化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3101-3111.DOI: 10.16085/j.issn.1000-6613.2024-1486
• 专栏:化工生态环境前沿交叉新技术 • 上一篇
表面修饰FeOCl活化过硫酸盐引发有机污染物非自由基降解
- 昆明理工大学建筑工程学院,云南 昆明 650500
-
收稿日期:2024-09-09修回日期:2024-10-11出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:徐西蒙 -
作者简介:王御豪(2000—),男,硕士研究生,研究方向为水体新兴污染物处理技术。E-mail:20222210109@stu.kust.edu.cn。 -
基金资助:云南省重点研发计划(202203AC100004)
Degradation of organic pollutants via non-radical pathway by surface modified FeOCl activating persulfate
WANG Yuhao( ), JIANG Qinli, XU Ximeng(
), JIANG Qinli, XU Ximeng( )
)
- Faculty of Civil Engineering and Mechanics, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2024-09-09Revised:2024-10-11Online:2025-06-25Published:2025-07-08 -
Contact:XU Ximeng
摘要:
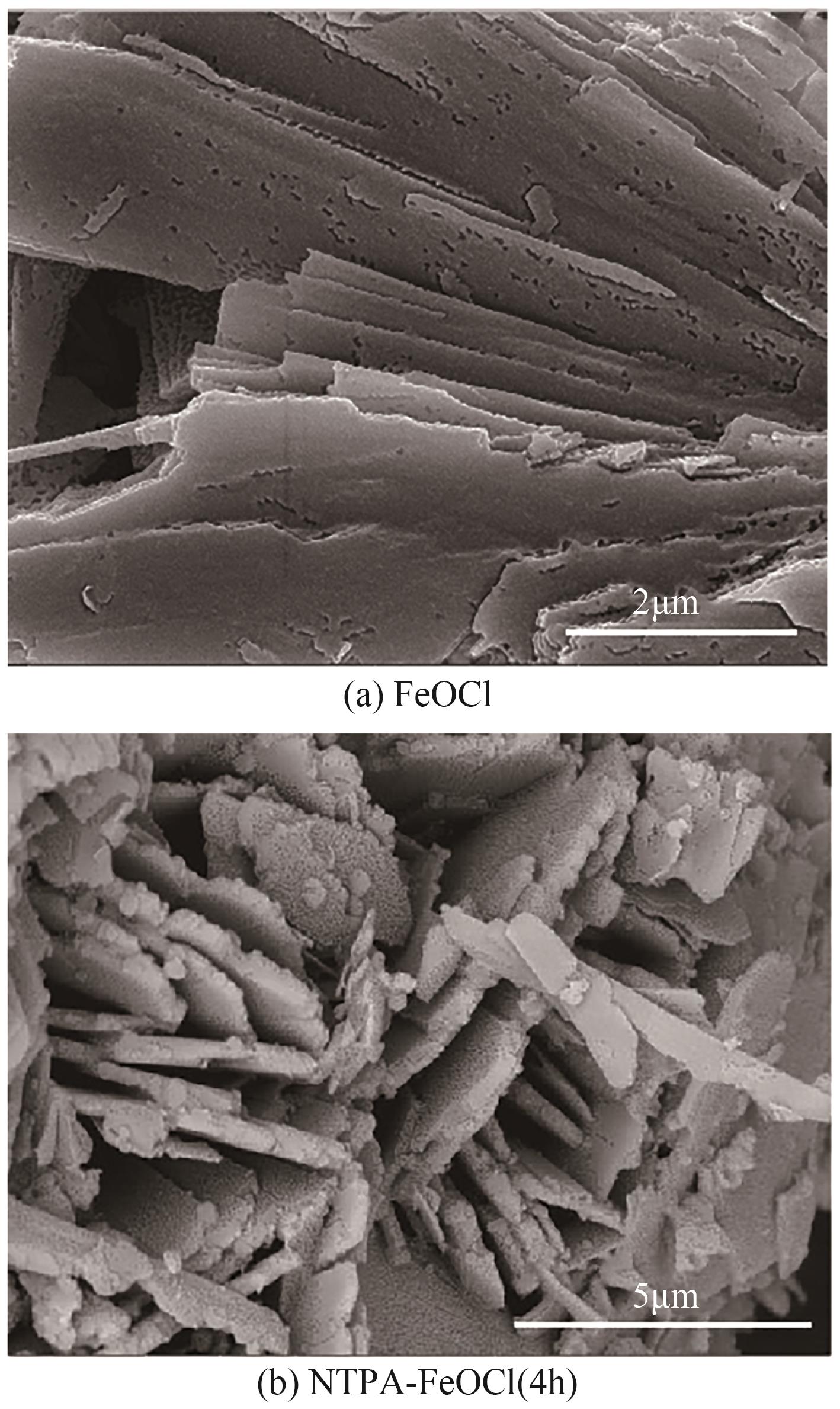
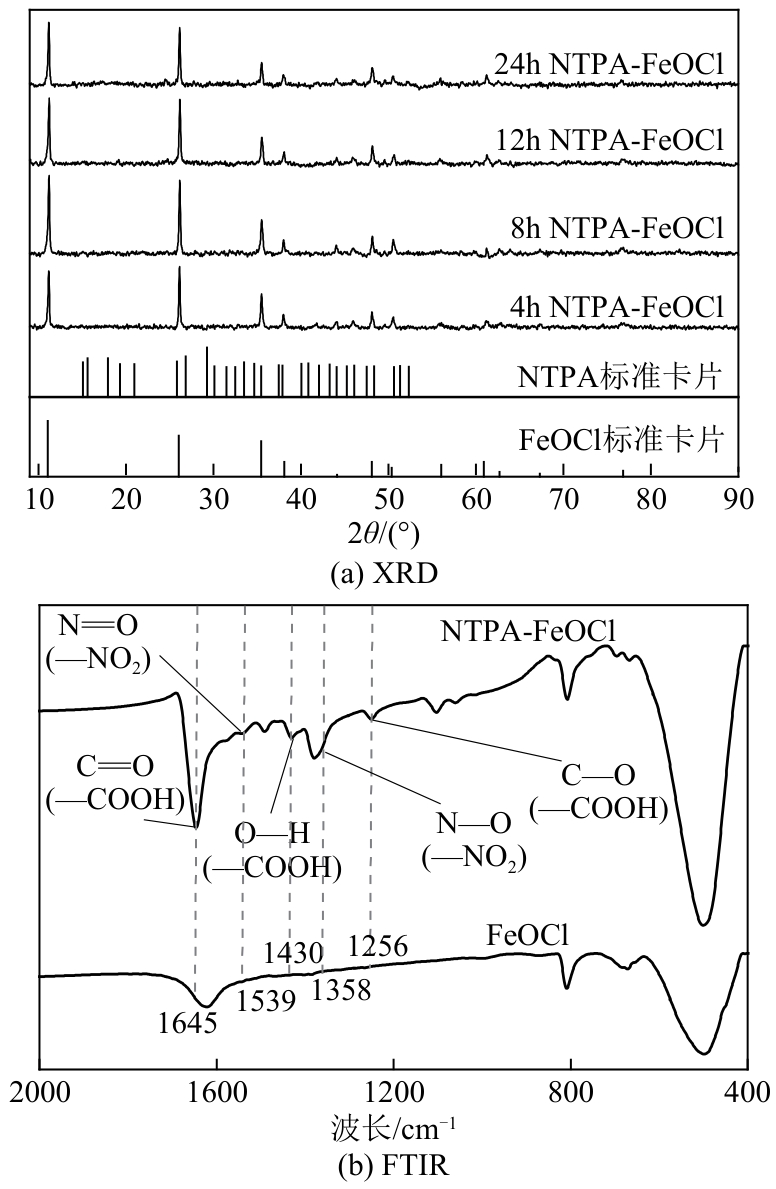
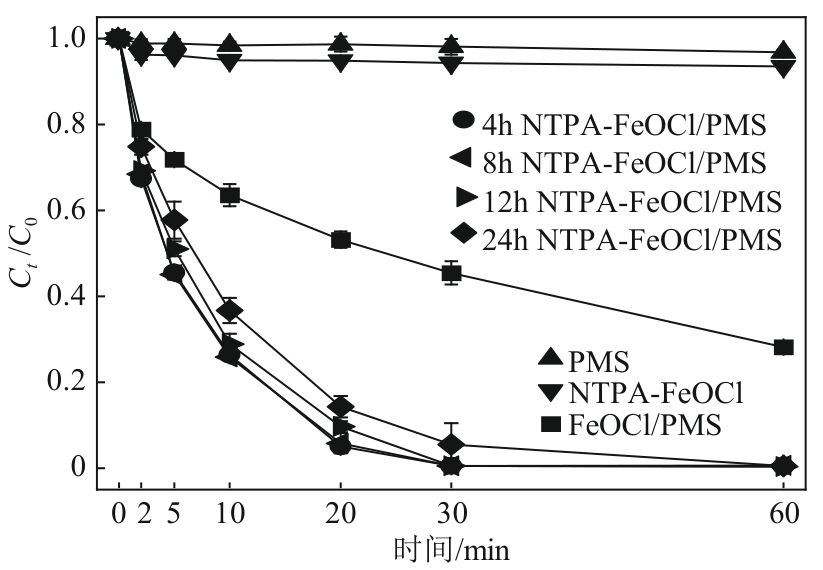
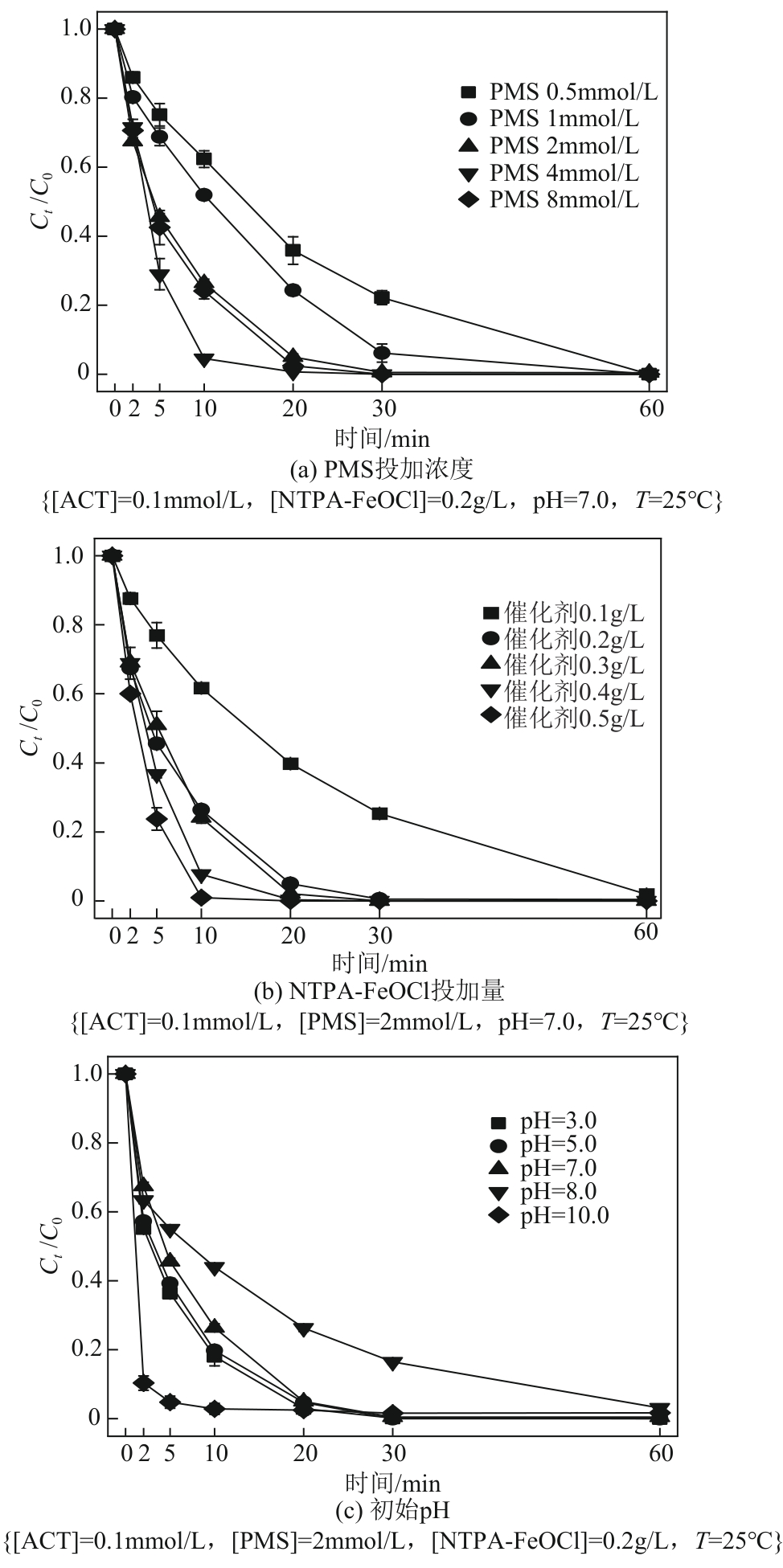
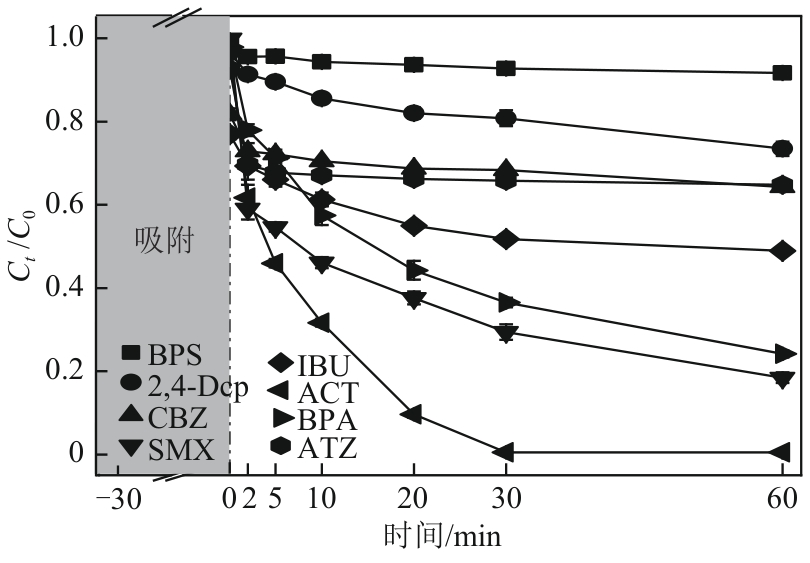
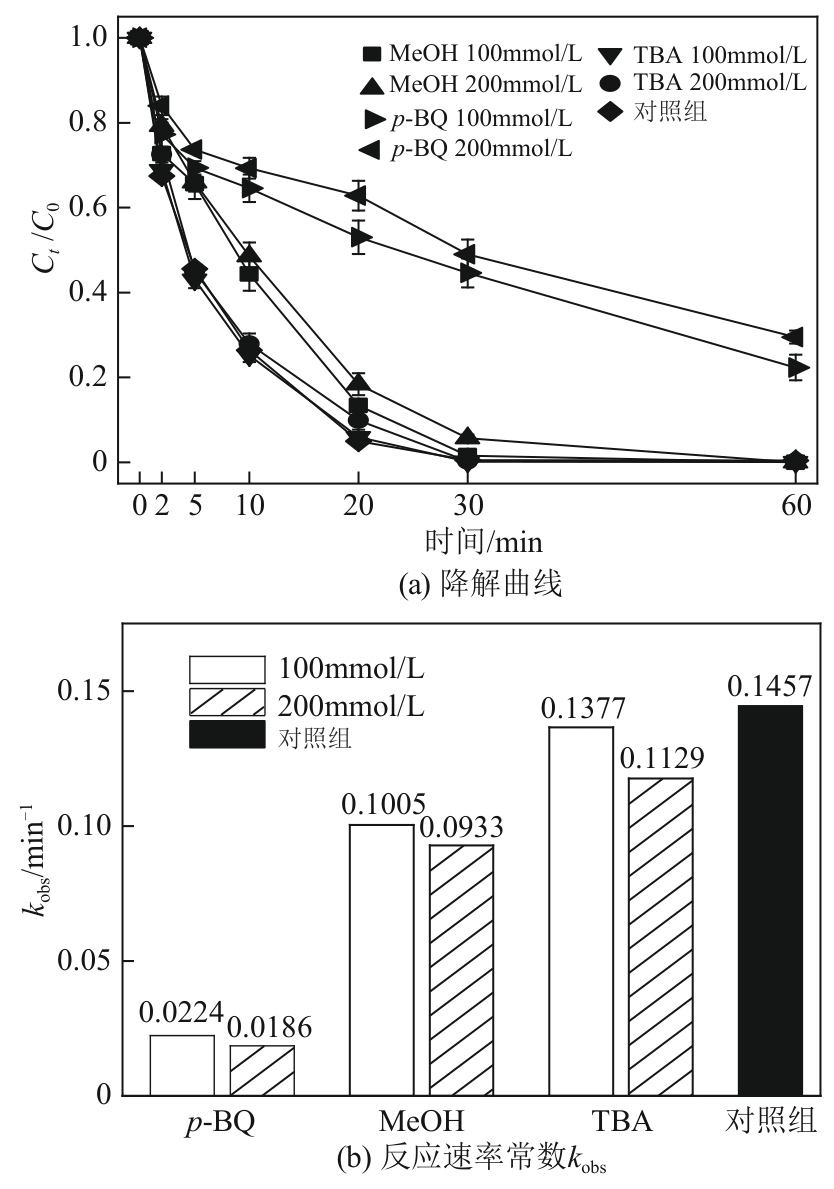
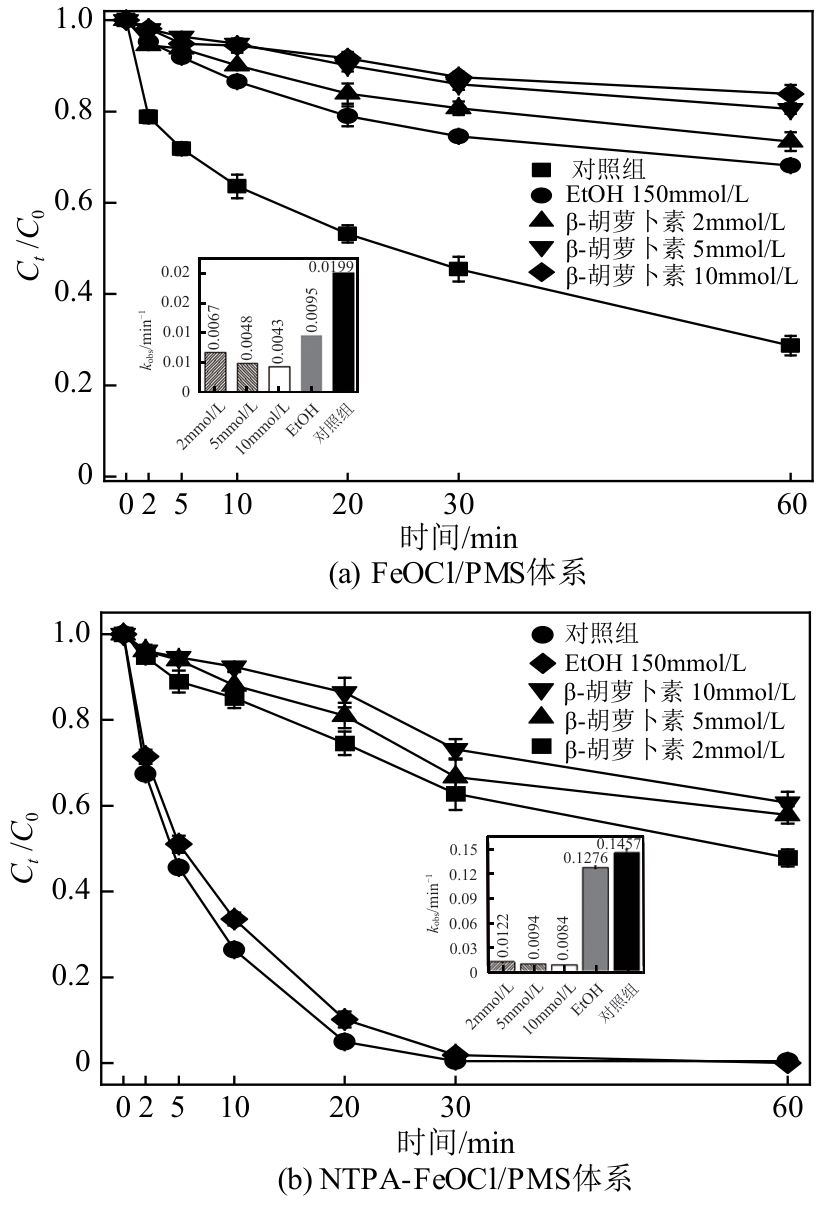
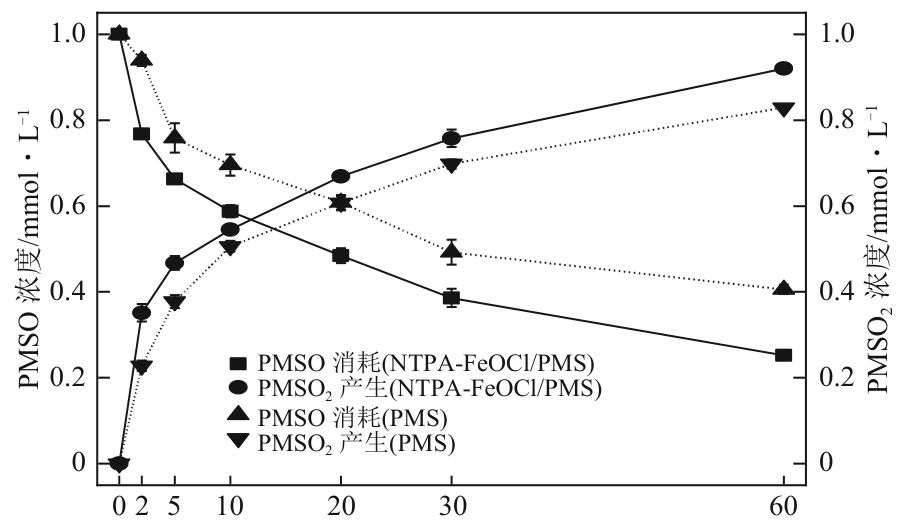
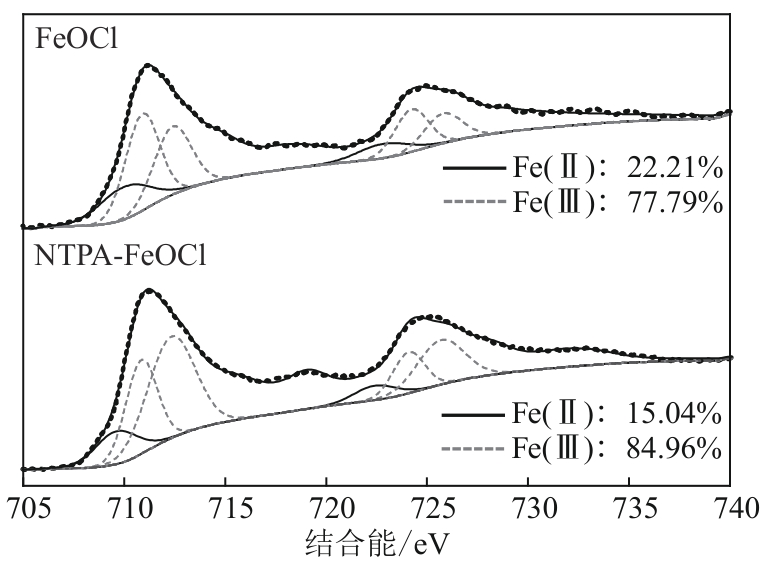
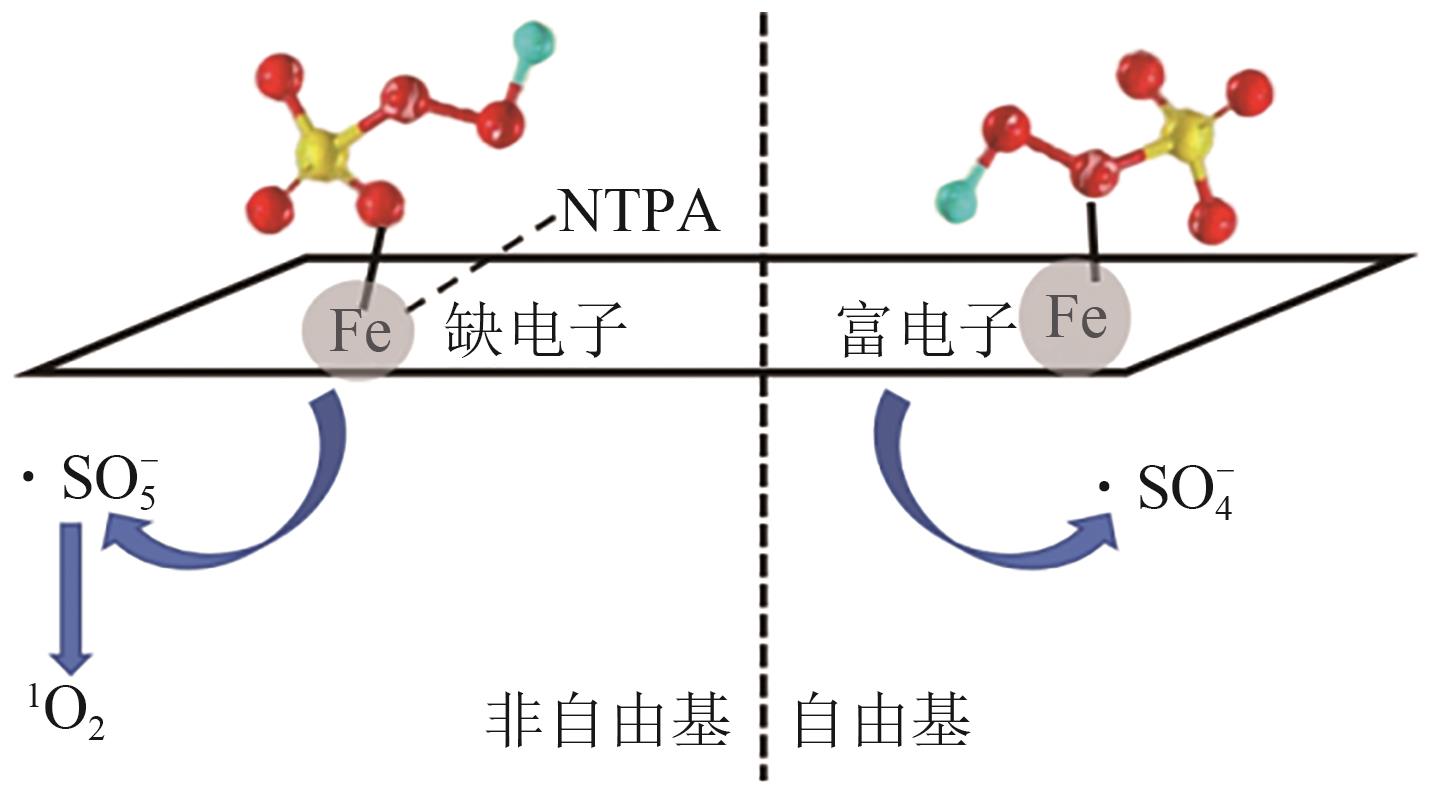
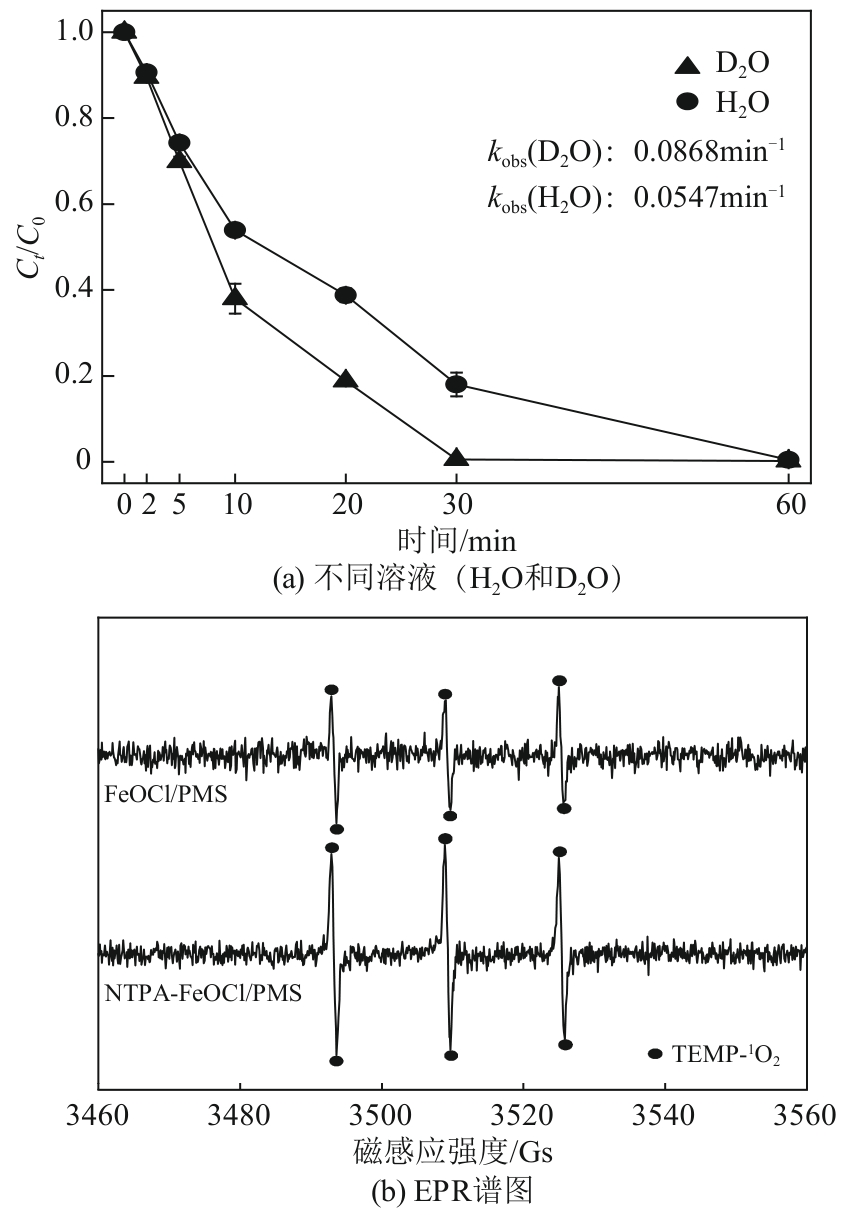
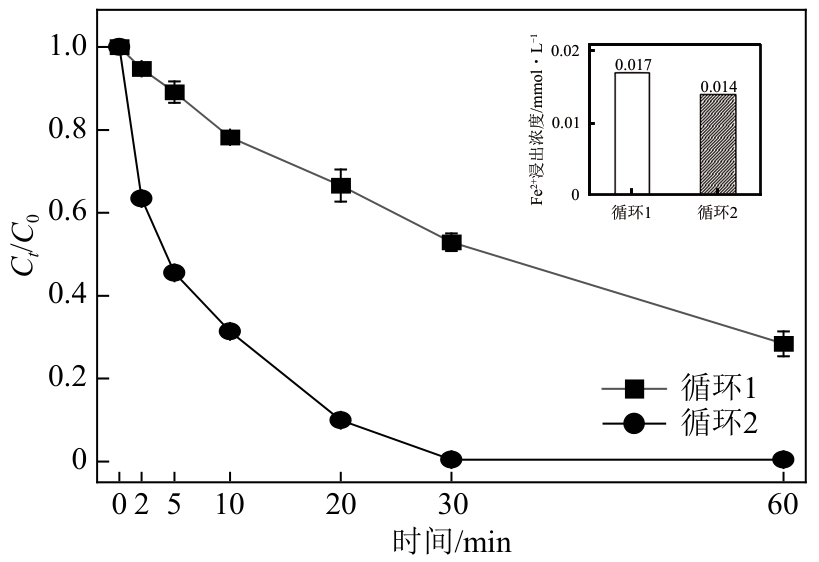
类芬顿反应中,铁基催化剂活性中心所在的化学环境对体系的反应机理具有深远的影响。基于该影响,本研究通过使用吸电子有机物硝基对苯二甲酸(NTPA)对二维层状铁基材料FeOCl表面进行修饰,制备出易分离回收的铁基催化剂(NTPA-FeOCl),利用SEM、XRD、XPS、FTIR表征分析其形貌和微观结构,通过活化过一硫酸盐(PMS)降解对乙酰氨基酚(ACT),来评价其催化性能。另外,通过控制实验变量和引入不同抗生素、内分泌干扰物、护理品类有机污染物,系统考察了NTPA-FeOCl活化PMS体系降解有机物的效能、特点。结果表明,在NTPA-FeOCl投加量0.2g/L,PMS浓度2mmol/L,以及初始pH=7.0的条件下,ACT(0.1mmol/L)可在30min内降解99.69%,且体系对不同有机物的降解表现出选择性。淬灭实验结果表明,体系内ACT的降解由自由基和非自由基共同完成,但单线氧是主导活性氧物种,因此进一步证明了体系对富电子型有机物的选择氧化性。NTPA修饰后,FeOCl表面部分Fe原子呈现缺电子状态,吸引PMS端头O原子发生反应,从而提高了体系内单线氧的生成量,强化了有机物降解的非自由基路径。
中图分类号:
引用本文
王御豪, 蒋沁利, 徐西蒙. 表面修饰FeOCl活化过硫酸盐引发有机污染物非自由基降解[J]. 化工进展, 2025, 44(6): 3101-3111.
WANG Yuhao, JIANG Qinli, XU Ximeng. Degradation of organic pollutants via non-radical pathway by surface modified FeOCl activating persulfate[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3101-3111.
| 污染物名称 | 体积分数/% | 检测波长/nm | 流速/mL·min-1 | 进样时间/min | |||
|---|---|---|---|---|---|---|---|
| 乙腈 | 甲醇 | 乙酸(1%)-水溶液 | 水 | ||||
| SMX | 40 | — | 60 | — | 264 | 1 | 5.5 |
| ACT | 15 | — | 85 | — | 254 | 1 | 7 |
| BPA | — | 75 | — | 25 | 225 | 1 | 6 |
| BPS | — | 55 | 45 | — | 258 | 0.8 | 5.5 |
| IBU | — | 80 | 20 | — | 220 | 1 | 7 |
| ATZ | — | 70 | — | 30 | 225 | 1 | 6 |
| CBZ | 60 | — | — | 40 | 286 | 1 | 5.5 |
| 2.4-DCP | 60 | — | 40 | — | 284 | 1 | 7 |
| PMSO | 28 | — | — | 72 | 215 | 1 | 8.5 |
| PMSO2 | 25 | — | — | 75 | 230 | 1 | 10 |
表1 各污染物高效液相色谱仪流动相参数
| 污染物名称 | 体积分数/% | 检测波长/nm | 流速/mL·min-1 | 进样时间/min | |||
|---|---|---|---|---|---|---|---|
| 乙腈 | 甲醇 | 乙酸(1%)-水溶液 | 水 | ||||
| SMX | 40 | — | 60 | — | 264 | 1 | 5.5 |
| ACT | 15 | — | 85 | — | 254 | 1 | 7 |
| BPA | — | 75 | — | 25 | 225 | 1 | 6 |
| BPS | — | 55 | 45 | — | 258 | 0.8 | 5.5 |
| IBU | — | 80 | 20 | — | 220 | 1 | 7 |
| ATZ | — | 70 | — | 30 | 225 | 1 | 6 |
| CBZ | 60 | — | — | 40 | 286 | 1 | 5.5 |
| 2.4-DCP | 60 | — | 40 | — | 284 | 1 | 7 |
| PMSO | 28 | — | — | 72 | 215 | 1 | 8.5 |
| PMSO2 | 25 | — | — | 75 | 230 | 1 | 10 |
| 单因素条件 | 变量 | kobs/min-1 | R2 |
|---|---|---|---|
| PMS投加浓度 | 0.5mmol/L | 0.0485 | 0.9932 |
| 1mmol/L | 0.0672 | 0.9929 | |
| 2mmol/L | 0.1457 | 0.9939 | |
| 4mmol/L | 0.2599 | 0.9763 | |
| 8mmol/L | 0.1852 | 0.9818 | |
| 初始pH | 3.0 | 0.1684 | 0.9938 |
| 5.0 | 0.1542 | 0.9943 | |
| 7.0 | 0.1457 | 0.9939 | |
| 8.0 | 0.0488 | 0.9998 | |
| 10.0 | 1.2322 | 0.9999 | |
| NTPA-FeOCl投加量 | 0.1g/L | 0.0451 | 0.9992 |
| 0.2g/L | 0.1457 | 0.9939 | |
| 0.3g/L | 0.1917 | 0.9776 | |
| 0.4g/L | 0.2882 | 0.9944 | |
| 0.5g/L | 0.4674 | 0.9698 |
表2 不同反应参数下NTPA-FeOCl/PMS体系降解ACT的拟一级动力学拟合参数
| 单因素条件 | 变量 | kobs/min-1 | R2 |
|---|---|---|---|
| PMS投加浓度 | 0.5mmol/L | 0.0485 | 0.9932 |
| 1mmol/L | 0.0672 | 0.9929 | |
| 2mmol/L | 0.1457 | 0.9939 | |
| 4mmol/L | 0.2599 | 0.9763 | |
| 8mmol/L | 0.1852 | 0.9818 | |
| 初始pH | 3.0 | 0.1684 | 0.9938 |
| 5.0 | 0.1542 | 0.9943 | |
| 7.0 | 0.1457 | 0.9939 | |
| 8.0 | 0.0488 | 0.9998 | |
| 10.0 | 1.2322 | 0.9999 | |
| NTPA-FeOCl投加量 | 0.1g/L | 0.0451 | 0.9992 |
| 0.2g/L | 0.1457 | 0.9939 | |
| 0.3g/L | 0.1917 | 0.9776 | |
| 0.4g/L | 0.2882 | 0.9944 | |
| 0.5g/L | 0.4674 | 0.9698 |
| [1] | WANG Jianlong, WANG Shizong. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| [2] | DUAN Xiaoguang, SUN Hongqi, SHAO Zongping, et al. Nonradical reactions in environmental remediation processes: Uncertainty and challenges[J]. Applied Catalysis B: Environmental, 2018, 224: 973-982. |
| [3] | LIANG Jun, DUAN Xiaoguang, XU Xiaoyun, et al. Persulfate oxidation of sulfamethoxazole by magnetic iron-char composites via nonradical pathways: Fe(Ⅳ) versus surface-mediated electron transfer[J]. Environmental Science & Technology, 2021, 55(14): 10077-10086. |
| [4] | LIU Lindong, LIU Qian, WANG Ying, et al. Nonradical activation of peroxydisulfate promoted by oxygen vacancy-laden NiO for catalytic phenol oxidative polymerization[J]. Applied Catalysis B: Environmental, 2019, 254: 166-173. |
| [5] | YAN Yiqi, WEI Zongsu, DUAN Xiaoguang, et al. Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes[J]. Environmental Science & Technology, 2023, 57(33): 12153-12179. |
| [6] | DOU Jibo, CHENG Jie, LU Zhijiang, et al. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process[J]. Applied Catalysis B: Environmental, 2022, 301: 120832. |
| [7] | WANG Jinling, HOU Kaipeng, WEN Yuzhen, et al. Interlayer structure manipulation of iron oxychloride by potassium cation intercalation to steer H2O2 activation pathway[J]. Journal of the American Chemical Society, 2022, 144(10): 4294-4299. |
| [8] | MA Yahui, WANG Dalin, XU Yin, et al. Nonradical electron transfer-based peroxydisulfate activation by a Mn-Fe bimetallic oxide derived from spent alkaline battery for the oxidation of bisphenol A[J]. Journal of Hazardous Materials, 2022, 436: 129172. |
| [9] | WU Que, ZHANG Yongqing, MENG Hong, et al. Cu/N co-doped biochar activating PMS for selective degrading paracetamol via a non-radical pathway dominated by singlet oxygen and electron transfer[J]. Chemosphere, 2024, 357: 141858. |
| [10] | YIN Yue, Ruolin LYU, ZHANG Weiming, et al. Exploring mechanisms of different active species formation in heterogeneous Fenton systems by regulating iron chemical environment[J]. Applied Catalysis B: Environmental, 2021, 295: 120282. |
| [11] | 王金岭, 温玉真, 汪华林, 等. FeOCl层状材料及其插层化合物: 结构、 性质与应用[J]. 化学进展, 2021, 33(2): 263-280. |
| WANG Jinling, WEN Yuzhen, WANG Hualin, et al. FeOCl and its intercalation compounds: Structures, properties and applications[J]. Progress in Chemistry, 2021, 33(2): 263-280. | |
| [12] | YANG Xuejing, XU Ximeng, XU Jing, et al. Iron oxychloride (FeOCl): An efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants[J]. Journal of the American Chemical Society, 2013, 135(43): 16058-16061. |
| [13] | XU Ximeng, ZHANG Shujing, WANG Yuhao, et al. 2D Surfaces twisted to enhance electron freedom toward efficient advanced oxidation processes[J]. Applied Catalysis B: Environment and Energy, 2024, 345: 123701. |
| [14] | 杨本谱. 铁(Ⅲ)-邻菲罗啉光化还原分光光度法测定铁[J]. 冶金分析, 1990, 10(4): 53-54. |
| YANG Benpu. Photoreduction spectrophotometric determination of iron (Ⅲ) with phenanthroline[J]. Metallurgical Analysis, 1990, 10(4): 53-54. | |
| [15] | BECHAMBI Olfa, JLAIEL Lobna, NAJJAR Wahiba, et al. Photocatalytic degradation of bisphenol A in the presence of Ce-ZnO: Evolution of kinetics, toxicity and photodegradation mechanism[J]. Materials Chemistry and Physics, 2016, 173: 95-105. |
| [16] | LI Yan, ZHANG Han, RASHID Azhar, et al. Bisphenol A attenuation in natural microcosm: Contribution of ecological components and identification of transformation pathways through stable isotope tracing[J]. Journal of Hazardous Materials, 2020, 385: 121584. |
| [17] | GAO Kaihua, CHEN Jitao, LIU Zhongmin, et al. Intensified redox co-conversion of As(Ⅲ) and Cr(Ⅵ) with MIL-125(Ti)-derived COOH functionalized TiO2: Performance and mechanism[J]. Chemical Engineering Journal, 2019, 360: 1223-1232. |
| [18] | ZHANG Xiaodong, YUE Ke, RAO Renzhi, et al. Synthesis of acidic MIL-125 from plastic waste: Significant contribution of N orbital for efficient photocatalytic degradation of chlorobenzene and toluene[J]. Applied Catalysis B: Environmental, 2022, 310: 121300. |
| [19] | 周宇辉, 林洋仟, 王御豪, 等. 磁性复合材料活化过硫酸盐去除水中双酚A[J]. 中国环境科学, 2024, 44(2): 832-840. |
| ZHOU Yuhui, LIN Yangqian, WANG Yuhao, et al. Removing bisphenol A with magnetic sandwich composite activated peroxymonosulfate[J]. China Environmental Science, 2024, 44(2): 832-840. | |
| [20] | XU Ximeng, ZONG Shaoyan, CHEN Weiming, et al. Comparative study of bisphenol A degradation via heterogeneously catalyzed H2O2 and persulfate: Reactivity, products, stability and mechanism[J]. Chemical Engineering Journal, 2019, 369: 470-479. |
| [21] | XU Ximeng, CHEN Weiming, ZONG Shaoyan, et al. Magnetic clay as catalyst applied to organics degradation in a combined adsorption and Fenton-like process[J]. Chemical Engineering Journal, 2019, 373: 140-149. |
| [22] | QI Chengdu, LIU Xitao, MA Jun, et al. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants[J]. Chemosphere, 2016, 151: 280-288. |
| [23] | LEI Yu, YU Yafei, LEI Xin, et al. Assessing the use of probes and quenchers for understanding the reactive species in advanced oxidation processes[J]. Environmental Science & Technology, 2023, 57(13): 5433-5444. |
| [24] | GARAVELLI Marco, BERNARDI Fernando, OLIVUCCI Massimo, et al. DFT study of the reactions between singlet-oxygen and a carotenoid model[J]. Journal of the American Chemical Society, 1998, 120(39): 10210-10222. |
| [25] | HUANG Bingkun, REN Xinyi, ZHAO Jian, et al. Modulating electronic structure engineering of atomically dispersed cobalt catalyst in Fenton-like reaction for efficient degradation of organic pollutants[J]. Environmental Science & Technology, 2023, 57(37): 14071-14081. |
| [26] | FANG Qianzhen, YANG Hailan, YE Shujing, et al. Generation and identification of 1O2 in catalysts/peroxymonosulfate systems for water purification[J]. Water Research, 2023, 245: 120614. |
| [27] | BI Guangyu, DING Rongrong, SONG Junsheng, et al. Discriminating the active Ru species towards the selective generation of singlet oxygen from peroxymonosulfate: Nanoparticles surpass single-atom catalysts[J]. Angewandte Chemie, 2024, 136(17): 2401551. |
| [28] | WANG Zhen, QIU Wei, PANG Suyan, et al. Further understanding the involvement of Fe(Ⅳ) in peroxydisulfate and peroxymonosulfate activation by Fe(Ⅱ) for oxidative water treatment[J]. Chemical Engineering Journal, 2019, 371: 842-847. |
| [29] | YAO Jiayi, WU Nannan, TANG Xiaosheng, et al. Methyl phenyl sulfoxide (PMSO) as a quenching agent for high-valent metal-oxo species in peroxymonosulfate based processes should be reconsidered[J]. Chemical Engineering Journal Advances, 2022, 12: 100378. |
| [30] | Wen-Da OH, DONG Zhili, Teik-Thye LIM. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects[J]. Applied Catalysis B: Environmental, 2016, 194: 169-201. |
| [31] | HU Jian, ZENG Xiangkang, WANG Gen, et al. Modulating mesoporous Co3O4 hollow nanospheres with oxygen vacancies for highly efficient peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 400: 125869. |
| [32] | YAO Yiyuan, WANG Chaohai, YAN Xin, et al. Rational regulation of Co-N-C coordination for high-efficiency generation of 1O2 toward nearly 100% selective degradation of organic pollutants[J]. Environmental Science & Technology, 2022, 56(12): 8833-8843. |
| [33] | WU Zelin, XIONG Zhaokun, LIU Wen, et al. Active center size-dependent Fenton-like chemistry for sustainable water decontamination[J]. Environmental Science & Technology, 2023, 57(50): 21416-21427. |
| [1] | 杨群, 李红艳, 张峰, 毛立波, 崔佳丽, 董颖虹, 郭紫瑞. 钴氮共掺杂废菌棒生物炭活化PMS去除水中加替沙星[J]. 化工进展, 2025, 44(2): 1088-1099. |
| [2] | 张政, 刘琳, 李子晨, 王梦琦, 黄春燕, 葛圆圆. 载铜地质聚合物微球的制备及其催化降解双酚S的性能[J]. 化工进展, 2024, 43(9): 5290-5301. |
| [3] | 李亚男, 郭凯, 王嘉琪, 武亚宁. 煤气化渣活化过二硫酸盐和过一硫酸盐降解苯酚的比较[J]. 化工进展, 2024, 43(6): 3503-3512. |
| [4] | 曾湘楚, 宁海潮, 武哲, 韦瑞松, 银秀菊. 耦合均相-异相类芬顿体系强化水体耐药伤寒沙门氏菌的杀灭[J]. 化工进展, 2024, 43(12): 7078-7094. |
| [5] | 孙鹏, 金子涵, 张连科. 向日葵秸秆基炭材料活化过一硫酸盐降解土壤中苯胺[J]. 化工进展, 2024, 43(11): 6493-6503. |
| [6] | 马超, 孙志华, 王蕾, 姬钰, 陈翠忠, 王健康, 赵纯. 电/高锰酸钾/过一硫酸盐体系降解活性黄K-RN及其机理[J]. 化工进展, 2024, 43(10): 5958-5968. |
| [7] | 季青豪, 范婷婷, 王春梅. MIL-100(FeⅡ/FeⅢ)/ACF复合材料的制备及光催化脱色性能[J]. 化工进展, 2024, 43(10): 5913-5921. |
| [8] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [9] | 潘杰, 王明新, 高生旺, 夏训峰, 韩雪. 氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑[J]. 化工进展, 2022, 41(8): 4204-4212. 唑[J]. 化工进展, 2022, 41(8): 4204-4212. |
| [10] | 何畅帆, 赵小航, 章雪莹, 何林, 隋红, 李鑫钢. 过一硫酸盐-高铁酸盐-FeS体系土柱淋洗修复邻二氯苯污染土壤[J]. 化工进展, 2022, 41(5): 2743-2752. |
| [11] | 许泽涛, 曹怡婷, 王俏, 王志红. 固相钴基催化剂活化过一硫酸盐在水处理中的研究进展[J]. 化工进展, 2022, 41(2): 730-739. |
| [12] | 吕朋, 何畅帆, 何林, 李鑫钢, 隋红. 含重质油污泥非均相氧化降解特性及其强化机制[J]. 化工进展, 2022, 41(11): 6149-6157. |
| [13] | 薛雨微, 叶校圳, 曾静, 王永全, 洪俊明. 纳米片层铁锰双金属催化剂活化过[J]. 化工进展, 2022, 41(10): 5661-5668. |
| [14] | 徐劼, 高仕谦, 夏晶, 张珂, 邵子纯, 王澜静, 田永静. Sr掺杂强化LaCo0.5Cu0.5O3型钙钛矿活化过一硫酸盐的性能[J]. 化工进展, 2020, 39(9): 3525-3534. |
| [15] | 孙鹏, 张凯凯, 张玉, 张延荣. 生物炭/过一硫酸盐体系同时去除Cu2+和对硝基苯胺[J]. 化工进展, 2020, 39(10): 4268-4274. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||