化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5290-5301.DOI: 10.16085/j.issn.1000-6613.2023-1322
• 资源与环境化工 • 上一篇
载铜地质聚合物微球的制备及其催化降解双酚S的性能
张政( ), 刘琳, 李子晨, 王梦琦, 黄春燕, 葛圆圆(
), 刘琳, 李子晨, 王梦琦, 黄春燕, 葛圆圆( )
)
- 广西大学化学化工学院,广西石化资源加工及过程强化技术重点实验室,广西 南宁 530004
-
收稿日期:2023-08-01修回日期:2023-08-28出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:葛圆圆 -
作者简介:张政(1998—),男,硕士研究生,研究方向为地聚物基环境功能材料。E-mail:zhangz0282@163.com。 -
基金资助:国家自然科学基金(22266006)
Preparation of copper-loaded geopolymer microspheres and their catalytic degradation of bisphenol S
ZHANG Zheng( ), LIU Lin, LI Zichen, WANG Mengqi, HUANG Chunyan, GE Yuanyuan(
), LIU Lin, LI Zichen, WANG Mengqi, HUANG Chunyan, GE Yuanyuan( )
)
- Guangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology, School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China
-
Received:2023-08-01Revised:2023-08-28Online:2024-09-15Published:2024-09-30 -
Contact:GE Yuanyuan
摘要:
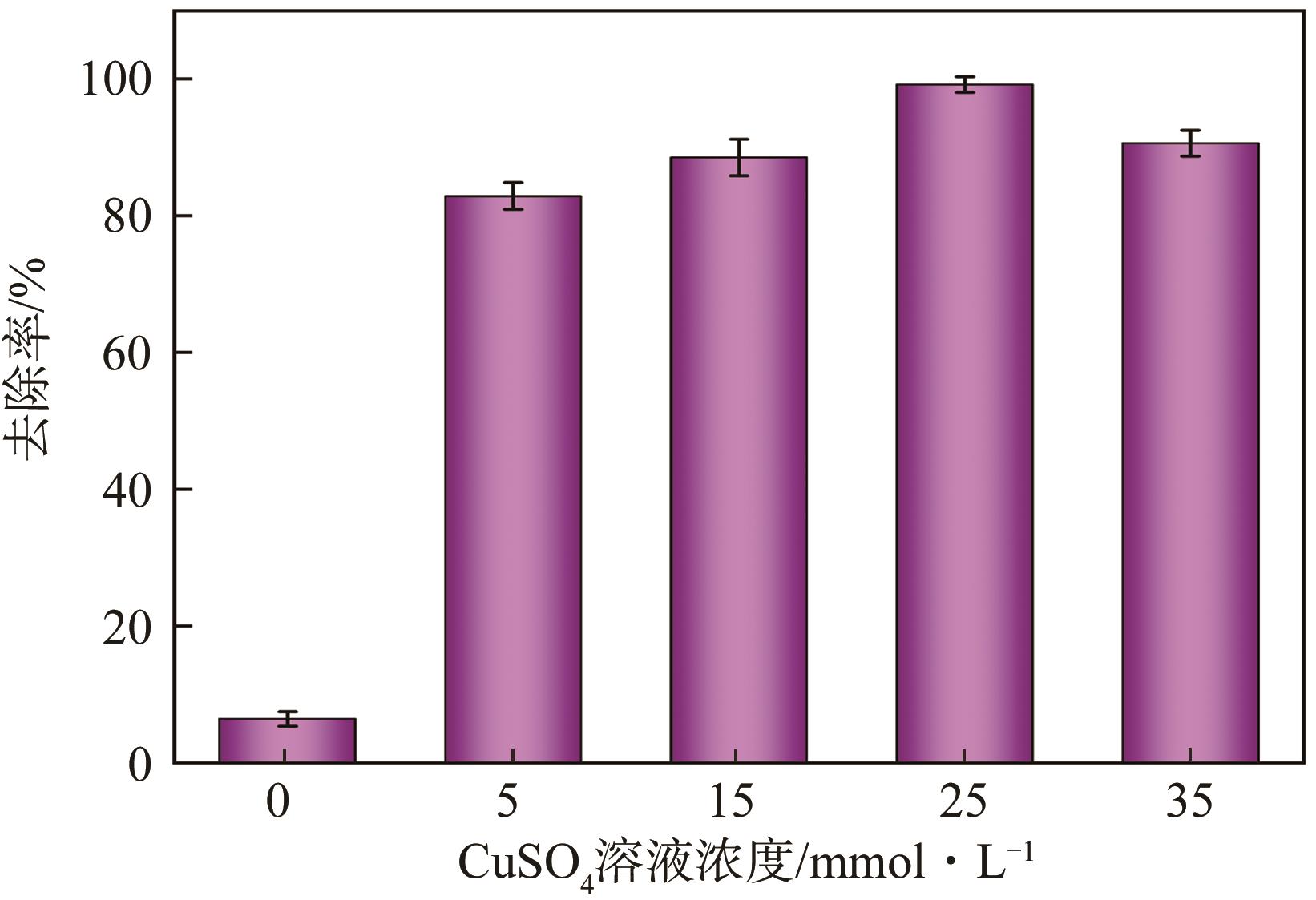
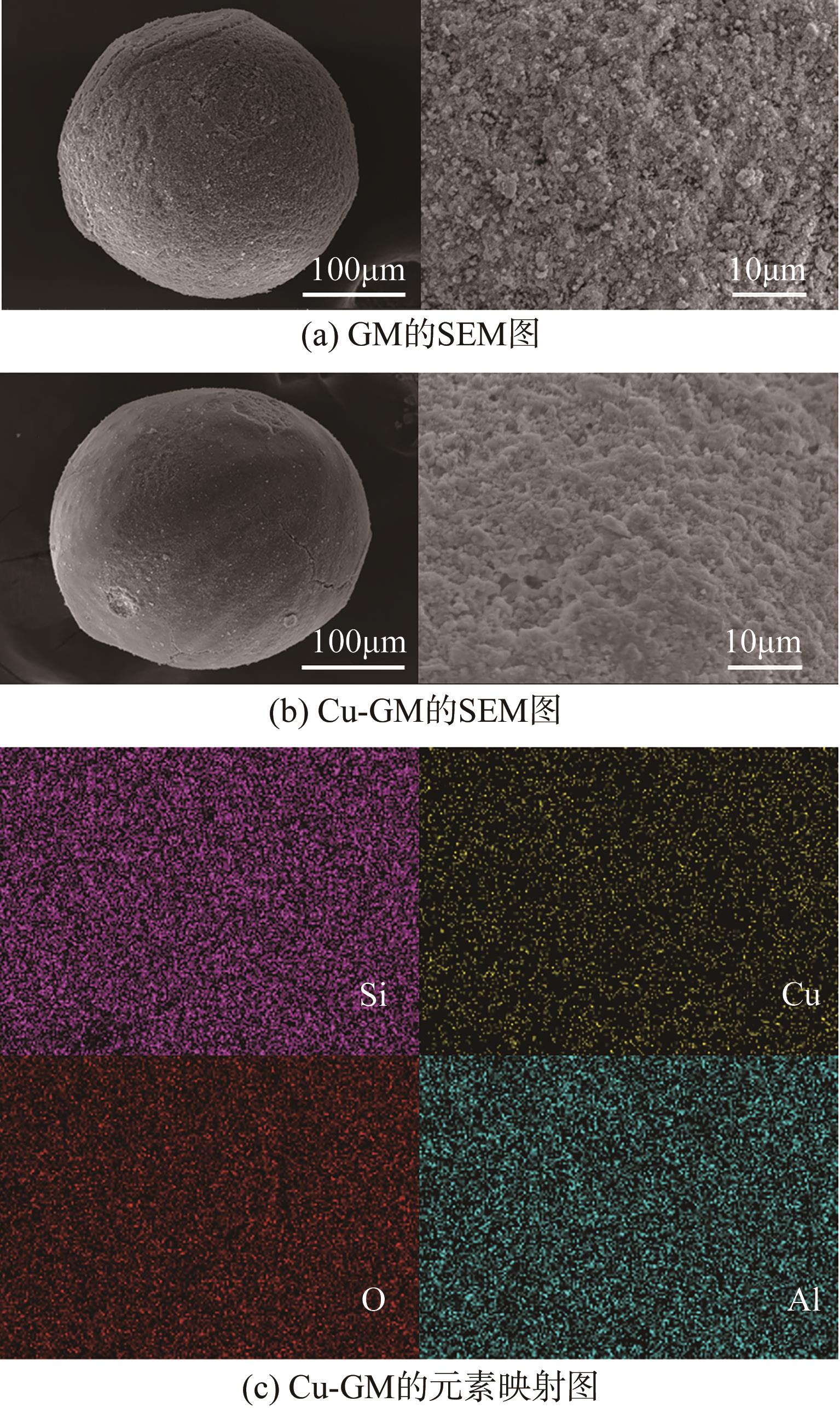
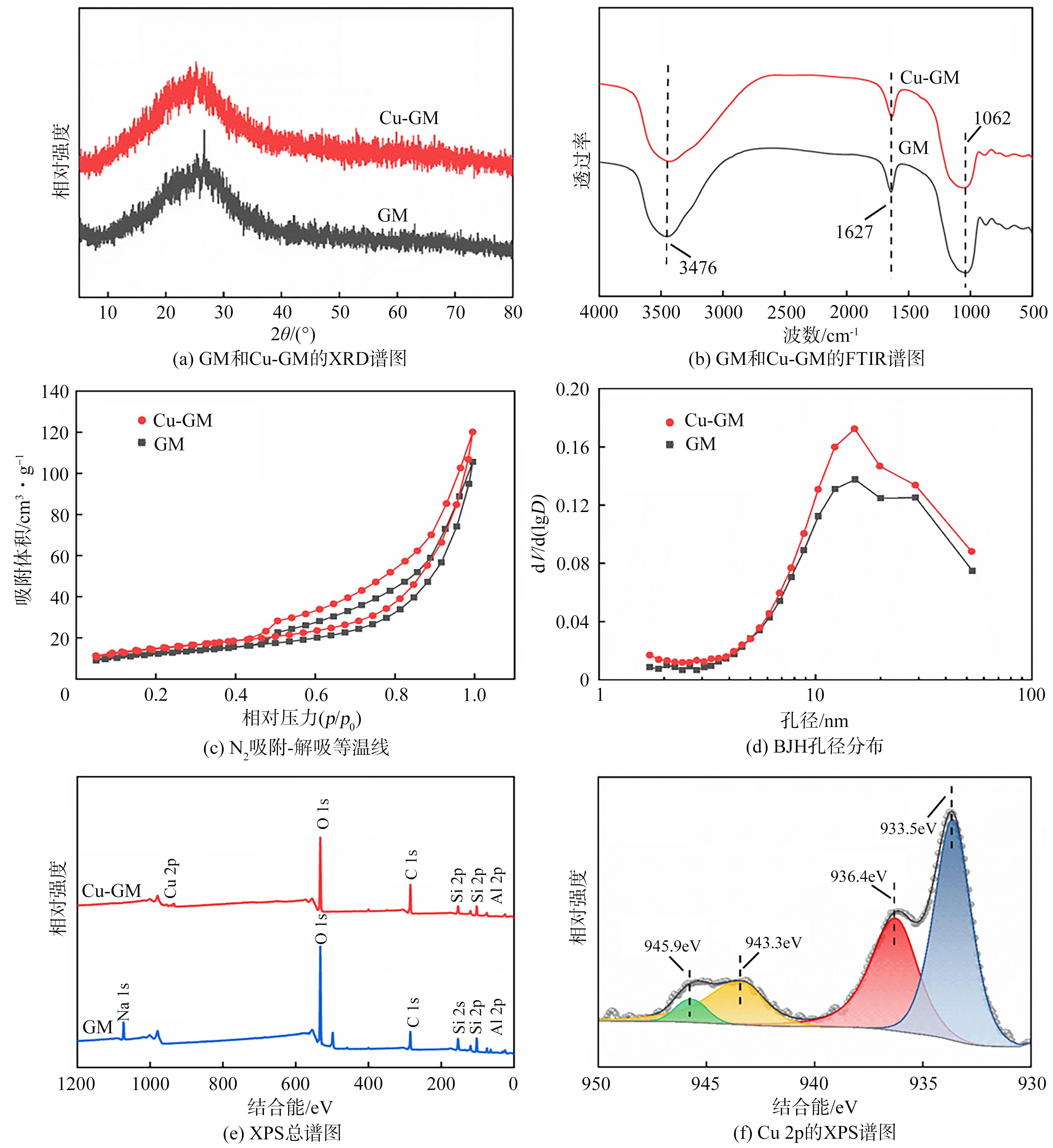
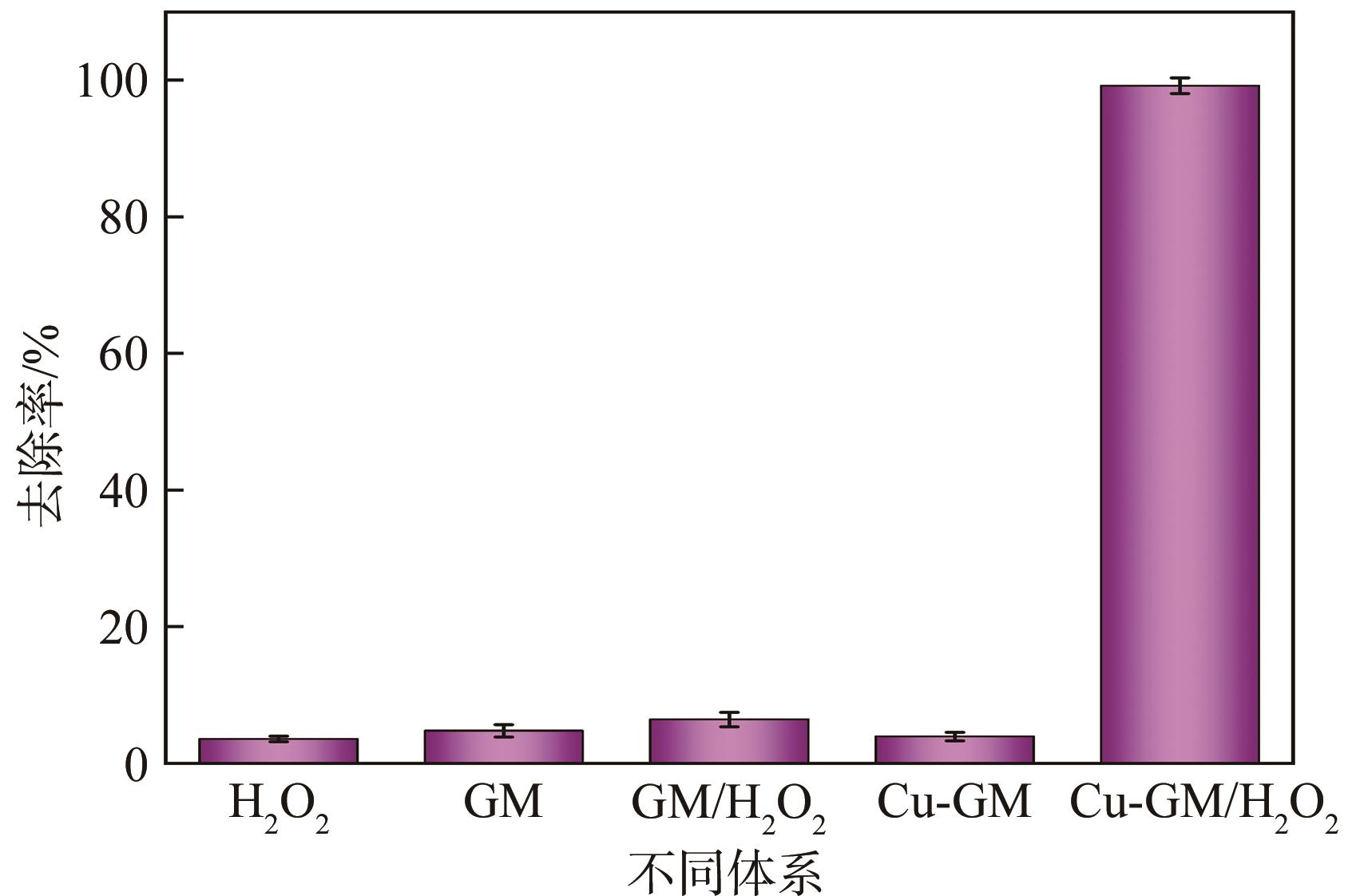
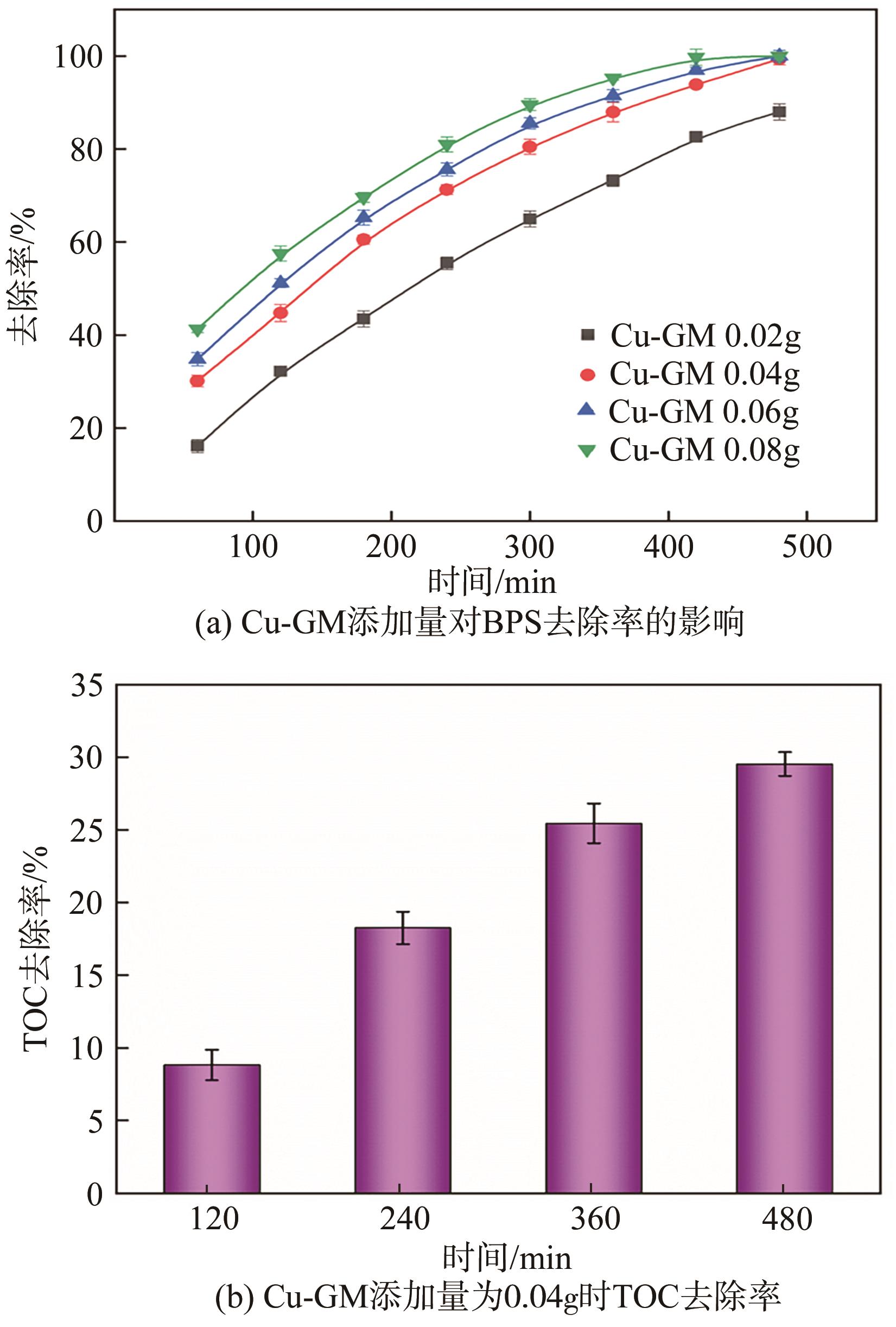
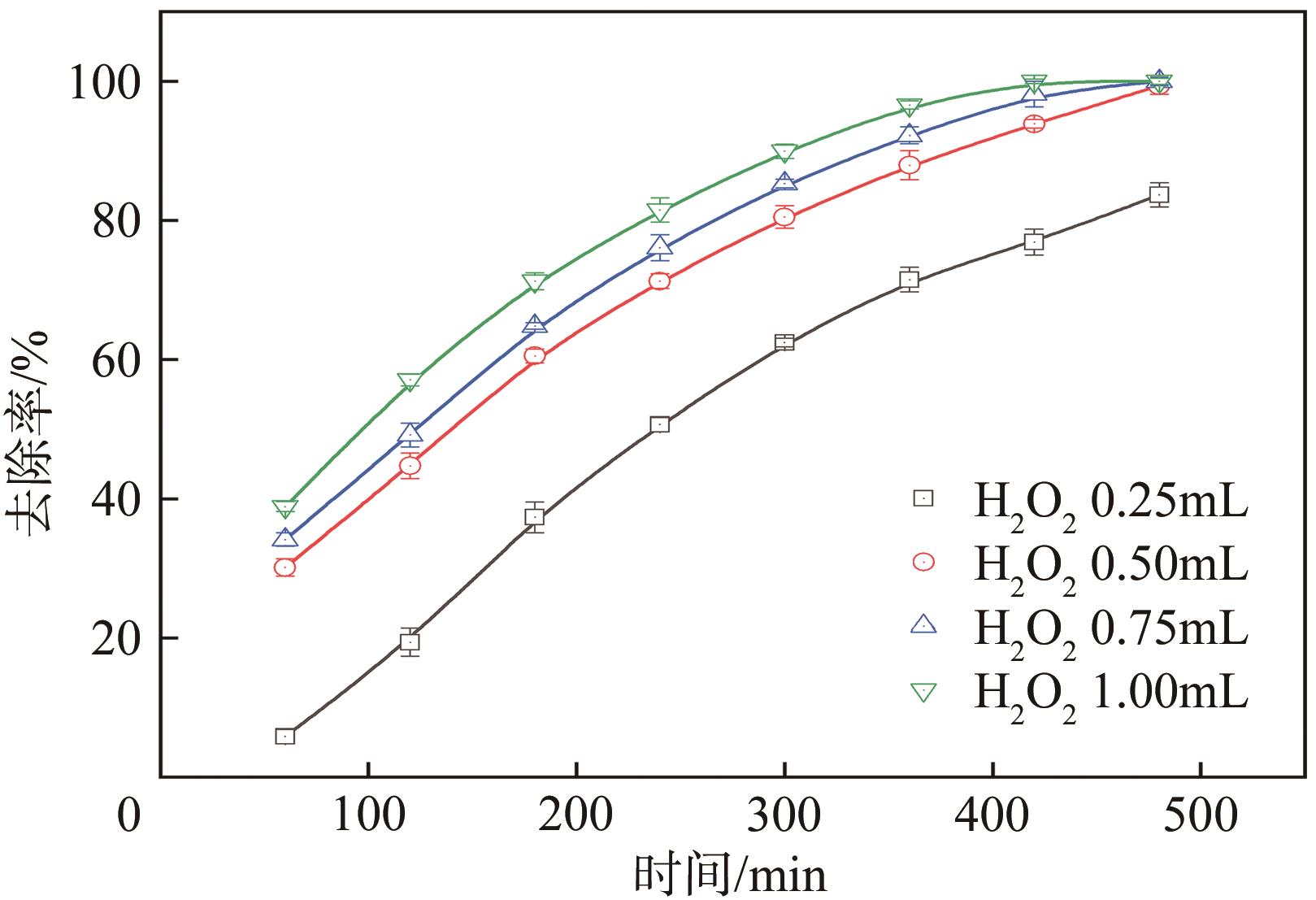
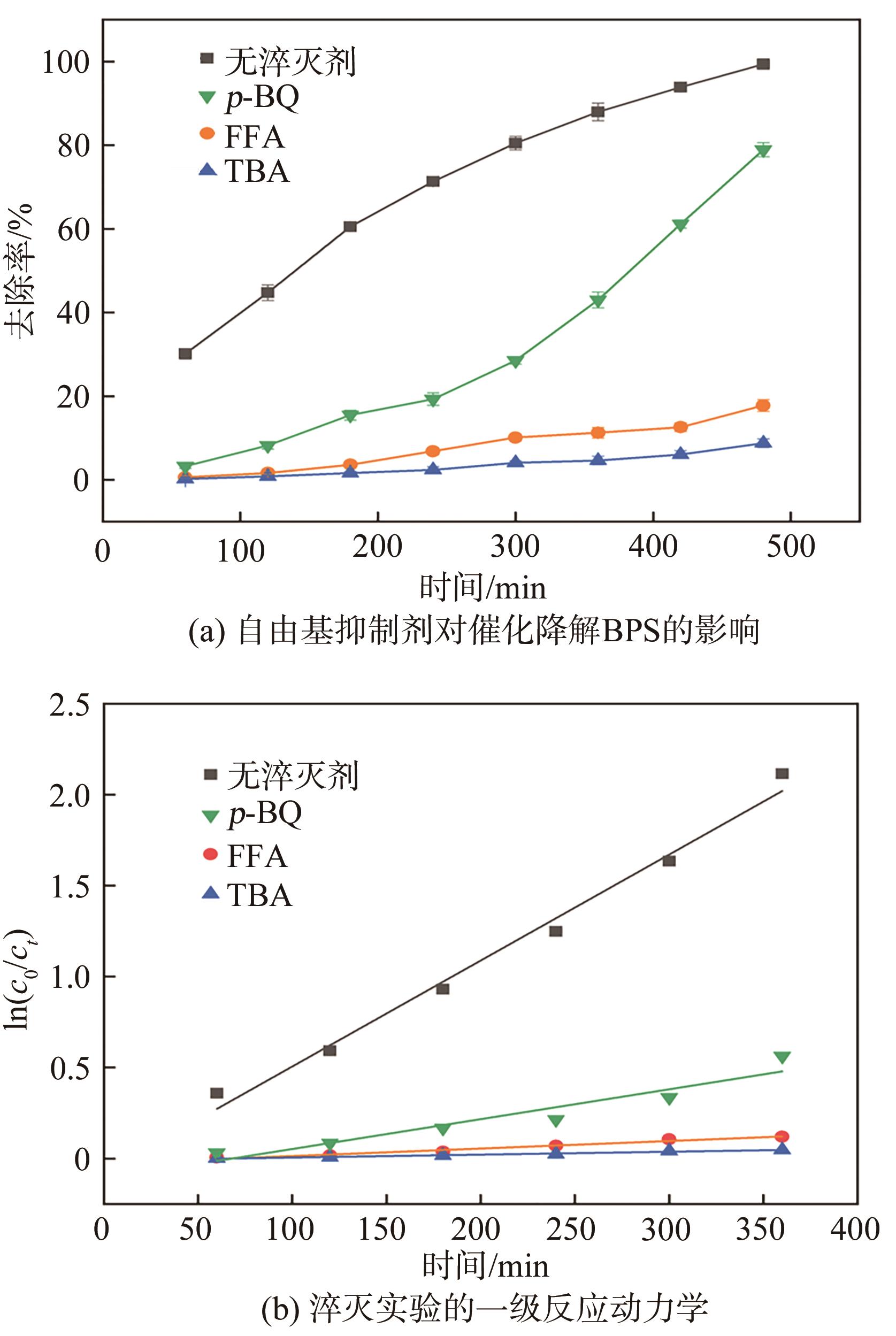
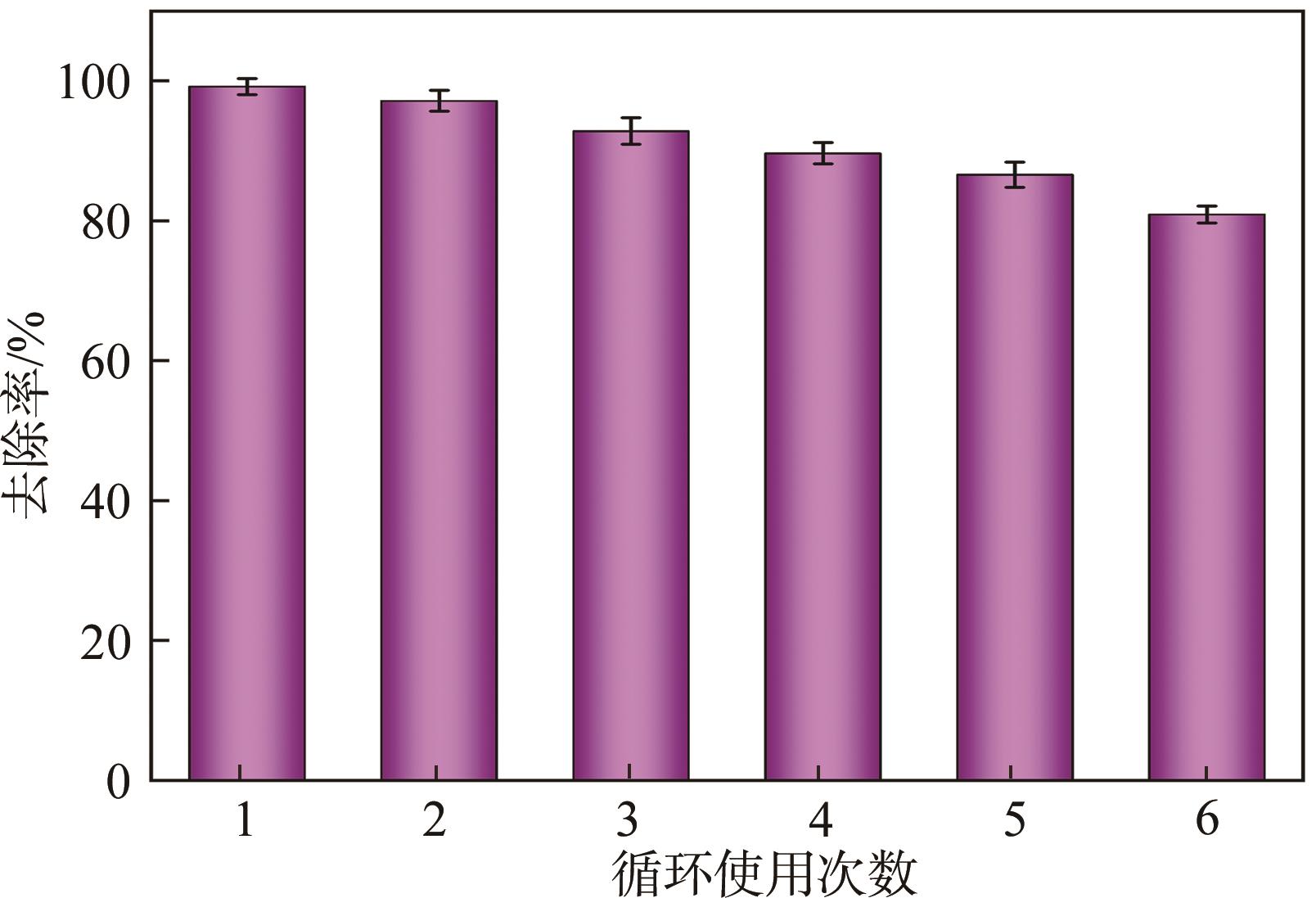
传统的芬顿反应因在废水处理过程中会产生大量富含金属污泥而应用受限。本研究采用悬浮固化法得到了一种低成本、易回收、绿色的多孔地质聚合物微球(GM),将其作为载体通过浸渍法制备了载铜地质聚合物微球(Cu-GM),作为类芬顿反应催化剂,催化H2O2降解水中的双酚S(BPS)。SEM、XRD、BET和XPS等一系列表征结果表明,Cu+/2+被稳定固定在GM表面。进一步探究了Cu-GM用量、H2O2用量、BPS浓度和溶液初始pH对催化降解的影响。结果表明,在优化条件下,Cu-GM在480min内对BPS的去除率可达99.3%,催化降解过程符合一级反应动力学。通过自由基淬灭实验发现,在催化降解过程中·OH和1O2是主要活性物质。循环实验表明Cu-GM具有良好的重复利用性,在去除水中有机污染物方面有着极大的应用潜力。
中图分类号:
引用本文
张政, 刘琳, 李子晨, 王梦琦, 黄春燕, 葛圆圆. 载铜地质聚合物微球的制备及其催化降解双酚S的性能[J]. 化工进展, 2024, 43(9): 5290-5301.
ZHANG Zheng, LIU Lin, LI Zichen, WANG Mengqi, HUANG Chunyan, GE Yuanyuan. Preparation of copper-loaded geopolymer microspheres and their catalytic degradation of bisphenol S[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5290-5301.
催化剂添加量 /g | 一级动力学模型参数 | 二级动力学模型参数 | ||
|---|---|---|---|---|
| k1×103/min -1 | R2 | k2×104/L·mg -1·min -1 | R2 | |
| 0.02 | 3.78 | 0.995 | 8.24 | 0.938 |
| 0.04 | 5.82 | 0.985 | 21.5 | 0.849 |
| 0.06 | 6.75 | 0.980 | 31.8 | 0.814 |
| 0.08 | 8.22 | 0.960 | 57.4 | 0.707 |
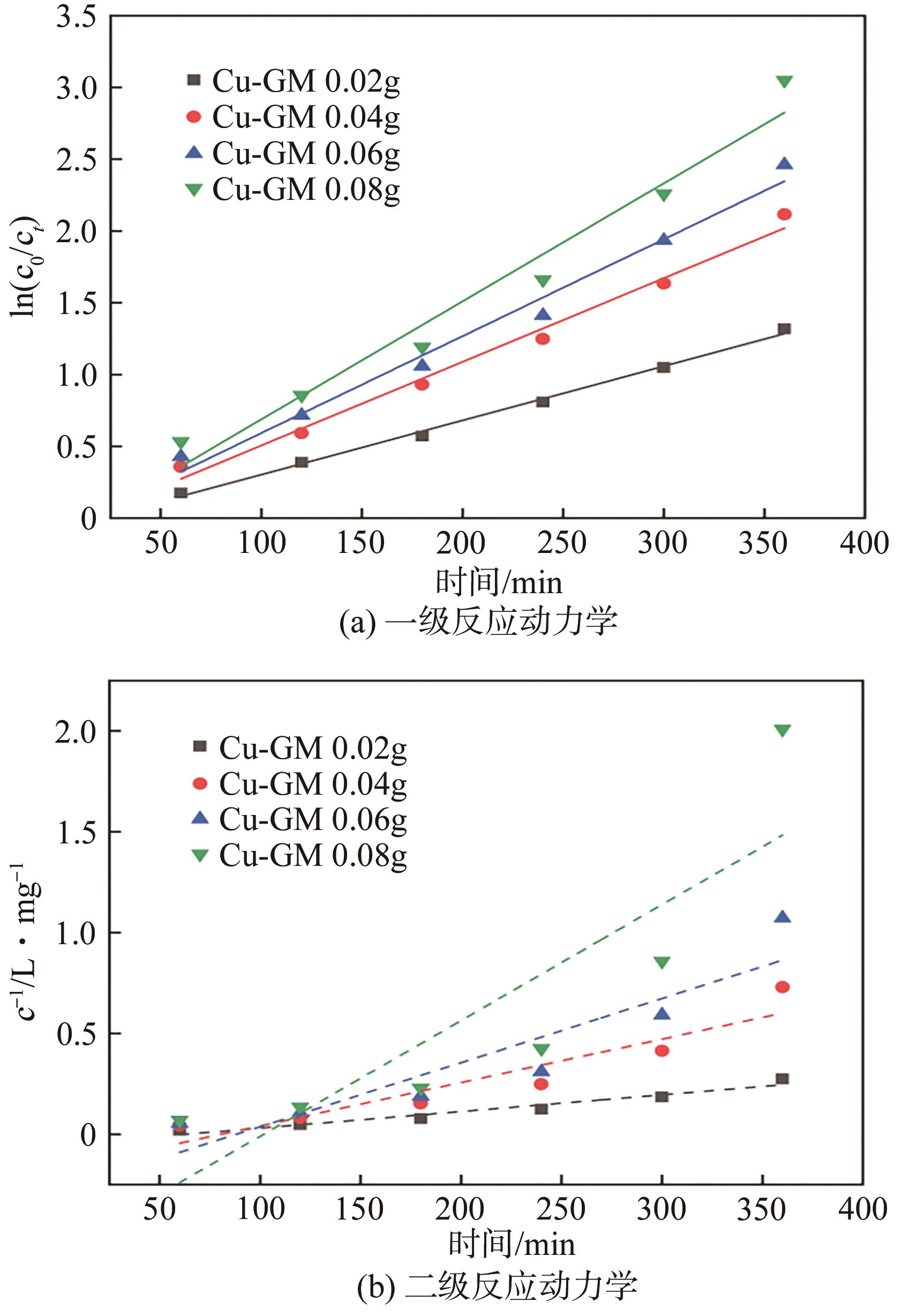
表1 不同Cu-GM添加量下催化降解BPS的动力学参数
催化剂添加量 /g | 一级动力学模型参数 | 二级动力学模型参数 | ||
|---|---|---|---|---|
| k1×103/min -1 | R2 | k2×104/L·mg -1·min -1 | R2 | |
| 0.02 | 3.78 | 0.995 | 8.24 | 0.938 |
| 0.04 | 5.82 | 0.985 | 21.5 | 0.849 |
| 0.06 | 6.75 | 0.980 | 31.8 | 0.814 |
| 0.08 | 8.22 | 0.960 | 57.4 | 0.707 |
H2O2添加量 /mL | 一级动力学模型 | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min -1 | R2 | k2×104/L·mg -1·min -1 | R2 | |
| 0.25 | 4.05 | 0.992 | 8.06 | 0.931 |
| 0.50 | 5.82 | 0.985 | 21.5 | 0.848 |
| 0.75 | 7.05 | 0.972 | 34. 6 | 0.774 |
| 1.00 | 9.16 | 0.937 | 77.9 | 0.605 |
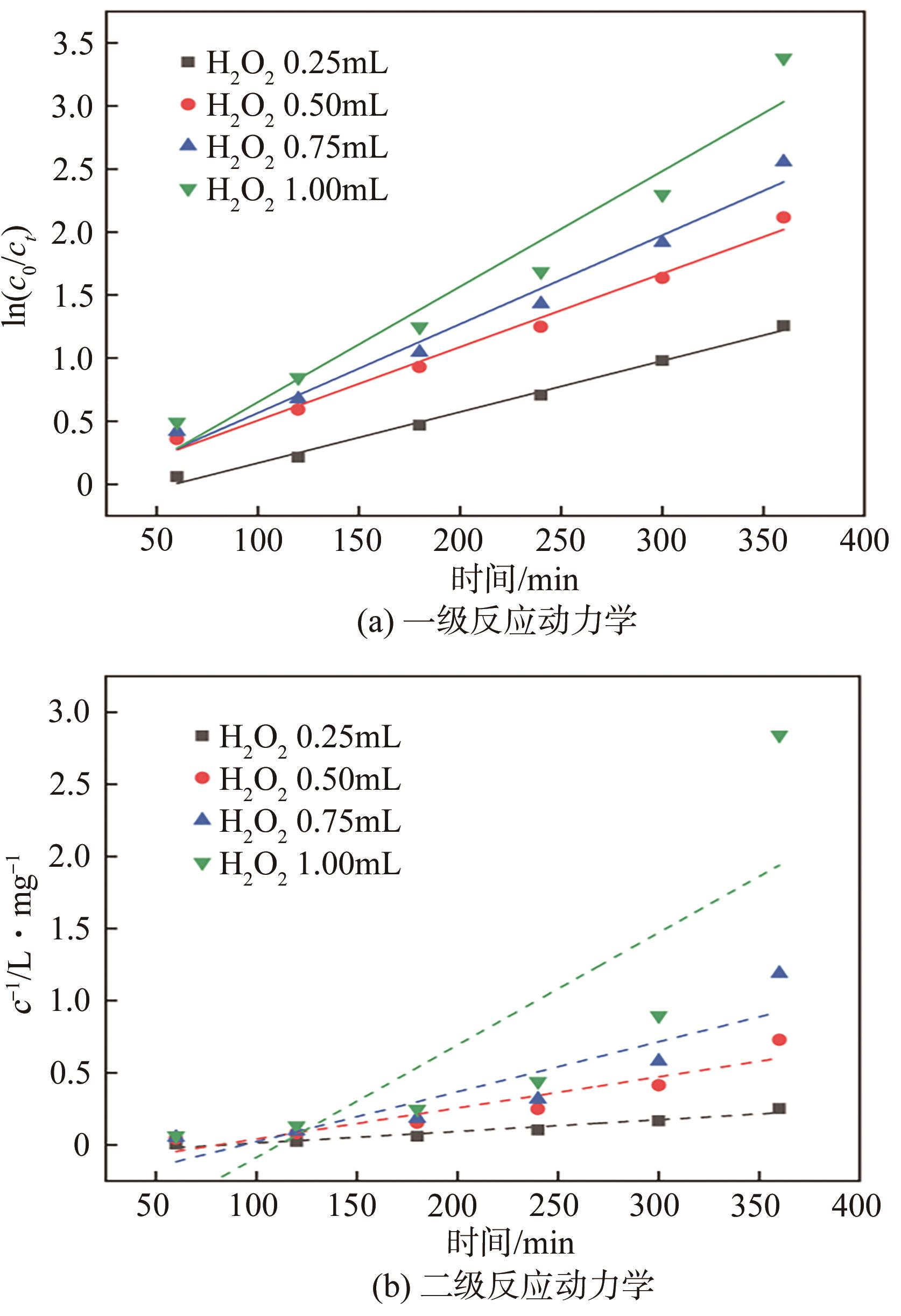
表2 不同H2O2添加量下催化降解BPS的动力学参数
H2O2添加量 /mL | 一级动力学模型 | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min -1 | R2 | k2×104/L·mg -1·min -1 | R2 | |
| 0.25 | 4.05 | 0.992 | 8.06 | 0.931 |
| 0.50 | 5.82 | 0.985 | 21.5 | 0.848 |
| 0.75 | 7.05 | 0.972 | 34. 6 | 0.774 |
| 1.00 | 9.16 | 0.937 | 77.9 | 0.605 |
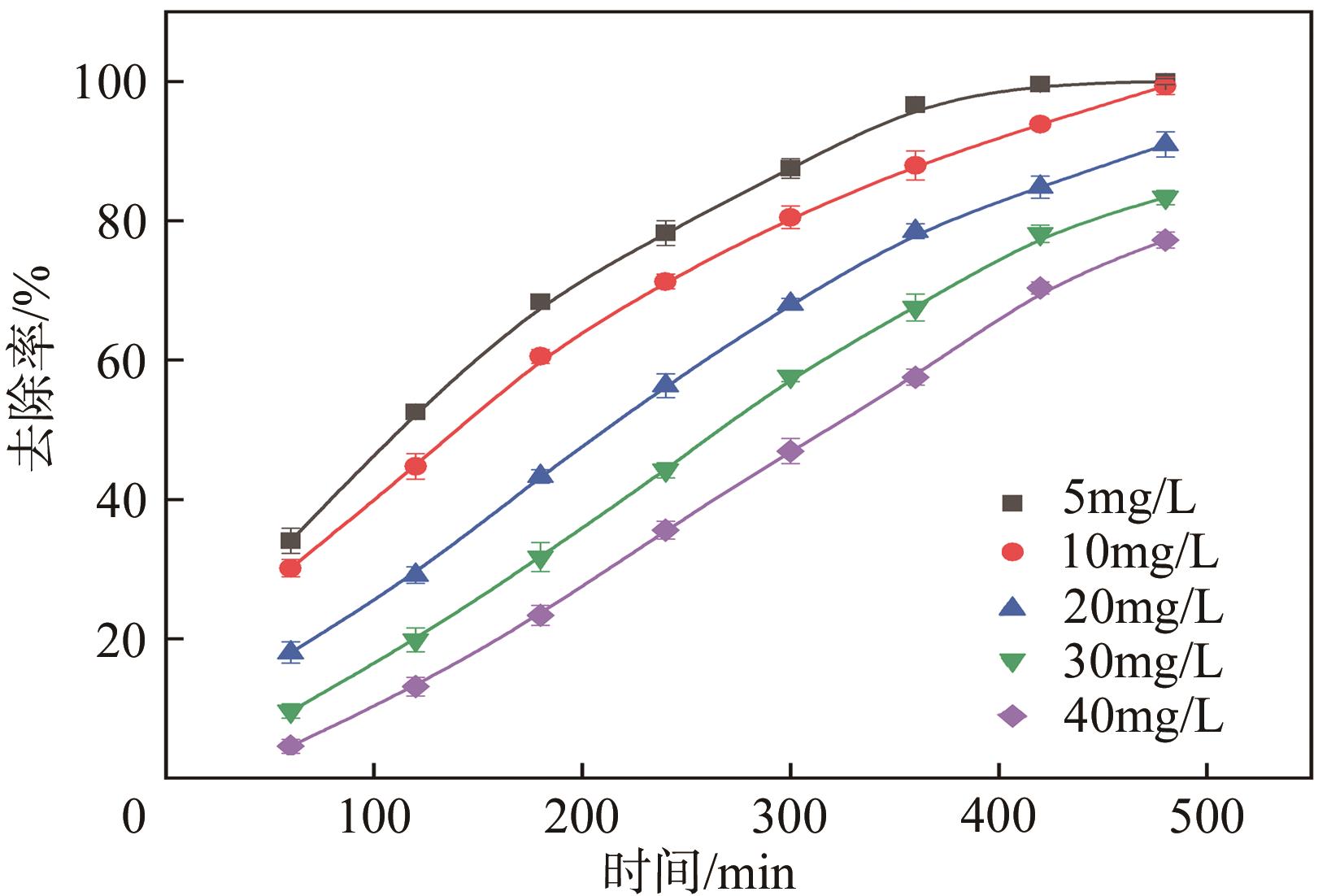
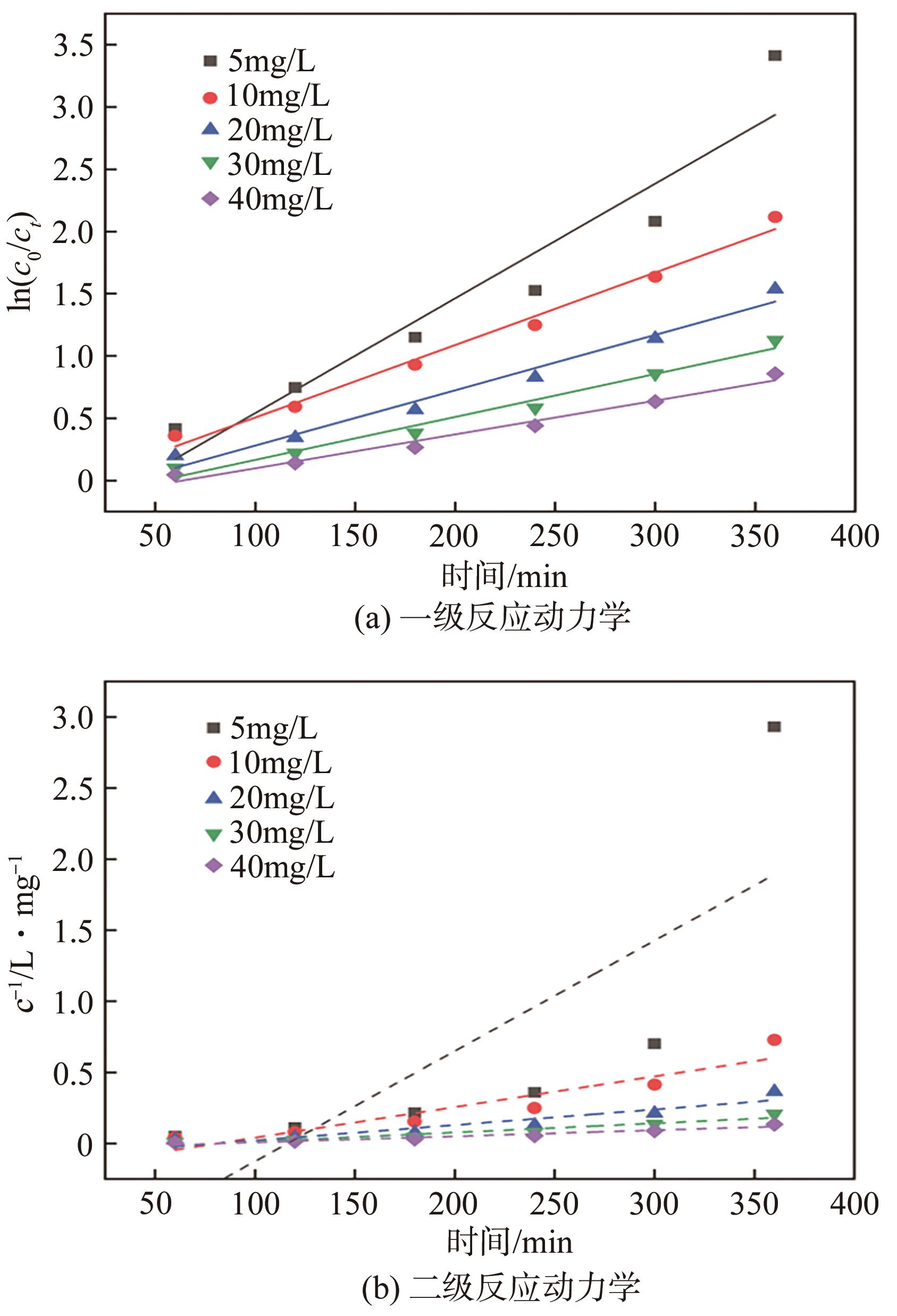
BPS浓度 /mg·L-1 | 一级动力学模型` | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min-1 | R2 | k2×104/L·mg-1·min-1 | R2 | |
| 5 | 9.22 | 0.896 | 77. 7 | 0.530 |
| 10 | 5.82 | 0.985 | 21.5 | 0.848 |
| 20 | 4.45 | 0.969 | 10.9 | 0.860 |
| 30 | 3.45 | 0.971 | 6.46 | 0.900 |
| 40 | 2.72 | 0.973 | 4.28 | 0.920 |
表3 不同BPS浓度下催化降解BPS的动力学参数
BPS浓度 /mg·L-1 | 一级动力学模型` | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min-1 | R2 | k2×104/L·mg-1·min-1 | R2 | |
| 5 | 9.22 | 0.896 | 77. 7 | 0.530 |
| 10 | 5.82 | 0.985 | 21.5 | 0.848 |
| 20 | 4.45 | 0.969 | 10.9 | 0.860 |
| 30 | 3.45 | 0.971 | 6.46 | 0.900 |
| 40 | 2.72 | 0.973 | 4.28 | 0.920 |
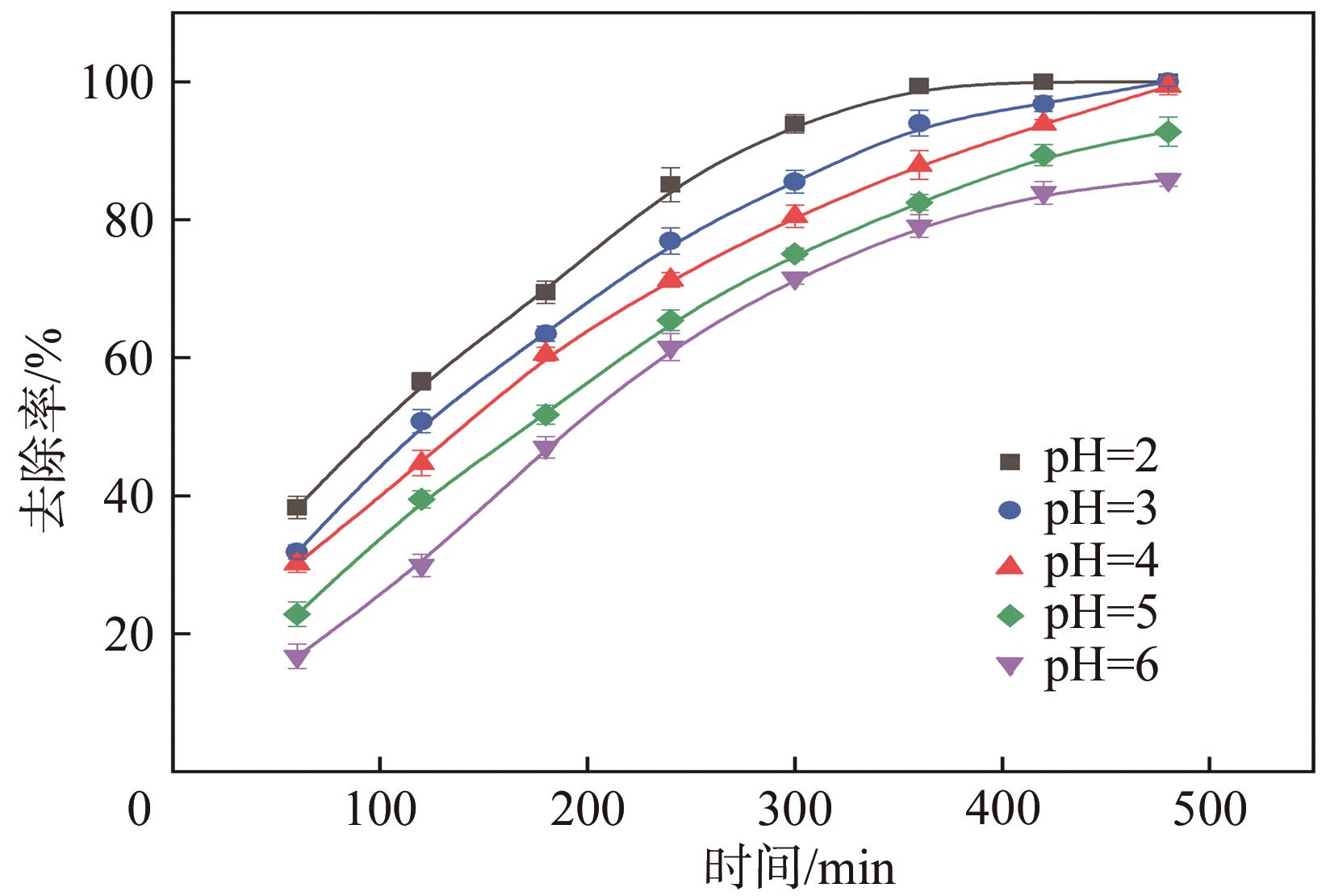
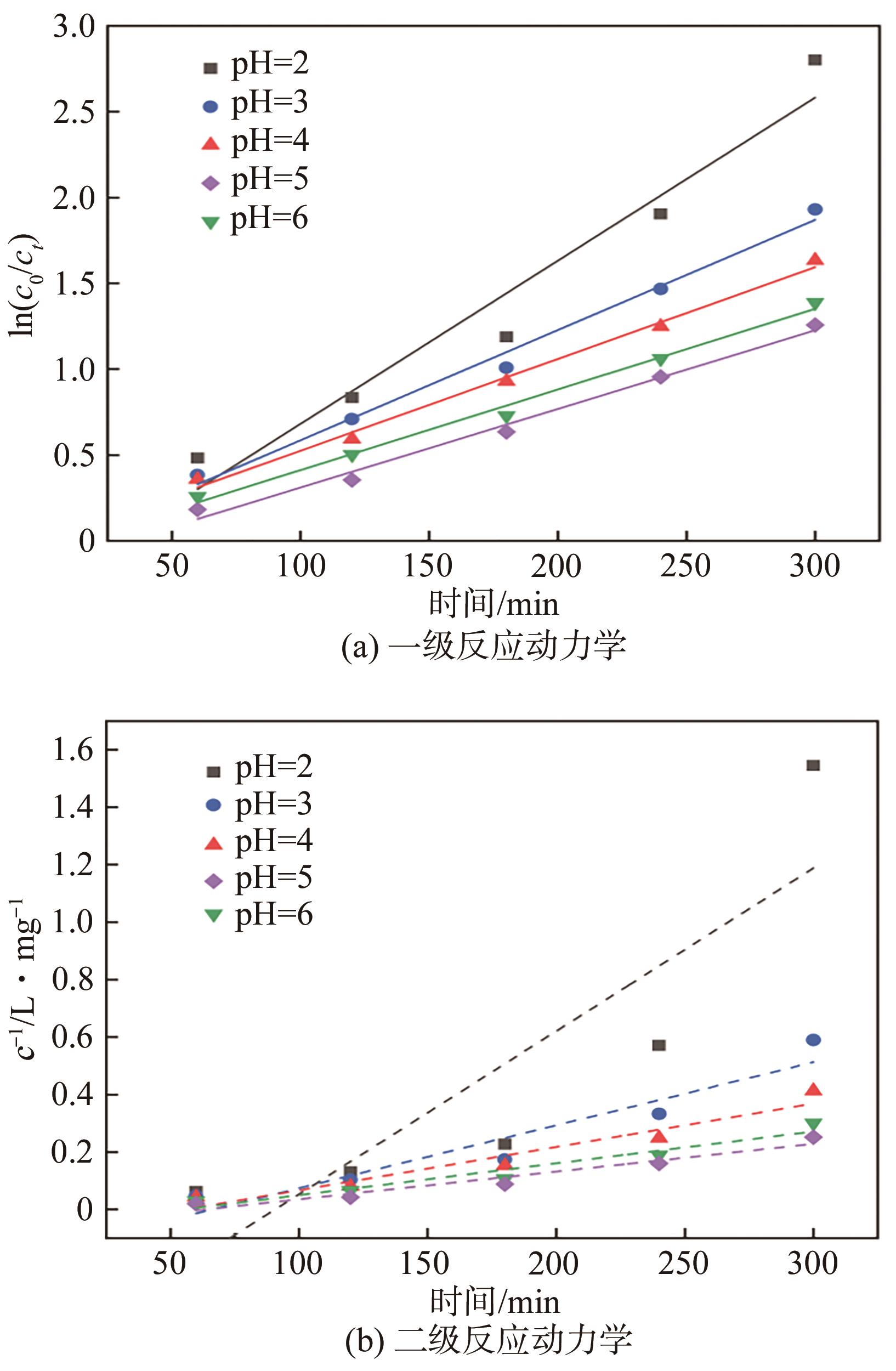
| pH | 一级动力学模型 | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min-1 | R2 | k2×104/L·mg-1·min-1 | R2 | |
| 2 | 9.51 | 0.938 | 414.8 | 0.376 |
| 3 | 6.42 | 0.986 | 44.0 | 0.676 |
| 4 | 5.35 | 0.991 | 21.5 | 0.848 |
| 5 | 4.70 | 0.989 | 1.68 | 0.899 |
| 6 | 4.58 | 0.985 | 4.87 | 0.921 |
表4 不同pH下催化降解BPS的动力学参数
| pH | 一级动力学模型 | 二级动力学模型 | ||
|---|---|---|---|---|
| k1×103/min-1 | R2 | k2×104/L·mg-1·min-1 | R2 | |
| 2 | 9.51 | 0.938 | 414.8 | 0.376 |
| 3 | 6.42 | 0.986 | 44.0 | 0.676 |
| 4 | 5.35 | 0.991 | 21.5 | 0.848 |
| 5 | 4.70 | 0.989 | 1.68 | 0.899 |
| 6 | 4.58 | 0.985 | 4.87 | 0.921 |
| 催化剂 | 外观形貌 | pH | 浓度/mg·L-1 | 去除率/% | 参考文献 |
|---|---|---|---|---|---|
| Cu-GM | 微米级球形 | 4 | 10 | 99.3 | 本文 |
| CuFe2O4/SBC | 纳米级颗粒 | 7 | 20 | 84.5 | [ |
| CoFe-PBA | 纳米级颗粒 | 5.89 | 20 | 73.77 | [ |
| A-boron | 纳米级颗粒 | 7 | 10 | 97.0 | [ |
| S-nZVI | 纳米级颗粒 | 5.6 | 5 | 92.8 | [ |
| CuCo2S4 | 纳米片 | 7.2 | 10μmol/L | 100 | [ |
表5 Cu-GM与文献中其他催化剂催化降解BPS实验效果的对比
| 催化剂 | 外观形貌 | pH | 浓度/mg·L-1 | 去除率/% | 参考文献 |
|---|---|---|---|---|---|
| Cu-GM | 微米级球形 | 4 | 10 | 99.3 | 本文 |
| CuFe2O4/SBC | 纳米级颗粒 | 7 | 20 | 84.5 | [ |
| CoFe-PBA | 纳米级颗粒 | 5.89 | 20 | 73.77 | [ |
| A-boron | 纳米级颗粒 | 7 | 10 | 97.0 | [ |
| S-nZVI | 纳米级颗粒 | 5.6 | 5 | 92.8 | [ |
| CuCo2S4 | 纳米片 | 7.2 | 10μmol/L | 100 | [ |
| 1 | XING Jianing, ZHANG Siyi, ZHANG Miaolian, et al. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A[J]. Comparative Biochemistry and Physiology C: Toxicology & Pharmacology, 2022, 254: 109275. |
| 2 | SUN Xiaoli, PENG Junyu, WANG Muhua, et al. Determination of nine bisphenols in sewage and sludge using dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography tandem mass spectrometry[J]. Journal of Chromatography A, 2018, 1552: 10-16. |
| 3 | LALWANI Dipa, RUAN Yuefei, TANIYASU Sachi, et al. Nationwide distribution and potential risk of bisphenol analogues in Indian waters[J]. Ecotoxicology and Environmental Safety, 2020, 200: 110718. |
| 4 | 侯爽, 隋倩. 华东地区某市重点行业优控环境激素的筛选及分布特征[J]. 化工进展, 2019, 38(2): 1140-1145. |
| HOU Shuang, SUI Qian. Screening and distribution of priority environmental hormones in the key industrial sectors in a city of East China[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 1140-1145. | |
| 5 | EDAES Felipe Sanches, DE SOUZA Cleide Barbieri. BPS and BPF are as carcinogenic as BPA and are not viable alternatives for its replacement[J]. Endocrine, Metabolic & Immune Disorders Drug Targets, 2022, 22(9): 927-934. |
| 6 | MENDY Angelico, SALO Pӓivi M, WILKERSON Jesse, et al. Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes[J]. Environmental Research, 2020, 183: 108944. |
| 7 | 国务院办公厅关于印发新污染物治理行动方案的通知[J]. 中华人民共和国国务院公报, 2022(16): 34-39. |
| Notice of The General Office of the State Council on printing and distributing the action plan for new pollutant control[J]. Gazette of the State Council of the People’s Republic of China, 2022(16): 34-39. | |
| 8 | WIEGAND Hanna Laura, Mischa JÜTTE, KLEIN Katharina, et al. Influences of pH, reagent dose, and water matrix components on the formation and utilization of hydroxyl radicals in the oxidation of bisphenol S and para-chlorobenzoic acid by the Fenton reaction[J]. ACS ES&T Water, 2023, 3(3): 629-638. |
| 9 | 胡德皓, 孙亮, 毛慧敏, 等. 芬顿氧化技术处理废水中难降解有机物的应用进展[J]. 山东化工, 2019, 48(7): 60-62. |
| HU Dehao, SUN Liang, MAO Huimin, et al. Research progress of the Fenton oxidation technology for treatment of refractory organics[J]. Shandong Chemical Industry, 2019, 48(7): 60-62. | |
| 10 | ZHAO Xiaoyu, ZHANG Zhenghua. FeOCl in advanced oxidization processes for water purification: A critical review[J]. Current Pollution Reports, 2023, 9(2): 143-164. |
| 11 | 李兴发, 胡浩栋, 王朝旭. 缓冲溶液法制备高效的铁基类芬顿催化剂[J]. 化工进展, 2020, 39(11): 4456-4461. |
| LI Xingfa, HU Haodong, WANG Chaoxu. Preparation of iron-based Fenton-like catalyst with high efficiency by buffer solution method[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4456-4461. | |
| 12 | THOMAS Nishanth, DIONYSIOU Dionysios D, PILLAI Suresh C. Heterogeneous Fenton catalysts: A review of recent advances[J]. Journal of Hazardous Materials, 2021, 404: 124082. |
| 13 | BONFIM Daniela P F, SANTANA Cássia S, BATISTA Marcelo S, et al. Catalytic evaluation of CuO/[Si]MCM-41 in Fenton-like reactions[J]. Chemical Engineering & Technology, 2019, 42(4): 882-888. |
| 14 | CAO Wenrui, HAN Muen, Lai LYU, et al. Efficient fenton-like process induced by fortified electron-rich O microcenter on the reduction state Cu-doped CNO polymer[J]. ACS Applied Materials & Interfaces, 2019, 11(18): 16496-16505. |
| 15 | LIAO Weixiang, Lai LYU, WANG Di, et al. Graphitized Cu-β-cyclodextrin polymer driving an efficient dual-reaction-center Fenton-like process by utilizing electrons of pollutants for water purification[J]. Journal of Environmental Sciences, 2023, 126: 565-574. |
| 16 | LIU Juan, CUI Jianan, ZHAO Tianyu, et al. Fe3O4-CeO2 loaded on modified activated carbon as efficient heterogeneous catalyst[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 565: 59-69. |
| 17 | LIU Hongrun, LIU Yuankun, LI Xing, et al. Adsorption and Fenton-like degradation of ciprofloxacin using corncob biochar-based magnetic iron-copper bimetallic nanomaterial in aqueous solutions[J]. Nanomaterials, 2022, 12(4): 579. |
| 18 | SHANGGUAN Zichen, YUAN Xingzhong, QIN Chencheng, et al. Improving the removal of tetracycline via carbonate-mediated triplet-excited state by the Cu-containing zeolites activated percarbonate[J]. Chemical Engineering Journal, 2023, 457: 141046. |
| 19 | SAZAMA Petr, BORTNOVSKY Oleg, Jiří DĚDEČEK, et al. Geopolymer based catalysts—New group of catalytic materials[J]. Catalysis Today, 2011, 164(1): 92-99. |
| 20 | THAKUR Nimisha, Farooq WAHAB M, KHANAL Durga D, et al. Synthetic aluminosilicate based geopolymers—Second generation geopolymer HPLC stationary phases[J]. Analytica Chimica Acta, 2019, 1081: 209-217. |
| 21 | TANG Qing, GE Yuanyuan, WANG Kaituo, et al. Preparation and characterization of porous metakaolin-based inorganic polymer spheres as an adsorbent[J]. Materials & Design, 2015, 88: 1244-1249. |
| 22 | THAKUR Nimisha, ARMSTRONG Daniel W. Arsenic sequestration by iron oxide coated geopolymer microspheres[J]. Journal of Cleaner Production, 2021, 291: 125931. |
| 23 | YU Zefeng, SONG Weifeng, LI Jiayao, et al. Improved simultaneous adsorption of Cu(Ⅱ) and Cr (Ⅵ) of organic modified metakaolin-based geopolymer[J]. Arabian Journa of Chemistry, 2020, 13(3): 4811-4823. |
| 24 | GE Yuanyuan, CUI Xuemin, KONG Yan, et al. Porous geopolymeric spheres for removal of Cu(Ⅱ) from aqueous solution: Synthesis and evaluation[J]. Journal of Hazardous Materials, 2015, 283: 244-251. |
| 25 | SHAN Haidi, WANG Xiaoyun, GE Yuanyuan, et al. Homologous amino acids promoted co-immobilization of laccase and mediator onto geopolymer microspheres for enhancing degradation of dyes in water[J]. Journal of Hazardous Materials, 2022, 423: 127107. |
| 26 | WANG Xiaoyun, ZHANG Zheng, GE Yuanyuan. Oleic acid-tailored geopolymer microspheres with tunable porous structure for enhanced removal from tetracycline in saline water[J]. Sustainability, 2022, 14(11): 6705. |
| 27 | UPADHYAY Jyoti, MISRA Swayam Prabha, IRUSTA Silvia, et al. Oxidation of aldehydes to carboxylic acids over geopolymer supported CuO[J]. Molecular Catalysis, 2023, 536: 112911. |
| 28 | SALAM Mohamed Abdel, MOKHTAR Mohamed, ALBUKHARI SOHA M, et al. Insight into the role of the zeolitization process in enhancing the adsorption performance of kaolinite/diatomite geopolymer for effective retention of Sr(Ⅱ) ions; batch and column studies[J]. Journal of Environmental Management, 2021, 294: 112984. |
| 29 | WU Honghai, XIE Hanrui, HE Guangping, et al. Effects of the pH and anions on the adsorption of tetracycline on iron-montmorillonite[J]. Applied Clay Science, 2016, 119: 161-169. |
| 30 | REN Yi, LIU Chao, LI Naiwen, et al. A bimetallic co-doped iron and copper in polymeric graphitic carbon nitride to enhance catalytic activity and reusability of Fenton-like oxidation[J]. Separation and Purification Technology, 2023, 316: 123782. |
| 31 | CHEN Aitao, XIAO Jun, KONG Xiao, et al. Regulating the charge density of Cu(Ⅰ) single sites enriched on the surface of N3c vacancies-engineered g-C3N4 for efficient Fenton-like reactions[J]. Separation and Purification Technology, 2023, 314: 123525. |
| 32 | KURODA Yasushige, ITADANI Atsushi, KUMASHIRO Ryotaro, et al. Anomalous valence changes and specific dinitrogen adsorption features of copper ion exchanged in ZSM-5 zeolite prepared from an aqueous solution of [Cu(NH3)2]+ [J]. Physical Chemistry Chemical Physics, 2004, 6(9): 2534-2541. |
| 33 | LIU Yong, FAN Qin, WANG Jianlong. Zn-Fe-CNTs catalytic in situ generation of H2O2 for Fenton-like degradation of sulfamethoxazole[J]. Journal of Hazardous Materials, 2018, 342: 166-176. |
| 34 | 刘创创, 王逸, 周丽华, 等. 纳米N-C/ZrO2活化过硫酸盐降解苯酚的机理研究[J]. 环境科学学报, 2021, 41(10): 3956-3968. |
| LIU Chuangchuang, WANG Yi, ZHOU Lihua, et al. The mechanism on activation of peroxymonosulfate using nano N-C/ZrO2 for phenol degradation[J]. Acta Scientiae Circumstantiae, 2021, 41(10): 3956-3968. | |
| 35 | WANG Lihong, JIANG Jin, MA Jun, et al. A review on advanced oxidation processes homogeneously initiated by copper(Ⅱ)[J]. Chemical Engineering Journal, 2022, 427: 131721. |
| 36 | YANG Youwei, GUO Changsheng, ZENG Yiting, et al. Peroxymonosulfate activation by CuFe-Prussian Blue analogues for the degradation of bisphenol S: Effect, mechanism, and pathway[J]. Chemosphere, 2023, 331: 138748. |
| 37 | WANG Bingyu, LI Qiaoqiao, Ying LYU, et al. Insights into the mechanism of peroxydisulfate activated by magnetic spinel CuFe2O4/SBC as a heterogeneous catalyst for bisphenol S degradation[J]. Chemical Engineering Journal, 2021, 416: 129162. |
| 38 | 杨有威, 曾亦婷, 郭昌胜, 登. 类普鲁士蓝的制备及其活化PMS降解双酚S[J]. 化工进展, 2023, 42(12): 6676-6686. |
| YANG Youwei, ZENG Yiting, GUO Changsheng, et al. Preparation of Prussian Blue and its activation of PMS for degrading bisphenol S[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6676-6686. | |
| 39 | SHAO Penghui, DUAN Xiaoguang, XU Jun, et al. Heterogeneous activation of peroxymonosulfate by amorphous boron for degradation of bisphenol S[J]. Journal of Hazardous Materials, 2017, 322: 532-539. |
| 40 | CAI Jing, ZHANG Yan. Enhanced degradation of bisphenol S by persulfate activated with sulfide-modified nanoscale zero-valent iron[J]. Environmental Science and Pollution Research, 2022, 29(6): 8281-8293. |
| 41 | XU Haodan, WANG Da, MA Jun, et al. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S[J]. Applied Catalysis B: Environmental, 2018, 238: 557-567. |
| [1] | 杨有威, 曾亦婷, 郭昌胜, 罗玉霞, 高艳, 王春英. 类普鲁士蓝的制备及其活化PMS降解双酚S[J]. 化工进展, 2023, 42(12): 6676-6686. |
| [2] | 付佳, 谌伦建, 徐冰, 华绍烽, 李从强, 杨明坤, 邢宝林, 仪桂云. 模拟煤炭气化废水中苯酚的微生物降解[J]. 化工进展, 2023, 42(1): 526-537. |
| [3] | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| [4] | 吕莹, 胡学武, 陈素素, 刘兴宇, 陈勃伟, 张明江. 多环芳烃污染土壤的微生物修复技术研究进展[J]. 化工进展, 2022, 41(6): 3249-3262. |
| [5] | 周永泉, 张艾, 刘亚男, 王铮. 等离子体射流耦合活性碳纤维去除水中糖皮质激素[J]. 化工进展, 2022, 41(4): 2209-2215. |
| [6] | 翟重渊, 赵丹荻, 何亚鹏, 黄惠, 陈步明, 郭忠诚. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626. |
| [7] | 王吉坤, 李阳, 陈贵锋, 刘敏, 寇丽红, 王琦, 何毅聪. 臭氧催化氧化降解煤化工高盐废水有机物的机理[J]. 化工进展, 2022, 41(1): 493-502. |
| [8] | 黄嘉绮, 葛圆圆, 李志礼, 王艺频, 崔学民. 生物炭/地聚物复合膜的制备及其对四环素的去除[J]. 化工进展, 2022, 41(1): 427-434. |
| [9] | 梁一尊, 葛艳清, 王驰, 李凯, 梅毅. 低维黑磷的制备及其在光催化降解领域的应用研究进展[J]. 化工进展, 2021, 40(2): 845-858. |
| [10] | 罗艳红, 岳秀萍, 姜悦如, 赵博玮, 高艳娟, 段燕青. 高级氧化技术降解吲哚的研究进展[J]. 化工进展, 2021, 40(2): 1025-1034. |
| [11] | 杨硕, 余薇薇, 杨伦, 杜邦昊, 谢明原, 赵晨菊, 万巧玲, 潘伟亮. 纳米零价铁降解水中17β-雌二醇的作用机制[J]. 化工进展, 2020, 39(9): 3826-3834. |
| [12] | 赵朝成, 吴光锐. MOFs复合材料催化降解水中有机污染物的应用研究进展[J]. 化工进展, 2019, 38(04): 1775-1784. |
| [13] | 崔喜, 刘冰灵, 赫崇衡, 田恒水. 脂肪族聚醚型聚氨酯弹性体热降解机理及热稳定性[J]. 化工进展, 2016, 35(11): 3585-3589. |
| [14] | 叶林静, 安小英, 姜韵婕, 闫超, 关卫省. ZnO/CdS复合光催化剂的制备及降解四环素类抗生素[J]. 化工进展, 2015, 34(11): 3944-3950. |
| [15] | 宫慧勇, 蒋晶晶, 刘韶泽, 郭永, 李作鹏. 纳米氧化亚铜的形貌控制合成及光催化降解有机染料的研究进展[J]. 化工进展, 2015, 34(11): 3915-3925. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||