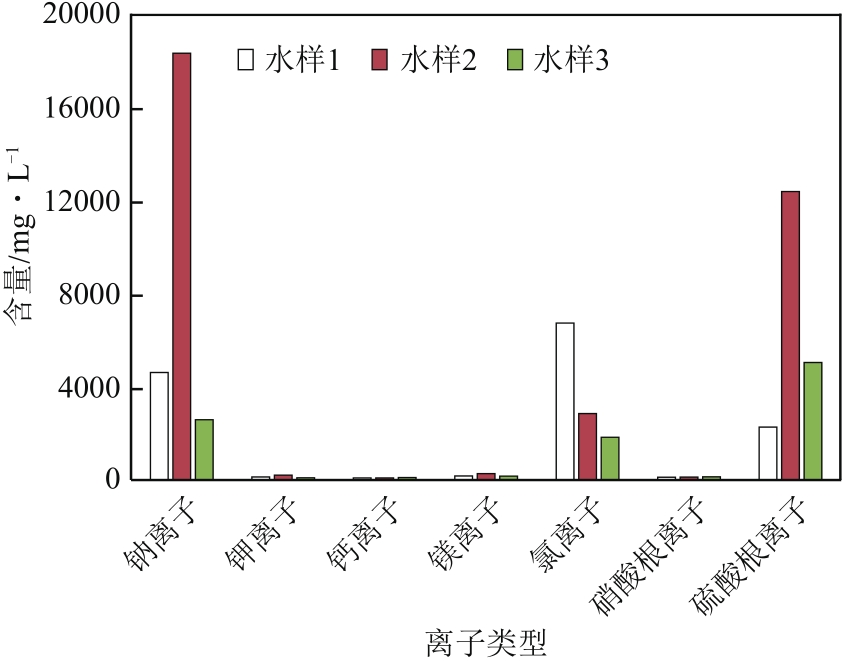
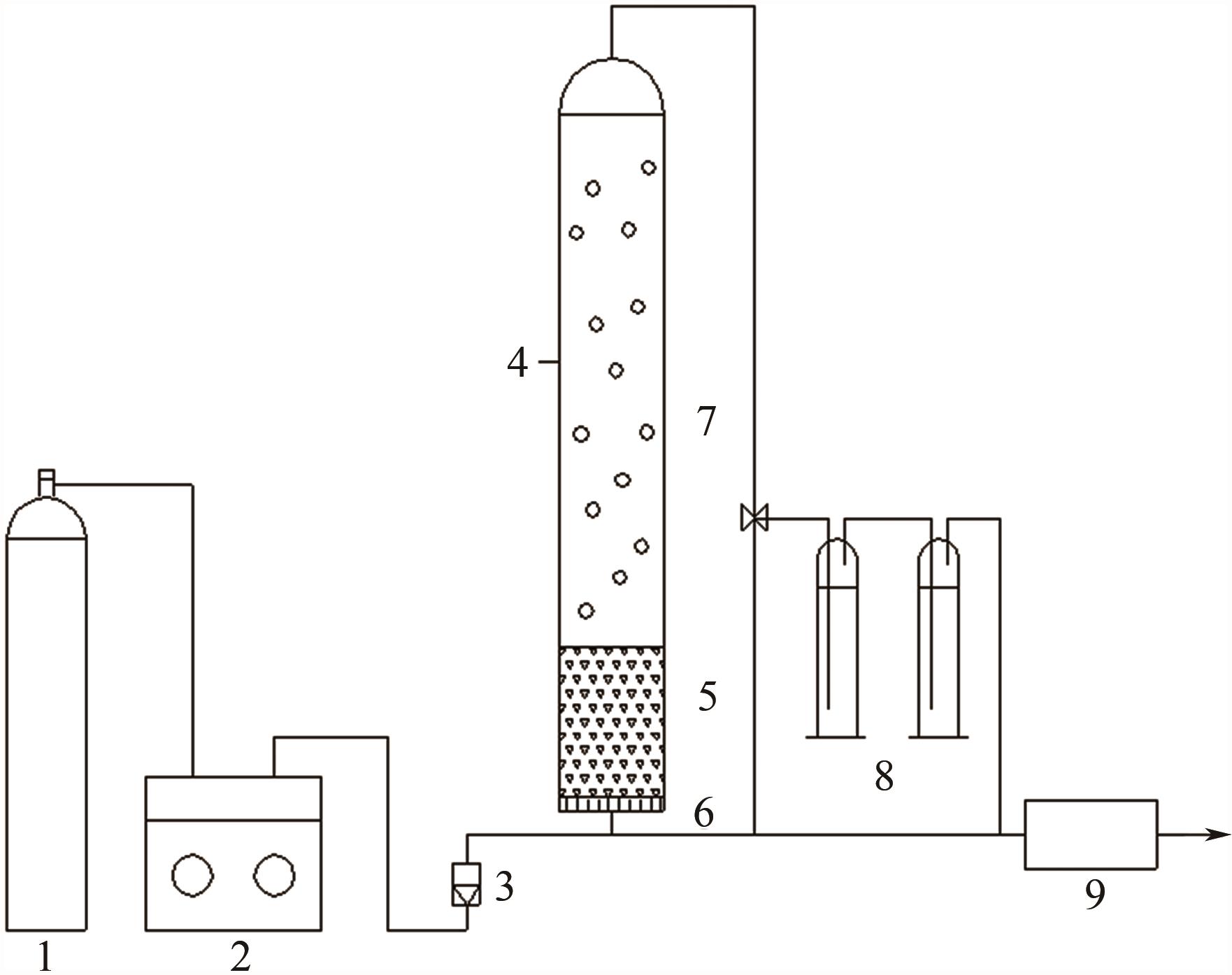
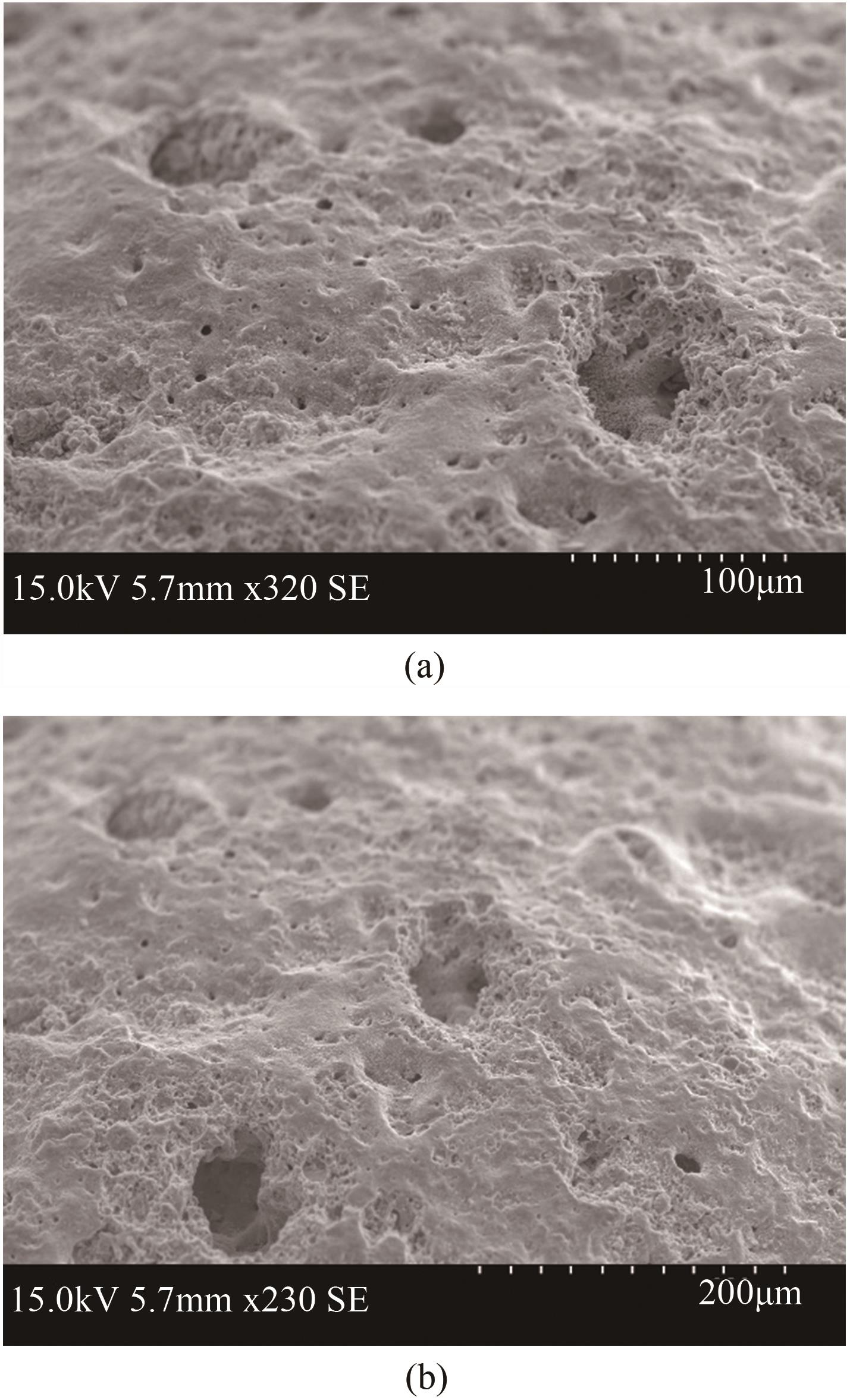
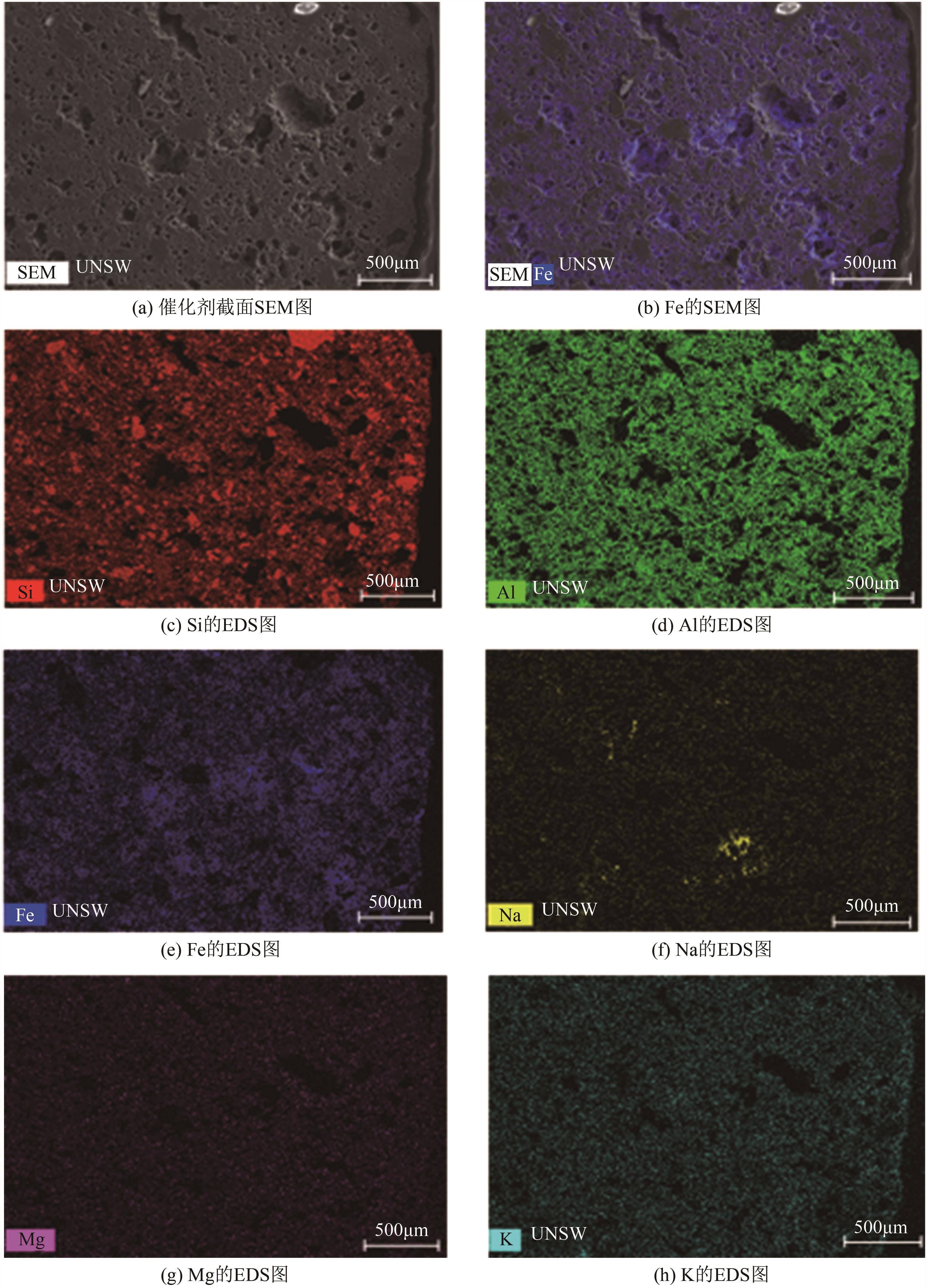
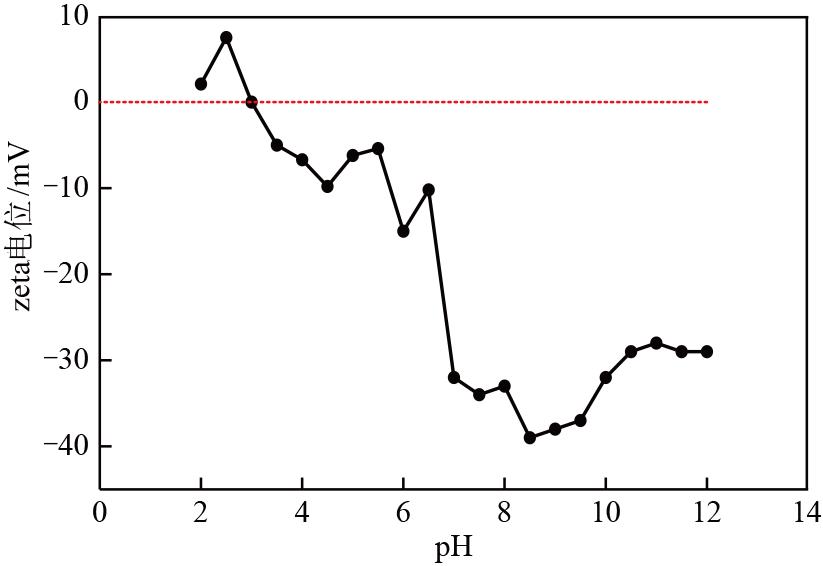
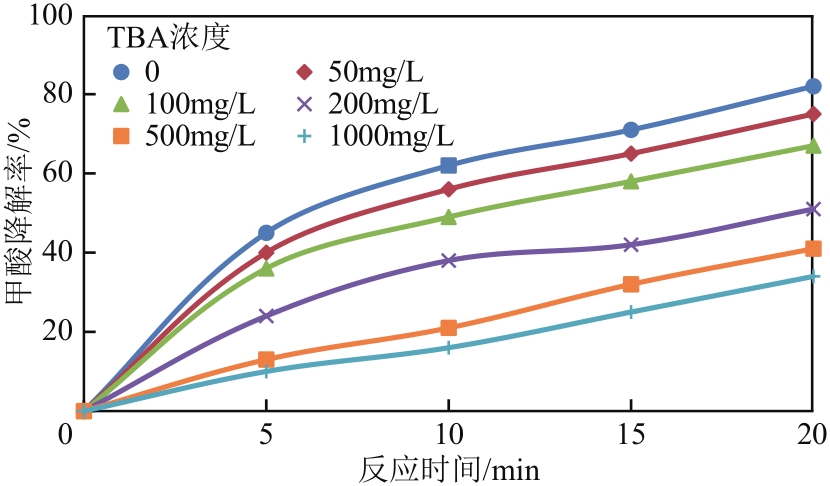
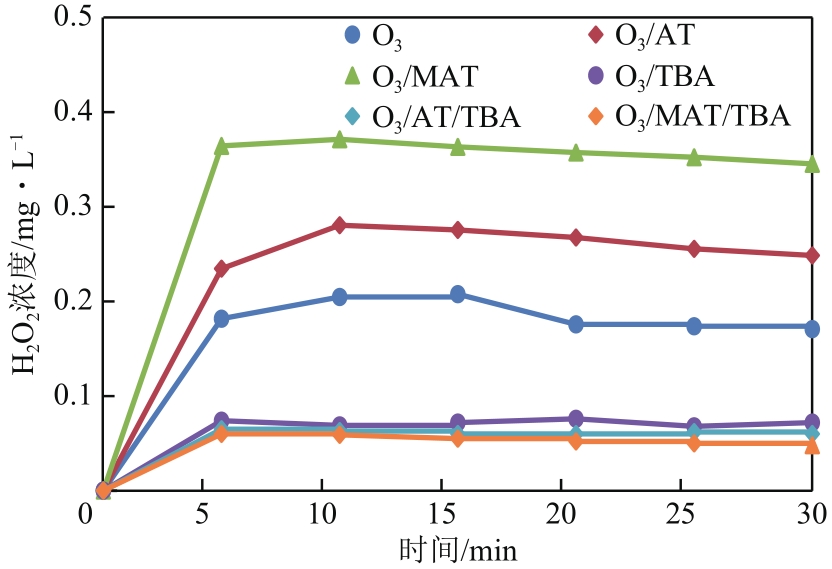
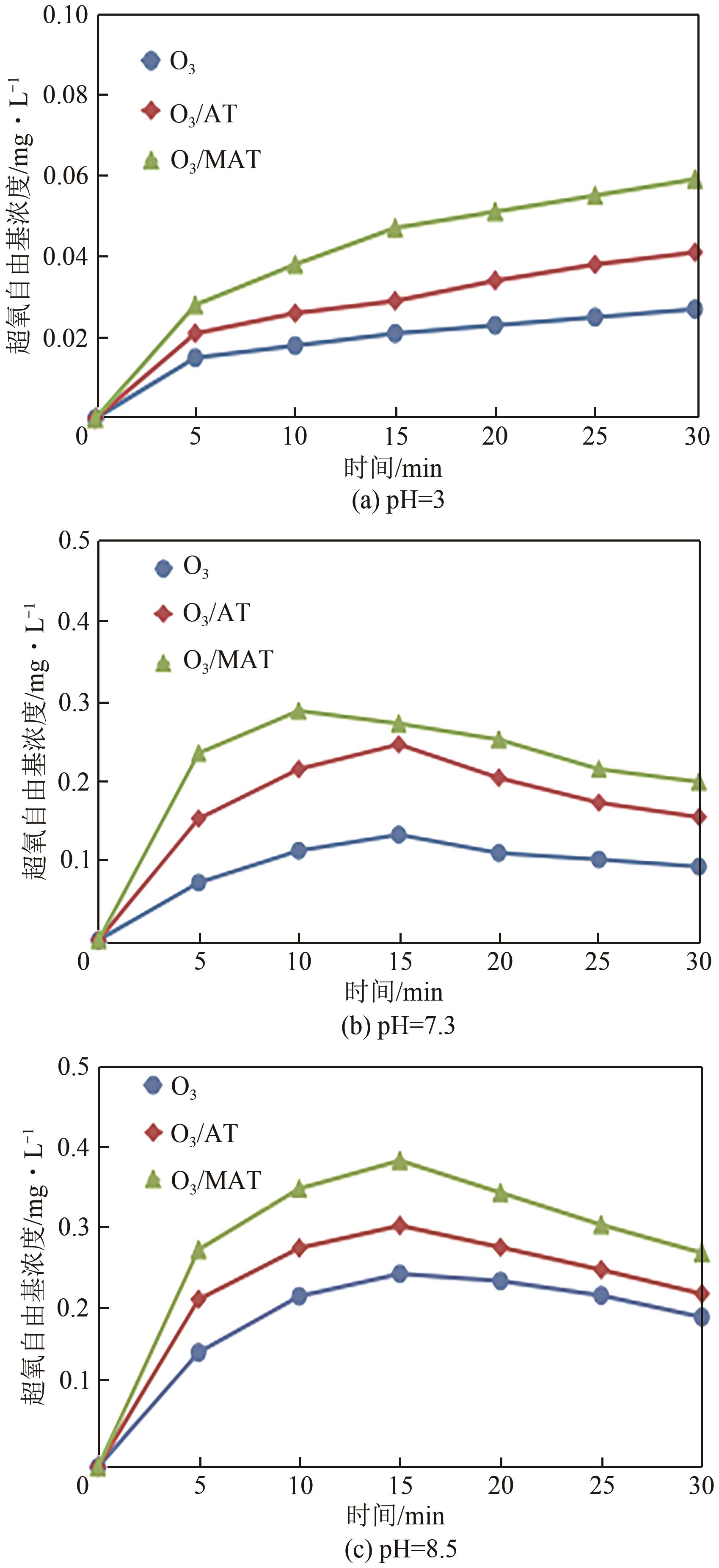
| 1 |
GHOSH S, DE S. Energy analysis of a cogeneration plant using coal gasification and solid oxide fuel cell[J]. Energy, 2006, 31(2/3): 345-363.
|
| 2 |
AKOB D M, COZZARELLI I M, DUNLAP D S, et al. Organic and inorganic composition and microbiology of produced waters from Pennsylvania shale gas wells[J]. Applied Geochemistry, 2015, 60: 116-125.
|
| 3 |
王姣.煤化工企业高含盐废水处理问题研究[J].煤炭技术, 2013, 32(11): 229-230.
|
|
WANG Jiao. Research on coal chemical enterprises with high salinity wastewater treatment[J]. Coal Technology, 2013, 32(11): 229-230.
|
| 4 |
何斌, 王亚娥. 蒸氨-脱酚-SBR处理兰炭废水的研究[J]. 广东化工, 2009, 36(12): 140-141.
|
|
HE Bin, WANG Ya’e. Research on coking waste water treatment by distillation ammonia nitrogen-elimination phenol-SBR[J]. Guangdong Chemical Industry, 2009, 36(12): 140-141.
|
| 5 |
王伟, 韩洪军, 张静, 等. 煤制气废水处理技术研究进展[J]. 化工进展, 2013, 32(3): 681-686.
|
|
WANG Wei, HAN Hongjun, ZHANG Jing, et al. Progress in treatment technologies of coal gasification wastewater[J]. Chemical Industry and Engineering Progress, 2013, 32(3): 681-686.
|
| 6 |
王文豪, 高健磊, 高镜清. 预处理+A/O+臭氧氧化+BAF深度处理煤化工废水[J]. 工业水处理, 2019, 39(6): 103-106.
|
|
WANG Wenhao, GAO Jianlei, GAO Jingqing. Pretreatment+A/O+ozonation+BAF process for advanced treatment of coal chemical wastewater[J]. Industrial Water Treatment, 2019, 39(6): 103-106.
|
| 7 |
熊日华, 何灿, 马瑞, 等. 高盐废水分盐结晶工艺及其技术经济分析[J]. 煤炭科学技术, 2018, 46(9): 37-43.
|
|
XIONG Rihua, HE Can, MA Rui, et al. Process introduction and techno-economic analysis on pure salt recovery crystallization for high salinity wastewater[J]. Coal Science and Technology, 2018, 46(9): 37-43.
|
| 8 |
纪钦洪, 于广欣, 张振家. 煤化工含盐废水处理与综合利用探讨[J].水处理技术, 2014, 40(11): 8-12.
|
| 18 |
王利平, 沈肖龙, 倪可, 等. 非均相催化臭氧氧化深度处理炼油废水[J]. 环境工程学报, 2015, 9(5): 2297-2302.
|
|
WANG Liping, SHEN Xiaolong, NI Ke, et al. Treatment of oil-refinery wastewater by heterogeneous catalytic ozonation process[J]. Chinese Journal of Environmental Engineering, 2015, 9(5): 2297-2302.
|
| 19 |
CHÁVEZ A M, REY A, BELTRÁN F J, et al. Solar photo-ozonation: a novel treatment method for the degradation of water pollutants[J]. Journal of Hazardous Materials, 2016, 317: 36-43.
|
| 20 |
MEHRJOUEI M, MÜLLER S, MÖLLER D. A review on photocatalytic ozonation used for the treatment of water and wastewater[J]. Chemical Engineering Journal, 2015, 263: 209-219.
|
| 21 |
PI Y Z, ERNST M, SCHROTTER J C. Effect of phosphate buffer upon CuO/Al2O3 and Cu(Ⅱ) catalyzed ozonation of oxalic acid solution[J]. Ozone: Science & Engineering, 2003, 25(5): 393-397.
|
| 22 |
ROSAL R, GONZALO M S, RODRÍGUEZ A, et al. Catalytic ozonation of atrazine and linuron on MnOx/Al2O3 and MnOx/SBA-15 in a fixed bed reactor[J]. Chemical Engineering Journal, 2010, 165(3): 806-812.
|
| 23 |
王吉坤, 陈贵锋, 李阳, 等. 臭氧催化氧化去除煤化工高盐废水难降解有机物工艺研究[J]. 煤化工, 2021, 49(3): 81-85.
|
|
WANG Jikun, CHEN Guifeng, LI Yang, et al. Study on removal of refractory organics from high salt wastewater of coal chemical industry by ozone catalytic oxidation[J]. Coal Chemical Industry, 2021, 49(3): 81-85.
|
| 24 |
马栋, 段锋. 煤化工高盐废水臭氧催化氧化脱除COD[J]. 环境工程学报, 2020, 14(4): 984-992.
|
|
MA Dong, DUAN Feng. COD removal from high-salt wastewater in coal chemical industry by ozone catalytic oxidation[J]. Chinese Journal of Environmental Engineering, 2020, 14(4): 984-992.
|
| 25 |
WEN G, PAN Z H, MA J, et al. Reuse of sewage sludge as a catalyst in ozonation—Efficiency for the removal of oxalic acid and the control of bromate formation[J]. Journal of Hazardous Materials, 2012, 239/240: 381-388.
|
| 26 |
QIANG Z M, LING W C, TIAN F. Kinetics and mechanism for omethoate degradation by catalytic ozonation with Fe(Ⅲ)-loaded activated carbon in water[J]. Chemosphere, 2013, 90(6): 1966-1972.
|
| 27 |
ZHAI X, CHEN Z L, ZHAO S Q, et al. Enhanced ozonation of dichloroacetic acid in aqueous solution using nanometer ZnO powders[J]. Journal of Environmental Sciences, 2010, 22(10): 1527-1533.
|
| 28 |
LYU A, HU C, NIE Y L, et al. Catalytic ozonation of toxic pollutants over magnetic cobalt-doped Fe3O4 suspensions[J]. Applied Catalysis B: Environmental, 2012, 117/118: 246-252.
|
| 8 |
JI Qinhong, YU Guangxin, ZHANG Zhenjia. Investigation of the treatment and comprehensive utilization of saline wastewater in coal chemical industry[J]. Technology of Water Treatment, 2014, 40(11): 8-12.
|
| 9 |
王亮, 蒋佩娟, 刘华杰, 等. 煤化工高含盐废水中有机物去除方法探究[J]. 工业用水与废水, 2017, 48(2): 24-27, 72.
|
|
WANG Liang, JIANG Peijuan, LIU Huajie, et al. Study on methods for organic pollutants removal from high saltcontaining wastewater of coal chemical industry[J]. Industrial Water & Wastewater, 2017, 48(2): 24-27, 72.
|
| 10 |
张文博, 刘发强, 牛进龙. 铁炭内电解法处理化工废水的研究[J]. 石化技术与应用, 2007, 25(1): 44-47.
|
|
ZHANG Wenbo, LIU Faqiang, NIU Jinlong. Chemical wastewater treatment by ferrum-carbon internal electrolysis[J]. Petrochemical Technology & Application, 2007, 25(1): 44-47.
|
| 11 |
曾小勇, 王红武, 马鲁铭, 等. 微曝气催化铁内电解法预处理化工废水[J]. 中国给水排水, 2005, 21(12): 1-4.
|
|
ZENG Xiaoyong, WANG Hongwu, MA Luming, et al. Pretreatment of chemical wastewater by micro-aeration catalyzed iron internal electrolysis process[J]. China Water & Wastewater, 2005, 21(12): 1-4.
|
| 12 |
张俊琪, 樊金红, 马鲁铭, 等. 催化铁内电解法处理酸性化工废水后混合印染废水进行混凝处理的研究[J]. 水处理技术, 2012, 38(9): 88-92.
|
|
ZHANG Junqi, FAN Jinhong, MA Luming, et al. Coagulation treatment of printing and dyeing wastewater mixed with acid chemical wastewater after catalyzed iron internal electrolysis treatment[J]. Technology of Water Treatment, 2012, 38(9): 88-92.
|
| 13 |
胡洁, 王乔, 周珉, 等. 芬顿和臭氧氧化法深度处理化工废水的对比研究[J]. 四川环境, 2015, 34(4): 23-26.
|
|
HU Jie, WANG Qiao, ZHOU Min, et al. Comparison study on the application of Fenton and ozone oxidation in chemical wastewater treatment[J]. Sichuan Environment, 2015, 34(4): 23-26.
|
| 14 |
任明, 孙淑英, 金艳, 等. 催化臭氧氧化法处理煤化工高盐废水[J]. 环境工程, 2018, 36(8): 54-59.
|
|
REN Ming, SUN Shuying, JIN Yan, et al. Treatment of high-salt wastewater from coal chemical industry by catalytic ozone oxidation[J]. Environmental Engineering, 2018, 36(8): 54-59.
|
| 15 |
钱媛媛, 王永杰, 杨雪晶. 臭氧相关水处理工艺及其体质特征研究进展[J]. 化工进展, 2021, 40(S1): 411-425.
|
|
QIAN Yuanyuan, WANG Yongjie, YANG Xuejing. Application of ozone for water treatment and implication of mass transfer characteristics[J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 411-425.
|
| 16 |
任斌, 金政伟, 李瑞龙, 等. 臭氧催化氧化降解煤化工高盐废水有机物研究[J]. 工业用水与废水, 2020, 51(6): 29-32, 53.
|
|
REN Bin, JIN Zhengwei, LI Ruilong, et al. Study on degradation of organic matters in coal chemical high-salt wastewater by catalytic ozonation[J]. Industrial Water & Wastewater, 2020, 51(6): 29-32, 53.
|
| 17 |
王吉坤. 活性氧化铝对矿井水氟化物的吸附性能研究[J]. 煤质技术, 2020, 35(5): 16-21, 26.
|
|
WANG Jikun. Study on the adsorption performance of dluoride in mine water by the activated alumina[J]. Coal Quality Technology, 2020, 35(5): 16-21, 26.
|
 ), 李阳1,2(
), 李阳1,2( ), 陈贵锋1,2, 刘敏1,2, 寇丽红1,2, 王琦1,2, 何毅聪1,2
), 陈贵锋1,2, 刘敏1,2, 寇丽红1,2, 王琦1,2, 何毅聪1,2
 ), LI Yang1,2(
), LI Yang1,2( ), CHEN Guifeng1,2, LIU Min1,2, KOU Lihong1,2, WANG Qi1,2, HE Yicong1,2
), CHEN Guifeng1,2, LIU Min1,2, KOU Lihong1,2, WANG Qi1,2, HE Yicong1,2