化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3520-3531.DOI: 10.16085/j.issn.1000-6613.2022-1577
光催化及其协同电化学降解VOCs的研究进展
- 太原理工大学土木工程学院,山西 太原 030024
-
收稿日期:2022-08-25修回日期:2023-01-12出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:张兴惠 -
作者简介:徐伟(1999—),男,硕士研究生,研究方向为光电催化降解VOCs。E-mail:llxwyu@163.com。
Research progress of photocatalysis and co-electrochemical degradation of VOCs
XU Wei( ), LI Kaijun, SONG Linye, ZHANG Xinghui(
), LI Kaijun, SONG Linye, ZHANG Xinghui( ), YAO Shunhua
), YAO Shunhua
- School of Civil Engineering, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-08-25Revised:2023-01-12Online:2023-07-15Published:2023-08-14 -
Contact:ZHANG Xinghui
摘要:
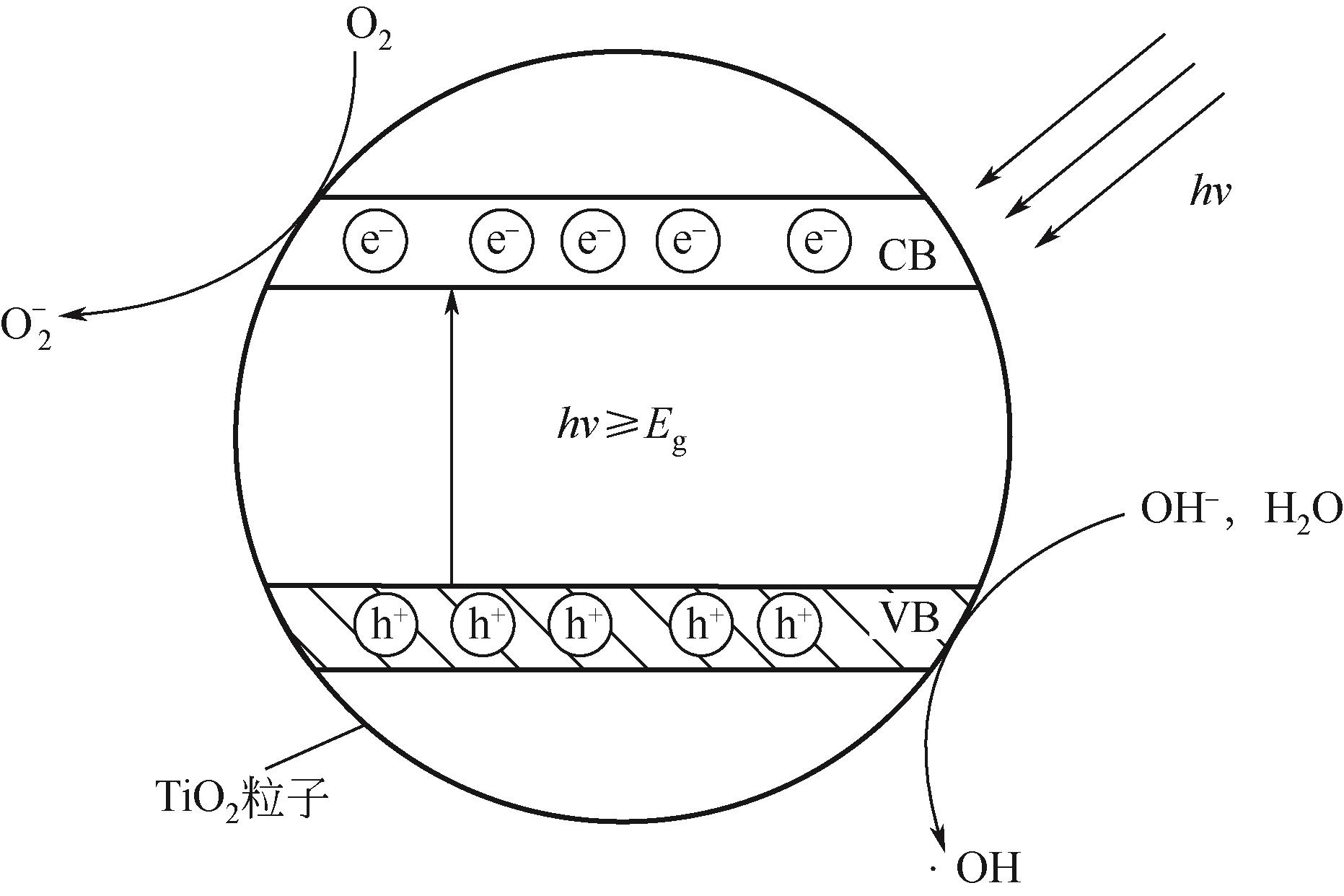
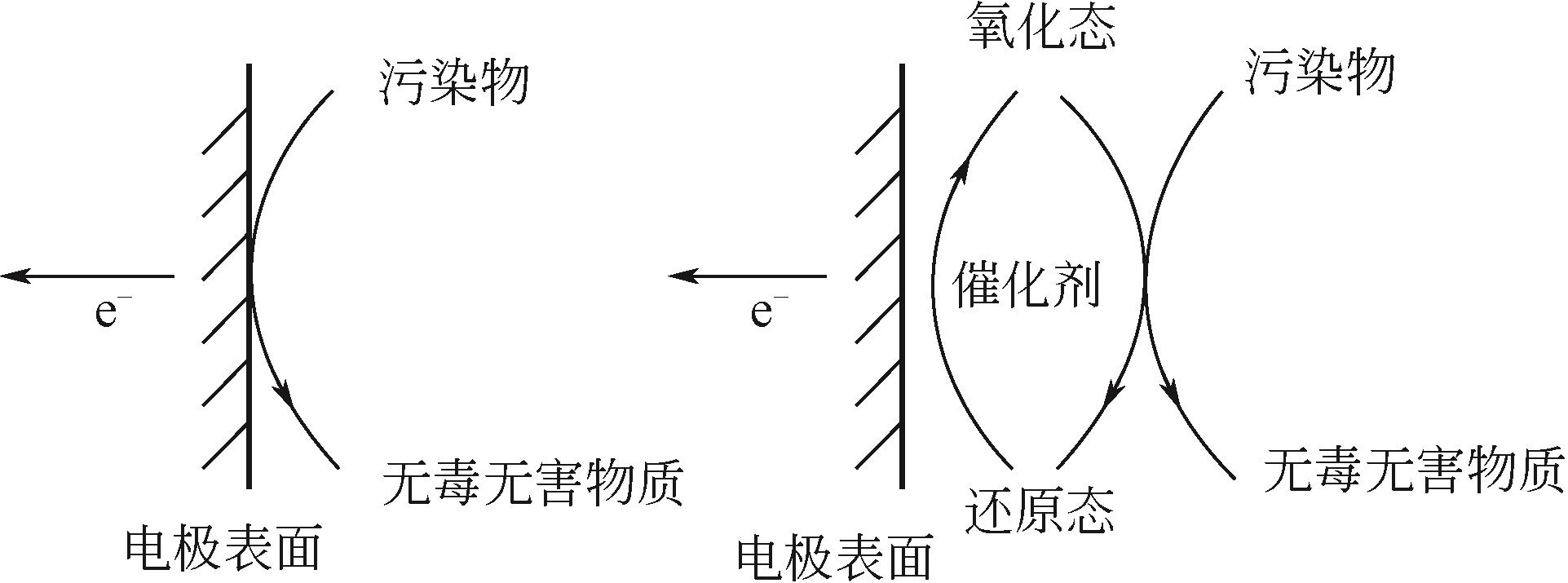
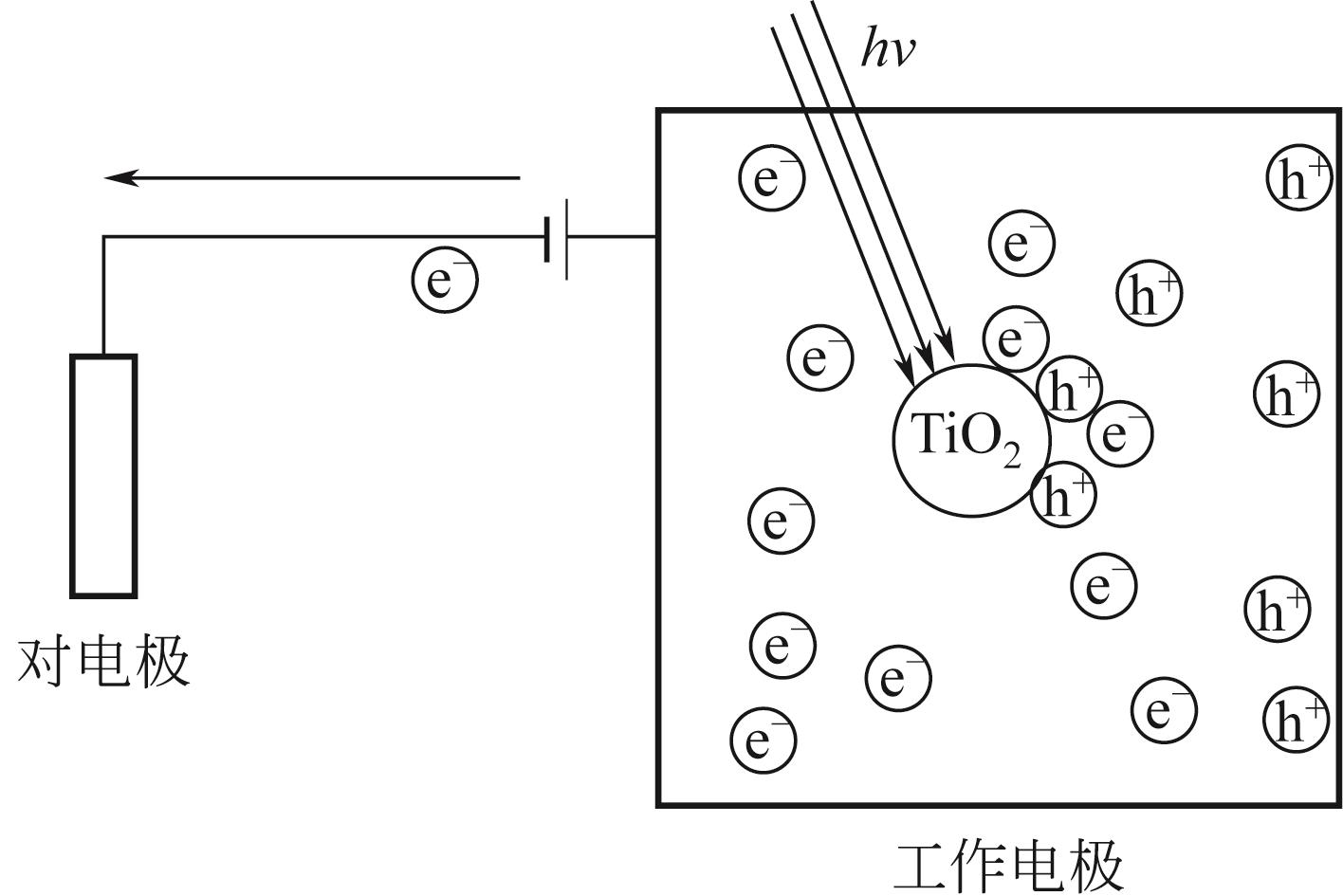
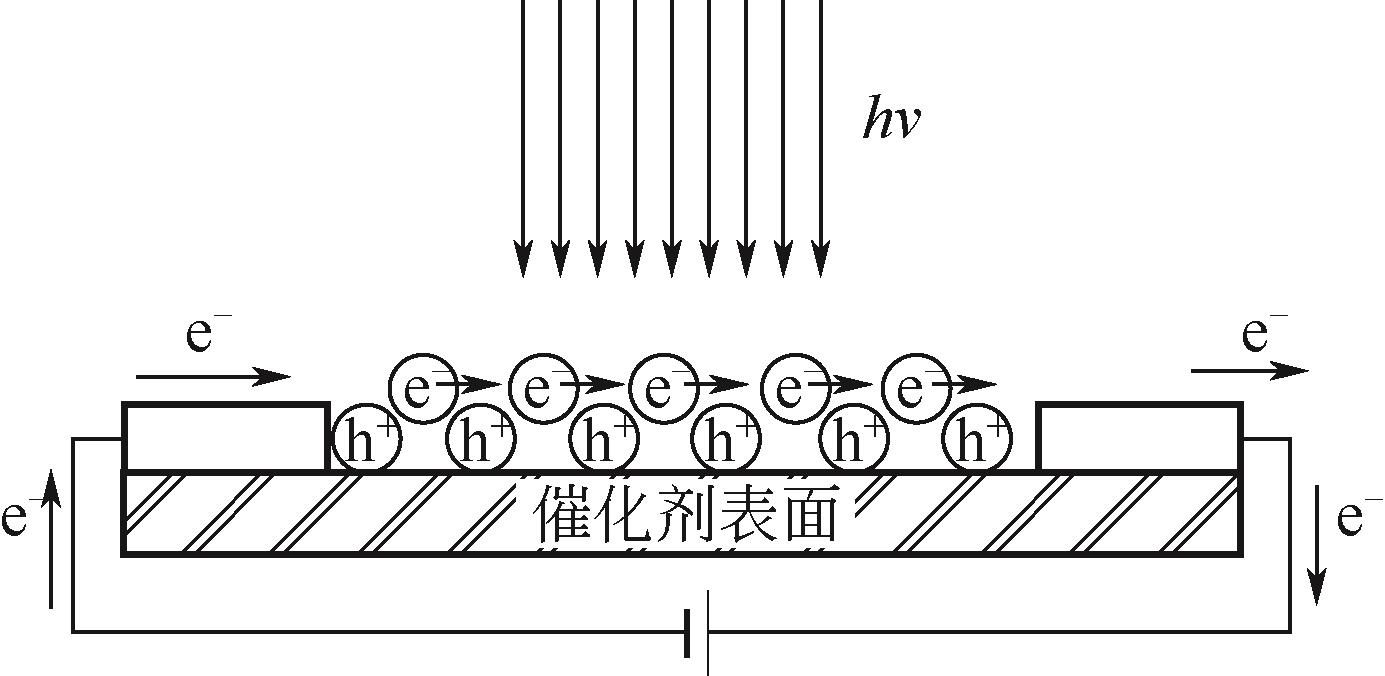
各类行业的废气排放导致环境污染问题严重。挥发性有机污染物(VOCs)作为工业废气中首要组成部分,因其成分的复杂性而难以处理,无选择性氧化的光催化高级氧化技术在VOCs降解领域引起广泛研究。为了解决光催化反应历程中存在的效率低问题,本文从VOCs的光催化工艺参数影响因素(温度、相对湿度、初始气体浓度、氧浓度和气体流速)展开描述,总结了各种工艺参数的影响机理和影响趋势。随着光化学技术和电化学技术的不断发展,将光电技术结合起来成为新的研究方向,外加偏压能够有效降低光催化反应历程中电子空穴对的复合率,本文总结了各类光电催化反应器中外加偏压对光电催化的影响机理和影响趋势。总结近五年的光/光电催化的实验研究进展,对于光/光电催化领域降解工业废气VOCs的工艺流程设计与优化具有借鉴意义。文中指出,未来进行与工业废气VOCs相契合参数范围的实验研究和简洁且高效的光/光电催化反应器研发将成为今后的发展趋势。
中图分类号:
引用本文
徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531.
XU Wei, LI Kaijun, SONG Linye, ZHANG Xinghui, YAO Shunhua. Research progress of photocatalysis and co-electrochemical degradation of VOCs[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3520-3531.
| 序号 | 污染物与 催化剂类型 | 实验条件变量 | 降解趋势 | 说明 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 促进 | 抑制 | 促进后 抑制 | 抑制后 促进 | 无明显 影响 | |||||
| 1 | 苯、甲苯 (Ag/TiO2) | RH=25%和50% | + | 两种湿度都适合在UVC照射下去除这些污染物 | [ | ||||
| 温度条件:120℃、150℃和180℃ | +(甲苯) | +(苯) | 温度的升高对光催化性能产生了不利影响 | ||||||
| 2 | 甲醛 (MoS2/OMC) | 初始浓度:0.5mg/m3、1.0mg/m3、1.5mg/m3、2.0mg/m3和2.5mg/m3 | + | 传质扩散和甲醛吸附作用明显减弱 | [ | ||||
| 催化剂用量:0.01g/m3、0.02g/m3、0.03g/m3、0.04g/m3和0.05g/m3 | + | 团聚现象和传质效率低 | |||||||
| 3 | 甲醛 (TiO2-GR/ACF) | 辐射照度:7.7W/m2、13.1W/m2和19.9W/m2 | + | 降解效率随着辐射照度的增强而增强 | [ | ||||
| 风量:70m3/h、130m3/h和183m3/h | + | 实验室整体甲醛污染环境的置换,风量越大置换量越高 | |||||||
| 初始浓度:0.25mg/m3、0.4mg/m3和1.0mg/m3 | + | 吸附位点还未饱和 | |||||||
| RH=27%、45%和60% | + | 甲醛与水分子竞争ACF的吸附活性点 | |||||||
| 4 | 二甲苯 (TiO2/ACF) | 初始质量浓度:28mg/m3、32mg/m3、38mg/m3和42mg/m3 | + | ACF的强烈吸附导致表面吸附活性位点达到饱和 | [ | ||||
| 迎面风速:0.15m/s、0.30m/s、0.45m/s、0.60m/s和0.75m/s | + | ACF与催化剂表面的传质 | |||||||
| RH=30%、40%、50%、60%和70% | + | ACF为催化剂表面提供更多的水,·OH也能从催化剂表面迁至ACF上 | |||||||
| 5 | 甲苯 (纳米管状TiO2) | 流速:75L/h、150L/h、225L/h和300L/h(标况下) | + | 低速下,传质速率略微增加;高速下,停留时间减少 | [ | ||||
| (RH=50%、150L/h,标况下)初始浓度:82.14mg/m3、143.75mg/m3和328.57mg/m3 | + | 催化剂表面自由基已经 饱和 | |||||||
| (82.14mg/m3,150L/h,标况下)RH=25%、50%和75% | + | 催化剂上吸附水可能会增加其活性 | |||||||
| (123.21mg/m3,150L/h,标况下)RH=25%、50%和75% | + | 相对湿度对降解率具有显著提升 | |||||||
| 6 | 苯 (负载式光催化氧化反应器) | 流速设置:0.028~0.3m/s | + | 停留时间变短 | [ | ||||
| 初始质量分数:0.00006增加到0.00032 | + | 低浓度下,活性位点未达到饱和 | |||||||
| 水的浓度:2000mg/m3增加到12000mg/m3 | + | 活性位点的竞争 | |||||||
| 7 | 苯乙烯(TiO2) | 初始浓度:425.97mg/m3、851.94mg/m3和1703.89mg/m3 | + | 累积的界面产物竞争活性位点,抑制了对光子的吸收 | [ | ||||
| RH=5%、50%和100% | + | 降解率先快速增加,水分子饱和后逐渐抑制 | |||||||
| 8 | 甲苯 (Bi缺陷型Bi2WO6) | 氧气体积分数:10%、15%、20%和25% | + | 氧气浓度使超氧自由基数量增加,但也参与活性位点竞争 | [ | ||||
| 甲苯浓度:112.88mg/m3、225.77mg/m3、376.28mg/m3和1128.83mg/m3 | + | 催化剂表面的活性位点的限制 | |||||||
| 9 | 乙苯(TiO2) | RH=26%增至80% | + | 水与乙苯竞争活性位点 | [ | ||||
| 流速:565mL/min、995mL/min、1425mL/min和2549mL/min | + | 催化剂表面的停留时间增加 | |||||||
| 臭氧体积分数:3.6%、4.2%和5.5% | + | O3分解成更强的氧化性物质 | |||||||
| 10 | 丙酮和甲苯(TiO2) | 丙酮浓度控制为:47.51mg/m3、95.02mg/m3和142.53mg/m3 甲苯浓度控制为:75.26mg/m3、150.51mg/m3和225.77mg/m3 | +(甲苯) | +(丙酮) | 甲苯氧化中间体通过与臭氧的非均相反应从而快速阻断其占据活性位点 | [ | |||
(O3条件下)丙酮浓度控制为:47.51mg/m3、95.02mg/m3和142.33mg/m3 甲苯浓度控制为:75.26mg/m3、150.51mg/m3和225.77mg/m3 | +(甲苯) | +(丙酮) | |||||||
| 停留时间:8s、16s、24s和32s | + | 停留时间变长 | |||||||
| RH=6%增至40% | + | 催化剂表面活性位点的 竞争 | |||||||
| (O3条件下)空气湿度:6%和40% | + | 臭氧的存在促进降解,但湿度增加,降解趋势仍是抑制 | |||||||
| 丙酮和甲苯混合物的降解 | +(甲苯) | +(丙酮) | 甲苯的降解中间产物阻碍了丙酮的吸附和氧化 | ||||||
| 11 | 甲苯、乙苯、二甲苯 (TiO2) | RH=26%增至60% | + | 活性位点的竞争 | [ | ||||
| O3体积分数:2.4%、3.5%、4.2%和5.3% | + | 可形成更多的自由基 | |||||||
| O3条件下的重复性实验 | + | 光催化剂失活率变低 | |||||||
| 停留时间:0~120s | + | VOC与催化剂的接触时间变长 | |||||||
| 12 | 苯(SnO2) | 潮湿空气(25%)、干燥空气、氮气环境下 | + | 干燥空气和氮气中羟基自由基机制消失,空穴直接氧化作用于苯的降解 | [ | ||||
| 13 | 气相甲酸(TiO2/ZnO) | 灯管数量(1、2、4根) | + | 随着光照强度的增加而提高 | [ | ||||
| (TiO2/ZnO/g-C3N4) | RH=30%、60%和90% | + | 活性位点的竞争 | ||||||
| RH=30%、60%和90% | + | ||||||||
| 14 | MTBE、丙酮、乙醛和庚烷(TiO2薄膜) | RH=6%增至40% | + | +(仅乙醛) | 降解率不仅取决于初始化合物的性质,还取决于中间产物的性质(吸附亲和力,降解的难易程度) | [ | |||
| 15 | 甲苯 (Ag/TiO2/CA) | 停留时间从7.8s增加到15.6s | + | 传质的强化 | |||||
| MTBE、丙酮、乙醛和庚烷的浓度分别由11.86mg/m3、18.03mg/m3、7.57mg/m3和13.52mg/m3增至23.72mg/m3、36.05mg/m3、13.33mg/m3和18.85mg/m3 | + | 浓度增加导致VOC的绝对降解量增加 | |||||||
| RH=25%~30%、45%~40%、50%~55%和60%~65% | + | 高湿度下效率降低的原因是水分子和污染物之间的竞争 | [ | ||||||
| 辐照强度:8mW/cm2、16mW/cm2、24mW/cm2和32mW/cm2 | + | 辐射强度的影响不是线性的 | |||||||
| 浓度:376.28mg/m3、752.56mg/m3和1128.83mg/m3 | + | 中间产物与甲苯的活性位点竞争 | |||||||
| 流速:0.3~1.2L/min | + | 污染物吸附和传质的不足 | |||||||
| 16 | 甲苯 (TiO2/GR) | RH=0、50%和80% | + | 过多的水对甲苯的降解贡献很小 | [ | ||||
| 非O3环境、O3环境 | + | 在臭氧的帮助下,甲苯的去除效率显著提高 | |||||||
| 17 | 甲胺 (TiO2) | RH=0、30%、60%和90% | + | 催化剂表面的竞争吸附 | [ | ||||
| 灯管:2根、3根和4根 | + | 气相甲胺的光催化降解速率与光照强度成正比 | |||||||
| 18 | 二甲苯 (Pt/TiO2) | O2体积分数为21%条件下RH=0、20%、50%、100% | + | 低氧气含量下,降解率在低RH水平下可以得到促进 | [ | ||||
| (O2体积分数为0、5%、10%条件下)RH=0、20%、50%、100% | + | ||||||||
| 19 | 乙烯(GR/TiO2) | 干燥氧气环境下、水蒸气的氮气环境下 | + | ·OH对乙烯的降解起主导作用 | [ | ||||
| 20 | 苯 (混合晶型TiO2) | 初始浓度由74.39mg/m3增至412.30mg/m3 | + | 活性位点有限 | [ | ||||
| 光照强度0.66klx、1.25klx、2.18klx和2.60klx | + | 光照强度进一步提高时,苯降解率增加较少 | |||||||
| 21 | 苯 (Bi2WO6/Pa) | 初始浓度:50mg/m3 增至200mg/m3 | + | 活性位点受到限制 | [ | ||||
| N2、O2环境下 | + | 空穴的直接氧化作用,氧气环境下 | |||||||
| 22 | 甲苯 (CuS-CdS/TiO2) | 温度:20℃、30℃、40℃和50℃ | + | 吸附与解吸之间的平衡决定了反应进程 | [ | ||||
| 流量:0.5L/min、1.0L/min、1.5L/min、3.0L/min和6.0L/min | + | 催化剂表面的传质速率与停留时间的平衡 | |||||||
| 浓度:3962mg/m3增至19822mg/m3 | + | 活性位点的数量有限 | |||||||
| 23 | 苯 (H3PW12O40/TiO2/palygorskite) | 初始浓度:60mg/m3增至1300mg/m3 | + | 光催化剂表面活性位点数量的限制 | [ | ||||
| 空气、O2和N2环境下 | + | N2环境下不存在自由基,降解主要依靠HPW和空穴氧化 | |||||||
表1 光催化实验中实验变量对气态污染物降解趋势的影响
| 序号 | 污染物与 催化剂类型 | 实验条件变量 | 降解趋势 | 说明 | 参考 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 促进 | 抑制 | 促进后 抑制 | 抑制后 促进 | 无明显 影响 | |||||
| 1 | 苯、甲苯 (Ag/TiO2) | RH=25%和50% | + | 两种湿度都适合在UVC照射下去除这些污染物 | [ | ||||
| 温度条件:120℃、150℃和180℃ | +(甲苯) | +(苯) | 温度的升高对光催化性能产生了不利影响 | ||||||
| 2 | 甲醛 (MoS2/OMC) | 初始浓度:0.5mg/m3、1.0mg/m3、1.5mg/m3、2.0mg/m3和2.5mg/m3 | + | 传质扩散和甲醛吸附作用明显减弱 | [ | ||||
| 催化剂用量:0.01g/m3、0.02g/m3、0.03g/m3、0.04g/m3和0.05g/m3 | + | 团聚现象和传质效率低 | |||||||
| 3 | 甲醛 (TiO2-GR/ACF) | 辐射照度:7.7W/m2、13.1W/m2和19.9W/m2 | + | 降解效率随着辐射照度的增强而增强 | [ | ||||
| 风量:70m3/h、130m3/h和183m3/h | + | 实验室整体甲醛污染环境的置换,风量越大置换量越高 | |||||||
| 初始浓度:0.25mg/m3、0.4mg/m3和1.0mg/m3 | + | 吸附位点还未饱和 | |||||||
| RH=27%、45%和60% | + | 甲醛与水分子竞争ACF的吸附活性点 | |||||||
| 4 | 二甲苯 (TiO2/ACF) | 初始质量浓度:28mg/m3、32mg/m3、38mg/m3和42mg/m3 | + | ACF的强烈吸附导致表面吸附活性位点达到饱和 | [ | ||||
| 迎面风速:0.15m/s、0.30m/s、0.45m/s、0.60m/s和0.75m/s | + | ACF与催化剂表面的传质 | |||||||
| RH=30%、40%、50%、60%和70% | + | ACF为催化剂表面提供更多的水,·OH也能从催化剂表面迁至ACF上 | |||||||
| 5 | 甲苯 (纳米管状TiO2) | 流速:75L/h、150L/h、225L/h和300L/h(标况下) | + | 低速下,传质速率略微增加;高速下,停留时间减少 | [ | ||||
| (RH=50%、150L/h,标况下)初始浓度:82.14mg/m3、143.75mg/m3和328.57mg/m3 | + | 催化剂表面自由基已经 饱和 | |||||||
| (82.14mg/m3,150L/h,标况下)RH=25%、50%和75% | + | 催化剂上吸附水可能会增加其活性 | |||||||
| (123.21mg/m3,150L/h,标况下)RH=25%、50%和75% | + | 相对湿度对降解率具有显著提升 | |||||||
| 6 | 苯 (负载式光催化氧化反应器) | 流速设置:0.028~0.3m/s | + | 停留时间变短 | [ | ||||
| 初始质量分数:0.00006增加到0.00032 | + | 低浓度下,活性位点未达到饱和 | |||||||
| 水的浓度:2000mg/m3增加到12000mg/m3 | + | 活性位点的竞争 | |||||||
| 7 | 苯乙烯(TiO2) | 初始浓度:425.97mg/m3、851.94mg/m3和1703.89mg/m3 | + | 累积的界面产物竞争活性位点,抑制了对光子的吸收 | [ | ||||
| RH=5%、50%和100% | + | 降解率先快速增加,水分子饱和后逐渐抑制 | |||||||
| 8 | 甲苯 (Bi缺陷型Bi2WO6) | 氧气体积分数:10%、15%、20%和25% | + | 氧气浓度使超氧自由基数量增加,但也参与活性位点竞争 | [ | ||||
| 甲苯浓度:112.88mg/m3、225.77mg/m3、376.28mg/m3和1128.83mg/m3 | + | 催化剂表面的活性位点的限制 | |||||||
| 9 | 乙苯(TiO2) | RH=26%增至80% | + | 水与乙苯竞争活性位点 | [ | ||||
| 流速:565mL/min、995mL/min、1425mL/min和2549mL/min | + | 催化剂表面的停留时间增加 | |||||||
| 臭氧体积分数:3.6%、4.2%和5.5% | + | O3分解成更强的氧化性物质 | |||||||
| 10 | 丙酮和甲苯(TiO2) | 丙酮浓度控制为:47.51mg/m3、95.02mg/m3和142.53mg/m3 甲苯浓度控制为:75.26mg/m3、150.51mg/m3和225.77mg/m3 | +(甲苯) | +(丙酮) | 甲苯氧化中间体通过与臭氧的非均相反应从而快速阻断其占据活性位点 | [ | |||
(O3条件下)丙酮浓度控制为:47.51mg/m3、95.02mg/m3和142.33mg/m3 甲苯浓度控制为:75.26mg/m3、150.51mg/m3和225.77mg/m3 | +(甲苯) | +(丙酮) | |||||||
| 停留时间:8s、16s、24s和32s | + | 停留时间变长 | |||||||
| RH=6%增至40% | + | 催化剂表面活性位点的 竞争 | |||||||
| (O3条件下)空气湿度:6%和40% | + | 臭氧的存在促进降解,但湿度增加,降解趋势仍是抑制 | |||||||
| 丙酮和甲苯混合物的降解 | +(甲苯) | +(丙酮) | 甲苯的降解中间产物阻碍了丙酮的吸附和氧化 | ||||||
| 11 | 甲苯、乙苯、二甲苯 (TiO2) | RH=26%增至60% | + | 活性位点的竞争 | [ | ||||
| O3体积分数:2.4%、3.5%、4.2%和5.3% | + | 可形成更多的自由基 | |||||||
| O3条件下的重复性实验 | + | 光催化剂失活率变低 | |||||||
| 停留时间:0~120s | + | VOC与催化剂的接触时间变长 | |||||||
| 12 | 苯(SnO2) | 潮湿空气(25%)、干燥空气、氮气环境下 | + | 干燥空气和氮气中羟基自由基机制消失,空穴直接氧化作用于苯的降解 | [ | ||||
| 13 | 气相甲酸(TiO2/ZnO) | 灯管数量(1、2、4根) | + | 随着光照强度的增加而提高 | [ | ||||
| (TiO2/ZnO/g-C3N4) | RH=30%、60%和90% | + | 活性位点的竞争 | ||||||
| RH=30%、60%和90% | + | ||||||||
| 14 | MTBE、丙酮、乙醛和庚烷(TiO2薄膜) | RH=6%增至40% | + | +(仅乙醛) | 降解率不仅取决于初始化合物的性质,还取决于中间产物的性质(吸附亲和力,降解的难易程度) | [ | |||
| 15 | 甲苯 (Ag/TiO2/CA) | 停留时间从7.8s增加到15.6s | + | 传质的强化 | |||||
| MTBE、丙酮、乙醛和庚烷的浓度分别由11.86mg/m3、18.03mg/m3、7.57mg/m3和13.52mg/m3增至23.72mg/m3、36.05mg/m3、13.33mg/m3和18.85mg/m3 | + | 浓度增加导致VOC的绝对降解量增加 | |||||||
| RH=25%~30%、45%~40%、50%~55%和60%~65% | + | 高湿度下效率降低的原因是水分子和污染物之间的竞争 | [ | ||||||
| 辐照强度:8mW/cm2、16mW/cm2、24mW/cm2和32mW/cm2 | + | 辐射强度的影响不是线性的 | |||||||
| 浓度:376.28mg/m3、752.56mg/m3和1128.83mg/m3 | + | 中间产物与甲苯的活性位点竞争 | |||||||
| 流速:0.3~1.2L/min | + | 污染物吸附和传质的不足 | |||||||
| 16 | 甲苯 (TiO2/GR) | RH=0、50%和80% | + | 过多的水对甲苯的降解贡献很小 | [ | ||||
| 非O3环境、O3环境 | + | 在臭氧的帮助下,甲苯的去除效率显著提高 | |||||||
| 17 | 甲胺 (TiO2) | RH=0、30%、60%和90% | + | 催化剂表面的竞争吸附 | [ | ||||
| 灯管:2根、3根和4根 | + | 气相甲胺的光催化降解速率与光照强度成正比 | |||||||
| 18 | 二甲苯 (Pt/TiO2) | O2体积分数为21%条件下RH=0、20%、50%、100% | + | 低氧气含量下,降解率在低RH水平下可以得到促进 | [ | ||||
| (O2体积分数为0、5%、10%条件下)RH=0、20%、50%、100% | + | ||||||||
| 19 | 乙烯(GR/TiO2) | 干燥氧气环境下、水蒸气的氮气环境下 | + | ·OH对乙烯的降解起主导作用 | [ | ||||
| 20 | 苯 (混合晶型TiO2) | 初始浓度由74.39mg/m3增至412.30mg/m3 | + | 活性位点有限 | [ | ||||
| 光照强度0.66klx、1.25klx、2.18klx和2.60klx | + | 光照强度进一步提高时,苯降解率增加较少 | |||||||
| 21 | 苯 (Bi2WO6/Pa) | 初始浓度:50mg/m3 增至200mg/m3 | + | 活性位点受到限制 | [ | ||||
| N2、O2环境下 | + | 空穴的直接氧化作用,氧气环境下 | |||||||
| 22 | 甲苯 (CuS-CdS/TiO2) | 温度:20℃、30℃、40℃和50℃ | + | 吸附与解吸之间的平衡决定了反应进程 | [ | ||||
| 流量:0.5L/min、1.0L/min、1.5L/min、3.0L/min和6.0L/min | + | 催化剂表面的传质速率与停留时间的平衡 | |||||||
| 浓度:3962mg/m3增至19822mg/m3 | + | 活性位点的数量有限 | |||||||
| 23 | 苯 (H3PW12O40/TiO2/palygorskite) | 初始浓度:60mg/m3增至1300mg/m3 | + | 光催化剂表面活性位点数量的限制 | [ | ||||
| 空气、O2和N2环境下 | + | N2环境下不存在自由基,降解主要依靠HPW和空穴氧化 | |||||||
| 1 | 北京大学统计科学中心环境统计组(2020). 空气质量评估报告(七): “2+66”城市2013—2019年区域污染状况评估[R/OL](2020-07-27). . |
| Environmental Statistics Group, Center for Statistical Sciences, University( Peking 2020). Air quality Assessment report(seven-): Assessment of regional pollution in "2+66" cities from 2013 to 2019[R/OL](2020-07-27). . | |
| 2 | 赵旭辉, 史天哲, 马啸, 等. 江淮地区城市O3污染过程的非典型特征及其前体物来源分析[J]. 环境化学, 2022, 41(3): 834-849. |
| ZHAO Xuhui, SHI Tianzhe, MA Xiao, et al. Atypical characteristics of urban O3 pollution process and the source analysis of precursor pollutants in Yangtze-Huaihe Region[J]. Environmental Chemistry, 2022, 41(3): 834-849. | |
| 3 | 李长英, 陈明功, 盛楠, 等. 挥发性有机物处理技术的特点与发展[J]. 化工进展, 2016, 35(3): 917-925. |
| LI Changying, CHEN Minggong, SHENG Nan, et al. The characteristics and development of volatile organic compounds treatment technology[J]. Chemical Industry and Engineering Progress, 2016, 35(3): 917-925. | |
| 4 | FUJISHIMA AKIRA, HONDA KENICHI. Electrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972, 238(5358): 37-38. |
| 5 | DIBBLE Lynette A, RAUPP Gregory B. Kinetics of the gas-solid heterogeneous photocatalytic oxidation of trichloroethylene by near UV illuminated titanium dioxide[J]. Catalysis Letters, 1990, 4(4): 345-354. |
| 6 | KHAN Hayat, ALALM Mohamed GAR, Marc LALONDE-LAVOIE, et al. Photocatalytic degradation of NO x and ethanol in the gas phase by spray dried Ce-TiO2 [J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106813. |
| 7 | DA SILVEIRA Arnaldo Efigênio Castro, Bárbara Maria Borges Ribeiro, Bianca Gvozdenovic Medina Bricio, et al. Influence of palladium content dispersed in the TiO2 photocatalyst for degradation of volatile organic compounds in gas emission[J]. Environmental Science and Pollution Research, 2022, 29(28): 42242-42250. |
| 8 | Kasimayan UMA, SINGARAVELU Chandra Mohan, KAVINKUMAR Veerappan, et al. Ultrasonically modified P25-TiO2/In2O3 heterostructured nanoparticles: An efficient dual- responsive photocatalyst for solution and gas phase reactions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 125: 257-266. |
| 9 | SONG Rui, CHI Haibo, MA Qiuling, et al. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-based photoelectrocatalysis[J]. Journal of the American Chemical Society, 2021, 143(34): 13664-13674. |
| 10 | MA Bingrui, XIN Shuaishuai, XIN Yanjun, et al. Visible-light-driven photoelectrocatalytic degradation of p-chloronitrobenzene by BiOBr/TiO2 nanotube arrays photoelectrodes: Mechanisms, degradation pathway and DFT calculation[J]. Separation and Purification Technology, 2021, 268: 118699. |
| 11 | WARD Michael D, BARD Allen J. Photocurrent enhancement via trapping of photogenerated electrons of titanium dioxide particles[J]. The Journal of Physical Chemistry, 1982, 86(18): 3599-3605. |
| 12 | KOTHAVALE V P, PATIL T S, PATIL P B, et al. Photoelectrocatalytic degradation of Rhodamine B using N doped TiO2 thin Films[J]. Materials Today: Proceedings, 2020, 23: 382-388. |
| 13 | ZHANG Yu, CUI Wenquan, AN Weijia, et al. Combination of photoelectrocatalysis and adsorption for removal of bisphenol A over TiO2-graphene hydrogel with 3D network structure[J]. Applied Catalysis B: Environmental, 2018, 221: 36-46. |
| 14 | SCHRECK Murielle, NIEDERBERGER Markus. Photocatalytic gas phase reactions[J]. Chemistry of Materials, 2019, 31(3): 597-618. |
| 15 | Dursun Sukru, Zeynep Cansu Ayturan. Simultaneous removal of gaseous benzene and toluene with photocatalytic oxidation process at high temperatures under UVC irradiation[J]. Environmental Science and Pollution Research International, 2022, 29(25): 38232-38247. |
| 16 | ABBAS Naseem, HUSSAIN Murid, RUSSO Nunzio, et al. Studies on the activity and deactivation of novel optimized TiO2 nanoparticles for the abatement of VOCs[J]. Chemical Engineering Journal, 2011, 175: 330-340. |
| 17 | SHAYEGAN Zahra, LEE Chang-Seo, HAGHIGHAT Fariborz. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review[J]. Chemical Engineering Journal, 2018, 334: 2408-2439. |
| 18 | ZHANG Lianfeng, ANDERSON William A, SAWELL Steven, et al. Mechanistic analysis on the influence of humidity on photocatalytic decomposition of gas-phase chlorobenzene[J]. Chemosphere, 2007, 68(3): 546-553. |
| 19 | 张金辉. TiO2/金属有机骨架纳米复合材料制备及其光催化氧化挥发性有机物性能研究[D]. 广州: 华南理工大学, 2020. |
| ZHANG Jinhui. Synthesis of TiO2/metal-organic frameworks and their applications in photocatalytic oxidation of volatile organic compounds[D]. Guangzhou: South China University of Technology, 2020. | |
| 20 | 张兴惠, 都亚茹, 张兴芳, 等. TiO2/ACF滤网在闭式循环系统中降解气相二甲苯的研究[J]. 太原理工大学学报, 2019, 50(3): 315-321. |
| ZHANG Xinghui, DU Yaru, ZHANG Xingfang, et al. Studies on the degradation of gaseous xylene in closed-cycle system by using TiO2/ACF[J]. Journal of Taiyuan University of Technology, 2019, 50(3): 315-321. | |
| 21 | RAO Zepeng, XIE Xiaofeng, WANG Xiao, et al. Defect chemistry of Er3+-doped TiO2 and its photocatalytic activity for the degradation of flowing gas-phase VOCs[J]. The Journal of Physical Chemistry C, 2019, 123(19): 12321-12334. |
| 22 | Umberto BELLÈ, INVERNIZZI Marzio, POLVARA Elisa, et al. A novel nanotubular TiO2-based plug-flow reactor for gas phase photocatalytic degradation of toluene[J]. Chemical Engineering Journal, 2022, 437: 135323. |
| 23 | 刘守新, 刘鸿. 光催化及光电催化基础与应用[M]. 北京: 化学工业出版社, 2006. |
| LIU Shouxin, LIU Hong. Foundation and application of photocatalysis and photoelectrocatalysis[M]. Beijing: Chemical Industry Press, 2006. | |
| 24 | CHEN Shi, SUN Zhiguo, ZHANG Li, et al. Photodegradation of gas phase benzene by SnO2 nanoparticles by direct hole oxidation mechanism[J]. Catalysts, 2020, 10(1): 117. |
| 25 | GAYA Umar Ibrahim, ABDULLAH Abdul Halim. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems[J]. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 2008, 9(1): 1-12. |
| 26 | 杜晶晶, 赵军伟, 程晓民, 等. 一维TiO2纳米管光催化降解气相苯的动力学研究[J]. 人工晶体学报, 2020, 49(12): 2308-2312, 2321. |
| DU Jingjing, ZHAO Junwei, CHENG Xiaomin, et al. Kinetic of photocatalytic degradation of gaseous benzene by one-dimensional TiO2 nanotubes[J]. Journal of Synthetic Crystals, 2020, 49(12): 2308-2312, 2321. | |
| 27 | HE Li, GUAN Wei, ZENG Yao, et al. Molybdenum sulfide (MoS2)/ordered mesoporous carbon (OMC) tubular mesochannel photocatalyst for enhanced photocatalytic oxidation for removal of volatile organic compounds (VOCs)[J]. Frontiers in Chemistry, 2022, 9: 748622. |
| 28 | ZHANG Lianfeng, MORALEJO Carol, ANDERSON William A. A review of the influence of humidity on photocatalytic decomposition of gaseous pollutants on TiO2-based catalysts[J]. The Canadian Journal of Chemical Engineering, 2020, 98(1): 263-273. |
| 29 | 朱瑜瑜. Bi2WO6基光催化剂的制备及可见光催化降解甲苯的性能研究[D]. 常州: 常州大学, 2021. |
| ZHU Yuyu. Preparation of Bi2WO6-based photocatalyst and study on its performance in the degradation of toluene under visible light[D]. CHangzhou: Changzhou University, 2021. | |
| 30 | Bárbara Maria Borges Ribeiro, Renato Carajelescov Nonato, Tânia Miyoko Fujimoto, et al. Toluene degradation by heterogeneous photocatalysis assisted with ozone in a tubular reactor: Analysis over the reactor length[J]. Environmental Science and Pollution Research, 2021, 28(19): 24216-24223. |
| 31 | SALDANHA Larissa Albuquerque Silva, SANTOS Nilza Tatiane das Graças, TOMAZ Edson. Photocatalytic ethylbenzene degradation associated with ozone (TiO2/UV/O3) under different percentages of catalytic coating area: Evaluation of process parameters[J]. Separation and Purification Technology, 2021, 263: 118344. |
| 32 | 卫鹏. MO x -TiO2(M=Cu, Mn, Ag)光催化臭氧氧化气相己烷同分异构体机理研究[D].广州: 广东工业大学, 2019. WEI Peng, Photocatalytic ozonation mechanism investigation of gaseous hexane isomers on MOx-TiO2(M=Cu, Mn, Ag) [D]. Guangzhou: Guangdong University of Technology, 2019. |
| 33 | SHEN Yungshuen, KU Young. Decomposition of gas-phase trichloroethene by the UV/TiO2 process in the presence of ozone[J]. Chemosphere, 2002, 46(1): 101-107. |
| 34 | ZHONG Lexuan, HAGHIGHAT Fariborz, LEE Chang-Seo, et al. Performance of ultraviolet photocatalytic oxidation for indoor air applications: Systematic experimental evaluation[J]. Journal of Hazardous Materials, 2013, 261: 130-138. |
| 35 | 彭嫚. TiO2-GR/ACF复合材料的制备及其降解甲醛特性试验研究[D]. 北京: 北京建筑大学, 2021. |
| PENG Man. Preparation of TiO2-GR/ACF composite and its formaldehyde degradation performance studies[D]. Beijing: Beijing University of Civil Engineering and Architecture, 2021. | |
| 36 | 曹鑫苗. 光催化氧化降解气相苯的数值模拟[D]. 大连: 大连理工大学, 2021. |
| CAO Xinmiao. Numerical simulation of photocatalytic oxidation degradation of gas phase benzene[D]. Dalian: Dalian University of Technology, 2021. | |
| 37 | 张力昀. 基于氧同位素标记的苯系物TiO2光催化降解机理研究[D]. 广州: 广东工业大学, 2021. |
| ZHANG Liyun. Photocatalytic degradation mechanism of benzene compounds by TiO2 based on oxygen isotope tracing[D]. Guangzhou: Guangdong University of Technology, 2021. | |
| 38 | KASK M, BOLOBAJEV J, KRICHEVSKAYA M. Gas-phase photocatalytic degradation of acetone and toluene, and their mixture in the presence of ozone in continuous multi-section reactor as possible air post-treatment for exhaust from pulsed corona discharge[J]. Chemical Engineering Journal, 2020, 399: 125815. |
| 39 | BORGES RIBEIRO Bárbara Maria, FUJIMOTO Tânia Miyoko, GVOZDENOVIC MEDINA BRICIO Bianca, et al. Gas-phase aromatic compounds degradation by a partially TiO2 coated photoreactor assisted with ozone[J]. Process Safety and Environmental Protection, 2020, 135: 265-272. |
| 40 | 杨洋. TiO2基薄膜的制备及其对气相甲酸的光催化降解研究[D]. 南京: 南京信息工程大学, 2022. |
| YANG Yang. Preparation and photocatalytic degradation of gaseous formic acid by TiO2 films[D]. Nanjing: Nanjing University of Information Science & Technology, 2022. | |
| 41 | DUNDAR Ibrahim, KRICHEVSKAYA Marina, KATERSKI Atanas, et al. Photocatalytic degradation of different VOCs in the gas-phase over TiO2 thin films prepared by ultrasonic spray pyrolysis[J]. Catalysts, 2019, 9(11): 915. |
| 42 | JONOIDI JAFARI Ahmad, KERMANI Majid, Ahmad HOSSEINI-BANDEGHARAEI, et al. Synthesis and characterization of Ag/TiO2/composite aerogel for enhanced adsorption and photo-catalytic degradation of toluene from the gas phase[J]. Chemical Engineering Research and Design, 2019, 150: 1-13. |
| 43 | YE Haoling, WANG Zhi, LIU Yiqiu, et al. Efficient degradation of gas-phase toluene by ozone-assisted photocatalytic oxidation on TiO2/graphene composites[J]. Catalysis Letters, 2019, 149(10): 2739-2748. |
| 44 | 姚胜男. TiO2基薄膜对气相甲胺的光催化降解研究[D]. 南京: 南京信息工程大学, 2019. |
| YAO Shengnan. Photocatalytic degradation of gaseous methylamine by TiO2 based thin films[D]. Nanjing: Nanjing University of Information Science & Technology, 2019. | |
| 45 | ZHANG Jinjian, VIKRANT Kumar, KIM Ki-Hyun, et al. Unveiling the collective effects of moisture and oxygen on the photocatalytic degradation of m-Xylene using a titanium dioxide supported platinum catalyst[J]. Chemical Engineering Journal, 2022, 439: 135747. |
| 46 | 代雪萍. 石墨烯/二氧化钛光催化材料对乙醛、乙烯的吸附降解特性研究[D]. 上海: 中国科学院大学(中国科学院上海硅酸盐研究所), 2019. |
| DAI Xueping. Study on adsorption and degradation performance of graphene/TiO2 photocatalyst towards acetaldehyde and ethylene[D]. Shanghai: Shanghai Institute of Ceramics, Chinese Academy of Sciences, 2019. | |
| 47 | 沈晓玲, 田立江, 李帅, 等. 锐钛矿-金红石混合晶型TiO2降解气相苯[J]. 化工环保, 2019, 39(2): 172-177. |
| SHEN Xiaoling, TIAN Lijiang, LI Shuai, et al. Degradation of gas phase benzene by anatase-rutile mixed phase TiO2 [J]. Environmental Protection of Chemical Industry, 2019, 39(2): 172-177. | |
| 48 | ZHANG Mengjie, LU Jun, ZHU Chengzhu, et al. Photocatalytic degradation of gaseous benzene with Bi2WO6/Palygorskite composite catalyst[J]. Solid State Sciences, 2019, 90: 76-85. |
| 49 | XIN Yanjun, CHEN Qinghua, ZHANG Guodong. Construction of ternary heterojunction CuS-CdS/TiO2 nanobelts for photocatalytic degradation of gaseous toluene[J]. Journal of Alloys and Compounds, 2018, 751: 231-240. |
| 50 | MA Jianzhong, ZHU Chengzhu, XU Yongpeng, et al. Photocatalytic degradation of gaseous benzene with H3PW12O40/TiO2/palygorskite composite catalyst[J]. Journal of Saudi Chemical Society, 2017, 21(2): 132-142. |
| 51 | LIU Yuan, XIE Changsheng, LI Huayao, et al. Low bias photoelectrocatalytic (PEC) performance for organic vapour degradation using TiO2/WO3 nanocomposite[J]. Applied Catalysis B: Environmental, 2011, 102(1/2): 157-162. |
| 52 | 蒋展鹏, 王海燕, 杨宏伟. 电助光催化技术研究进展[J]. 化学进展, 2005, 17(4): 622-630. |
| JIANG Zhanpeng, WANG Haiyan, YANG Hongwei. Progress in electrically assisted photocatalysis[J]. Progress in Chemistry, 2005, 17(4): 622-630. | |
| 53 | 冷文华, 张昭, 成少安, 等. 光电催化降解苯胺的研究——外加电压的影响[J]. 环境科学学报, 2001, 21(6): 710-714. |
| LENG Wenhua, ZHANG Zhao, CHENG Shaoan, et al. Effect of applied potential on the photocatalytic degradation of aniline[J]. Acta Scientiae Circumstantiae, 2001, 21(6): 710-714. | |
| 54 | DAI Wenrui, TAO Ying, ZOU Hangjun, et al. Gas-phase photoelectrocatalytic oxidation of NO via TiO2 nanorod array/FTO photoanodes[J]. Environmental Science & Technology, 2020, 54(9): 5902-5912. |
| 55 | YE Shengying, TIAN Qingmei, SONG Xianliang, et al. Photoelectrocatalytic degradation of ethylene by a combination of TiO2 and activated carbon felts[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2009, 208(1): 27-35. |
| 56 | YE Shengying, ZHENG Senhong, SONG Xianliang, et al. Photoelectrocatalytic decomposition of ethylene using TiO2/activated carbon fiber electrode with applied pulsed direct current square-wave potential[J]. Applied Surface Science, 2015, 341: 61-68. |
| 57 | WANG Xiaoguang, PAN Honghui, MURUGANANTHAN Muthu, et al. Gas-phase photoelectrocatalytic oxidation of volatile organic compounds using defective WO3/TiO2 nanotubes mesh[J]. Environmental Science: Nano, 2022, 9(6): 2172-2181. |
| 58 | YAN Cai, ZHONG Meifang, HAN Jianqing, et al. Efficient degradation of trimethylamine in gas phase by petal-shaped Co-MoS2 catalyst in the photo-electrochemical system[J]. Chemical Engineering Journal, 2021, 405: 127034. |
| 59 | NIE Cheng, LIU Lifen, HE Rui. Pt/TiO2-ZnO in a circuit Photo-electro-catalytically removed HCHO for outstanding indoor air purification[J]. Separation and Purification Technology, 2018, 206: 316-323. |
| 60 | DONG Jing, LI Qing, XIA Wenjie, et al. Improvement of water resistance by Fe2O3/TiO2 photoelectrocatalysts for formaldehyde removal: Experimental and theoretical investigation[J]. Environmental Science and Pollution Research, 2022, 29(10): 13805-13821. |
| 61 | 聂成. 金属负载TiO2/ZnO光电催化净化室内空气甲醛[D]. 大连: 大连理工大学, 2018. |
| NIE Cheng. Metal doped titania/zinc oxide photo-electro-catalytic removal of HCHO in indoor air[D]. Dalian: Dalian University of Technology, 2018. | |
| 62 | Yihan LYU, LIU Haiyang, JIN Dexin, et al. Effective degradation of norfloxacin on Ag3PO4/CNTs photoanode: Z-scheme mechanism, reaction pathway, and toxicity assessment[J]. Chemical Engineering Journal, 2022, 429: 132092. |
| 63 | 王海燕, 蒋展鹏, 杨宏伟. 溶液pH对电助光催化氧化苯甲酰胺的影响研究[J]. 环境科学研究, 2008, 21(5): 14-18. |
| WANG Haiyan, JIANG Zhanpeng, YANG Hongwei. Study on effect of solution pH on electrically assisted photocatalytic degradation of benzamide[J]. Research of Environmental Sciences, 2008, 21(5): 14-18. | |
| 64 | 吴毅杰. 光电化学池中阴、阳极材料的制备及其光催化分解水的研究[D]. 苏州: 苏州大学, 2015. |
| WU Yijie. Preparation of photoelectrode materials in photoelectrochemical cell and their photocatalytic water splitting properties[D]. Suzhou: Soochow University, 2015. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [5] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [6] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [7] | 吕程远, 张函, 杨明旺, 杜健军, 樊江莉. 生物成像用二氧杂环丁烷余辉发光体系的研究进展[J]. 化工进展, 2023, 42(8): 4108-4122. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [10] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [11] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [12] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [13] | 储甜甜, 刘润竹, 杜高华, 马嘉浩, 张孝阿, 王成忠, 张军营. 有机胍催化脱氢型RTV硅橡胶的制备和可降解性能[J]. 化工进展, 2023, 42(7): 3664-3673. |
| [14] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [15] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||