化工进展 ›› 2023, Vol. 42 ›› Issue (12): 6676-6686.DOI: 10.16085/j.issn.1000-6613.2023-0803
• 资源与环境化工 • 上一篇
类普鲁士蓝的制备及其活化PMS降解双酚S
杨有威1,2,3( ), 曾亦婷1,2, 郭昌胜3, 罗玉霞1,2, 高艳1,2, 王春英1,2(
), 曾亦婷1,2, 郭昌胜3, 罗玉霞1,2, 高艳1,2, 王春英1,2( )
)
- 1.矿冶环境污染防控江西省重点实验室,江西 赣州 341000
2.江西理工大学资源与环境工程学院, 江西 赣州 341000
3.中国环境科学研究院环境基准与风险评估国家重点实验室, 北京 100012
-
收稿日期:2023-05-15修回日期:2023-07-21出版日期:2023-12-25发布日期:2024-01-08 -
通讯作者:王春英 -
作者简介:杨有威(1998—),男,硕士研究生,研究方向为废水处理与资源化技术。E-mail:1287476942@qq.com。 -
基金资助:江西省自然科学基金面上项目(20232BAB203040);江西理工大学“青年优秀人才支持计划”(JXUSTQJYX2016003);2020年国家大学生创新创业训练计划(202010407004)
Preparation of Prussian blue and its activation of PMS for degrading bisphenol S
YANG Youwei1,2,3( ), ZENG Yiting1,2, GUO Changsheng3, LUO Yuxia1,2, GAO Yan1,2, WANG Chunying1,2(
), ZENG Yiting1,2, GUO Changsheng3, LUO Yuxia1,2, GAO Yan1,2, WANG Chunying1,2( )
)
- 1.Jiangxi Provincial Key Laboratory of Environmental Pollution Prevention and Control in Mining and Metallurgy, Ganzhou 341000, Jiangxi, China
2.School of Resources and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
3.State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
-
Received:2023-05-15Revised:2023-07-21Online:2023-12-25Published:2024-01-08 -
Contact:WANG Chunying
摘要:
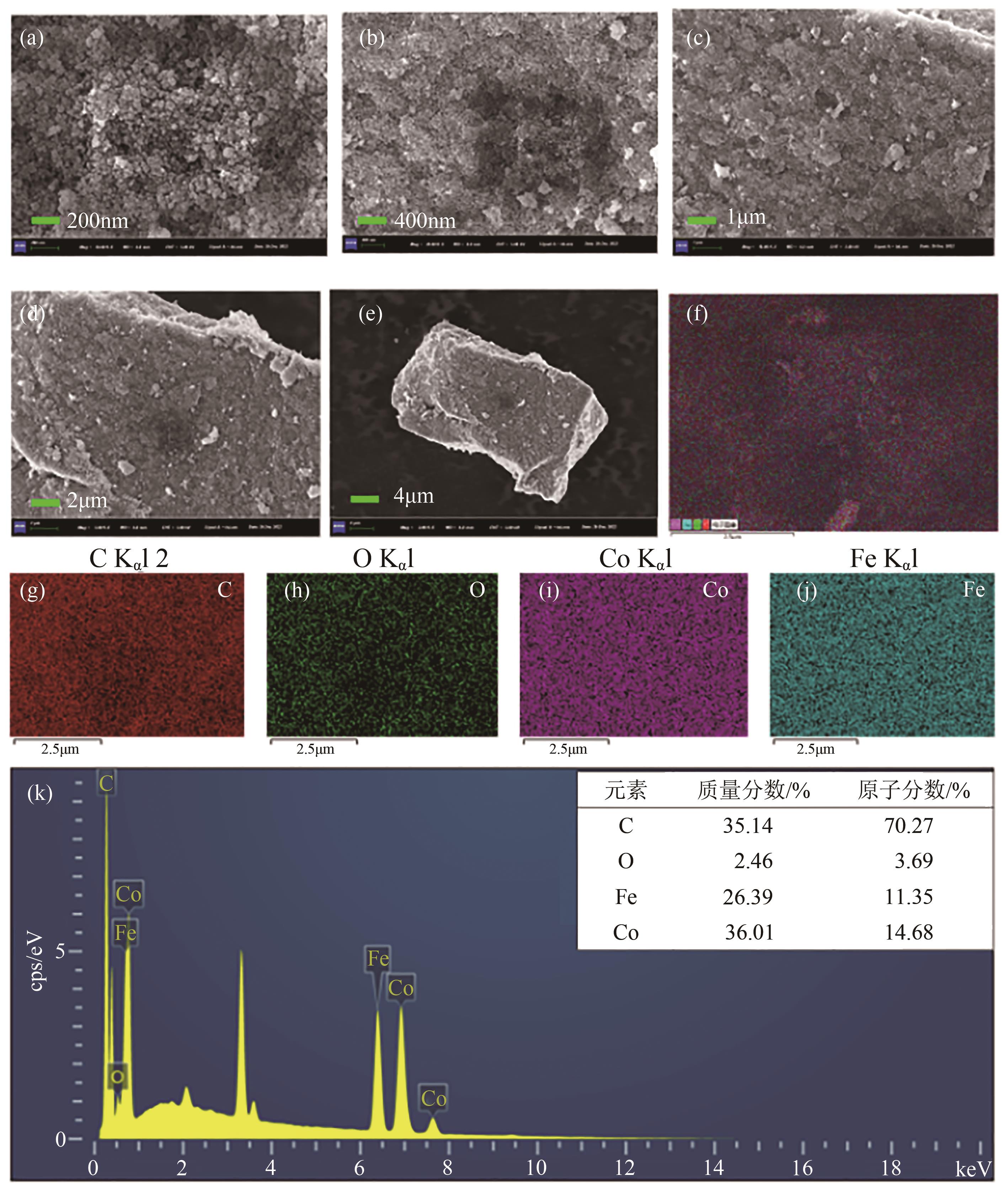
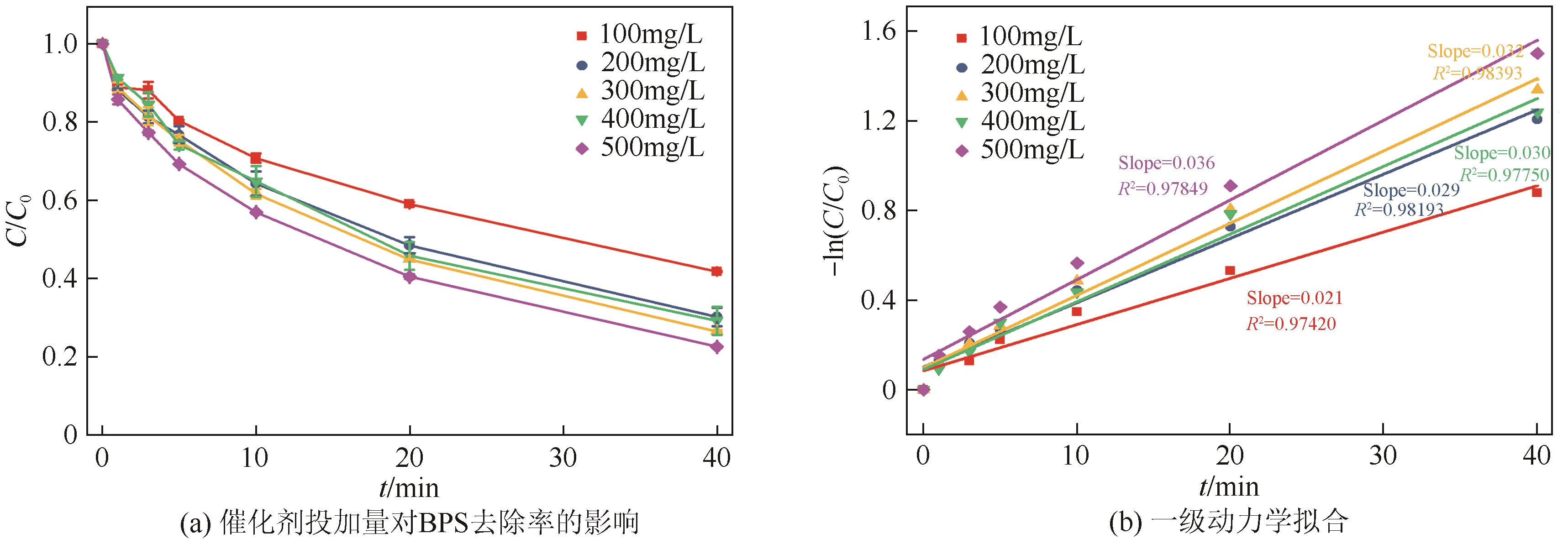
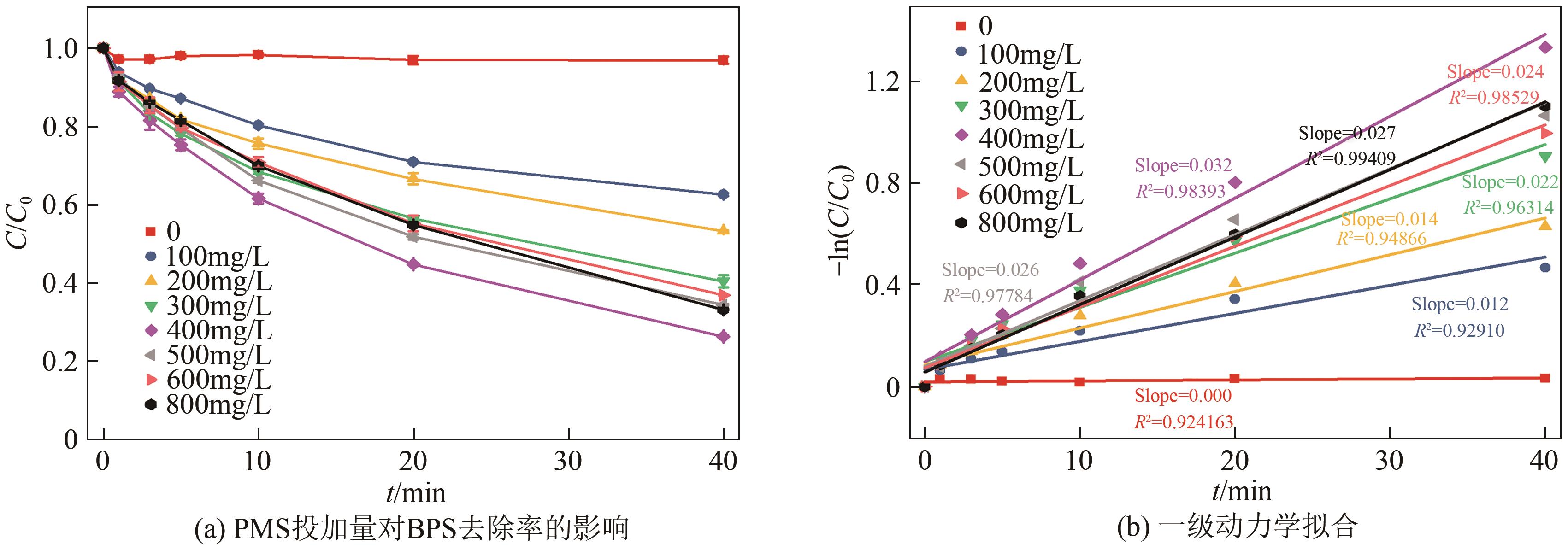
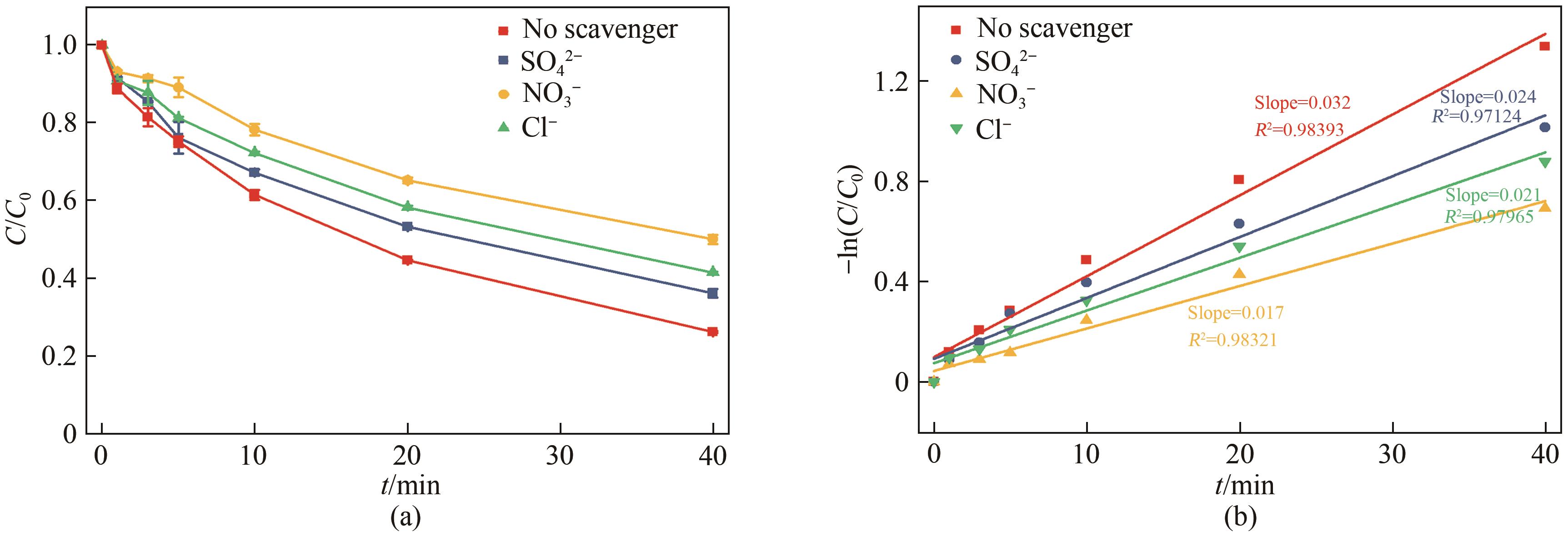
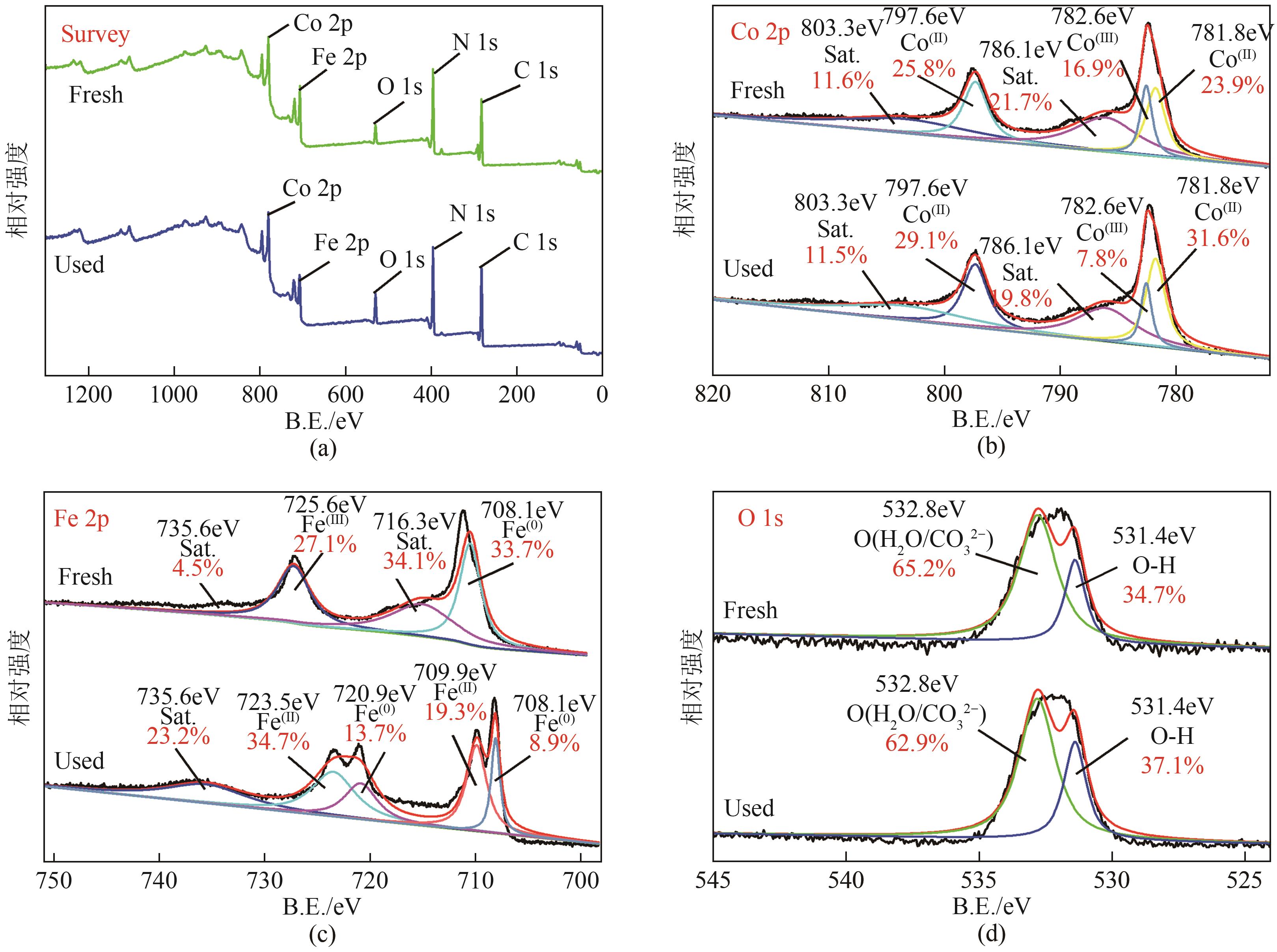
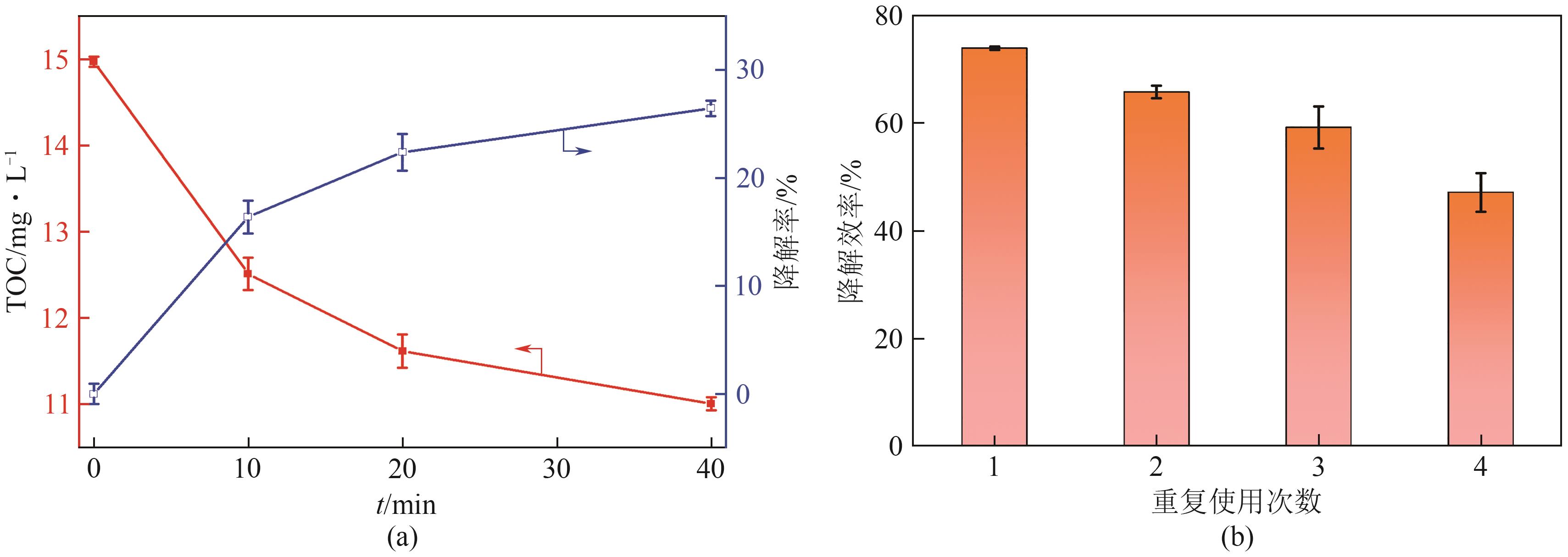
通过简单共沉淀法合成了类普鲁士蓝化合物(CoFe-PBA),用于活化过一硫酸盐(PMS)降解有机污染物双酚S(BPS)。使用扫描电镜、X射线衍射、X射线光电子能谱等手段对CoFe-PBA进行表征,结果表明CoFe-PBA由紧密结合的Co3[Fe(CN)6]2构成,为纳米级,表面均匀分布着C、Fe、Co、O元素,具有丰富的活性位点。催化剂投加量300mg/L、PMS投加量400mg/L、pH=5.89条件下,CoFe-PBA/PMS降解体系40min内去除73.77%的BPS,对酸性和共存离子(SO42-、NO3-和Cl-)敏感,碱性环境能促进PMS快速活化,重复实验显示该体系具有良好稳定性,使用4次后仅下降26.70%,活化性能优于其他材料。机理分析表明,CoFe-PBA与PMS相互作用,作用过程中改变了金属位点价态,发生电子转移,产生各种活性物质降解BPS,其主要作用活性物种为1O2;产物分析表明,在CoFe-PBA活化PMS系统中,BPS可历经三种路径最终转化为开环产物及CO2和H2O。本研究通过低耗能、低成本、快速简易的方法制备CoFe-PBA,可为活化PMS绿色降解BPS提供思路。
中图分类号:
引用本文
杨有威, 曾亦婷, 郭昌胜, 罗玉霞, 高艳, 王春英. 类普鲁士蓝的制备及其活化PMS降解双酚S[J]. 化工进展, 2023, 42(12): 6676-6686.
YANG Youwei, ZENG Yiting, GUO Changsheng, LUO Yuxia, GAO Yan, WANG Chunying. Preparation of Prussian blue and its activation of PMS for degrading bisphenol S[J]. Chemical Industry and Engineering Progress, 2023, 42(12): 6676-6686.
| 污染物 | 浓度 /mg·L-1 | 去除时间 /min | 去除率 /% | 去除速率 /mg·min-1 | 参考文献 |
|---|---|---|---|---|---|
| 双酚S | 20.0 | 40 | 73.77 | 0.3689 | 本文 |
| 双酚S | 20.0 | 120 | 84.5 | 0.1408 | [ |
| 双酚S | 5.0 | 30 | 97.7 | 0.1628 | [ |
| 双酚S | 5.0 | 60 | 92.8 | 0.0773 | [ |
| 双酚S | 2.5 | 90 | 100.0 | 0.0278 | [ |
| 双酚S | 10.0 | 150 | 97.0 | 0.0647 | [ |
表1 其他类可活化过硫酸盐降解双酚S的实验对比
| 污染物 | 浓度 /mg·L-1 | 去除时间 /min | 去除率 /% | 去除速率 /mg·min-1 | 参考文献 |
|---|---|---|---|---|---|
| 双酚S | 20.0 | 40 | 73.77 | 0.3689 | 本文 |
| 双酚S | 20.0 | 120 | 84.5 | 0.1408 | [ |
| 双酚S | 5.0 | 30 | 97.7 | 0.1628 | [ |
| 双酚S | 5.0 | 60 | 92.8 | 0.0773 | [ |
| 双酚S | 2.5 | 90 | 100.0 | 0.0278 | [ |
| 双酚S | 10.0 | 150 | 97.0 | 0.0647 | [ |
| 1 | 余翠, 武琳, 陈忠, 等. 双酚S对高脂饮食斑马鱼脂代谢的影响及机制[J]. 南京医科大学学报(自然科学版), 2020, 40(7): 981-985, 1020. |
| YU Cui, WU Lin, CHEN Zhong, et al. Effects and mechanism of BPS on lipid metabolism of zebrafish with high-fat diet[J]. Journal of Nanjing Medical University (Natural Sciences), 2020, 40(7): 981-985, 1020. | |
| 2 | YAMAZAKI Eriko, YAMASHITA Nobuyoshi, TANIYASU Sachi, et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India[J]. Ecotoxicology and Environmental Safety, 2015, 122: 565-572. |
| 3 | JIN Hangbiao, ZHU Lingyan. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China[J]. Water Research, 2016, 103: 343-351. |
| 4 | JI Kyunghee, HONG Seongjin, Younglim KHO, et al. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish[J]. Environmental Science & Technology, 2013, 47(15): 8793-8800. |
| 5 | HUANG Wei, ZHU Lin, ZHAO Chao, et al. Integration of proteomics and metabolomics reveals promotion of proliferation by exposure of bisphenol S in human breast epithelial MCF-10A cells[J]. The Science of the Total Environment, 2020, 712: 136453. |
| 6 | GOYAL Nitin, BARMAN Sanghamitra, BULASARA Vijaya Kumar. Efficient removal of bisphenol S from aqueous solution by synthesized nano-zeolite secony mobil-5[J]. Microporous and Mesoporous Materials, 2018, 259: 184-194. |
| 7 | PELAIA Tiana, RUBIN Alexander M, SEEBACHER Frank. Bisphenol S reduces locomotor performance and modifies muscle protein levels but not mitochondrial bioenergetics in adult zebrafish[J]. Aquatic Toxicology, 2023, 257: 106440. |
| 8 | IKE M, CHEN M Y, DANZL E, et al. Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions[J]. Water Science and Technology, 2006, 53(6): 153-159. |
| 9 | XUE Jingchuan, KANNAN Kurunthachalam. Mass flows and removal of eight bisphenol analogs, bisphenol A diglycidyl ether and its derivatives in two wastewater treatment plants in New York State, USA[J]. Science of the Total Environment, 2019, 648: 442-449. |
| 10 | 李林, 朱登贵, 孙淑敏, 等. 普鲁士蓝及其类似物作为钠离子电池正极材料的研究进展[J]. 分子科学学报, 2023, 39(1): 1-10. |
| LI Lin, ZHU Denggui, SUN Shumin, et al. Research progress of Prussian blue and its analogues as cathode materials for sodium ion batteries[J]. Journal of Molecular Science, 2023, 39(1): 1-10. | |
| 11 | 董沛沛, 冯永强, 王潇, 等. 多孔普鲁士蓝类似物的合成及电催化析氧性能[J]. 精细化工, 2021, 38(4): 823-829. |
| DONG Peipei, FENG Yongqiang, WANG Xiao, et al. Synthesis of porous Prussian-blue analogues and electrocatalytic properties for oxygen evolution reaction[J]. Fine Chemicals, 2021, 38(4): 823-829. | |
| 12 | 黎素, 张博, 谢春生, 等. Bi-FeC2O4复合催化剂活化过硫酸盐降解罗丹明B[J]. 环境科学学报, 2021, 41(7): 2796-2805. |
| LI Su, ZHANG Bo, XIE Chunsheng, et al. Catalytic degradation of Rhodamine B by Bi-FeC2O4 composite activated persulfate[J]. Acta Scientiae Circumstantiae, 2021, 41(7): 2796-2805. | |
| 13 | 李英豪, 郑向前, 高晓亚, 等. CoFe2O4的制备及其活化过一硫酸盐降解磺胺甲噁唑[J]. 精细化工, 2022, 39(5): 1020-1027. |
| LI Yinghao, ZHENG Xiangqian, GAO Xiaoya, et al. Preparation of CoFe2O4 and its peroxymonosulfate activation for degradation of sulfamethoxazole[J]. Fine Chemicals, 2022, 39(5): 1020-1027. | |
| 14 | NIU Lijun, ZHANG Guangming, XIAN Guang, et al. Tetracycline degradation by persulfate activated with magnetic γ-Fe2O3/CeO2 catalyst: Performance, activation mechanism and degradation pathway[J]. Separation and Purification Technology, 2021, 259: 118156. |
| 15 | YANG Youwei, GUO Changsheng, ZENG Yiting, et al. Peroxymonosulfate activation by CuFe-Prussian blue analogues for the degradation of bisphenol S: Effect, mechanism, and pathway[J]. Chemosphere, 2023, 331: 138748. |
| 16 | LAI Leiduo, YAN Jianfei, LI Jun, et al. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism[J]. Chemical Engineering Journal, 2018, 343: 676-688. |
| 17 | 王磊, 成先雄, 连军锋, 等. 尖晶石型c-CuFe2O4催化过硫酸盐降解偶氮染料[J]. 精细化工, 2021, 38(10): 2117-2124. |
| WANG Lei, CHENG Xianxiong, LIAN Junfeng, et al. Degradation of azo dye by catalyzed persulfate with spinel c-CuFe2O4 [J]. Fine Chemicals, 2021, 38(10): 2117-2124. | |
| 18 | GHANBARI Farshid, MORADI Mahsa. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 19 | 甄建政,聂士松,潘世元, 等.多维度碳基负载金属催化剂活化PMS降解水中污染物的研究进展[J].化工进展,2022,41(4):1858-1872. |
| ZHEN Jianzheng, NIE Shisong, PAN Shiyuan, et al. Research progress on advanced activation of peroxymonosulfate by multidimensional carbon-supported metal catalyst for degradation of organic pollutants in water [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1858-1872. | |
| 20 | HUANG Yaohui, HUANG Yi-Fong, HUANG Chuning, et al. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst[J]. Journal of Hazardous Materials, 2009, 170(2/3): 1110-1118. |
| 21 | 董康妮, 谢更新, 晏铭, 等. 磺化生物炭活化过硫酸盐去除水中盐酸四环素[J]. 中国环境科学, 2022, 42(8): 3650-3657. |
| DONG Kangni, XIE Gengxin, YAN Ming, et al. Removal of tetracycline hydrochloride from aqueous solutions by sulfonated biochar-activated persulfate[J]. China Environmental Science, 2022, 42(8): 3650-3657. | |
| 22 | HAMMOUDA Samia Ben, ZHAO Feiping, SAFAEI Zahra, et al. Sulfate radical-mediated degradation and mineralization of bisphenol F in neutral medium by the novel magnetic Sr2CoFeO6 double perovskite oxide catalyzed peroxymonosulfate: Influence of co-existing chemicals and UV irradiation[J]. Applied Catalysis B: Environmental, 2018, 233: 99-111. |
| 23 | LOU Xiaoyi, WU Liuxi, GUO Yaoguang, et al. Peroxymonosulfate activation by phosphate anion for organics degradation in water[J]. Chemosphere, 2014, 117: 582-585. |
| 24 | ZHANG Tao, ZHU Haibo, Jean-Philippe CROUÉ. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism[J]. Environmental Science & Technology, 2013, 47(6): 2784-2791. |
| 25 | 朱紫琦, 李立, 徐铭骏, 等. 菱形片状铁锰催化剂活化过硫酸盐降解四环素[J]. 中国环境科学, 2021, 41(11): 5142-5152. |
| ZHU Ziqi, LI Li, XU Mingjun, et al. Rhombic sheet iron-manganese catalyst-activating peroxymonosulfate for tetracycline degradation[J]. China Environmental Science, 2021, 41(11): 5142-5152. | |
| 26 | 徐铭骏, 郭兆春, 李立, 等. 纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料[J]. 化工进展, 2022, 41(2): 1043-1053. |
| XU Mingjun, GUO Zhaochun, LI Li, et al. Degradation of azo dyes by sodium percarbonate activated with nanosheet Mn2O3@α-Fe3O4 [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1043-1053. | |
| 27 | LU Hongtao, SUI Minghao, YUAN Bojie, et al. Efficient degradation of nitrobenzene by Cu-Co-Fe-LDH catalyzed peroxymonosulfate to produce hydroxyl radicals[J]. Chemical Engineering Journal, 2019, 357: 140-149. |
| 28 | XU Haodan, WANG Da, MA Jun, et al. A superior active and stable spinel sulfide for catalytic peroxymonosulfate oxidation of bisphenol S[J]. Applied Catalysis B: Environmental, 2018, 238: 557-567. |
| 29 | HU Enlai, FENG Yafei, NAI Jianwei, et al. Construction of hierarchical Ni-Co-P hollow nanobricks with oriented nanosheets for efficient overall water splitting[J]. Energy & Environmental Science, 2018, 11(4): 872-880. |
| 30 | LI Miaoqing, LUO Rui, WANG Chaohai, et al. Iron-tannic modified cotton derived Fe0/graphitized carbon with enhanced catalytic activity for bisphenol A degradation[J]. Chemical Engineering Journal, 2019, 372: 774-784. |
| 31 | ZHU Shijun, WANG Wei, XU Yongpeng, et al. Iron sludge-derived magnetic Fe0/Fe3C catalyst for oxidation of ciprofloxacin via peroxymonosulfate activation[J]. Chemical Engineering Journal, 2019, 365: 99-110. |
| 32 | GUO Ruonan, CHEN Ying, NENGZI Lichao, et al. In situ preparation of carbon-based Cu-Fe oxide nanoparticles from CuFe Prussian blue analogues for the photo-assisted heterogeneous peroxymonosulfate activation process to remove lomefloxacin[J]. Chemical Engineering Journal, 2020, 398: 125556. |
| 33 | LU Sen, WANG Guanlong, CHEN Shuo, et al. Heterogeneous activation of peroxymonosulfate by LaCo1- x Cu x O3 perovskites for degradation of organic pollutants[J]. Journal of Hazardous Materials, 2018, 353: 401-409. |
| 34 | 胡明珠. 钴铁双金属催化剂活化过硫酸盐降解有机污染物的性能与机理[D]. 杭州: 浙江大学, 2021. |
| HU Mingzhu. Performance and mechanism of cobalt-iron bimetallic catalyst activating persulfate to degrade organic pollutants[D]. Hangzhou: Zhejiang University, 2021. | |
| 35 | WANG Bingyu, LI Qiaoqiao, LV Ying, et al. Insights into the mechanism of peroxydisulfate activated by magnetic spinel CuFe2O4/SBC as a heterogeneous catalyst for bisphenol S degradation[J]. Chemical Engineering Journal, 2021, 416: 129162. |
| 36 | LIU Yang, GUO Hongguang, ZHANG Yongli, et al. Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation[J]. Environmental Pollution, 2019, 252: 1042-1050. |
| 37 | CAI Jing, ZHANG Yan. Enhanced degradation of bisphenol S by persulfate activated with sulfide-modified nanoscale zero-valent iron[J]. Environmental Science and Pollution Research, 2022, 29(6): 8281-8293. |
| 38 | WEI Junyan, YIN Linning, QU Ruijuan, et al. Experimental and quantum chemical study on the transformation behavior of bisphenol S by radical-driven persulfate oxidation[J]. Environmental Science: Water Research & Technology, 2022, 8(1): 116-126. |
| 39 | SHAO Penghui, DUAN Xiaoguang, XU Jun, et al. Heterogeneous activation of peroxymonosulfate by amorphous boron for degradation of bisphenol S[J]. Journal of Hazardous Materials, 2017, 322: 532-539. |
| 40 | HUANG Quanlong, CHEN Congjin, ZHAO Xilian, et al. Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: Efficiency, stability and mechanism[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105800. |
| [1] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [2] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [3] | 殷成阳, 侯铭, 杨爽, 毛迪, 刘俊言. 过渡金属改性Cu-SSZ-13分子筛脱硝催化剂研究进展[J]. 化工进展, 2023, 42(6): 2963-2974. |
| [4] | 张国春, 周志辉, 吴红丹. 基于α-Al2O3载体管的新型MXene膜异丙醇脱水性能[J]. 化工进展, 2023, 42(10): 5381-5389. |
| [5] | 李亚男, 年佩, 徐楠, 罗海玉, 魏逸彬. 面向精密流体分离的MXene基膜材料研究进展[J]. 化工进展, 2023, 42(10): 5249-5258. |
| [6] | 毛梦雷, 孙丹阳, 孟子晖, 刘文芳. 氧化石墨烯和过渡金属碳/氮化合物固定化酶[J]. 化工进展, 2022, 41(4): 1941-1955. |
| [7] | 贾艳萍, 薛东奇, 刘启帆, 张海丰, 李正, 张兰河. 亚硫酸盐活化技术及其在废水处理中的应用[J]. 化工进展, 2022, 41(1): 418-426. |
| [8] | 田婷婷, 李朝阳, 王召东, 陆慧, 李新冬, 毛艳丽, 宋忠贤, 朱新锋. 过渡金属活化过硫酸盐降解有机废水技术研究进展[J]. 化工进展, 2021, 40(6): 3480-3488. |
| [9] | 黄国勇, 李毅, 屈辰玮, 孙晓华, 李勃天, 戈磊, 叶海木, 张红梅. 热电池用过渡金属二硫化物及其复合材料的研究进展[J]. 化工进展, 2021, 40(4): 2161-2174. |
| [10] | 石彩, 史峻铭, 滕敏, 王维聪, 额其马林, 黄占华. 过渡金属磷化物在光催化机理方面的研究进展[J]. 化工进展, 2021, 40(11): 6079-6093. |
| [11] | 王春霞, 宋兆毅, 倪基平, 潘宗卫, 黄国勇. 电催化析氢催化剂研究进展[J]. 化工进展, 2021, 40(10): 5523-5534. |
| [12] | 朱子翼,董鹏,张举峰,黎永泰,肖杰,曾晓苑,李雪,张英杰. 新一代储能钠离子电池正极材料的改性研究进展[J]. 化工进展, 2020, 39(3): 1043-1056. |
| [13] | 谷霞, 王若飞, 邵怀启, 姜涛. AlCl3/促进剂协同催化1-癸烯齐聚制备聚α-烯烃合成油[J]. 化工进展, 2020, 39(11): 4497-4502. |
| [14] | 高宁,周玉康,沈树宝,陈英文. 含镉化合物在光催化领域应用的研究进展[J]. 化工进展, 2019, 38(12): 5372-5379. |
| [15] | 李刚,张穗穗,张妮娜,高婷,兰婷玮,马晓迅. MeMo/USY催化剂对神东煤热解产物的调控[J]. 化工进展, 2019, 38(03): 1329-1337. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||