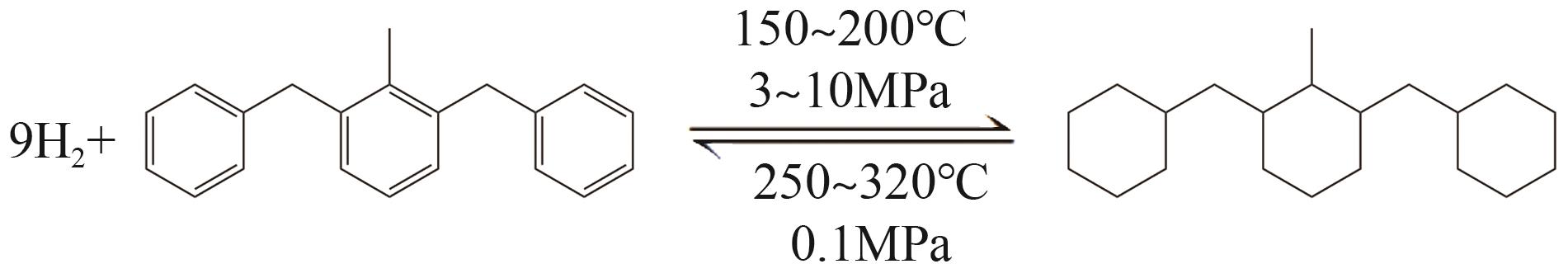化工进展 ›› 2024, Vol. 43 ›› Issue (4): 1731-1741.DOI: 10.16085/j.issn.1000-6613.2023-0515
• 能源加工与技术 • 上一篇
液态有机储氢技术研究现状与展望
刘若璐1( ), 汤海波1, 何翡翡2, 罗凤盈1, 王金鸽2, 杨娜2, 李洪伟2, 张锐明1,2,3(
), 汤海波1, 何翡翡2, 罗凤盈1, 王金鸽2, 杨娜2, 李洪伟2, 张锐明1,2,3( )
)
- 1.先进能源科学与技术广东省实验室佛山分中心(佛山仙湖实验室),广东 佛山 528200
2.广东瀚锐氢能 科技有限公司,广东 佛山 528200
3.广东省武理工氢能产业技术研究院,广东 佛山 528200
-
收稿日期:2023-04-04修回日期:2023-08-08出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:张锐明 -
作者简介:刘若璐(1997—),女,硕士,研究方向为氢能及燃料电池。E-mail:liuruolu@xhlab.cn。
Recent research and prospect of liquid organic hydrogen carries technology
LIU Ruolu1( ), TANG Haibo1, HE Feifei2, LUO Fengying1, WANG Jinge2, YANG Na2, LI Hongwei2, ZHANG Ruiming1,2,3(
), TANG Haibo1, HE Feifei2, LUO Fengying1, WANG Jinge2, YANG Na2, LI Hongwei2, ZHANG Ruiming1,2,3( )
)
- 1.Foshan Xianhu Laboratory of the Advanced Energy Science and Technology Guangdong Laboratory, Foshan 528200, Guangdong, China
2.Guangdong Hanrui Hydrogen Energy Technology Company Limited, Foshan 528200, Guangdong, China
3.Guangdong Hydrogen Energy Institute of WHUT, Foshan 528200, Guangdong, China
-
Received:2023-04-04Revised:2023-08-08Online:2024-04-15Published:2024-05-13 -
Contact:ZHANG Ruiming
摘要:
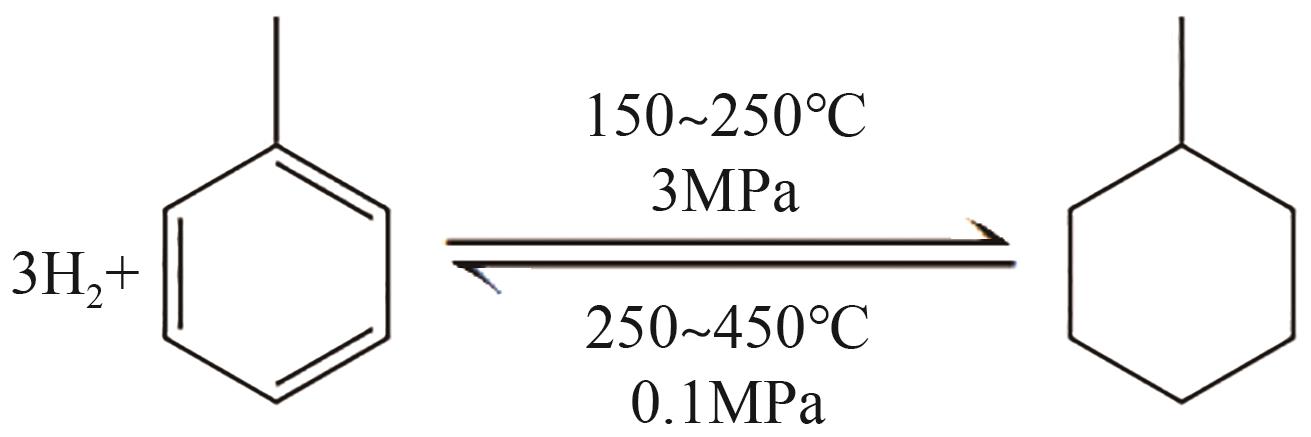
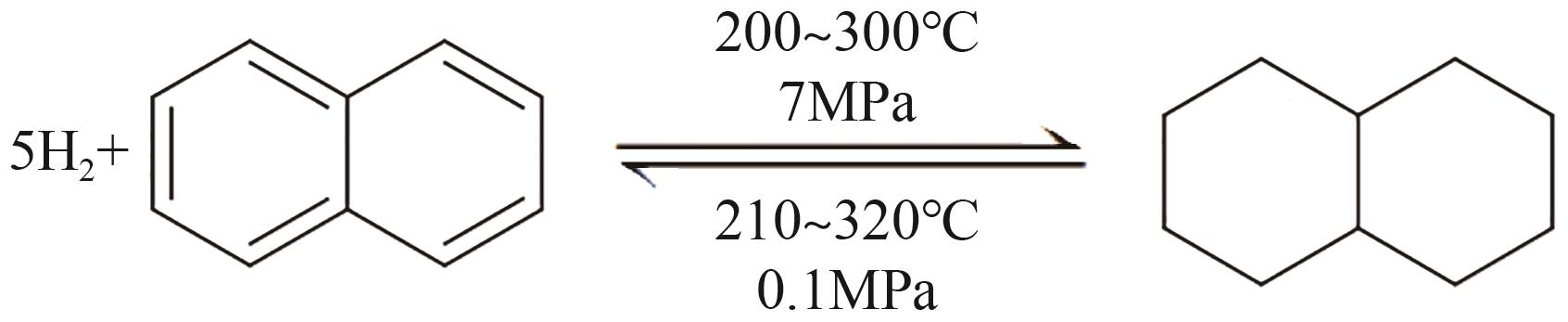
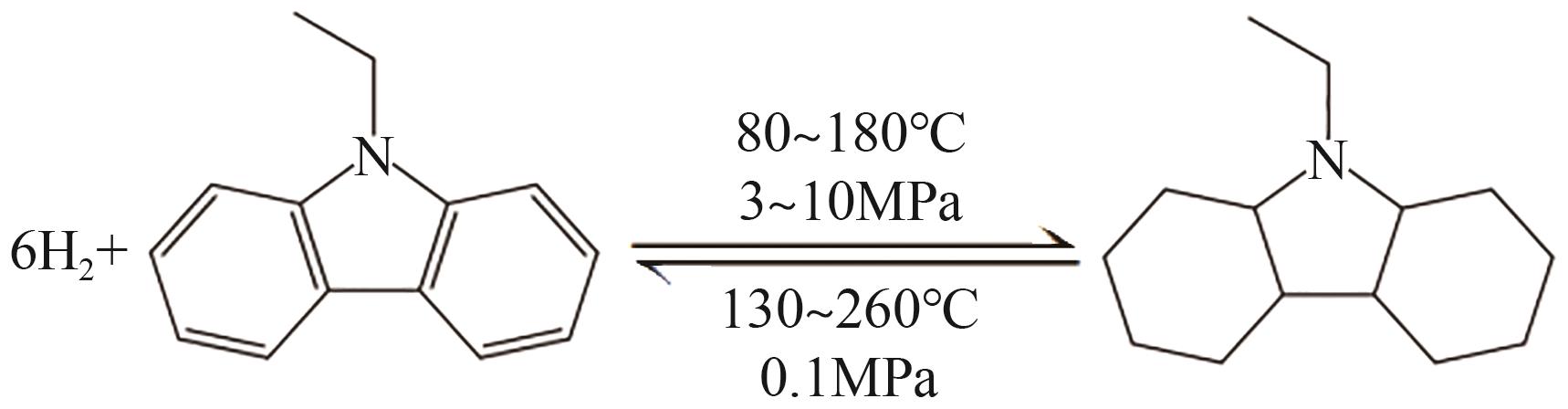
随着构建绿色氢能社会愿景的提出,氢能的需求量将会大规模增长,氢能的储运就成为了制约产业规模化发展的瓶颈。液态有机储氢技术在氢能大规模储存和长距离运输上具有成本低、安全性高等传统高压储氢无法比拟的优势,由于这项技术目前仍处于发展初期,国内相关的报道较少。本文综述了芳香烃类和氮杂环芳香烃类等主要的液态有机储氢材料,并对其储氢性能、优势、存在问题及发展现状展开了分析;阐述了液态有机储氢技术中加氢和脱氢过程所涉及的各种金属催化剂的性能。基于目前的研究,对液态有机储氢技术在未来氢能规模化应用方面的发展前景进行了展望,同时指出液态有机储氢技术在诸多氢能应用领域的可行性及其极高的经济价值。但是若要实现大规模应用,则需选择更优的有机储氢材料,开发高选择性、高催化活性及低成本的新型催化剂,进一步优化加氢和脱氢技术。
中图分类号:
引用本文
刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741.
LIU Ruolu, TANG Haibo, HE Feifei, LUO Fengying, WANG Jinge, YANG Na, LI Hongwei, ZHANG Ruiming. Recent research and prospect of liquid organic hydrogen carries technology[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1731-1741.
| 序号 | 名称 | 分子结构 | 富氢分子结构 | 质量储氢密度/% | 体积储氢密度/kg·m-3 | 熔点/℃ | 沸点/℃ | 脱氢焓/kJ·mol-1 |
|---|---|---|---|---|---|---|---|---|
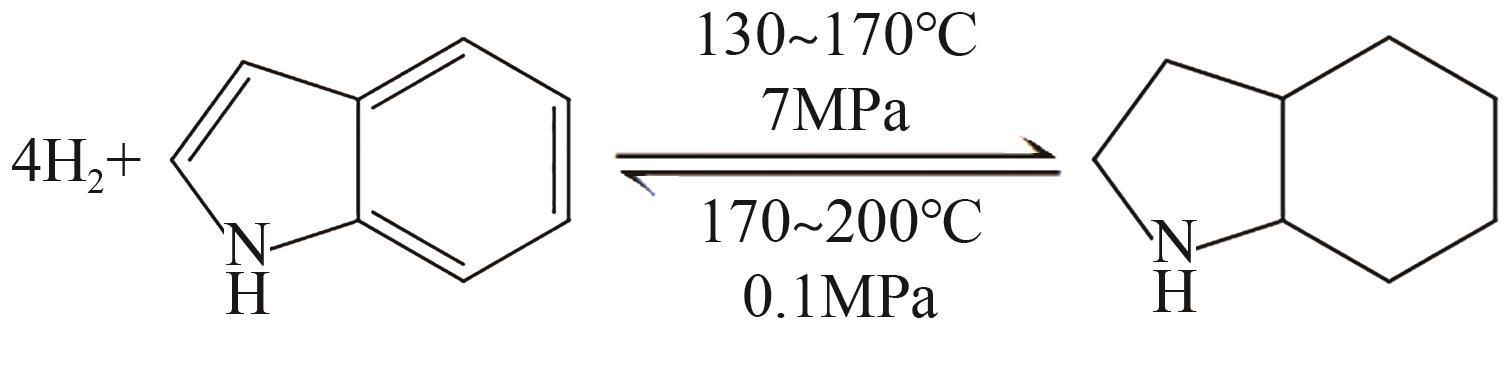
| 1 | 咔唑 | 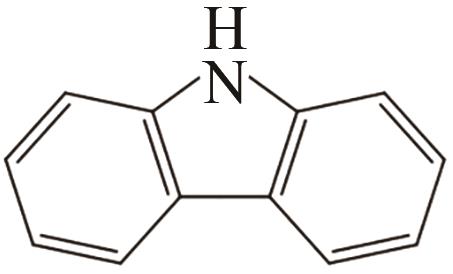 |  | 6.7 | 87 | 48.12 | 355 | 48.12 |
| 2 | N-甲基咔唑 | 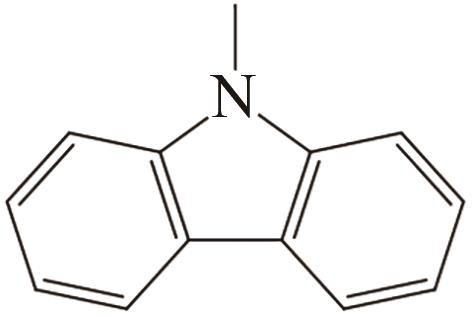 | 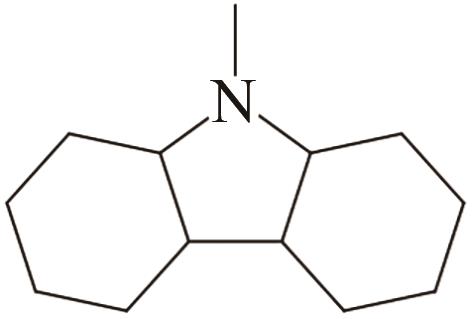 | 6.2 | — | 89 | 337.8 | 49.1 |
| 3 | N-乙基咔唑 | 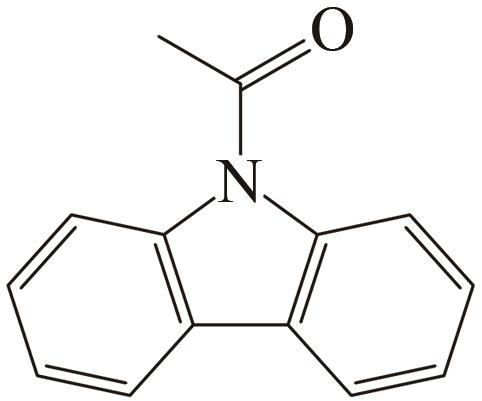 | 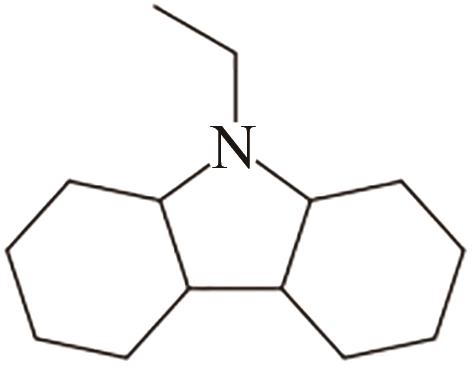 | 5.8 | 54.0 | 68 | 348 | 50.6 |
| 4 | N-乙酰咔唑 | 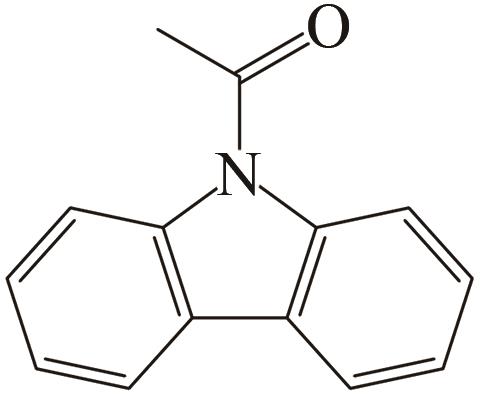 | 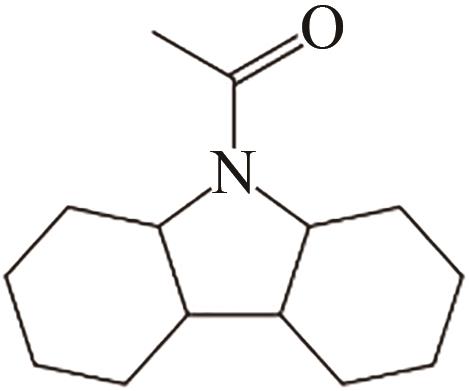 | 6.32 | 63.83 | 77 | 315.5 | — |
| 5 | N-苯甲酰咔唑 | 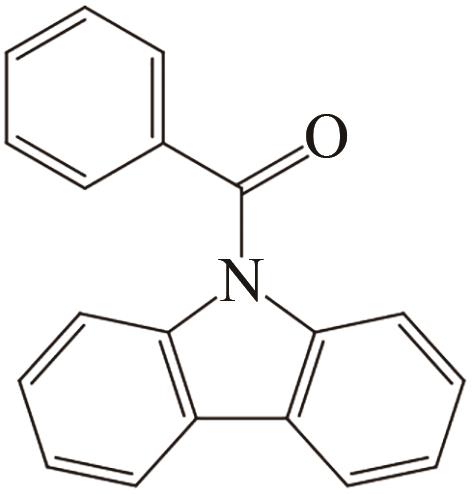 | 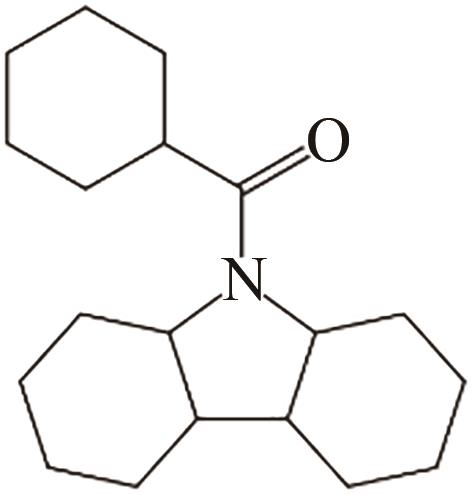 | 6.92 | 68.92 | 101 | 391.2 | — |
| 6 | N-苯基咔唑 |  | 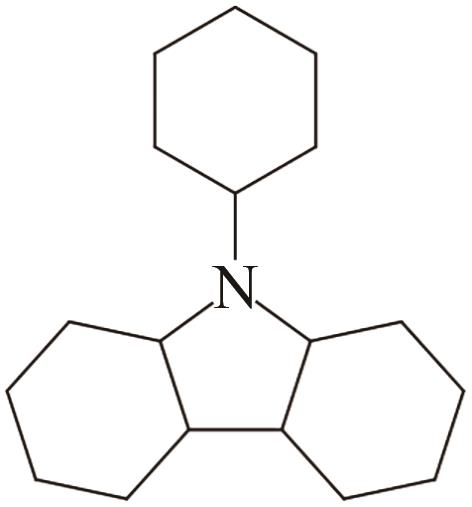 | 6.94 | 67.11 | 70 | 415.3 | 57.7 |
表1 咔唑及其衍生物的物理参数和储氢性能
| 序号 | 名称 | 分子结构 | 富氢分子结构 | 质量储氢密度/% | 体积储氢密度/kg·m-3 | 熔点/℃ | 沸点/℃ | 脱氢焓/kJ·mol-1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 咔唑 |  |  | 6.7 | 87 | 48.12 | 355 | 48.12 |
| 2 | N-甲基咔唑 |  |  | 6.2 | — | 89 | 337.8 | 49.1 |
| 3 | N-乙基咔唑 |  |  | 5.8 | 54.0 | 68 | 348 | 50.6 |
| 4 | N-乙酰咔唑 |  |  | 6.32 | 63.83 | 77 | 315.5 | — |
| 5 | N-苯甲酰咔唑 |  |  | 6.92 | 68.92 | 101 | 391.2 | — |
| 6 | N-苯基咔唑 |  |  | 6.94 | 67.11 | 70 | 415.3 | 57.7 |
| 序号 | 名称 | 分子结构 | 富氢分子结构 | 质量储氢密度/% | 体积储氢密度/kg·m-3 | 熔点/℃ | 沸点/℃ | 脱氢焓/kJ·mol-1 |
|---|---|---|---|---|---|---|---|---|
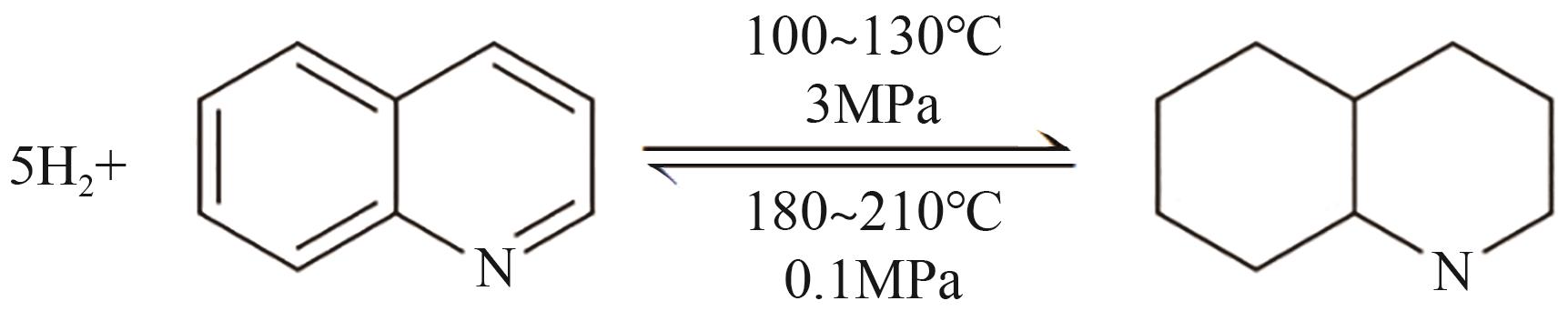
| 1 | 吲哚 | 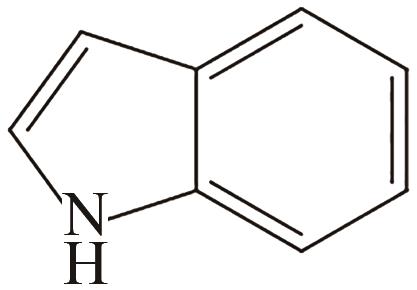 | 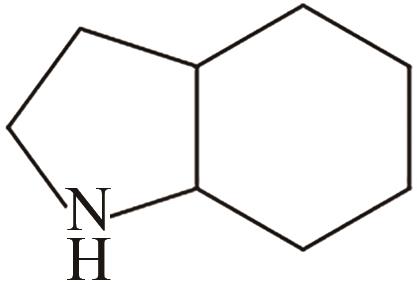 | 6.4 | 56 | 53 | 253 | 53.6 |
| 2 | N-甲基吲哚 | 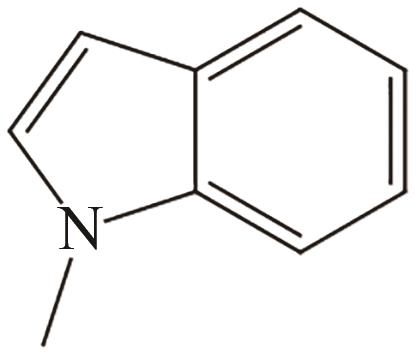 | 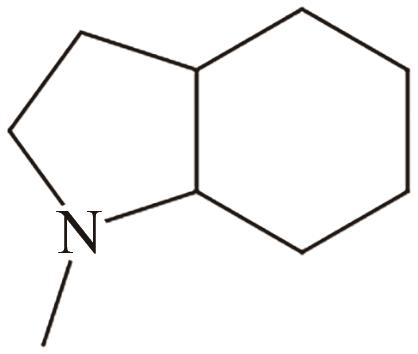 | 5.76 | 59.1 | <-20 | 239 | — |
| 3 | 2-甲基吲哚 | 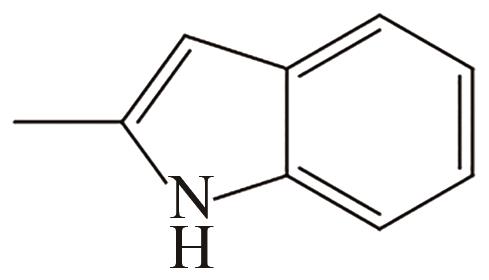 | 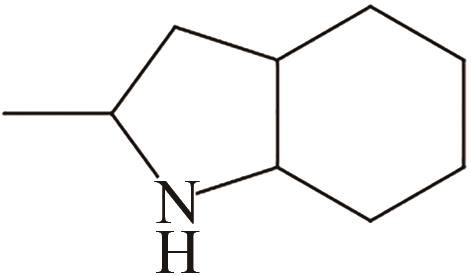 | 5.76 | — | 57 | 273 | 51.7 |
| 4 | N-乙基吲哚 | 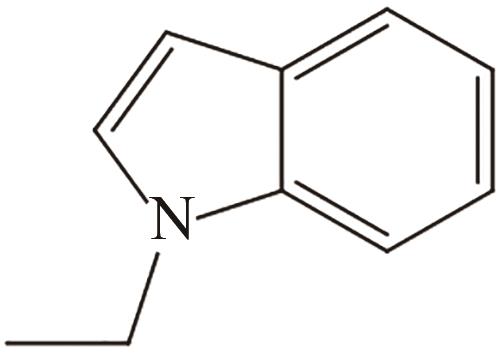 | 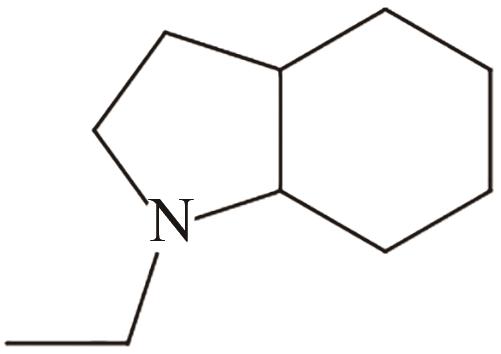 | 5.23 | — | -17.8 | 253.5 | — |
| 5 | 1,2-二甲基吲哚 | 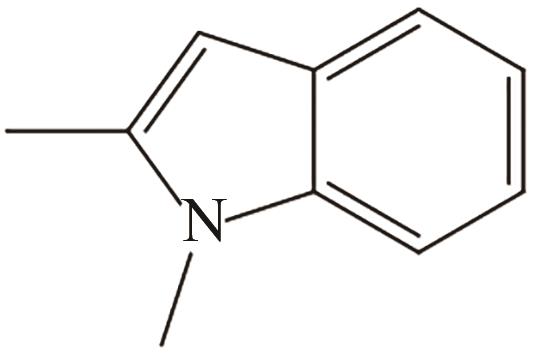 | 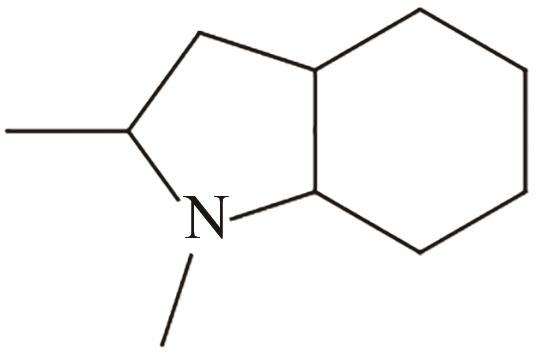 | 5.23 | — | 55 | 260.5 | — |
表2 吲哚及其衍生物的物理参数和储氢性能
| 序号 | 名称 | 分子结构 | 富氢分子结构 | 质量储氢密度/% | 体积储氢密度/kg·m-3 | 熔点/℃ | 沸点/℃ | 脱氢焓/kJ·mol-1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 吲哚 |  |  | 6.4 | 56 | 53 | 253 | 53.6 |
| 2 | N-甲基吲哚 |  |  | 5.76 | 59.1 | <-20 | 239 | — |
| 3 | 2-甲基吲哚 |  |  | 5.76 | — | 57 | 273 | 51.7 |
| 4 | N-乙基吲哚 |  |  | 5.23 | — | -17.8 | 253.5 | — |
| 5 | 1,2-二甲基吲哚 |  |  | 5.23 | — | 55 | 260.5 | — |
| 序号 | 催化剂 | 反应物 | 完全加氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5% Ru/Al2O3 | 1-甲基吲哚 | 8H-1-甲基吲哚 | 130 | 6 | — | 100 | 100 | [ |
| 2 | 5% Ru/Al2O3 | 2-甲基吲哚 | 8H-2-甲基吲哚 | 170 | 7 | 0.5 | >99 | >99 | [ |
| 3 | 5% Ru/Al2O3 | 1-乙基吲哚 | 8H-1-乙基吲哚 | 190 | 9 | 1.33 | 100 | 100 | [ |
| 4 | 5% Ru/Al2O3 | 1-乙基吲哚 | 8H-1-乙基吲哚 | 160 | 9 | 4 | 100 | 100 | [ |
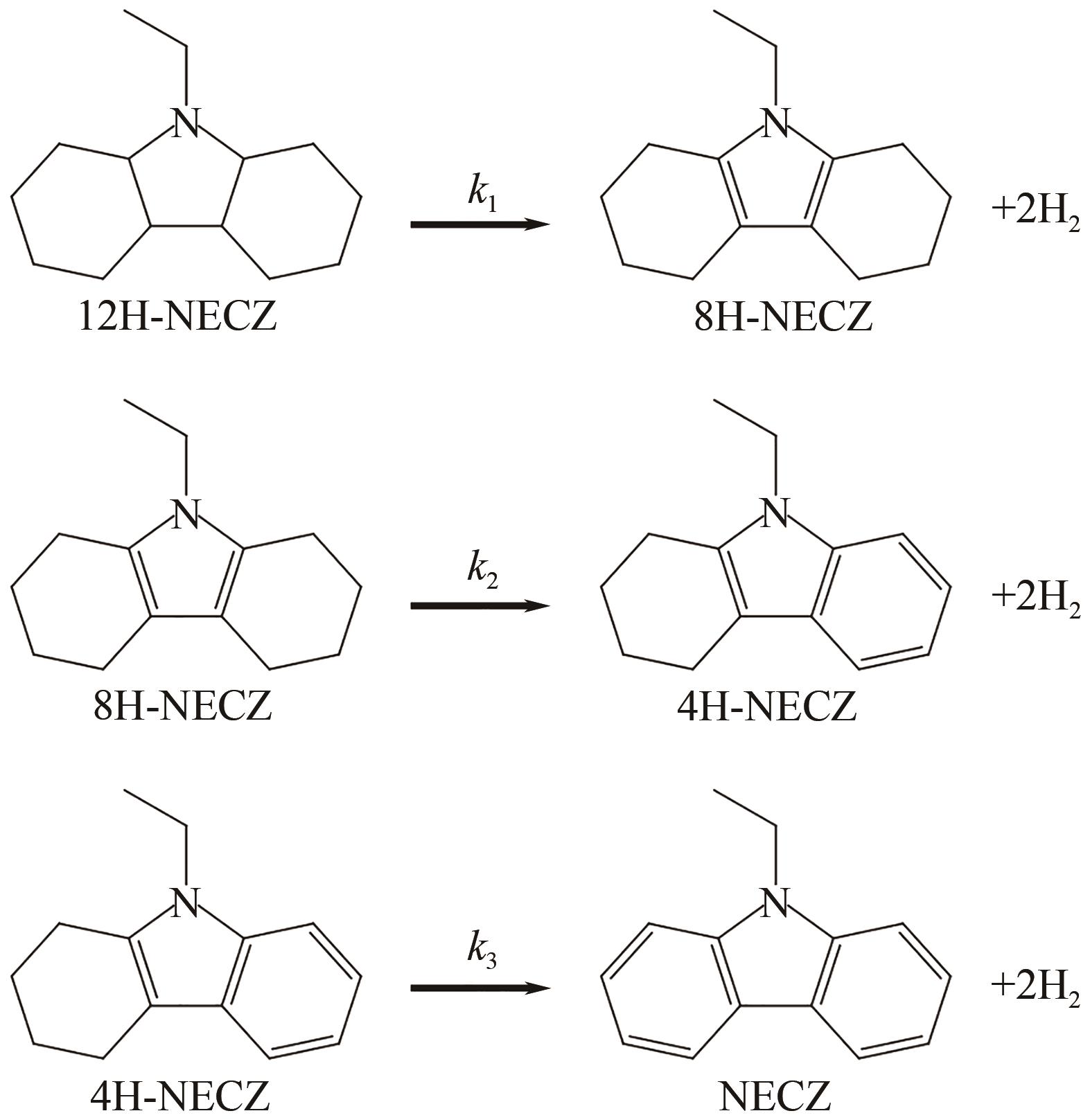
| 5 | 4.6% Ru/Al2O3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 130 | 7 | 10 | — | 100 | [ |
| 6 | 1.3% Ru/YH3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 130 | 7 | 10 | — | 100 | [ |
| 7 | 1.3% Ru/YH3 | 2-甲基吲哚 | 8H-2-甲基吲哚 | 130 | 7 | 10 | 100 | 100 | [ |
| 8 | 0.62% Ru0.2 | 喹啉 | 十氢喹啉 | 60 | 2 | 24 | 100 | — | [ |
| 9 | 3% Pt/ Al2O3-P-O2 | 二苄基甲苯 | 18H-二苄基甲苯 | 140 | 4 | 0.5 | >99 | — | [ |
| 10 | 1% Pt/WO3-500 | 萘 | 十氢化萘 | 70 | 3 | 1 | 100 | 100 | [ |
| 11 | 2% Ir3 | 喹啉 | 1,2,3,4-四氢喹啉 | 80 | 0.1 | 17 | 99 | 98 | [ |
| 12 | Ru-Ni/C | 2-萘酚 | 5,6,7,8-四氢-2-萘酚 | 90 | 4 | 3 | 100 | 71.08 | [ |
| 13 | 1% Pt-WO3/α-Al2O3 | 萘 | 十氢化萘 | 70 | 3 | 1 | 100 | 100 | [ |
| 14 | 1% Pd-Rh/γ-Al2O3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 160 | 6 | 1 | 100 | 100 | [ |
| 15 | Ru-Pd/Al2O3 | N-丙基咔唑 | 12H-N-丙基咔唑 | 150 | 7 | 7 | >99 | >99 | [ |
表3 贵金属加氢催化剂参数
| 序号 | 催化剂 | 反应物 | 完全加氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5% Ru/Al2O3 | 1-甲基吲哚 | 8H-1-甲基吲哚 | 130 | 6 | — | 100 | 100 | [ |
| 2 | 5% Ru/Al2O3 | 2-甲基吲哚 | 8H-2-甲基吲哚 | 170 | 7 | 0.5 | >99 | >99 | [ |
| 3 | 5% Ru/Al2O3 | 1-乙基吲哚 | 8H-1-乙基吲哚 | 190 | 9 | 1.33 | 100 | 100 | [ |
| 4 | 5% Ru/Al2O3 | 1-乙基吲哚 | 8H-1-乙基吲哚 | 160 | 9 | 4 | 100 | 100 | [ |
| 5 | 4.6% Ru/Al2O3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 130 | 7 | 10 | — | 100 | [ |
| 6 | 1.3% Ru/YH3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 130 | 7 | 10 | — | 100 | [ |
| 7 | 1.3% Ru/YH3 | 2-甲基吲哚 | 8H-2-甲基吲哚 | 130 | 7 | 10 | 100 | 100 | [ |
| 8 | 0.62% Ru0.2 | 喹啉 | 十氢喹啉 | 60 | 2 | 24 | 100 | — | [ |
| 9 | 3% Pt/ Al2O3-P-O2 | 二苄基甲苯 | 18H-二苄基甲苯 | 140 | 4 | 0.5 | >99 | — | [ |
| 10 | 1% Pt/WO3-500 | 萘 | 十氢化萘 | 70 | 3 | 1 | 100 | 100 | [ |
| 11 | 2% Ir3 | 喹啉 | 1,2,3,4-四氢喹啉 | 80 | 0.1 | 17 | 99 | 98 | [ |
| 12 | Ru-Ni/C | 2-萘酚 | 5,6,7,8-四氢-2-萘酚 | 90 | 4 | 3 | 100 | 71.08 | [ |
| 13 | 1% Pt-WO3/α-Al2O3 | 萘 | 十氢化萘 | 70 | 3 | 1 | 100 | 100 | [ |
| 14 | 1% Pd-Rh/γ-Al2O3 | N-乙基咔唑 | 12H-N-乙基咔唑 | 160 | 6 | 1 | 100 | 100 | [ |
| 15 | Ru-Pd/Al2O3 | N-丙基咔唑 | 12H-N-丙基咔唑 | 150 | 7 | 7 | >99 | >99 | [ |
| 序号 | 催化剂 | 反应物 | 完全脱氢产物 | 温度 /℃ | 压力 /MPa | 时间 /h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5% Pd/Al2O3 | 8H-1-甲基吲哚 | 1-甲基吲哚 | 190 | 0.1 | 5 | 100 | 100 | [ |
| 2 | 5% Pd/Al2O3 | 8H-2-甲基吲哚 | 2-甲基吲哚 | 190 | 0.1 | 4 | 100 | 100 | [ |
| 3 | 5% Pd/Al2O3 | 8H-1-乙基吲哚 | 1-乙基吲哚 | 190 | 0.1 | 6 | 100 | 100 | [ |
| 4 | 3% Pd@MIL-101 | 12H-N-丙基咔唑 | N-丙基咔唑 | 190 | — | 6 | 100 | — | [ |
| 5 | 1% Pd/Al2O3 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 1.2 | — | — | [ |
| 6 | 10% PdO/AC | LOHC混合物(40% 8H-甲基吲哚,36% 12H-N-丙基咔唑,24% 12H-乙基咔唑) | LOHC混合物(40%甲基吲哚,36% N-丙基咔唑,24%乙基咔唑) | 100 | — | 60 | 100 | 95.8 | [ |
| 7 | 10% PdO/AC | LOHC混合物(40% 8H-甲基吲哚,36% 12H-N-丙基咔唑,24% 12H-乙基咔唑) | LOHC混合物(40%甲基吲哚,36t% N-丙基咔唑,24% N-乙基咔唑) | 140 | — | 8 | 100 | 100 | [ |
| 8 | Pd/rGO | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 7 | >99 | 97.65 | [ |
| 9 | Pd/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 97.4 | [ |
| 10 | 3% Pt/ Al2O3-P-H2 | 18H-二苄基甲苯 | 二苄基甲苯 | 270 | 0.1 | 5 | 70.8 | — | [ |
| 11 | 0.15% Pt/CeO2 | 18H-二苄基甲苯 | 二苄基甲苯 | 300 | 0.1 | 2.5 | 80.5 | — | [ |
| 12 | Ru-Pd/Al2O3 | 12H-N-丙基咔唑 | N-丙基咔唑 | 180 | 0.1 | 4 | >99 | 97.1 | [ |
| 13 | Pd-Ni/Al2O3 | 12H-N-丙基咔唑 | N-丙基咔唑 | 180 | 0.1 | 8 | >99 | >99 | [ |
| 14 | Pd4Ni1/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 99.1 | [ |
| 15 | Pd1Co3/SiO2 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 8 | 100 | 71.66 | [ |
表4 贵金属脱氢催化剂参数
| 序号 | 催化剂 | 反应物 | 完全脱氢产物 | 温度 /℃ | 压力 /MPa | 时间 /h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5% Pd/Al2O3 | 8H-1-甲基吲哚 | 1-甲基吲哚 | 190 | 0.1 | 5 | 100 | 100 | [ |
| 2 | 5% Pd/Al2O3 | 8H-2-甲基吲哚 | 2-甲基吲哚 | 190 | 0.1 | 4 | 100 | 100 | [ |
| 3 | 5% Pd/Al2O3 | 8H-1-乙基吲哚 | 1-乙基吲哚 | 190 | 0.1 | 6 | 100 | 100 | [ |
| 4 | 3% Pd@MIL-101 | 12H-N-丙基咔唑 | N-丙基咔唑 | 190 | — | 6 | 100 | — | [ |
| 5 | 1% Pd/Al2O3 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 1.2 | — | — | [ |
| 6 | 10% PdO/AC | LOHC混合物(40% 8H-甲基吲哚,36% 12H-N-丙基咔唑,24% 12H-乙基咔唑) | LOHC混合物(40%甲基吲哚,36% N-丙基咔唑,24%乙基咔唑) | 100 | — | 60 | 100 | 95.8 | [ |
| 7 | 10% PdO/AC | LOHC混合物(40% 8H-甲基吲哚,36% 12H-N-丙基咔唑,24% 12H-乙基咔唑) | LOHC混合物(40%甲基吲哚,36t% N-丙基咔唑,24% N-乙基咔唑) | 140 | — | 8 | 100 | 100 | [ |
| 8 | Pd/rGO | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 7 | >99 | 97.65 | [ |
| 9 | Pd/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 97.4 | [ |
| 10 | 3% Pt/ Al2O3-P-H2 | 18H-二苄基甲苯 | 二苄基甲苯 | 270 | 0.1 | 5 | 70.8 | — | [ |
| 11 | 0.15% Pt/CeO2 | 18H-二苄基甲苯 | 二苄基甲苯 | 300 | 0.1 | 2.5 | 80.5 | — | [ |
| 12 | Ru-Pd/Al2O3 | 12H-N-丙基咔唑 | N-丙基咔唑 | 180 | 0.1 | 4 | >99 | 97.1 | [ |
| 13 | Pd-Ni/Al2O3 | 12H-N-丙基咔唑 | N-丙基咔唑 | 180 | 0.1 | 8 | >99 | >99 | [ |
| 14 | Pd4Ni1/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 99.1 | [ |
| 15 | Pd1Co3/SiO2 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | — | 8 | 100 | 71.66 | [ |
| 序号 | 催化剂 | 反应物 | 完全加氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni70/AlSiO-1/1 | 二苄基甲苯 | 18H-二苄基甲苯 | 150 | 7 | 1.5 | 100 | 100 | [ |
| 2 | Ni70/AlSiO-1/1 | N-乙基咔唑 | 12H-N-乙基咔唑 | 150 | 7 | 1.5 | 100 | 100 | [ |
| 3 | Ni70/AlSiO-1/1 | N-丙基咔唑 | 12H-N-丙基咔唑 | 150 | 7 | 1 | 100 | 100 | [ |
| 4 | Ni/MCM-41 | N-乙基咔唑 | 12H-N-乙基咔唑 | — | — | — | 92 | 78 | [ |
| 5 | Raney-Ni | 二苄基甲苯 | 18H-二苄基甲苯 | 170 | 0.8 | 10 | — | — | [ |
| 6 | 1% CoCl2 + 10% NaBhH4 | 喹啉 | 1,2,3,4-四氢喹啉 | 130 | 3 | 17 | 100 | 100 | [ |
| 7 | CoO x @CN + MeOH | 喹啉 | 十氢喹啉 | 120 | 3 | 4 | 100 | 100 | [ |
| 8 | 4% Co/AlN | 喹啉 | 1,2,3,4-喹啉 | 100 | 4 | 12 | >99 | 100 | [ |
| 9 | 富镍LaNi6 | 二苄基甲苯 | 18H-二苄基甲苯 | 280 | 6 | 20 | >99 | — | [ |
| 10 | 4% NiO + 20% MoO3/Al2O3 | 萘 | 四氢化萘 | 200 | 6 | 8 | 95.62 | 99.75 | [ |
表5 非贵金属加氢催化剂参数
| 序号 | 催化剂 | 反应物 | 完全加氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni70/AlSiO-1/1 | 二苄基甲苯 | 18H-二苄基甲苯 | 150 | 7 | 1.5 | 100 | 100 | [ |
| 2 | Ni70/AlSiO-1/1 | N-乙基咔唑 | 12H-N-乙基咔唑 | 150 | 7 | 1.5 | 100 | 100 | [ |
| 3 | Ni70/AlSiO-1/1 | N-丙基咔唑 | 12H-N-丙基咔唑 | 150 | 7 | 1 | 100 | 100 | [ |
| 4 | Ni/MCM-41 | N-乙基咔唑 | 12H-N-乙基咔唑 | — | — | — | 92 | 78 | [ |
| 5 | Raney-Ni | 二苄基甲苯 | 18H-二苄基甲苯 | 170 | 0.8 | 10 | — | — | [ |
| 6 | 1% CoCl2 + 10% NaBhH4 | 喹啉 | 1,2,3,4-四氢喹啉 | 130 | 3 | 17 | 100 | 100 | [ |
| 7 | CoO x @CN + MeOH | 喹啉 | 十氢喹啉 | 120 | 3 | 4 | 100 | 100 | [ |
| 8 | 4% Co/AlN | 喹啉 | 1,2,3,4-喹啉 | 100 | 4 | 12 | >99 | 100 | [ |
| 9 | 富镍LaNi6 | 二苄基甲苯 | 18H-二苄基甲苯 | 280 | 6 | 20 | >99 | — | [ |
| 10 | 4% NiO + 20% MoO3/Al2O3 | 萘 | 四氢化萘 | 200 | 6 | 8 | 95.62 | 99.75 | [ |
| 序号 | 催化剂 | 反应物 | 完全脱氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4% Co3O4/AlN | 1,2,3,4-四氢喹啉 | 喹啉 | 120 | 0.5 | 12 | >99 | 88 | [ |
| 2 | Ni/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 6.0 | [ |
表6 非贵金属脱氢催化剂参数
| 序号 | 催化剂 | 反应物 | 完全脱氢产物 | 温度/℃ | 压力/MPa | 时间/h | 转换率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4% Co3O4/AlN | 1,2,3,4-四氢喹啉 | 喹啉 | 120 | 0.5 | 12 | >99 | 88 | [ |
| 2 | Ni/K6 | 12H-N-乙基咔唑 | N-乙基咔唑 | 180 | 0.1 | 6 | 100 | 6.0 | [ |
| 1 | YASUSHI Sekine, TAKUMA Higo. Recent trends on the dehydrogenation catalysis of liquid organic hydrogen carrier (LOHC): A review[J]. Topics in Catalysis, 2021, 64(7): 470-480. |
| 2 | 周一鸣, 齐随涛, 周宇亮, 等. 多环芳烃类液体有机氢载体储放氢技术研究进展[J]. 化工进展, 2023, 42(2): 1000-1007. |
| ZHOU Yiming, QI Suitao, ZHOU Yuliang, et al. Research progress in the hydrogenation and dehydrogenation technology of polycyclic aromatic hydrocarbon liquid organic hydrogen carriers[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1000-1007. | |
| 3 | 张媛媛, 赵静, 鲁锡兰, 等. 有机液体储氢材料的研究进展[J]. 化工进展, 2016, 35(9): 2869-2874. |
| ZHANG Yuanyuan, ZHAO Jing, LU Xilan, et al. Progress in liquid organic hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2869-2874. | |
| 4 | MODISHA Phillimon M, OUMA Cecil N M, GARIDZIRAI Rudaviro, et al. The prospect of hydrogen storage using liquid organic hydrogen carriers[J]. Energy & Fuels, 2019, 33(4): 2778-2796. |
| 5 | 宋鹏飞, 侯建国, 王秀林. 甲基环己烷-甲苯液体有机物储氢技术的研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(S1): 18-23. |
| SONG Pengfei, HOU Jianguo, WANG Xiulin. Research progress on methylcyclohexane-toluene liquid organic hydrogen storage technology[J]. Natural Gas Chemical Industry, 2021, 46(S1): 18-23. | |
| 6 | 王蒙恩, 田亚飞, 张智芳, 等. 环己烷、甲基环己烷和乙基环己烷脱氢反应的热力学计算[J]. 石化技术与应用, 2022, 40(4): 238-242. |
| WANG Meng’en, TIAN Yafei, ZHANG Zhifang, et al. Thermodynamic calculation of dehydrogenation of cyclohexane, methyl cyclohexane and ethyl cyclohexane[J]. Petrochemical Technology & Application, 2022, 40(4): 238-242. | |
| 7 | Nicole BRÜCKNER, OBESSER Katharina, Andreas BÖSMANN, et al. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems[J]. ChemSusChem, 2014, 7(1): 229-235. |
| 8 | BOLLMANN Jonas, SCHMIDT Nikolas, BECK Dominik, et al. A path to a dynamic hydrogen storage system using a liquid organic hydrogen carrier (LOHC): Burner-based direct heating of the dehydrogenation unit[J]. International Journal of Hydrogen Energy, 2023, 48(3): 1011-1023. |
| 9 | DÜRR S, ZILM S, GEIßELBRECHT M, et al. Experimental determination of the hydrogenation/dehydrogenation-Equilibrium of the LOHC system H0/H18-dibenzyltoluene[J]. International Journal of Hydrogen Energy, 2021, 46(64): 32583-32594. |
| 10 | MA Jialing, YIN Lifei, LING Lixia, et al. The formation of high energy density fuel via the hydrogenation of naphthalene over Ni catalyst: The combined DFT and microkinetic analysis[J]. Fuel, 2023, 333: 126307. |
| 11 | ZHAO Tong, ZHAO Binbin, NIU Yufeng, et al. Hydrogenation of naphthalene to decalin catalyzed by Pt supported on WO3 of different crystallinity at low temperature[J]. Journal of Fuel Chemistry and Technology, 2021, 49(8): 1181-1189. |
| 12 | SHIN Byeong Soo, YOON Chang Won, KWAK Sang Kyu, et al. Thermodynamic assessment of carbazole-based organic polycyclic compounds for hydrogen storage applications via a computational approach[J]. International Journal of Hydrogen Energy, 2018, 43(27): 12158-12167. |
| 13 | ZHU Qilong, XU Qiang. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage[J]. Energy & Environmental Science, 2015, 8(2): 478-512. |
| 14 | EMEL’YANENKO Vladimir N, ZAITSAU Dzmitry H, PIMERZIN Andrey A, et al. N-phenyl-carbazole as a potential liquid organic hydrogen carrier: Thermochemical and computational study[J]. The Journal of Chemical Thermodynamics, 2019, 132: 122-128. |
| 15 | WU Yong, YU Hongen, GUO Yanru, et al. A rare earth hydride supported ruthenium catalyst for the hydrogenation of N-heterocycles: Boosting the activity via a new hydrogen transfer path and controlling the stereoselectivity[J]. Chemical Science, 2019, 10(45): 10459-10465. |
| 16 | STARK Katharina, KEIL Philipp, SCHUG Sebastian, et al. Melting points of potential liquid organic hydrogen carrier systems consisting of N-alkylcarbazoles[J]. Journal of Chemical & Engineering Data, 2016, 61(4): 1441-1448. |
| 17 | VOSTRIKOV Sergey V, KONNOVA Maria E, TUROVTZEV Vladimir V, et al. Thermodynamics of hydrogen storage: Equilibrium study of the LOHC system indole/octahydroindole[J]. Fuel, 2023, 335: 127025. |
| 18 | YANG Ming, CHENG Guoe, XIE Dandan, et al. Study of hydrogenation and dehydrogenation of 1-methylindole for reversible onboard hydrogen storage application[J]. International Journal of Hydrogen Energy, 2018, 43(18): 8868-8876. |
| 19 | LI Linlin, YANG Ming, DONG Yuan, et al. Hydrogen storage and release from a new promising liquid organic hydrogen storage carrier (LOHC): 2-methylindole[J]. International Journal of Hydrogen Energy, 2016, 41(36): 16129-16134. |
| 20 | DONG Yuan, YANG Ming, YANG Zihua, et al. Catalytic hydrogenation and dehydrogenation of N-ethylindole as a new heteroaromatic liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2015, 40(34): 10918-10922. |
| 21 | DONG Yuan, YANG Ming, LI Linlin, et al. Study on reversible hydrogen uptake and release of 1,2-dimethylindole as a new liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2019, 44(10): 4919-4929. |
| 22 | PREUSTER Patrick, PAPP Christian, WASSERSCHEID Peter. Liquid organic hydrogen carriers (LOHCs): Toward a hydrogen-free hydrogen economy[J]. Accounts of Chemical Research, 2017, 50(1): 74-85. |
| 23 | SAFRONOV Sergey P, VOSTRIKOV Sergey V, SAMAROV Artemiy A, et al. Comprehensive thermodynamic study of substituted indoles/perhydro indoles as potential liquid organic hydrogen carrier system[J]. Fuel, 2023, 331: 125764. |
| 24 | SCHWARZ Matthias, BACHMANN Philipp, SILVA Thais Nascimento, et al. Model catalytic studies of novel liquid organic hydrogen carriers: Indole, indoline and octahydroindole on Pt(111)[J]. Chemistry, 2017, 23(59): 14806-14818. |
| 25 | WEI Zhongzhe, SHAO Fangjun, WANG Jianguo. Recent advances in heterogeneous catalytic hydrogenation and dehydrogenation of N-heterocycles[J]. Chinese Journal of Catalysis, 2019, 40(7): 980-1002. |
| 26 | SAFRONOV Sergey P, VOSTRIKOV Sergey V, SAMAROV Artemiy A, et al. Reversible storage and release of hydrogen with LOHC: Evaluation of thermochemical data for methyl-quinolines with complementary experimental and computational methods[J]. Fuel, 2022, 317: 123501. |
| 27 | CHAUHAN Arzoo, KAR Ashish Kumar, SRIVASTAVA Rajendra. Ru-decorated N-doped carbon nanoflakes for selective hydrogenation of levulinic acid to γ-valerolactone and quinoline to tetrahydroquinoline with HCOOH in water[J]. Applied Catalysis A: General, 2022, 636: 118580. |
| 28 | SUN Feifei, AN Yue, LEI Lecheng, et al. Identification of the starting reaction position in the hydrogenation of (N-ethyl)carbazole over Raney-Ni[J]. Journal of Energy Chemistry, 2015, 24(2): 219-224. |
| 29 | JIANG Zhao, GUO Shuyi, FANG Tao. Enhancing the catalytic activity and selectivity of PdAu/SiO2 bimetallic catalysts for dodecahydro-N-ethylcarbazole dehydrogenation by controlling the particle size and dispersion[J]. ACS Applied Energy Materials, 2019, 2(10): 7233-7243. |
| 30 | Francisco MARTINEZ-ESPINAR, BLONDEAU Pascal, NOLIS Pau, et al. NHC-stabilised Rh nanoparticles: Surface study and application in the catalytic hydrogenation of aromatic substrates[J]. Journal of Catalysis, 2017, 354: 113-127. |
| 31 | SHI Libin, ZHOU Yiming, QI Suitao, et al. Pt catalysts supported on H2 and O2 plasma-treated Al2O3 for hydrogenation and dehydrogenation of the liquid organic hydrogen carrier pair dibenzyltoluene and perhydrodibenzyltoluene[J]. ACS Catalysis, 2020, 10(18): 10661-10671. |
| 32 | WANG Shengdong, HUANG Haiyun, BRUNEAU Christian, et al. Iridium-catalyzed hydrogenation and dehydrogenation of N-heterocycles in water under mild conditions[J]. ChemSusChem, 2019, 12(11): 2350-2354. |
| 33 | 明卫星, 李明璇, 纪璐, 等. 2-萘酚选择性加氢催化剂及工艺研究[J]. 染料与染色, 2022, 59(5): 44-46, 43. |
| MING Weixing, LI Mingxuan, JI Lu, et al. Study on catalyst and process for selective hydrogenation of 2-naphthol[J]. Dyestuffs and Coloration, 2022, 59(5): 44-46, 43. | |
| 34 | 梁瑜, 赵彤, 赵斌彬, 等. WO3对Pt/α-Al2O3催化萘深度加氢的促进作用[J]. 化工学报, 2021, 72(11): 5643-5652. |
| LIANG Yu, ZHAO Tong, ZHAO Binbin, et al. Promotion of WO3 species on Pt/α-Al2O3 for the deep hydrogenation of naphthalene[J]. CIESC Journal, 2021, 72(11): 5643-5652. | |
| 35 | XUE Wenjie, LIU Hongxia, MAO Baohua, et al. Reversible hydrogenation and dehydrogenation of N-ethylcarbazole over bimetallic Pd-Rh catalyst for hydrogen storage[J]. Chemical Engineering Journal, 2021, 421: 127781. |
| 36 | ZHU Ting, YANG Ming, CHEN Xuedi, et al. A highly active bifunctional Ru-Pd catalyst for hydrogenation and dehydrogenation of liquid organic hydrogen carriers[J]. Journal of Catalysis, 2019, 378: 382-391. |
| 37 | DING Chenghan, ZHU Ting, WANG Fanyi, et al. High active Pd@MIL-101 catalyst for dehydrogenation of liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2020, 45(32): 16144-16152. |
| 38 | 袁胜楠, 张龙龙, 郭锦平, 等. 催化剂钯负载量对液态有机物脱氢性能的影响[J]. 当代化工研究, 2022(11): 27-29. |
| YUAN Shengnan, ZHANG Longlong, GUO Jinping, et al. Effect of palladium loading of catalyst on dehydrogenation of liquid organic hydrogen carriers[J]. Modern Chemical Research, 2022(11): 27-29. | |
| 39 | SHUANG Huili, CHEN Hao, WU Fei, et al. Catalytic dehydrogenation of hydrogen-rich liquid organic hydrogen carriers by palladium oxide supported on activated carbon[J]. Fuel, 2020, 275: 117896. |
| 40 | WANG Bin, CHANG Tieyan, JIANG Zhao, et al. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7317-7325. |
| 41 | FENG Zhaolu, BAI Xuefeng. Enhanced activity of bimetallic Pd-Ni nanoparticles on KIT-6 for production of hydrogen from dodecahydro-N-ethylcarbazole[J]. Fuel, 2022, 329: 125473. |
| 42 | LEE Sanghun, LEE Jaemyung, KIM Taehong, et al. Pt/CeO2 catalyst synthesized by combustion method for dehydrogenation of perhydro-dibenzyltoluene as liquid organic hydrogen carrier: Effect of pore size and metal dispersion[J]. International Journal of Hydrogen Energy, 2021, 46(7): 5520-5529. |
| 43 | CHEN Xuedi, LI Gen, GAO Min, et al. Wet-impregnated bimetallic Pd-Ni catalysts with enhanced activity for dehydrogenation of perhydro-N-propylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(56): 32168-32178. |
| 44 | 龚翔, 李林森, 姜召. PdCo/SiO2双金属催化剂用于杂环储氢载体的高效脱氢[J]. 化工学报, 2022, 73(10): 4448-4460. |
| GONG Xiang, LI Linsen, JIANG Zhao. Employing PdCo/SiO2 catalyst in high activity dehydrogenation reaction of heterocyclic H2 storage carrier[J]. CIESC Journal, 2022, 73(10): 4448-4460. | |
| 45 | VICERICH María A, BENITEZ Viviana M, SÁNCHEZ María A, et al. Ru-Pt catalysts supported on Al2O3 and SiO2-Al2O3 for the selective ring opening of naphthenes[J]. The Canadian Journal of Chemical Engineering, 2020, 98(3): 749-756. |
| 46 | MIAO Lei, YAN Jing, WANG Weiyan, et al. Dehydrogenation of methylcyclohexane over Pt supported on Mg-Al mixed oxides catalyst: The effect of promoter Ir[J]. Chinese Journal of Chemical Engineering, 2020, 28(9): 2337-2342. |
| 47 | OUMA C N M, OBODO K O, MODISHA Phillimon M, et al. Effect of chalcogen (S, Se and Te) surface additives on the dehydrogenation of a liquid organic hydrogen carrier system, octahydroindole-indole, on a Pt (111) surface[J]. Applied Surface Science, 2021, 566: 150636. |
| 48 | CHEN Ben, HUI Bowen, DONG Yuting, et al. Distributions of Ni in MCM-41 for the hydrogenation of N-ethylcarbazole[J]. Fuel, 2022, 324: 124405. |
| 49 | DING Yuhang, DONG Yuan, ZHANG Heshun, et al. A highly adaptable Ni catalyst for Liquid Organic Hydrogen Carriers hydrogenation[J]. International Journal of Hydrogen Energy, 2021, 46(53): 27026-27036. |
| 50 | Ahsan ALI, Udaya KUMAR G, LEE Hee Joon. Investigation of hydrogenation of Dibenzyltoluene as liquid organic hydrogen carrier[J]. Materials Today: Proceedings, 2021, 45: 1123-1127. |
| 51 | 冯小阳, 蒋利军, 李志念, 等. 富镍镧镍合金催化二苄基甲苯加氢性能研究[J]. 太阳能学报, 2022, 43(6): 382-388. |
| FENG Xiaoyang, JIANG Lijun, LI Zhinian, et al. Hydrogenation performance of dibenzyltoluene catalyzed by Ni-rich La-Ni alloys[J]. Acta Energiae Solaris Sinica, 2022, 43(6): 382-388. | |
| 52 | SU Xiaoping, AN Pu, GAO Junwen, et al. Selective catalytic hydrogenation of naphthalene to tetralin over a Ni-Mo/Al2O3 catalyst[J]. Chinese Journal of Chemical Engineering, 2020, 28(10): 2566-2576. |
| 53 | HERVOCHON Julien, DORCET Vincent, JUNGE Kathrin, et al. Convenient synthesis of cobalt nanoparticles for the hydrogenation of quinolines in water[J]. Catalysis Science & Technology, 2020, 10(14): 4820-4826. |
| 54 | WEI Zhongzhe, CHEN Yiqing, WANG Jing, et al. Cobalt encapsulated in N-doped graphene layers: An efficient and stable catalyst for hydrogenation of quinoline compounds[J]. ACS Catalysis, 2016, 6(9): 5816-5822. |
| 55 | HE Zhenhong, SUN Yongchang, WANG Kuan, et al. Reversible aerobic oxidative dehydrogenation/hydrogenation of N-heterocycles over AlN supported redox cobalt catalysts[J]. Molecular Catalysis, 2020, 496: 111192. |
| 56 | LEE Jusung, CHERIF Ali, YOON Hajun, et al. Large-scale overseas transportation of hydrogen: Comparative techno-economic and environmental investigation[J]. Renewable and Sustainable Energy Reviews, 2022, 165: 112556. |
| 57 | BULGARIN Alexander, JORSCHICK Holger, PREUSTER Patrick, et al. Purity of hydrogen released from the liquid organic hydrogen carrier compound perhydro dibenzyltoluene by catalytic dehydrogenation[J]. International Journal of Hydrogen Energy, 2020, 45(1): 712-720. |
| 58 | JORSCHICK Holger, VOGL Marius, PREUSTER Patrick, et al. Hydrogenation of liquid organic hydrogen carrier systems using multicomponent gas mixtures[J]. International Journal of Hydrogen Energy, 2019, 44(59): 31172-31182. |
| [1] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [2] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [3] | 谷星朋, 马红钦, 刘嘉豪. 雷尼镍的磷量子点改性及其催化加氢脱硫性能[J]. 化工进展, 2024, 43(3): 1293-1301. |
| [4] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [5] | 陈风, 王宣德, 黄伟, 王晓东, 王琰. HZSM-22的粒径调控及Pt/HZSM-22的正十二烷加氢异构催化性能[J]. 化工进展, 2024, 43(3): 1309-1317. |
| [6] | 萧垚鑫, 张军, 单锐, 袁浩然, 陈勇. Pt/CaO材料催化糠醇加氢制备戊二醇[J]. 化工进展, 2024, 43(3): 1318-1327. |
| [7] | 李伟杰, 康金灿, 张传明, 林丽娜, 李昌鑫, 朱红平. 锆改性Cu/SiO2催化剂催化3-羟基丙酸甲酯选择性加氢[J]. 化工进展, 2024, 43(3): 1328-1341. |
| [8] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| [9] | 刘斌, 王勇军, 吕汪洋, 陈文兴. 高稳定性钛系聚酯催化剂TiOC@SiO2的制备及应用[J]. 化工进展, 2024, 43(3): 1395-1402. |
| [10] | 王雄, 康文倩, 任悦, 乔彤森, 张鹏, 黄安平, 李广全. 多孔有机聚合物中试制备及其在聚烯烃催化剂中的应用[J]. 化工进展, 2024, 43(3): 1412-1417. |
| [11] | 闫守成, 张慧华, 徐倩倩, 王煜坤. 石墨烯复合载体催化剂在柴油车尾气NO脱除中的应用[J]. 化工进展, 2024, 43(3): 1456-1465. |
| [12] | 董冰岩, 李贞栋, 王佩祥, 涂文娟, 谭艳雯, 张芹. DBD等离子体耦合BiOI催化材料降解苯甲羟肟酸的特性与机制[J]. 化工进展, 2024, 43(3): 1565-1575. |
| [13] | 胡洪远, 张洋, 张贺东, 范兵强, 郑诗礼, 汤吉海. 硫酸钠制备碳酸氢钠过程中Na2SO4-NH3-CO2-H2O体系相平衡规律[J]. 化工进展, 2024, 43(3): 1621-1629. |
| [14] | 黄晟, 杨振丽, 李振宇. 氢产业链发展的路径分析[J]. 化工进展, 2024, 43(2): 882-893. |
| [15] | 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 氧化铝基加氢脱硫催化剂研究进展[J]. 化工进展, 2024, 43(2): 948-961. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||