(21) : 12873-12881. |
| 58 | YE Jiexu, JIANG Chenkai, CHEN Han, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8) : 4470-4479. |
| 59 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Research on alkanolamine-based physical-chemical solutions as biphasic solvents for CO2 capture[J]. Energy & Fuels, 2019, 33(11): 11389-11398. |
| 60 | 周小斌, 荆国华, 周作明, 等. 2-氨基-2-甲基-1-丙醇(AMP)活化相变吸收剂捕集CO2特性[C]//2017中国环境科学学会科学与技术年会论文集. 厦门, 2017: 876-883. |
| ZHOU Xiaobin, JING Guohua, ZHOU Zuoming, et al. Capture of CO2 with 2-amino-2-methyl-1-propanol (AMP) activated phase change absorber[C]//2017 Chinese Society for Environmental Science Science Annual Conference Proceedings Xiamen, 2017: 876-883. | |
| 61 | 孙亚伟, 谢美连, 刘庆岭, 等. 膜法分离燃煤电厂烟气中CO2的研究现状及进展[J]. 化工进展, 2017, 36(5): 1880-1889. |
| SUN Yawei, XIE Meilian, LIU Qingling, et al. Membrane-based carbon dioxide separation from flue gases of coal-fired power plant—current status and developments[J]. Chemical Industry and Engineering Progress, 2017, 36(5): 1880-1889. | |
| 62 | YI Ningtong, FANG Mengxiang, DI Wentao, et al. Aerosol emissions of amine-based CO2 absorption system: effects of condensation nuclei and operating conditions.[J]. Environmental Science & Technology, 2021, 55(8): 5152-5160. |
| 63 | 杨林军, 张琳, 孙莹. 燃煤净烟气中共存杂质对膜法捕集CO2影响现状[J]. 化工进展, 2019, 38(4): 1996-2002. |
| YANG Linjun, ZHANG Lin, SUN Ying. Present situation of the effect of coexisting impurities in coal fired flue gas on CO2 capture by membranes[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1996-2002. | |
| 64 | NGUYEN T, HILLIARD M, ROCHELLE G T. Amine volatility in CO2 capture[J]. International Journal of Greenhouse Gas Control, 2010, 4(5) : 707-715. |
| 65 | HILLIARD M D. A predictive thermodynamic model for an aqueous blend of potassium carbonate, piperazine, and monoethanolamine for carbon dioxide capture from flue gas[J]. Carbon Dioxide—Absorption and Adsorption, 2008: 1083. |
| 66 | LIU F, ROCHELLE G T, FANG M X, et al. Volatility of 2-(diethylamino)-ethanol and 2-((2-aminoethyl) amino) ethanol, a biphasic solvent for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2021, 106 : 103257. |
| 67 | 李红, 吉轲, 齐天勤机, 等. 复配醇胺溶液对CO2的吸收解吸性能及其降解性[J]. 化工进展, 2021, 45(5): 1-17. |
| LI Hong, JI Ke, qinji QITIAN, et al. CO2 absorption and desorption properties of mixed alcohol amine solution[J]. Chemical Industry and Engineering Progress, 2021, 45(5): 1-17. | |
| 68 | 储可弘, 陈绍云, 李强, 等. 基于N-乙基乙醇胺非水CO2吸收剂的抗氧化剂[J]. 化工进展, 2019, 38(12): 5565-5571. |
| CHU Kehong, CHEN Shaoyun, LI Qiang, et al. Oxidation inhibitor for thylethanolamine based non-aqueous CO2 absorbent[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5565-5571. |
| [1] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [4] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [5] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [6] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [7] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| [8] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [9] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [10] | 杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859. |
| [11] | 李玲, 马超峰, 卢春山, 于万金, 石能富, 金佳敏, 张建君, 刘武灿, 李小年. 新型含氟替代品1,1,2-三氟乙烯的合成工艺与催化剂研究进展[J]. 化工进展, 2023, 42(4): 1822-1831. |
| [12] | 殷铭, 郭晋, 庞纪峰, 吴鹏飞, 郑明远. 铜催化剂在涉氢反应中的失活机制和稳定策略[J]. 化工进展, 2023, 42(4): 1860-1868. |
| [13] | 李云闯, 谢方明, 席亚男, 万新月, 孙玉虎, 赵永峰, 李根, 刘宏海, 高雄厚, 刘洪涛. 高水热稳定性介孔分子筛的低成本合成研究进展[J]. 化工进展, 2023, 42(4): 1877-1884. |
| [14] | 王钰琢, 李刚. 硫、氮共掺杂三维石墨烯的全固态超级电容器[J]. 化工进展, 2023, 42(4): 1974-1982. |
| [15] | 谭德新, 曾佳欣, 梁莉敏, 申思慧, 曾子倩, 王艳丽. 取代烷基变化对芳炔单体及其聚合物性能影响[J]. 化工进展, 2023, 42(4): 2031-2037. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||