化工进展 ›› 2024, Vol. 43 ›› Issue (10): 5958-5968.DOI: 10.16085/j.issn.1000-6613.2023-1697
• 资源与环境化工 • 上一篇
电/高锰酸钾/过一硫酸盐体系降解活性黄K-RN及其机理
马超1,2( ), 孙志华1(
), 孙志华1( ), 王蕾1(
), 王蕾1( ), 姬钰3, 陈翠忠1, 王健康1, 赵纯1,2
), 姬钰3, 陈翠忠1, 王健康1, 赵纯1,2
- 1.石河子大学水利建筑工程学院,新疆 石河子 832003
2.重庆大学环境与生态学院,重庆 400045
3.哈尔滨工程大学航天与建筑工程学院,黑龙江 哈尔滨 150001
-
收稿日期:2023-09-25修回日期:2024-01-05出版日期:2024-10-15发布日期:2024-10-29 -
通讯作者:孙志华,王蕾 -
作者简介:马超(1999—),男,硕士研究生,研究方向为高级氧化技术。E-mail:641345455@qq.cn。 -
基金资助:石河子大学高层次人才科研启动项目(RCZK202314);国家自然科学基金(22076015);兵团区域创新引导计划(2022BB007)
Degradation of reactive yellow K-RN by electricity/potassium permanganate/peroxymonosulfate system and its mechanism
MA Chao1,2( ), SUN Zhihua1(
), SUN Zhihua1( ), WANG Lei1(
), WANG Lei1( ), JI Yu3, CHEN Cuizhong1, WANG Jiankang1, ZHAO Chun1,2
), JI Yu3, CHEN Cuizhong1, WANG Jiankang1, ZHAO Chun1,2
- 1.College of Water Conservancy & Architectural Engineering, Shihezi University, Shihezi 832003, Xinjiang, China
2.College of Environment and Ecology, Chongqing University, Chongqing 400045, China
3.College of Aerospace and Civil Engineering, Harbin Engineering University,Harbin 150001, Heilongjiang, China
-
Received:2023-09-25Revised:2024-01-05Online:2024-10-15Published:2024-10-29 -
Contact:SUN Zhihua, WANG Lei
摘要:
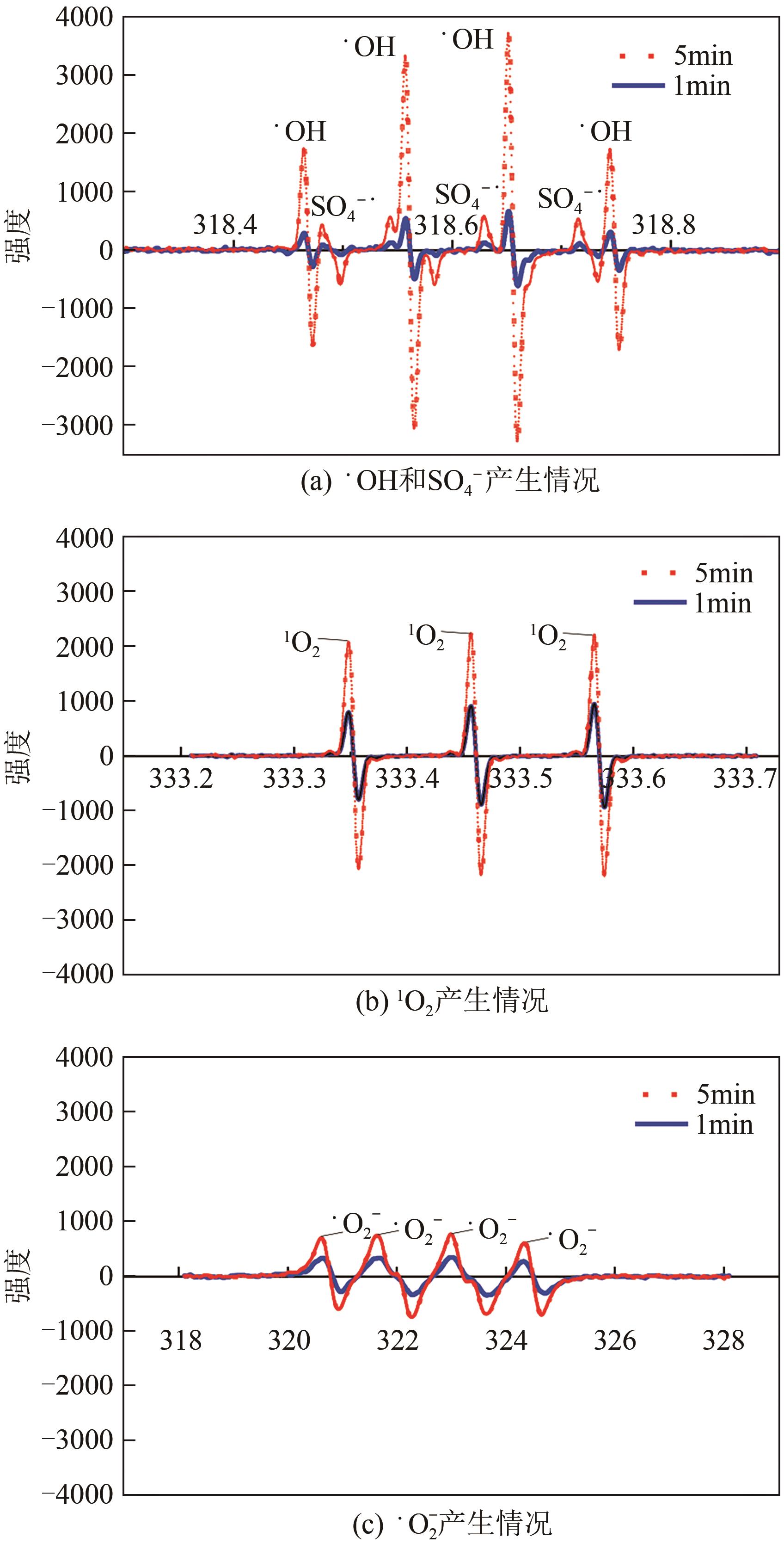
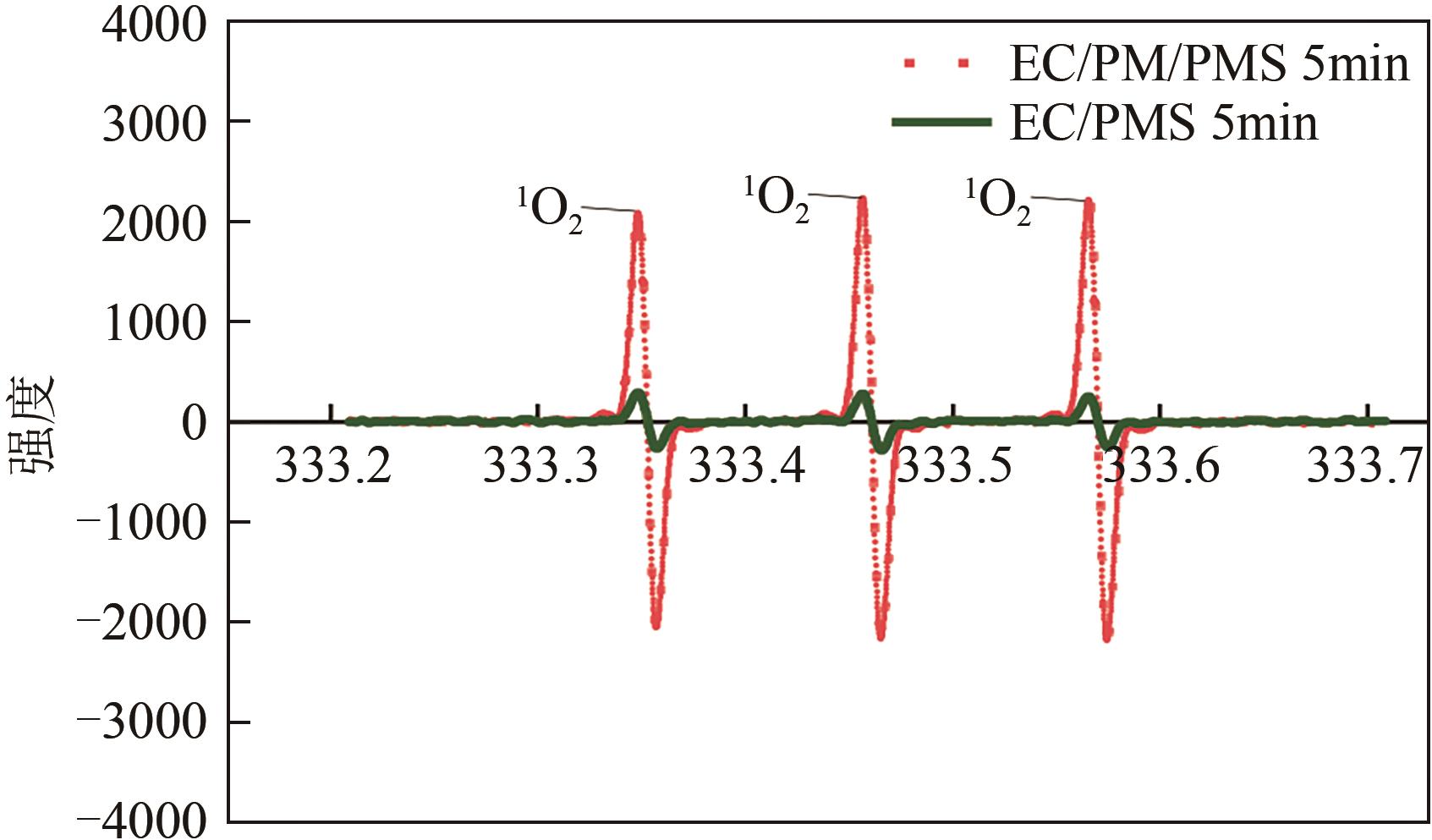
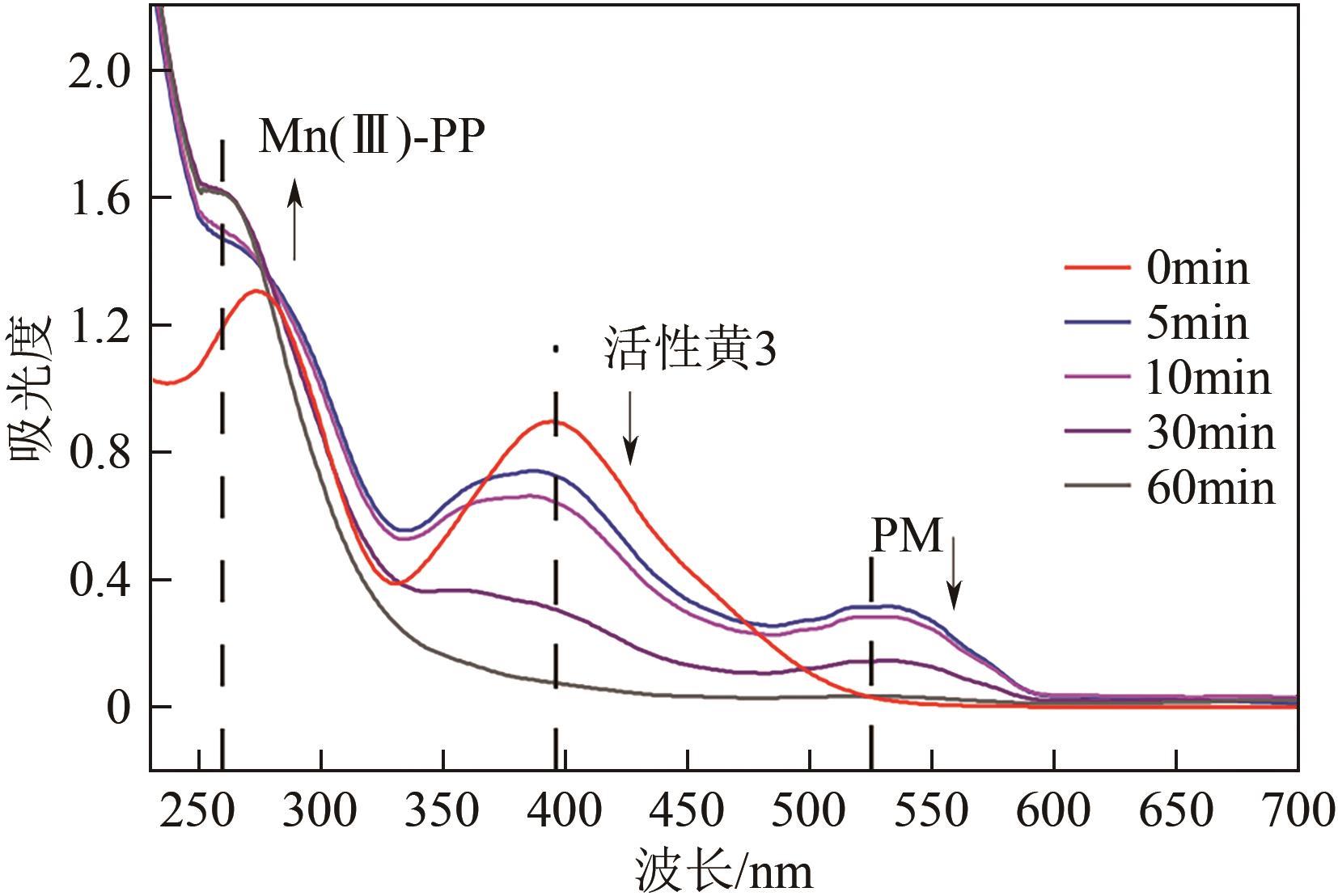
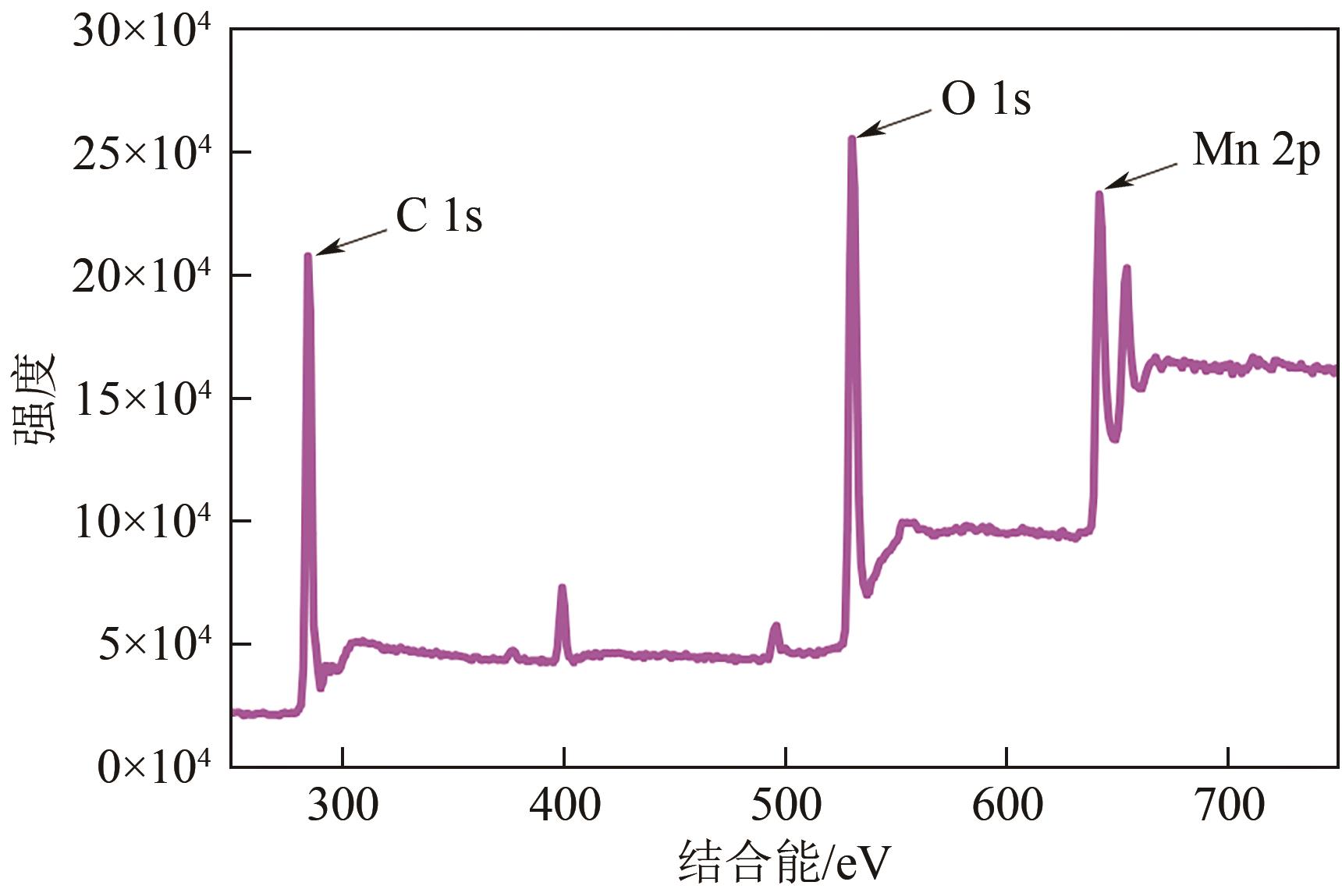
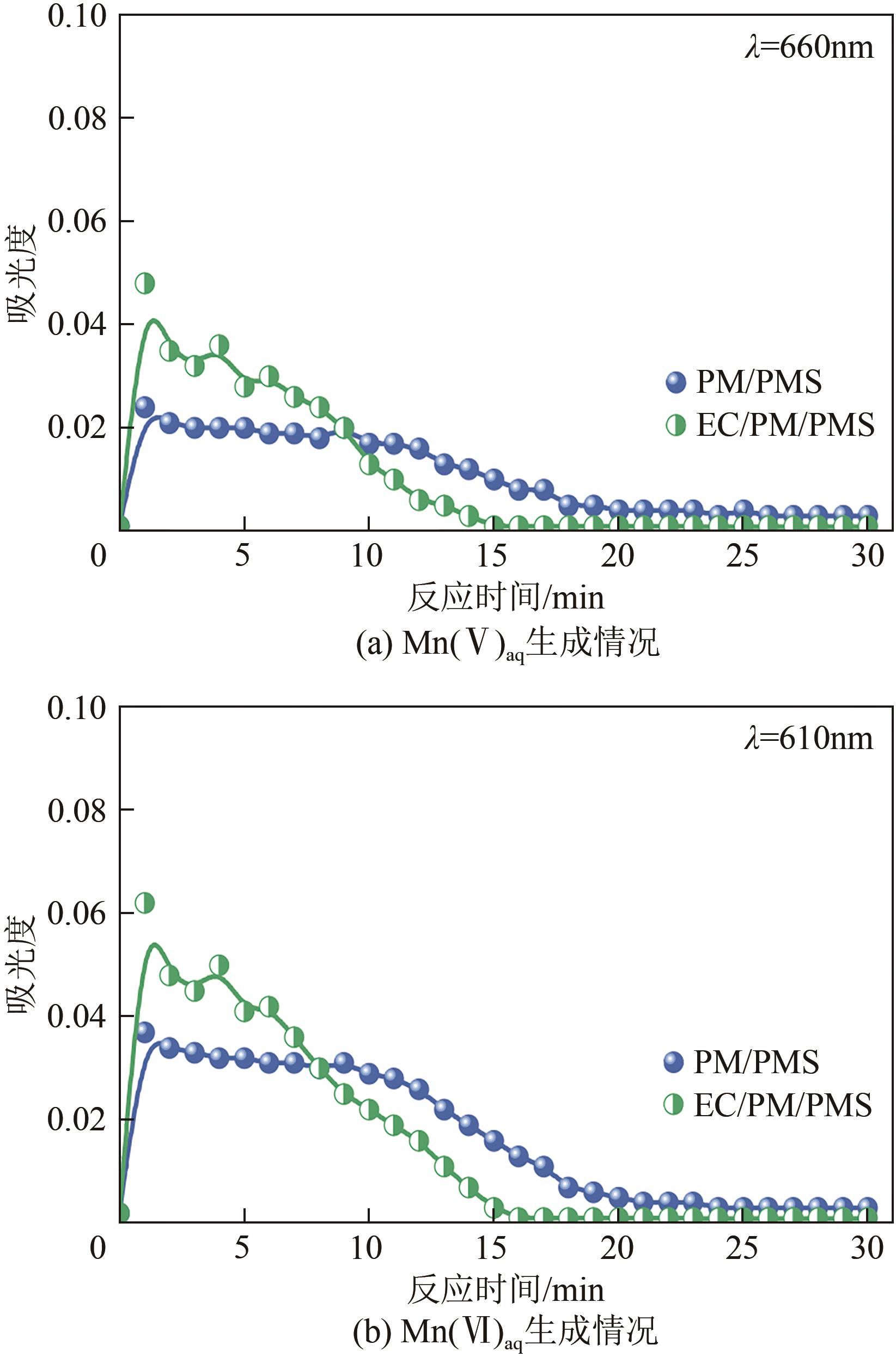
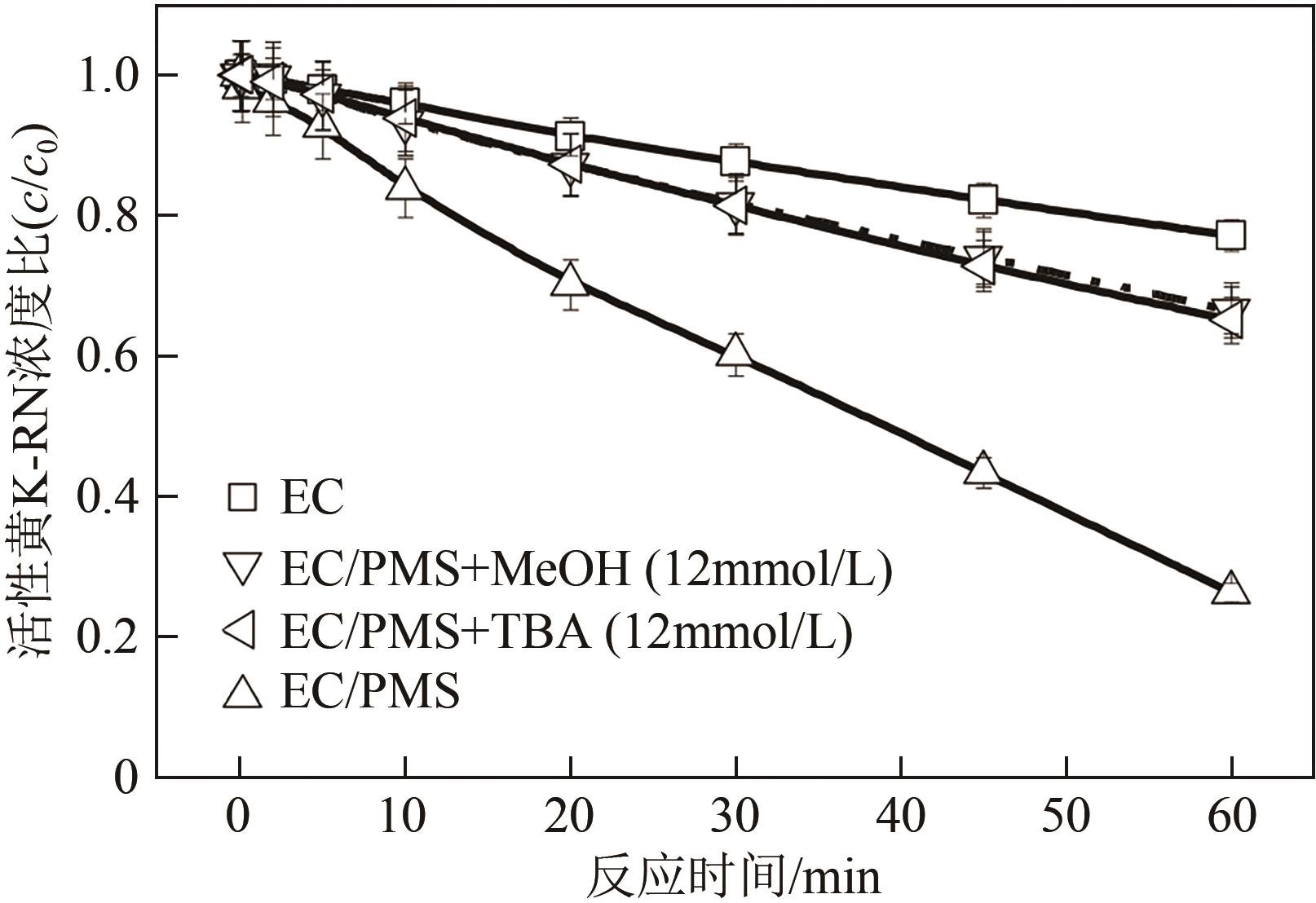
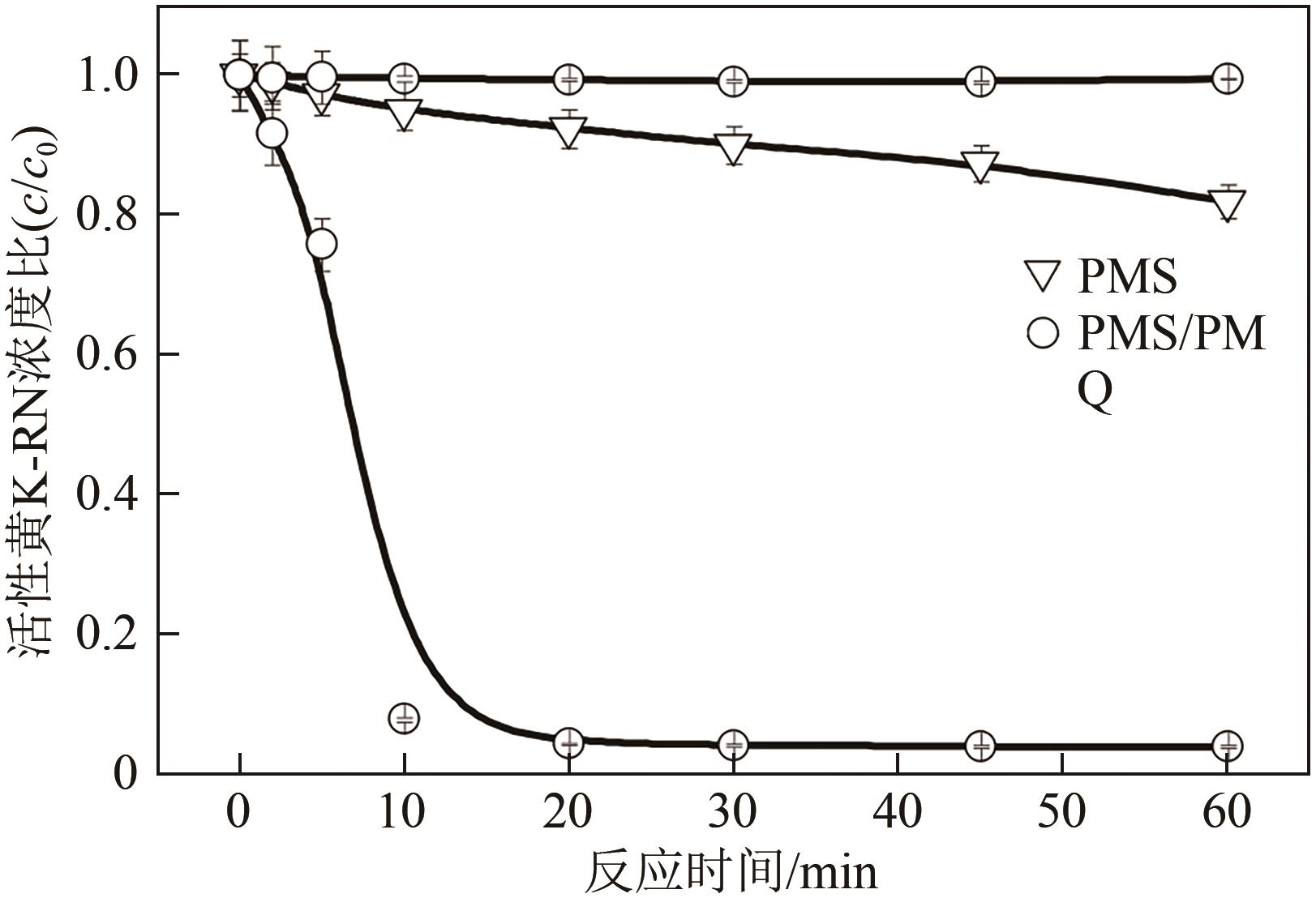
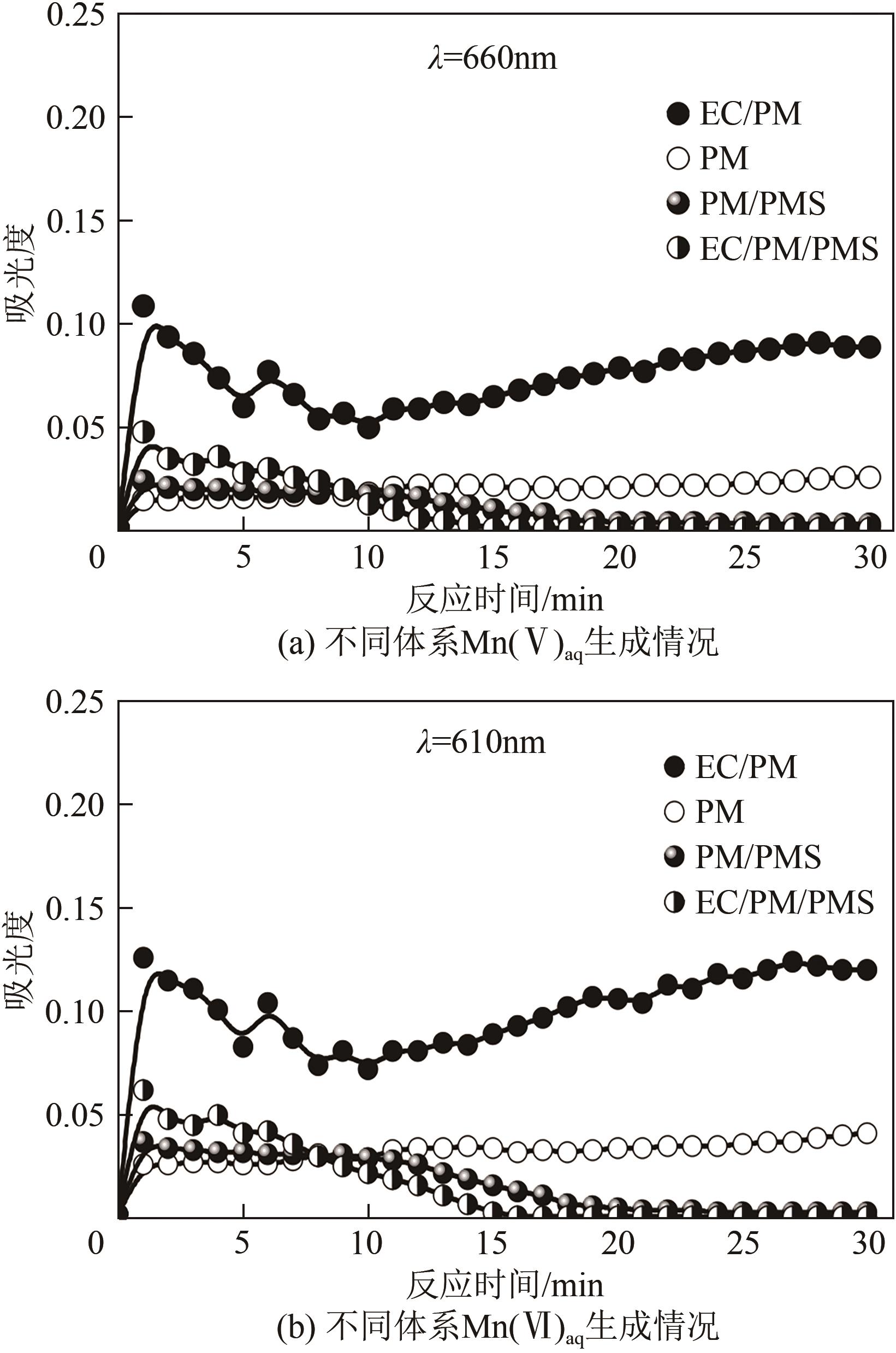
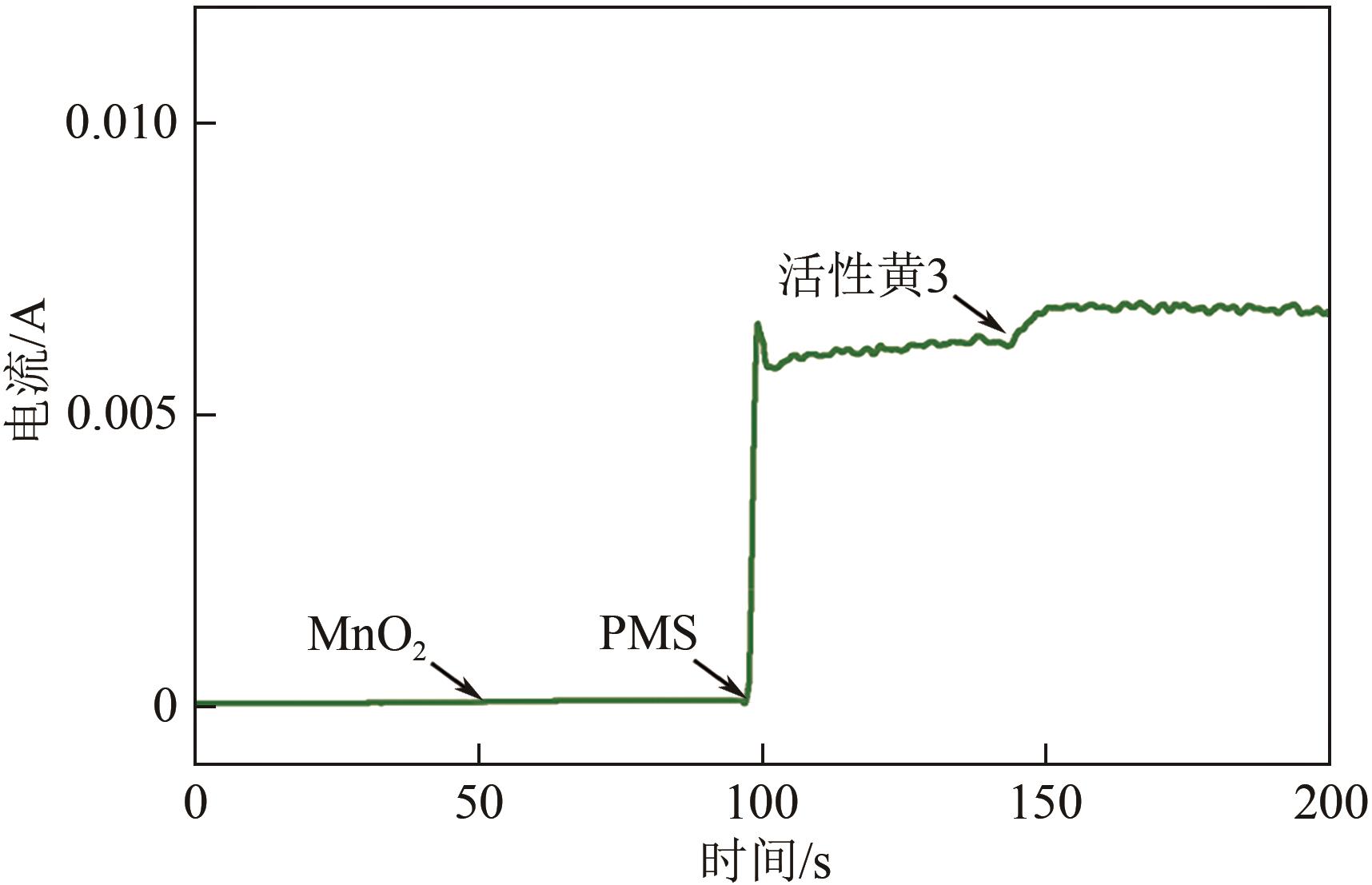
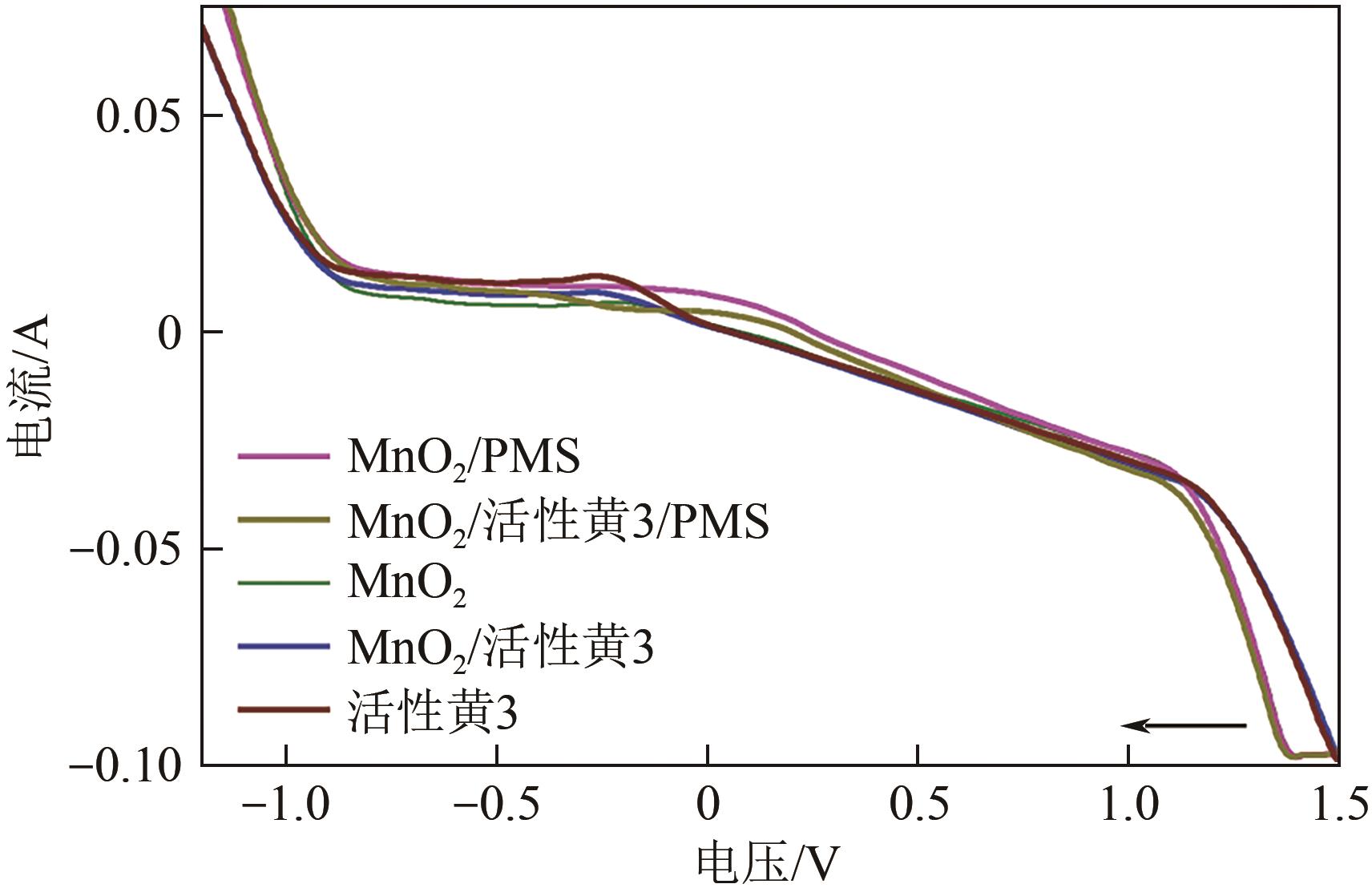
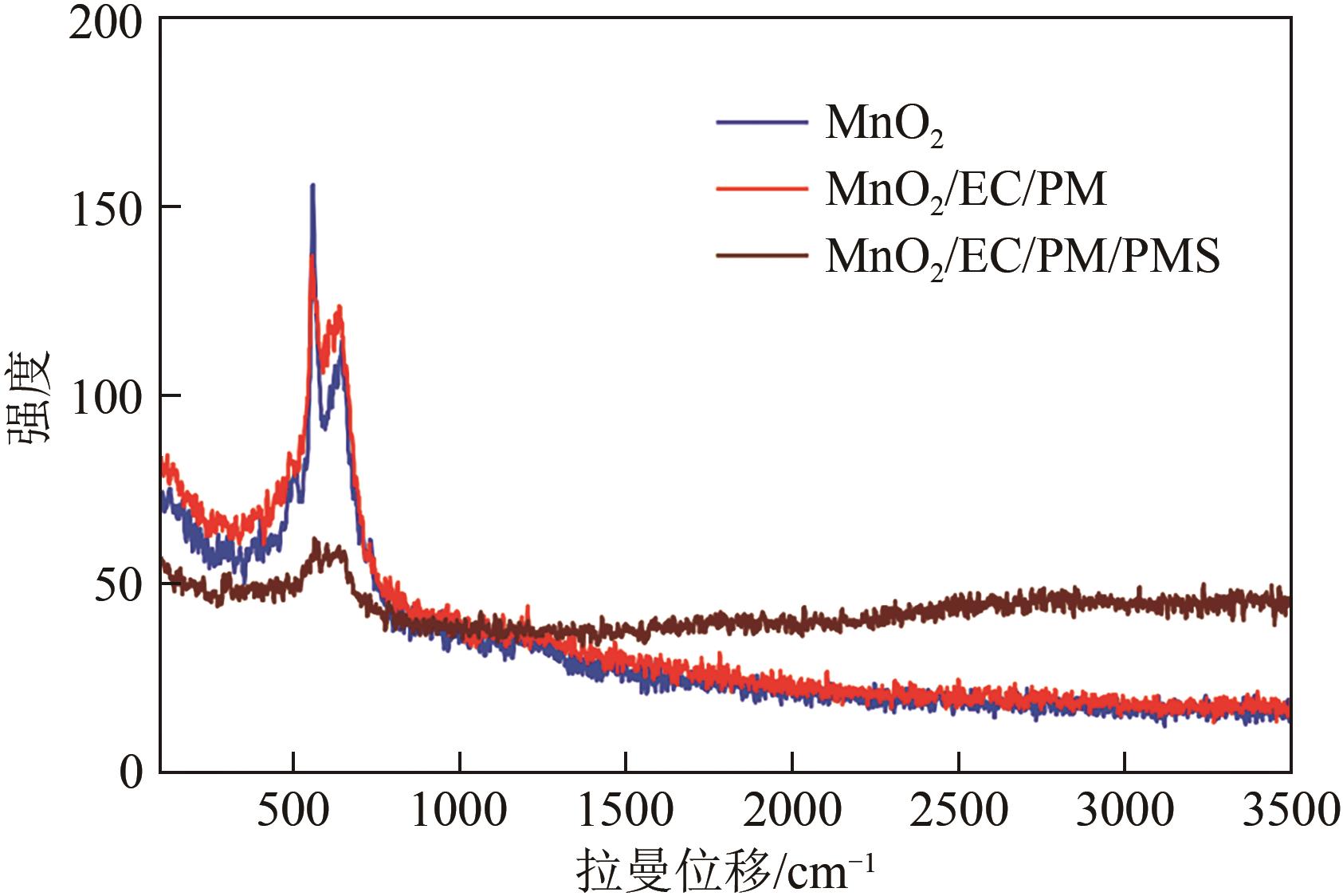
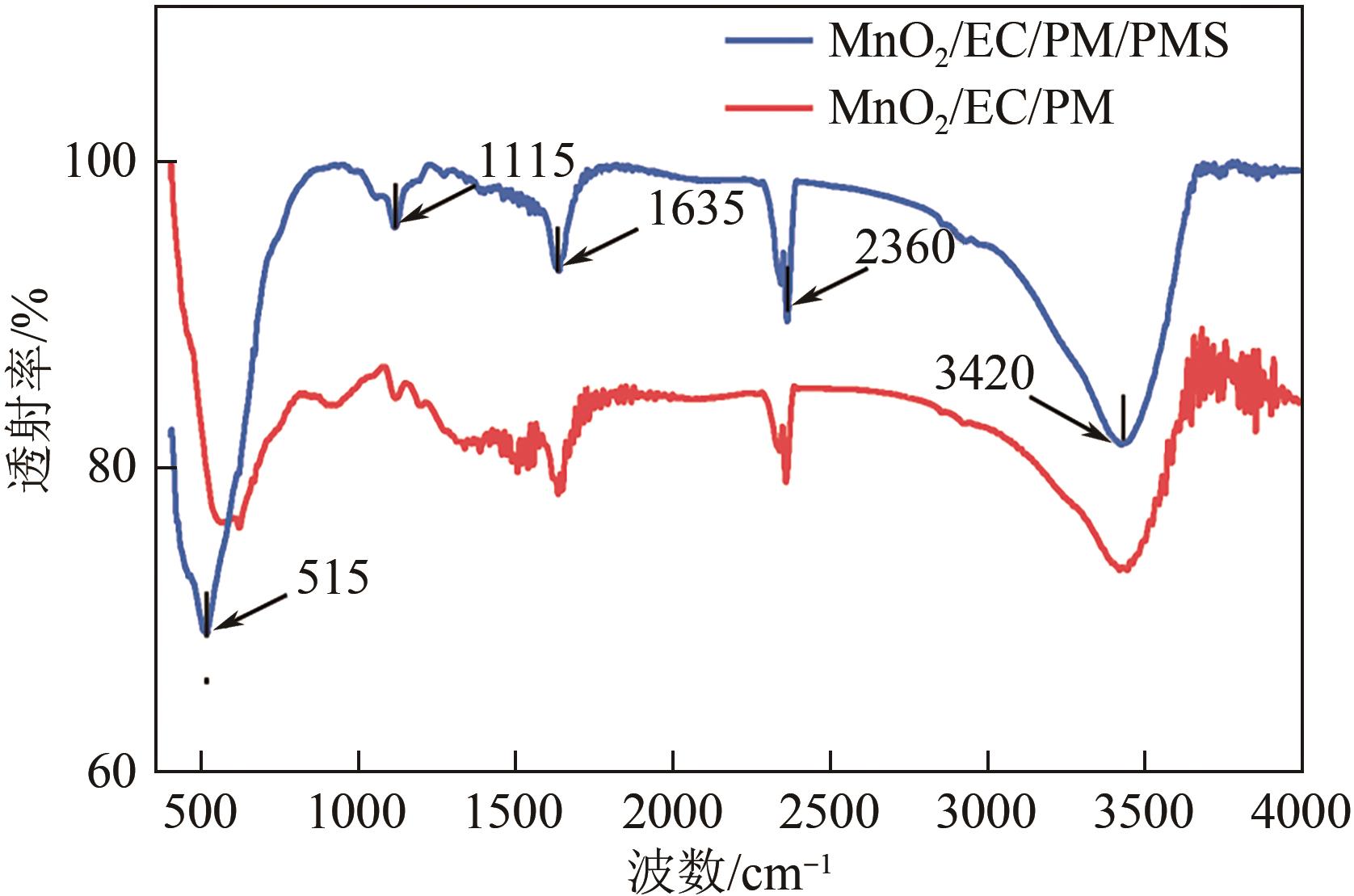
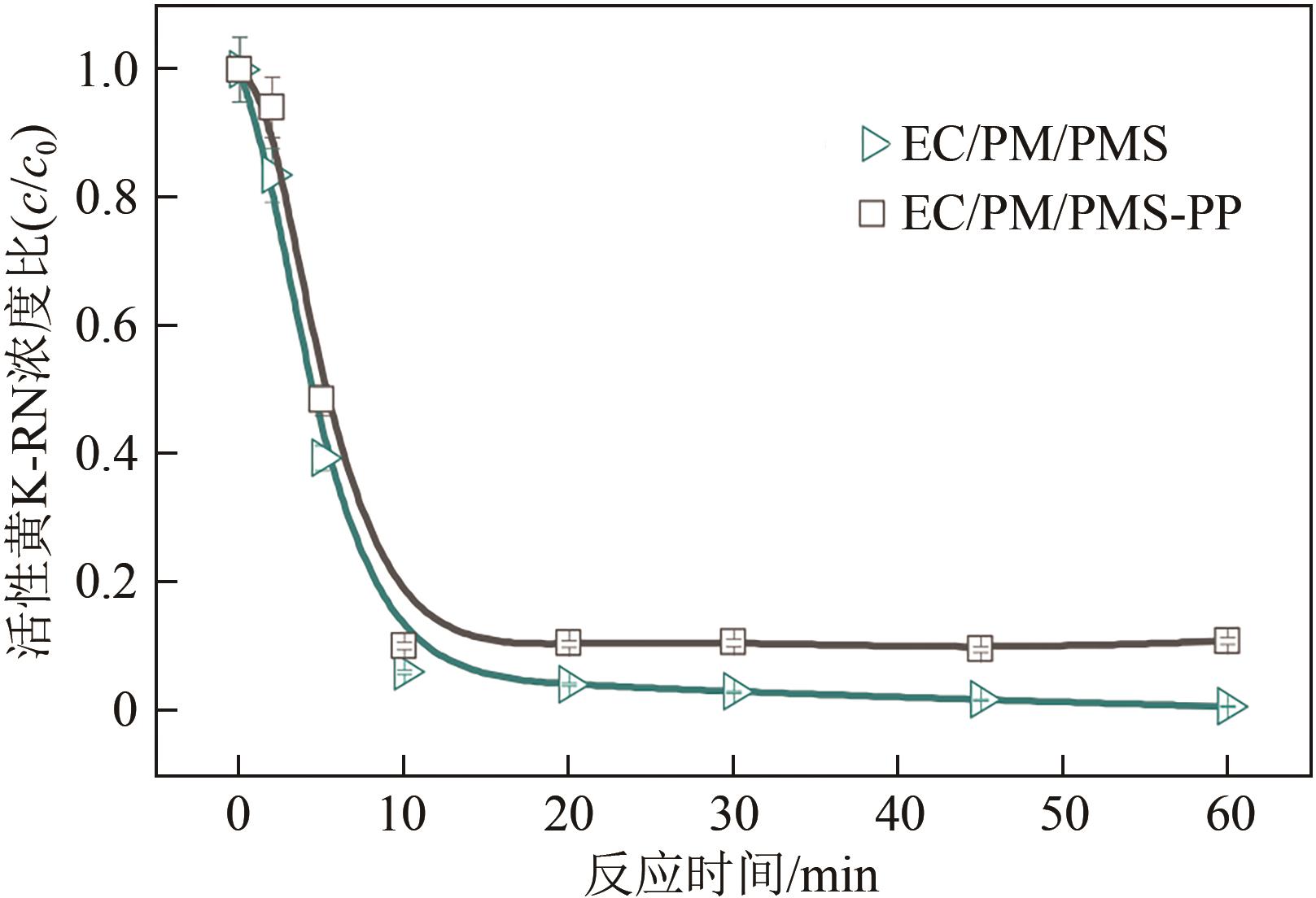
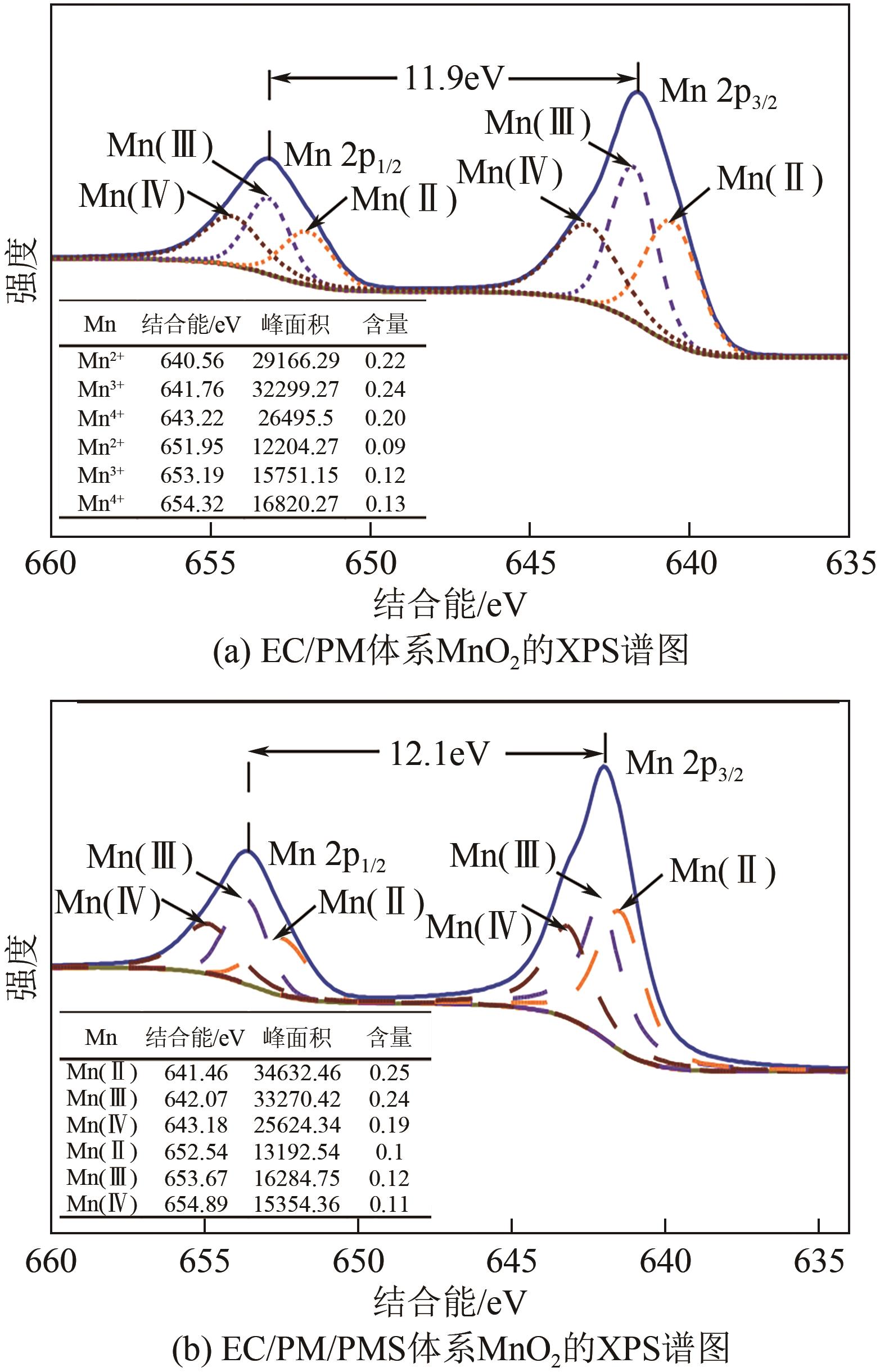
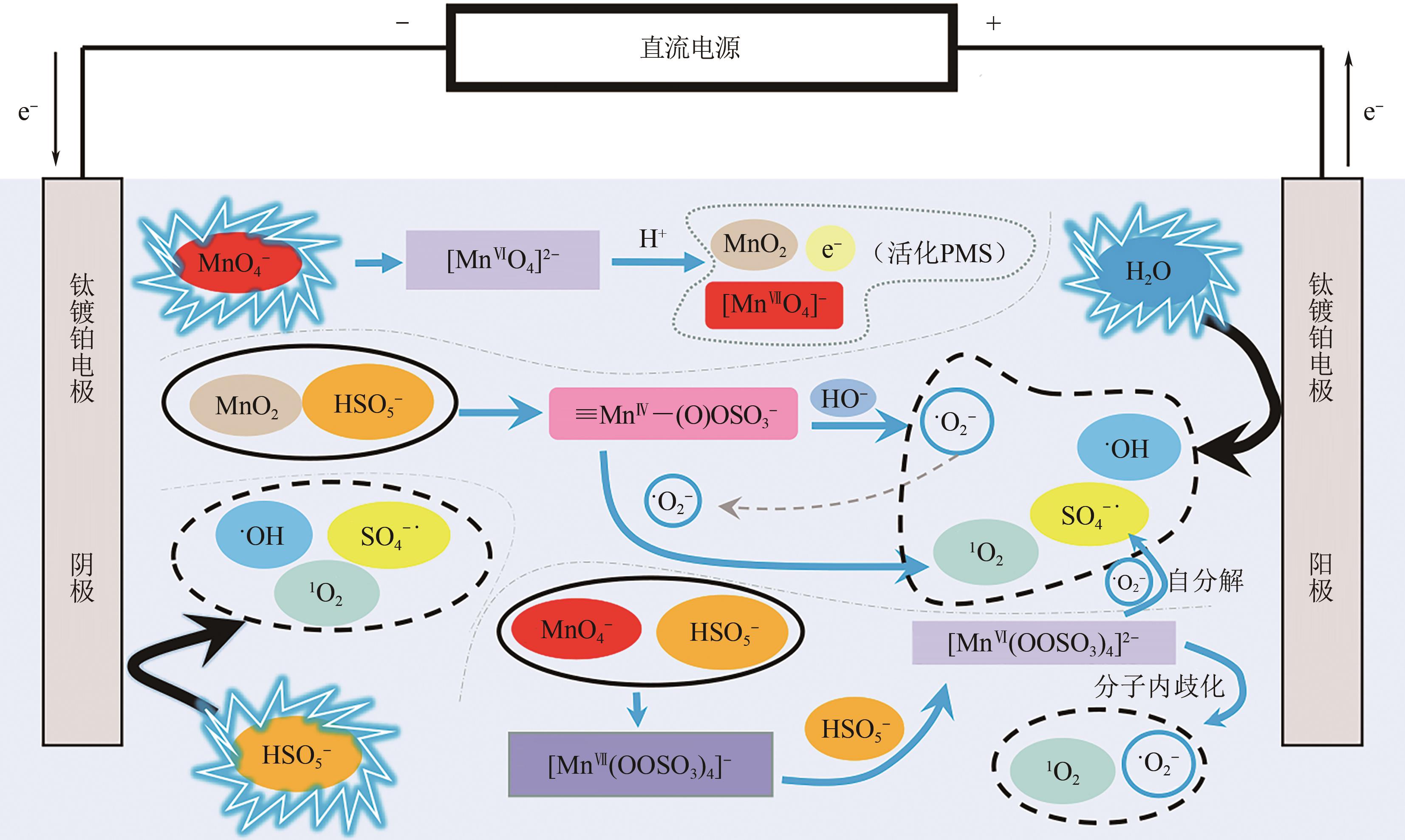
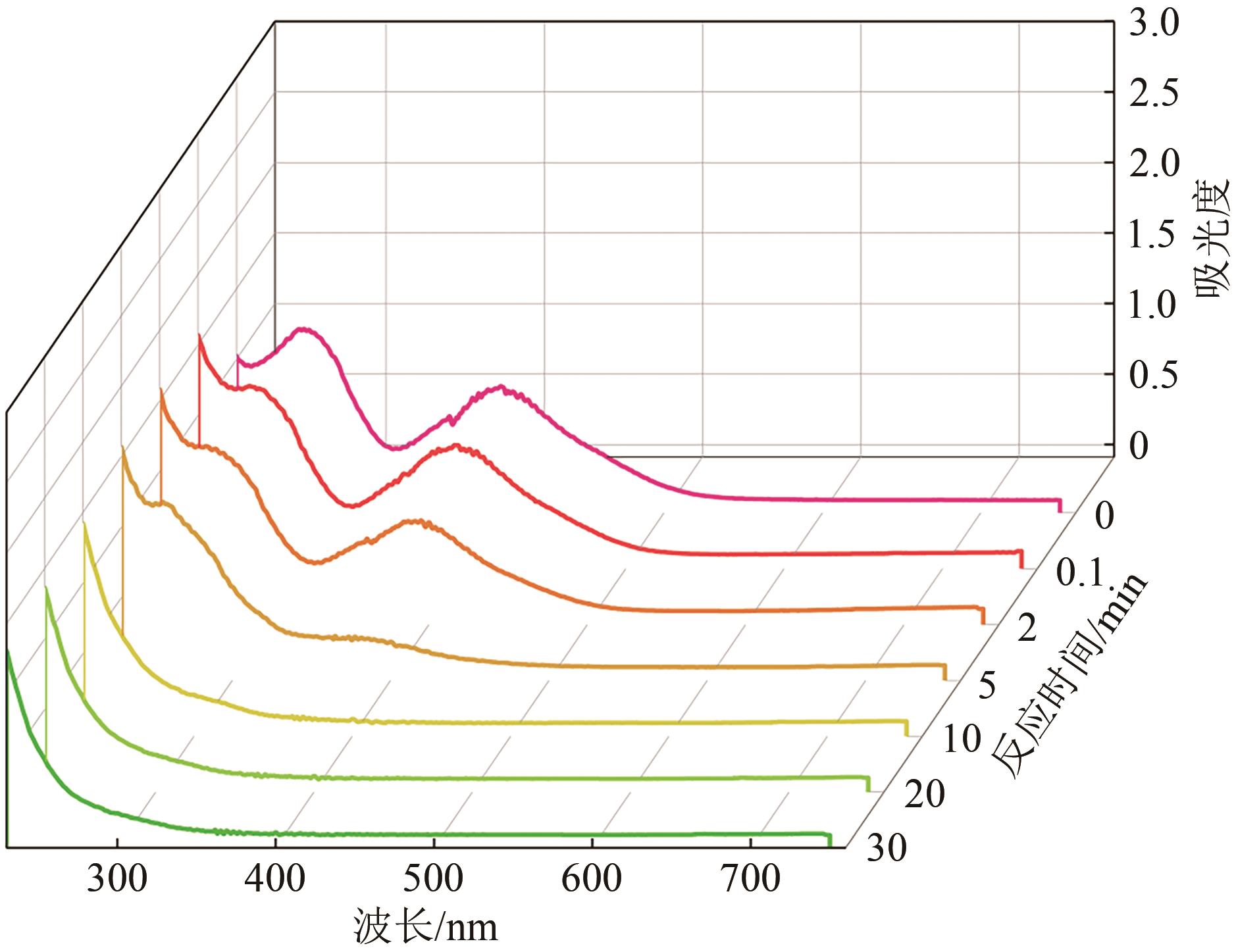
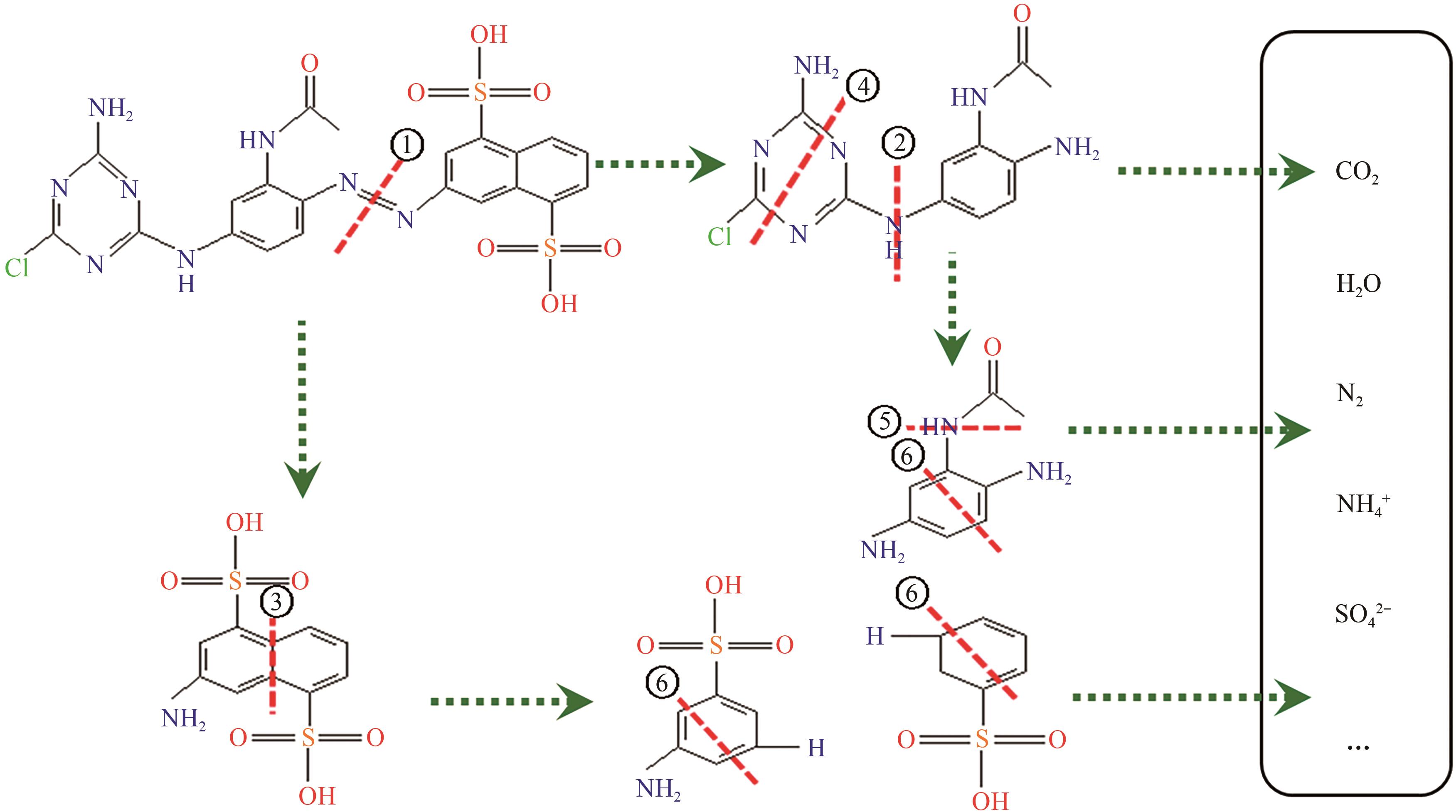
印染废水中的有机染料处理在水处理技术中长久以来都是一大难题,电/高锰酸钾/过一硫酸盐体系(EC/PM/PMS体系)可以高效地降解活性黄K-RN等难降解有机污染物,但其降解机制还尚未明确,且锰类物质能否活化PMS产生活性物质以及PMS活化机理还需进一步探究。因此,本文研究了EC/PM/PMS体系在降解印染废水时活性物质的类别和产生途径,同时确定了锰类物质在其中的作用机理和PMS的活化过程。结果表明,EC/PM/PMS体系是一种包含自由基氧化和非自由基氧化的复合体系,体系中由PM还原生成五价锰、六价锰和无定形二氧化锰活化PMS。此外,明确了活性氧类自由基氧化是活性黄K-RN降解的主要路径,占比为95.3%,电极直接氧化占比2.1%,非自由基氧化占比仅为2.6%。
中图分类号:
引用本文
马超, 孙志华, 王蕾, 姬钰, 陈翠忠, 王健康, 赵纯. 电/高锰酸钾/过一硫酸盐体系降解活性黄K-RN及其机理[J]. 化工进展, 2024, 43(10): 5958-5968.
MA Chao, SUN Zhihua, WANG Lei, JI Yu, CHEN Cuizhong, WANG Jiankang, ZHAO Chun. Degradation of reactive yellow K-RN by electricity/potassium permanganate/peroxymonosulfate system and its mechanism[J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5958-5968.
| 体系 | 5min去除率/% | 120min矿化率/% |
|---|---|---|
| EC | 2.06 | 1.7 |
| PM | 0.23 | 0.47 |
| PMS | 1.83 | 3.60 |
| EC/PM | 4.57 | 6.69 |
| EC/PMS | 5.64 | 4.54 |
| PM/PMS | 24.18 | 12.41 |
| EC/PM/PMS | 60.53 | 47.96 |
表1 各体系对活性黄K-RN的5min降解率和120min矿化率
| 体系 | 5min去除率/% | 120min矿化率/% |
|---|---|---|
| EC | 2.06 | 1.7 |
| PM | 0.23 | 0.47 |
| PMS | 1.83 | 3.60 |
| EC/PM | 4.57 | 6.69 |
| EC/PMS | 5.64 | 4.54 |
| PM/PMS | 24.18 | 12.41 |
| EC/PM/PMS | 60.53 | 47.96 |
| 捕获剂 | 反应速率常数/L2·mol-1·s-1 | ||||
|---|---|---|---|---|---|
| •OH | SO4-• | 1O2 | •O2- | PM | |
| MeOH | 9.7×108 | 2.5×107 | 3.0×103 | 1.1×107 | — |
| TBA | 6.0×108 | 7.6×105 | 1.8×103 | <103 | — |
表2 捕获剂与活性物质的反应速率常数
| 捕获剂 | 反应速率常数/L2·mol-1·s-1 | ||||
|---|---|---|---|---|---|
| •OH | SO4-• | 1O2 | •O2- | PM | |
| MeOH | 9.7×108 | 2.5×107 | 3.0×103 | 1.1×107 | — |
| TBA | 6.0×108 | 7.6×105 | 1.8×103 | <103 | — |
| 反应体系 | 降解路径 | kobs/10-3min-1 | 占比/% |
|---|---|---|---|
| EC | — | 5.30 | — |
| EC(MeOH) | 甲 | 5.30 | 2.1 |
| — | 乙 | — | 95.3 |
| — | 丙 | — | 2.6 |
| EC/PM/PMS(MeOH) | 甲+丙 | 11.84 | 4.8 |
| EC/PM/PMS | 甲+乙+丙 | 249.52 | 100 |
表3 不同体系的降解路径及占比
| 反应体系 | 降解路径 | kobs/10-3min-1 | 占比/% |
|---|---|---|---|
| EC | — | 5.30 | — |
| EC(MeOH) | 甲 | 5.30 | 2.1 |
| — | 乙 | — | 95.3 |
| — | 丙 | — | 2.6 |
| EC/PM/PMS(MeOH) | 甲+丙 | 11.84 | 4.8 |
| EC/PM/PMS | 甲+乙+丙 | 249.52 | 100 |
| 1 | LIU Haizhou, BRUTON Thomas A, LI Wei, et al. Oxidation of benzene by persulfate in the presence of Fe(Ⅲ)- and Mn(Ⅳ)-containing oxides: Stoichiometric efficiency and transformation products[J]. Environmental Science & Technology, 2016, 50(2): 890-898. |
| 2 | MA Zhifei, YANG Yu, JIANG Yonghai, et al. Enhanced degradation of 2,4-dinitrotoluene in groundwater by persulfate activated using iron-carbon micro-electrolysis[J]. Chemical Engineering Journal, 2017, 311: 183-190. |
| 3 | QIAN Yajie, GUO Xin, ZHANG Yalei, et al. Perfluorooctanoic acid degradation using UV-persulfate process: Modeling of the degradation and chlorate formation[J]. Environmental Science & Technology, 2016, 50(2): 772-781. |
| 4 | SINGH Nirmal, LEE Donald G. Permanganate: A green and versatile industrial oxidant[J]. Organic Process Research & Development, 2001, 5(6): 599-603. |
| 5 | LI Jing, LIN Heng, ZHU Kangmeng, et al. Degradation of Acid Orange 7 using peroxymonosulfate catalyzed by granulated activated carbon and enhanced by electrolysis[J]. Chemosphere, 2017, 188: 139-147. |
| 6 | 朱云华. 电化学活化高锰酸钾产生Mn(Ⅲ)去除水中双氯芬酸的效能与机理研究[D]. 重庆: 重庆大学, 2019. |
| ZHU Yunhua. Kinetics and mechanisms on the degradation of diclofenac from aqueous solution by Mn(Ⅲ) generated in electrochemical activation of permanganate[D]. Chongqing: Chongqing University, 2019. | |
| 7 | 闵一珏. ZE-1号印染废水脱色菌群的选育及适用条件的研究[J]. 环境污染与防治, 1992, 14(6): 14-16. |
| MIN Yijue. Breeding and application conditions of decolorizing bacteria in ZE-1 printing and dyeing wastewater[J]. Environmental Pollution & Control, 1992, 14(6): 14-16. | |
| 8 | 饶丹丹, 孙波, 乔俊莲, 等. 三价锰的性质、产生及环境意义[J]. 化学进展, 2017, 29(9): 1142-1153. |
| RAO Dandan, SUN Bo, QIAO Junlian, et al. The properties, generation and environmental significance of Mn(Ⅲ)[J]. Progress in Chemistry, 2017, 29(9): 1142-1153. | |
| 9 | WEBB Samuel M, DICK Gregory J, BARGAR John R, et al. Evidence for the presence of Mn(Ⅲ) intermediates in the bacterial oxidation of Mn(Ⅱ)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(15): 5558-5563. |
| 10 | 任灏. 以三价状态的锰进行锰的测定[J]. 化学世界, 1965, 6(2): 79-80. |
| REN Hao. Determination of manganese by trivalent manganese[J]. Chemical World, 1965, 6(2): 79-80. | |
| 11 | HASSAN E M, BELAL F. Kinetic spectrophotometric determination of nizatidine and ranitidine in pharmaceutical preparations[J]. Journal of Pharmaceutical and Biomedical Analysis, 2002, 27(1/2): 31-38. |
| 12 | SIMANDI Laszlo I, JAKY Miklos, SCHELLY Z A. Short-lived manganate (Ⅵ) and manganate (Ⅴ) intermediates in the permanganate oxidation of sulfite ion[J]. Journal of the American Chemical Society, 1984, 106(22): 6866-6867. |
| 13 | Sanja PAPIĆ, Dinko VUJEVIĆ, KOPRIVANAC Natalija, et al. Decolourization and mineralization of commercial reactive dyes by using homogeneous and heterogeneous Fenton and UV/Fenton processes[J]. Journal of Hazardous Materials, 2009, 164(2/3): 1137-1145. |
| 14 | 唐孟姣, 薛秀玲, 赖小丽. Al0-O2体系降解活性黄3RS溶液[J]. 环境化学, 2015, 34(7): 1350-1355. |
| TANG Mengjiao, XUE Xiuling, LAI Xiaoli. Degradation of reactive yellow 3RS by the Al0-O2 system[J]. Environmental Chemistry, 2015, 34(7): 1350-1355. | |
| 15 | 吴永杭, 胡方明, 张珊, 等. 商用TiO2催化臭氧降解活性黄3RE的研究[J]. 广东化工, 2022, 49(15): 125-127. |
| WU Yonghang, HU Fangming, ZHANG Shan, et al. Catalytic ozonation of aqueous reactive yellow 3RE with commercial TiO2 [J]. Guangdong Chemical Industry, 2022, 49(15): 125-127. | |
| 16 | 朱驯, 项东升, 刘德驹, 等. 内电解-超声波耦合处理活性黄3RX染料废水的研究[J]. 工业水处理, 2010, 30(3):25-27, 71. |
| ZHU Xun, XIANG Dongsheng, LIU Deju, et al. Experimental study on the treatment of active yellow 3RX wastewater by internal electrolysis and ultrasonic irradiation[J]. Industrial Water Treatment, 2010, 30(3): 25-27, 71. | |
| 17 | YANG Yi, JIANG Jin, LU Xinglin, et al. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process[J]. Environmental Science & Technology, 2015, 49(12): 7330-7339. |
| 18 | 陈军超. TEMPO捕获过氧自由基的机理研究[D]. 哈尔滨: 哈尔滨工业大学, 2018. |
| CHEN Junchao. Study on the mechanism of TEMPO capturing peroxy radicals[D]. Harbin: Harbin Institute of Technology, 2018. | |
| 19 | 周柳霞. 电池用二氧化锰的生产方法与研究进展[J]. 中国锰业, 2010, 28(3): 1-7. |
| ZHOU Liuxia. A research on manufacturing method of MnO2 for battery[J]. China’s Manganese Industry, 2010, 28(3): 1-7. | |
| 20 | WANG Shizong, WANG Jianlong. Degradation of carbamazepine by radiation-induced activation of peroxymonosulfate[J]. Chemical Engineering Journal, 2018, 336: 595-601. |
| 21 | WANG Y R, CHU W. Degradation of 2,4,5-trichlorophenoxyacetic acid by a novel Electro-Fe(Ⅱ)/Oxone process using iron sheet as the sacrificial anode[J]. Water Research, 2011, 45(13): 3883-3889. |
| 22 | WANG Y R, CHU W. Photo-assisted degradation of 2,4,5-trichlorophenoxyacetic acid by Fe(Ⅱ)-catalyzed activation of oxone process: The role of UV irradiation, reaction mechanism and mineralization[J]. Applied Catalysis B: Environmental, 2012, 123/124: 151-161. |
| 23 | ZHANG Hui, WANG Zhe, LIU Chiachi, et al. Removal of COD from landfill leachate by an electro/Fe2+/peroxydisulfate process[J]. Chemical Engineering Journal, 2014, 250: 76-82. |
| 24 | 刘臻. 活性炭纤维阴极电活化过硫酸盐去除水中难降解有机污染物研究[D]. 重庆: 重庆大学, 2019. |
| LIU Zhen. Removal of refractory organic pollutants in water by electrochemical activation of perisulfate with ACF cathode[D].Chongqing: Chongqing University, 2019. | |
| 25 | BUXTON George V, GREENSTOCK Clive L, Phillips HELMAN W, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O-) in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(2): 513-886. |
| 26 | CHU W, WANG Y R, LEUNG H F. Synergy of sulfate and hydroxyl radicals in UV/S2O8 2-/H2O2 oxidation of iodinated X-ray contrast medium iopromide[J]. Chemical Engineering Journal, 2011, 178: 154-160. |
| 27 | MOHAMMAD M, KHAN A Y, SUBHANI M S, et al. Kinetics and electrochemical studies on superoxide[J]. Research on Chemical Intermediates, 2001, 27(3): 259-267. |
| 28 | NETA P, HUIE Robert E, ROSS Alberta B. Rate constants for reactions of inorganic radicals in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. |
| 29 | SONG Haoran, YAN Linxia, MA Jun, et al. Nonradical oxidation from electrochemical activation of peroxydisulfate at Ti/Pt anode: Efficiency, mechanism and influencing factors[J]. Water Research, 2017, 116: 182-193. |
| 30 | YANG Yi, BANERJEE Gourab, BRUDVIG Gary W, et al. Oxidation of organic compounds in water by unactivated peroxymonosulfate[J]. Environmental Science & Technology, 2018, 52(10): 5911-5919. |
| 31 | 万阳芳, 李慧颖, 刘俊果, 等. 电解离子水中羟自由基的产生规律[J]. 食品工业科技, 2015, 36(5): 49-52. |
| WAN Yangfang, LI Huiying, LIU Junguo, et al. Forming rules of hydroxyl radicals produced in electrolyzed water[J]. Science and Technology of Food Industry, 2015, 36(5): 49-52. | |
| 32 | WANG Lihong, JIANG Jin, PANG Suyan, et al. Further insights into the combination of permanganate and peroxymonosulfate as an advanced oxidation process for destruction of aqueous organic contaminants[J]. Chemosphere, 2019, 228: 602-610. |
| 33 | JIANG Jin, PANG Suyan, MA Jun. Oxidation of triclosan by permanganate (Mn(Ⅶ)): Importance of ligands and in situ formed manganese oxides[J]. Environmental Science & Technology, 2009, 43(21): 8326-8331. |
| 34 | 张璐虹, 唐有根, 张丽, 等. MgAl-LDH吸附甲基橙性能研究[J]. 功能材料, 2012, 43(18): 2469-2472. |
| ZHANG Luhong, TANG Yougen, ZHANG Li, et al. Studies on adsorption of methyl orange from aqueous solutions onto Mg/Al-LDH[J]. Journal of Functional Materials, 2012, 43(18): 2469-2472. | |
| 35 | ANIPSITAKIS George P, DIONYSIOU Dionysios D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| 36 | ANIPSITAKIS George P, STATHATOS Elias, DIONYSIOU Dionysios D. Heterogeneous activation of oxone using Co3O4 [J]. The Journal of Physical Chemistry B, 2005, 109(27): 13052-13055. |
| 37 | Wen-Da OH, DONG Zhili, HU Zhongting, et al. A novel quasi-cubic CuFe2O4-Fe2O3 catalyst prepared at low temperature for enhanced oxidation of bisphenol A via peroxymonosulfate activation[J]. Journal of Materials Chemistry A, 2015, 3(44): 22208-22217. |
| 38 | KHAN Aimal, ZHANG Kaikai, SUN Peng, et al. High performance of the A-Mn2O3 nanocatalyst for persulfate activation: Degradation process of organic contaminants via singlet oxygen[J]. Journal of Colloid and Interface Science, 2021, 584: 885-899. |
| 39 | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 2017, 113: 80-88. |
| 40 | ZHU Shishu, LI Xiaojie, KANG Jian, et al. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science & Technology, 2019, 53(1): 307-315. |
| 41 | 白格, 陈茂清, 蔡楠, 等. 高级氧化技术中自由基的检测技术和方法研究进展[J]. 分析测试学报, 2021, 40(7): 1109-1118. |
| BAI Ge, CHEN Maoqing, CAI Nan, et al. Advances on determination methods of free radicals in advanced oxidation processes[J]. Journal of Instrumental Analysis, 2021, 40(7): 1109-1118. | |
| 42 | 吴祖望, 张蓉, 荣泽明. 论偶氮染料的偶氮-腙互变异构[J]. 化工学报, 2015, 66(1): 52-59. |
| WU Zuwang, ZHANG Rong, RONG Zeming. Azo-hydrazone tautomerism of azo dyes[J]. CIESC Journal, 2015, 66(1): 52-59. | |
| 43 | 曾兆敏. 偶氮-腙体互变异构及其在染料分子设计中的预报[J]. 北京化纤工学院学报, 1987, 7(1): 90-100. |
| ZENG Zhaomin. Azo-hydrazone tautomerism and its prediction in dye-molecular designing[J]. Journal of Beijing Institute of Fashion Technology, 1987, 7(1): 90-100. | |
| 44 | FREYRIA F S, BONELLI B, SETHI R, et al. Reactions of acid orange 7 with iron nanoparticles in aqueous solutions[J]. The Journal of Physical Chemistry C, 2011, 115(49): 24143-24152. |
| 45 | CHIANG Li-Choung, CHANG Juu-En, WEN Ten-Chin. Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate[J]. Water Research, 1995, 29(2): 671-678. |
| 46 | ZHAO Xiaodan, MA Jun, JIANG Jin, et al. Phenols and anilines degradation by permanganate in the absence/presence of carbon nanotubes: Oxidation and dehalogenation[J]. Separation and Purification Technology, 2016, 170: 344-352. |
| 47 | CLIFTON Carol L, HUIE Robert E. Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4 -· alcohols[J]. International Journal of Chemical Kinetics, 1989, 21(8): 677-687. |
| 48 | PADMAJA S, ALFASSI Z B, NETA P, et al. Rate constants for reactions of SO4 ˙– radicals in acetonitrile[J]. International Journal of Chemical Kinetics, 1993, 25(3): 193-198. |
| 49 | CZAPLICKA Marianna. Photo-degradation of chlorophenols in the aqueous solution[J]. Journal of Hazardous Materials, 2006, 134(1/2/3): 45-59. |
| 50 | KOBAYASHI S, ANDO W. Co-oxidations of 1,3-dephenylisobenzofuran by the Haber-Weiss reaction. Is singlet oxygen concerned in this oxidation?[J]. Biochemical and Biophysical Research Communications, 1979, 88(2): 676-681. |
| 51 | 刘占才, 牛俊英. 超氧阴离子自由基对生物体的作用机理研究[J]. 焦作教育学院学报, 2002, 18(4): 48-51. |
| LIU Zhancai, NIU Junying. Study on the mechanism of superoxide anion free radical on organism[J]. Journal of Jiaozuo Teachers College, 2002, 18(4): 48-51. | |
| 52 | SU Pingru, ZHU Huicen, SHEN Zhemin. QSAR models for removal rates of organic pollutants adsorbed by in situ formed manganese dioxide under acid condition[J]. Environmental Science and Pollution Research International, 2016, 23(4): 3609-3620. |
| [1] | 王正峰, 谢雨杭, 李伟科, 范永春, 康钟尹, 付乾. 多孔炭修饰的吸附催化一体化电极高效电解碳酸氢盐[J]. 化工进展, 2024, 43(9): 4892-4899. |
| [2] | 梁宏成, 赵冬妮, 权银, 李敬妮, 胡欣怡. SEI膜形貌与结构对锂离子电池性能的影响[J]. 化工进展, 2024, 43(9): 5049-5062. |
| [3] | 吴剑扬, 王汝娜, 陈耀, 申兰耀, 于永利, 蒋宁, 邱景义, 周恒辉. 锂离子电池高镍正极材料前体的制备工艺[J]. 化工进展, 2024, 43(9): 5079-5085. |
| [4] | 李美萱, 成建凤, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王俊莲, 王春霞, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 高电压镍锰酸锂正极材料的合成与电化学机理[J]. 化工进展, 2024, 43(9): 5086-5094. |
| [5] | 屈芸, 成丽媛, 代国亮, 王刚, 郭羽晴, 孙洁. PAN/MXene同轴纤维电极的制备及性能[J]. 化工进展, 2024, 43(9): 5113-5122. |
| [6] | 安芳芳, 曹少磊, 连增帅, 舒大武, 张岩, 李万新, 韩博. 十二烷基甜菜碱对热活化过硫酸钠降解C.I.活性黑5的影响[J]. 化工进展, 2024, 43(9): 5302-5308. |
| [7] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
| [8] | 靳立军, 刘铮铮, 李扬, 杨赫, 胡浩权. 耦合富氢小分子催化活化的煤热解提高焦油产率策略与实践[J]. 化工进展, 2024, 43(7): 3613-3619. |
| [9] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| [10] | 何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088. |
| [11] | 罗臻, 王庆吉, 王占生, 杨雪莹, 谢加才, 王浩. 炼化污染场地抽出水强氧化短程处理工艺[J]. 化工进展, 2024, 43(7): 4155-4163. |
| [12] | 刘梦凡, 王华伟, 王亚楠, 张艳茹, 蒋旭彤, 孙英杰. Bio-FeMnCeO x 活化PMS降解四环素效能与机制[J]. 化工进展, 2024, 43(6): 3492-3502. |
| [13] | 李亚男, 郭凯, 王嘉琪, 武亚宁. 煤气化渣活化过二硫酸盐和过一硫酸盐降解苯酚的比较[J]. 化工进展, 2024, 43(6): 3503-3512. |
| [14] | 李莹莹, 刘安, 姜乐妍, 李晖, 陈春钰, 居殿春. 过渡金属硫化物Co9S8的制备及电化学性能研究进展[J]. 化工进展, 2024, 43(6): 3114-3127. |
| [15] | 孙悦, 邢宝林, 张耀杰, 冯来宏, 曾会会, 蒋振东, 徐冰, 贾建波, 张传祥, 谌伦建, 张越, 张文豪. B掺杂多孔碳纳米片的制备及其储锂性能[J]. 化工进展, 2024, 43(6): 3209-3220. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||