化工进展 ›› 2022, Vol. 41 ›› Issue (2): 1043-1053.DOI: 10.16085/j.issn.1000-6613.2021-0556
纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料
徐铭骏1,2( ), 郭兆春3, 李立1,2, 朱紫琦1,2, 张倩1,2, 洪俊明1,2(
), 郭兆春3, 李立1,2, 朱紫琦1,2, 张倩1,2, 洪俊明1,2( )
)
- 1.华侨大学化工学院,福建 厦门 361021
2.福建省工业废水生化处理工程技术研究中心,福建 厦门 361021
3.厦门烟草工业有限责任公司,福建 厦门 361021
-
收稿日期:2021-03-19修回日期:2021-04-14出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:洪俊明 -
作者简介:徐铭骏(1998—),男,硕士研究生,主要研究方向为水处理高级氧化。E-mail:mingjunxu1998@163.com 。 -
基金资助:国家自然科学基金(51978291);华侨大学研究生科研创新基金(20014087038)
Degradation of azo dyes by sodium percarbonate activated with nanosheet Mn2O3@α-Fe3O4
XU Mingjun1,2( ), GUO Zhaochun3, LI Li1,2, ZHU Ziqi1,2, ZHANG Qian1,2, HONG Junming1,2(
), GUO Zhaochun3, LI Li1,2, ZHU Ziqi1,2, ZHANG Qian1,2, HONG Junming1,2( )
)
- 1.College of Chemical Engineering, Huaqiao University, Xiamen 361021, Fujian, China
2.Fujian Province Engineering Research Center of Industrial Wastewater Biochemical Treatment, Xiamen 361021, Fujian, China
3.Xiamen Tobacco Industrial Company Limited, Xiamen 361021, Fujian, China
-
Received:2021-03-19Revised:2021-04-14Online:2022-02-05Published:2022-02-23 -
Contact:HONG Junming
摘要:
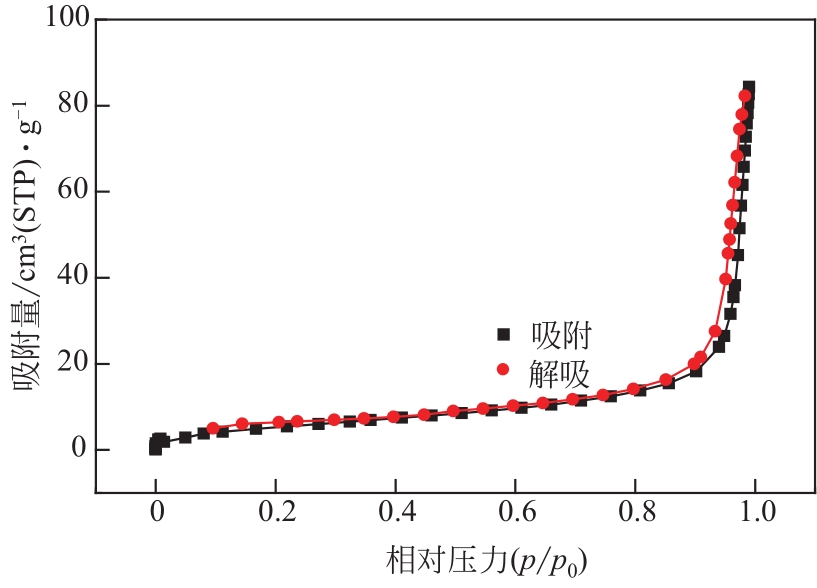
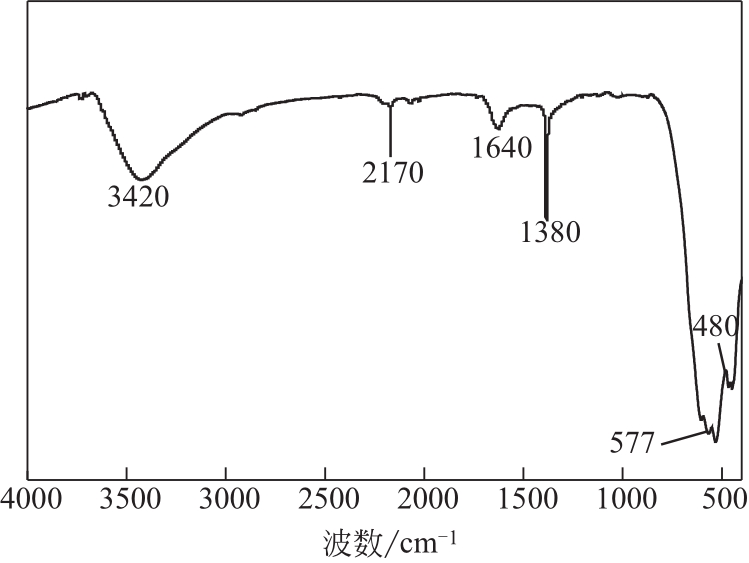
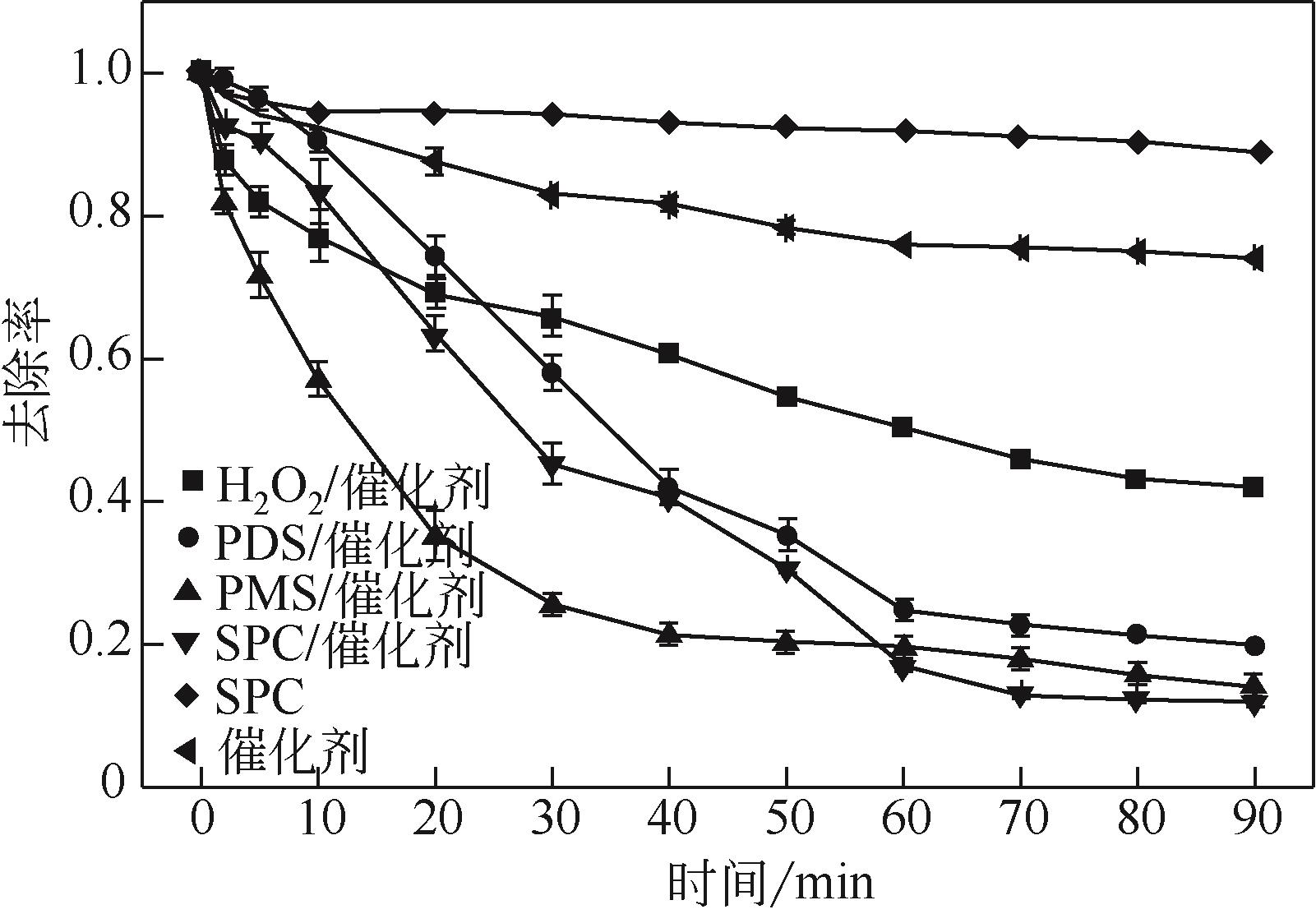
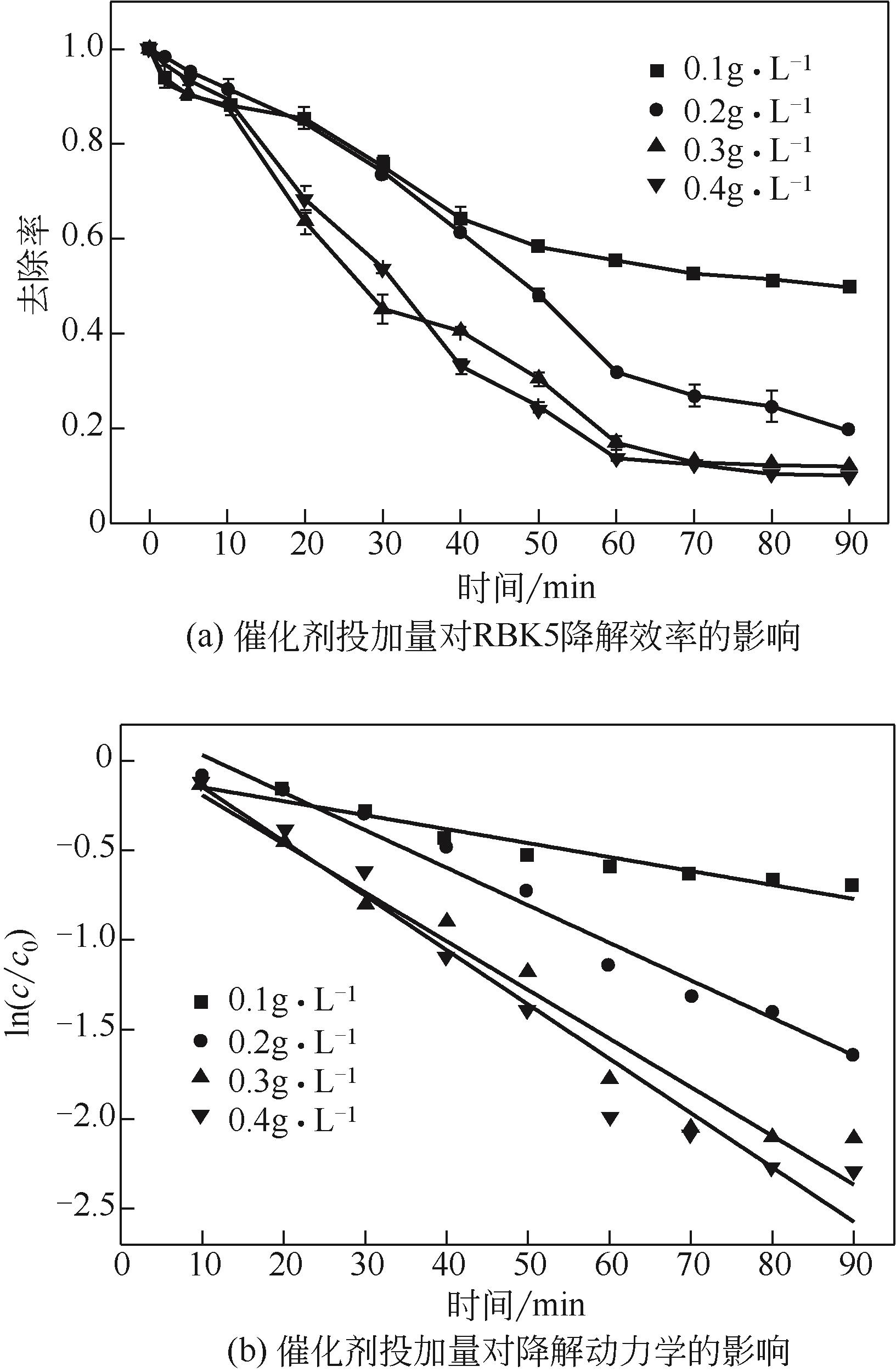
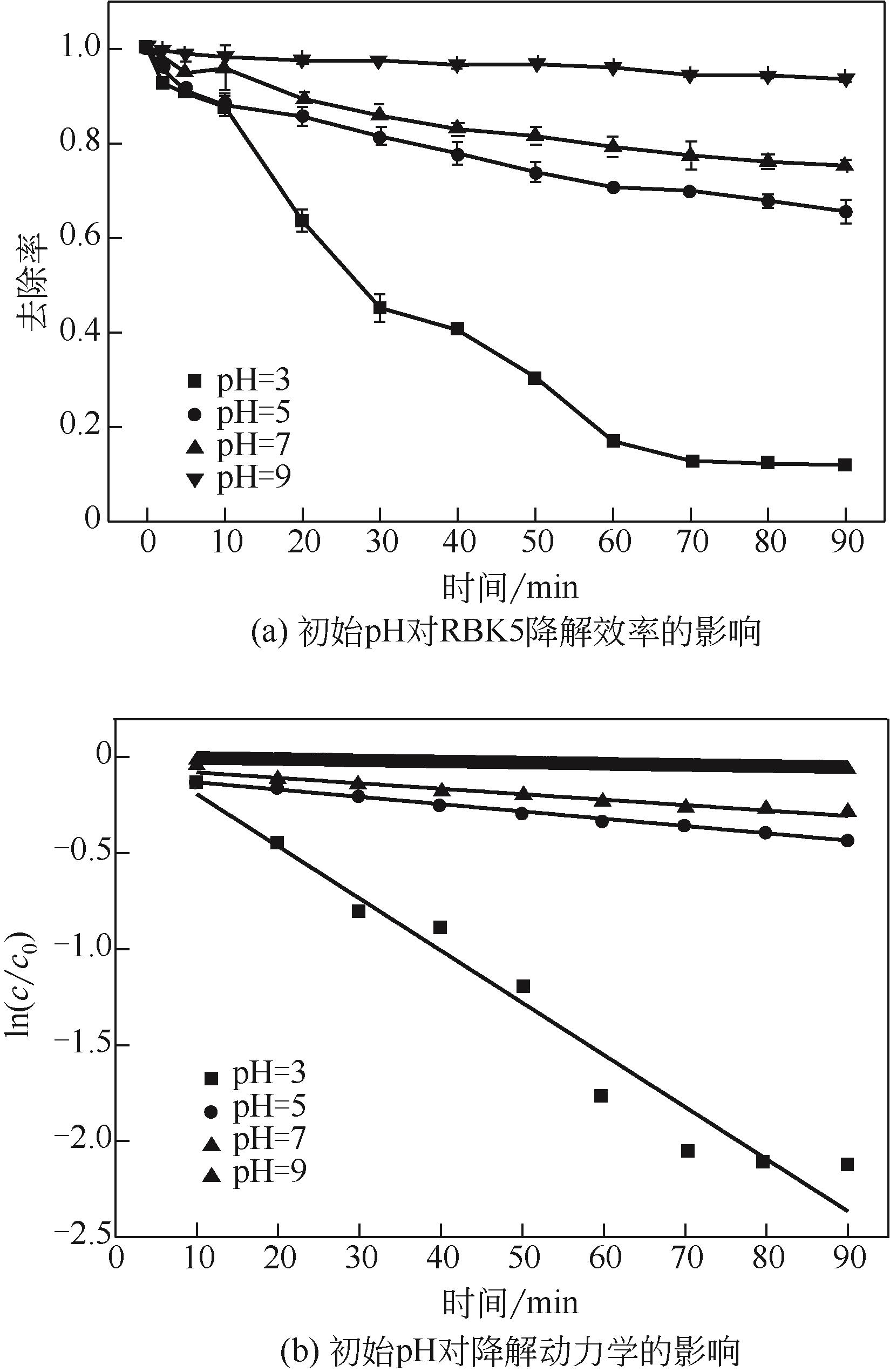
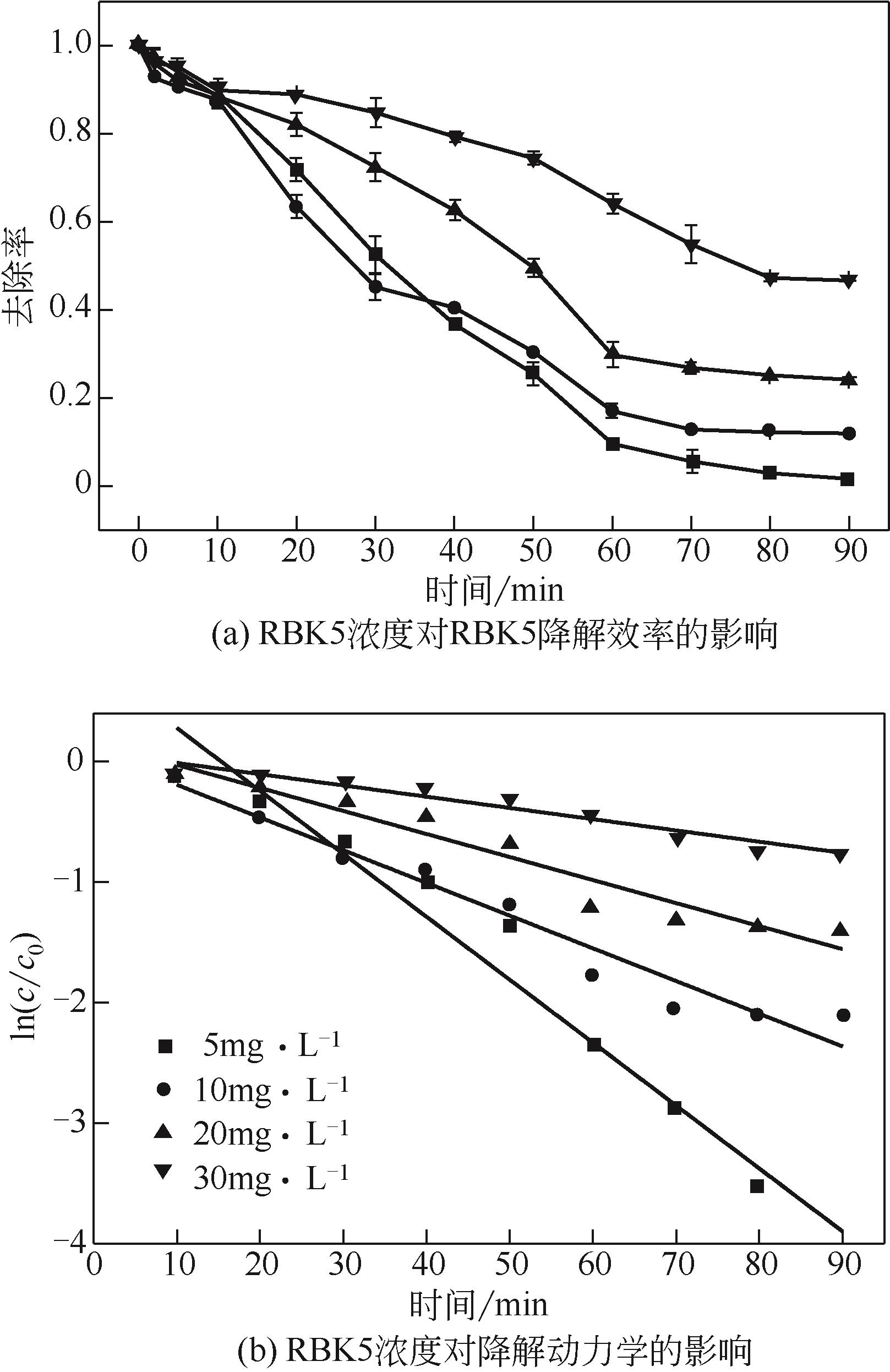
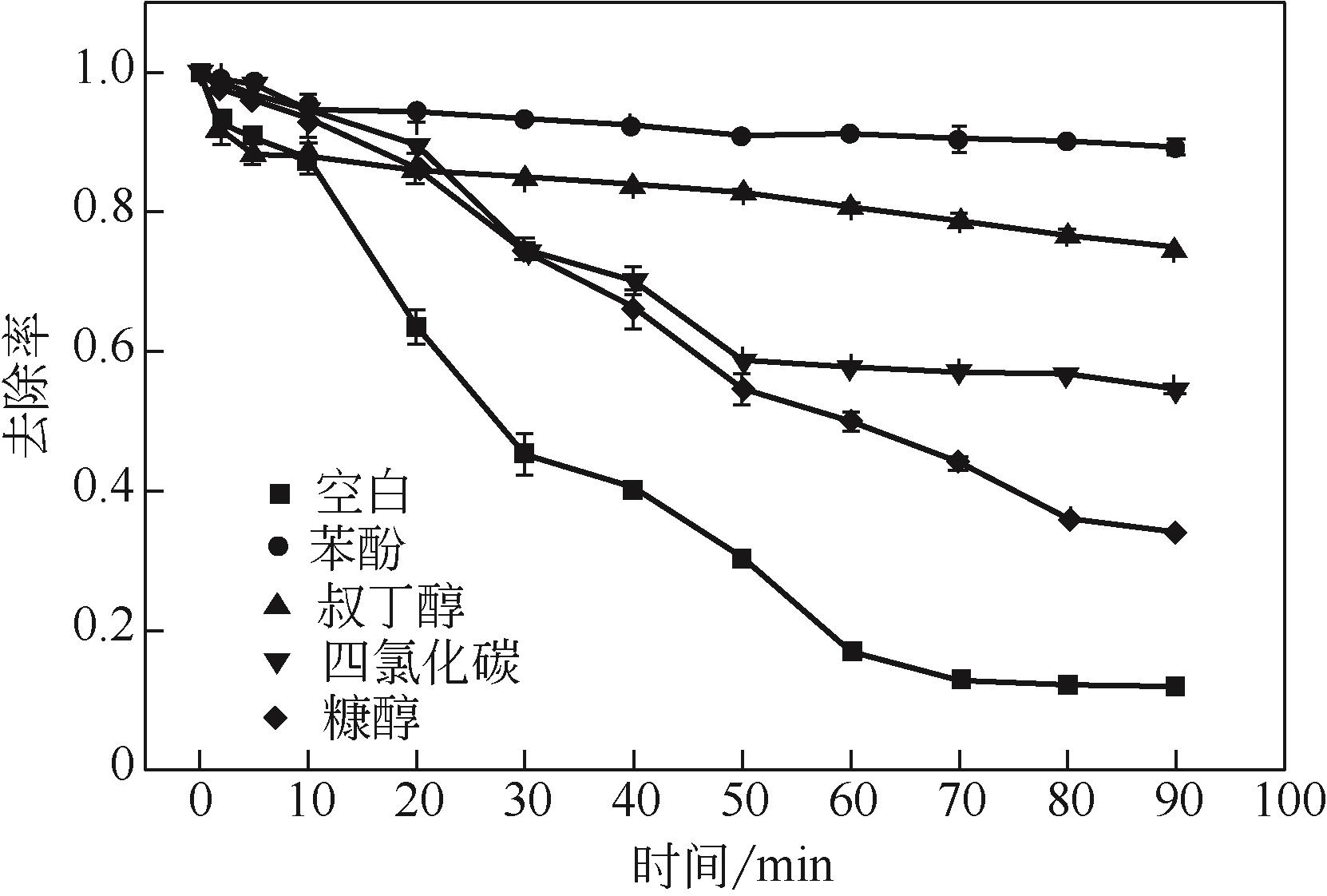
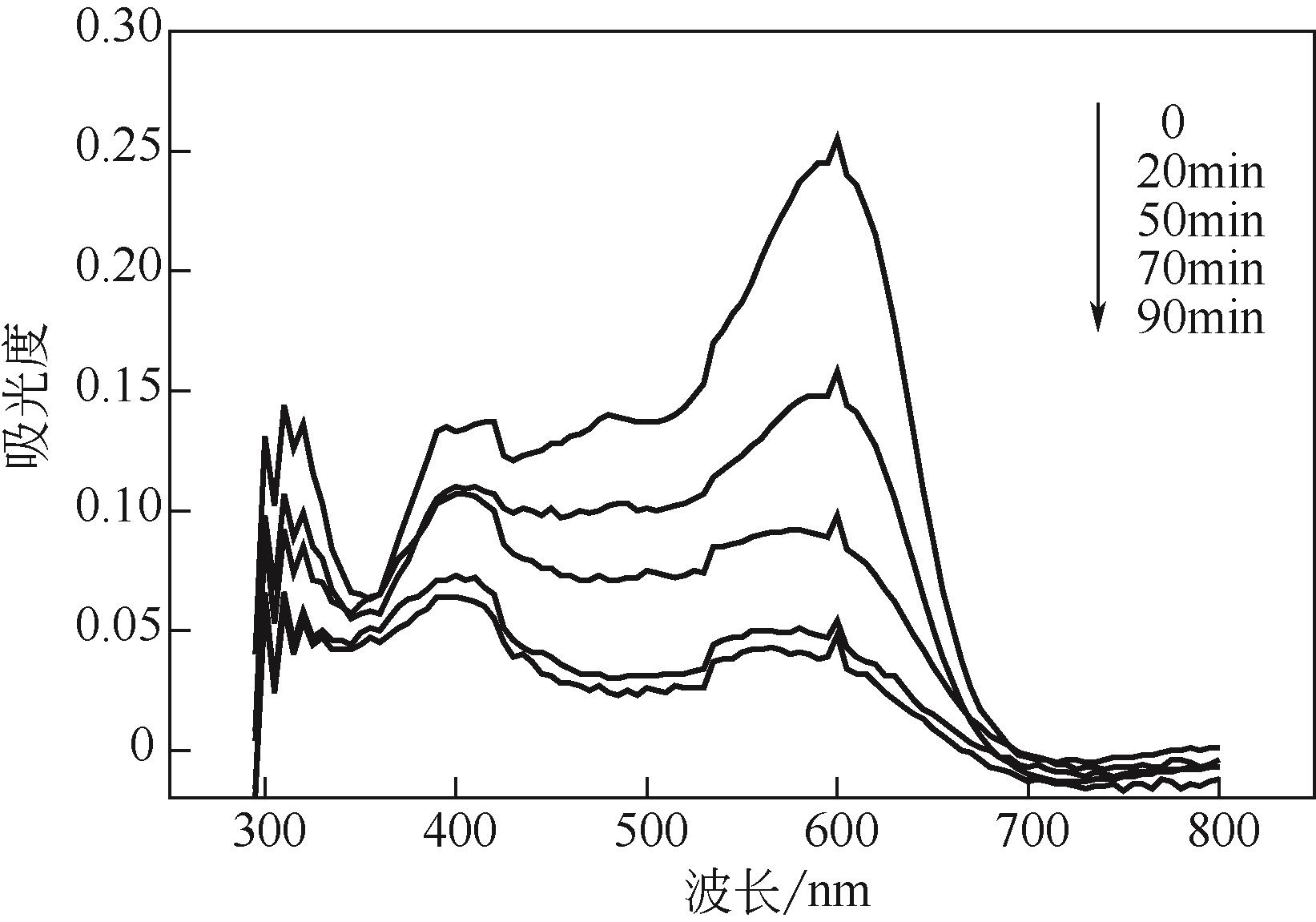
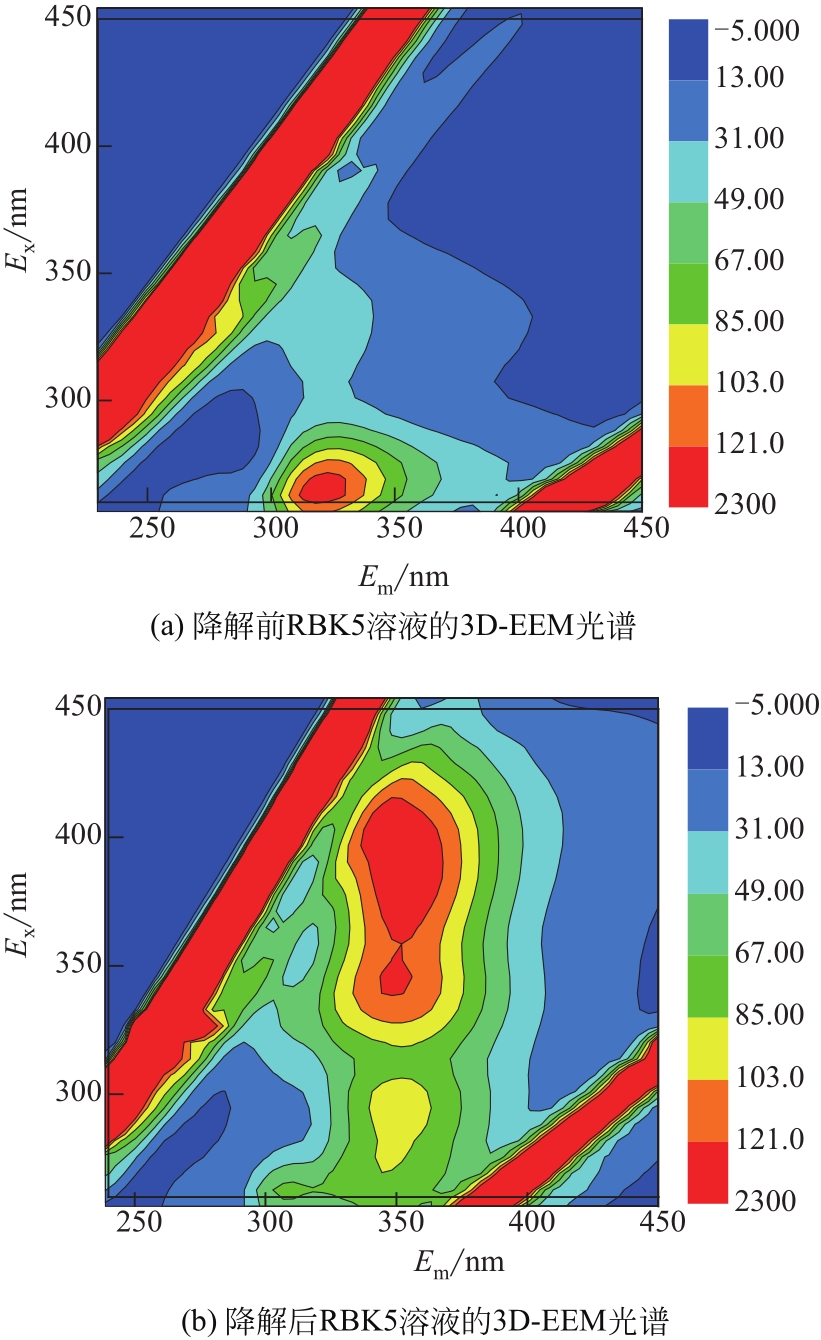
过碳酸钠是过氧化氢与碳酸钠的加成化合物,具有在存储、运输和使用过程中安全稳定的优点。本文采用共沉淀-高温煅烧法制备纳米片状Mn2O3@α-Fe3O4,活化过碳酸钠(SPC)产生自由基氧化降解偶氮染料活性黑5(RBK5)。采用透射电子显微镜(TEM)、X射线粉末衍射仪(XRD)、扫描电子显微镜(SEM)、傅里叶变换红外光谱仪(FTIR)、X射线光电子能谱(XPS)及比表面积测试(BET)表征制备的纳米片状Mn2O3@α-Fe3O4催化剂,分别探究催化剂投加量、过碳酸钠浓度、初始pH及RBK5溶液浓度对降解效率的影响。当催化剂投加量为0.3g/L、过碳酸钠浓度为1.0mmol/L、初始pH为3、反应时间为90min时,RBK5的降解效率达88%,反应过程符合拟一级动力学(R2>0.9)。Mn2O3@α-Fe3O4/过碳酸钠体系中起氧化降解作用的活性物种为·OH、CO
中图分类号:
引用本文
徐铭骏, 郭兆春, 李立, 朱紫琦, 张倩, 洪俊明. 纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料[J]. 化工进展, 2022, 41(2): 1043-1053.
XU Mingjun, GUO Zhaochun, LI Li, ZHU Ziqi, ZHANG Qian, HONG Junming. Degradation of azo dyes by sodium percarbonate activated with nanosheet Mn2O3@α-Fe3O4[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1043-1053.
| 催化剂投加量/g·L-1 | K/min-1 | R2 |
|---|---|---|
| 0.1 | 0.0078 | 0.926 |
| 0.2 | 0.0210 | 0.973 |
| 0.3 | 0.0272 | 0.953 |
| 0.4 | 0.0303 | 0.955 |
表1 不同催化剂投加量下RBK5的降解动力学参数
| 催化剂投加量/g·L-1 | K/min-1 | R2 |
|---|---|---|
| 0.1 | 0.0078 | 0.926 |
| 0.2 | 0.0210 | 0.973 |
| 0.3 | 0.0272 | 0.953 |
| 0.4 | 0.0303 | 0.955 |
| 过碳酸钠浓度/mmol·L-1 | K/min-1 | R2 |
|---|---|---|
| 1 | 0.0272 | 0.953 |
| 2 | 0.0254 | 0.953 |
| 3 | 0.0235 | 0.900 |
| 4 | 0.0025 | 0.986 |
表2 不同过碳酸钠浓度下RBK5的降解动力学参数
| 过碳酸钠浓度/mmol·L-1 | K/min-1 | R2 |
|---|---|---|
| 1 | 0.0272 | 0.953 |
| 2 | 0.0254 | 0.953 |
| 3 | 0.0235 | 0.900 |
| 4 | 0.0025 | 0.986 |
| pH | K/min-1 | R2 |
|---|---|---|
| 3 | 0.0272 | 0.953 |
| 5 | 0.0038 | 0.961 |
| 7 | 0.0025 | 0.985 |
| 9 | 0.0006 | 0.936 |
表3 不同初始pH下RBK5的降解动力学参数
| pH | K/min-1 | R2 |
|---|---|---|
| 3 | 0.0272 | 0.953 |
| 5 | 0.0038 | 0.961 |
| 7 | 0.0025 | 0.985 |
| 9 | 0.0006 | 0.936 |
| RBK5浓度/mmol·L-1 | K/min-1 | R2 |
|---|---|---|
| 5 | 0.0522 | 0.964 |
| 10 | 0.0272 | 0.953 |
| 20 | 0.0191 | 0.934 |
| 30 | 0.0093 | 0.936 |
表4 不同RBK5浓度下的降解动力学参数
| RBK5浓度/mmol·L-1 | K/min-1 | R2 |
|---|---|---|
| 5 | 0.0522 | 0.964 |
| 10 | 0.0272 | 0.953 |
| 20 | 0.0191 | 0.934 |
| 30 | 0.0093 | 0.936 |
| 1 | WU J N, WANG T W. Ozonation of aqueous azo dye in a semi-batch reactor[J]. Water Research, 2001, 35(4): 1093-1099. |
| 2 | BANERJEE P, DASGUPTA S, DE S. Removal of dye from aqueous solution using a combination of advanced oxidation process and nanofiltration[J]. Journal of Hazardous Materials, 2007, 140(1/2): 95-103. |
| 3 | 王艳, 宋祖德, 冯明明, 等. 铁氧化物联合低含量亚铁离子催化H2O2降解偶氮染料活性黑5[J]. 水处理技术, 2014, 40(2): 35-38. |
| WANG Yan, SONG Zude, FENG Mingming, et al. Degradation of azo dye reactive black 5 by low ferrous ions concentration catalyzed H2O2 in combination with waste iron oxide[J]. Technology of Water Treatment, 2014, 40(2): 35-38. | |
| 4 | HUNG C M, HUANG C P, CHEN C W, et al. Activation of percarbonate by water treatment sludge-derived biochar for the remediation of PAH-contaminated sediments[J]. Environmental Pollution, 2020, 265: 114914. |
| 5 | FU X R, GU X G, LU S G, et al. Benzene depletion by Fe2+-catalyzed sodium percarbonate in aqueous solution[J]. Chemical Engineering Journal, 2015, 267: 25-33. |
| 6 | VIISIMAA M, GOI A. Use of hydrogen peroxide and percarbonate to treat chlorinated aromatic hydrocarbon-contaminated soil[J]. Journal of Environmental Engineering and Landscape Management, 2014, 22(1): 30-39. |
| 7 | SAJJADI S, KHATAEE A, DARVISHI CHESHMEH SOLTANI R, et al. Implementation of magnetic Fe3O4@ZIF-8 nanocomposite to activate sodium percarbonate for highly effective degradation of organic compound in aqueous solution[J]. Journal of Industrial and Engineering Chemistry, 2018, 68: 406-415. |
| 8 | DANISH M, GU X G, LU S G, et al. Efficient transformation of trichloroethylene activated through sodium percarbonate using heterogeneous zeolite supported nano zero valent iron-copper bimetallic composite[J]. Chemical Engineering Journal, 2017, 308: 396-407. |
| 9 | VIGNESH R H, SANKAR K V, AMARESH S, et al. Synthesis and characterization of MnFe2O4 nanoparticles for impedometric ammonia gas sensor[J]. Sensors and Actuators B: Chemical, 2015, 220: 50-58. |
| 10 | 李立, 吴丽颖, 董正玉, 等. 高晶度Mn-Fe LDH催化剂活化过一硫酸盐降解偶氮染料RBK5[J]. 环境科学, 2020, 41(6): 2736-2745. |
| LI Li, WU Liying, DONG Zhengyu, et al. Degradation of RBK5 by high crystallinity Mn-Fe LDH catalyst activating peroxymonosulfate[J]. Environmental Science, 2020, 41(6): 2736-2745. | |
| 11 | 佘月城, 董正玉, 吴丽颖, 等. MnFe2O4活化过一硫酸盐降解废水中LAS[J]. 中国环境科学, 2019, 39(8): 3323-3331. |
| SHE Yuecheng, DONG Zhengyu, WU Liying, et al. Degradation of LAS in wastewater by peroxymonosulfate activated by MnFe2O4[J]. China Environmental Science, 2019, 39(8): 3323-3331. | |
| 12 | DU J K, XIAO G F, XI Y X, et al. Periodate activation with manganese oxides for sulfanilamide degradation[J]. Water Research, 2020, 169: 115278. |
| 13 | ZHENG H, BAO J G, HUANG Y, et al. Efficient degradation of atrazine with porous sulfurized Fe2O3 as catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2019, 259: 118056. |
| 14 | CHEN L, JIANG X, XIE R Z, et al. A novel porous biochar-supported Fe-Mn composite as a persulfate activator for the removal of acid red 88[J]. Separation and Purification Technology, 2020, 250: 117232. |
| 15 | CHEN Z Q, WANG L Y, XU H D, et al. Efficient heterogeneous activation of peroxymonosulfate by modified CuFe2O4 for degradation of tetrabromobisphenol A[J]. Chemical Engineering Journal, 2020, 389: 124345. |
| 16 | WAN Z, WANG J L. Degradation of sulfamethazine antibiotics using Fe3O4-Mn3O4 nanocomposite as a Fenton-like catalyst[J]. Journal of Chemical Technology & Biotechnology, 2017, 92(4): 874-883. |
| 17 | MA Z, ZHAO D, CHANG Y, et al. Synthesis of MnFe2O4@Mn-Co oxide core-shell nanoparticles and their excellent performance for heavy metal removal[J]. Dalton Transactions, 2013, 42(39): 14261-14267. |
| 18 | MAGAGULA B, NHLAPO N, FOCKE W W. Mn2Al-LDH- and Co2Al-LDH-stearate as photodegradants for LDPE film[J]. Polymer Degradation and Stability, 2009, 94(6): 947-954. |
| 19 | WANG G, ZHAO D Y, KOU F Y, et al. Removal of norfloxacin by surface Fenton system(MnFe2O4/H2O2): kinetics, mechanism and degradation pathway[J]. Chemical Engineering Journal, 2018, 351: 747-755. |
| 20 | XU X J, YANG Y, JIA Y F, et al. Heterogeneous catalytic degradation of 2,4-dinitrotoluene by the combined persulfate and hydrogen peroxide activated by the as-synthesized Fe-Mn binary oxides[J]. Chemical Engineering Journal, 2019, 374: 776-786. |
| 21 | WU L Y, YU Y B, ZHANG Q, et al. A novel magnetic heterogeneous catalyst oxygen-defective CoFe2O4-x for activating peroxymonosulfate[J]. Applied Surface Science, 2019, 480: 717-726. |
| 22 | WU L Y, ZHANG Q, HONG J M, et al. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4-x[J]. Chemosphere, 2019, 221: 412-422. |
| 23 | 王艳, 尹超, 戴明星, 等. Fe/Mn/Al2O3催化H2O2降解偶氮染料废水的研究[J]. 应用化工, 2013, 42(11): 2002-2004. |
| WANG Yan, YIN Chao, DAI Mingxing, et al. Degradation of azo dyes wastewater by Fe/Mn/Al2O3 catalytic H2O2[J]. Applied Chemical Industry, 2013, 42(11): 2002-2004. | |
| 24 | LYU Y C, LYU S G, TANG P, et al. Degradation of trichloroethylene in aqueous solution by sodium percarbonate activated with Fe(Ⅱ)-citric acid complex in the presence of surfactant Tween-80[J]. Chemosphere, 2020, 257: 127223. |
| 25 | 徐劼, 王琳, 陈家斌, 等. 磁性Fe3O4-CuO非均相活化过碳酸钠降解AO7[J]. 环境科学, 2020, 41(4): 1734-1742. |
| XU Jie, WANG Lin, CHEN Jiabin, et al. Degradation of AO7 with magnetic Fe3O4-CuO heterogeneous catalyzed sodium percarbonate system[J]. Environmental Science, 2020, 41(4): 1734-1742. | |
| 26 | FU X R, GU X G, LU S G, et al. Enhanced degradation of benzene in aqueous solution by sodium percarbonate activated with chelated- Fe(Ⅱ)[J]. Chemical Engineering Journal, 2016, 285: 180-188. |
| 27 | LIN K Y A, LIN J T, LIN Y F. Heterogeneous catalytic activation of percarbonate by ferrocene for degradation of toxic amaranth dye in water[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 78: 144-149. |
| 28 | GAO J, DUAN X D, O’SHEA K, et al. Degradation and transformation of bisphenol A in UV/sodium percarbonate: dual role of carbonate radical anion[J]. Water Research, 2020, 171: 115394. |
| 29 | LIANG H Y, ZHANG Y Q, HUANG S B, et al. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate[J]. Chemical Engineering Journal, 2013, 218: 384-391. |
| 30 | WAN Z, WANG J L. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst[J]. Journal of Hazardous Materials, 2017, 324: 653-664. |
| 31 | 于永波, 黄湾, 董正玉, 等. N原子杂化石墨烯高效活化过一硫酸盐降解RBk5染料废水[J]. 环境科学, 2019, 40(7): 3154-3161. |
| YU Yongbo, HUANG Wan, DONG Zhengyu, et al. Degradation of RBk5 with peroxymonosulfate efficiently activated by N-doped graphene[J]. Environmental Science, 2019, 40(7): 3154-3161. | |
| 32 | LI Z, LUO S Q, YANG Y, et al. Highly efficient degradation of trichloroethylene in groundwater based on peroxymonosulfate activation by bentonite supported Fe/Ni bimetallic nanoparticle[J]. Chemosphere, 2019, 216: 499-506. |
| 33 | DONG Z Y, ZHANG Q, CHEN B Y, et al. Oxidation of bisphenol A by persulfate via Fe3O4-α-MnO2 nanoflower-like catalyst: mechanism and efficiency[J]. Chemical Engineering Journal, 2019, 357: 337-347. |
| 34 | Abdellatif EL-GHENYMY, CENTELLAS Francesc, GARRIDO Jose Antonio, et al. Decolorization and mineralization of Orange G azo dye solutions by anodic oxidation with a boron-doped diamond anode in divided and undivided tank reactors[J]. Electrochimica Acta, 2014, 130: 568-576. |
| 35 | CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. |
| 36 | SONI B D, RUPARELIA J P. Decolourization and mineralization of reactive black-5 with transition metal oxide coated electrodes by electrochemical oxidation[J]. Procedia Engineering, 2013, 51: 335-341. |
| [1] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [2] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [3] | 刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103. |
| [4] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [5] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [6] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [7] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [8] | 曾天续, 张永显, 严渊, 刘宏, 马娇, 党鸿钟, 吴新波, 李维维, 陈永志. 羟胺对硝化菌活性及其动力学参数的影响[J]. 化工进展, 2023, 42(6): 3272-3280. |
| [9] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [10] | 张成松, 张静, 龚斌, 李明洋, 袁佳新, 李宏业. 自吸射流柔性搅拌桨振动特性[J]. 化工进展, 2023, 42(4): 1728-1738. |
| [11] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [12] | 王唯, 张东旭, 李遵照, 王晓霖, 黄启玉. 油包水乳状液体系中水合物生长行为研究进展[J]. 化工进展, 2023, 42(3): 1155-1166. |
| [13] | 李芸, 崔楠, 熊星星, 黄志远, 王东亮, 许丹, 李军, 李泽兵. 稀土Er(Ⅲ)对短程硝化性能的影响及其抑制动力学特性[J]. 化工进展, 2023, 42(3): 1659-1668. |
| [14] | 闫兴清, 戴行涛, 喻健良, 李岳, 韩冰, 胡军. 高压氢气泄漏射流研究进展[J]. 化工进展, 2023, 42(3): 1118-1128. |
| [15] | 宋叶, 陈玉卓, 宋云彩, 冯杰. 有机固废合成气原位净化催化剂设计及反应器分析[J]. 化工进展, 2023, 42(3): 1383-1396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||