化工进展 ›› 2021, Vol. 40 ›› Issue (1): 427-439.DOI: 10.16085/j.issn.1000-6613.2020-0439
废弃物衍生分级多孔炭的制备及吸附应用进展
- 浙江大学能源清洁利用国家重点实验室,浙江 杭州 310027
-
收稿日期:2020-03-23出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:黄群星 -
作者简介:杨宇轩(1997—),男,博士研究生,研究方向为废弃物基材料制备及应用。E-mail:11827034@zju.edu.cn 。 -
基金资助:国家重点研发计划(2018YFC1901300)
Progress on preparation and adsorption application of solid waste derived hierarchical porous carbon
Yuxuan YANG( ), Chenxi ZHU, Qunxing HUANG(
), Chenxi ZHU, Qunxing HUANG( )
)
- State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, Zhejiang, China
-
Received:2020-03-23Online:2021-01-05Published:2021-01-12 -
Contact:Qunxing HUANG
摘要:
分级多孔炭因其高比表面积、大孔容及分级孔结构,目前广泛应用于超级电容器、锂离子电池、催化及吸附等领域。废弃物在热解气化过程中残留的碳基材料则是制备分级多孔炭很好的前体。本文根据废弃物来源及自身特性间的差异,对生物质和非生物质废弃物作为原料制备的分级多孔炭的特性及应用进行了综述及总结。并对不同制备方法的优劣及适用对象进行了比较。对分级多孔炭在挥发性有机物(VOCs)吸附、CO2吸附捕集、染料吸附、抗生素以及酚类物质的吸附过程进行分析,总结出废弃物基多孔炭在孔径结构及表面杂原子掺杂情况下的优势能够增强这几类物质的吸附效果。结合已有文献,对废弃物基分级多孔炭的制备、孔径设计及表面官能团设计提出展望。
中图分类号:
引用本文
杨宇轩, 朱晨曦, 黄群星. 废弃物衍生分级多孔炭的制备及吸附应用进展[J]. 化工进展, 2021, 40(1): 427-439.
Yuxuan YANG, Chenxi ZHU, Qunxing HUANG. Progress on preparation and adsorption application of solid waste derived hierarchical porous carbon[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 427-439.
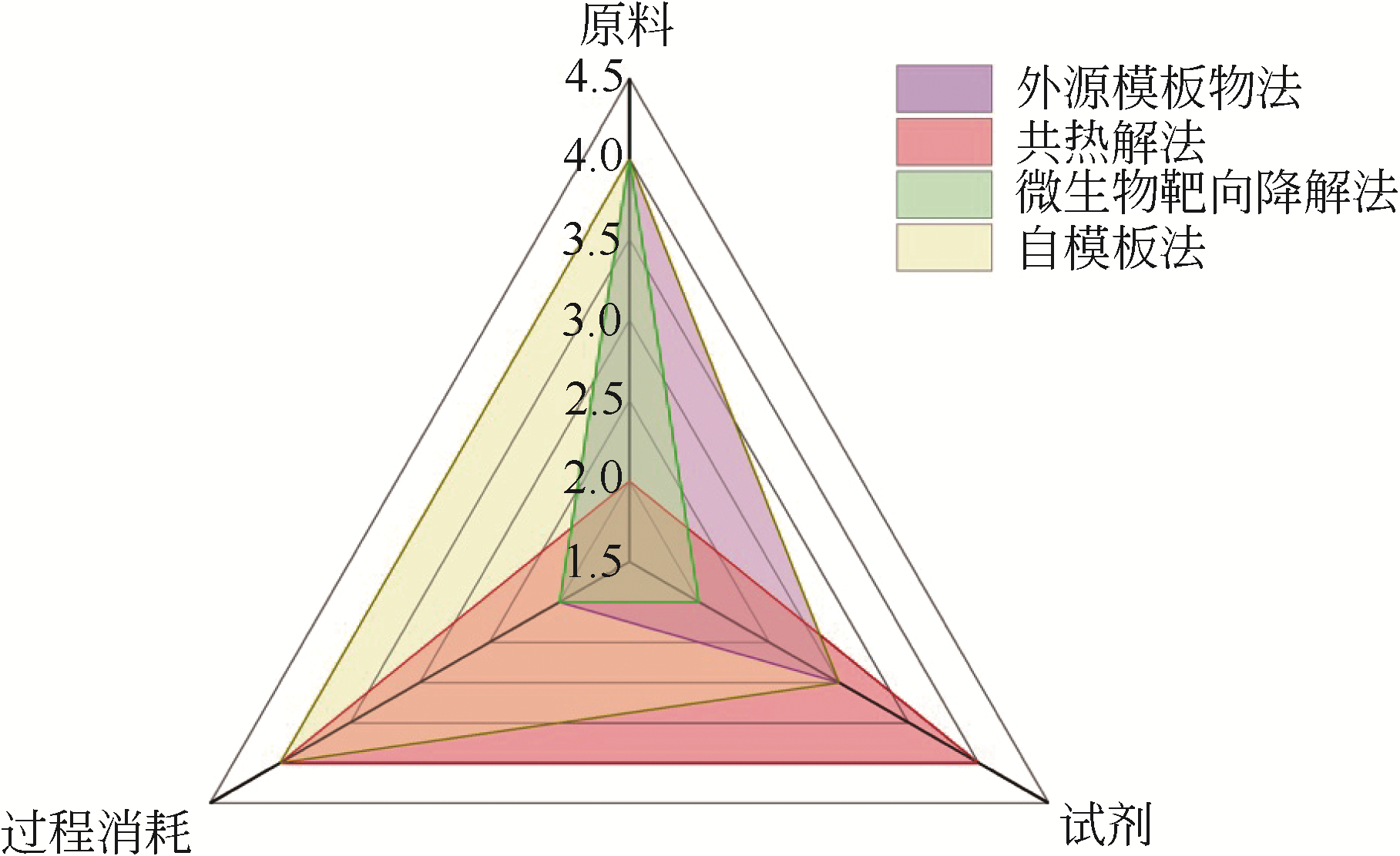
| 制备方法 | 适用原材料范围 | 制备所得材料特征 | 优点 | 缺点 |
|---|---|---|---|---|
| 外源模板物法 | 适用范围广,基本适合全部有机固体废弃物 | 介孔孔径均匀,孔径分布具有较明显特征峰 | 孔径可控,可根据模板添加量调节孔径分布及微孔/介孔比例 | 成本相对较高,制备工艺复杂 |
共热解法 | 适用于组分间存在交互作用,或一方存在较多灰分的两种/多种废弃物 | 介孔孔径分布不确定,比表面积比单独热解高 | 废弃物利用率高,工艺简单 | 组分间交互作用复杂,难以实现对孔径分布的定向调控 |
微生物靶向 预降解法 | 适用于植物基生物质废弃物 | 比表面积较大,根据组分不同,孔径分布存在差异 | 比表面积大,孔容大 | 适用范围小,仅适用于植物基生物质 |
| 自模板法 | 适用于灰分含量高,自身有机/无机结构较为规整的废弃物 | 比表面积大,孔容大,多数为介孔/大孔主导材料 | 制备工艺简单 | 产率较低,经济性相对较差 |
表1 废弃物基分级多孔炭制备方法的适用范围及优缺点
| 制备方法 | 适用原材料范围 | 制备所得材料特征 | 优点 | 缺点 |
|---|---|---|---|---|
| 外源模板物法 | 适用范围广,基本适合全部有机固体废弃物 | 介孔孔径均匀,孔径分布具有较明显特征峰 | 孔径可控,可根据模板添加量调节孔径分布及微孔/介孔比例 | 成本相对较高,制备工艺复杂 |
共热解法 | 适用于组分间存在交互作用,或一方存在较多灰分的两种/多种废弃物 | 介孔孔径分布不确定,比表面积比单独热解高 | 废弃物利用率高,工艺简单 | 组分间交互作用复杂,难以实现对孔径分布的定向调控 |
微生物靶向 预降解法 | 适用于植物基生物质废弃物 | 比表面积较大,根据组分不同,孔径分布存在差异 | 比表面积大,孔容大 | 适用范围小,仅适用于植物基生物质 |
| 自模板法 | 适用于灰分含量高,自身有机/无机结构较为规整的废弃物 | 比表面积大,孔容大,多数为介孔/大孔主导材料 | 制备工艺简单 | 产率较低,经济性相对较差 |
| 制备方法 | 原料 | 试剂 | 过程消耗 | 总得分 | |||
|---|---|---|---|---|---|---|---|
| 描述 | 得分 | 描述 | 得分 | 描述 | 得分 | ||
| 外源模板物法 | 单种废弃物 | 4 | 无机模板物,活化剂,酸,去离子水 | 3 | 模板加入材料,高温活化,去灰后烘干 | 2 | 0.675 |
| 共热解法 | 多种废弃物 | 2 | 活化剂,酸,去离子水 | 4 | 高温活化,去灰后烘干 | 4 | 0.85 |
| 微生物靶向降解法 | 单种废弃物 | 4 | 活化剂,酸,去离子水 | 2 | 恒温培养微生物,高温活化,去灰后烘干 | 2 | 0.65 |
| 自模板法 | 单种废弃物 | 4 | 微生物,活化剂,酸(用量大),去离子水 | 3 | 高温活化,去灰后烘干 | 4 | 0.925 |
表2 4种制备方法的指标描述及评价
| 制备方法 | 原料 | 试剂 | 过程消耗 | 总得分 | |||
|---|---|---|---|---|---|---|---|
| 描述 | 得分 | 描述 | 得分 | 描述 | 得分 | ||
| 外源模板物法 | 单种废弃物 | 4 | 无机模板物,活化剂,酸,去离子水 | 3 | 模板加入材料,高温活化,去灰后烘干 | 2 | 0.675 |
| 共热解法 | 多种废弃物 | 2 | 活化剂,酸,去离子水 | 4 | 高温活化,去灰后烘干 | 4 | 0.85 |
| 微生物靶向降解法 | 单种废弃物 | 4 | 活化剂,酸,去离子水 | 2 | 恒温培养微生物,高温活化,去灰后烘干 | 2 | 0.65 |
| 自模板法 | 单种废弃物 | 4 | 微生物,活化剂,酸(用量大),去离子水 | 3 | 高温活化,去灰后烘干 | 4 | 0.925 |
| 原料 | 测试工况 | 比表面积/m2·g-1 | 微孔占总孔比例/% | VOCs吸附量 | 参考文献 |
|---|---|---|---|---|---|
| 蔬菜根 | <440mg·m-3,20℃,250mL·min-1 | 1599 | 72.5 | 甲苯:700mg·g-1 | [ |
| 荷叶 | 1100mg·m-3,100mL·min-1 | 2290 | 56.6 | 甲苯:446mg·g-1 | [ |
| 稻壳 | 440mg·m-3,109mL·min-1 | 3714 | — | 甲苯:708mg·g-1 | [ |
| 木质素与PVC | 1760mg·m-3,30mL·min-1 | 1513 | 90 | 甲苯:263.4mg·g-1 | [ |
| 废弃竹料 | 相对压力为1,25℃ | 2272 | — | 甲苯:约10mmol·g-1 | [ |
污泥 | 甲苯:<440mg·m-3,20℃,250mL·min-1 柠檬烯:<607mg·m-3,20℃,250mL·min-1 2-丁酮:<321mg·m-3,20℃,250mL·min-1 | 990 | 54 | 甲苯:350mg·g-1 柠檬烯:220mg·g-1 2-丁酮:640mg·g-1 | [ |
| 高含硅稻壳 | 甲苯:20℃,1320mg·m-3,30mL·min-1 苯酚:20℃,252mg·m-3,1mL·min-1 | 1818 | 93.3 | 甲苯:263.6mg·g-1 苯酚:6.53mg·g-1 | [ |
| 榴莲壳 | 1320mg·m-3 | 1404 | — | 甲苯:57.14mg·g-1 | [ |
木质素 | 相对压力为0.9,25℃ | 2250 | — | 苯:581.43mg·g-1 甲苯:622.03mg·g-1 二甲苯:609.35mg·g-1 | [ |
| 花生壳 | 相对压力为0.99,25℃ | 1025 | 75 | 甲苯:368mg·g-1 | [ |
| 木屑 | 约10kPa,30℃ | 1284 | 62 | 甲苯:185mg·g-1 | [ |
| 废弃焦炭 | 浓度:836mg·m-3,流量1000mL·min-1 | 534 | 51 | 甲苯:254mg·g-1 | [ |
| 牛骨 | 相对压力为0.9,25℃ | 2312 | 14 | 甲苯:1198mg·g-1 | [ |
表3 不同废弃物基分级多孔炭材料对VOCs吸附性能的对比
| 原料 | 测试工况 | 比表面积/m2·g-1 | 微孔占总孔比例/% | VOCs吸附量 | 参考文献 |
|---|---|---|---|---|---|
| 蔬菜根 | <440mg·m-3,20℃,250mL·min-1 | 1599 | 72.5 | 甲苯:700mg·g-1 | [ |
| 荷叶 | 1100mg·m-3,100mL·min-1 | 2290 | 56.6 | 甲苯:446mg·g-1 | [ |
| 稻壳 | 440mg·m-3,109mL·min-1 | 3714 | — | 甲苯:708mg·g-1 | [ |
| 木质素与PVC | 1760mg·m-3,30mL·min-1 | 1513 | 90 | 甲苯:263.4mg·g-1 | [ |
| 废弃竹料 | 相对压力为1,25℃ | 2272 | — | 甲苯:约10mmol·g-1 | [ |
污泥 | 甲苯:<440mg·m-3,20℃,250mL·min-1 柠檬烯:<607mg·m-3,20℃,250mL·min-1 2-丁酮:<321mg·m-3,20℃,250mL·min-1 | 990 | 54 | 甲苯:350mg·g-1 柠檬烯:220mg·g-1 2-丁酮:640mg·g-1 | [ |
| 高含硅稻壳 | 甲苯:20℃,1320mg·m-3,30mL·min-1 苯酚:20℃,252mg·m-3,1mL·min-1 | 1818 | 93.3 | 甲苯:263.6mg·g-1 苯酚:6.53mg·g-1 | [ |
| 榴莲壳 | 1320mg·m-3 | 1404 | — | 甲苯:57.14mg·g-1 | [ |
木质素 | 相对压力为0.9,25℃ | 2250 | — | 苯:581.43mg·g-1 甲苯:622.03mg·g-1 二甲苯:609.35mg·g-1 | [ |
| 花生壳 | 相对压力为0.99,25℃ | 1025 | 75 | 甲苯:368mg·g-1 | [ |
| 木屑 | 约10kPa,30℃ | 1284 | 62 | 甲苯:185mg·g-1 | [ |
| 废弃焦炭 | 浓度:836mg·m-3,流量1000mL·min-1 | 534 | 51 | 甲苯:254mg·g-1 | [ |
| 牛骨 | 相对压力为0.9,25℃ | 2312 | 14 | 甲苯:1198mg·g-1 | [ |
| 原料 | 比表面积/m2·g-1 | 微孔孔容/cm3·g-1 | 杂原子掺杂情况 | 测试工况 | 吸附量/mmol·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 杨絮 | 1455 | 0.38 | N掺杂 | 0℃,1bar 25℃,1bar | 6.2 4.1 | [ |
| 脲醛树脂 | 4547 | 0.26 | N掺杂 | 30℃,1bar 50℃,1bar | 2.4 1.4 | [ |
| 稻壳 | 1190 | 0.42 | 无 | 25℃,15kPa | 1.9 | [ |
| 酶解木质素 | 2870 | 0.7 | 无 | 30℃,1bar 75℃,1bar | 1.3 0.6 | [ |
| 荷花梗 | 2893 | 0.7 | 无 | 0℃,1bar 25℃,1bar | 6.2 3.9 | [ |
| 含油污泥 | 1224 | — | N掺杂 | 25℃,1bar | 3 | [ |
芦竹 | 2232 | 0.5 | 无 | 0℃,1bar 10℃,1bar 25℃,1bar | 4.1 3.3 3.2 | [ |
| 可口可乐 | 1994 | 0.77 | N掺杂 | 0℃,1bar 25℃,1bar | 6.3 5.2 | [ |
| 枣 | 3337 | 0.55 | 无 | 0℃,1bar 25℃,1bar | 6.4 4.4 | [ |
| 蒜皮 | 1262 | 0.65 | 无 | 0℃,1bar 25℃,1bar | 6.2 4.2 | [ |
| 茶叶渣 | 354 | — | 无 | 0℃,1bar | 1.9 | [ |
表4 不同废弃物基分级多孔炭材料对CO2吸附性能的对比
| 原料 | 比表面积/m2·g-1 | 微孔孔容/cm3·g-1 | 杂原子掺杂情况 | 测试工况 | 吸附量/mmol·g-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 杨絮 | 1455 | 0.38 | N掺杂 | 0℃,1bar 25℃,1bar | 6.2 4.1 | [ |
| 脲醛树脂 | 4547 | 0.26 | N掺杂 | 30℃,1bar 50℃,1bar | 2.4 1.4 | [ |
| 稻壳 | 1190 | 0.42 | 无 | 25℃,15kPa | 1.9 | [ |
| 酶解木质素 | 2870 | 0.7 | 无 | 30℃,1bar 75℃,1bar | 1.3 0.6 | [ |
| 荷花梗 | 2893 | 0.7 | 无 | 0℃,1bar 25℃,1bar | 6.2 3.9 | [ |
| 含油污泥 | 1224 | — | N掺杂 | 25℃,1bar | 3 | [ |
芦竹 | 2232 | 0.5 | 无 | 0℃,1bar 10℃,1bar 25℃,1bar | 4.1 3.3 3.2 | [ |
| 可口可乐 | 1994 | 0.77 | N掺杂 | 0℃,1bar 25℃,1bar | 6.3 5.2 | [ |
| 枣 | 3337 | 0.55 | 无 | 0℃,1bar 25℃,1bar | 6.4 4.4 | [ |
| 蒜皮 | 1262 | 0.65 | 无 | 0℃,1bar 25℃,1bar | 6.2 4.2 | [ |
| 茶叶渣 | 354 | — | 无 | 0℃,1bar | 1.9 | [ |
| 原料 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 微孔占总孔比例/% | 杂原子掺杂情况 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
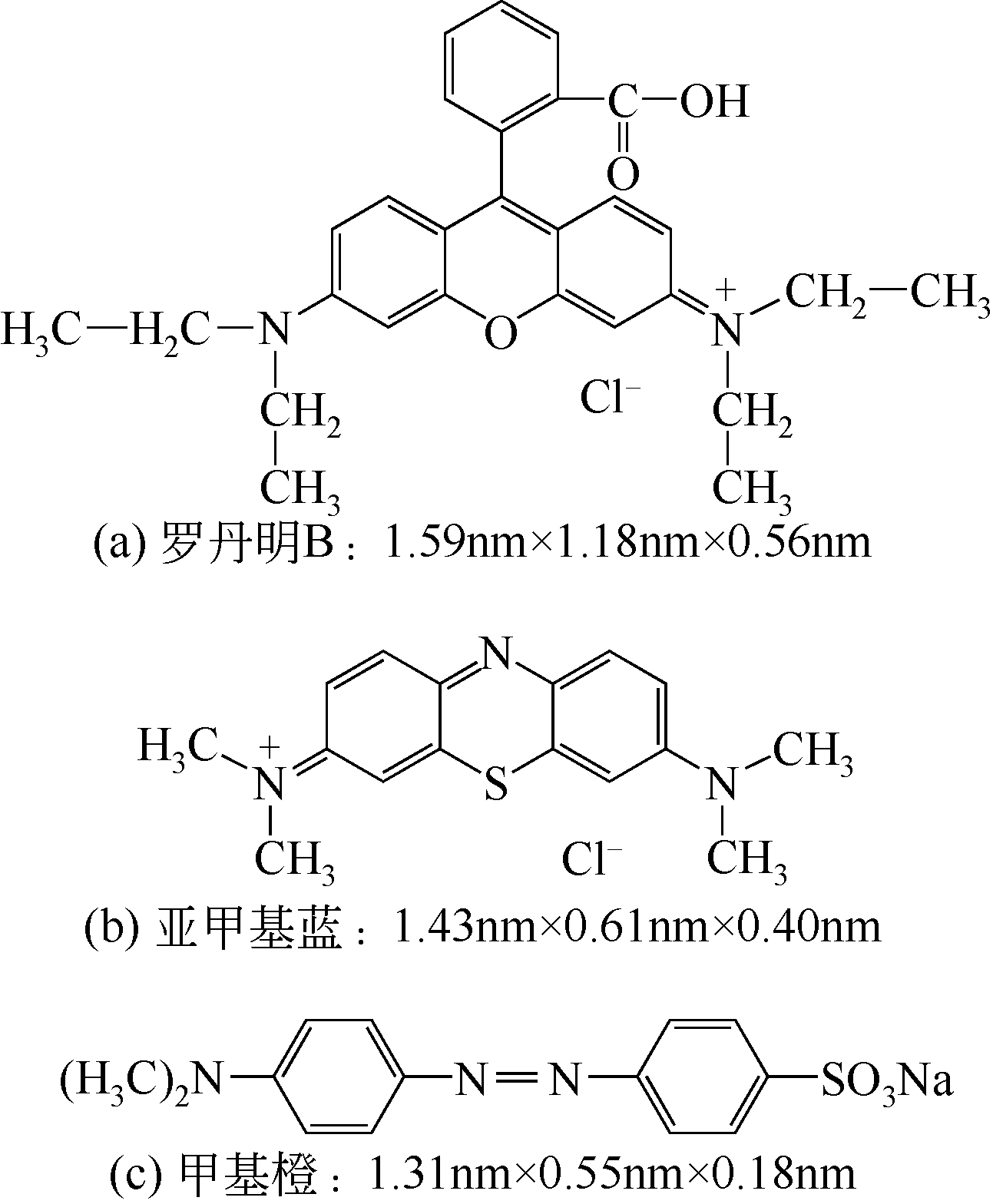
| 香蕉皮 | 1238 | — | 0.64 | — | N掺杂 | 亚甲基蓝:1584.95 | [ |
| 纤维素纤维 | 1295 | 4.2 | 2.69 | 15 | N掺杂 | 甲基橙:337.8 | [ |
| 轮胎热解重质油 | 868 | 6.1 | 1.34 | 8 | 无 | 亚甲基蓝:843.5 | [ |
| 含油污泥+稻壳 | 2575 | 2.49 | 1.61 | 67 | 无 | 亚甲基蓝:757.8 | [ |
| 爆米花 | 3074 | 3.42 | 2.15 | — | 无 | 罗丹明B:7543 | [ |
| 废锯材 | 725 | 0.29 | 4.6 | — | 无 | 亚甲基蓝:269 | [ |
| 竹笋壳 | 489 | 3.31 | 0.67 | 52 | N掺杂 | 甲基橙:140 罗丹明B:103 | [ |
| 甘蔗渣 | 644 | 微孔:0.86 介孔:5.5 | 0.41 | 41 | 无 | 亚甲基蓝:101 | [ |
| 白糖 | 1144 | 2.17 | 0.53 | — | 无 | 罗丹明B:123.46 | [ |
| 含油污泥 | 324 | 1.53 | 0.25 | 25 | 无 | 亚甲基蓝:316.02 直接蓝6:124.24 | [ |
表5 不同废弃物基分级多孔炭材料对染料吸附性能的对比
| 原料 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 微孔占总孔比例/% | 杂原子掺杂情况 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 香蕉皮 | 1238 | — | 0.64 | — | N掺杂 | 亚甲基蓝:1584.95 | [ |
| 纤维素纤维 | 1295 | 4.2 | 2.69 | 15 | N掺杂 | 甲基橙:337.8 | [ |
| 轮胎热解重质油 | 868 | 6.1 | 1.34 | 8 | 无 | 亚甲基蓝:843.5 | [ |
| 含油污泥+稻壳 | 2575 | 2.49 | 1.61 | 67 | 无 | 亚甲基蓝:757.8 | [ |
| 爆米花 | 3074 | 3.42 | 2.15 | — | 无 | 罗丹明B:7543 | [ |
| 废锯材 | 725 | 0.29 | 4.6 | — | 无 | 亚甲基蓝:269 | [ |
| 竹笋壳 | 489 | 3.31 | 0.67 | 52 | N掺杂 | 甲基橙:140 罗丹明B:103 | [ |
| 甘蔗渣 | 644 | 微孔:0.86 介孔:5.5 | 0.41 | 41 | 无 | 亚甲基蓝:101 | [ |
| 白糖 | 1144 | 2.17 | 0.53 | — | 无 | 罗丹明B:123.46 | [ |
| 含油污泥 | 324 | 1.53 | 0.25 | 25 | 无 | 亚甲基蓝:316.02 直接蓝6:124.24 | [ |
| 原料 | 比表面积 /m2·g-1 | 平均孔径 /nm | 孔容 /cm3·g-1 | 微孔占 总孔比例/% | 杂原子 掺杂情况 | 吸附量/mg·g-1 | 文献来源 |
|---|---|---|---|---|---|---|---|
| 牛骨 | 3231 | 2.45 | 1.98 | 74 | 无 | 磺胺甲 氯霉素:1240 | [ |
| 含油污泥+生物质 | 1342 | 1.81 | 0.61 | 85 | 无 | 磺胺甲 唑:362 唑:362 | [ |
| 木质素 | 2320 | 1.34 | 2.31 | 78 | 无 | 四环素:1297 氯霉素:1067 | [ |
| 虾壳 | 3171 | 1.93 | 2.44 | 49 | N掺杂 | 磺胺甲 氯霉素:1240 | [ |
| 杏仁壳 | 1274 | 2.82 | 1.67 | — | 无 | 磺胺甲 唑:345 唑:345 | [ |
壳聚糖 | 2607 | 1.97 | 1.28 | — | 无 | 氯霉素:786 氟洛芬:752 甲砜霉素:692 | [ |
| 可食用菌渣 | 3342 | — | 1.84 | — | N掺杂 | 双酚A:1249 2,4-二氯苯酚:1155 | [ |
| 牛骨 | 2687 | — | 2.1 | — | N掺杂 | 苯酚:431 | [ |
| 半焦 | 121 | — | — | — | 无 | 硝基苯酚:94 | [ |
| 土豆皮 | 1041 | — | 1.22 | — | 无 | 双酚A:446 | [ |
| 花椒籽废渣 | 1210 | — | 0.66 | — | 无 | 硝基苯酚:406 | [ |
表6 不同废弃物基分级多孔炭材料对抗生素/酚类有机物吸附性能的对比
| 原料 | 比表面积 /m2·g-1 | 平均孔径 /nm | 孔容 /cm3·g-1 | 微孔占 总孔比例/% | 杂原子 掺杂情况 | 吸附量/mg·g-1 | 文献来源 |
|---|---|---|---|---|---|---|---|
| 牛骨 | 3231 | 2.45 | 1.98 | 74 | 无 | 磺胺甲 氯霉素:1240 | [ |
| 含油污泥+生物质 | 1342 | 1.81 | 0.61 | 85 | 无 | 磺胺甲 唑:362 唑:362 | [ |
| 木质素 | 2320 | 1.34 | 2.31 | 78 | 无 | 四环素:1297 氯霉素:1067 | [ |
| 虾壳 | 3171 | 1.93 | 2.44 | 49 | N掺杂 | 磺胺甲 氯霉素:1240 | [ |
| 杏仁壳 | 1274 | 2.82 | 1.67 | — | 无 | 磺胺甲 唑:345 唑:345 | [ |
壳聚糖 | 2607 | 1.97 | 1.28 | — | 无 | 氯霉素:786 氟洛芬:752 甲砜霉素:692 | [ |
| 可食用菌渣 | 3342 | — | 1.84 | — | N掺杂 | 双酚A:1249 2,4-二氯苯酚:1155 | [ |
| 牛骨 | 2687 | — | 2.1 | — | N掺杂 | 苯酚:431 | [ |
| 半焦 | 121 | — | — | — | 无 | 硝基苯酚:94 | [ |
| 土豆皮 | 1041 | — | 1.22 | — | 无 | 双酚A:446 | [ |
| 花椒籽废渣 | 1210 | — | 0.66 | — | 无 | 硝基苯酚:406 | [ |
| 1 | 杜春林, 黄涛珍. 从政府主导到多元共治:城市生活垃圾分类的治理困境与创新路径[J]. 行政论坛, 2019, 26(4): 116-121. |
| DU Chunlin, HUANG Taozhen. From government-led to pluralistic co-governance: governance dilemma and innovation path of urban domestic waste classification[J]. Administrative Tribune, 2019, 26(4): 116-121. | |
| 2 | ZHOU H, MENG A L, LONG Y Q, et al. An overview of characteristics of municipal solid waste fuel in China: physical, chemical composition and heating value[J]. Renew. Sustain. Energy Rev., 2014, 36: 107-122. |
| 3 | ZHOU H, LONG Y Q, MENG A H, et al. Thermogravimetric characteristics of typical municipal solid waste fractions during co-pyrolysis[J]. Waste Management, 2015, 38: 194-200. |
| 4 | 袁浩然, 鲁涛, 熊祖鸿, 等. 城市生活垃圾热解气化技术研究进展[J]. 化工进展, 2012, 31(2): 421-427. |
| YUAN Haoran, LU Tao, XIONG Zuhong, et al. Advance in pyrolysis and gasification of municipal solid waste study[J]. Chemical Industry and Engineering Progress, 2012, 31(2): 421-427. | |
| 5 | BAE W G, KIM H N, KIM D, et al. 25th Anniversary article: scalable multiscale patterned structures inspired by nature: the role of hierarchy[J]. Advanced Materials, 2014, 26(5): 675-700. |
| 6 | SUN M H, HUANG S Z, CHEN L H, et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine[J]. Chemical Society Reviews, 2016, 45: 3479-3563. |
| 7 | GIL R R, RUIZ B, LOZANO M S, et al. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning[J]. Chemical Engineering Journal, 2014, 245: 80-88. |
| 8 | YU D, WANG L, WU M. Simultaneous removal of dye and heavy metal by banana peels derived hierarchically porous carbons[J]. J. Taiwan Inst. Chem. Eng., 2018, 93: 543-553. |
| 9 | NIU J, SHAO R, LIANG J, et al. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors[J]. Nano Energy, 2017, 36: 322-330. |
| 10 | DAI J, QIN L, ZHANG R, et al. Sustainable bovine bone-derived hierarchically porous carbons with excellent adsorption of antibiotics: equilibrium, kinetic and thermodynamic investigation[J]. Powder Technol., 2018, 331: 162-170. |
| 11 | LIU M, NIU J, ZHANG Z, et al. Porous carbons with tailored heteroatom doping and well-defined porosity as high-performance electrodes for robust Na-ion capacitors[J]. J. Power Sources, 2019, 414: 68-75. |
| 12 | SHAN B, CUI Y, LIU W, et al. Fibrous bio-carbon foams: a new material for lithium-ion hybrid supercapacitors with ultrahigh integrated energy/power density and ultralong cycle life [J]. ACS Sustain. Chem. Eng., 2018, 6: 14989-15000. |
| 13 | 张伟霞. 真菌调控生物质基多级孔炭的制备及其甲苯吸附性能[D]. 广州: 华南理工大学, 2019. |
| ZHANG Weixia. Preparation of fungus modified hierarchical porous carbon and its adsorption performance for toluene[D]. Guangzhou: South China University of Technology, 2019. | |
| 14 | WANG P, YE H, YIN Y X, et al. Fungi-enabled synthesis of ultrahigh-surface-area porous carbon[J]. Adv. Mater., 2019, 31: 1-7. |
| 15 | ZHANG W, CHENG H, NIU Q, et al. Microbial targeted degradation pretreatment: a novel approach to preparation of activated carbon with specific hierarchical porous structures, high surface areas, and satisfactory toluene adsorption performance[J]. Environ. Sci. Technol., 2019, 53: 7632-7640. |
| 16 | CHENG H, SUN Y, WANG X, et al. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: ultrahigh surface area and impressive improvement in toluene adsorption[J]. J. Hazard. Mater., 2020, 392: 122298. |
| 17 | CHENG J, GU J J, TAO W, et al. Edible fungus slag derived nitrogen-doped hierarchical porous carbon as a high-performance adsorbent for rapid removal of organic pollutants from water[J]. Bioresour. Technol., 2019, 294: 122149. |
| 18 | SUN B, YUAN Y, LI H, et al. Waste-cellulose-derived porous carbon adsorbents for methyl orange removal[J]. Chemical Engineering Journal, 2019, 371: 55-63. |
| 19 | CHANG B, SHI W, YIN H, et al. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture[J]. Chemical Engineering Journal, 2019, 358: 1507-1518. |
| 20 | 查飞, 于霞, 朱钰, 等. 花椒籽废渣制备活性炭及其对对硝基苯酚的吸附行为[J]. 西北师范大学学报(自然科学版), 2016, 52(2): 60-67. |
| ZHA Fei, YU Xia, ZHU Yu, et al. Preparation of activated carbon from waste residue of Chinese prickly ash seeds and its adsorption properties of p-nitrophenol[J]. Journal of Northwest Normal University(Natural Science Edtion), 2016, 52(2): 60-67. | |
| 21 | MURALI G, HARISH S, PONNUSAMY, et al. Hierarchically porous structured carbon derived from peanut shell as an enhanced high rate anode for lithium ion batteries[J]. Appl. Surf. Sci., 2019, 492: 464-472. |
| 22 | WU J, XIA M, ZHANG X, et al. Hierarchical porous carbon derived from wood tar using crab as the template: performance on supercapacitor[J]. J. Power Sources, 2020, 455: 227982. |
| 23 | WEI L, SEVILLA M, FUERTES A B, et al. Polypyrrole-derived activated carbons for high-performance electrical double-layer capacitors with ionic liquid electrolyte[J]. Adv. Funct. Mater., 2012, 22: 827-834. |
| 24 | QIE L, CHEN W, XU H, et al. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors[J]. Energy Environ. Sci., 2013, 6: 2497-2504. |
| 25 | TIWARI D, BHUNIA H, BAJPAI P K. Development of chemically activated N-enriched carbon adsorbents from urea-formaldehyde resin for CO2 adsorption: kinetics, isotherm, and thermodynamics[J]. J. Environ. Manage., 2018, 218: 579-592. |
| 26 | ZHANG H, ZHOU X L, SHAO L M, et al. Hierarchical porous carbon spheres from low-density polyethylene for high-performance supercapacitors[J]. ACS Sustain. Chem. Eng., 2019, 7: 3801-3810. |
| 27 | ZHANG N, SHEN Y. One-step pyrolysis of lignin and polyvinyl chloride for synthesis of porous carbon and its application for toluene sorption[J]. Bioresour. Technol., 2019, 284: 325-332. |
| 28 | ZHANG Y, JI G, LI C, et al. Templating synthesis of hierarchical porous carbon from heavy residue of tire pyrolysis oil for methylene blue removal[J]. Chemical Engineering Journal, 2020, 390: 124398. |
| 29 | KONIKKARA N, KENNEDEY L J, VIJAYA J J. Preparation and characterization of hierarchical porous carbons derived from solid leather waste for supercapacitor applications[J]. J. Hazard. Mater., 2016, 318: 173-185. |
| 30 | WANG J, SUN C, LIN B C, et al. Micro- and mesoporous-enriched carbon materials prepared from a mixture of petroleum-derived oily sludge and biomass[J]. Fuel Process Technology, 2018, 171: 140-147. |
| 31 | WANG A Y, SUN K, WU L, et al. Co-carbonization of biomass and oily sludge to prepare sulfamethoxazole super-adsorbent materials[J]. Sci. Total Environ., 2020, 698: 134238. |
| 32 | 王亮. 氧化钙模板法制备分级多孔炭及其对废水中染料的吸附性能研究[D]. 马鞍山: 安徽工业大学, 2018. |
| WANG Liang. Preparation of hierarchical porous carbon using CaO template and its adsorption properties for dyes in wastewater[D]. Ma’anshan: Anhui University of Technology, 2018. | |
| 33 | DAI Q, JIANG X, JIANG Y, et al. Formation of PAHs during the pyrolysis of dry sewage sludge[J]. Fuel, 2014, 30: 92-99. |
| 34 | MOHSENI-BANDPEI A, MAJLESI M, RAFIEE M, et al. Polycyclic aromatic hydrocarbons (PAHs) formation during the fast pyrolysis of hazardous health-care waste[J]. Chemosphere, 2019, 227: 277-288. |
| 35 | HALE S E, LEHMANN J, RUTHERFORD D. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars[J]. Environ. Sci. Technol., 2012, 46: 2830-2838. |
| 36 | GHIDOTTI M, FABBRI D, HORMUNG A. Profiles of volatile organic compounds in biochar: insights into process conditions and quality assessment[J]. ACS Sustain. Chem. Eng., 2017, 5: 510-517. |
| 37 | 陈亮, 傅闯, 李兴源. 基于加权平均组合评价法的电网直流融冰计划决策[J]. 电网技术, 2014, 38(10): 2913-2918. |
| CHEN Liang, FU Chuang, LI Xingyuan. Decision-making of power grid DC de-icing plan based on weighted average combinative evaluation[J]. Power System Technology, 2014, 38(10): 2913-2918. | |
| 38 | 钟隆春. 沥青基活性炭汞吸附剂的开发与机理研究[D]. 北京: 华北电力大学, 2018. |
| ZHONG Longchun. Development and mechanisms study of mercury removal with activated carbon adsorbent from asphalt[D]. Beijing: North China Electric Power University, 2018. | |
| 39 | HUANG L, QIAN H, DENG S, et al. Urban residential indoor volatile organic compounds in summer, Beijing: profile, concentration and source characterization[J]. Atmos. Environ., 2018, 188: 1-11. |
| 40 | CHENG J, ZHANG Y, WANG T, et al. Emission of volatile organic compounds (VOCs) during coal combustion at different heating rates[J]. Fuel, 2018, 225: 554-562. |
| 41 | ZHANG X, GAO B, CREAMER A E, et al. Adsorption of VOCs onto engineered carbon materials: a review[J]. J. Hazard. Mater., 2017, 338: 102-123. |
| 42 | 张辉, 杨元涛, 马静红, 等. 甲苯在多级孔丝光沸石上的吸附平衡和吸附动力学[J]. 燃料化学学报, 2018, 46(6): 710-716. |
| ZHANG Hui, YANG Yuantao, MA Jinghong, et al. Adsorption equilibrium and kinetics of toluene on hierarchical mordenite[J]. Journal of Fuel Chemistry and Technology, 2018, 46(6): 710-716. | |
| 43 | LIU S, PENG Y, CHEN J, et al. A new insight into adsorption state and mechanism of adsorbates in porous materials[J]. J. Hazard. Mater., 2020, 382: 121103. |
| 44 | ANFRUNS A, MARTIN M J, MONTES-MORAN M A. Removal of odourous VOCs using sludge-based adsorbents[J]. Chemical Engineering Journal, 2011, 166: 1022-1031. |
| 45 | SHEN Y, ZHANG N. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption[J]. Bioresour. Technol., 2019, 282: 294-300. |
| 46 | THAM Y J, LATIF P A, ABDULLAH A M, et al. Performances of toluene removal by activated carbon derived from durian shell[J]. Bioresour. Technol., 2011, 102: 724-728. |
| 47 | SAHA D, MIRANDO N, LEVCHENKO A. Liquid and vapor phase adsorption of BTX in lignin derived activated carbon: equilibrium and kinetics study[J]. J. Clean. Prod., 2018, 182: 372-378. |
| 48 | BEDANE A H, GUO T, EIC M, et al. Adsorption of volatile organic compounds on peanut shell activated carbon[J]. Can. J. Chem. Eng., 2018, 97: 238-246. |
| 49 | YANG X, YI H, TANG X, et al. Behaviors and kinetics of toluene adsorption‐desorption on activated carbons with varying pore structure[J]. J. Environ. Sci. (China), 2018, 67: 104-114. |
| 50 | QIE Z, ZHANG Z, SUN F, et al. Effect of pore hierarchy and pore size on the combined adsorption of SO2 and toluene in activated coke[J]. Fuel, 2019, 257: 116090. |
| 51 | YANG Y, SUN C, LIN B, et al. Surface modified and activated waste bone char for rapid and efficient VOCs adsorption[J]. Chemosphere, 2020, 256: 127054. |
| 52 | LIU X, SUN C, LIU H, et al. Developing hierarchically ultra-micro/mesoporous biocarbons for highly selective carbon dioxide adsorption[J]. Chemical Engineering Journal, 2019, 361: 199-208. |
| 53 | CHEN W, WANG X, HASHISHO Z, et al. Template-free and fast one-step synthesis from enzymatic hydrolysis lignin to hierarchical porous carbon for CO2 capture[J]. Microporous Mesoporous Mater., 2019, 280: 57-65. |
| 54 | WU X X, ZHANG C Y, TIAN Z W, et al. Large-surface-area carbons derived from lotus stem waste for efficient CO2 capture[J]. New Carbon Mater, 2019, 33: 252-261. |
| 55 | MENG F, GONG Z, WANG Z, et al. Study on a nitrogen-doped porous carbon from oil sludge for CO2 adsorption[J]. Fuel, 2019, 251: 562-571. |
| 56 | SINGH G, LAKHI K S, RAMADASS K, et al. A combined strategy of acid-assisted polymerization and solid state activation to synthesize functionalized nanoporous activated biocarbons from biomass for CO2 capture[J]. Microporous Mesoporous Mater., 2018, 271: 23-32. |
| 57 | BOYJOO Y, CHENG Y, ZHONG H, et al. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors[J]. Carbon, 2017, 116: 490-499. |
| 58 | LI J, MICHALKIEWICZ B, MIN J, et al. Selective preparation of biomass-derived porous carbon with controllable pore sizes toward highly efficient CO2 capture[J]. Chemical Engineering Journal, 2019, 360: 250-259. |
| 59 | HUANG G G, LIU Y F, WU X X, et al. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance[J]. New Carbon Mater., 2019, 34: 247-257. |
| 60 | TAO D J, MAO F F, LUO J J, et al. Mesoporous N-doped carbon derived from tea waste for high-performance CO2 capture and conversion[J]. Mater. Today Commun., 2020, 22: 100849. |
| 61 | SETHIS G, SAYARI A. Comprehensive study of ultra-microporous nitrogen-doped activated carbon for CO2 capture[J]. Carbon, 2015, 93: 68-80. |
| 62 | HAN J, ZHANG L, ZHAO B, et al. The N-doped activated carbon derived from sugarcane bagasse for CO2 adsorption[J]. Ind. Crops Prod., 2019, 128: 290-297. |
| 63 | LI X, JIN Y, XUE Q, et al. Ultra-high selective capture of CO2 on one-sided N-doped carbon nanoscrolls[J]. J. CO2 Util., 2017, 18: 275-282. |
| 64 | SHI J, YAN N, CUI H, et al. Sulfur doped microporous carbons for CO2 adsorption[J]. J. Environ. Chem. Eng., 2017, 5: 4605-4611. |
| 65 | YU Y, QIAO N, WANG D, et al. Fluffy honeycomb-like activated carbon from popcorn with high surface area and well-developed porosity for ultra-high efficiency adsorption of organic dyes[J]. Bioresour. Technol., 2019, 285: 121340. |
| 66 | CHEN L, JI T, MU L, et al. Pore size dependent molecular adsorption of cationic dye in biomass derived hierarchically porous carbon[J]. J. Environ. Manage., 2017, 196: 168-177. |
| 67 | HOU Y, YAN S, HUANG G, et al. Fabrication of N-doped carbons from waste bamboo shoot shell with high removal efficiency of organic dyes from water[J]. Bioresour. Technol., 2020, 303: 122939. |
| 68 | KUEASOOK R, RATTANACHUESKUL N, CHANLEK N, et al. Green and facile synthesis of hierarchically porous carbon monoliths via surface self-assembly on sugarcane bagasse scaffold: influence of mesoporosity on efficiency of dye adsorption[J]. Microporous Mesoporous Mater., 2020, 296: 110005. |
| 69 | XIAO W, GARBA Z N, SUN S, et al. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye[J]. J. Clean. Prod., 2020, 253: 119989. |
| 70 | LI X, HAN D, ZHANG M, et al. Removal of toxic dyes from aqueous solution using new activated carbon materials developed from oil sludge waste[J]. Colloids Surfaces A: Physicochem. Eng. Asp., 2019, 578: 123505. |
| 71 | MICHAEL I, RIZZO L, MCARDELL CS, et al. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review[J]. Water Res., 2013, 47: 957-995. |
| 72 | MA Y, LI M, WU M, et al. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge[J]. Sci. Total Environ., 2015, 518/519: 498-506. |
| 73 | WANG P, XIAO P, ZHONG S, et al. Bamboo-like carbon nanotubes derived from colloidal polymer nanoplates for efficient removal of bisphenol A[J]. J. Mater. Chem. A, 2016, 4: 15450-15456. |
| 74 | DU W, SUN J, ZAN Y, et al. Biomass-derived nitrogen-doped hierarchically porous carbon networks as efficient absorbents for phenol removal from wastewater over a wide pH range[J]. RSC Adv., 2017, 7: 46629-46635. |
| 75 | XIE A, DAI J, CHEN X, et al. Ultrahigh adsorption of typical antibiotics onto novel hierarchical porous carbons derived from renewable lignin via halloysite nanotubes-template and in-situ activation[J]. Chemical Engineering Journal, 2016, 304: 609-620. |
| 76 | QIN L, ZHOU Z, DAI J, et al. Novel N-doped hierarchically porous carbons derived from sustainable shrimp shell for high-performance removal of sulfamethazine and chloramphenicol[J]. J. Taiwan Inst. Chem. Eng., 2016, 62: 228-238. |
| 77 | ZBAIR M, ABSAINE H AIT, ANFAR Z. Porous carbon by microwave assisted pyrolysis: an effective and low-cost adsorbent for sulfamethoxazole adsorption and optimization using response surface methodology[J]. J. Clean. Prod., 2018, 202: 571-581. |
| 78 | LIU H, WEI Y, LUO J, et al. 3D hierarchical porous-structured biochar aerogel for rapid and efficient phenicol antibiotics removal from water[J]. Chemical Engineering Journal, 2019, 368: 639-648. |
| 79 | YANG X X, HOU X F, GAO X M, et al. Hierarchical porous carbon from semi-coke via a facile preparation method for p-nitrophenol adsorption[J]. Colloids Surfaces A: Physicochem. Eng. Asp., 2019, 563: 50-58. |
| 80 | ARAMPATZIDOU A C, DELIYANNI E A. Comparison of activation media and pyrolysis temperature for activated carbons development by pyrolysis of potato peels for effective adsorption of endocrine disruptor bisphenol-A[J]. J. Colloid Interface Sci., 2016, 466: 101-112. |
| 81 | 王亚非, 于霞, 朱钰, 等. K2CO3活化制备花椒籽废渣的活性炭及其对对硝基苯酚的吸附性能[J]. 应用化学, 2017, 34(5): 597-605. |
| WANG Yafei, YU Xia, ZHU Yu, et al. Activated carbon prepared from prickly ash seed residue by K2CO3 activation and its adsorption performance for p-nitrophenol[J]. Chinese Journal of Applied Chemistry, 2017, 34(5): 597-605. | |
| 82 | ABOULKAS A, HAMMANI H, Achaby M EL, et al. Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char[J]. Bioresour. Technol., 2017, 243: 400-408. |
| 83 | YI L, ZUO L, WEI C, et al. Enhanced adsorption of bisphenol A, tylosin, and tetracycline from aqueous solution to nitrogen-doped multiwall carbon nanotubes via cation-π and π-π electron-donor-acceptor (EDA) interactions[J]. Sci. Total Environ., 2020, 719: 137389. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [9] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [10] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [11] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [12] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [13] | 王知彩, 刘伟伟, 周璁, 潘春秀, 闫洪雷, 李占库, 颜井冲, 任世彪, 雷智平, 水恒福. 基于煤基腐殖酸的高效减水剂合成与性能表征[J]. 化工进展, 2023, 42(7): 3634-3642. |
| [14] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [15] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||


 唑:1194
唑:1194 唑:643.5
唑:643.5