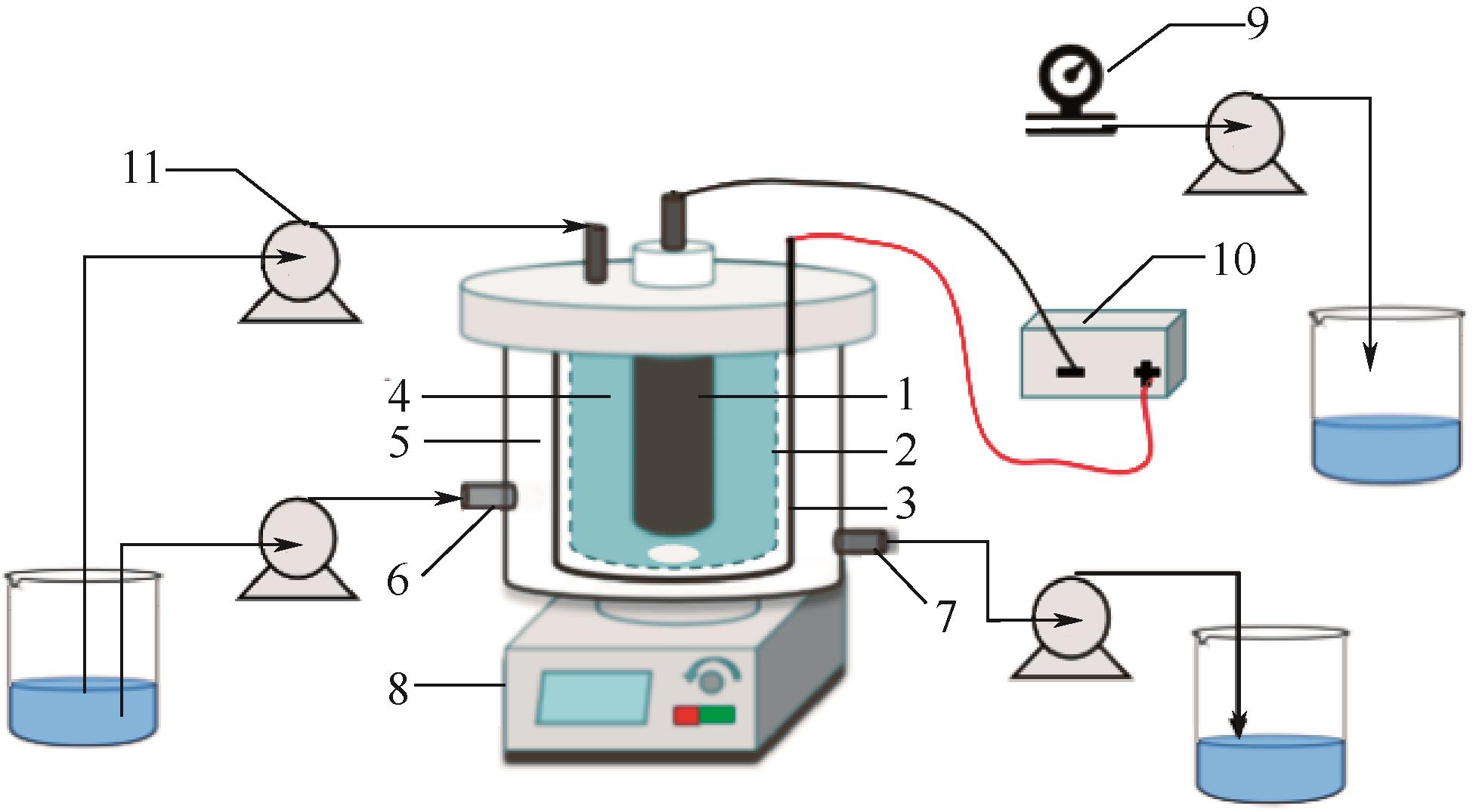
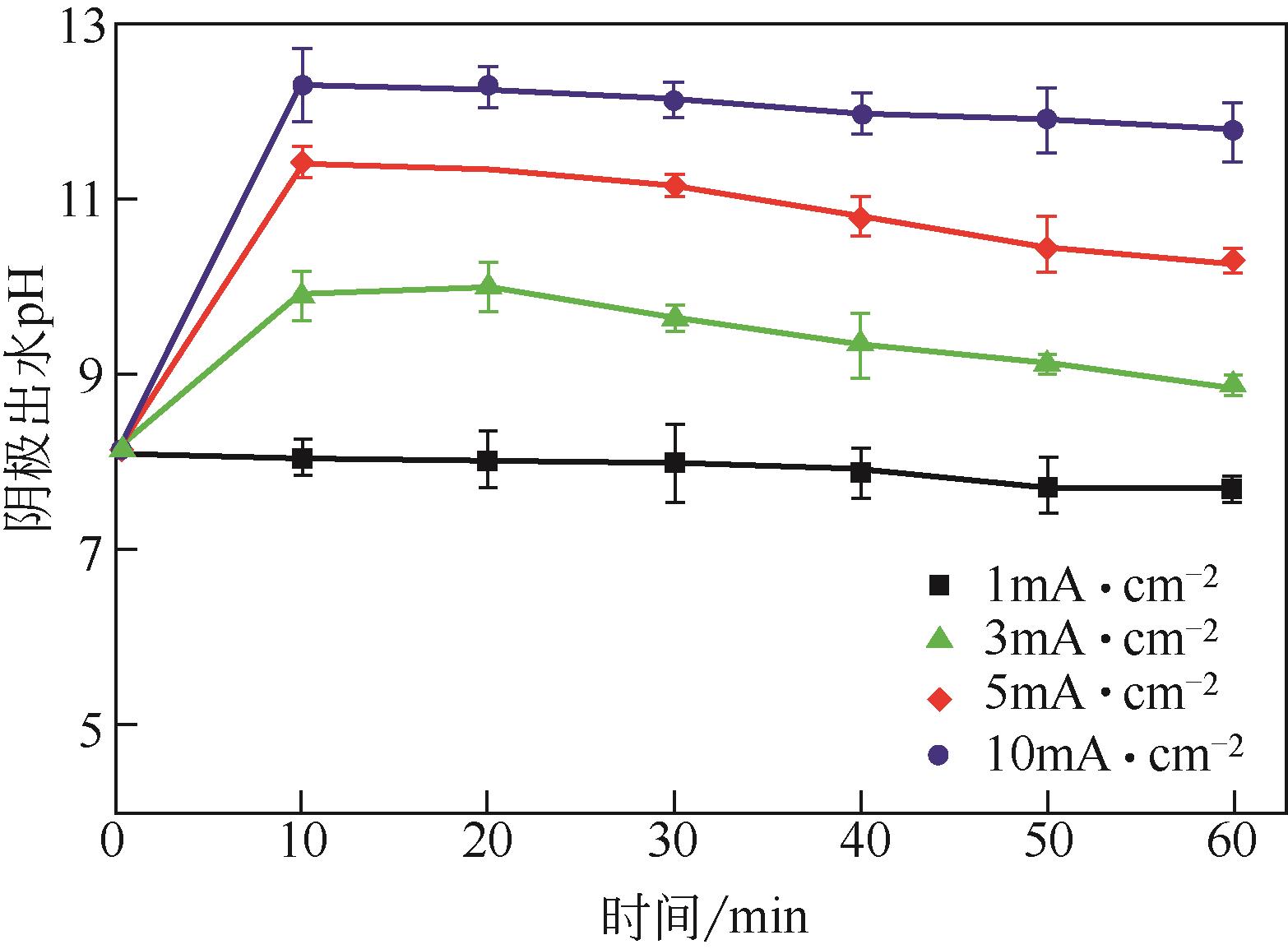
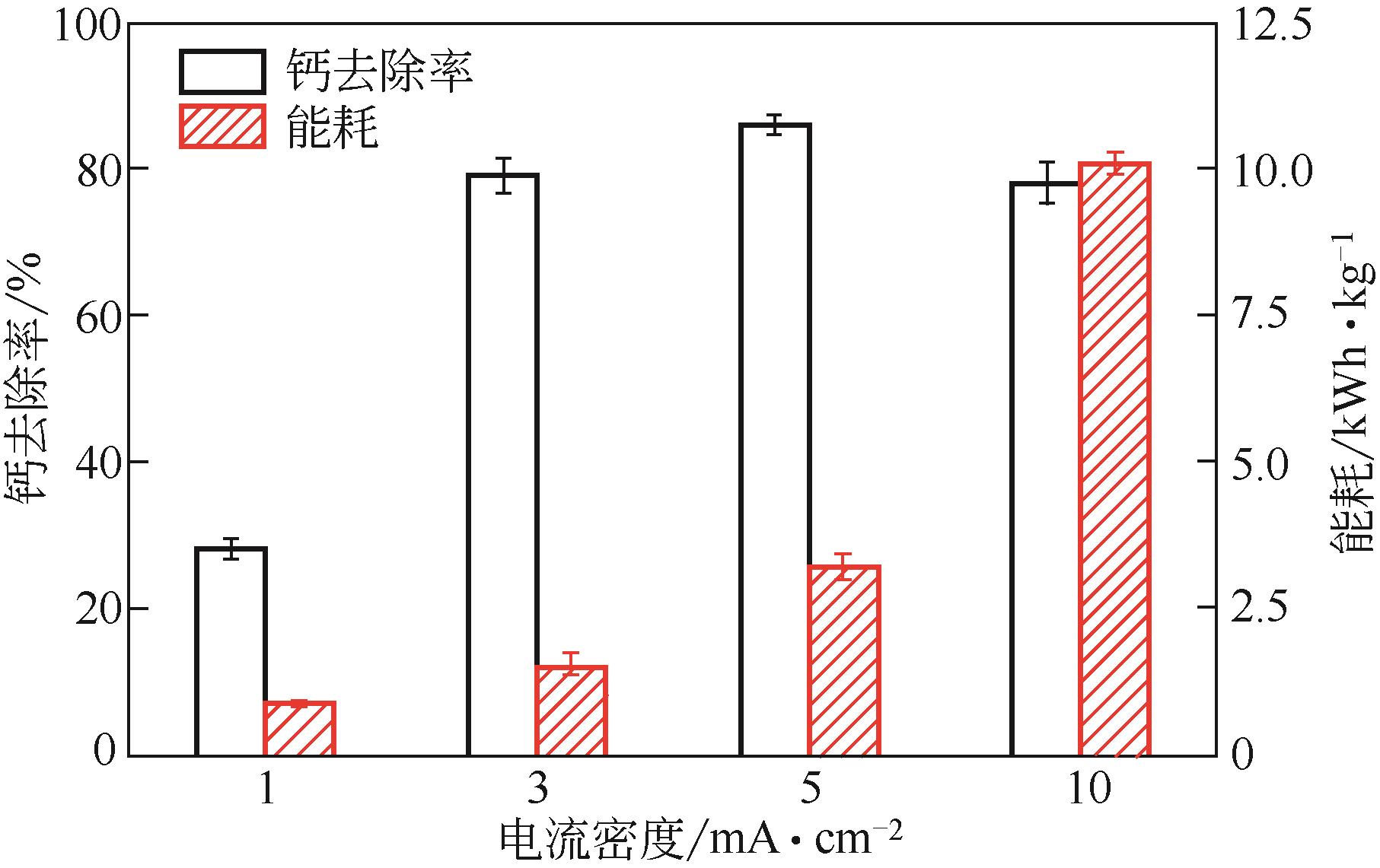
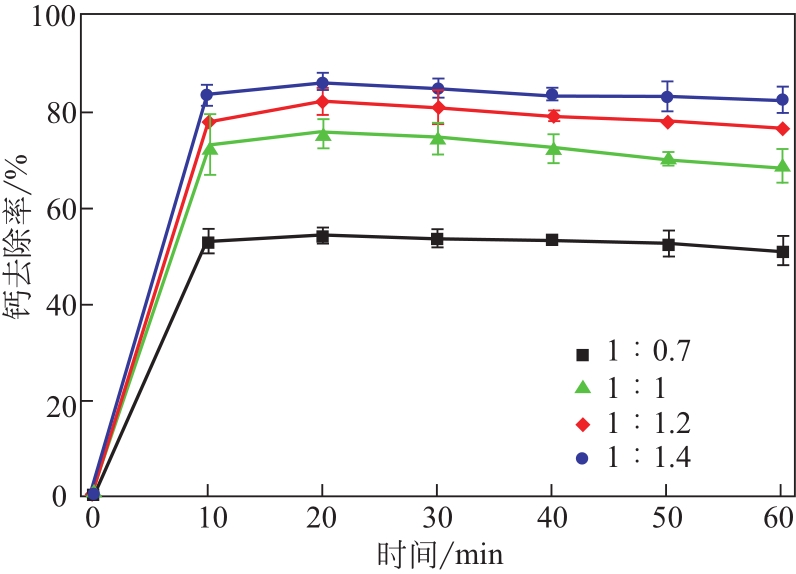
| 1 |
SEO Seok Jun, JEON Hongrae, LEE Jae Kwang, et al. Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications[J]. Water Research, 2010, 44(7): 2267-2275.
|
| 2 |
PING Q Y, HE Z. Improving the flexibility of microbial desalination cells through spatially decoupling anode and cathode[J]. Bioresource Technology, 2013, 144: 304-310.
|
| 3 |
HASSON David, CORNEL Andrei. Effect of residence time on the degree of CaCO3 precipitation in the presence of an anti-scalant[J]. Desalination, 2017, 401: 64-67.
|
| 4 |
ZHI Suli, ZHANG Keqiang. Hardness removal by a novel electrochemical method[J]. Desalination, 2016, 381: 8-14.
|
| 5 |
HASSON David, SIDORENKO Georgiy, SEMIAT Raphael. Calcium carbonate hardness removal by a novel electrochemical seeds system[J]. Desalination, 2010, 263(1/2/3): 285-289.
|
| 6 |
SANJUAN Ignacio, BENAVENTE David, Vicente GARCIA-GARCIA, et al. Electrochemical softening of concentrates from an electrodialysis brackish water desalination plant: efficiency enhancement using a three-dimensional cathode[J]. Separation and Purification Technology, 2019, 208: 217-226.
|
| 7 |
ZASLAVSCHI Irina, SHEMER Hilla, HASSON David, et al. Electrochemical CaCO3 scale removal with a bipolar membrane system[J]. Journal of Membrane Science, 2013, 445: 88-95.
|
| 8 |
王建国, 陆帅, 李松. 电磁场对碳酸钙反应结晶成核影响的研究[J]. 工业水处理, 2014, 34(9): 67-69.
|
|
WANG Jianguo, LU Shuai, LI Song. Research on the effect of electromagnetic field on calcium carbonate crystallization nucleation[J]. Industrial Water Treatment, 2014, 34(9): 67-69.
|
| 9 |
王蛟平. 循环冷却水电化学除垢动力学特性与工艺研究[D]. 济南:济南大学, 2019.
|
|
WANG Jiaoping. Kinetic performance of electrochemical process applied in circulating cooling water scaling[D]. Jinan: University of Jinan, 2019.
|
| 10 |
沈雪. 复合混凝剂对混凝-超滤工艺水处理效能和膜污染的影响[D]. 济南:山东大学, 2020.
|
|
SHEN Xue. Effects of composite coagulant on water treatment efficiency and membrane fouling of coagulation-ultrafiltration process[D]. Jinan: Shandong University, 2020.
|
| 11 |
张鑫. 管式膜微滤过程强化及水力学特性研究[D]. 大连:大连理工大学, 2014.
|
|
ZHANG Xin. Tubular membrane microfiltration process intensification and hydraulic characteristics study[D]. Dalian: Dalian University of Technology, 2014.
|
| 12 |
田岳林, 袁栋栋, 李汝琪. 陶瓷膜污染过程分析与膜清洗方法优化[J]. 环境工程学报, 2013, 7(1): 253-257.
|
|
TIAN Yuelin, YUAN Dongdong, LI Ruqi. Analysis on ceramic membrane fouling procedure and optimization of membrane flushing method[J].Chinese Journal of Environmental Engineering, 2013, 7(1): 253-257.
|
| 13 |
张国俊, 刘忠洲. 膜过程中膜清洗技术研究进展[J]. 水处理技术, 2003, 29(4): 187-190.
|
|
ZHANG Guojun, LIU Zhongzhou. Progress in membrane cheaning techniques[J]. Technology of Water Treatment, 2003, 29(4): 187-190.
|
 ), YAN Wei1, TANG Yizhen1, LIU Di2, JIANG Bo1(
), YAN Wei1, TANG Yizhen1, LIU Di2, JIANG Bo1( )
)