化工进展 ›› 2024, Vol. 43 ›› Issue (3): 1621-1629.DOI: 10.16085/j.issn.1000-6613.2023-0472
• 资源与环境化工 • 上一篇
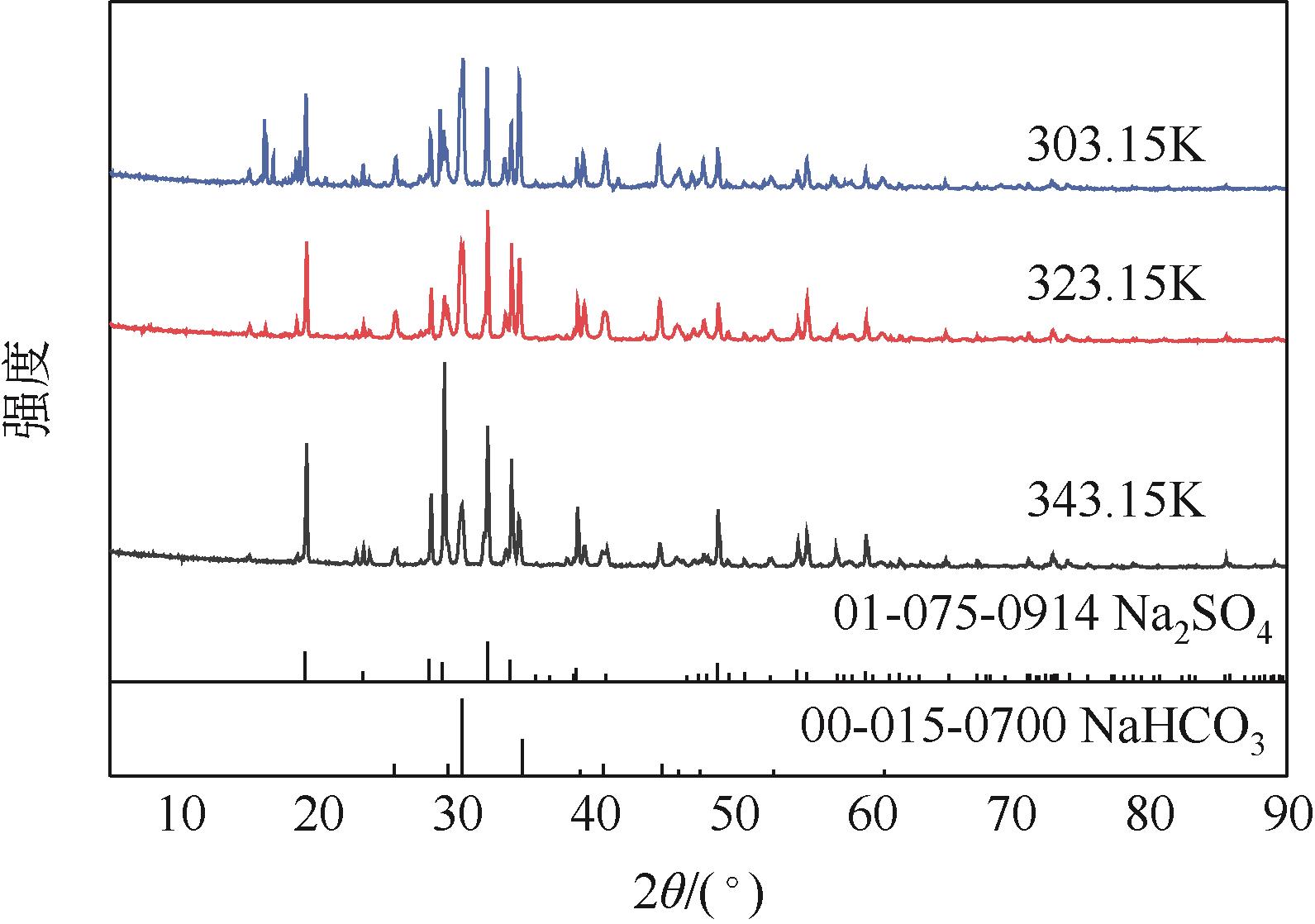
硫酸钠制备碳酸氢钠过程中Na2SO4-NH3-CO2-H2O体系相平衡规律
胡洪远1,2( ), 张洋2(
), 张洋2( ), 张贺东3, 范兵强2, 郑诗礼2, 汤吉海1
), 张贺东3, 范兵强2, 郑诗礼2, 汤吉海1
- 1.南京工业大学化工学院,江苏 南京 211816
2.中国科学院过程工程研究所绿色过程与工程重点实验室,战略金属资源绿色循环利用国家工程研究中心,北京 100190
3.郑州中科新兴产业技术研究院,河南 郑州 450000
-
收稿日期:2023-03-27修回日期:2023-04-26出版日期:2024-03-10发布日期:2024-04-11 -
通讯作者:张洋 -
作者简介:胡洪远(1994—),男,硕士研究生,研究方向为水盐体系。E-mail:hyhu2020@163.com。 -
基金资助:国家重点研发计划(2022YFC3901303)
Phase equilibrium of Na2SO4-NH3-CO2-H2O system in preparation of sodium bicarbonate from sodium sulfate
HU Hongyuan1,2( ), ZHANG Yang2(
), ZHANG Yang2( ), ZHANG Hedong3, FAN Bingqiang2, ZHENG Shili2, TANG Jihai1
), ZHANG Hedong3, FAN Bingqiang2, ZHENG Shili2, TANG Jihai1
- 1.College of Chemical Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu, China
2.Key Laboratory of Green Process and Engineering, National Engineering Research Center of Green Recycling for Strategic Metal Resources, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
3.Zhengzhou Institute of Emerging Industrial Technology, Zhengzhou 450000, Henan, China
-
Received:2023-03-27Revised:2023-04-26Online:2024-03-10Published:2024-04-11 -
Contact:ZHANG Yang
摘要:
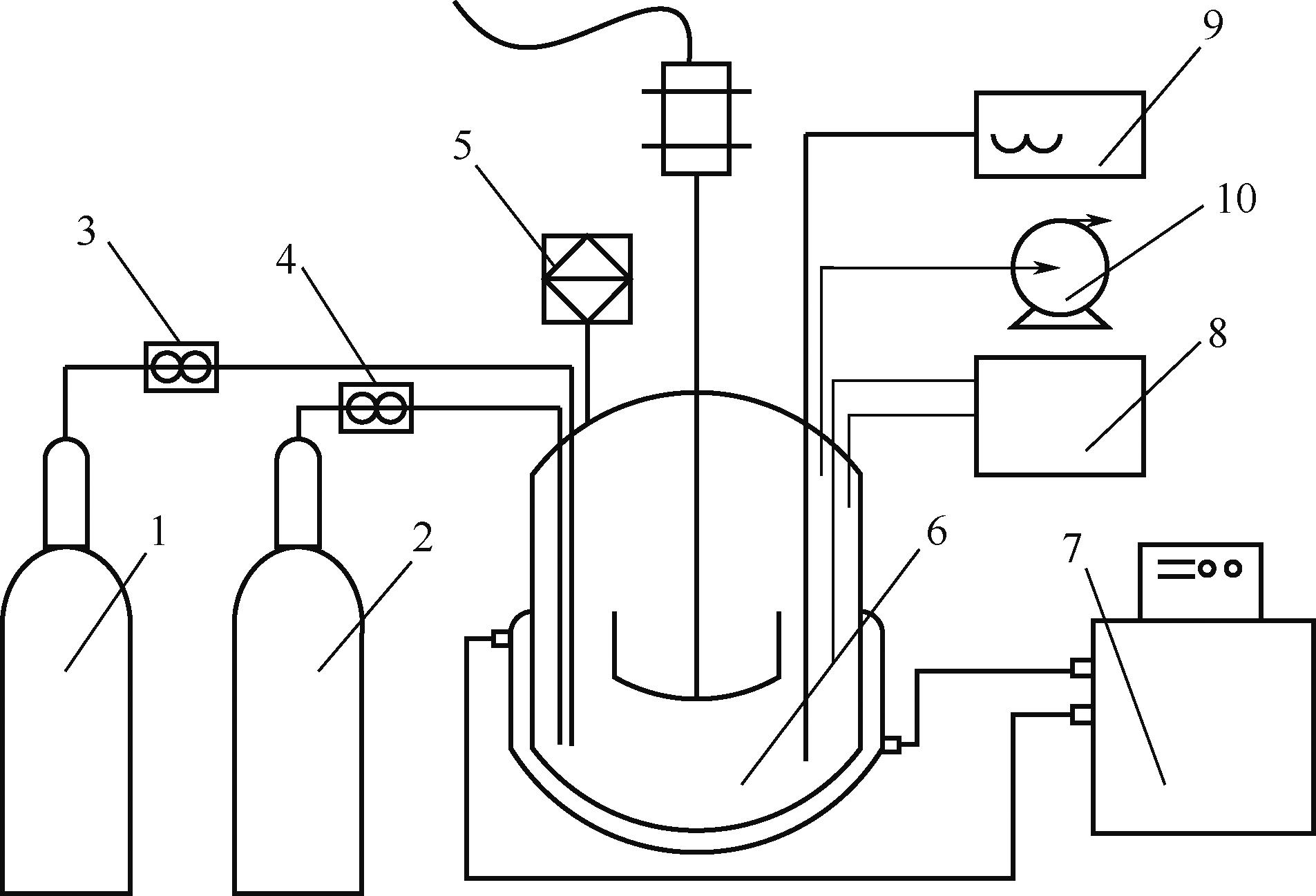
Na2SO4-NH3-CO2-H2O体系相平衡规律的掌握,对硫酸钠高值利用制备碳酸氢钠过程的工艺优化有重要意义。本文采用等温溶解平衡法在101.3~1099.0kPa下303.15K、323.15K和343.15K时研究了Na2SO4-NH3-CO2-H2O体系以及其子体系Na2SO4-NH3-H2O的液-固相平衡关系,并获得了Na2SO4-NH3-H2O体系以及SO
中图分类号:
引用本文
胡洪远, 张洋, 张贺东, 范兵强, 郑诗礼, 汤吉海. 硫酸钠制备碳酸氢钠过程中Na2SO4-NH3-CO2-H2O体系相平衡规律[J]. 化工进展, 2024, 43(3): 1621-1629.
HU Hongyuan, ZHANG Yang, ZHANG Hedong, FAN Bingqiang, ZHENG Shili, TANG Jihai. Phase equilibrium of Na2SO4-NH3-CO2-H2O system in preparation of sodium bicarbonate from sodium sulfate[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1621-1629.
| 点 | T/K | ρ/g·cm-3 | 液相组成(质量分数)/% | 平衡固相 | ||
|---|---|---|---|---|---|---|
| NH3 | Na2SO4 | H2O | ||||
| F | 303.15 | 1.2560 | 0.00 | 24.42 | 75.58 | Na2SO4 |
| 303.15 | 1.2500 | 1.09 | 24.17 | 74.74 | Na2SO4 | |
| 303.15 | 1.2388 | 1.78 | 23.59 | 74.64 | Na2SO4 | |
| 303.15 | 1.2020 | 2.65 | 22.22 | 75.13 | Na2SO4 | |
| 303.15 | 1.1694 | 3.68 | 20.60 | 75.73 | Na2SO4 | |
| 303.15 | 1.1434 | 4.89 | 18.99 | 76.12 | Na2SO4 | |
| 303.15 | 1.1171 | 5.81 | 17.66 | 76.53 | Na2SO4 | |
| 303.15 | 1.0815 | 8.09 | 14.37 | 77.54 | Na2SO4 | |
| 303.15 | 1.0274 | 10.98 | 11.06 | 77.96 | Na2SO4 | |
| 303.15 | 1.0023 | 13.15 | 7.84 | 79.01 | Na2SO4 | |
| 303.15 | 0.9599 | 16.37 | 5.82 | 77.81 | Na2SO4 | |
| 303.15 | 0.9435 | 20.10 | 3.95 | 75.95 | Na2SO4 | |
| 303.15 | 0.9176 | 23.48 | 2.75 | 73.77 | Na2SO4 | |
| 303.15 | 0.9140 | 26.01 | 2.08 | 71.90 | Na2SO4 | |
| 303.15 | 0.9152 | 27.47 | 1.66 | 70.87 | Na2SO4 | |
| 303.15 | 0.9043 | 28.35 | 1.53 | 70.13 | Na2SO4 | |
| E | 303.15 | 0.8926 | 29.00 | 1.59 | 69.41 | Na2SO4 |
| D | 303.15 | 0.8954 | 29.20 | 0 | 70.80 | — |
| F' | 323.15 | 1.2808 | 0.00 | 25.64 | 74.36 | Na2SO4 |
| 323.15 | 1.2363 | 1.12 | 23.70 | 75.18 | Na2SO4 | |
| 323.15 | 1.1244 | 4.87 | 18.09 | 77.04 | Na2SO4 | |
| 323.15 | 1.0730 | 6.83 | 15.72 | 77.45 | Na2SO4 | |
| 323.15 | 1.0475 | 8.03 | 14.00 | 77.97 | Na2SO4 | |
| 323.15 | 1.0128 | 10.71 | 11.82 | 77.47 | Na2SO4 | |
| 323.15 | 1.0072 | 11.17 | 10.87 | 77.96 | Na2SO4 | |
| 323.15 | 0.9984 | 11.97 | 10.05 | 77.98 | Na2SO4 | |
| 323.15 | 0.9891 | 12.68 | 9.67 | 77.65 | Na2SO4 | |
| E' | 323.15 | 0.9875 | 12.77 | 9.49 | 77.74 | Na2SO4 |
| 323.15 | 0.9862 | 13.12 | 8.93 | 77.95 | — | |
| 323.15 | 0.9732 | 13.68 | 8.90 | 77.42 | — | |
| 323.15 | 0.9610 | 14.89 | 7.54 | 77.57 | — | |
| 323.15 | 0.9542 | 15.93 | 5.65 | 78.42 | — | |
| 323.15 | 0.9402 | 16.56 | 4.68 | 78.76 | — | |
| 323.15 | 0.9367 | 17.35 | 2.43 | 80.22 | — | |
| D' | 323.15 | 0.9252 | 18.37 | 0.00 | 81.63 | — |
| F'' | 343.15 | 1.2804 | 0.00 | 24.71 | 75.29 | Na2SO4 |
| 343.15 | 1.2464 | 0.71 | 23.67 | 75.62 | Na2SO4 | |
| 343.15 | 1.2034 | 2.06 | 22.05 | 75.89 | Na2SO4 | |
| 343.15 | 1.1662 | 3.01 | 21.01 | 75.98 | Na2SO4 | |
| 343.15 | 1.1583 | 3.18 | 20.54 | 76.28 | Na2SO4 | |
| 343.15 | 1.1626 | 3.15 | 20.06 | 76.78 | Na2SO4 | |
| E'' | 343.15 | 1.1631 | 3.15 | 19.94 | 76.91 | Na2SO4 |
| 343.15 | 1.1612 | 3.17 | 19.54 | 77.29 | — | |
| 343.15 | 1.1512 | 3.35 | 19.30 | 77.35 | — | |
| 343.15 | 1.0993 | 3.92 | 15.29 | 80.78 | — | |
| 343.15 | 1.0763 | 4.33 | 13.28 | 82.39 | — | |
| 343.15 | 1.0434 | 4.86 | 11.61 | 83.53 | — | |
| 343.15 | 1.0172 | 5.68 | 8.31 | 86.01 | — | |
| 343.15 | 0.9906 | 6.50 | 5.71 | 87.79 | — | |
| D'' | 343.15 | 0.9426 | 8.69 | 0.00 | 91.31 | — |
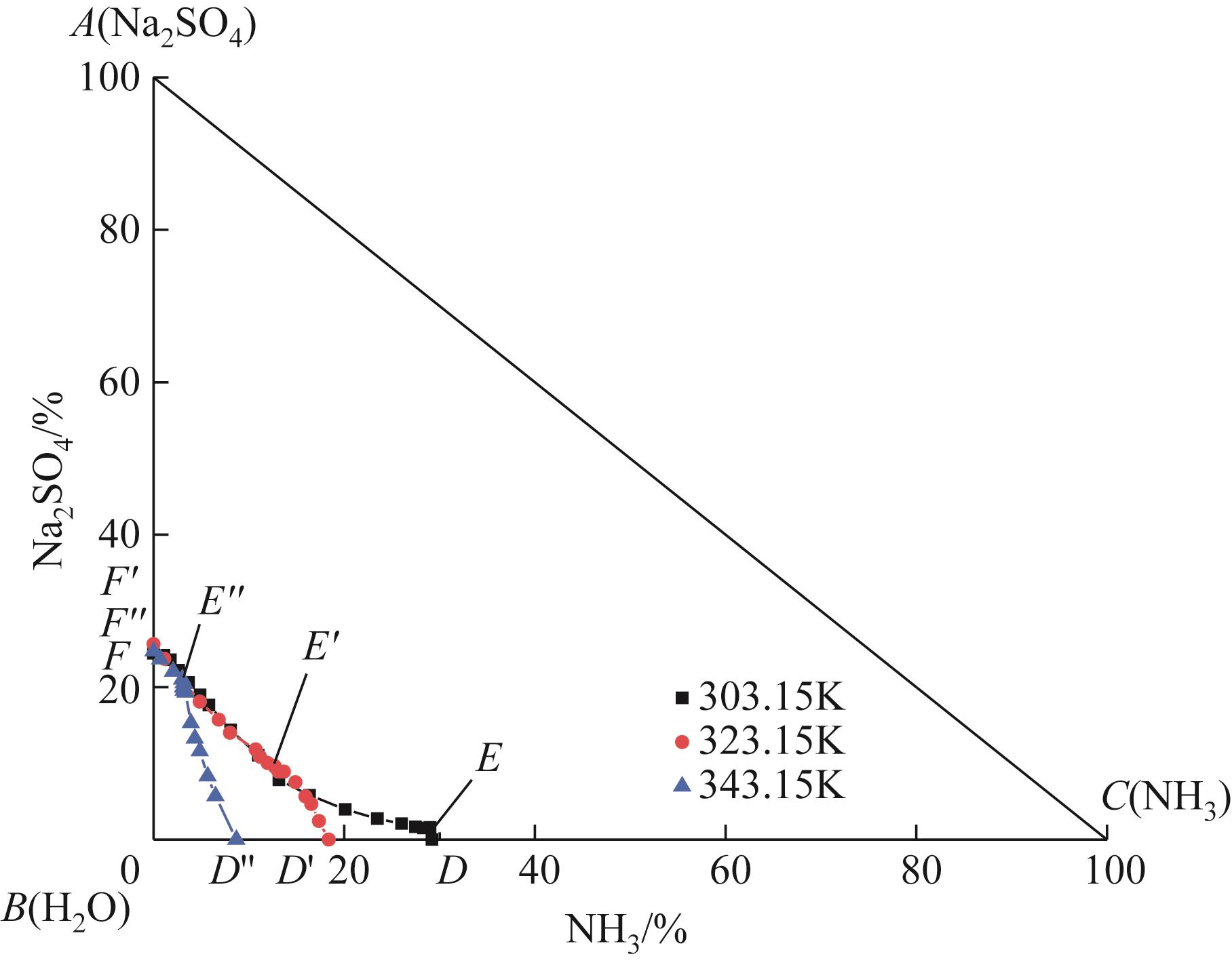
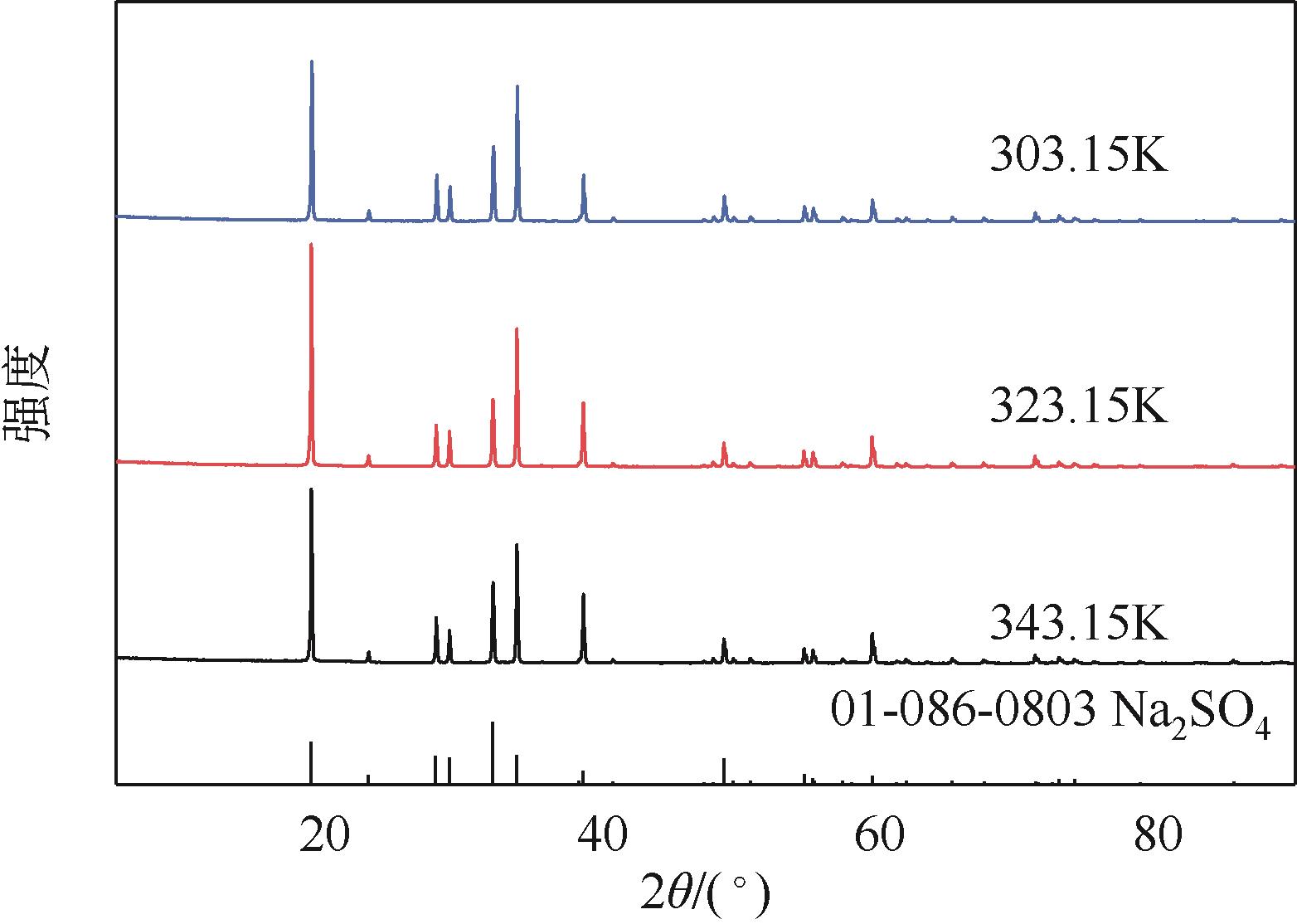
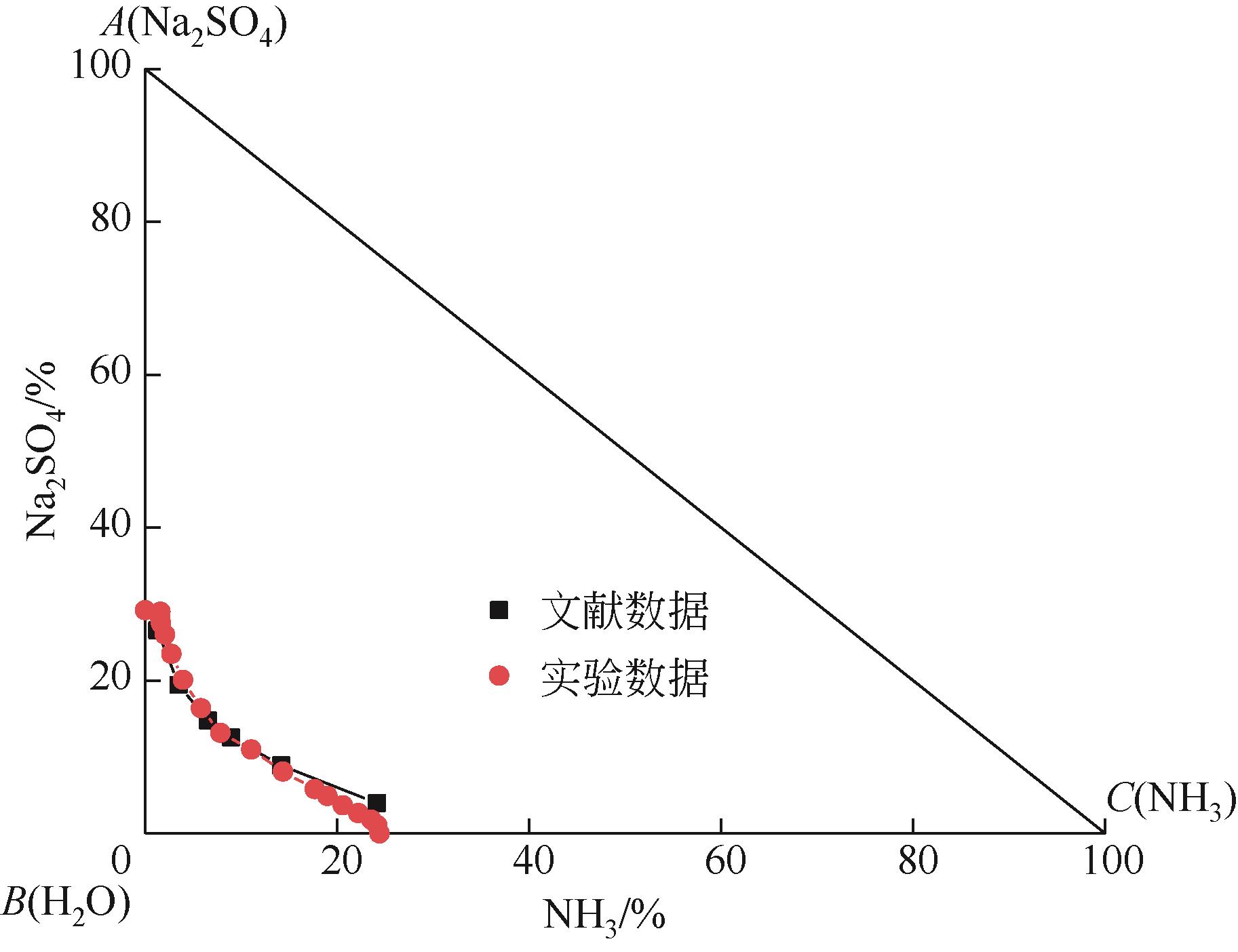
表1 101.3kPa下Na2SO4-NH3-H2O体系在303.15K、323.15K和343.15K时溶解度数据
| 点 | T/K | ρ/g·cm-3 | 液相组成(质量分数)/% | 平衡固相 | ||
|---|---|---|---|---|---|---|
| NH3 | Na2SO4 | H2O | ||||
| F | 303.15 | 1.2560 | 0.00 | 24.42 | 75.58 | Na2SO4 |
| 303.15 | 1.2500 | 1.09 | 24.17 | 74.74 | Na2SO4 | |
| 303.15 | 1.2388 | 1.78 | 23.59 | 74.64 | Na2SO4 | |
| 303.15 | 1.2020 | 2.65 | 22.22 | 75.13 | Na2SO4 | |
| 303.15 | 1.1694 | 3.68 | 20.60 | 75.73 | Na2SO4 | |
| 303.15 | 1.1434 | 4.89 | 18.99 | 76.12 | Na2SO4 | |
| 303.15 | 1.1171 | 5.81 | 17.66 | 76.53 | Na2SO4 | |
| 303.15 | 1.0815 | 8.09 | 14.37 | 77.54 | Na2SO4 | |
| 303.15 | 1.0274 | 10.98 | 11.06 | 77.96 | Na2SO4 | |
| 303.15 | 1.0023 | 13.15 | 7.84 | 79.01 | Na2SO4 | |
| 303.15 | 0.9599 | 16.37 | 5.82 | 77.81 | Na2SO4 | |
| 303.15 | 0.9435 | 20.10 | 3.95 | 75.95 | Na2SO4 | |
| 303.15 | 0.9176 | 23.48 | 2.75 | 73.77 | Na2SO4 | |
| 303.15 | 0.9140 | 26.01 | 2.08 | 71.90 | Na2SO4 | |
| 303.15 | 0.9152 | 27.47 | 1.66 | 70.87 | Na2SO4 | |
| 303.15 | 0.9043 | 28.35 | 1.53 | 70.13 | Na2SO4 | |
| E | 303.15 | 0.8926 | 29.00 | 1.59 | 69.41 | Na2SO4 |
| D | 303.15 | 0.8954 | 29.20 | 0 | 70.80 | — |
| F' | 323.15 | 1.2808 | 0.00 | 25.64 | 74.36 | Na2SO4 |
| 323.15 | 1.2363 | 1.12 | 23.70 | 75.18 | Na2SO4 | |
| 323.15 | 1.1244 | 4.87 | 18.09 | 77.04 | Na2SO4 | |
| 323.15 | 1.0730 | 6.83 | 15.72 | 77.45 | Na2SO4 | |
| 323.15 | 1.0475 | 8.03 | 14.00 | 77.97 | Na2SO4 | |
| 323.15 | 1.0128 | 10.71 | 11.82 | 77.47 | Na2SO4 | |
| 323.15 | 1.0072 | 11.17 | 10.87 | 77.96 | Na2SO4 | |
| 323.15 | 0.9984 | 11.97 | 10.05 | 77.98 | Na2SO4 | |
| 323.15 | 0.9891 | 12.68 | 9.67 | 77.65 | Na2SO4 | |
| E' | 323.15 | 0.9875 | 12.77 | 9.49 | 77.74 | Na2SO4 |
| 323.15 | 0.9862 | 13.12 | 8.93 | 77.95 | — | |
| 323.15 | 0.9732 | 13.68 | 8.90 | 77.42 | — | |
| 323.15 | 0.9610 | 14.89 | 7.54 | 77.57 | — | |
| 323.15 | 0.9542 | 15.93 | 5.65 | 78.42 | — | |
| 323.15 | 0.9402 | 16.56 | 4.68 | 78.76 | — | |
| 323.15 | 0.9367 | 17.35 | 2.43 | 80.22 | — | |
| D' | 323.15 | 0.9252 | 18.37 | 0.00 | 81.63 | — |
| F'' | 343.15 | 1.2804 | 0.00 | 24.71 | 75.29 | Na2SO4 |
| 343.15 | 1.2464 | 0.71 | 23.67 | 75.62 | Na2SO4 | |
| 343.15 | 1.2034 | 2.06 | 22.05 | 75.89 | Na2SO4 | |
| 343.15 | 1.1662 | 3.01 | 21.01 | 75.98 | Na2SO4 | |
| 343.15 | 1.1583 | 3.18 | 20.54 | 76.28 | Na2SO4 | |
| 343.15 | 1.1626 | 3.15 | 20.06 | 76.78 | Na2SO4 | |
| E'' | 343.15 | 1.1631 | 3.15 | 19.94 | 76.91 | Na2SO4 |
| 343.15 | 1.1612 | 3.17 | 19.54 | 77.29 | — | |
| 343.15 | 1.1512 | 3.35 | 19.30 | 77.35 | — | |
| 343.15 | 1.0993 | 3.92 | 15.29 | 80.78 | — | |
| 343.15 | 1.0763 | 4.33 | 13.28 | 82.39 | — | |
| 343.15 | 1.0434 | 4.86 | 11.61 | 83.53 | — | |
| 343.15 | 1.0172 | 5.68 | 8.31 | 86.01 | — | |
| 343.15 | 0.9906 | 6.50 | 5.71 | 87.79 | — | |
| D'' | 343.15 | 0.9426 | 8.69 | 0.00 | 91.31 | — |
| 点 | T/K | ρ/g·cm-3 | 液相组成/g·L-1 | 固相 | ||||
|---|---|---|---|---|---|---|---|---|
| SO | OH- | CO | HCO | NH | ||||
| 303.15 | 1.1351 | 132 | 78 | 84 | 0 | 116 | Na2SO4 | |
| 303.15 | 1.1700 | 151 | 66 | 97 | 0 | 117 | Na2SO4 | |
| 303.15 | 1.2063 | 170 | 55 | 109 | 0 | 117 | Na2SO4 | |
| 303.15 | 1.2235 | 184 | 43 | 124 | 0 | 118 | Na2SO4 | |
| 303.15 | 1.2412 | 197 | 32 | 139 | 0 | 119 | Na2SO4 | |
| 303.15 | 1.2543 | 209 | 16 | 150 | 0 | 118 | Na2SO4 | |
| 303.15 | 1.2677 | 221 | 0 | 162 | 15 | 117 | Na2SO4 | |
| G | 303.15 | 1.2680 | 240 | 0 | 135 | 23 | 118 | Na2SO4 |
| G' | 303.15 | 1.2695 | 259 | 0 | 109 | 31 | 119 | Na2SO4+NaHCO3 |
| 303.15 | 1.2730 | 277 | 0 | 85 | 39 | 119 | Na2SO4+NaHCO3 | |
| 303.15 | 1.2767 | 295 | 0 | 62 | 47 | 119 | Na2SO4+NaHCO3 | |
| 323.15 | 1.1643 | 151 | 61 | 66 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.1905 | 164 | 54 | 78 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2175 | 178 | 48 | 90 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2340 | 195 | 37 | 106 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2527 | 211 | 27 | 121 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2515 | 219 | 13 | 136 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2509 | 235 | 0 | 151 | 9 | 115 | Na2SO4 | |
| H | 323.15 | 1.2573 | 250 | 0 | 122 | 17 | 115 | Na2SO4 |
| H' | 323.15 | 1.2627 | 264 | 0 | 93 | 26 | 114 | Na2SO4+NaHCO3 |
| 323.15 | 1.2610 | 281 | 0 | 72 | 32 | 114 | Na2SO4+NaHCO3 | |
| 323.15 | 1.2590 | 297 | 0 | 50 | 38 | 114 | Na2SO4+NaHCO3 | |
| 343.15 | 1.1577 | 149 | 58 | 42 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.1833 | 168 | 49 | 54 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2110 | 186 | 41 | 66 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2250 | 197 | 33 | 81 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2421 | 208 | 26 | 97 | 0 | 108 | Na2SO4 | |
| 343.15 | 1.2450 | 223 | 13 | 109 | 0 | 108 | Na2SO4 | |
| 343.15 | 1.2518 | 231 | 0 | 121 | 5 | 109 | Na2SO4 | |
| 343.15 | 1.2554 | 249 | 0 | 103 | 11 | 109 | Na2SO4 | |
| 343.15 | 1.2544 | 266 | 0 | 84 | 17 | 109 | Na2SO4 | |
| J | 343.15 | 1.2540 | 279 | 0 | 63 | 21 | 108 | Na2SO4 |
| J' | 343.15 | 1.2522 | 291 | 0 | 42 | 25 | 108 | Na2SO4+NaHCO3 |
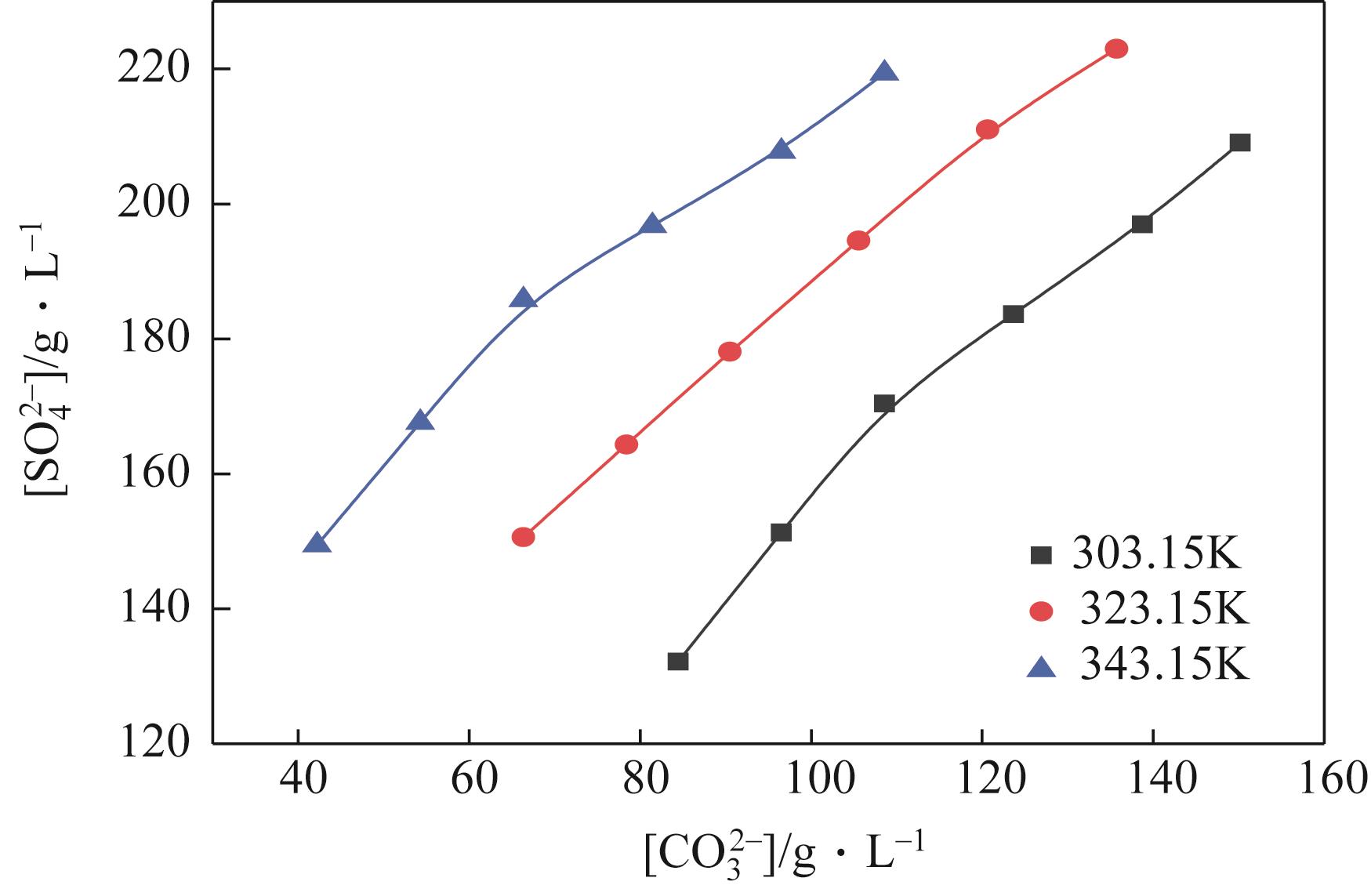
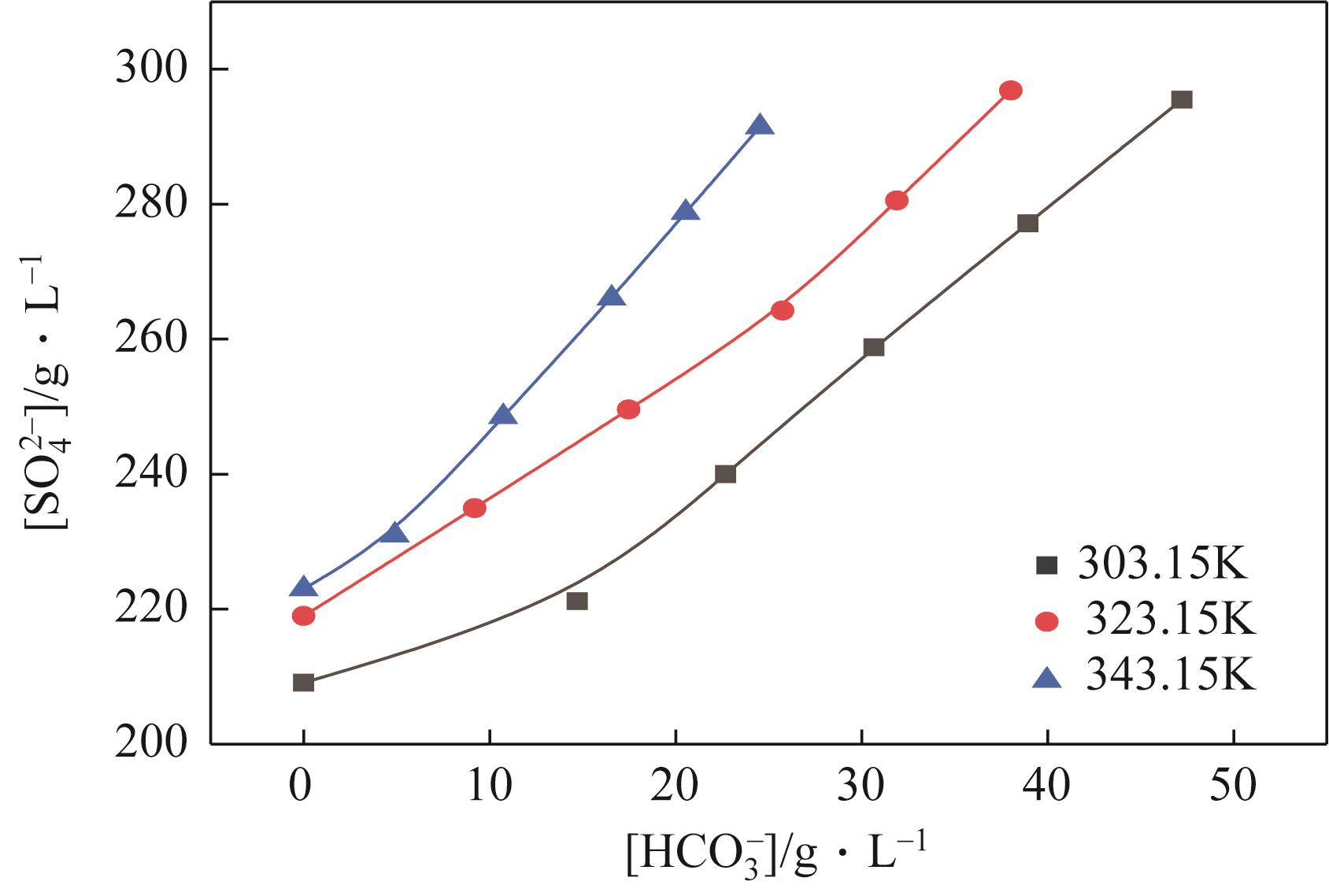
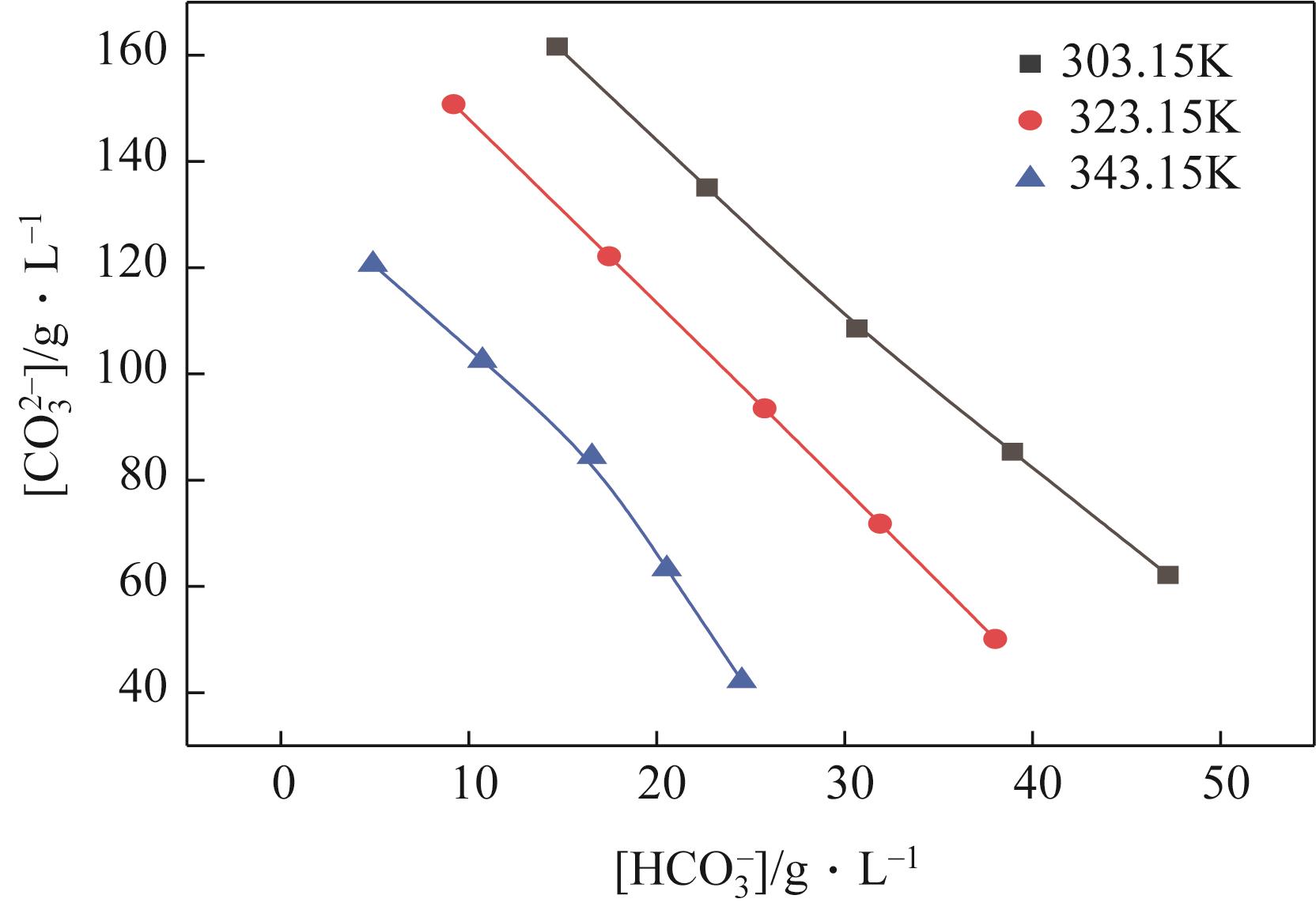
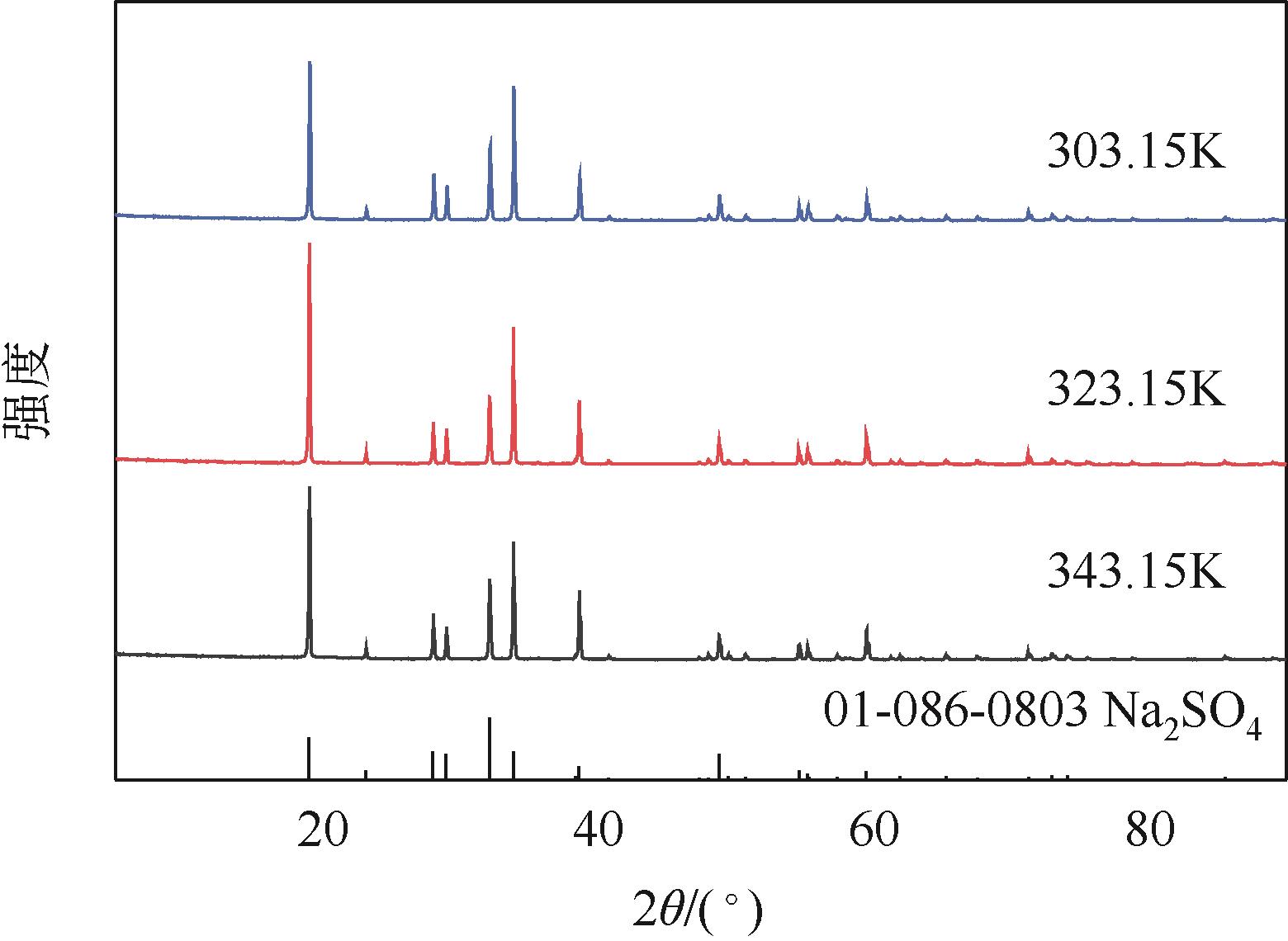
表2 101.3kPa下Na2SO4-NH3-CO2-H2O体系在303.15K、323.15K和343.15K时相平衡数据
| 点 | T/K | ρ/g·cm-3 | 液相组成/g·L-1 | 固相 | ||||
|---|---|---|---|---|---|---|---|---|
| SO | OH- | CO | HCO | NH | ||||
| 303.15 | 1.1351 | 132 | 78 | 84 | 0 | 116 | Na2SO4 | |
| 303.15 | 1.1700 | 151 | 66 | 97 | 0 | 117 | Na2SO4 | |
| 303.15 | 1.2063 | 170 | 55 | 109 | 0 | 117 | Na2SO4 | |
| 303.15 | 1.2235 | 184 | 43 | 124 | 0 | 118 | Na2SO4 | |
| 303.15 | 1.2412 | 197 | 32 | 139 | 0 | 119 | Na2SO4 | |
| 303.15 | 1.2543 | 209 | 16 | 150 | 0 | 118 | Na2SO4 | |
| 303.15 | 1.2677 | 221 | 0 | 162 | 15 | 117 | Na2SO4 | |
| G | 303.15 | 1.2680 | 240 | 0 | 135 | 23 | 118 | Na2SO4 |
| G' | 303.15 | 1.2695 | 259 | 0 | 109 | 31 | 119 | Na2SO4+NaHCO3 |
| 303.15 | 1.2730 | 277 | 0 | 85 | 39 | 119 | Na2SO4+NaHCO3 | |
| 303.15 | 1.2767 | 295 | 0 | 62 | 47 | 119 | Na2SO4+NaHCO3 | |
| 323.15 | 1.1643 | 151 | 61 | 66 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.1905 | 164 | 54 | 78 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2175 | 178 | 48 | 90 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2340 | 195 | 37 | 106 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2527 | 211 | 27 | 121 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2515 | 219 | 13 | 136 | 0 | 115 | Na2SO4 | |
| 323.15 | 1.2509 | 235 | 0 | 151 | 9 | 115 | Na2SO4 | |
| H | 323.15 | 1.2573 | 250 | 0 | 122 | 17 | 115 | Na2SO4 |
| H' | 323.15 | 1.2627 | 264 | 0 | 93 | 26 | 114 | Na2SO4+NaHCO3 |
| 323.15 | 1.2610 | 281 | 0 | 72 | 32 | 114 | Na2SO4+NaHCO3 | |
| 323.15 | 1.2590 | 297 | 0 | 50 | 38 | 114 | Na2SO4+NaHCO3 | |
| 343.15 | 1.1577 | 149 | 58 | 42 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.1833 | 168 | 49 | 54 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2110 | 186 | 41 | 66 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2250 | 197 | 33 | 81 | 0 | 107 | Na2SO4 | |
| 343.15 | 1.2421 | 208 | 26 | 97 | 0 | 108 | Na2SO4 | |
| 343.15 | 1.2450 | 223 | 13 | 109 | 0 | 108 | Na2SO4 | |
| 343.15 | 1.2518 | 231 | 0 | 121 | 5 | 109 | Na2SO4 | |
| 343.15 | 1.2554 | 249 | 0 | 103 | 11 | 109 | Na2SO4 | |
| 343.15 | 1.2544 | 266 | 0 | 84 | 17 | 109 | Na2SO4 | |
| J | 343.15 | 1.2540 | 279 | 0 | 63 | 21 | 108 | Na2SO4 |
| J' | 343.15 | 1.2522 | 291 | 0 | 42 | 25 | 108 | Na2SO4+NaHCO3 |
| p/kPa | ρ/g·cm-3 | 液相组成/g·L-1 | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|
| SO | CO | HCO | NH | Na+ | |||
| 115.0 | 1.2291 | 246 | 36 | 37 | 79 | 41 | NaHCO3 |
| 307.0 | 1.2278 | 244 | 33 | 40 | 79 | 39 | NaHCO3 |
| 563.0 | 1.2234 | 246 | 26 | 48 | 81 | 38 | NaHCO3 |
| 793.0 | 1.2244 | 243 | 23 | 54 | 81 | 35 | NaHCO3 |
| 1058.0 | 1.2248 | 245 | 18 | 60 | 81 | 34 | NaHCO3 |
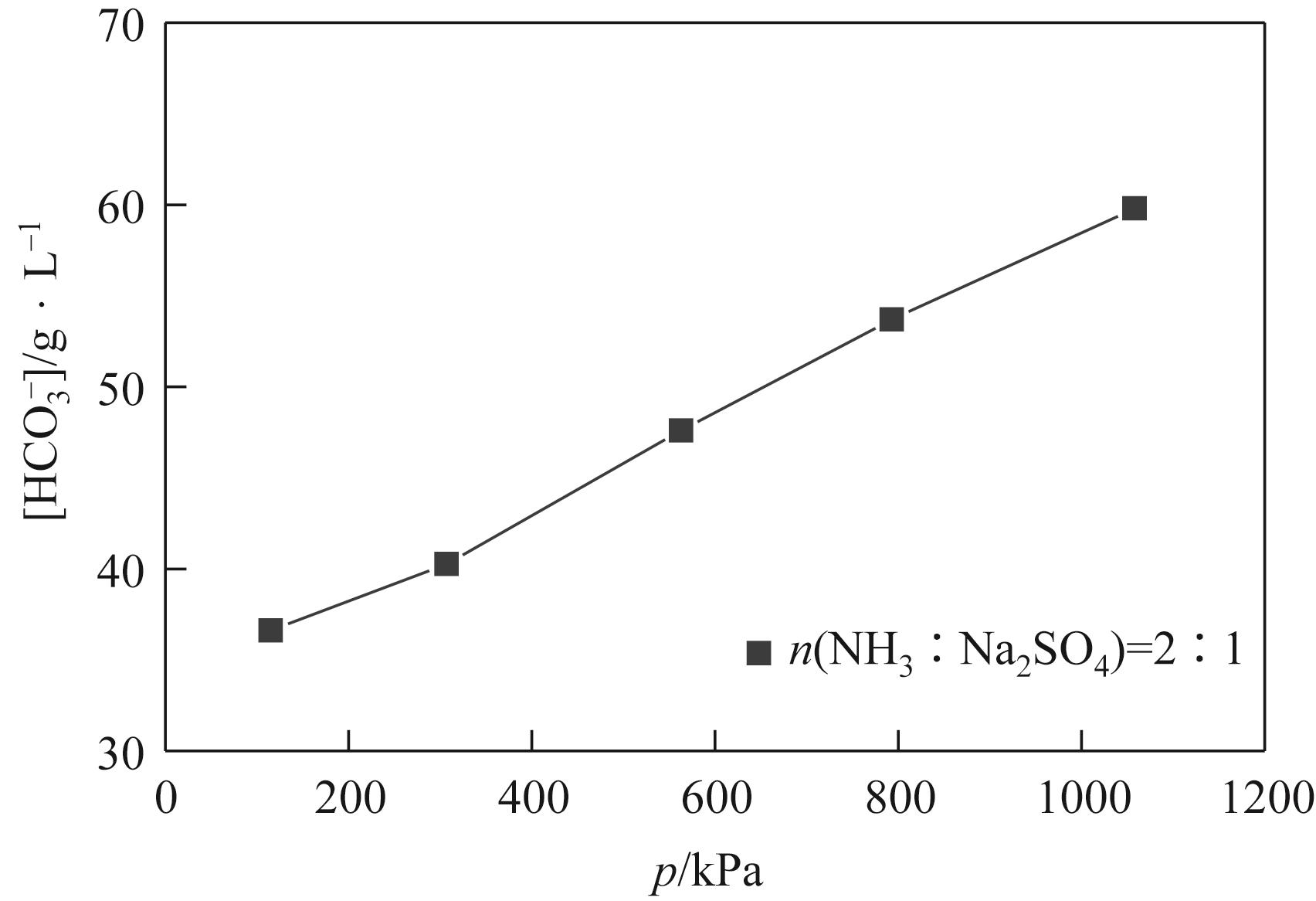
表3 115.0~1058.0kPa下Na2SO4-NH3-CO2-H2O体系在303.15K时相平衡数据
| p/kPa | ρ/g·cm-3 | 液相组成/g·L-1 | 平衡固相 | ||||
|---|---|---|---|---|---|---|---|
| SO | CO | HCO | NH | Na+ | |||
| 115.0 | 1.2291 | 246 | 36 | 37 | 79 | 41 | NaHCO3 |
| 307.0 | 1.2278 | 244 | 33 | 40 | 79 | 39 | NaHCO3 |
| 563.0 | 1.2234 | 246 | 26 | 48 | 81 | 38 | NaHCO3 |
| 793.0 | 1.2244 | 243 | 23 | 54 | 81 | 35 | NaHCO3 |
| 1058.0 | 1.2248 | 245 | 18 | 60 | 81 | 34 | NaHCO3 |
| 1 | HAN D, HE W F, JI C, et al. Thermodynamic analysis of a novel evaporation and crystallization system based on humidification processes at ambient temperature[J]. Desalination, 2018, 439: 108-118. |
| 2 | 范嘉昊, 张洋, 范兵强, 等. (NH4)2SO4和Na2SO4混合溶液中 (NH4)2SO4结晶动力学及铁/铝/锰/铬等离子对(NH4)2SO4结晶的影响规律[J]. 化工进展, 2023, 42(1): 488-496. |
| FAN Jiahao, ZHANG Yang, FAN Bingqiang, et al. Crystallization kinetics of (NH4)2SO4 in mixed solution of (NH4)2SO4 and Na2SO4 and the influence of Fe/Al/Mn/Cr ions on crystallization[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 488-496. | |
| 3 | 李绪宾, 刘会娥, 陈爽, 等. 工业废盐的流态化行为[J]. 化工进展, 2017, 36(1): 81-90. |
| LI Xubin, LIU Huie, CHEN Shuang, et al. Fluidization behavior of industrial waste salt[J]. Chemical Industry and Engineering Progress, 2017, 36(1): 81-90. | |
| 4 | WANG Li, YAO Yaochun, LIANG Feng, et al. Study on factors of vanadium extraction from low-grade vanadium slag with high silicon content by roasting[J]. Silicon, 2020, 12(7): 1691-1698. |
| 5 | HAGEMANN J. Unlocking rustenburg base metals refiners sulphur removal section[J]. Journal of the Southern African Institute of Mining and Metallurgy, 2016, 116(6): 569-574. |
| 6 | CHOUBEY P K, CHUNG Kang-Sup, KIM Min-seuk, et al. Advance review on the exploitation of the prominent energy-storage element lithium. Part Ⅱ: From sea water and spent lithium ion batteries (LIBs)[J]. Minerals Engineering, 2017, 110: 104-121. |
| 7 | 杨文振, 熊萍, 孙秀云, 等. 医药废硫酸钠盐燃烧特性及低温炭化除杂研究[J]. 无机盐工业, 2021, 53(9): 76-82. |
| YANG Wenzhen, XIONG Ping, SUN Xiuyun, et al. Study on combustion characteristics of waste pharmaceutical Na2SO4 and impurity removal by low-temperature carbonization[J]. Inorganic Chemicals Industry, 2021, 53(9): 76-82. | |
| 8 | 袁晋亭, 曾锐, 莎莉, 等. 高盐高有机制药废水污泥电渗透高干脱水[J]. 化工进展, 2020, 39(8): 3380-3385. |
| YUAN Jinting, ZENG Rui, SHA Li, et al. Electroosmotic dewatering of high salinity organic pharmaceutical wastewater sludge[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3380-3385. | |
| 9 | 中科院过程所成功实现硫酸钠制纯碱工业化[J]. 盐科学与化工, 2020, 49(12): 24. |
| Industrialization of sodium sulfate to soda ash was successfully realized in the Institute of Process Engineering of Chinese Academy of Sciences[J]. Journal of Salt Science and Chemical Industry, 2020, 49(12): 24. | |
| 10 | 王炼, 陈利芳, 高静静, 等. 化工行业废盐资源化现状及发展趋势[J]. 科技导报, 2021, 39(17): 9-16. |
| WANG Lian, CHEN Lifang, GAO Jingjing, et al. Status quo of industrial waste salt resource utilization and its development trend[J]. Science & Technology Review, 2021, 39(17): 9-16. | |
| 11 | 王吉坤, 李阳, 陈贵锋, 等. 臭氧催化氧化降解煤化工高盐废水有机物的机理[J]. 化工进展, 2022, 41(1): 493-502. |
| WANG Jikun, LI Yang, CHEN Guifeng, et al. Catalytic oxidation mechanism of organics degradation by ozone in high-salt wastewater of coal chemical industry[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 493-502. | |
| 12 | 张群, 陈重军, 谢嘉玮, 等. 高盐废水微生物脱盐池处理研究进展[J]. 化工进展, 2022, 41(2): 974-980. |
| ZHANG Qun, CHEN Chongjun, XIE Jiawei, et al. Research progress on the microbial desalination cell for high-salt wastewater treatment[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 974-980. | |
| 13 | KHLISSA F, M’NIF A, ROKBANI R. Application of the conductimetry to the study of the transformation of KCl and Na2SO4 into K2SO4 between 5 and 30℃[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(7): 929-934. |
| 14 | WANG Xu, DU Yawei, LIU Jie, et al. Modeling and simulation of continuous electrodialysis metathesis process for conversion of Na2SO4 to K2SO4 [J]. Desalination, 2022, 528: 115605. |
| 15 | 廖恩鑫, 陈丽芳, 张泽亚, 等. 硫酸钠与氯化钾制备硫酸钾实验研究[J]. 无机盐工业, 2020, 52(10): 106-109. |
| LIAO Enxin, CHEN Lifang, ZHANG Zeya, et al. Experimental study on preparing potassium sulfate by sodium sulfate and potassium chloride[J]. Inorganic Chemicals Industry, 2020, 52(10): 106-109. | |
| 16 | PISARSKA B, JAROSZEK H, MIKOŁAJCZAK W, et al. Application of electro-electrodialysis for processing of sodium sulphate waste solutions containing organic compounds: Preliminary study[J]. Journal of Cleaner Production, 2017, 142: 3741-3747. |
| 17 | LIU Jie, XU Fan, YUAN Junsheng, et al. High-value conversion of Na2SO4 wastewater by a continuous electrodialytic metathesis process: Effects of coexisting ions[J]. Journal of Membrane Science, 2020, 615: 118584. |
| 18 | KULDEEP, BADENHORST W D, KAURANEN P, et al. Bipolar membrane electrodialysis for sulfate recycling in the metallurgical industries[J]. Membranes, 2021, 11(9): 718. |
| 19 | 颜海洋, 汪耀明, 蒋晨啸, 等. 离子膜电渗析在高盐废水“零排放”中的应用、机遇与挑战[J]. 化工进展, 2019, 38(1): 672-681. |
| YAN Haiyang, WANG Yaoming, JIANG Chenxiao, et al. Ion exchange membrane electrodialysis for high salinity wastewater ‘zero liquid discharge’: Applications, opportunities and challenges[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 672-681. | |
| 20 | 张洋, 张贺东, 范兵强, 等. 一种利用氯化钠废盐制备碳酸氢钠联产氯化铵的方法: CN114715919B[P]. 2022-09-06. |
| ZHANG Yang, ZHANG Hedong, FAN Bingqiang, et al. Method for preparing sodium bicarbonate and co-producing ammonium chloride by using sodium chloride waste salt: CN114715919B[P]. 2022-09-06. | |
| 21 | ZHANG Yang, XU Hongbin, ZHANG Yi, et al. Phase equilibria of the Na+, NH 4 + //SO 4 2 - , Cl--H2O system[J]. Journal of Chemical & Engineering Data, 2013, 58(4): 1050-1053. |
| 22 | ZHANG Yang, XU Hongbin, LIU Changlin, et al. Phase equilibria of Na+, NH 4 + //SO 4 2 - , HCO 3 - , Cl--H2O quinary system[J]. Journal of Chemical & Engineering Data, 2013, 58(7): 2095-2099. |
| 23 | KURZ F, RUMPF B, MAURER G. Simultaneous solubility of ammonia and carbon dioxide in aqueous solutions of ammonium sulfate and (ammonium sulfate + sodium sulfate) at temperatures from 313K to 393K and pressures to 10MPa[J]. The Journal of Chemical Thermodynamics, 1996, 28(5): 497-520. |
| 24 | RUMPF B, MAURER G. Solubility of ammonia in aqueous solutions of sodium sulfate and ammonium sulfate at temperatures from 333.15K to 433.15K and pressures up to 3MPa[J]. Industrial & Engineering Chemistry Research, 1993, 32(8): 1780-1789. |
| 25 | BIELING V, KURZ F, RUMPF B, et al. Simultaneous solubility of ammonia and carbon dioxide in aqueous solutions of sodium sulfate in the temperature range 313—393K and pressures up to 3MPa[J]. Industrial & Engineering Chemistry Research, 1995, 34(4): 1449-1460. |
| 26 | 吕秉玲. Na+、NH 4 + //HCO 3 - 、Cl–、SO 4 2 - 、H2O体系相图及其在氨化-碳酸化法开发天然盐碱湖中的应用[J]. 纯碱工业, 1990(1): 13-16. |
| Bingling LYU. Na+, NH 4 + //HCO 3 - , Cl–, SO 4 2 - , H2O system phase diagram and its application in natural saline-alkali lake development by ammoniation-carbonation method[J]. Soda Industry, 1990(1): 13-16. | |
| 27 | CORTI H R, KRENZER M E, DE PABLO J J, et al. Effect of a dissolved gas on the solubility of an electrolyte in aqueous solution[J]. Industrial & Engineering Chemistry Research, 1990, 29(6): 1043-1050. |
| 28 | STEPHEN H, STEPHEN T, SILCOCK H. Solubilities of inorganic and organic compounds[M]. Oxford: Pergamon Press, 1979. |
| [1] | 郭迎春, 梁晓怿. 柠檬酸改性球形活性炭对氨气吸附性能的影响[J]. 化工进展, 2024, 43(2): 1082-1088. |
| [2] | 王秋华, 吴嘉帅, 张卫风. 碱性工业固废矿化封存二氧化碳研究进展[J]. 化工进展, 2023, 42(3): 1572-1582. |
| [3] | 谷凯, 吴寅凯, 尹俊权, 李卫华, 孙英杰, 张庆建, 葛燕辰, 何依洋, 赵灵燕, 王华伟. 多元浸沥场景下固化/稳定飞灰中重金属浸出行为[J]. 化工进展, 2023, 42(11): 6113-6125. |
| [4] | 岳子瀚, 龙臻, 周雪冰, 臧小亚, 梁德青. sⅡ型水合物储氢研究进展[J]. 化工进展, 2023, 42(10): 5121-5134. |
| [5] | 何民宇, 刘维燥, 刘清才, 秦治峰. CO2矿物封存技术研究进展[J]. 化工进展, 2022, 41(4): 1825-1833. |
| [6] | 郑鹏, 李蔚玲, 郭亚飞, 孙健, 王瑞林, 赵传文. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| [7] | 贾文龙, 宋硕硕, 李长俊, 吴瑕, 杨帆, 张员瑞. 超临界CO2萃取含油污泥研究现状与进展[J]. 化工进展, 2022, 41(12): 6573-6585. |
| [8] | 杨晋, 殷勇高, 陈万河, 王静远, 陈九法. 硫酸钠水合盐相变蓄冷材料的制备及性能优化[J]. 化工进展, 2022, 41(11): 5977-5985. |
| [9] | 王英梅, 牛爱丽, 张兆慧, 展静, 张学民. 二氧化碳水合物快速生成方法研究进展[J]. 化工进展, 2021, 40(S2): 117-125. |
| [10] | 王逸伟, 刘智琪, 孙强, 刘爱贤, 杨兰英, 宫敬, 郭绪强. 聚苯乙烯磺酸钠作用下Ⅰ型水合物的生成热力学与动力学[J]. 化工进展, 2021, 40(S1): 168-181. |
| [11] | 陈沛坤, 张袁斌, 崔希利, 邢华斌. 氨气深度脱除材料与技术研究进展[J]. 化工进展, 2021, 40(7): 3957-3975. |
| [12] | 胡倩, 周诗岽, 郭宇, 张雪艳, 王娇娇, 王国栋, 姬浩洋. 蜡晶析出对CO2水合物相平衡及诱导特性的影响[J]. 化工进展, 2021, 40(5): 2452-2460. |
| [13] | 王中辉, 苏胜, 尹子骏, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327. |
| [14] | 耿雅雯, 刘锋, 冯震, 陈俊, 张雪智. 硫自养/异养协同反硝化深度脱氮处理三氯蔗糖生产废水[J]. 化工进展, 2021, 40(10): 5829-5836. |
| [15] | 代辉祥, 陆文静, 李超, 王前. 双介质阻挡放电低温等离子体对模拟堆肥气体中氨气的去除[J]. 化工进展, 2020, 39(9): 3801-3809. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||