化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2318-2327.DOI: 10.16085/j.issn.1000-6613.2020-0981
CO2矿化及吸收-矿化一体化(IAM)方法研究进展
王中辉( ), 苏胜(
), 苏胜( ), 尹子骏, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军
), 尹子骏, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军
- 华中科技大学能源与动力工程学院,煤燃烧国家重点实验室,湖北 武汉 430074
-
收稿日期:2020-06-02出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:苏胜 -
作者简介:王中辉(1997—),男,硕士研究生,研究方向为CO2矿化减排。E-mail:751107735@qq.com 。 -
基金资助:国家自然科学基金(U20A20303)
Research progress of CO2 mineralization and integrated absorption-mineralization (IAM) method
WANG Zhonghui( ), SU Sheng(
), SU Sheng( ), YIN Zijun, AN Xiaoxue, ZHAO Zhigang, CHEN Yifeng, LIU Tao, WANG Yi, HU Song, XIANG Jun
), YIN Zijun, AN Xiaoxue, ZHAO Zhigang, CHEN Yifeng, LIU Tao, WANG Yi, HU Song, XIANG Jun
- State Key Laboratory of Coal Combustion, School of Energy and Power Engineering, Huazhong University of Science and Technology, Wuhan 430074, Hubei, China
-
Received:2020-06-02Online:2021-04-05Published:2021-04-14 -
Contact:SU Sheng
摘要:
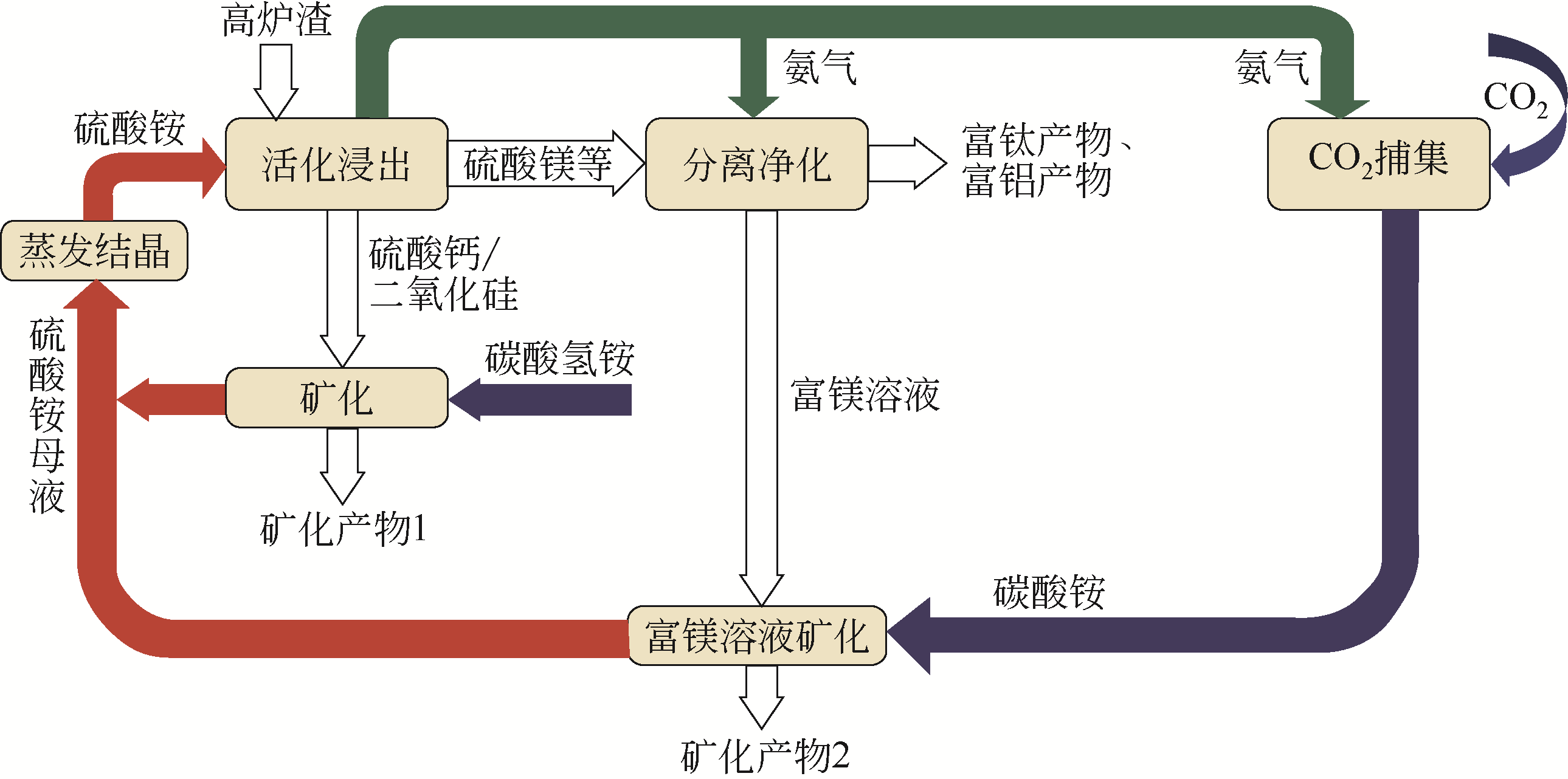
为避免温室效应带来的负面影响,CO2减排已成为目前的当务之急。CO2矿物碳酸化作为一种有潜力的CO2减排技术,受到了学者们的广泛关注。CO2矿物碳酸化方法主要包括直接干法碳酸化、直接湿法碳酸化以及间接碳酸化等不同工艺过程。目前,CO2直接或间接碳酸化方法面临的关键挑战是提升CO2碳酸化反应动力学特性;反应速率慢、碳酸化效率较低是当前该技术的主要问题。传统CO2胺类化学吸收法具有吸收速率快、吸收容量大和吸收剂能循环再生的优点,但能耗和运行成本较高。将CO2胺类化学吸收法与CO2碳酸化过程结合而开发的CO2吸收-矿化一体化技术(IAM)不仅解决了传统工艺高能耗、低转化率的问题,而且使工艺流程简化、成本降低,有利于应用于工业化。本文主要综述了近年来CO2矿化技术的研究进展,对比了各种工艺技术路线的不同特点,并分析指出加强对IAM工艺反应机理的研究以及开发出高效、经济的吸收剂和矿化原料,将是该工艺未来研究的重点和关键。
中图分类号:
引用本文
王中辉, 苏胜, 尹子骏, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军. CO2矿化及吸收-矿化一体化(IAM)方法研究进展[J]. 化工进展, 2021, 40(4): 2318-2327.
WANG Zhonghui, SU Sheng, YIN Zijun, AN Xiaoxue, ZHAO Zhigang, CHEN Yifeng, LIU Tao, WANG Yi, HU Song, XIANG Jun. Research progress of CO2 mineralization and integrated absorption-mineralization (IAM) method[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2318-2327.
| 矿化原料 | 主要成分 | 主要化学反应方程式 |
|---|---|---|
| 天然矿物 | ||
| 橄榄石 | Mg2SiO4 | Mg2SiO4+2CO2 |
| 蛇纹石 | Mg3Si2O5(OH)4 | Mg3Si2O5(OH)4+3CO2 |
| 硅灰石 | CaSiO3 | CaSiO3+CO2 |
| 工业固废 | ||
| 粉煤灰 | CaO | CaO+CO2 CaO+H2O Ca(OH)2(aq)+CO2 |
| 钢渣 | CaO、MgO | CaO+CO2 MgO+CO2 |
| 磷石膏 | CaSO4·2H2O | CaSO4·2H2O+2NH3·H2O+CO2 CaSO4(s)+2KAlSi3O8+CO2+2H2O |
| 电石渣 | Mg(OH)2 | Ca(OH)2(aq)+CO2 |
| 盐湖苦卤 | MgCl2 | MgCl2·6H2O(aq)+2NH3·H2O Mg(OH)2+CO2+2H2O |
表1 矿化原料及其主要化学反应过程
| 矿化原料 | 主要成分 | 主要化学反应方程式 |
|---|---|---|
| 天然矿物 | ||
| 橄榄石 | Mg2SiO4 | Mg2SiO4+2CO2 |
| 蛇纹石 | Mg3Si2O5(OH)4 | Mg3Si2O5(OH)4+3CO2 |
| 硅灰石 | CaSiO3 | CaSiO3+CO2 |
| 工业固废 | ||
| 粉煤灰 | CaO | CaO+CO2 CaO+H2O Ca(OH)2(aq)+CO2 |
| 钢渣 | CaO、MgO | CaO+CO2 MgO+CO2 |
| 磷石膏 | CaSO4·2H2O | CaSO4·2H2O+2NH3·H2O+CO2 CaSO4(s)+2KAlSi3O8+CO2+2H2O |
| 电石渣 | Mg(OH)2 | Ca(OH)2(aq)+CO2 |
| 盐湖苦卤 | MgCl2 | MgCl2·6H2O(aq)+2NH3·H2O Mg(OH)2+CO2+2H2O |
| 参数 | CO2捕获 | ||
|---|---|---|---|
| 化学再生(CaO) | 化学再生(粉煤灰) | 传统热再生 | |
| 吸收剂浓度/mol·L-1 | 2 | 2 | 2 |
| 烟气CO2压力/kPa | 9 | 9 | 9 |
| 吸收温度/℃ | 40 | 40 | 40 |
| 再生压力/atm | 1 | 1 | 2 |
| 再生温度/℃ | 40 | 40 | 116 |
| 换热器换热温差/℃ | — | — | 10 |
| CO2循环负荷/mol·mol-1 | 0.21 | 0.21 | 0.21 |
| 再生消耗能量/MJ·kg-1 CO2 | 0 | 0 | 4.7 |
| 产物 | CaCO3 | 富含CaCO3的飞灰 | 高纯度CO2 |
表2 IAM工艺与传统MEA工艺实验条件及结果[48,51,59]
| 参数 | CO2捕获 | ||
|---|---|---|---|
| 化学再生(CaO) | 化学再生(粉煤灰) | 传统热再生 | |
| 吸收剂浓度/mol·L-1 | 2 | 2 | 2 |
| 烟气CO2压力/kPa | 9 | 9 | 9 |
| 吸收温度/℃ | 40 | 40 | 40 |
| 再生压力/atm | 1 | 1 | 2 |
| 再生温度/℃ | 40 | 40 | 116 |
| 换热器换热温差/℃ | — | — | 10 |
| CO2循环负荷/mol·mol-1 | 0.21 | 0.21 | 0.21 |
| 再生消耗能量/MJ·kg-1 CO2 | 0 | 0 | 4.7 |
| 产物 | CaCO3 | 富含CaCO3的飞灰 | 高纯度CO2 |
| 参数 | 传统MEA工艺能耗 | IAM工艺能耗 | IAM工艺节能 |
|---|---|---|---|
| 汽提塔再沸器/kJ·(kg CO2)-1 | 760 | — | 760 |
| 压缩装置/kJ·(kg CO2)-1 | 397 | — | 397 |
| 泵/kJ·(kg CO2)-1 | 9.9 | 9.9 | 0 |
| 过滤装置/kJ·(kg CO2)-1 | — | 51.7 | -51.7 |
| 总计/kJ·(kg CO2)-1 | 1166.9 | 61.6 | 1115.3 |
| 年能耗成本/ | 142865 | 233 | 142632 |
| 设备投入成本/ | 42460 | 5537 | 36923 |
表3 IAM工艺与传统MEA工艺能耗和资本投入
| 参数 | 传统MEA工艺能耗 | IAM工艺能耗 | IAM工艺节能 |
|---|---|---|---|
| 汽提塔再沸器/kJ·(kg CO2)-1 | 760 | — | 760 |
| 压缩装置/kJ·(kg CO2)-1 | 397 | — | 397 |
| 泵/kJ·(kg CO2)-1 | 9.9 | 9.9 | 0 |
| 过滤装置/kJ·(kg CO2)-1 | — | 51.7 | -51.7 |
| 总计/kJ·(kg CO2)-1 | 1166.9 | 61.6 | 1115.3 |
| 年能耗成本/ | 142865 | 233 | 142632 |
| 设备投入成本/ | 42460 | 5537 | 36923 |
| 1 | ALLEN M R, FRAME D J, HUNTINGFORD C, et al. Warming caused by cumulative carbon emissions towards the trillionth tonne[J]. Nature, 2009, 458: 163-1166. |
| 2 | ROGELJ J, ELZEN M D, HOHNE N, et al. Paris agreement climate proposals need a boost to keep warming well below 2℃[J]. Nature, 2016, 534: 631-639. |
| 3 | BERGER J, CHRISTENSEN J, DUBASH N K, et al. Emissions gap report 2019[R]. Nairobi: UNEP, 2019. |
| 4 | SANNA A, UIBU M, CARAMANNA G, et al. A review of mineral carbonation technologies to sequester CO2[J]. Chemical Society Reviews, 2014, 43: 8049-8080. |
| 5 | 康丽娜, 尚会建, 郑学明. CO2的捕集封存技术进展及在我国的应用前景[J]. 化工进展, 2010, 29(S1): 24-27. |
| KANG Lina, SHANG Huijian, ZHENG Xueming. Progress of CO2 capture and storage technology and its application prospects in China[J]. Chemical Industry and Engineering Progress, 2010, 29(S1): 24-27. | |
| 6 | LOCKWOOD T. A comparative review of next-generation carbon capture technologies for coal-fired power plant[J]. Energy Procedia, 2017, 114: 2658-2670. |
| 7 | ROMANOV V, SOONG Y, CARNEY C, et al. Mineralization of carbon dioxide: a literature review[J]. ChemBioEng Reviews, 2015, 2: 231-256. |
| 8 | PARK S, LEE M G, PARK J, et al. CO2 (carbon dioxide) fixation by applying new chemical absorption-precipitation methods[J]. Energy, 2013, 59: 737-742. |
| 9 | NARAHARISETTI P K, YEO T Y, BU J. New classification of CO2 mineralization processes and economic evaluation[J]. Renewable and Sustainable Energy Reviews, 2019, 99: 220-233. |
| 10 | GEERLINGS H, ZEVENHOVEN R. CO2 mineralization-bridge between storage and utilization of CO2[J]. Annual Review of Chemical and Biomolecular Engineering, 2013, 4: 103-117. |
| 11 | SEIFRITZ W. CO2 disposal by means of silicates[J]. Nature, 1990, 345(6275): 486-486. |
| 12 | BOBICKI E R, LIU Qingxia, XU Zhenghe, et al. Carbon capture and storage using alkaline industrial wastes[J]. Progress in Energy and Combustion Science, 2012, 38: 302-320. |
| 13 | OLAJIRE A A. A review of mineral carbonation technology in sequestration of CO2[J]. Journal of Petroleum Science and Engineering, 2013, 109: 364-392. |
| 14 | 张兵兵, 王慧敏, 曾尚红, 等. 二氧化碳矿物封存技术现状及展望[J]. 化工进展, 2012, 31(9): 2075-2083. |
| ZHANG Bingbing, WANG Huimin, ZENG Shanghong, et al. Present situation and prospect of carbon dioxide mineral storage technology[J]. Chemical Industry and Engineering Progress, 2012, 31(9): 2075-2083. | |
| 15 | PAN S, CHIANG A, CHANG E E, et al. An innovative approach to integrated carbon mineralization and waste utilization: a review[J]. Aerosol and Air Quality Research, 2015, 15: 1072-1091. |
| 16 | FAGERLUND J, TEIR S, NDUAGU E, et al. Carbonation of magnesium silicate mineral using a pressurized gas-solid process[J].Energy Procedia, 2009, 1: 4907-4914. |
| 17 | RENFORTH P, WASHBOURNE C L, TAYLDER J, et al. Silicate production and availability for mineral carbonation[J]. Environmental Science & Technology, 2011, 45: 2035-2041. |
| 18 | ZHAO Jihui, WANG Dongmin, LIAO Shucong. Effect of mechanical grinding on physical and chemical characteristics of circulating fluidized bed fly ash from coal gangue power plant[J]. Construction and Building Materials, 2015, 101: 851-860. |
| 19 | 王晓龙, 郜时旺, 刘练波, 等. 捕集并利用燃煤电厂二氧化碳生产高附加值产品的新工艺[J]. 中国电机工程学报, 2012, 32(S1): 164-167. |
| WANG Xiaolong, GAO Shiwang, LIU Lianbo, et al. New process for capturing and using carbon dioxide from coal-fired power plants to produce high value-added products[J]. Proceedings of the CSEE, 2012, 32(S1): 164-167. | |
| 20 | PAN S Y, SHAH K J, CHEN Y H, et al. Deployment of accelerated carbonation using alkaline solid wastes for carbon mineralization and utilization toward a circular economy[J]. ACS Sustainable Chemistry & Engineering, 2017, 5: 6429-6437. |
| 21 | 谢和平, 谢凌志, 王昱飞, 等. 全球二氧化碳减排不应是CCS, 应是CCU[J]. 四川大学学报(工程科学版), 2012, 44(4): 1-5. |
| XIE Heping, XIE Lingzhi, WANG Yufei, et al. Global carbon dioxide emission reduction should not be CCS, but CCU[J]. Journal of Sichuan University (Engineering Science Edition), 2012, 44(4): 1-5. | |
| 22 | AZDARPOUR A, ASADULLAH M, MOHAMMADIAN E, et al. A review on carbon dioxide mineral carbonation through pH-swing process[J]. Chemical Engineering Journal, 2015, 279: 615-630. |
| 23 | LACKNER K S, BUTT D P, WENDT C H. Progress on binding CO2 in mineral substrates[J]. Energy Conversion and Management, 1997, 38: S259-264. |
| 24 | LIU Wei, SU Sheng, XU Kai, et al. CO2 sequestration by direct gas-solid carbonation of fly ash with steam addition[J]. Journal of Cleaner Production, 2018, 178: 98-107. |
| 25 | O’CONNOR W K, DAHLIN D C, NILSEN R P, et al. Carbon dioxide sequestration by direct mineral carbonation with carbonic acid[C]//TURNER P C. Albany Research Center Proceedings of the 25th International Technical Conference on Coal Utilization & Fuel Systems, Oregon: Albany Research Center, 2000: 1-15. |
| 26 | KWAK J H, HU Jianzhi, HOYT D W, et al. Metal carbonation of forsterite in supercritical CO2 and H2O using solid state 29Si, 13C NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2010, 114: 4126-4134. |
| 27 | 绳昊一, 吕莉, 梁斌, 等. 焙烧温度对硅酸钙矿化CO2的影响[J]. 矿产综合利用, 2015, 10(5): 76-80. |
| SHENG Haoyi, Li LYU, LIANG Bin, et al. Effect of roasting temperature on mineralized CO2 of calcium silicate[J]. Comprehensive Utilization of Minerals, 2015, 10(5): 76-80. | |
| 28 | 栗明宏, 赵瑞红, 柴彤, 等. 烟道气碳化水镁石副产碱式碳酸镁碳捕集技术[J]. 煤炭转化, 2017, 40(3): 76-80. |
| LI Minghong, ZHAO Ruihong, CAI Tong, et al. Flue gas carbonized brucite by-product basic magnesium carbonate carbon capture technology[J]. Coal Conversion, 2017, 40(3): 76-80. | |
| 29 | WANG Tao, HUANG Hao, HU Xutao, et al. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate[J]. Chemical Engineering Journal, 2017, 323: 320-329. |
| 30 | HUANG Hao, GUO Ruonan, WANG Tao, et al. Carbonation curing for wollastonite-Portland cementitious materials: CO2 sequestration potential and feasibility assessment[J]. Journal of Cleaner Production, 2019, 211: 830-841. |
| 31 | YI Zhenwei, WANG Tao, GUO Ruonan, et al. Sustainable building material from CO2 mineralization slag: aggregate for concretes and effect of CO2 curing[J]. Journal of CO2 Utilization, 2020, 40: 101196. |
| 32 | GUO Ruonan, CHEN Qiyang, HUANG Hao, et al. Carbonation curing of industrial solid waste-based aerated concretes[J]. Greenhouse Gases Science and Technology, 2019, 9: 433-443. |
| 33 | 黄浩, 王涛, 方梦祥. 二氧化碳矿化养护混凝土技术及新型材料研究进展[J]. 化工进展, 2019, 38(10): 4363-4373. |
| HUANG Hao, WANG Tao, FANG Mengxiang. Review on carbon dioxide mineral carbonation curing technology of concrete and novel material development[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4363-4373. | |
| 34 | HUIGEN W J J. Carbon dioxide sequestration by mineral carbonation[D]. Netherlands: Wageningen University, 2007. |
| 35 | GADIKOTA G, MATTER J, KELEMEN P, et al. Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3[J]. Physical Chemistry Chemical Physics, 2014, 16: 4679-4693. |
| 36 | WANG Fei, DREISINGERA D, JARVIS M, et al. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration[J]. Mineral Engineering, 2019, 131: 185-197. |
| 37 | SARAN R K, ARORA V, YADAV S. CO2 sequestration by mineral carbonation: a review[J]. Global NEST Journal, 2018, 20(3): 497-503. |
| 38 | 李春, 刘维燥, 岳海荣, 等. CO2矿化非碱性矿的离子迁移规律及过程强化基础[J]. 中国基础科学, 2018(4): 49-54. |
| LI Chun, LIU Weizao, YUE Hairong, et al. Ion migration rule and process reinforcement basis of CO2 mineralization of non-alkaline ores[J]. China Basic Science, 2018(4): 49-54. | |
| 39 | YIN Shu, ALDAHRI T, ROHANI S, et al. Insights into the roasting kinetics and mechanism of blast furnace slag with ammonium sulfate for CO2 mineralization[J]. Industrial Engineering Chemistry Research, 2019, 58: 14036-14036. |
| 40 | LIU Qiang, LIU Weizao, HU Jinpeng, et al. Energy-efficient mineral carbonation of blast furnace slag with high value-added products[J]. Journal of Cleaner Production, 2018, 197: 242-252. |
| 41 | LIU Weizao, YIN Shu, LUO Dongmei, et al. Optimizing the recovery of high-value-added ammonium alum during mineral carbonation of blast furnace slag[J]. Journal of Alloys and Compounds, 2019, 774: 1151-1159. |
| 42 | MAROTO-VALER M M, FAUTH D J, KUCHTA M E, et al. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration[J]. Fuel Process Technology, 2005, 86: 1627-1645. |
| 43 | TEIR S, REVITZER H, ELONEVA S, et al. Dissolution of natural serpentinite in mineral and organic acids[J]. International Journal of Mineral Processing, 2007, 83: 36-46. |
| 44 | BLENCOE J, PALMER D, ANOVITZ L, et al. Carbonation of metal silicates for long-term CO2 sequestration: US 8673256[P]. 2014-03-18. |
| 45 | 包炜军, 李会泉, 张懿. 强化碳酸化固定CO2反应过程分析与机理探讨[J]. 化工学报, 2009,60(9): 2332-2338. |
| BAO Weijun, LI Huiquan, ZHANG Yi. Process analysis and mechanism discussion of enhanced carbonation fixation of CO2[J]. CIESC Journal, 2009, 60(9): 2332-2338. | |
| 46 | WANG X L, MAROTO-VALER M M. Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation[J]. Fuel, 2011, 90: 1229-1237. |
| 47 | WANG X L, MAROTO-VALER M M. Integration of CO2 capture and mineral carbonation by using recyclable ammonium salts[J]. ChemSusChem, 2011, 4: 1291-1300. |
| 48 | JI Long, YU Hai, YU Bing, et al. Integrated absorption-mineralization for energy-efficient CO2 sequestration: reaction mechanism and feasibility of using fly ash as a feedstock[J]. Chemical Engineering Journal, 2018, 352: 151-162. |
| 49 | 林海周, 裴爱国, 方梦祥. 燃煤电厂烟气二氧化碳胺法捕集工艺改进研究进展[J]. 化工进展, 2018, 37(12): 4874-4886. |
| LIN Haizhou, PEI Aiguo, FANG Mengxiang. Progress of research on process modifications for amine solvent-based post combustion CO2 capture from coal-fired power plant[J]. Chemical Industry and Engineering Progress, 2018, 37(12): 4874-4886. | |
| 50 | YU C H, HUANG C H, TAN C S, et al. A review of CO2 capture by absorption and adsorption[J]. Aerosol and Air Quality Research, 2012, 12: 745-769. |
| 51 | LI Kangkang, LEIGH W, FERON P, et al. Systematic study of aqueous monoethanolamine (MEA)-based CO2 capture process: techno-economic assessment of the MEA process and its improvements[J]. Applied Energy, 2016, 165: 648-659. |
| 52 | SHAKERIAN F, KIM K H, SZULEJKO J E, et al. A comparative review between amines and ammonia as sorptive media for post-combustion CO2 capture[J]. Applied Energy, 2015, 148: 10-22. |
| 53 | HANG Deng, BIELICK J M, OPPENHEIMER M, et al. Leakage risks of geologic CO2 storage and the impacts on the global energy system and climate change mitigation[J]. Climatic Change, 2017, 144: 151-163. |
| 54 | PARK S, MIN J, LEE M G, et al. Characteristics of CO2 fixation by chemical conversion to carbonate salts[J]. Chemical Engineering Journal, 2013, 231: 287-293. |
| 55 | KANG J M, MURNANDARI A, YOUN M H, et al. Energy-efficient chemical regeneration of AMP using calcium hydroxide for operating carbon dioxide capture process[J]. Chemical Engineering Journal, 2018, 335: 338-344. |
| 56 | ARTI M, YOUN M H, PARK K T, et al. Single process for CO2 capture and mineralization in various alkanolamines using calcium chloride[J]. Energy & Fuels, 2017, 31(1): 763-769. |
| 57 | LIU Meishen, GADIKOTA G. Integrated CO2 capture, conversion, and storage to produce calcium carbonate using an amine looping strategy[J]. Energy & Fuels, 2019, 33: 1722-1733. |
| 58 | JI Long, YU Hai, LI Kangkang, et al. Integrated absorption-mineralization for low-energy CO2 capture and sequestration[J]. Applied Energy, 2018, 225: 356-366. |
| 59 | YU Bing, LI Kangkang, JI Long, et al. Coupling a sterically hindered amine-based absorption and coal fly ash triggered amine regeneration: a high energy-saving process for CO2 absorption and sequestration[J]. International Journal of Greenhouse Gas Control, 2019, 87: 58-65. |
| [1] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [2] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [3] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [6] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [7] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [8] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [9] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [12] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [13] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [14] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [15] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||