化工进展 ›› 2022, Vol. 41 ›› Issue (2): 730-739.DOI: 10.16085/j.issn.1000-6613.2021-0489
固相钴基催化剂活化过一硫酸盐在水处理中的研究进展
- 广东工业大学土木与交通工程学院,广东 广州 510006
-
收稿日期:2021-03-11修回日期:2021-04-20出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:王俏 -
作者简介:许泽涛(1996—),男,硕士研究生,研究方向为水污染处理技术。E-mail:2111909014@mail2.gdut.edu.cn 。 -
基金资助:国家自然科学基金青年基金(22006200);广东工业大学青年百人A启动项目(220413320)
Research progress of peroxymonosulfate activated by solid-phase cobalt-based catalyst in water treatment
XU Zetao( ), CAO Yiting, WANG Qiao(
), CAO Yiting, WANG Qiao( ), WANG Zhihong
), WANG Zhihong
- School of Civil and Traffic Engineering,Guangdong University of Technology, Guangzhou 510006, Guangdong, China
-
Received:2021-03-11Revised:2021-04-20Online:2022-02-05Published:2022-02-23 -
Contact:WANG Qiao
摘要:
固相钴基催化剂驱动的过一硫酸盐(PMS)高级氧化技术,由于其高催化活性和易于从水中分离的优势,近年来受到了研究人员的广泛关注。本文回顾了近年来用于过一硫酸盐活化的固相钴基催化剂的研究进展,总结了钴基催化剂的种类和催化剂开发过程中采取的改性措施,汇总了钴基过一硫酸盐高级氧化技术在难降解有机污染物削减方面的应用,并分析了多种水质环境因素对削减效能的影响作用,同时阐述了钴基催化剂活化过一硫酸盐的氧化机理。最后,展望了固相钴基催化剂在未来研究中有望往大尺度、低泄漏、高循环、低能耗、具有磁效应或与膜反应器相结合的方向进一步发展。
中图分类号:
引用本文
许泽涛, 曹怡婷, 王俏, 王志红. 固相钴基催化剂活化过一硫酸盐在水处理中的研究进展[J]. 化工进展, 2022, 41(2): 730-739.
XU Zetao, CAO Yiting, WANG Qiao, WANG Zhihong. Research progress of peroxymonosulfate activated by solid-phase cobalt-based catalyst in water treatment[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 730-739.
| 污染物 | 污染物浓度 | PMS浓度 | 催化剂 | 浓度/g·L-1 | pH | 温度/℃ | 时间/min | 去除率/% | 动力学常数/min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 罗丹明B | 40mg/L | 0.1mol/L | Co-HAP-2 | 0.2 | 5.5 | 25 | 12 | 93.30 | 0.0332 | [ |
| 罗丹明B | 10mg/L | 0.05mg/L | Co3O4-Bi2O3 | 0.05 | 6 | 25 | 30 | 100 | — | [ |
| 亮红3BF | 0.1mmol/L | 0.4mmol/L | AC-CuCo2O4 | 0.2 | 10 | 25 | 10 | 98 | 0.476 | [ |
| 双酚A | 20mg/L | 0.3mmol/L | CoS | 0.05 | 7 | 25 | 10 | 90 | 0.37 | [ |
| 双酚A | 20mg/L | 0.3g/L | Co@NC-ZS-700 | 0.1 | 6.2 | 25 | 20 | 95 | — | [ |
| 双酚A | 80mg/L | 0.3g/L | Co-N-C-900 | 0.5 | — | 30 | 3 | 100 | 2.81 | [ |
| 2,4-二氯苯氧基乙酸 | 20mg/L | 1mmol/L | CuO-Co3O4@CeO2 | 0.07 | 6 | 25 | 45 | 97.50 | 0.1344 | [ |
| 2,4-二氯苯酚 | 50mg/L | 6mmol/L | CoOOH | 0.20 | 7 | 25 | 120 | 100 | 0.0462 | [ |
| 2,4-二氯苯酚 | 50mg/L | 1.26g/L | Co3Fe7-CoFe2O4 | 0.05 | 7.7 | 30 | 30 | 97.10 | 0.119 | [ |
| 阿特拉津 | 10μmol/L | 1mmol/L | CoBC500 | 0.1 | 5.3 | 25 | 10 | 99 | 0.76 | [ |
| 阿特拉津 | 23μmol/L | 0.3mmol/L | 3Co@Ⅰ | 0.2 | 7 | 25 | 30 | 96 | 0.1034 | [ |
| 二氢呋喃 | 10mg/L | 0.65mmol/L | Co-S@NC | 0.1 | 4.8 | 25 | 90 | 100.00 | 0.054 | [ |
| 喹克洛拉克 | 50mg/L | 20mmol/L | Co/NAC | 0.08 | — | 25 | 30 | 93 | 0.0022 | [ |
| 氯霉素 | 30mg/L | 10mmol/L | Co3O4-BC | 0.2 | 7 | 26 | 10 | 100 | 0.3361 | [ |
| 环丙沙星 | 10mg/L | 1.62mmol/L | Co-Fe/SiO2LC | 0.2 | 7 | 25 | 60 | 99.60 | 0.686 | [ |
| 环丙沙星 | 10mg/L | 1.3mmol/L | CoS2(HNSs) | 0.08 | 8 | 25 | 3 | 100.00 | 0.1209 | [ |
| 萘普生 | 0.043mmol/L | 2.5mmol/L | CoCNx/SBA-15 | 0.0375 | 6.4 | 25 | 55 | 100 | 0.0877 | [ |
| 5-磺基水杨酸 | 20mg/L | 150mg/L | CoTS | 0.1 | 7 | 30 | 60 | 100 | 0.0784 | [ |
| 四环素 | 30mg/L | 0.4g/L | ALCo-LDH | 0.2 | — | 25 | 30 | 96.10 | 0.980 | [ |
| 四环素 | 30mg/L | 0.3g/L | CoSx | 0.2 | 5 | 25 | 30 | 100 | 0.151 | [ |
| 三氯生 | 10mg/L | 0.05g/L | Co2Mn1O4 | 0.02 | 6.8 | 25 | 30 | 96.40 | 0.112 | [ |
表1 固相钴基催化剂在水处理中的应用
| 污染物 | 污染物浓度 | PMS浓度 | 催化剂 | 浓度/g·L-1 | pH | 温度/℃ | 时间/min | 去除率/% | 动力学常数/min-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 罗丹明B | 40mg/L | 0.1mol/L | Co-HAP-2 | 0.2 | 5.5 | 25 | 12 | 93.30 | 0.0332 | [ |
| 罗丹明B | 10mg/L | 0.05mg/L | Co3O4-Bi2O3 | 0.05 | 6 | 25 | 30 | 100 | — | [ |
| 亮红3BF | 0.1mmol/L | 0.4mmol/L | AC-CuCo2O4 | 0.2 | 10 | 25 | 10 | 98 | 0.476 | [ |
| 双酚A | 20mg/L | 0.3mmol/L | CoS | 0.05 | 7 | 25 | 10 | 90 | 0.37 | [ |
| 双酚A | 20mg/L | 0.3g/L | Co@NC-ZS-700 | 0.1 | 6.2 | 25 | 20 | 95 | — | [ |
| 双酚A | 80mg/L | 0.3g/L | Co-N-C-900 | 0.5 | — | 30 | 3 | 100 | 2.81 | [ |
| 2,4-二氯苯氧基乙酸 | 20mg/L | 1mmol/L | CuO-Co3O4@CeO2 | 0.07 | 6 | 25 | 45 | 97.50 | 0.1344 | [ |
| 2,4-二氯苯酚 | 50mg/L | 6mmol/L | CoOOH | 0.20 | 7 | 25 | 120 | 100 | 0.0462 | [ |
| 2,4-二氯苯酚 | 50mg/L | 1.26g/L | Co3Fe7-CoFe2O4 | 0.05 | 7.7 | 30 | 30 | 97.10 | 0.119 | [ |
| 阿特拉津 | 10μmol/L | 1mmol/L | CoBC500 | 0.1 | 5.3 | 25 | 10 | 99 | 0.76 | [ |
| 阿特拉津 | 23μmol/L | 0.3mmol/L | 3Co@Ⅰ | 0.2 | 7 | 25 | 30 | 96 | 0.1034 | [ |
| 二氢呋喃 | 10mg/L | 0.65mmol/L | Co-S@NC | 0.1 | 4.8 | 25 | 90 | 100.00 | 0.054 | [ |
| 喹克洛拉克 | 50mg/L | 20mmol/L | Co/NAC | 0.08 | — | 25 | 30 | 93 | 0.0022 | [ |
| 氯霉素 | 30mg/L | 10mmol/L | Co3O4-BC | 0.2 | 7 | 26 | 10 | 100 | 0.3361 | [ |
| 环丙沙星 | 10mg/L | 1.62mmol/L | Co-Fe/SiO2LC | 0.2 | 7 | 25 | 60 | 99.60 | 0.686 | [ |
| 环丙沙星 | 10mg/L | 1.3mmol/L | CoS2(HNSs) | 0.08 | 8 | 25 | 3 | 100.00 | 0.1209 | [ |
| 萘普生 | 0.043mmol/L | 2.5mmol/L | CoCNx/SBA-15 | 0.0375 | 6.4 | 25 | 55 | 100 | 0.0877 | [ |
| 5-磺基水杨酸 | 20mg/L | 150mg/L | CoTS | 0.1 | 7 | 30 | 60 | 100 | 0.0784 | [ |
| 四环素 | 30mg/L | 0.4g/L | ALCo-LDH | 0.2 | — | 25 | 30 | 96.10 | 0.980 | [ |
| 四环素 | 30mg/L | 0.3g/L | CoSx | 0.2 | 5 | 25 | 30 | 100 | 0.151 | [ |
| 三氯生 | 10mg/L | 0.05g/L | Co2Mn1O4 | 0.02 | 6.8 | 25 | 30 | 96.40 | 0.112 | [ |
| 1 | ZHAO Q, LU D, JIANG H, et al. Peroxymonosulfate-based cleaning technology for metal oxide-coated ceramic ultrafiltration membrane polluted by Alcian Blue 8GX dye: radical and non-radical oxidation cleaning mechanism[J]. Journal of Membrane Science, 2019, 573: 210-217. |
| 2 | XU H, WANG D, MA J, et al. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S[J]. Applied Catalysis B: Environmental, 2018, 238: 557-567. |
| 3 | CHEN Z, BI S, ZHAO G, et al. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: mechanisms and intermediates identification[J]. Science of the Total Environment, 2020, 711: 134715. |
| 4 | ZHANG Q, HE D, LI X, et al. Mechanism and performance of singlet oxygen dominated peroxymonosulfate activation on CoOOH nanoparticles for 2,4-dichlorophenol degradation in water[J]. Journal of Hazardous Materials, 2020, 384: 121350. |
| 5 | CAO J, SUN S, LI X, et al. Efficient charge transfer in aluminum-cobalt layered double hydroxide derived from Co-ZIF for enhanced catalytic degradation of tetracycline through peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 382: 122802. |
| 6 | WANG Y X, ZHOU L, DUAN X G, et al. Photochemical degradation of phenol solutions on Co3O4 nanorods with sulfate radicals[J]. Catalysis Today, 2015, 258: 576-584. |
| 7 | CAI H, LI J, YIN H, et al. Degradation of atrazine in aqueous solution through peroxymonosulfate activated by Co-modified nano-titanium dioxide[J]. Water Environment Research, 2020, 92 (9): 1363-1375. |
| 8 | DENG J, FENG S, ZHANG K, et al. Heterogeneous activation of peroxymonosulfate using ordered mesoporous Co3O4 for the degradation of chloramphenicol at neutral pH[J]. Chemical Engineering Journal, 2017, 308: 505-515. |
| 9 | ZHU C, ZHANG Y, FAN Z, et al. Carbonate-enhanced catalytic activity and stability of Co3O4 nanowires for 1O2-driven bisphenol A degradation via peroxymonosulfate activation: critical roles of electron and proton acceptors[J]. Journal of Hazardous Materials, 2020, 393: 122395. |
| 10 | XIE M, TANG J, FANG G, et al. Biomass Schiff base polymer-derived N-doped porous carbon embedded with CoO nanodots for adsorption and catalytic degradation of chlorophenol by peroxymonosulfate[J]. Journal of Hazardous Materials, 2020, 384: 121345. |
| 11 | WEI J, FENG Y, LIU Y, et al. MxCo3-xO4(M = Co, Mn, Fe) porous nanocages derived from metal–organic frameworks as efficient water oxidation catalysts[J]. Journal of Materials Chemistry A, 2015, 3 (44): 22300-22310. |
| 12 | YANG Q, CHOI H, AL-ABED S R, et al. Iron–cobalt mixed oxide nanocatalysts: heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications[J]. Applied Catalysis B: Environmental, 2009, 88 (3/4): 462-469. |
| 13 | YAO Y, CAI Y, WU G, et al. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water[J]. Journal of Hazardous Materials, 2015, 296: 128-137. |
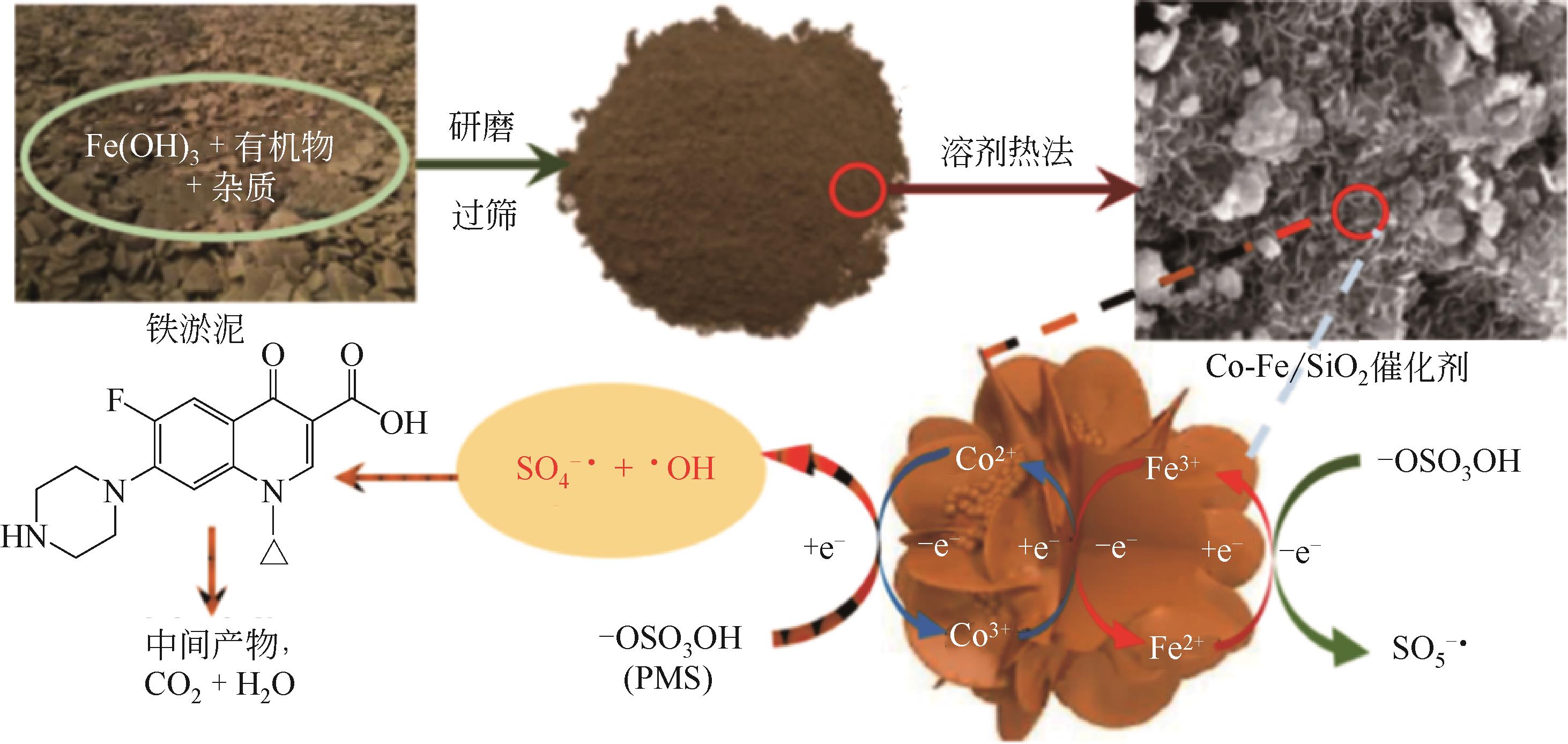
| 14 | ZHU S, XU Y, ZHU Z, et al. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation[J]. Chemical Engineering Journal, 2020, 384: 123298. |
| 15 | LI W, LI Y, ZHANG D, et al. CuO-Co3O4@CeO2 as a heterogeneous catalyst for efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate[J]. Journal of Hazardous Materials, 2020, 381: 121209. |
| 16 | XU H, ZHANG Y, LI J, et al. Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols[J]. Environmental Pollution, 2020, 257: 113610. |
| 17 | LIU B, GUO W, WANG H, et al. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: the pivotal roles of persistent free radicals and ecotoxicity assessment[J]. Journal of Hazardous Materials, 2020, 398: 122768. |
| 18 | CHEN S, LIU X, GAO S, et al. CuCo2O4 supported on activated carbon as a novel heterogeneous catalyst with enhanced peroxymonosulfate activity for efficient removal of organic pollutants[J]. Environmental Research, 2020, 183: 109245. |
| 19 | 杨世迎, 张翱, 任腾飞, 等. 炭基材料催化过氧化物降解水中有机污染物:表面作用机制[J]. 化学进展, 2017, 29 (5): 539-552. |
| YANG Shiying, ZHANG Ao, REN Tengfei, et al. Surface mechanism of carbon-based materials for catalyzing peroxide degradation of organic pollutants in water[J]. Progress in Chemistry, 2017, 29 (5): 539-552. | |
| 20 | ZHOU N, ZU J, YANG L, et al. Cobalt (0/Ⅱ) incorporated N-doped porous carbon as effective heterogeneous peroxymonosulfate catalyst for quinclorac degradation[J]. Journal of Colloid and Interface Science, 2020, 563: 197-206. |
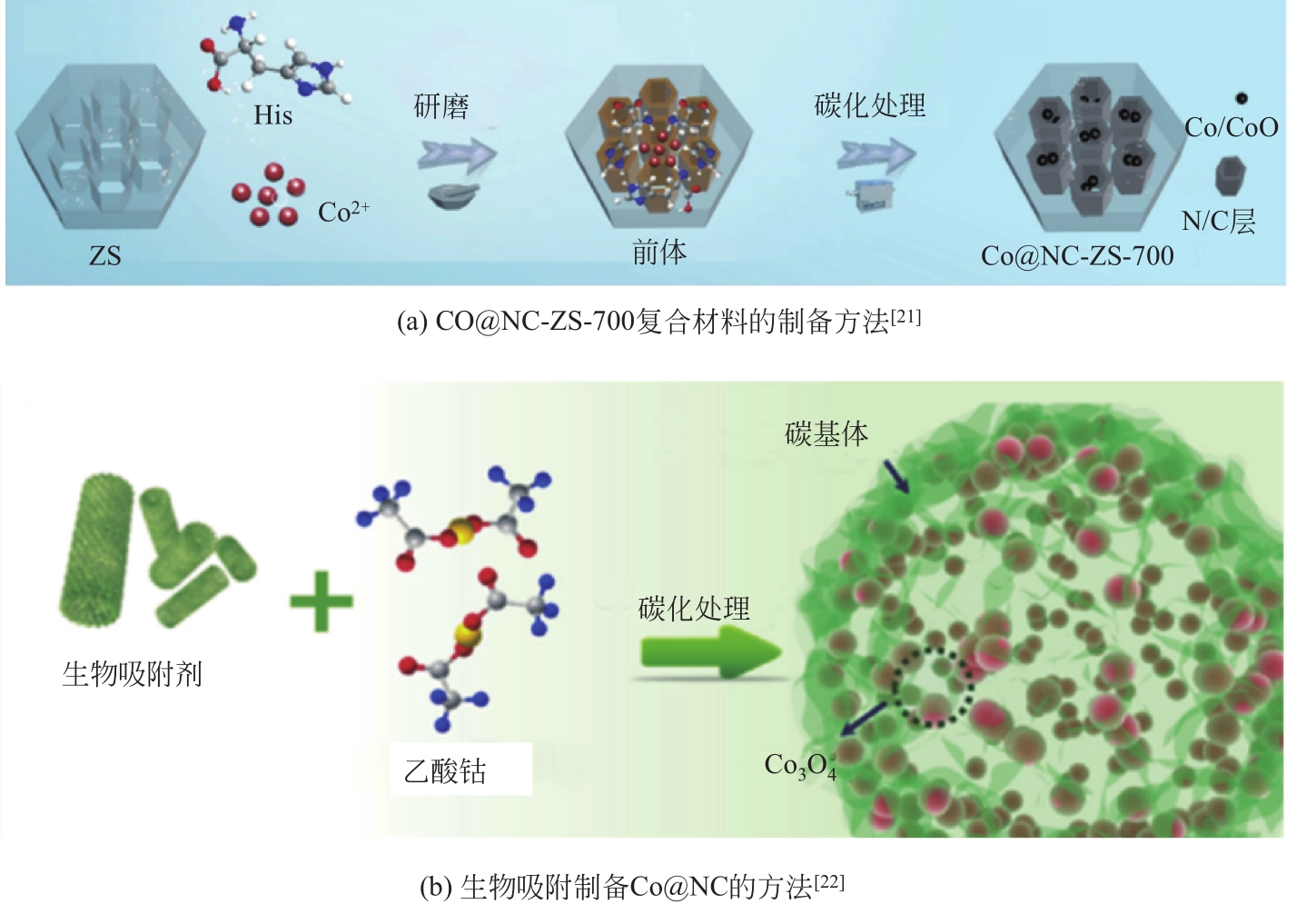
| 21 | LUO J, BO S, AN Q, et al. Designing ordered composites with confined Co-N/C layers for efficient pollutant degradation: structure-dependent performance and PMS activation mechanism[J]. Microporous and Mesoporous Materials, 2020, 293: 109810. |
| 22 | DU W, ZHANG Q, SHANG Y, et al. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2020, 262: 118302. |
| 23 | 赵朝成, 吴光锐. MOFs复合材料催化降解水中有机污染物的应用研究进展[J]. 化工进展, 2019, 38 (4): 1775-1784. |
| ZHAO Chaocheng, WU Guangrui. Research progress on the mechanism and applications of MOFs composite materials for catalytic degradation of organic pollutants in the solution[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1775-1784. | |
| 24 | ZENG T, ZHANG X, WANG S, et al. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants[J]. Environmental Science & Technology, 2015, 49 (4): 2350-2357. |
| 25 | LI Z, TANG X, HUANG G, et al. Bismuth MOFs based hierarchical Co3O4-Bi2O3 composite: an efficient heterogeneous peroxymonosulfate activator for azo dyes degradation[J]. Separation and Purification Technology, 2020, 242: 116825. |
| 26 | WU X, ZHAO W, HUANG Y, et al. A mechanistic study of amorphous CoSx cages as advanced oxidation catalysts for excellent peroxymonosulfate activation towards antibiotics degradation[J]. Chemical Engineering Journal, 2020, 381: 122768. |
| 27 | PANG Y, KONG L, CHEN D, et al. Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B[J]. Journal of Hazardous Materials, 2020, 384: 121447. |
| 28 | DING Y, HU Y, PENG X, et al. Micro-nano structured CoS: an efficient catalyst for peroxymonosulfate activation for removal of bisphenol A[J]. Separation and Purification Technology, 2020, 233: 116022. |
| 29 | CHEN M, WANG N, ZHU L. Single-atom dispersed Co-N-C: a novel adsorption-catalysis bifunctional material for rapid removing bisphenol A[J]. Catalysis Today, 2020, 348: 187-193. |
| 30 | ZHOU Y, ZHANG Y, HU X. Synergistic coupling Co3Fe7 alloy and CoFe2O4 spinel for highly efficient removal of 2,4-dichlorophenol by activating peroxymonosulfate[J]. Chemosphere, 2020, 242: 125244. |
| 31 | LI W, LI S, TANG Y, et al. Highly efficient activation of peroxymonosulfate by cobalt sulfide hollow nanospheres for fast ciprofloxacin degradation[J]. Journal of Hazardous Materials, 2020, 389: 121856. |
| 32 | DONG X, DUAN X, SUN Z, et al. Natural illite-based ultrafine cobalt oxide with abundant oxygen-vacancies for highly efficient Fenton-like catalysis[J]. Applied Catalysis B: Environmental, 2020, 261: 118214. |
| 33 | HOU J, LIN J, FU H, et al. Vitamin B12 derived CoCNx composite confined in SBA-15 as highly effective catalyst to activate peroxymonosulfate for naproxen degradation[J]. Chemical Engineering Journal, 2020, 389: 124344. |
| 34 | LI Meng-Chia, GHANBARI Farshid, CHANG Fang-Chih, et al. Enhanced degradation of 5-sulfosalicylic acid using peroxymonosulfate activated by ordered porous silica-confined Co3O4 prepared via a solvent-free confined space strategy[J]. Separation and Purification Technology, 2020, 249: 116972. |
| 35 | GHANBARI F, AHMADI M, GOHARI F. Heterogeneous activation of peroxymonosulfate via nanocomposite CeO2-Fe3O4 for organic pollutants removal: the effect of UV and US irradiation and application for real wastewater[J]. Separation and Purification Technology, 2019, 228: 115732. |
| 36 | FARSHID Ghanbaria, Martínez-Huitleb CARLOS A. Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: A comparative study[J]. Journal of Electroanalytical Chemistry, 2019, 847: 113182. |
| 37 | YUAN R, JIANG M, GAO S, et al. 3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam: a new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation[J]. Chemical Engineering Journal, 2020, 380: 122447. |
| 38 | JAAFARZADEH N, GHANBARI F, AHMADI M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: a novel combination of advanced oxidation processes[J]. Chemical Engineering Journal, 2017, 320: 436-447. |
| 39 | BACHA A U R, NABI I, CHENG H, et al. Photoelectrocatalytic degradation of endocrine-disruptor bisphenol-A with significantly activated peroxymonosulfate by Co-BiVO4 photoanode[J]. Chemical Engineering Journal, 2020, 389: 124482. |
| 40 | HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications[J]. Applied Catalysis B: Environmental, 2016, 181: 103-117. |
| 41 | WU Y, FANG Z, SHI Y, et al. Activation of peroxymonosulfate by BiOCl@Fe3O4 catalyst for the degradation of atenolol: kinetics, parameters, products and mechanism[J]. Chemosphere, 2019, 216: 248-257. |
| 42 | WANG Q, SHAO Y, GAO N, et al. Activation of peroxymonosulfate by Al2O3-based CoFe2O4 for the degradation of sulfachloropyridazine sodium: Kinetics and mechanism[J]. Separation and Purification Technology, 2017, 189: 176-185. |
| 43 | DUAN X, SU C, MIAO J, et al. Insights into perovskite-catalyzed peroxymonosulfate activation: maneuverable cobalt sites for promoted evolution of sulfate radicals[J]. Applied Catalysis B: Environmental, 2018, 220: 626-634. |
| 44 | LI Y, MA S, XU S, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: emphasizing the synergistic effect between graphitized structure and CoFe2O4[J]. Chemical Engineering Journal, 2020, 387: 124094. |
| 45 | LI H, WAN J, MA Y, et al. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: mechanism, performance, and stability[J]. Journal of Hazardous Materials, 2016, 318: 154-163. |
| [1] | 吴锋振, 刘志炜, 谢文杰, 游雅婷, 赖柔琼, 陈燕丹, 林冠烽, 卢贝丽. 生物质基铁/氮共掺杂多孔炭的制备及其活化过一硫酸盐催化降解罗丹明B[J]. 化工进展, 2023, 42(6): 3292-3301. |
| [2] | 陈邦富, 欧阳平, 李宇涵, 段有雨, 董帆. ZnSn(OH)6 基纳米材料在环境光催化中的应用[J]. 化工进展, 2023, 42(2): 756-764. |
| [3] | 潘杰, 王明新, 高生旺, 夏训峰, 韩雪. 氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑[J]. 化工进展, 2022, 41(8): 4204-4212. 唑[J]. 化工进展, 2022, 41(8): 4204-4212. |
| [4] | 何畅帆, 赵小航, 章雪莹, 何林, 隋红, 李鑫钢. 过一硫酸盐-高铁酸盐-FeS体系土柱淋洗修复邻二氯苯污染土壤[J]. 化工进展, 2022, 41(5): 2743-2752. |
| [5] | 吕朋, 何畅帆, 何林, 李鑫钢, 隋红. 含重质油污泥非均相氧化降解特性及其强化机制[J]. 化工进展, 2022, 41(11): 6149-6157. |
| [6] | 薛雨微, 叶校圳, 曾静, 王永全, 洪俊明. 纳米片层铁锰双金属催化剂活化过[J]. 化工进展, 2022, 41(10): 5661-5668. |
| [7] | 徐劼, 高仕谦, 夏晶, 张珂, 邵子纯, 王澜静, 田永静. Sr掺杂强化LaCo0.5Cu0.5O3型钙钛矿活化过一硫酸盐的性能[J]. 化工进展, 2020, 39(9): 3525-3534. |
| [8] | 孙鹏, 张凯凯, 张玉, 张延荣. 生物炭/过一硫酸盐体系同时去除Cu2+和对硝基苯胺[J]. 化工进展, 2020, 39(10): 4268-4274. |
| [9] | 叶江明,梁绍华,张思文,孙荣岳,毕小龙. 失活钙基吸收剂环境中自活化机理[J]. 化工进展, 2019, 38(9): 4302-4307. |
| [10] | 聂利富, 徐喆, 柯善明, 曾燮榕, 林鹏. Au对TiO2光电催化材料的改性策略研究进展[J]. 化工进展, 2019, 38(07): 3274-3284. |
| [11] | 徐煜轩, 杨继年, 聂士斌. 功能化层状硅酸镍在磁、电及催化领域的应用[J]. 化工进展, 2019, 38(06): 2835-2846. |
| [12] | 刘丽艳, 孙至柔, 叶文博, 谭蔚. 超声辅助Fe3O4活化过一硫酸盐降解酸性红B[J]. 化工进展, 2016, 35(11): 3663-3668. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||