化工进展 ›› 2023, Vol. 42 ›› Issue (4): 2068-2080.DOI: 10.16085/j.issn.1000-6613.2022-1019
新型相变有机胺吸收捕集CO2技术研究进展
符乐1( ), 杨阳2, 徐文青2(
), 杨阳2, 徐文青2( ), 耿錾卜2,3, 朱廷钰2, 郝润龙1(
), 耿錾卜2,3, 朱廷钰2, 郝润龙1( )
)
- 1.华北电力大学环境科学与工程系,河北 保定 071003
2.中国科学院过程工程研究所,北京 100190
3.中国科学院大学,北京 100049
-
收稿日期:2020-05-31修回日期:2022-09-23出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:徐文青,郝润龙 -
作者简介:符乐(1999—),男,硕士研究生,研究方向为CO2吸收捕集。E-mail:lfu@ipe.ac.cn。 -
基金资助:国家自然科学基金(52100133)
Research progress in CO2 capture technology using novel biphasic organic amine absorbent
FU Le1( ), YANG Yang2, XU Wenqing2(
), YANG Yang2, XU Wenqing2( ), GENG Zanbu2,3, ZHU Tingyu2, HAO Runlong1(
), GENG Zanbu2,3, ZHU Tingyu2, HAO Runlong1( )
)
- 1.Department of Environmental Science and Engineering, North China Electric Power University, Baoding 071003, Hebei, China
2.Institute of Process Engineering, Chinese Academy of Science, Beijing 100190, China
3.University of ChineseAcademy of Sciences, Beijing 100049, China
-
Received:2020-05-31Revised:2022-09-23Online:2023-04-25Published:2023-05-08 -
Contact:XU Wenqing, HAO Runlong
摘要:
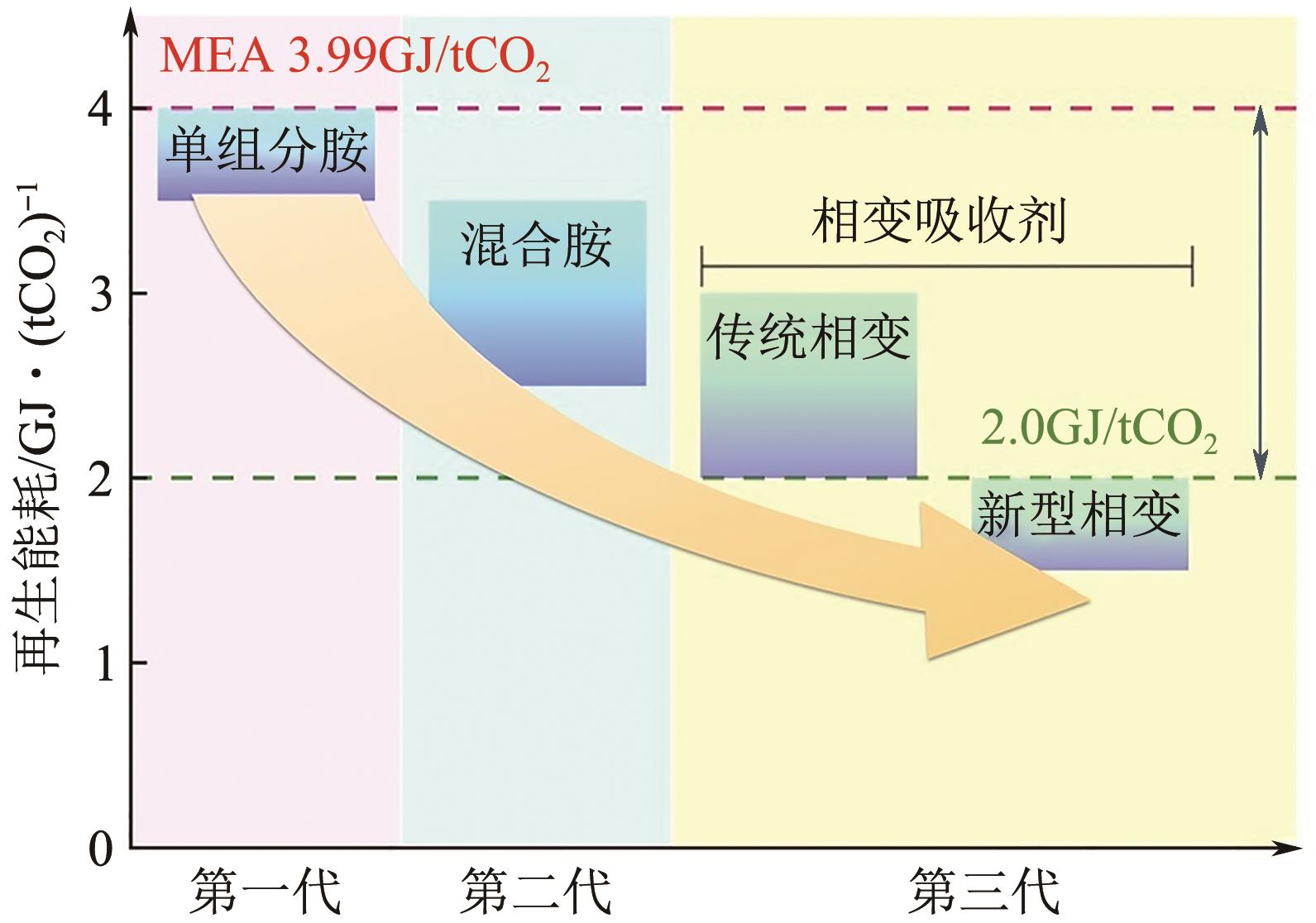
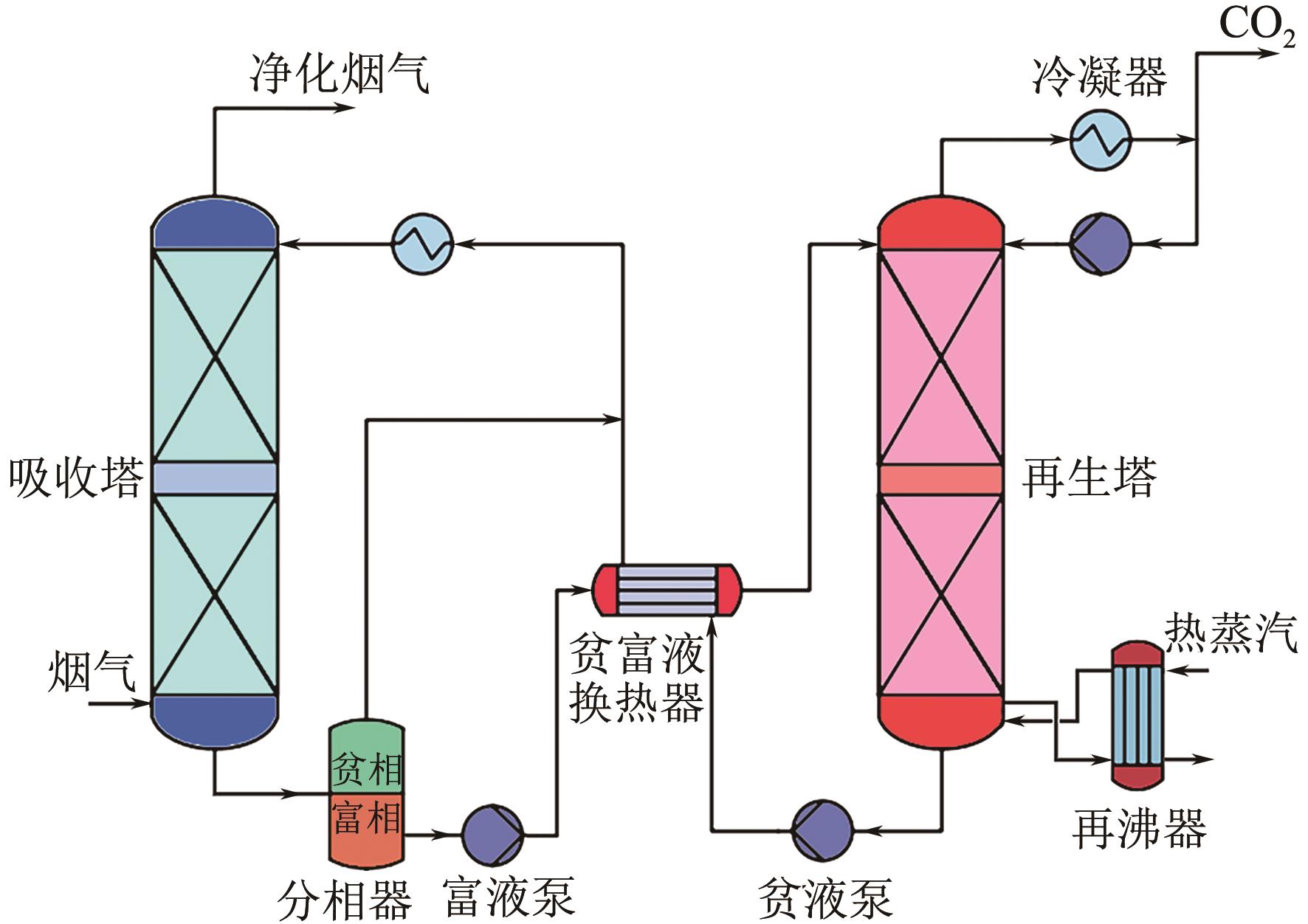
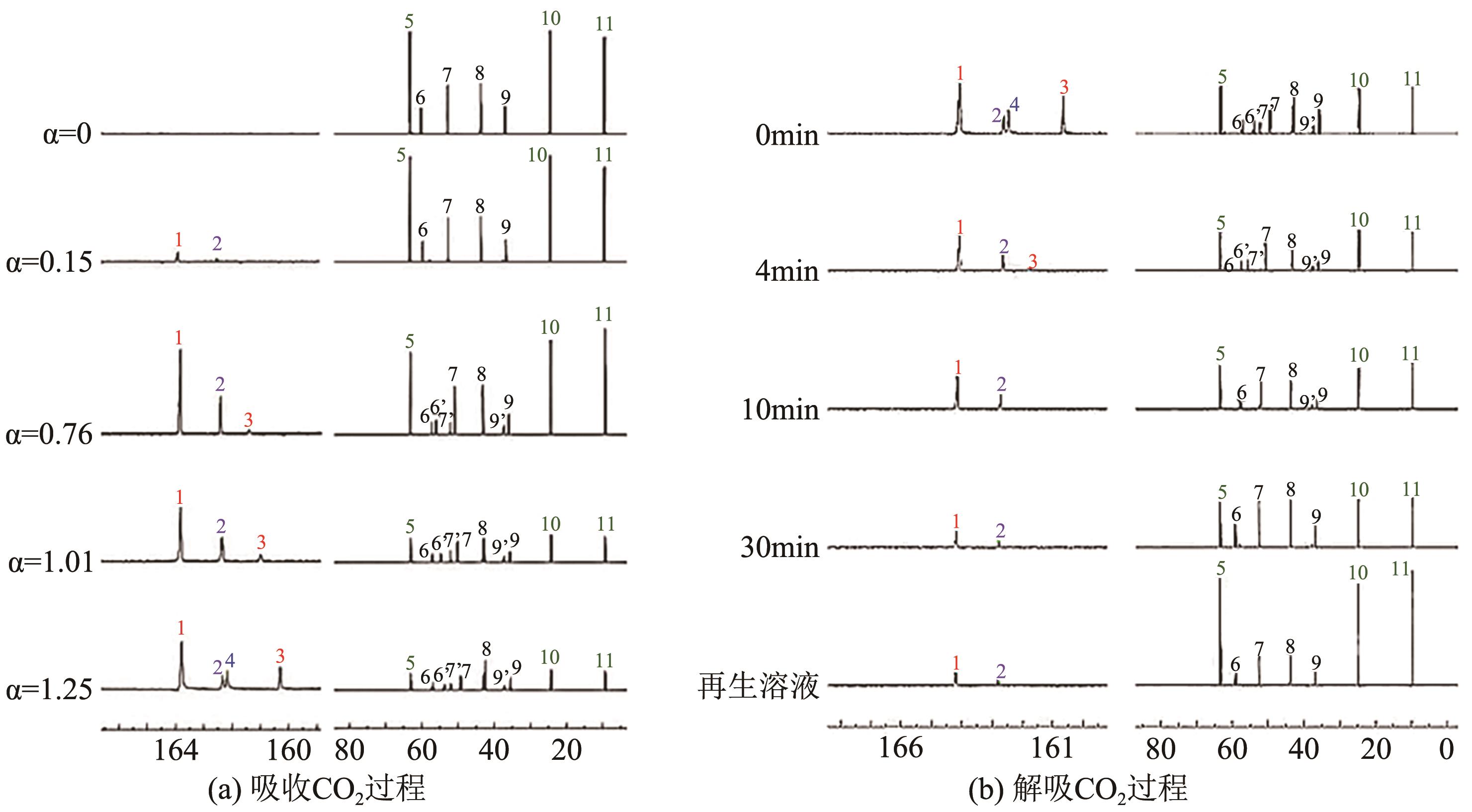
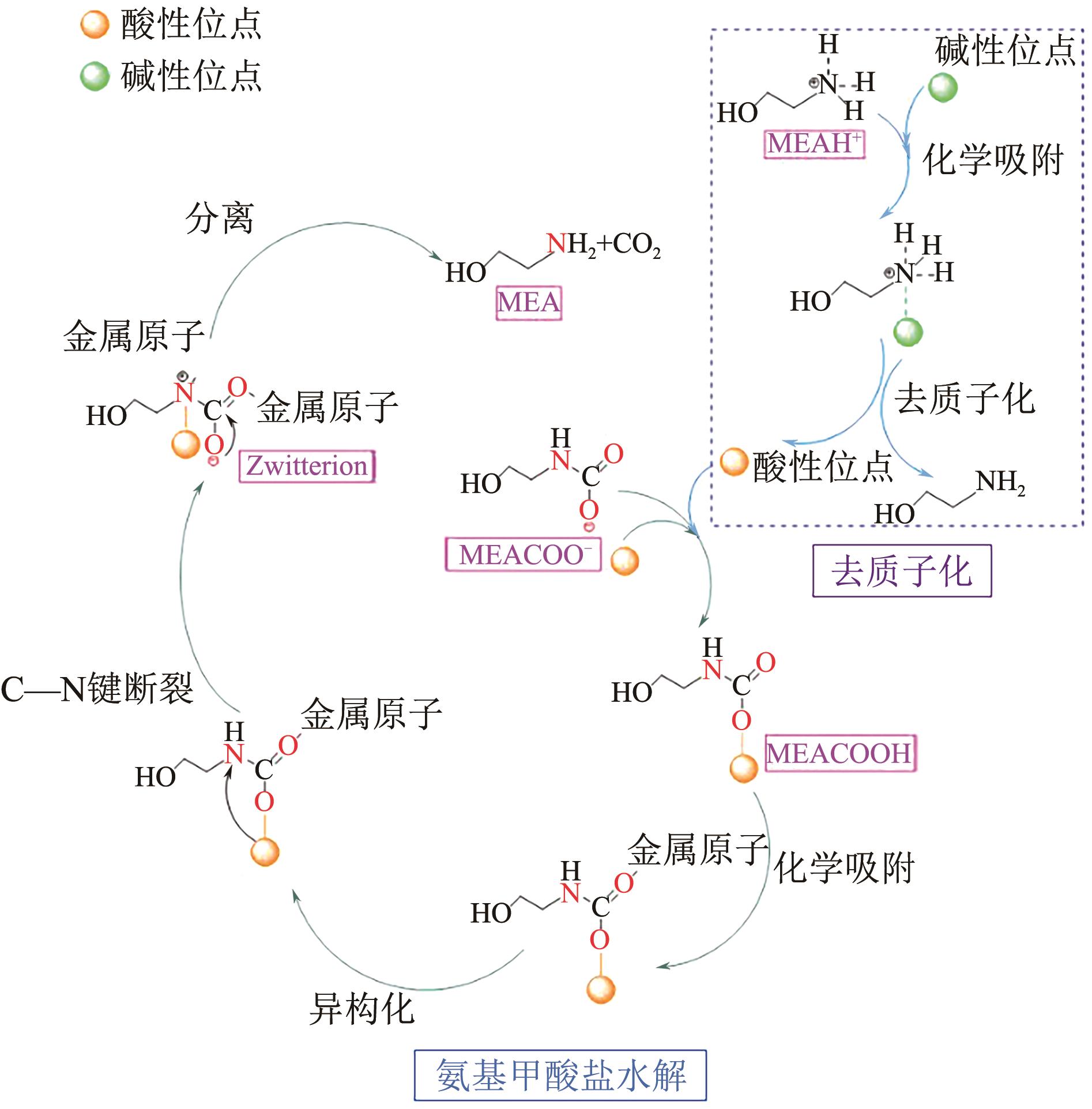
CO2捕集、利用与封存(CCUS)技术是实现碳减排的关键技术之一,有机胺吸收技术是目前研究最广泛、最成熟的CO2捕集技术,已有少数工业应用案例。吸收剂是吸收技术的核心,吸收剂的研发创新是该领域的热点方向。相比于单一相的有机胺吸收剂,相变吸收剂在吸收CO2后产生相变行为,仅需对富相进行再生,可大幅减少再生体积,降低再生能耗。本文介绍了传统混合胺相变吸收体系的典型工艺、吸收机理和吸收剂研究进展,分析了吸收剂吸收CO2后富相黏度高、富相体积占比大及其导致的再生能耗增加的问题。本文系统梳理了为解决上述问题而研发的四种新型的相变吸收体系,分别为空间位阻胺混合型相变吸收剂、物理溶剂混合型相变吸收剂、醇胺混合型相变吸收剂、催化剂-有机胺复合型相变吸收剂,对各类新型相变吸收体系的设计构建原理及性能强化机制进行了分析。最后,基于对研究进展的深入分析,提出了相变吸收剂的未来研究方向。
中图分类号:
引用本文
符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080.
FU Le, YANG Yang, XU Wenqing, GENG Zanbu, ZHU Tingyu, HAO Runlong. Research progress in CO2 capture technology using novel biphasic organic amine absorbent[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2068-2080.
| 吸收剂 | 实验条件 | 富相体积分数 /% | 吸收负荷 /mol CO2·(mol 胺)-1 | 富相黏度 /mPa·s | 再生能耗 /GJ·(t CO2)-1 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 气体流速 /mL·min-1 | CO2体积分数 /% | 吸收温度 /℃ | 再生温度 /℃ | |||||
| EEDA | DEEA | — | — | 30 | — | — | 0.53 | — | — | [ |
| BDA | DEEA | 463 | 12 | 40 | — | 78.0 | 0.51 | — | — | [ |
| MAPA | DEEA | 5000 | 1~20 | 40 | — | 68.0 | 0.50 | — | 2.20 | [ |
| DMBA | DEEA | — | — | 30 | — | 85.0 | 0.43 | — | — | [ |
| DETA | DEEA | 3000 | 15 | 40 | — | 80.0 | 0.83 | — | — | [ |
| DETA | PMDETA | 60 | 100 | 50 | 120 | 57.0 | 0.613 | 254.0 | 2.40 | [ |
| TETA | DEEA | — | 12 | 30 | 120 | 86.7 | 0.95 | 277.5 | 2.46 | [ |
| TETA | DMCA | 200 | 13 | 40 | 120 | 65.0 | 0.88 | — | 2.98 | [ |
| AEEA | DEEA | 2000 | 12 | 40 | 120 | 70.0 | 3.15① | 114.3 | 2.58 | [ |
表1 传统混合胺相变吸收剂在CO2吸收解吸中的相关研究
| 吸收剂 | 实验条件 | 富相体积分数 /% | 吸收负荷 /mol CO2·(mol 胺)-1 | 富相黏度 /mPa·s | 再生能耗 /GJ·(t CO2)-1 | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 气体流速 /mL·min-1 | CO2体积分数 /% | 吸收温度 /℃ | 再生温度 /℃ | |||||
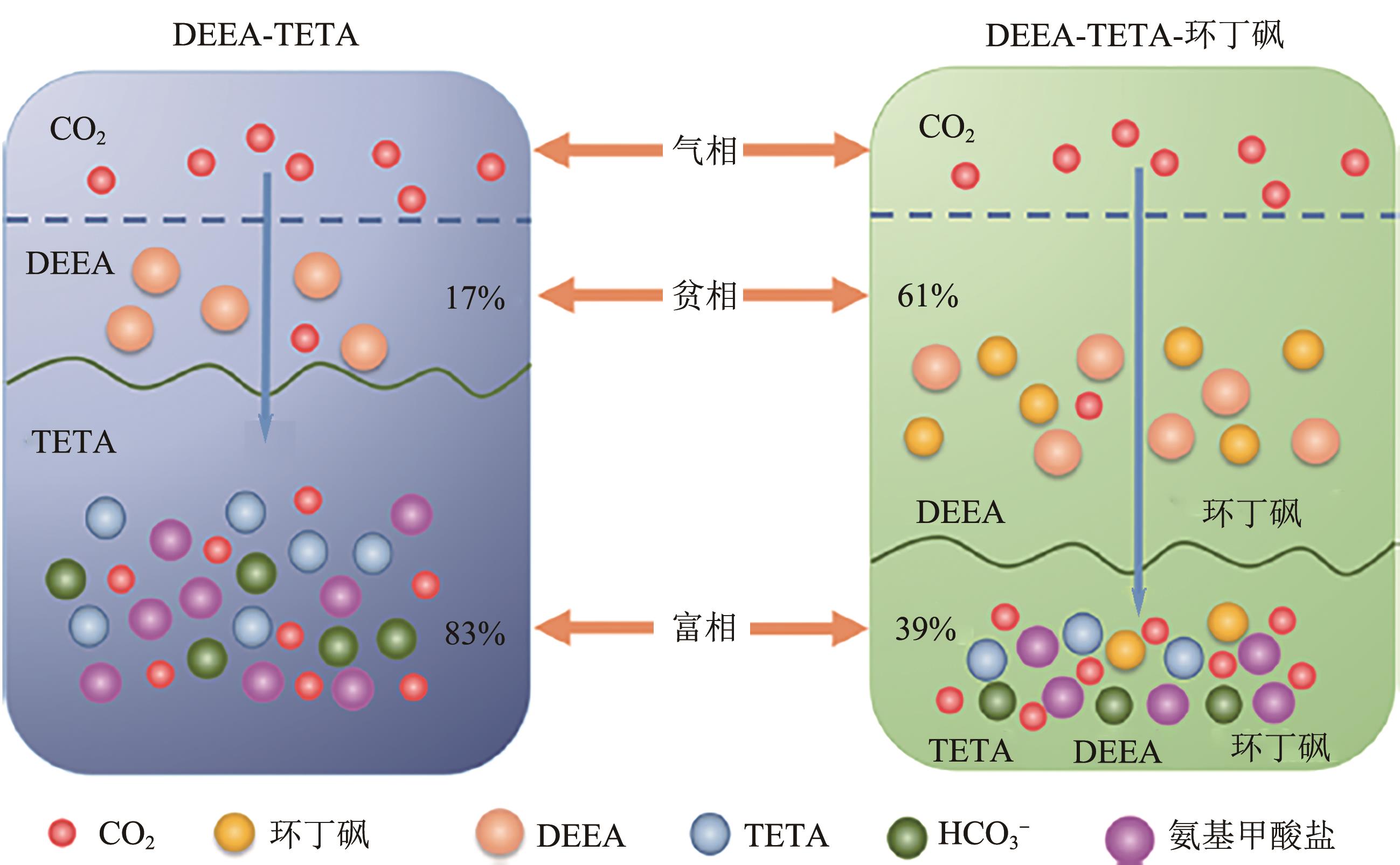
| EEDA | DEEA | — | — | 30 | — | — | 0.53 | — | — | [ |
| BDA | DEEA | 463 | 12 | 40 | — | 78.0 | 0.51 | — | — | [ |
| MAPA | DEEA | 5000 | 1~20 | 40 | — | 68.0 | 0.50 | — | 2.20 | [ |
| DMBA | DEEA | — | — | 30 | — | 85.0 | 0.43 | — | — | [ |
| DETA | DEEA | 3000 | 15 | 40 | — | 80.0 | 0.83 | — | — | [ |
| DETA | PMDETA | 60 | 100 | 50 | 120 | 57.0 | 0.613 | 254.0 | 2.40 | [ |
| TETA | DEEA | — | 12 | 30 | 120 | 86.7 | 0.95 | 277.5 | 2.46 | [ |
| TETA | DMCA | 200 | 13 | 40 | 120 | 65.0 | 0.88 | — | 2.98 | [ |
| AEEA | DEEA | 2000 | 12 | 40 | 120 | 70.0 | 3.15① | 114.3 | 2.58 | [ |
| 吸收剂 | 实验条件 | 富相体积分数/% | 吸收负荷/mol CO2·(mol胺)-1 | 富相黏度/mPa·s | 再生效率/% | 再生能耗/GJ·(t CO2)-1 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 空间位阻胺 | 气体流速/mL·min-1 | CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | ||||||
| TETA | DEEA | — | 60 | 100 | 40 | — | 44.0 | 0.95 | 1505.0 | 59.9 | — | [ |
| TETA | DEEA | AMP | 60 | 100 | 40 | — | 67.2 | 3.32① | 185.5 | 92.3 | — | [ |
| DETA | PMDETA | — | 60 | 100 | 40 | 120 | 38.0 | 0.613 | 541.0 | 25.1 | 2.40 | [ |
| DETA | PMDETA | AMP | 60 | 100 | 40 | 120 | 43.0 | 0.634 | 152.0 | 80.9 | 1.83 | [ |
表2 添加空间位阻胺前后吸收性能和再生性能比较
| 吸收剂 | 实验条件 | 富相体积分数/% | 吸收负荷/mol CO2·(mol胺)-1 | 富相黏度/mPa·s | 再生效率/% | 再生能耗/GJ·(t CO2)-1 | 参考文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 空间位阻胺 | 气体流速/mL·min-1 | CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | ||||||
| TETA | DEEA | — | 60 | 100 | 40 | — | 44.0 | 0.95 | 1505.0 | 59.9 | — | [ |
| TETA | DEEA | AMP | 60 | 100 | 40 | — | 67.2 | 3.32① | 185.5 | 92.3 | — | [ |
| DETA | PMDETA | — | 60 | 100 | 40 | 120 | 38.0 | 0.613 | 541.0 | 25.1 | 2.40 | [ |
| DETA | PMDETA | AMP | 60 | 100 | 40 | 120 | 43.0 | 0.634 | 152.0 | 80.9 | 1.83 | [ |
| 吸收剂 | 实验条件 | 富相 体积分数/% | 吸收负荷 /molCO2·(mol胺)-1 | 再生能耗 /GJ·(tCO2)-1 | 参考 文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 物理溶剂 | 气体流速 /mL·min-1 | CO2体积 分数/% | 吸收温度 /℃ | 再生温度 /℃ | ||||
| MEA | — | 正丙醇 | 500 | 100 | 45 | 110 | 56.4 | 0.48 | 2.40 | [ |
| AEP | — | 正丙醇 | 240 | 100 | 40 | 120 | 58.0 | 1.26 | 2.74 | [ |
| DETA | — | 正丙醇 | 100 | 100 | 30 | 110 | 41.7 | 1.08 | 2.12 | [ |
| AMP/MEA | — | DEGDME | 2000 | 12 | 40 | 120 | — | 0.40 | 2.70 | [ |
| MEA | — | 环丁砜 | 110 | 15 | 40 | 120 | 49.1 | 0.485 | 2.67 | [ |
| TETA | DEEA | 环丁砜 | 120 | 100 | 30 | 110 | 39.0 | 0.984 | 1.81 | [ |
| DETA | PMDETA | 环丁砜 | 120 | 100 | 30 | 110 | 27.1 | 1.22 | 1.86 | [ |
| AMP/MEA | PMDETA | DMSO | 80 | 100 | 40 | 120 | 56.8 | 0.88 | 2.30 | [ |
| TETA | — | 1-MI | 200 | 100 | 45 | 120 | 41.1 | 1.75 | 2.26 | [ |
表3 物理溶剂在CO2吸收解吸中的相关研究
| 吸收剂 | 实验条件 | 富相 体积分数/% | 吸收负荷 /molCO2·(mol胺)-1 | 再生能耗 /GJ·(tCO2)-1 | 参考 文献 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 叔胺 | 物理溶剂 | 气体流速 /mL·min-1 | CO2体积 分数/% | 吸收温度 /℃ | 再生温度 /℃ | ||||
| MEA | — | 正丙醇 | 500 | 100 | 45 | 110 | 56.4 | 0.48 | 2.40 | [ |
| AEP | — | 正丙醇 | 240 | 100 | 40 | 120 | 58.0 | 1.26 | 2.74 | [ |
| DETA | — | 正丙醇 | 100 | 100 | 30 | 110 | 41.7 | 1.08 | 2.12 | [ |
| AMP/MEA | — | DEGDME | 2000 | 12 | 40 | 120 | — | 0.40 | 2.70 | [ |
| MEA | — | 环丁砜 | 110 | 15 | 40 | 120 | 49.1 | 0.485 | 2.67 | [ |
| TETA | DEEA | 环丁砜 | 120 | 100 | 30 | 110 | 39.0 | 0.984 | 1.81 | [ |
| DETA | PMDETA | 环丁砜 | 120 | 100 | 30 | 110 | 27.1 | 1.22 | 1.86 | [ |
| AMP/MEA | PMDETA | DMSO | 80 | 100 | 40 | 120 | 56.8 | 0.88 | 2.30 | [ |
| TETA | — | 1-MI | 200 | 100 | 45 | 120 | 41.1 | 1.75 | 2.26 | [ |
| 吸收剂 | 实验条件 | 吸收负荷 /mol CO2·(mol胺)-1 | 再生效率 /% | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 添加剂 | 气体流速/mL·min-1 | CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | |||
| EMEA | H2O | 265 | 100 | 40 | 110 | 0.799 | 38.8 | [ |
| EMEA | 乙醇 | 265 | 100 | 40 | 110 | 0.547 | 100.0 | [ |
| TETA/AMP | H2O | 80 | 100 | 40 | 120 | 0.95 | 38.8 | [ |
| TETA/AMP | 乙醇 | 80 | 100 | 40 | 120 | 1.00 | 95.4 | [ |
表4 胺水和醇胺混合型吸收剂吸收性能和再生性能比较
| 吸收剂 | 实验条件 | 吸收负荷 /mol CO2·(mol胺)-1 | 再生效率 /% | 参考文献 | ||||
|---|---|---|---|---|---|---|---|---|
| 伯/仲胺 | 添加剂 | 气体流速/mL·min-1 | CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | |||
| EMEA | H2O | 265 | 100 | 40 | 110 | 0.799 | 38.8 | [ |
| EMEA | 乙醇 | 265 | 100 | 40 | 110 | 0.547 | 100.0 | [ |
| TETA/AMP | H2O | 80 | 100 | 40 | 120 | 0.95 | 38.8 | [ |
| TETA/AMP | 乙醇 | 80 | 100 | 40 | 120 | 1.00 | 95.4 | [ |
| 有机胺 | 催化剂 | 实验条件 | 总酸位点 /µmol·g-1 | 性能提升 | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | |||||
| 3.33mol/L MEA | TiO(OH)2 | 15 | 40 | 30~88 | — | DR提高4500%,DA提高68.8% | [ |
| 5mol/L MEA | SZMF | 15 | 40 | 98 | — | RE降低28%~40% | [ |
| 5mol/L MEA | Al2O3 | — | — | 65~96 | 935 | RE降低27% | [ |
| Al2O3/HZSM-5 | 1050 | RE降低23%~34% | |||||
| 5mol/L MEA | ZrO2 | 15 | 40 | 40-88 | 126.3 | DR提高54% | [ |
| ZnO | 122.7 | ||||||
| 5mol/L MEA | AgO-Ag2CO3 | 15 | 40 | 40~82 | — | DR提高1010%,CC提高52% | [ |
| 5mol/L MEA | SO42-/ZrO2 | — | — | 98 | 2796.1 | RE降低9.8% | [ |
| MEA-AMP-PZ | HZSM-5 | 15 | 40 | 90~98 | 9502.1 | DR提高121%,RE降低61.6% | [ |
| 5mol/L MEA | SO42-/ZrO2-HZSM-5 | 50 | 25 | 30~98 | 1596.0 | DR提高37%,DA提高40% RE降低31% | [ |
| 5mol/L MEA | Fe-Zr@BS | 15 | 40 | 55~98 | 610 | RE降低33% | [ |
表5 催化剂在CO2吸收解吸中的相关研究
| 有机胺 | 催化剂 | 实验条件 | 总酸位点 /µmol·g-1 | 性能提升 | 参考文献 | ||
|---|---|---|---|---|---|---|---|
| CO2体积分数/% | 吸收温度/℃ | 再生温度/℃ | |||||
| 3.33mol/L MEA | TiO(OH)2 | 15 | 40 | 30~88 | — | DR提高4500%,DA提高68.8% | [ |
| 5mol/L MEA | SZMF | 15 | 40 | 98 | — | RE降低28%~40% | [ |
| 5mol/L MEA | Al2O3 | — | — | 65~96 | 935 | RE降低27% | [ |
| Al2O3/HZSM-5 | 1050 | RE降低23%~34% | |||||
| 5mol/L MEA | ZrO2 | 15 | 40 | 40-88 | 126.3 | DR提高54% | [ |
| ZnO | 122.7 | ||||||
| 5mol/L MEA | AgO-Ag2CO3 | 15 | 40 | 40~82 | — | DR提高1010%,CC提高52% | [ |
| 5mol/L MEA | SO42-/ZrO2 | — | — | 98 | 2796.1 | RE降低9.8% | [ |
| MEA-AMP-PZ | HZSM-5 | 15 | 40 | 90~98 | 9502.1 | DR提高121%,RE降低61.6% | [ |
| 5mol/L MEA | SO42-/ZrO2-HZSM-5 | 50 | 25 | 30~98 | 1596.0 | DR提高37%,DA提高40% RE降低31% | [ |
| 5mol/L MEA | Fe-Zr@BS | 15 | 40 | 55~98 | 610 | RE降低33% | [ |
| 1 | 蔡博峰, 李琦, 张贤, 等. 中国二氧化碳捕集利用与封存(CCUS) 年度报告(2021)——中国CCUS 路径研究[R]. 生态环境部环境规划院, 中国科学院武汉岩土力学研究所, 中国21世纪议程管理中心, 2021. |
| CAI bofeng, LI Qi, ZHANG Xian, et al. China’s carbon dioxide capture use and storage (CCUS) annual report (2021)—China CCUS path[R]. Ecological Environment Conditions of Sarft, Wuhan Institute of Rock and Soil Mechanics, Chinese Academy of Sciences, China’s Agenda 21 Management Center, 2021. | |
| 2 | International Energy Agency. Global Energy Review: CO2 Emissions in 2021[R]. IEA, Paris, 2022. |
| 3 | 胡其会, 李玉星, 张建, 等. “双碳”战略下中国CCUS技术现状及发展建议[J]. 油气储运, 2022, 41(4): 361-371. |
| HU Qihui, LI Yuxing, ZHANG Jian, et al. Current status and development suggestions of CCUS technology in China under the “Double Carbon” strategy[J]. Oil & Gas Storage and Transportation, 2022, 41(4): 361-371. | |
| 4 | BABAR Muhammad, MUKHTAR Ahmad, MUBASHIR Muhammad, et al. Development of a novel switched packed bed process for cryogenic CO2 capture from natural gas[J]. Process Safety and Environmental Protection, 2021, 147: 878-887. |
| 5 | LIANG Dan, HU Yunfeng, BAO Qiang, et al. A suitable zeolite Rho for separating CO2/CH4 in pressure swing adsorption (PSA) process[J]. Inorganic Chemistry Communications, 2021, 127: 108547. |
| 6 | LI Gang, XIAO Penny, WEBLEY Paul. Binary adsorption equilibrium of carbon dioxide and water vapor on activated alumina[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2009, 25(18): 10666-10675. |
| 7 | MACDOWELL Niall, FLORIN Nick, BUCHARD Antoine, et al. An overview of CO2 capture technologies[J]. Energy & Environmental Science, 2010, 3(11): 1645-1669. |
| 8 | MANDAL B P, BISWAS A K, BANDYOPADHYAY S S. Selective absorption of H2S from gas streams containing H2S and CO2 into aqueous solutions of N-methyldiethanolamine and 2-amino-2-methyl-1-propanol[J]. Separation and Purification Technology, 2004, 35(3): 191-202. |
| 9 | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 10 | LI Qiangwei, GAO Ge, WANG Rujie, et al. Role of 1-methylimidazole in regulating the CO2 capture performance of triethylenetetramine-based biphasic solvents[J]. International Journal of Greenhouse Gas Control, 2021, 108: 103330. |
| 11 | SMITH Kathryn, XIAO Gongkui, MUMFORD Kathryn, et al. Demonstration of a concentrated potassium carbonate process for CO2 capture[J]. Energy & Fuels, 2014, 28(1): 299-306. |
| 12 | LI Yannan, CHENG Jun, HU Leiqing, et al. Regulating crystal structures of EDA-carbamates in solid-liquid phase-changing CO2 capture solutions[J]. Fuel, 2019, 252: 47-54. |
| 13 | ZHANG Zheng, ZHAO Wenbo, NONG Jiejing, et al. Liquid-solid phase-change behavior of diethylenetriamine in nonaqueous systems for carbon dioxide absorption[J]. Energy Technology, 2017, 5(3): 461-468. |
| 14 | BARZAGLI Francesco, MANI Fabrizio, PERUZZINI Maurizio. Novel water-free biphasic absorbents for efficient CO2 capture[J]. International Journal of Greenhouse Gas Control, 2017, 60: 100-109. |
| 15 | WANG Rujie, LIU Shanshan, WANG Lidong, et al. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas[J]. Applied Energy, 2019, 242: 302-310. |
| 16 | ZHANG Shihan, SHEN Yao, WANG Lidong, et al. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897. |
| 17 | RAYNAL Ludovic, ALIX Pascal, BOUILLON Pierre-Antoine, et al. The DMX™ process: An original solution for lowering the cost of post-combustion carbon capture[J]. Energy Procedia, 2011, 4: 779-786. |
| 18 | ZHANG Jiafei, QIAO Yu, WANG Wanzhong, et al. Development of an energy-efficient CO2 capture process using thermomorphic biphasic solvents[J]. Energy Procedia, 2013, 37: 1254-1261. |
| 19 | PERINU Cristina, ARSTAD Bjørnar, JENS Klaus-Joachim. NMR spectroscopy applied to amine-CO2-H2O systems relevant for post-combustion CO2 capture: A review[J]. International Journal of Greenhouse Gas Control, 2014, 20: 230-243. |
| 20 | ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6): 3660-3668. |
| 21 | XU Zhicheng, WANG Shujuan, ZHAO Bo, et al. Study on potential biphasic solvents: Absorption capacity, CO2 loading and reaction rate[J]. Energy Procedia, 2013, 37: 494-498. |
| 22 | XU Zhicheng, WANG Shujuan, CHEN Changhe. CO2 absorption by biphasic solvents: Mixtures of 1,4-butanediamine and 2-(diethylamino)-ethanol[J]. International Journal of Greenhouse Gas Control, 2013, 16: 107-115. |
| 23 | PINTO Diego D D, ZAIDY Syed A H, HARTONO Ardi, et al. Evaluation of a phase change solvent for CO2 capture: Absorption and desorption tests[J]. International Journal of Greenhouse Gas Control, 2014, 28: 318-327. |
| 24 | WANG Lidong, AN Shanlong, LI Qiangwei, et al. Phase change behavior and kinetics of CO2 absorption into DMBA/DEEA solution in a wetted-wall column[J]. Chemical Engineering Journal, 2017, 314: 681-687. |
| 25 | YOU Jong Kyun, LEE Woon Youn, KIM Je Young, et al. Screening of biphasic solvents for energy efficient CO2 capture[J]. Energy Procedia, 2017, 114: 2096-2102. |
| 26 | LEE Jun, Ki Hong Yeon, Kyun You Jong. Phase separation characteristics in biphasic solvents based on mutually miscible amines for energy efficient CO2 capture[J]. Korean Journal of Chemical Engineering, 2017, 34(6): 1840-1845. |
| 27 | ZHOU Xiaobin, LIU Fan, Bihong LYU, et al. Evaluation of the novel biphasic solvents for CO2 capture: Performance and mechanism[J]. International Journal of Greenhouse Gas Control, 2017, 60: 120-128. |
| 28 | LI Yaoyao, LIU Changjun, PARNAS Richard, et al. The CO2 absorption and desorption performance of the triethylenetetramine + N, N-diethylethanolamine + H2O system[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2351-2360. |
| 29 | LIU Fei, FANG Mengxiang, DONG Wenfeng, et al. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation[J]. Applied Energy, 2019, 233: 468-477. |
| 30 | ZHOU Xiaobin, JING Guohua, Bihong LYU, et al. Low-viscosity and efficient regeneration of carbon dioxide capture using a biphasic solvent regulated by 2-amino-2-methyl-1-propanol[J]. Applied Energy, 2019, 235: 379-390. |
| 31 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Biphasic behaviors and regeneration energy of a 2-(diethylamino)-ethanol and 2-((2-aminoethyl)amino) ethanol blend for CO2 capture[J]. Sustainable Energy & Fuels, 2019, 3(12): 3594-3602. |
| 32 | LIU Fei, FANG Mengxiang, YI Ningtong, et al. Research on alkanolamine-based physical-chemical solutions As biphasic solvents for CO2 capture[J]. Energy & Fuels, 2019, 33(11): 11389-11398. |
| 33 | LI Le, VOICE Alexander K, LI Han, et al. Amine blends using concentrated piperazine[J]. Energy Procedia, 2013, 37: 353-369. |
| 34 | LI Han, FRAILIE Peter T, ROCHELLE Gary T, et al. Thermodynamic modeling of piperazine/2-aminomethylpropanol/CO2/water[J]. Chemical Engineering Science, 2014, 117: 331-341. |
| 35 | YE Qing, WANG Xinlei, LU Yongqi. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| 36 | GUO Chao, CHEN Shaoyun, ZHANG Yongchun. A 13C NMR study of carbon dioxide absorption and desorption in pure and blended 2-(2-aminoethylamine)ethanol (AEEA) and 2-amino-2-methyl-1-propanol (AMP) solutions[J]. International Journal of Greenhouse Gas Control, 2014, 28: 88-95. |
| 37 | ZHANG Pei, SHI Yao, WEI Jianwen, et al. Regeneration of 2-amino-2-methyl-1-propanol used for carbon dioxide absorption[J]. Journal of Environmental Sciences, 2008, 20(1): 39-44. |
| 38 | KHAN Anoar Ali, HALDER G N, SAHA A K. Carbon dioxide capture characteristics from flue gas using aqueous 2-amino-2-methyl-1-propanol (AMP) and monoethanolamine (MEA) solutions in packed bed absorption and regeneration columns[J]. International Journal of Greenhouse Gas Control, 2015, 32: 15-23. |
| 39 | Saw Khun WAI, SAIWAN Chintana, IDEM Rapheal, et al. Carbon dioxide (CO2) solubility in diethylenetriamine and 2-amino-2-methyl-1-proponal (DETA-AMP) solvent system for amine-based CO2 capture in flue gas from coal combustion[J]. Energy Procedia, 2017, 114: 1973-1979. |
| 40 | NWAOHA Chikezie, SAIWAN Chintana, SUPAP Teeradet, et al. Carbon dioxide (CO2) capture performance of aqueous tri-solvent blends containing 2-amino-2-methyl-1-propanol (AMP) and methyldiethanolamine (MDEA) promoted by diethylenetriamine (DETA)[J]. International Journal of Greenhouse Gas Control, 2016, 53: 292-304. |
| 41 | LIU Fan, JING Guohua, ZHOU Xiaobin, et al. Performance and mechanisms of triethylene tetramine (TETA) and 2-amino-2-methyl-1-propanol (AMP) in aqueous and nonaqueous solutions for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 1352-1361. |
| 42 | 周小斌, 荆国华, 周作明, 等. 2-氨基-2-甲基-1-丙醇(AMP)活化相变吸收剂捕集CO2特性[C]//2017中国环境科学学会科学与技术年会论文集(第四卷). 厦门, 2017: 876-883. |
| 43 | Bihong LYU, ZHOU Xiaobin, ZHOU Zuoming, et al. Kinetics and thermodynamics of CO2 absorption into a novel DETA-AMP-PMDETA biphasic solvent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 13400-13410. |
| 44 | SHEN Li, LIU Fan, SHEN Yao, et al. Novel biphasic solvent of AEP/1-propanol/H2O for CO2 capture with efficient regeneration performance and low energy consumption[J]. Separation and Purification Technology, 2021, 270: 118700. |
| 45 | WANG Rujie, YANG Yuying, WANG Mengfan, et al. Energy efficient diethylenetriamine-1-propanol biphasic solvent for CO2 capture: Experimental and theoretical study[J]. Applied Energy, 2021, 290: 116768. |
| 46 | WANG Lidong, ZHANG Yifeng, WANG Rujie, et al. Advanced monoethanolamine absorption using sulfolane as a phase splitter for CO2 capture[J]. Environmental Science & Technology, 2018, 52(24): 14556-14563. |
| 47 | WANG Lidong, LIU Shanshan, WANG Rujie, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science & Technology, 2019, 53(21): 12873-12881. |
| 48 | WANG Rujie, JIANG Lei, LI Qiangwei, et al. Energy-saving CO2 capture using sulfolane-regulated biphasic solvent[J]. Energy, 2020, 211: 118667. |
| 49 | LI Xiaoling, ZHOU Xiaobin, WEI Jianwen, et al. Reducing the energy penalty and corrosion of carbon dioxide capture using a novel nonaqueous monoethanolamine-based biphasic solvent[J]. Separation and Purification Technology, 2021, 265: 118481. |
| 50 | TAMAJÓN F J, Estrella ÁLVAREZ, CERDEIRA F, et al. Comparative study of CO2 absorption in aqueous mixtures of methyldiethanolamine (MDEA) and methanol, focusing on the temperature and concentration influence over the absorption rate[J]. Defect and Diffusion Forum, 2014, 353: 193-198. |
| 51 | ZHANG Weidong, JIN Xianhang, TU Weiwei, et al. Development of MEA-based CO2 phase change absorbent[J]. Applied Energy, 2017, 195: 316-323. |
| 52 | YANG Fushen, JIN Xianhang, FANG Jiawei, et al. Development of CO2 phase change absorbents by means of the cosolvent effect[J]. Green Chemistry, 2018, 20(10): 2328-2336. |
| 53 | WANG Lidong, YU Songhua, LI Qiangwei, et al. Performance of sulfolane/DETA hybrids for CO2 absorption: Phase splitting behavior, kinetics and thermodynamics[J]. Applied Energy, 2018, 228: 568-576. |
| 54 | LIU Sen, LING Hao, Juan LYU, et al. New insights and assessment of primary alkanolamine/sulfolane biphasic solutions for post-combustion CO2 capture: Absorption, desorption, phase separation, and technological process[J]. Industrial & Engineering Chemistry Research, 2019, 58(44): 20461-20471. |
| 55 | ASADI Ehsan, HAGHTALAB Ali, SHIRAZIZADEH Habib Allah. Experimental measurement of carbon dioxide solubility in aqueous N-methyldiethanolamine + 2-(2-aminoethylamino) ethanol + sulfolane and diethanolamine + 2-(2-aminoethylamino) ethanol + sulfolane hybrid solvents at various temperatures and high pressure[J]. Journal of Chemical & Engineering Data, 2021, 66(1): 415-426. |
| 56 | YE Jiexu, JIANG Chenkai, CHEN Han, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: Role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8): 4470-4479. |
| 57 | ZHENG Shudong, TAO Mengna, LIU Qing, et al. Capturing CO2 into the precipitate of a phase-changing solvent after absorption[J]. Environmental Science & Technology, 2014, 48(15): 8905-8910. |
| 58 | Bihong LYU, GUO Bingsong, ZHOU Zuoming, et al. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes[J]. Environmental Science & Technology, 2015, 49(17): 10728-10735. |
| 59 | CHEN Siming, CHEN Shaoyun, ZHANG Yongchun, et al. Species distribution of CO2 absorption/desorption in aqueous and non-aqueous N-ethylmonoethanolamine solutions[J]. International Journal of Greenhouse Gas Control, 2016, 47: 151-158. |
| 60 | BARBAROSSA Vincenzo, BARZAGLI Francesco, MANI Fabrizio, et al. Efficient CO2 capture by non-aqueous 2-amino-2-methyl-1-propanol (AMP) and low temperature solvent regeneration[J]. RSC Advances, 2013, 3(30): 12349-12355. |
| 61 | BARZAGLI Francesco, MANI Fabrizio, PERUZZINI Maurizio. Efficient CO2 absorption and low temperature desorption with non-aqueous solvents based on 2-amino-2-methyl-1-propanol (AMP)[J]. International Journal of Greenhouse Gas Control, 2013, 16: 217-223. |
| 62 | ALIVAND Masood S, MAZAHERI Omid, WU Yue, et al. Catalytic solvent regeneration for energy-efficient CO2 Capture[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(51): 18755-18788. |
| 63 | LAI Qinghua, TOAN Sam, ASSIRI Mohammed A, et al. Catalyst- TiO(OH)2 could drastically reduce the energy consumption of CO2 capture[J]. Nature Communications, 2018, 9(1): 1-7. |
| 64 | ZHANG Xiaowen, ZHU Zhiqing, SUN Xiaoyu, et al. Reducing energy penalty of CO2 capture using Fe promoted SO4 2–/ZrO2/MCM-41 catalyst[J]. Environmental Science & Technology, 2019, 53(10): 6094-6102. |
| 65 | ZHANG Xiaowen, LIU Helei, LIANG Zhiwu, et al. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts[J]. Applied Energy, 2018, 229: 562-576. |
| 66 | BHATTI Umair H, Sungchan NAM, PARK Sungyoul, et al. Performance and mechanism of metal oxide catalyst-aided amine solvent regeneration[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12079-12087. |
| 67 | BHATTI Umair H, SIVANESAN Dharmalingam, Sungchan NAM, et al. Efficient Ag2O-Ag2CO3 catalytic cycle and its role in minimizing the energy requirement of amine solvent regeneration for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10234-10240. |
| 68 | LIU Helei, ZHANG Xin, GAO Hongxia, et al. Investigation of CO2 regeneration in single and blended amine solvents with and without catalyst[J]. Industrial & Engineering Chemistry Research, 2017, 56(27): 7656-7664. |
| 69 | ZHANG Xiaowen, ZHANG Rui, LIU Helei, et al. Evaluating CO2 desorption performance in CO2-loaded aqueous tri-solvent blend amines with and without solid acid catalysts[J]. Applied Energy, 2018, 218: 417-429. |
| 70 | XING Lei, WEI Kexin, LI Qiangwei, et al. One-step synthesized SO4 2-/ZrO2-HZSM-5 solid acid catalyst for carbamate decomposition in CO2 capture[J]. Environmental Science & Technology, 2020, 54(21): 13944-13952. |
| 71 | HUANG Yufei, ZHANG Xiaowen, LUO Xiao, et al. Catalytic performance and mechanism of meso-microporous material β-SBA-15-supported FeZr catalysts for CO2 desorption in CO2-loaded aqueous amine solution[J]. Industrial & Engineering Chemistry Research, 2021, 60(6): 2698-2709. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [3] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [4] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [5] | 桑伟, 唐建峰, 花亦怀, 陈洁, 孙培源, 许义飞. 物理溶剂及有机胺的性质对相变吸收性能的影响[J]. 化工进展, 2023, 42(4): 2151-2159. |
| [6] | 尚玉, 肖满, 崔秋芳, 涂特, 晏水平. CO2捕集工艺中热再生气余热的PVDF/BN-OH平板复合膜回收特性[J]. 化工进展, 2023, 42(3): 1618-1628. |
| [7] | 王璐, 张磊, 都健. 机器学习高效筛选用于CO2/N2选择性吸附分离的沸石材料[J]. 化工进展, 2023, 42(1): 148-158. |
| [8] | 沈天绪, 沈来宏. 基于3kW塔式串行流化床差异燃料的化学链燃烧解析[J]. 化工进展, 2023, 42(1): 138-147. |
| [9] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [10] | 周红军, 周颖, 徐春明. 中国碳达峰碳中和目标下炼化一体化新路径与实践[J]. 化工进展, 2022, 41(4): 2226-2230. |
| [11] | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| [12] | 郑鹏, 李蔚玲, 郭亚飞, 孙健, 王瑞林, 赵传文. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| [13] | 唐思扬, 李星宇, 鲁厚芳, 钟山, 梁斌. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| [14] | 张凡, 王树众, 李艳辉, 杨健乔, 孙圣瀚. 中国制造业碳排放问题分析与减排对策建议[J]. 化工进展, 2022, 41(3): 1645-1653. |
| [15] | 孔祥宇, 谢亮, 王延民, 翟尚鹏, 王建国. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||