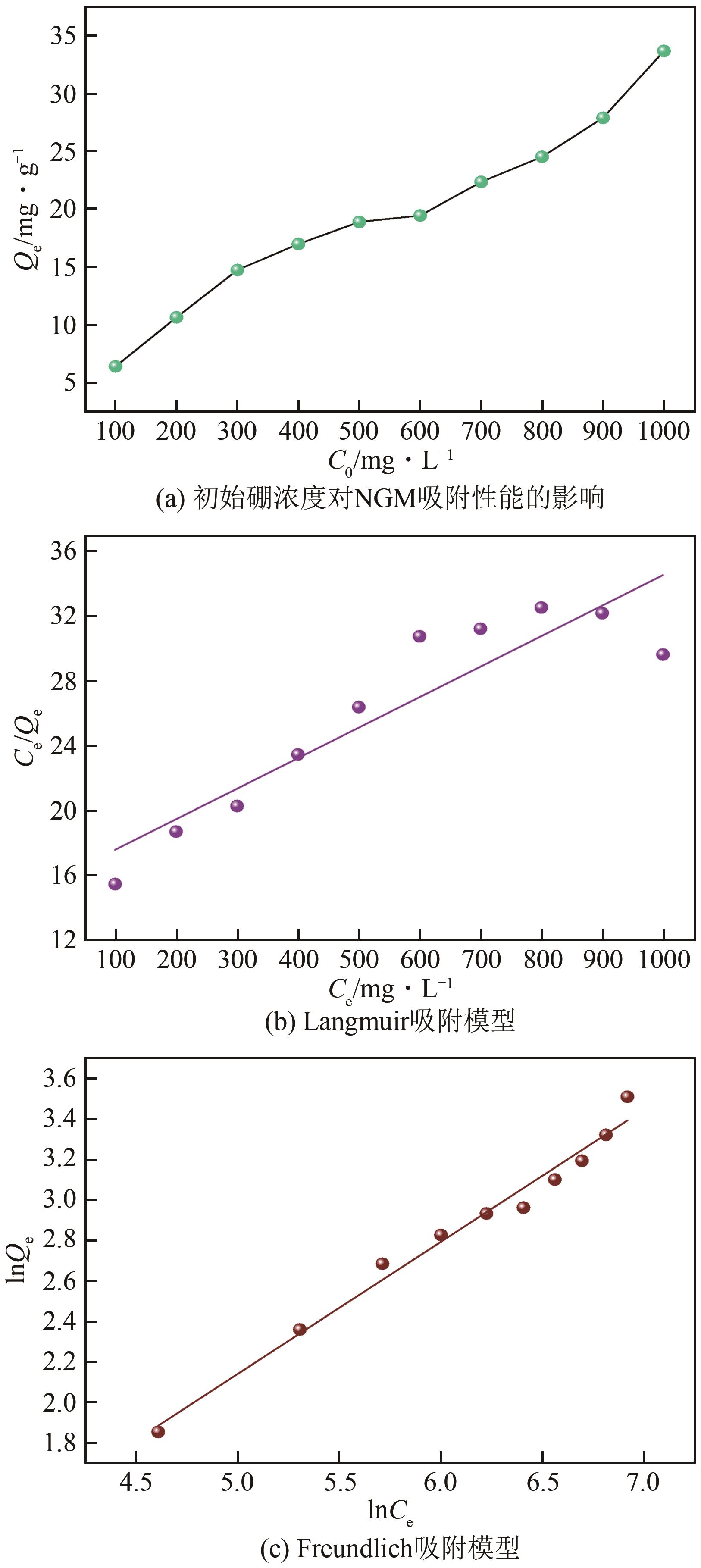
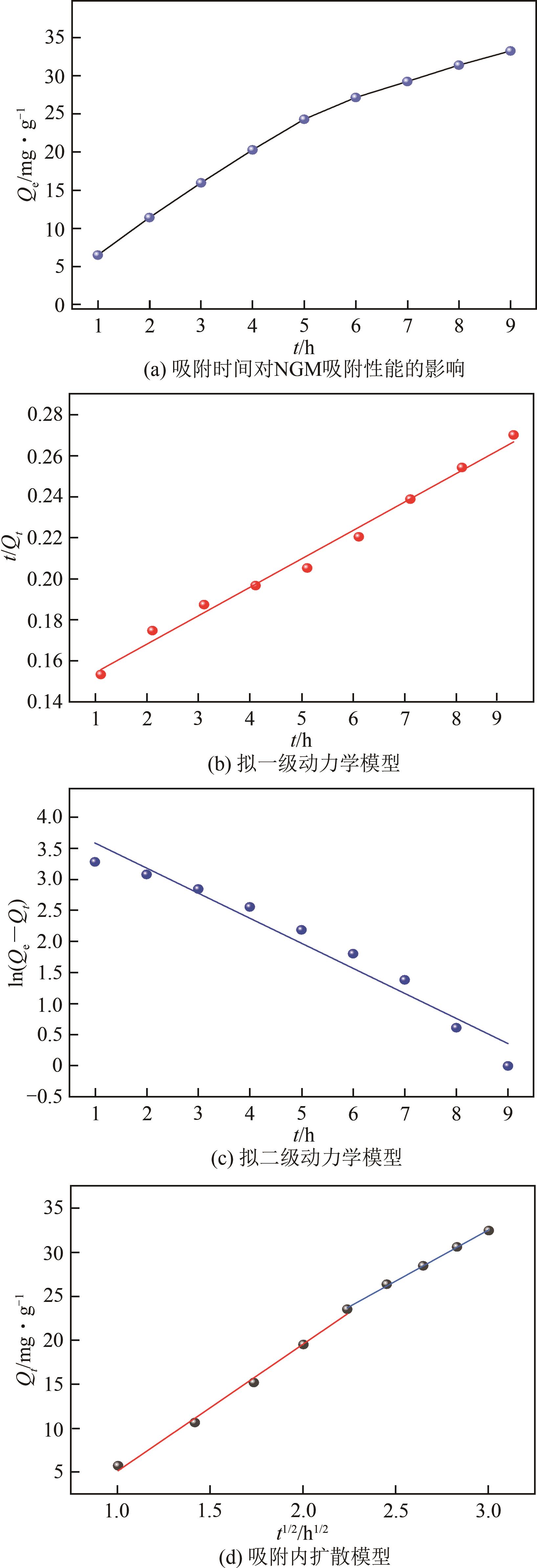
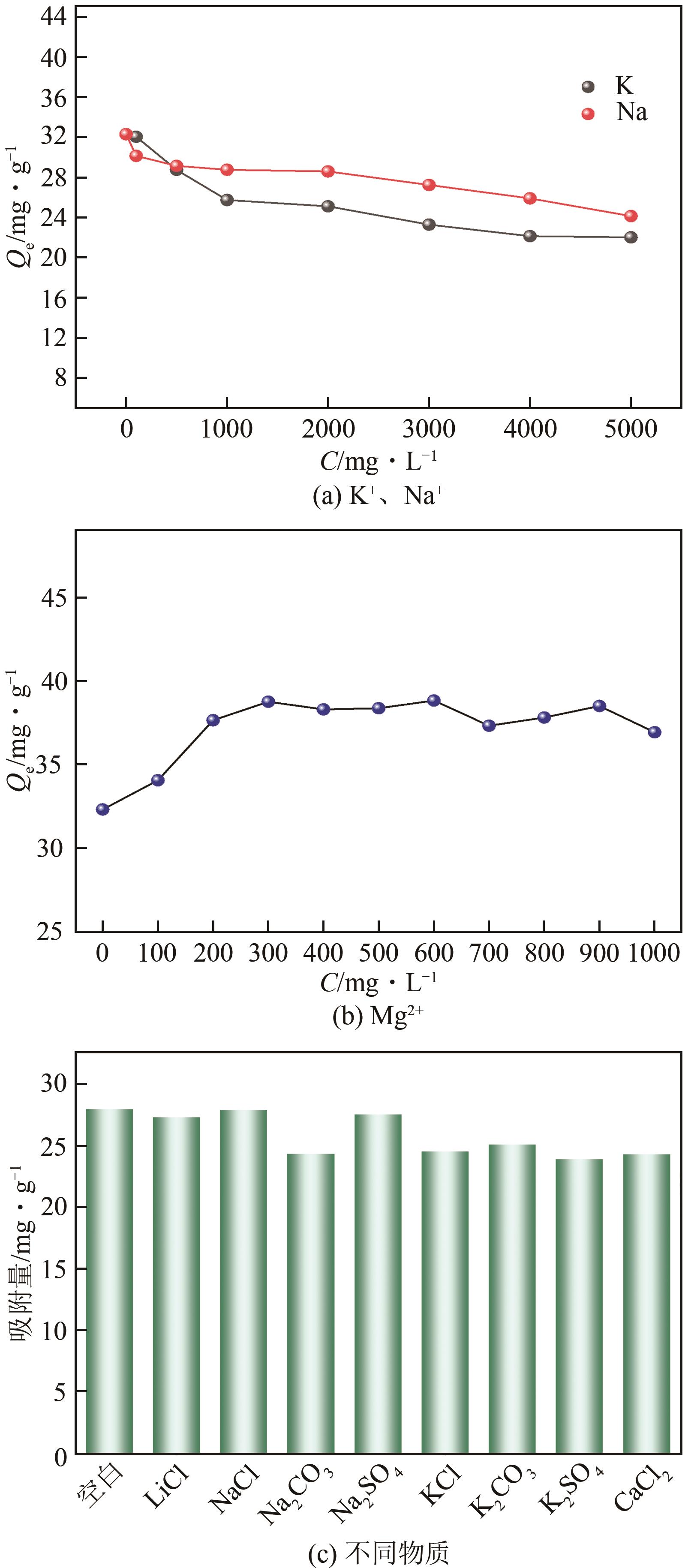
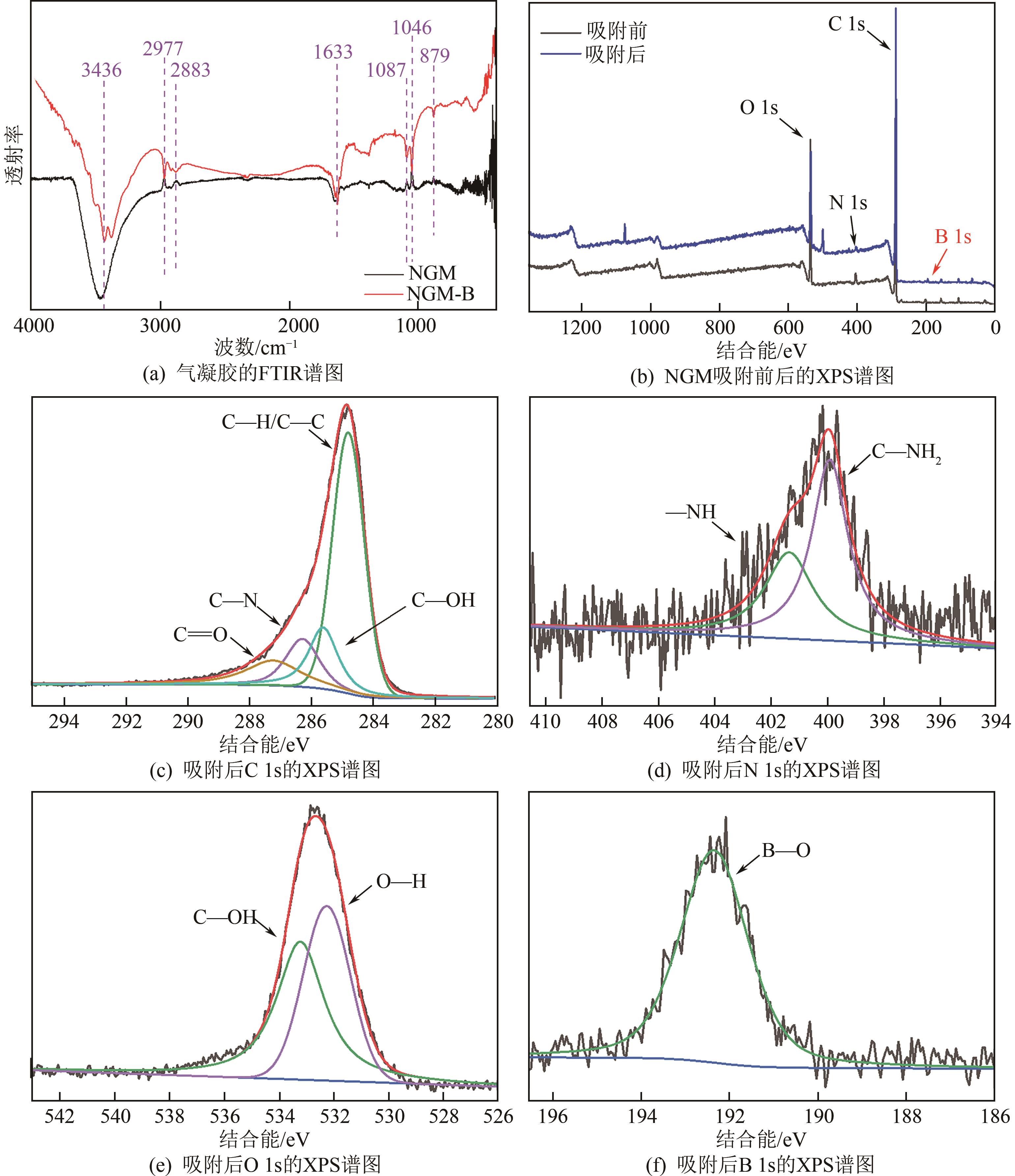
| 16 |
PINOTTI Camila N, DE SOUZA Luana M, MARQUES Willbrynner P, et al. A new magnetic composite with potential application in boron adsorption: Development, characterization, and removal tests[J]. Materials Chemistry and Physics, 2022, 277: 125368.
|
| 17 |
周彩云. 改性氧化石墨烯吸附染料的性能研究[D]. 苏州: 苏州科技学院, 2015.
|
|
ZHOU Caiyun. Study on adsorption properties of modified graphene oxide for dyes[D]. Suzhou: Suzhou University of Science
|
|
and Technology, 2015.
|
| 18 |
ASARE Emmanuel Agyapong, DARTEY Emmanuel, SARPONG Kofi, et al. Adsorption isotherm, kinetic and thermodynamic modelling of Bacillus subtilis ATCC13952 mediated adsorption of arsenic in groundwaters of selected gold mining communities in the wassa west municipality of the western region of Ghana[J]. American Journal of Analytical Chemistry, 2021, 12(5): 121-161.
|
| 19 |
LUO Qinglong, ZENG Meiting, WANG Xueying, et al. Glycidol-functionalized macroporous polymer for boron removal from aqueous solution[J]. Reactive and Functional Polymers, 2020, 150: 104543.
|
| 20 |
常娟, 程爱华. FeS/壳聚糖基碳气凝胶复合材料的制备及对Cr(Ⅵ)的吸附[J]. 化工进展, 2023, 42(11): 6042-6052.
|
|
CHANG Juan, CHENG Aihua. Preparation and Cr(Ⅵ) adsorption properties of FeS/chitosan-based carbon aerogel composites[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6042-6052.
|
| 21 |
CAI Liang, ZHANG Yuze, PENG Xiaowu, et al. Preparation of layered double hydroxide intercalated by Gallic acid for boron adsorption[J]. Journal of Water Process Engineering, 2021, 44: 102394.
|
| 22 |
LIN Juiyen, MAHASTI Nicolaus N N, HUANG Yaohui. Recent advances in adsorption and coagulation for boron removal from wastewater: A comprehensive review[J]. Journal of Hazardous Materials, 2021, 407: 124401.
|
| 23 |
ZHANG Xi, WANG Jiawei, CHEN Shaofeng, et al. A spherical N-methyl-D-glucamine-based hybrid adsorbent for highly efficient adsorption of boric acid from water[J]. Separation and Purification Technology, 2017, 172: 43-50.
|
| 24 |
Pelin DEMIRÇIVI, SAYGıLı Gülhayat Nasün. Comparative study of modified expanded perlite with hexadecyltrimethylammonium-bromide and gallic acid for boron adsorption[J]. Journal of Molecular Liquids, 2018, 254: 383-390.
|
| 25 |
Jiafei LYU, LIU Hongxu, ZENG Zhouliangzi, et al. Metal–organic framework UiO-66 as an efficient adsorbent for boron removal from aqueous solution[J]. Industrial & Engineering Chemistry Research, 2017, 56(9): 2565-2572.
|
| 26 |
INCE Ahmet, KARAGOZ Bunyamin, BICAK Niyazi. Solid tethered imino-bis-propanediol and quaternary amine functional copolymer brushes for rapid extraction of trace boron[J]. Desalination, 2013, 310: 60-66.
|
| 27 |
DE LA FUENTE GARCÍA-SOTO M M, MUÑOZ CAMACHO E. Boron removal by means of adsorption processes with magnesium oxide—Modelization and mechanism[J]. Desalination, 2009, 249(2): 626-634.
|
| 28 |
杨丽娟, 王刚, 张航, 等. 响应面法优化重金属絮凝剂MAMPAM除Cu(Ⅱ)的絮凝条件[J]. 环境科学研究, 2022, 35(3): 789-795.
|
|
YANG Lijuan, WANG Gang, ZHANG Hang, et al. Response surface methodology to optimize flocculation conditions of heavy metal flocculant MAMPAM for removing Cu(Ⅱ)[J]. Research of Environmental Sciences, 2022, 35(3): 789-795.
|
| 29 |
李艳. 响应面法优化等离子体掺氮改性TiO2纳米管的制备条件[J]. 应用化工, 2022, 51(2): 466-470.
|
|
LI Yan. Optimization of preparation factors for plasma nitrogen-doped modified TiO2 nanotubes by response surface methodology[J]. Applied Chemical Industry, 2022, 51(2): 466-470.
|
| 30 |
SUN Tao, ZHUO Qin, LIU Xin, et al. Hydrophobic silica aerogel reinforced with carbon nanotube for oils removal[J]. Journal of Porous Materials, 2014, 21(6): 967-973.
|
| 31 |
ISAACS-PAEZ E D, LEYVA-RAMOS R, JACOBO-AZUARA A, et al. Adsorption of boron on calcined AlMg layered double hydroxide from aqueous solutions: Mechanism and effect of operating conditions[J]. Chemical Engineering Journal, 2014, 245: 248-257.
|
| 32 |
LI Ping, LIU Chuang, ZHANG Li, et al. Enhanced boron adsorption onto synthesized MgO nanosheets by ultrasonic method[J]. Ultrasonics Sonochemistry, 2017, 34: 938-946.
|
| 33 |
ELJAMAL Osama, MAAMOUN Ibrahim, ALKHUDHAYRI Sami, et al. Insights into boron removal from water using Mg-Al-LDH: Reaction parameters optimization & 3D-RSM modeling[J]. Journal of Water Process Engineering, 2022, 46: 102608.
|
| 34 |
LI Xufeng, ZHANG Dian, XIANG Kewei, et al. Synthesis of polyborosiloxane and its reversible physical crosslinks[J]. RSC Advances, 2014, 4(62): 32894-32901.
|
| 1 |
WANG Boyang, GUO Xianghai, BAI Peng. Removal technology of boron dissolved in aqueous solutions: A review[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 444: 338-344.
|
| 2 |
TANG Yupan, LUO Lin, THONG Zhiwei, et al. Recent advances in membrane materials and technologies for boron removal[J]. Journal of Membrane Science, 2017, 541: 434-446.
|
| 3 |
TU Kha L, NGHIEM Long D, CHIVAS Allan R. Boron removal by reverse osmosis membranes in seawater desalination applications[J]. Separation and Purification Technology, 2010, 75(2): 87-101.
|
| 4 |
SHENG Rui, ZHANG Yang, KANG Jingjing, et al. Nanorod-like polymer adsorbents with intermediate dihydroxy functional groups for efficient boron removal[J]. ChemistrySelect, 2021, 6(24): 6197-6201.
|
| 5 |
JULIANE Schott, JEROME Kretzschmar, MARGRET Acker, et al. Formation of a Eu(Ⅲ) borate solid species from a weak Eu(Ⅲ) borate complex in aqueous solution[J]. Dalton Transactions, 2014, 43(30): 11516-11528.
|
| 6 |
WANG Chunchun, YANG Sudong, MA Qing, et al. Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds[J]. Carbon, 2017, 118: 765-771.
|
| 7 |
PEYDAYESH Mohammad, VOGT Julia, CHEN Xiulin, et al. Amyloid-based carbon aerogels for water purification[J]. Chemical Engineering Journal, 2022, 449: 137703.
|
| 8 |
LI Kexin, DING Dong, FANG Dezhen, et al. Hydrothermal deposition of titanate on biomass carbonaceous aerogel to prepare novel biomass adsorbents for Rb+ and Cs+ [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 590: 124501.
|
| 9 |
AI Lunhong, JIANG Jing. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene-carbon nanotube hybrid[J]. Chemical Engineering Journal, 2012, 192: 156-163.
|
| 10 |
XU Ting, YANG Dongzhi, FAN Zhuangjun, et al. Reduced graphene oxide/carbon nanotube hybrid fibers with narrowly distributed mesopores for flexible supercapacitors with high volumetric capacitances and satisfactory durability[J]. Carbon, 2019, 152: 134-143.
|
| 11 |
XU Hui, SONG Guojun, ZHANG Lina, et al. Preparation and performance evolution of enhancement polystyrene composites with graphene oxide/carbon nanotube hybrid aerogel: Mechanical properties, electrical and thermal conductivity[J]. Polymer Testing, 2021, 101: 107283.
|
| 12 |
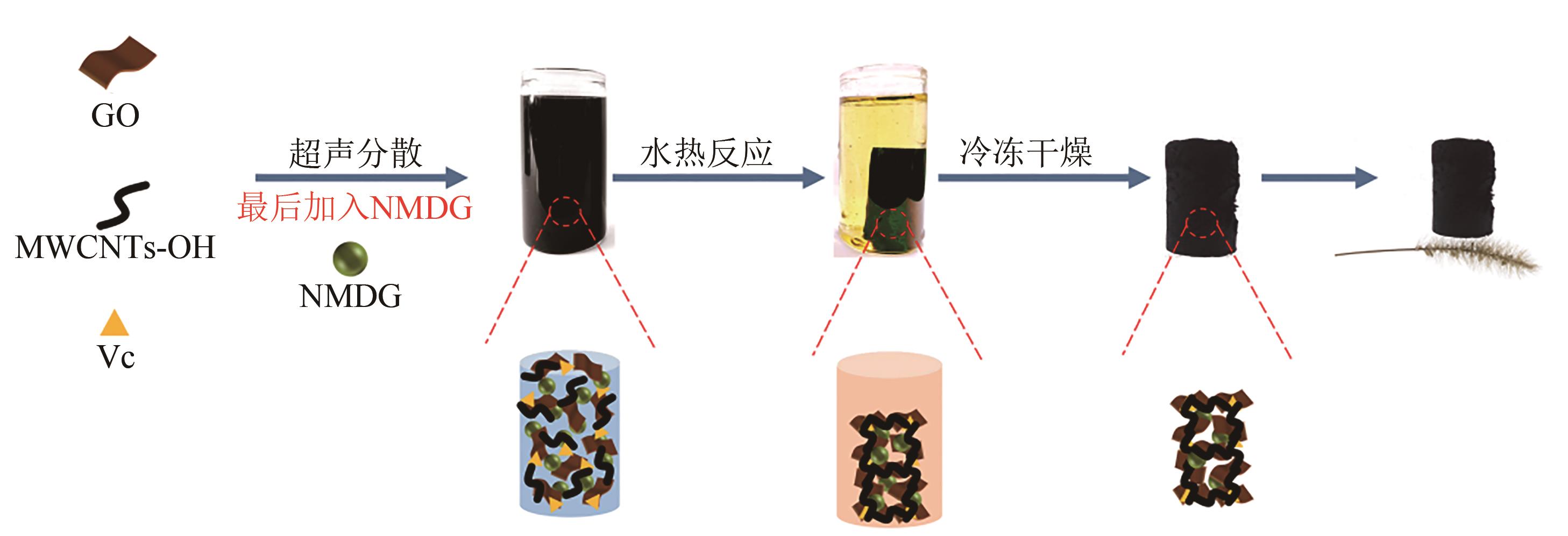
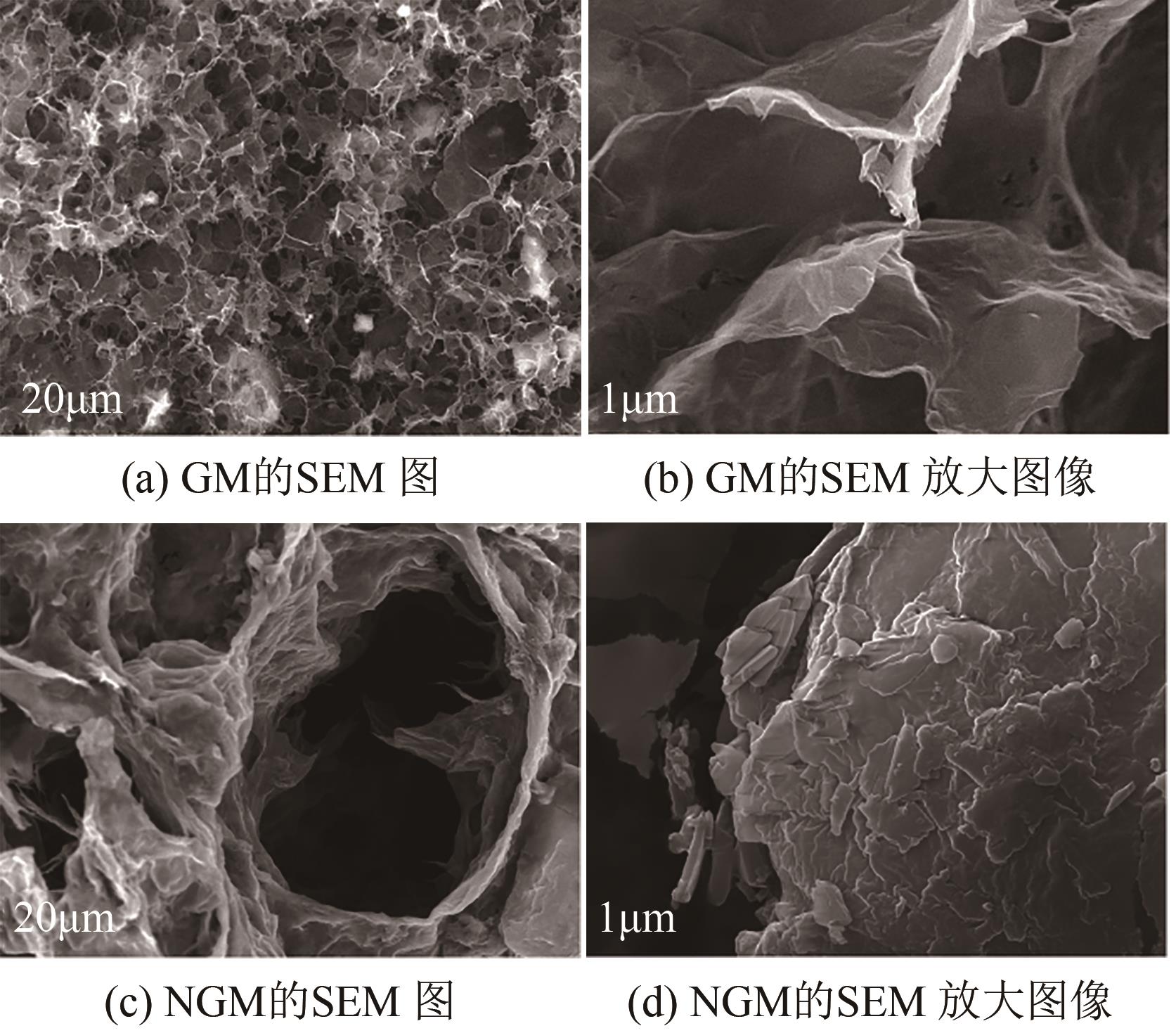
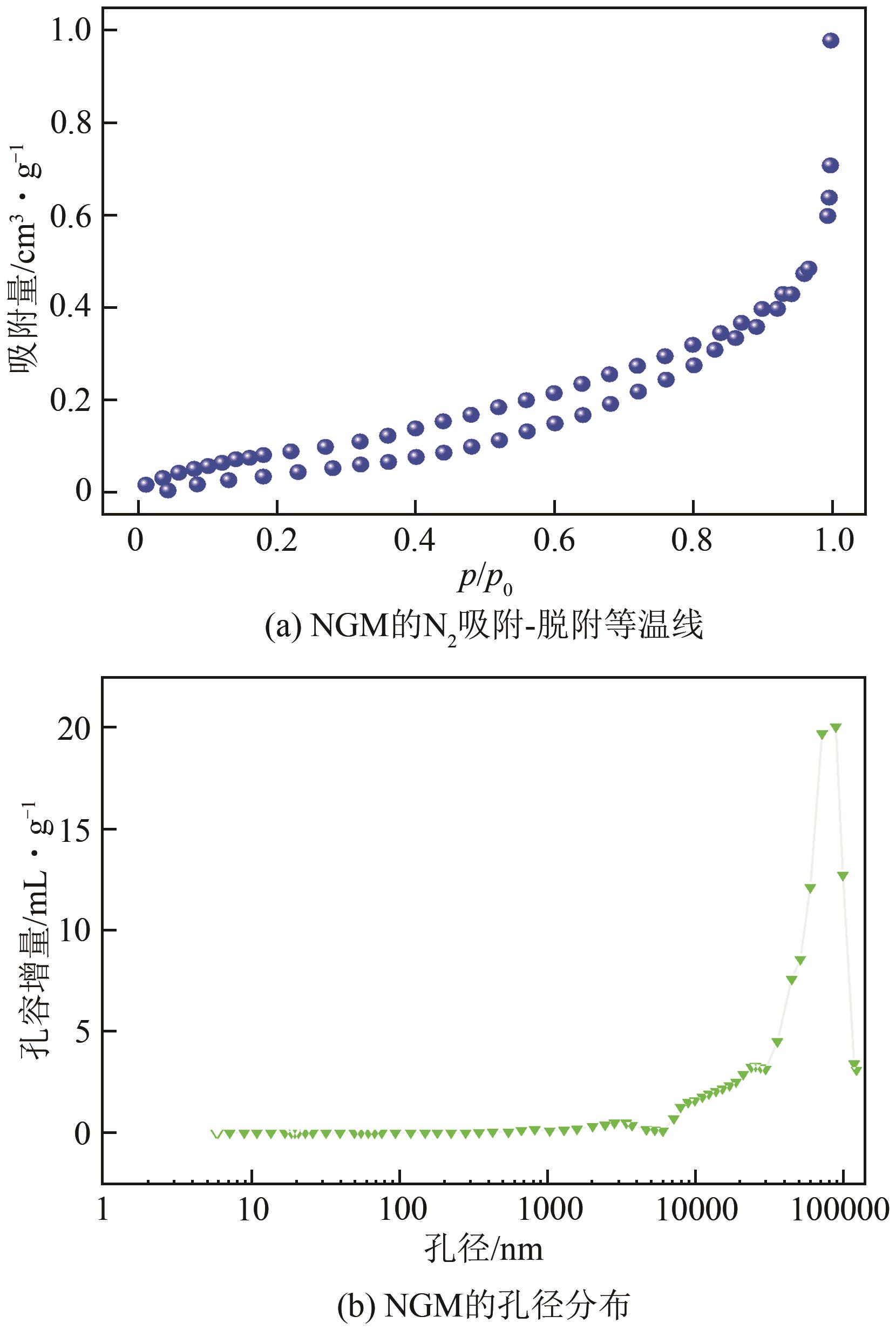
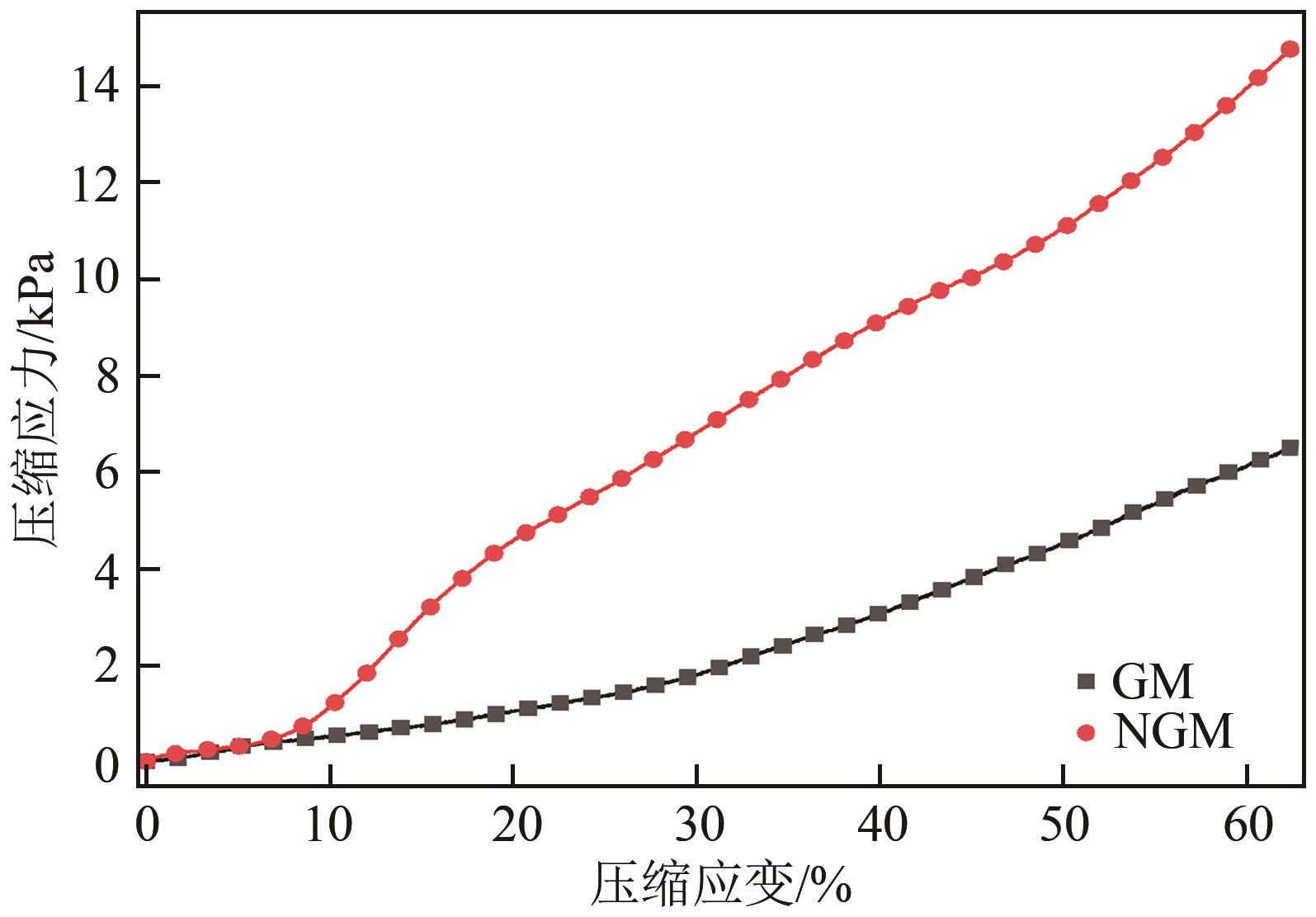
SUN Li, HUANG Jiancheng, LIU Haining, et al. Adsorption of boron by CA@KH-550@EPH@NMDG (CKEN) with biomass carbonaceous aerogels as substrate[J]. Journal of Hazardous Materials, 2018, 358: 10-19.
|
| 13 |
NASEF Mohamed Mahmoud, NALLAPPAN Madana, UJANG Zaini. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review[J]. Reactive and Functional Polymers, 2014, 85: 54-68.
|
| 14 |
KAMBOH Muhammad Afzal, YILMAZ Mustafa. Synthesis of N-methylglucamine functionalized calix[4]arene based magnetic sporopollenin for the removal of boron from aqueous environment[J]. Desalination, 2013, 310: 67-74.
|
| 15 |
AFOLABI Haruna Kolawole, NASEF Mohamed Mahmoud, NORDIN Nik Abdul Hadi Md, et al. Isotherms, kinetics, and thermodynamics of boron adsorption on fibrous polymeric chelator containing glycidol moiety optimized with response surface method[J]. Arabian Journal of Chemistry, 2021, 14(12): 103453.
|
| 35 |
PENG Haihao, XIONG Weiping, YANG Zhaohui, et al. Facile fabrication of three-dimensional hierarchical porous ZIF-L/gelatin aerogel: Highly efficient adsorbent with excellent recyclability towards antibiotics[J]. Chemical Engineering Journal, 2021, 426: 130798.
|
| 36 |
WANG Zhe, WU Zhongyu, ZHANG Yufeng, et al. Hyperbranched-polyol-tethered poly (amic acid) electrospun nanofiber membrane with ultrahigh adsorption capacity for boron removal[J]. Applied Surface Science, 2017, 402: 21-30.
|
| 37 |
JING Ying, JIA Meiying, XU Zhengyong, et al. Facile synthesis of recyclable 3D gelatin aerogel decorated with MIL-88B(Fe) for activation peroxydisulfate degradation of norfloxacin[J]. Journal of Hazardous Materials, 2022, 424: 127503.
|
| 38 |
AFOLABI Haruna Kolawole, NASEF Mohamed Mahmoud, NORDIN Nik Abdul Hadi Md, et al. Facile preparation of fibrous glycidol-containing adsorbent for boron removal from solutions by radiation-induced grafting of poly(vinylamine) and functionalisation[J]. Radiation Physics and Chemistry, 2021, 188: 109596.
|
| 39 |
LUO Qinglong, WANG Yinqiu, LI Liang, et al. Hydrothermal synthesis of hydroxyl terminated polymer boron adsorbents[J]. Journal of Solid State Chemistry, 2021, 296: 121977.
|
| 40 |
CHORGHE Darpan, SARI Mutiara Ayu, CHELLAM Shankararaman. Boron removal from hydraulic fracturing wastewater by aluminum and iron coagulation: Mechanisms and limitations[J]. Water Research, 2017, 126: 481-487.
|
| 41 |
TIAN Xiaojuan, WU Ni, ZHANG Bing, et al. Glycine functionalized boron nitride nanosheets with improved dispersibility and enhanced interaction with matrix for thermal composites[J]. Chemical Engineering Journal, 2021, 408: 127360.
|
 ), CUI Xiangmei(
), CUI Xiangmei( )
)