化工进展 ›› 2022, Vol. 41 ›› Issue (4): 2171-2179.DOI: 10.16085/j.issn.1000-6613.2021-0839
过碳酸钠修复石油污染土壤及其环境效应
汪林1( ), 蒲思淇1, 王明新1,2(
), 蒲思淇1, 王明新1,2( ), 薛金娟1,2, 韩莹1,2
), 薛金娟1,2, 韩莹1,2
- 1.常州大学环境与安全工程学院,江苏 常州 213164
2.江苏省石油化工安全与环保工程研究中心,江苏 常州 213164
-
收稿日期:2021-04-19修回日期:2021-07-21出版日期:2022-04-23发布日期:2022-04-25 -
通讯作者:王明新 -
作者简介:汪林(1993—),男,硕士研究生,主要研究方向为污染场地修复。E-mail:1332452258@qq.com 。 -
基金资助:国家自然科学基金(41772240);江苏省重点研发(社会发展)项目;江苏省研究生科研创新计划(KYCX21_2870)
Remediation of petroleum-contaminated soil by sodium percarbonate and its environmental effects
WANG Lin1( ), PU Siqi1, WANG Mingxin1,2(
), PU Siqi1, WANG Mingxin1,2( ), XUE Jinjuan1,2, HAN Ying1,2
), XUE Jinjuan1,2, HAN Ying1,2
- 1.College of Environment and Safety Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
2.Jiangsu Petrochemical Safety and Environmental Engineering Research Center, Changzhou 213164, Jiangsu, China
-
Received:2021-04-19Revised:2021-07-21Online:2022-04-23Published:2022-04-25 -
Contact:WANG Mingxin
摘要:
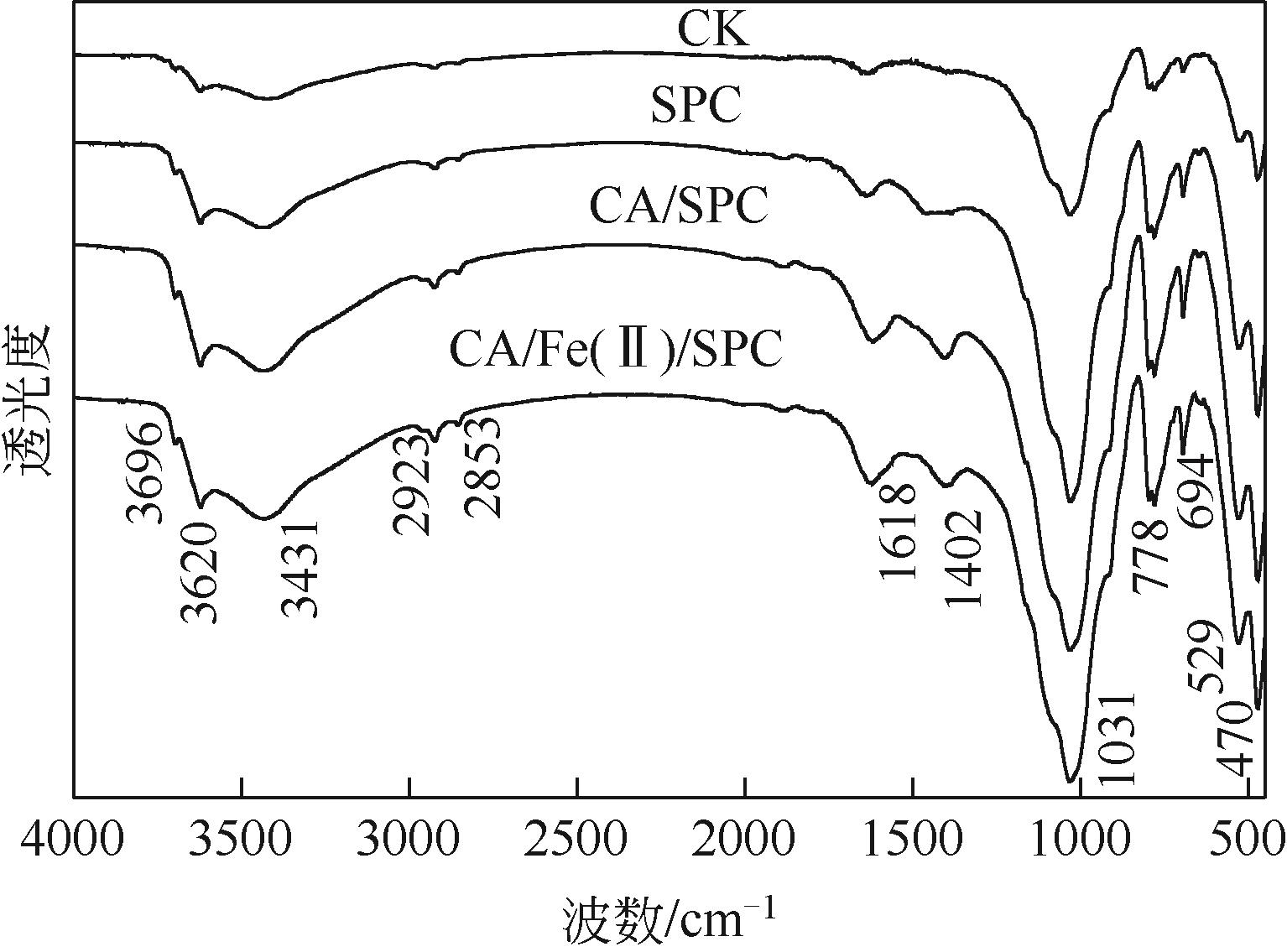
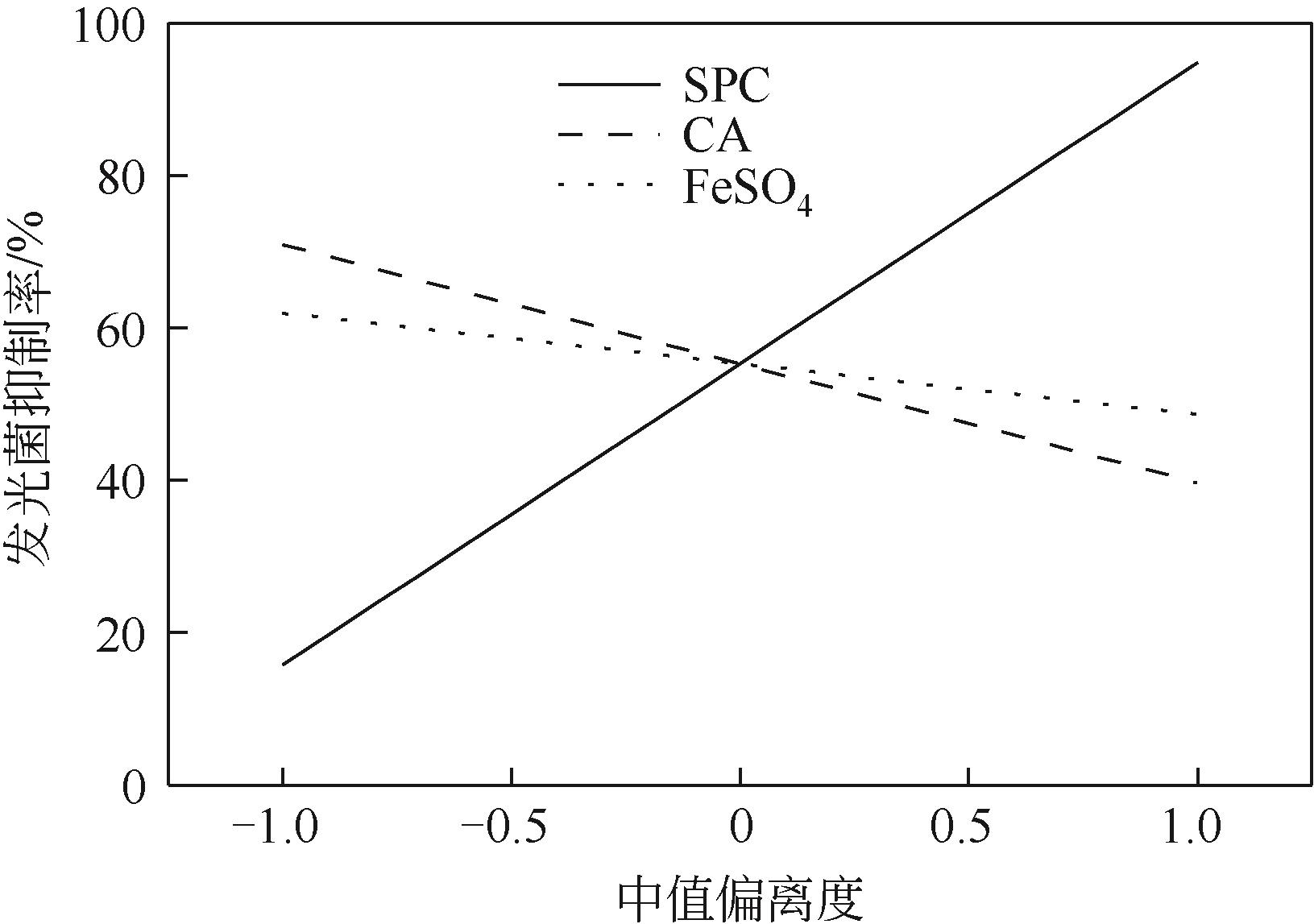
化学氧化可快速高效修复石油污染土壤,但很少关注研究其对土壤质量的影响以及残留污染物的环境风险。本文以过碳酸钠(SPC)为氧化剂,以柠檬酸(CA)/硫酸亚铁[Fe(Ⅱ)]为催化剂,分析了其对柴油污染土壤的修复效率,分析了柴油中不同组分的降解特征,通过残留初始总石油烃(TPH)有效性和浸提液生物毒性变化提示不同处理的环境风险,通过有机碳和傅里叶变换红外光谱(FTIR)分析修复前后土壤特性变化。结果表明,SPC单独处理效率较低,CA/Fe(Ⅱ)显著提高了TPH去除率。FTIR光谱表明,处理后土壤样品的Si—O—Si、C—H和—OH振动增强。气相色谱-质谱联用(GC/MS)图谱表明,残留TPH组分主要为长链烷烃(C16~C21)。羟丙基-β-环糊精(HPCD)浸提液发光抑制率随着浸提液pH的增加而增加,表明SPC投加量过多产生的强碱性对土壤生物毒性具有显著影响。增加CA投加量对TPH去除率的促进幅度大于SPC和FeSO4,且有助于降低残留TPH的生物有效性和提升土壤总有机碳(TOC)含量。采用化学氧化修复有机污染土壤应进行环境风险分析并对修复条件进行优化。
中图分类号:
引用本文
汪林, 蒲思淇, 王明新, 薛金娟, 韩莹. 过碳酸钠修复石油污染土壤及其环境效应[J]. 化工进展, 2022, 41(4): 2171-2179.
WANG Lin, PU Siqi, WANG Mingxin, XUE Jinjuan, HAN Ying. Remediation of petroleum-contaminated soil by sodium percarbonate and its environmental effects[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2171-2179.
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| X1 SPC投加量/% | 1 | 3 | 5 |
| X2 CA投加量/% | 0.5 | 1.5 | 2.5 |
| X3 FeSO4投加量/% | 0.5 | 1.5 | 2.5 |
表1 响应面法实验设计因素与水平
| 因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| X1 SPC投加量/% | 1 | 3 | 5 |
| X2 CA投加量/% | 0.5 | 1.5 | 2.5 |
| X3 FeSO4投加量/% | 0.5 | 1.5 | 2.5 |
| 控制因素 | 实验结果 | ||||||
|---|---|---|---|---|---|---|---|
| SPC投加量/% | CA投加量/% | FeSO4投加量/% | TPH去除率/% | 有效态TPH/mg·kg-1 | 浸提液发光菌抑制率/% | 浸提液pH | TOC/% |
| 3.00 | 2.50 | 0.50 | 68.88 | 1028.04 | 37 | 7.95 | 3.08 |
| 3.00 | 2.50 | 2.50 | 76.37 | 765.2 | 35 | 7.59 | 3.05 |
| 5.00 | 0.50 | 1.50 | 49.35 | 1379.6 | 99 | 9.45 | 1.86 |
| 1.00 | 1.50 | 2.50 | 62.86 | 1073.4 | 20 | 6.76 | 2.86 |
| 1.00 | 2.50 | 1.50 | 65.29 | 1017.26 | 0 | 6.45 | 2.98 |
| 5.00 | 1.50 | 0.50 | 64.62 | 1173.475 | 99 | 10.11 | 2.29 |
| 5.00 | 2.50 | 1.50 | 72.70 | 991.74 | 90 | 9.35 | 3.11 |
| 3.00 | 1.50 | 1.50 | 66.86 | 1151.95 | 51 | 8.71 | 2.67 |
| 1.00 | 0.50 | 1.50 | 29.99 | 3317.86 | 31 | 7.71 | 1.95 |
| 3.00 | 1.50 | 1.50 | 66.86 | 1059.452 | 52 | 8.61 | 2.58 |
| 3.00 | 1.50 | 1.50 | 66.16 | 1073.82 | 57 | 8.64 | 2.55 |
| 3.00 | 0.50 | 0.50 | 38.98 | 2011.03 | 90 | 10.01 | 2.25 |
| 5.00 | 1.50 | 2.50 | 70.75 | 853.433 | 95 | 9.55 | 2.60 |
| 3.00 | 1.50 | 1.50 | 65.96 | 1138.44 | 51 | 8.75 | 2.32 |
| 3.00 | 0.50 | 2.50 | 56.56 | 1355.67 | 60 | 8.83 | 2.56 |
| 3.00 | 1.50 | 1.50 | 67.26 | 1406.75 | 53 | 8.55 | 2.74 |
| 1.00 | 1.50 | 0.50 | 45.73 | 1608.605 | 28 | 7.54 | 2.87 |
表2 实验设计与响应值
| 控制因素 | 实验结果 | ||||||
|---|---|---|---|---|---|---|---|
| SPC投加量/% | CA投加量/% | FeSO4投加量/% | TPH去除率/% | 有效态TPH/mg·kg-1 | 浸提液发光菌抑制率/% | 浸提液pH | TOC/% |
| 3.00 | 2.50 | 0.50 | 68.88 | 1028.04 | 37 | 7.95 | 3.08 |
| 3.00 | 2.50 | 2.50 | 76.37 | 765.2 | 35 | 7.59 | 3.05 |
| 5.00 | 0.50 | 1.50 | 49.35 | 1379.6 | 99 | 9.45 | 1.86 |
| 1.00 | 1.50 | 2.50 | 62.86 | 1073.4 | 20 | 6.76 | 2.86 |
| 1.00 | 2.50 | 1.50 | 65.29 | 1017.26 | 0 | 6.45 | 2.98 |
| 5.00 | 1.50 | 0.50 | 64.62 | 1173.475 | 99 | 10.11 | 2.29 |
| 5.00 | 2.50 | 1.50 | 72.70 | 991.74 | 90 | 9.35 | 3.11 |
| 3.00 | 1.50 | 1.50 | 66.86 | 1151.95 | 51 | 8.71 | 2.67 |
| 1.00 | 0.50 | 1.50 | 29.99 | 3317.86 | 31 | 7.71 | 1.95 |
| 3.00 | 1.50 | 1.50 | 66.86 | 1059.452 | 52 | 8.61 | 2.58 |
| 3.00 | 1.50 | 1.50 | 66.16 | 1073.82 | 57 | 8.64 | 2.55 |
| 3.00 | 0.50 | 0.50 | 38.98 | 2011.03 | 90 | 10.01 | 2.25 |
| 5.00 | 1.50 | 2.50 | 70.75 | 853.433 | 95 | 9.55 | 2.60 |
| 3.00 | 1.50 | 1.50 | 65.96 | 1138.44 | 51 | 8.75 | 2.32 |
| 3.00 | 0.50 | 2.50 | 56.56 | 1355.67 | 60 | 8.83 | 2.56 |
| 3.00 | 1.50 | 1.50 | 67.26 | 1406.75 | 53 | 8.55 | 2.74 |
| 1.00 | 1.50 | 0.50 | 45.73 | 1608.605 | 28 | 7.54 | 2.87 |
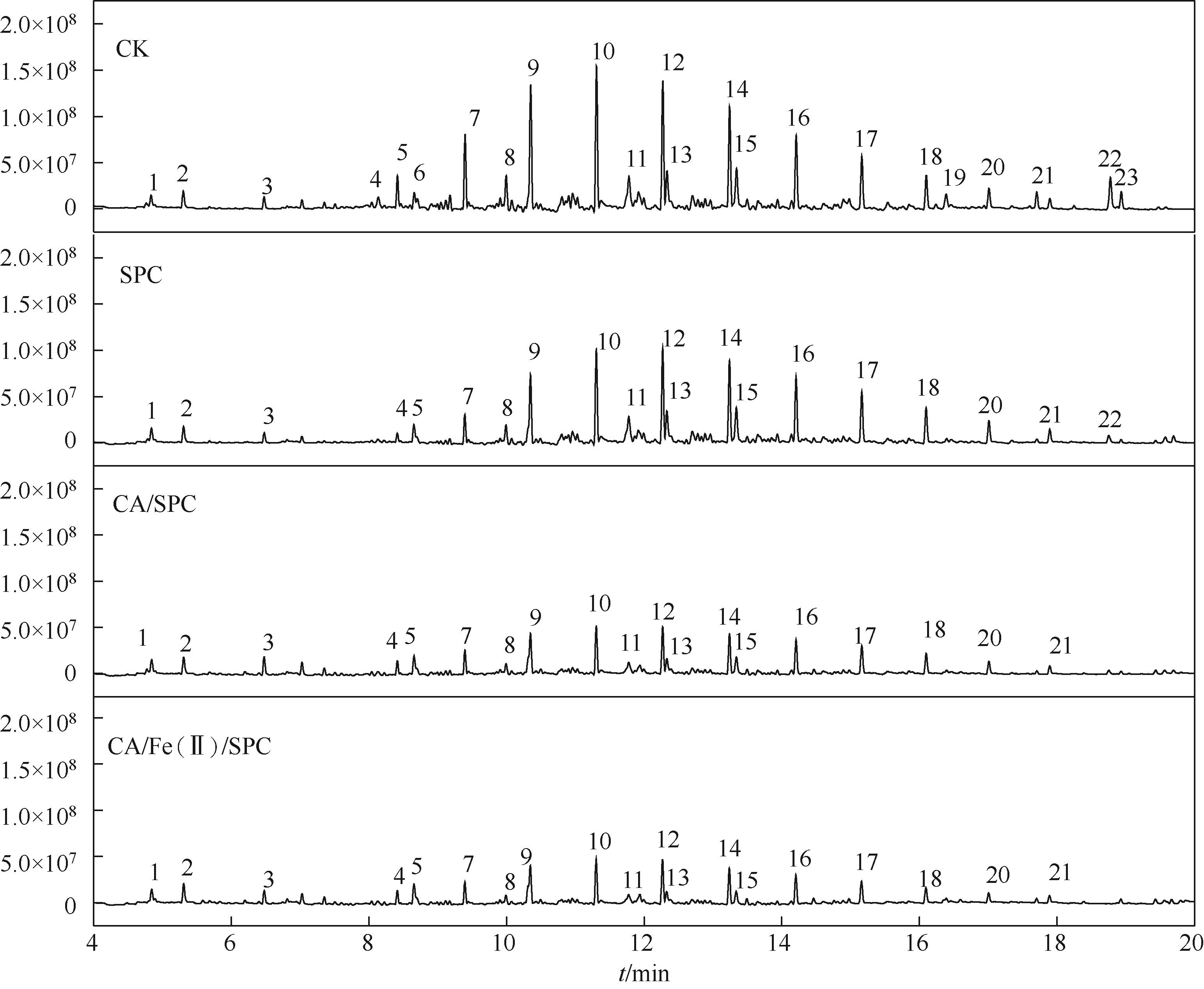
| 物质类型 | 编号 | 保留时间 /min | 物质名称 |
|---|---|---|---|
| 正构烷烃 | 1 | 4.83 | 十一烷 |
| 3 | 6.49 | 十二烷 | |
| 4 | 8.16 | 十三烷 | |
| 5 | 8.45 | 十四烷 | |
| 6 | 9.41 | 十五烷 | |
| 8 | 10.35 | 十六烷 | |
| 11 | 11.78 | 十七烷 | |
| 14 | 13.26 | 十八烷 | |
| 15 | 13.36 | 十九烷 | |
| 17 | 15.17 | 二十烷 | |
| 18 | 16.11 | 二十一烷 | |
| 20 | 18.85 | 二十二烷 | |
| 21 | 17.03 | 二十三烷 | |
| 22 | 18.79 | 二十四烷 | |
| 23 | 18..93 | 二十五烷 | |
| 异构烷烃 | 9 | 10.35 | 2-甲基十七烷 |
| 12 | 12.27 | 2,6,10,14-四甲基十五烷 | |
| 环烷烃 | 10 | 11.32 | 环十四烷 |
| 13 | 12.33 | 1-辛基-1-壬基苯环己烷 | |
| 烯烃 | 19 | 16.39 | 1-二十烯 |
| 芳香烃 | 2 | 5.30 | 1-甲基-2-丙基苯 |
| 7 | 9.41 | 1,6,7-三甲基萘 | |
| 16 | 14.21 | 1-甲基蒽 |
表3 柴油污染土壤中石油烃的主要物质
| 物质类型 | 编号 | 保留时间 /min | 物质名称 |
|---|---|---|---|
| 正构烷烃 | 1 | 4.83 | 十一烷 |
| 3 | 6.49 | 十二烷 | |
| 4 | 8.16 | 十三烷 | |
| 5 | 8.45 | 十四烷 | |
| 6 | 9.41 | 十五烷 | |
| 8 | 10.35 | 十六烷 | |
| 11 | 11.78 | 十七烷 | |
| 14 | 13.26 | 十八烷 | |
| 15 | 13.36 | 十九烷 | |
| 17 | 15.17 | 二十烷 | |
| 18 | 16.11 | 二十一烷 | |
| 20 | 18.85 | 二十二烷 | |
| 21 | 17.03 | 二十三烷 | |
| 22 | 18.79 | 二十四烷 | |
| 23 | 18..93 | 二十五烷 | |
| 异构烷烃 | 9 | 10.35 | 2-甲基十七烷 |
| 12 | 12.27 | 2,6,10,14-四甲基十五烷 | |
| 环烷烃 | 10 | 11.32 | 环十四烷 |
| 13 | 12.33 | 1-辛基-1-壬基苯环己烷 | |
| 烯烃 | 19 | 16.39 | 1-二十烯 |
| 芳香烃 | 2 | 5.30 | 1-甲基-2-丙基苯 |
| 7 | 9.41 | 1,6,7-三甲基萘 | |
| 16 | 14.21 | 1-甲基蒽 |
| 1 | PINEDO J, IBÁÑEZ R, LIJZEN J P A, et al. Assessment of soil pollution based on total petroleum hydrocarbons and individual oil substances[J]. Journal of Environmental Management, 2013, 130: 72-79. |
| 2 | 刘五星, 骆永明, 滕应, 等. 我国部分油田土壤及油泥的石油污染初步研究[J]. 土壤, 2007, 39(2): 247-251. |
| LIU Wuxing, LUO Yongming, TENG Ying, et al. A survey of petroleum contamination in several Chinese oilfield soils[J]. Soils, 2007, 39(2):247-251. | |
| 3 | 潘云飞, 唐正, 彭欣怡, 等. 石油烃污染土壤微生物修复技术研究现状及进展[J]. 化工进展, 2021, 40(8): 4562-4572. |
| PAN Yunfei, TANG Zheng, PENG Xinyi, et al. Research status and progress of microbial remediation technology for petroleum hydrocarbon contaminated soil[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4562-4572. | |
| 4 | FALCIGLIA P P, GIUSTRA M G, VAGLIASINDI F. Low-temperature thermal desorption of diesel polluted soil: influence of temperature and soil texture on contaminant removal kinetics[J]. Journal of Hazardous Materials, 2011, 185(1): 392-400. |
| 5 | SONG W, VIDONISH J E, KAMATH R, et al. Pilot-scale pyrolytic remediation of crude-oil-contaminated soil in a continuously-fed reactor: treatment intensity trade-offs[J]. Environmental Science & Technology, 2019, 53(4): 2045-2053. |
| 6 | HAN Z, JIAO W, TIAN Y, et al. Lab-scale removal of PAHs in contaminated soil using electrical resistance heating: removal efficiency and alteration of soil properties[J]. Chemosphere, 2020, 239: 124496. |
| 7 | ALTENBURGER A, BENDER M, EKELUND F, et al. Steam-treatment-based soil remediation promotes heat-tolerant, potentially pathogenic microbiota[J]. Environmental Technology, 2014, 35(6): 773-780. |
| 8 | LOMINCHAR M A, SANTOS A, MIGUEL E D, et al. Remediation of aged diesel contaminated soil by alkaline activated persulfate [J]. Science of the Total Environment, 2018, 622/623: 41-48. |
| 9 | OURIACHE H, ARRAR J, NAMANE A, et al. Treatment of petroleum hydrocarbons contaminated soil by Fenton like oxidation[J]. Chemosphere, 2019, 232: 377-386. |
| 10 | LI Y T, LI D, LAI L J, et al. Remediation of petroleum hydrocarbon contaminated soil by using activated persulfate with ultrasound and ultrasound/Fe[J]. Chemosphere, 2020, 238: 124657. |
| 11 | MENA Esperanza, RUIZ Clara, José VILLASEÑOR, et al. Biological permeable reactive barriers coupled with electrokinetic soil flushing for the treatment of diesel-polluted clay soil[J]. Journal of Hazardous Materials, 2015, 283(11): 131-139. |
| 12 | SON Y, CHA J, LIM M, et al. Comparison of ultrasonic and conventional mechanical soil-washing processes for diesel-contaminated sand[J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 2400-2407. |
| 13 | 杨建刚, 刘翔, 余刚, 等. 非离子表面活性剂溶液中多环芳烃的溶解特性[J]. 环境科学, 2003, 24(6): 79-82. |
| YANG Jiangang, LIU Xiang, YU Gang, et al. Characteristics of polycyclic aromatic hydrocarbons dissolved in nonionic surfactants[J]. Environmental Science,2003, 24(6): 79-82. | |
| 14 | LU M, ZHANG Z, SUN S, et al. Enhanced degradation of bioremediation residues in petroleum-contaminated soil using a two-liquid-phase bioslurry reactor[J]. Chemosphere, 2009, 77(2): 161-168. |
| 15 | DIAZ-MARTINEZ M E, ALARCON A, FERRERA-CERRATO R. Casuarina Equisetifolia (Casuarinaceae) growth in soil with diesel and application of biostimulation and bioaugmentation[J]. Revista De Biologia Tropical, 2013, 61(3): 1039-1052. |
| 16 | 刘少卿, 姜林, 黄喆, 等. 挥发及半挥发有机物污染场地蒸汽抽提修复技术原理与影响因素[J]. 环境科学, 2011, 32(3): 825-833. |
| LIU Shaoqing, JIANG Lin, HUANG Zhe, et al. Principles and influencing factors of steam extraction and remediation technology for volatile and semi-volatile organics contaminated sites[J]. Environmental Science, 2011, 32(3): 825-833. | |
| 17 | HALMEMIES S, GROENDAHL S, ARFFMAN M, et al. Vacuum extraction based response equipment for recovery of fresh fuel spills from soil[J]. Journal of Hazardous Materials, 2003, 97(1/2/3): 127-143. |
| 18 | BACIOCCHI R, BONI M R, D'APRILE L. Hydrogen peroxide lifetime as an indicator of the efficiency of 3-chlorophenol Fenton’s and Fenton-like oxidation in soils[J]. Journal of Hazardous Materials, 2003, 96(2/3): 305-329. |
| 19 | MA J, XIA X, MA Y, et al. Stability of dissolved percarbonate and its implications for groundwater remediation[J]. Chemosphere, 2018, 205: 41-44. |
| 20 | MIAO Z W, GU X G, LU S G, et al. Perchloroethylene (PCE) oxidation by percarbonate in Fe2+-catalyzed aqueous solution: PCE performance and its removal mechanism[J]. Chemosphere, 2015, 119: 1120-1125. |
| 21 | BUNDY J G, PATON G I, CAMPBELL C D. Combined microbial community level and single species biosensor responses to monitor recovery of oil polluted soil[J]. Soil Biology and Biochemistry, 2004, 36(7): 1149-1159. |
| 22 | 张亚楠, 杨兴伦, 卞永荣, 等. 化学提取法表征污染土壤中PAHs老化规律和蚯蚓富集特征[J]. 环境科学, 2015, 36(12): 4582-4590. |
| ZHANG Yanan, YANG Xinglun, BIAN Yongrong, et al. Characterization of PAHs aging and earthworm enrichment characteristics in contaminated soil by chemical extraction method[J]. Environmental Science, 2015, 36(12): 4582-4590. | |
| 23 | STOKES J D, WILKINSON A, REID B J, et al. Prediction of polycyclic aromatic hydrocarbon biodegradation in contaminated soils using an aqueous hydroxypropyl-beta-cyclodextrin extraction technique[J]. Environmental Toxicology and Chemistry, 2005, 24(6): 1325-1330. |
| 24 | 张金永, 叶倩, 王明新, 等. 机械化学法修复柴油污染土壤的效率、产物及影响[J]. 环境科学学报, 2021, 41(3): 1058-1065. |
| ZHANG Jinyong, YE Qian, WANG Mingxin, et al. Remediation of diesel-contaminated soil by mechanochemical treatment: efficiency, products and impacts[J]. Acta Scientiae Circumstantiae, 2021, 41(3): 1058-1065. | |
| 25 | JIANG W, JOENS J A, DIONYSIOU D D, et al. Optimization of photocatalytic performance of TiO2 coated glass microspheres using response surface methodology and the application for degradation of dimethyl phthalate[J]. Journal of Photochemistry & Photobiology A: Chemistry, 2013, 262: 7-13. |
| 26 | SUN B, GUAN X, FANG J, et al. Activation of manganese oxidants with bisulfite for enhanced oxidation of organic contaminants: the involvement of Mn(Ⅲ)[J]. Environmental Science & Technology, 2015, 49(20): 12414-12421. |
| 27 | CHOW C H, SZE-YIN LEUNG Y. Transformations of organic micropollutants undergoing permanganate/bisulfite treatment: kinetics, pathways and toxicity[J]. Chemosphere, 2019, 237: 124524. |
| 28 | YUAN D L, ZHANG C, TANG S F, et al. Fe3+-sulfite complexation enhanced persulfate Fenton-like process for antibiotic degradation based on response surface optimization[J]. Science of the Total Environment, 2020, 727: 138773. |
| 29 | 殷雪妍, 张艾, 刘亚男. 过氧化钙去除水中糖皮质激素的响应面分析[J]. 中国环境科学, 2018, 38(2): 608-615. |
| YIN Xueyan, ZHANG Ai, LIU Yanan. Response surface analysis of calcium peroxide to remove glucocorticoids in water [J]. China Environmental Science, 2018, 38(2): 608-615. | |
| 30 | BOKARE A D, CHOI W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes[J]. Journal of Hazardous Materials, 2014, 275: 121-135. |
| 31 | SHEN Y F, ZHANG N Y. Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption [J]. Bioresource Technology, 2019, 282: 294-300. |
| 32 | ADAMO I, GHISOLI C, CAUCIA F. A contribution to the study of FTIR spectra of opals[J]. Neues Jahrbuch für Mineralogie-Abhandlungen, 2010, 187(1): 63-68. |
| 33 | KRAMER M G, SANDERMAN J, CHADWICK O A, et al. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil[J]. Global Change Biology, 2012, 18(8): 2594-2605. |
| 34 | SALVADÓ J, TESI T, ANDERSSON A, et al. Organic carbon remobilized from thawing permafrost is resequestered by reactive iron on the Eurasian Arctic Shelf[J]. Geophysical Research Letters, 2015, 42(19): 8122-8130. |
| 35 | ZHAO Q, POULSON S R, OBRIST D, et al. Iron-bound organic carbon in forest soils: quantification and characterization[J]. Biogeosciences, 2016, 13(16): 1104-1108. |
| 36 | HUANG X, FENG C, ZHAO G, et al. Carbon sequestration potential promoted by oxalate extractable iron oxides through organic fertilization[J]. Soil Science Society of America Journal, 2017, 81(6): 1359-1370. |
| 37 | DROSOS Marios, PICCOLO Alessandro. The molecular dynamics of soil humus as a function of tillage[J]. Land Degradation & Development, 2018, 29(6): 1792-1805. |
| 38 | KRAMER M G, CHADWICK O A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale[J]. Nature Climate Change, 2018, 8(12): 1104-1108. |
| 39 | BARRAL M T, ARIAS M, GUERIF J. Effects of iron and organic matter on the porosity and structural stability of soil aggregates[J]. Soil & Tillage Research, 1998, 46(3/4): 261-272. |
| 40 | CHEN C, DYNES J J, WANG J, et al. Properties of Fe-organic matter associations via coprecipitation versus adsorption[J]. Environmental Science and Technology, 2014, 48(23): 13751-13759. |
| 41 | KLAUS Kaiser, GEORG Guggenberger. The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils[J]. Organic Geochemistry, 2000, 31(7/8): 711-725. |
| [1] | 王敏, 毛玉红, 陈超, 白丹. 水处理工艺中铝盐水解物的毒性、形态及控制研究进展[J]. 化工进展, 2023, 42(S1): 479-488. |
| [2] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [3] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [4] | 李卫华, 吴寅凯, 孙英杰, 尹俊权, 辛明学, 赵友杰. 垃圾焚烧飞灰重金属毒性浸出评价方法研究进展[J]. 化工进展, 2023, 42(5): 2666-2677. |
| [5] | 陆诗建, 刘玲, 刘滋武, 郭伯文, 俞徐林, 梁艳, 赵东亚, 朱全民. AEP-DPA-CuO相变纳米流体吸收CO2稳定性[J]. 化工进展, 2022, 41(8): 4555-4561. |
| [6] | 李坡, 张珊珊, 施锦秋, 高航, 王明新. 活化过硫酸盐修复苯胺污染地下水及其环境风险[J]. 化工进展, 2022, 41(5): 2753-2760. |
| [7] | 徐铭骏, 郭兆春, 李立, 朱紫琦, 张倩, 洪俊明. 纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料[J]. 化工进展, 2022, 41(2): 1043-1053. |
| [8] | 丁鑫, 张栋铭, 焦纬洲, 刘有智. 直接甲醇燃料电池阳极催化剂研究进展[J]. 化工进展, 2021, 40(9): 4918-4930. |
| [9] | 方智煌, 刘祥, 余阳, 钱雅洁, 薛罡. 高铁酸盐对水中H2受体拮抗剂的降解特性[J]. 化工进展, 2021, 40(8): 4647-4655. |
| [10] | 潘云飞, 唐正, 彭欣怡, 高品. 石油烃污染土壤微生物修复技术研究现状及进展[J]. 化工进展, 2021, 40(8): 4562-4572. |
| [11] | 陈兴兴, 刘敏, 陈滢. 淡水环境中微塑料污染研究进展[J]. 化工进展, 2020, 39(8): 3333-3343. |
| [12] | 焦莉, 徐金妹, 张秋亚, 彭慧, 许霞, 王利平. 氨基修饰片状氮化碳的制备及光催化性能[J]. 化工进展, 2020, 39(5): 1866-1874. |
| [13] | 王霜,王友昊,李法社,王文超,隋猛. 基于紫外吸光度的生物柴油氧化降解程度分析[J]. 化工进展, 2020, 39(2): 506-512. |
| [14] | 姜岩,周和平,张哲,刘红兵,沈顺祥. 石油烃污染场地低温修复机制研究进展[J]. 化工进展, 2020, 39(2): 419-428. |
| [15] | 贾艳萍,张真,毕朕豪,张健,张兰河. 铁碳微电解处理印染废水的效能及生物毒性变化[J]. 化工进展, 2020, 39(2): 790-797. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||