| 1 |
TURNER J A. Sustainable hydrogen production[J]. Science, 2004, 305(5686): 972-974.
|
| 2 |
KELLY T G, CHEN J G. Metal overlayer on metal carbide substrate: Unique bimetallic properties for catalysis and electrocatalysis[J]. Chemical Society Reviews, 2012, 41(24): 8021-8034.
|
| 3 |
姚淇露, 杜红霞, 卢章辉. 氨硼烷催化水解制氢[J]. 化学进展, 2020, 32(12): 1930-1951.
|
|
YAO Qilu, DU Hongxia, LU Zhanghui. Catalytic hydrolysis of ammonia borane for hydrogen production[J]. Progress in Chemistry, 2020, 32(12): 1930-1951.
|
| 4 |
YANG Jun, SUDIK Andrea, WOLVERTON Christopher, et al. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery[J]. Chemical Society Reviews, 2010, 39(2): 656-675.
|
| 5 |
LEI Weiwei, ZHANG Hui, WU Ying, et al. Oxygen-doped boron nitride nanosheets with excellent performance in hydrogen storage[J]. Nano Energy, 2014, 6: 219-224.
|
| 6 |
ADENIRAN Beatrice, MOKAYA Robert. Compactivation: A mechanochemical approach to carbons with superior porosity and exceptional performance for hydrogen and CO2 storage[J]. Nano Energy, 2015, 16: 173-185.
|
| 7 |
MARDER T B. Will we soon be fueling our automobiles with ammonia-borane?[J]. Angewandte Chemie International Edition, 2007, 46(43): 8116-8118.
|
| 8 |
ZHAO Jianzhi, SHI Jifu, ZHANG Xiaowei, et al. A soft hydrogen storage material: Poly(methyl acrylate)-confined ammonia borane with controllable dehydrogenation[J]. Advanced Materials, 2010, 22(3): 394-397.
|
| 9 |
PENG Bo, CHEN Jun. Ammonia borane as an efficient and lightweight hydrogenstorage medium[J]. Energy & Environmental Science, 2008, 1(4): 479-483.
|
| 10 |
ZHAN Wenwen, ZHU Qilong, XU Qiang. Dehydrogenation of ammonia borane by metal nanoparticle catalysts[J]. ACS Catalysis, 2016, 6(10): 6892-6905.
|
| 11 |
CHANDRA Manish, XU Qiang. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane[J]. Journal of Power Sources, 2006, 156(2): 190-194.
|
| 12 |
JIANG Hailong, XU Qiang. Catalytic hydrolysis of ammonia borane for chemical hydrogen storage[J]. Catalysis Today, 2011, 170(1): 56-63.
|
| 13 |
李燕, 邓雨真, 俞晶铃, 等. 氨硼烷分解制氢及其再生的研究进展[J]. 化工进展, 2019, 38(12): 5330-5338.
|
|
LI Yan, DENG Yuzhen, YU Jingling, et al. Research progress in hydrogen production from decomposition of ammonia borane and its regeneration[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5330-5338.
|
| 14 |
AKBAYRAK Serdar, KAYA Murat, Mürvet VOLKAN, et al. Palladium(0) nanoparticles supported on silica-coated cobalt ferrite: A highly active, magnetically isolable and reusable catalyst for hydrolytic dehydrogenation of ammonia borane[J]. Applied Catalysis B: Environmental, 2014, 147: 387-393.
|
| 15 |
KHALILY M A, EREN H, AKBAYRAK S, et al. Facile synthesis of three-dimensional Pt-TiO2 nano-networks: A highly active catalyst for the hydrolytic dehydrogenation of ammonia-borane[J]. Angewandte Chemie International Edition, 2016, 55(40): 12257-12261.
|
| 16 |
任杨斌, 范燕平, 刘宪云, 等. 氨硼烷水解制氢贵金属催化剂研究进展[J]. 当代化工研究, 2022(3): 1-4.
|
|
REN Yangbin, FAN Yanping, LIU Xianyun, et al. Research progress of hydrolytic noble metal catalyst for hydrogen production from ammonia borane[J]. Modern Chemical Research, 2022(3): 1-4.
|
| 17 |
CHEN Wenyao, JI Jian, DUAN Xuezhi, et al. Unique reactivity in Pt/CNT catalyzed hydrolytic dehydrogenation of ammonia borane[J]. Chemical Communications, 2014, 50(17): 2142-2144.
|
| 18 |
KIM S K, HAN W S, KIM T J, et al. Palladium catalysts for dehydrogenation of ammonia borane with preferential B-H activation[J]. Journal of the American Chemical Society, 2010, 132(29): 9954-9955.
|
| 19 |
BLAQUIERE N, DIALLO-GARCIA S, GORELSKY S I, et al. Ruthenium-catalyzed dehydrogenation of ammonia boranes[J]. Journal of the American Chemical Society, 2008, 130(43): 14034-14035.
|
| 20 |
CHEN Guozhu, DESINAN Stefano, ROSEI Renzo, et al. Hollow ruthenium nanoparticles with small dimensions derived from Ni@Ru core@shell structure: Synthesis and enhanced catalytic dehydrogenation of ammonia borane[J]. Chemical Communications, 2012, 48(64): 8009-8011.
|
| 21 |
PARK J H, KIM S K, KIM H S, et al. Convenient metal embedment into mesoporous silica channels for high catalytic performance in AB dehydrogenation[J]. Chemical Communications, 2013, 49(92): 10832-10834.
|
| 22 |
YANG Q, CHEN Y, WANG Z U., et al. One-pot tandem catalysis over Pd@MIL-101: Boosting the efficiency of nitro compound hydrogenation by coupling with ammonia borane dehydrogenation[J]. Chemical Communications, 2015, 51(52): 10419-10422.
|
| 23 |
XU Qiang, CHANDRA Manish. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia–borane at room temperature[J]. Journal of Power Sources, 2006, 163(1): 364-370.
|
| 24 |
任杨斌, 范燕平, 刘宪云, 等. 镍基催化剂催化氨硼烷水解产氢研究进展[J]. 中国材料进展, 2022, 41(4): 288-295.
|
|
REN Yangbin, FAN Yanping, LIU Xianyun, et al. Research progress on hydrogen generation by catalytic hydrolysis of ammonia borane over Ni catalysts[J]. Materials China, 2022, 41(4): 288-295.
|
| 25 |
GRÄTZEL M. Photoelectrochemical cells[J]. Nature, 2001, 414(6861): 338-344.
|
| 26 |
GRAY H B. Powering the planet with solar fuel[J]. Nature Chemistry, 2009, 1(1): 7.
|
| 27 |
KANAN M W, NOCERA D G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ [J]. Science, 2008, 321(5892): 1072-1075.
|
| 28 |
COBO S, HEIDKAMP J, JACQUES P A, et al. A Janus cobalt-based catalytic material for electro-splitting of water[J]. Nature Materials, 2012, 11(9): 802-807.
|
| 29 |
WANG Jiahai, CUI Wei, LIU Qian, et al. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting[J]. Advanced Materials, 2016, 28(2): 215-230.
|
| 30 |
WAN Jun, YAO Xu, GAO Xiang, et al. Microwave combustion for modification of transition metal oxides[J]. Advanced Functional Materials, 2016, 26(40): 7263-7270.
|
| 31 |
GONG Qiufang, WANG Yu, HU Qi, et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution[J]. Nature Communications, 2016, 7(1): 1-8.
|
| 32 |
LEVY R B, BOUDART M. Platinum-like behavior of tungsten carbide in surface catalysis[J]. Science, 1973, 181(4099): 547-549.
|
| 33 |
KOU Zongkui, MENG Tian, GUO Beibei, et al. A generic conversion strategy: From 2D metal carbides (M x C y ) to M-self-doped graphene toward high-efficiency energy applications[J]. Advanced Functional Materials, 2017, 27(8): 1604904.
|
| 34 |
LI Jisen, WANG Yu, LIU Chunhui, et al. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution[J]. Nature Communications, 2016, 7(1): 1-8.
|
| 35 |
HU Wenhui, SHANG Xiao, HAN Guanqun, et al. MoS x supported graphene oxides with different degree of oxidation as efficient electrocatalysts for hydrogen evolution[J]. Carbon, 2016, 100: 236-242.
|
| 36 |
HE Chunyong, TAO Juzhou. Exploration of the electrochemical mechanism of ultrasmall multiple phases molybdenum carbides nanocrystals for hydrogen evolution reaction[J]. RSC Advances, 2016, 6(11): 9240-9246.
|
| 37 |
REN Yangbin, DUAN Jizhuan, LIU Xianyun, et al. Ni-Mo2C nanocomposites as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane[J]. Energy & Fuels, 2021, 35(19): 16222-16231.
|
| 38 |
GE Yuzhen, QIN Xuetao, LI Aowen, et al. Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production[J]. Journal of the American Chemical Society, 2021, 143(2): 628-633.
|
| 39 |
WAN Jun, WU Jiabin, GAO Xiang, et al. Structure confined porous Mo2C for efficient hydrogen evolution[J]. Advanced Functional Materials, 2017, 27(45): 1-7.
|
| 40 |
ZHOU Xiangyang, LI Liang, YANG Juan, et al. Cobalt and molybdenum carbide nanoparticles grafted on nitrogen-doped carbon nanotubes as efficient chemical anchors and polysulfide conversion catalysts for lithium-sulfur batteries[J]. ChemElectroChem, 2020, 7(18): 3767-3775.
|
| 41 |
REN Xiang, ZHAO Jinxiu, WEI Qin, et al. High-performance N2-to-NH3 conversion electrocatalyzed by Mo2C nanorod[J]. ACS Central Science, 2019, 5(1): 116-121.
|
| 42 |
ZHANG Pengfei, LIU Yaoda, LIANG Tingting, et al. Nitrogen-doped carbon wrapped Co-Mo2C dual Mott-Schottky nanosheets with large porosity for efficient water electrolysis[J]. Applied Catalysis B: Environmental, 2021, 284: 119738.
|
| 43 |
LIU Jing, HODES Gary, YAN Junqing, et al. Metal-doped Mo2C (metal = Fe, Co, Ni, Cu) as catalysts on TiO2 for photocatalytic hydrogen evolution in neutral solution[J]. Chinese Journal of Catalysis, 2021, 42(1): 205-216.
|
| 44 |
DUAN Jizhuan, LIU Xianyun, BIAN Linyan, et al. Controllable synthesis of MoC and Mo2C to boost hydrogen generation from ammonia borane hydrolysis[J]. ACS Applied Energy Materials, 2023, 6(3): 1753-1762.
|
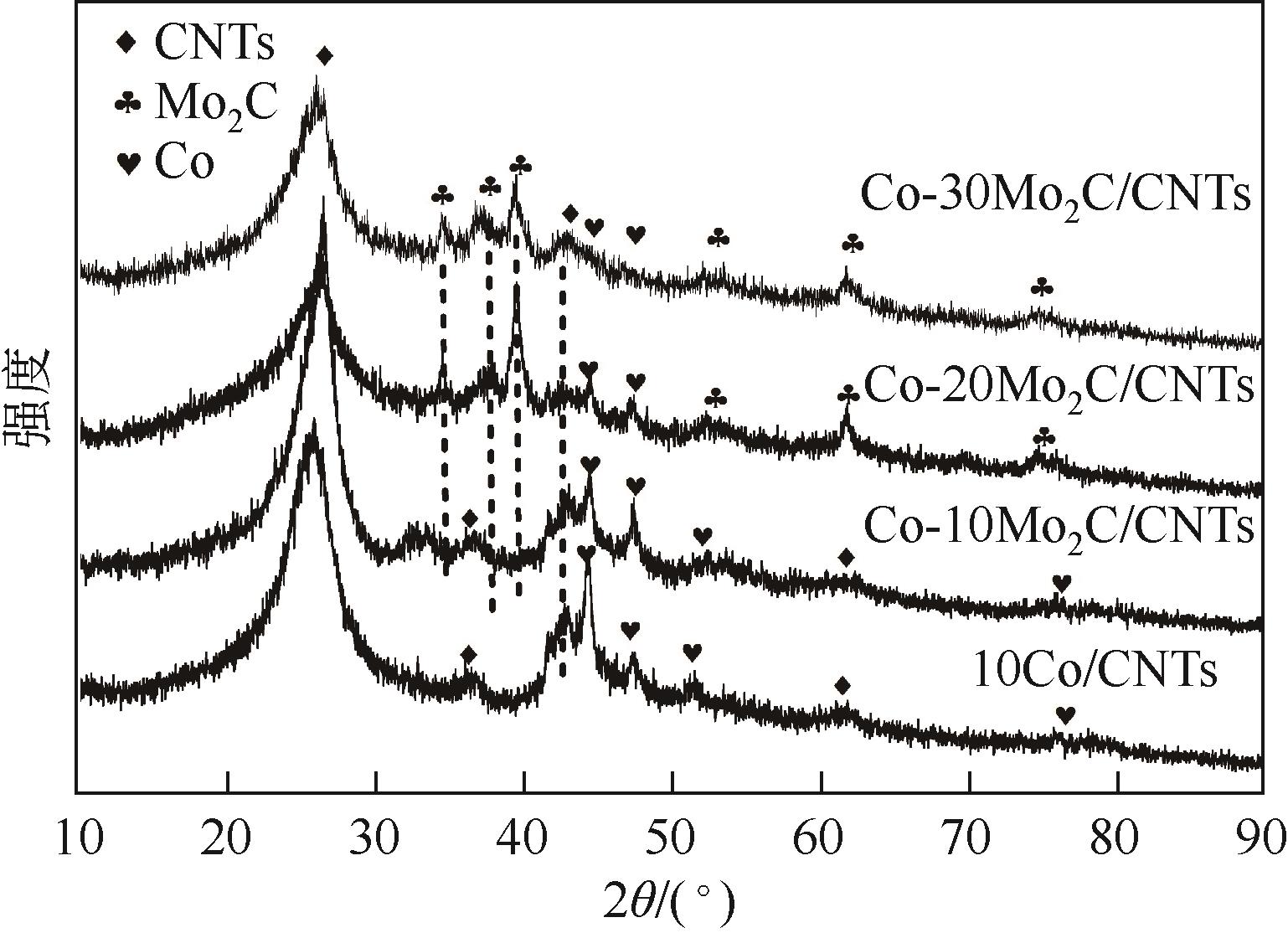
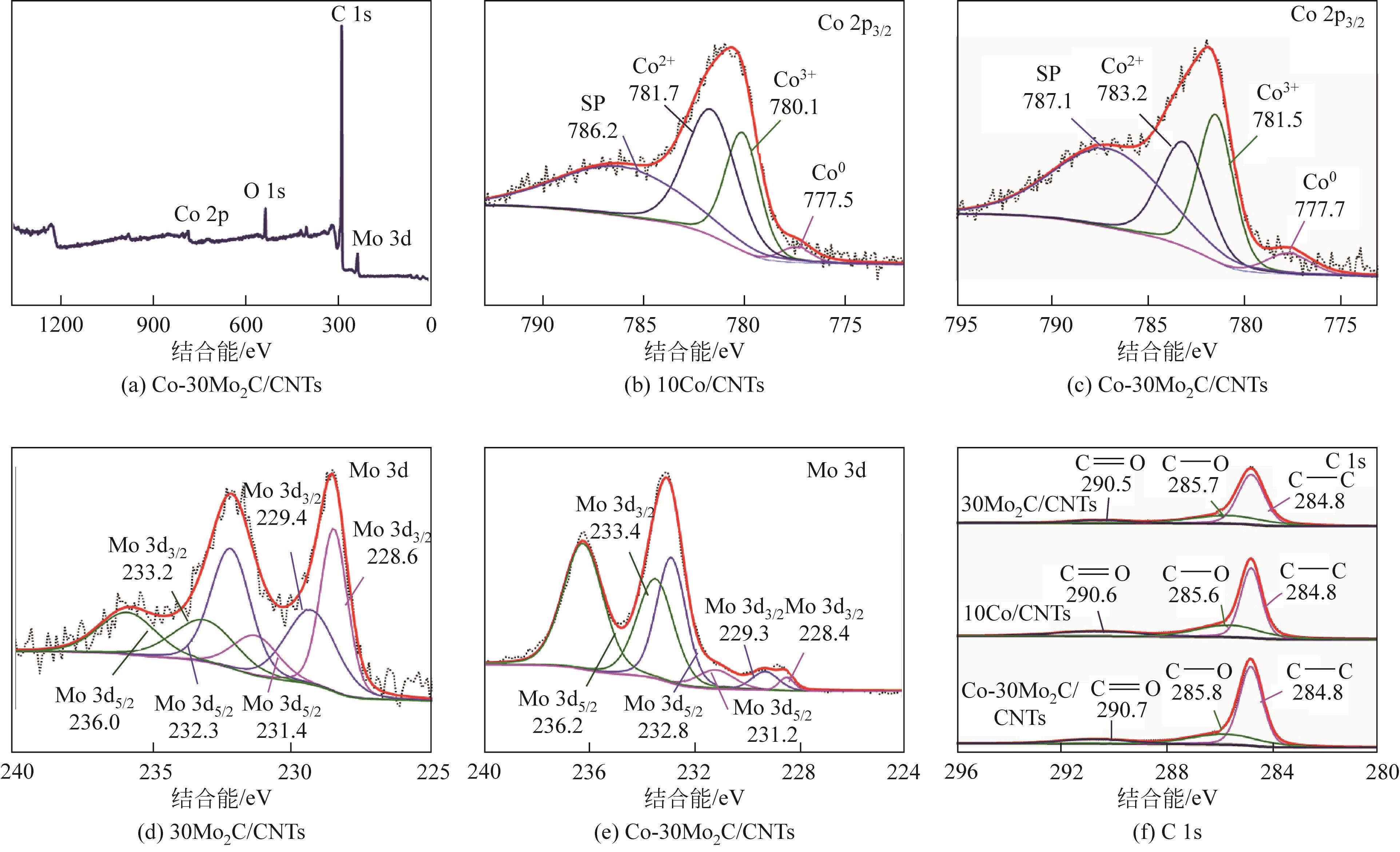
 ,这优异的催化活性归结于Co和Mo2C之间的协同作用,Mo2C的添加增强了对H2O分子的活化。经过五圈稳定性测试后,活性依然可以保留75%,这对非贵金属催化剂的可回收投入使用具有一定的研究意义。
,这优异的催化活性归结于Co和Mo2C之间的协同作用,Mo2C的添加增强了对H2O分子的活化。经过五圈稳定性测试后,活性依然可以保留75%,这对非贵金属催化剂的可回收投入使用具有一定的研究意义。