化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2328-2337.DOI: 10.16085/j.issn.1000-6613.2020-1049
燃煤烟气中SO3与NH4HSO4生成特性及其控制方法研究进展
尹子骏1( ), 苏胜1(
), 苏胜1( ), 王中辉1, 王乐乐2, 安晓雪1, 赵志刚1, 陈逸峰1, 刘涛1, 汪一1, 胡松1, 向军1
), 王中辉1, 王乐乐2, 安晓雪1, 赵志刚1, 陈逸峰1, 刘涛1, 汪一1, 胡松1, 向军1
- 1.华中科技大学煤燃烧国家重点实验室,湖北 武汉 430074
2.西安热工研究院有限公司苏州分公司,江苏 苏州 215153
-
收稿日期:2020-06-10出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:苏胜 -
作者简介:尹子骏(1996—),男,硕士研究生,研究方向为SO3生成控制。E-mail:291300330@qq.com 。 -
基金资助:国家自然科学基金(51976072)
Research progress on the characteristics and control methods of SO3 and NH4HSO4 formation in coal-fired flue gas
YIN Zijun1( ), SU Sheng1(
), SU Sheng1( ), WANG Zhonghui1, WANG Lele2, AN Xiaoxue1, ZHAO Zhigang1, CHEN Yifeng1, LIU Tao1, WANG Yi1, HU Song1, XIANG Jun1
), WANG Zhonghui1, WANG Lele2, AN Xiaoxue1, ZHAO Zhigang1, CHEN Yifeng1, LIU Tao1, WANG Yi1, HU Song1, XIANG Jun1
- 1.State Key Laboratory of Coal Combustion, Huazhong University of Science & Technology, Wuhan 430074, Hubei, China
2.Xi’an Thermal Power Research Institute Company Limited, Suzhou Branch, Suzhou 215153, Jiangsu, China
-
Received:2020-06-10Online:2021-04-05Published:2021-04-14 -
Contact:SU Sheng
摘要:
在燃煤烟气中选择性催化还原(SCR)技术由于脱硝效率高、选择性好被广泛应用,然而SCR催化剂的催化作用会使烟气中的SO2氧化成SO3,SO3会与NH3等反应生成硫酸氢铵(ABS)和硫酸铵(AS),当烟气温度低于硫酸铵盐的凝结温度时,其会沉积在催化剂、空预器及其附属设备上,引发诸多严重的问题,对电厂的运行和环境造成了不利影响。本文综述了燃煤烟气中SO3与硫酸氢铵的生成特性及其控制方法最新进展,分析了SO3在锅炉和SCR系统中的形成机理、迁徙转化特性,阐述了控制SO3与硫酸氢铵生成的方法,介绍了不同活性组分对催化剂表面SO3和硫酸氢铵生成的影响。最后提出了开发新型催化剂是燃煤烟气中SO3与硫酸氢铵生成控制的重点研究方向。
中图分类号:
引用本文
尹子骏, 苏胜, 王中辉, 王乐乐, 安晓雪, 赵志刚, 陈逸峰, 刘涛, 汪一, 胡松, 向军. 燃煤烟气中SO3与NH4HSO4生成特性及其控制方法研究进展[J]. 化工进展, 2021, 40(4): 2328-2337.
YIN Zijun, SU Sheng, WANG Zhonghui, WANG Lele, AN Xiaoxue, ZHAO Zhigang, CHEN Yifeng, LIU Tao, WANG Yi, HU Song, XIANG Jun. Research progress on the characteristics and control methods of SO3 and NH4HSO4 formation in coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2328-2337.
| 机组编号 | 装机容量/MW | SO3质量浓度/mg·m-3 | |||||
|---|---|---|---|---|---|---|---|
| SCR入口 | SCR出口 | 空预器出口 | 电除尘器出口 | 脱硫装置出口 | 湿式电除尘器出口 | ||
| A | 600 | 19.82 | 30.61 | 28.54 | 24.73 | 18.00 | — |
| B | 600 | 33.14 | 67.45 | 62.62 | 50.61 | 30.65 | 8.22 |
| C | 600 | 16.61 | 32.52 | 30.21 | 22.69 | 16.68 | — |
| D | 660 | 34.55 | 74.23 | 70.22 | 53.41 | 40.88 | — |
| E | 660 | 29373 | 68.66 | 65.76 | 50.91 | 33.76 | — |
表1 SO3质量浓度测试结果
| 机组编号 | 装机容量/MW | SO3质量浓度/mg·m-3 | |||||
|---|---|---|---|---|---|---|---|
| SCR入口 | SCR出口 | 空预器出口 | 电除尘器出口 | 脱硫装置出口 | 湿式电除尘器出口 | ||
| A | 600 | 19.82 | 30.61 | 28.54 | 24.73 | 18.00 | — |
| B | 600 | 33.14 | 67.45 | 62.62 | 50.61 | 30.65 | 8.22 |
| C | 600 | 16.61 | 32.52 | 30.21 | 22.69 | 16.68 | — |
| D | 660 | 34.55 | 74.23 | 70.22 | 53.41 | 40.88 | — |
| E | 660 | 29373 | 68.66 | 65.76 | 50.91 | 33.76 | — |
| 吸附剂 | 锅炉内 | SCR | 空预器 | |||||
|---|---|---|---|---|---|---|---|---|
| 优点 | 缺点 | 优点 | 缺点 | 优点 | 缺点 | |||
| 石灰石 | 可同时脱除SO2和SO3 | 导致催化剂中毒,影响ESP除尘效率 | — | — | — | — | ||
| Ca(OH)2 | 可同时脱除SO2和SO3 | 易结焦 | 延缓空预器堵塞,对飞灰质量影响小 | SO3脱除效率低,影响ESP除尘效率 | SO3脱除效率高 | SO3脱除效率低,影响ESP除尘效率 | ||
| MgO | SO3脱除产物可通过吹灰设备去除 | 脱除效率低于Na基和Ca基吸附剂 | 对ESP除尘效率影响小,延缓空预器堵塞 | 脱除效率低于Na基和Ca基吸附剂 | — | — | ||
| Mg(OH)2 | 不结焦,对催化剂影响小 | 吸附剂用量大,成本高 | — | — | — | — | ||
天然碱 NaHCO3 | 可同时脱除SO2和SO3 | 易结焦,导致催化剂中毒 | 对ESP除尘效率影响小 | 需增加研磨设备,成本高 | 避免NaHSO4在空预器沉积 | 与上游相比脱除效率降低 | ||
SBS Na2CO3 | SCR宽负荷运行,脱出效率高 | 导致催化剂中毒 | 对ESP除尘效率影响小,SO3脱除效率高 | NaHSO4会在空预器和烟道沉积 | 避免NaHSO4在空预器沉积 | NaHSO4会烟道沉积,对飞灰质量影响大 | ||
表2 不同碱性吸附剂的比较
| 吸附剂 | 锅炉内 | SCR | 空预器 | |||||
|---|---|---|---|---|---|---|---|---|
| 优点 | 缺点 | 优点 | 缺点 | 优点 | 缺点 | |||
| 石灰石 | 可同时脱除SO2和SO3 | 导致催化剂中毒,影响ESP除尘效率 | — | — | — | — | ||
| Ca(OH)2 | 可同时脱除SO2和SO3 | 易结焦 | 延缓空预器堵塞,对飞灰质量影响小 | SO3脱除效率低,影响ESP除尘效率 | SO3脱除效率高 | SO3脱除效率低,影响ESP除尘效率 | ||
| MgO | SO3脱除产物可通过吹灰设备去除 | 脱除效率低于Na基和Ca基吸附剂 | 对ESP除尘效率影响小,延缓空预器堵塞 | 脱除效率低于Na基和Ca基吸附剂 | — | — | ||
| Mg(OH)2 | 不结焦,对催化剂影响小 | 吸附剂用量大,成本高 | — | — | — | — | ||
天然碱 NaHCO3 | 可同时脱除SO2和SO3 | 易结焦,导致催化剂中毒 | 对ESP除尘效率影响小 | 需增加研磨设备,成本高 | 避免NaHSO4在空预器沉积 | 与上游相比脱除效率降低 | ||
SBS Na2CO3 | SCR宽负荷运行,脱出效率高 | 导致催化剂中毒 | 对ESP除尘效率影响小,SO3脱除效率高 | NaHSO4会在空预器和烟道沉积 | 避免NaHSO4在空预器沉积 | NaHSO4会烟道沉积,对飞灰质量影响大 | ||
| 1 | 李高磊, 郭沂权, 张世博, 等. 超低排放燃煤电厂SO3生成及控制的试验研究[J]. 中国电机工程学报, 2019, 39(4): 1079-1086. |
| LI G L, GUO Y Q, ZHANG S B, et al. Experimental research on SO3 generation and control in ultra-low emission coal-fired power plant[J]. Proceedings of the CSEE, 2019, 39(4): 1079-1086. | |
| 2 | 束航, 张玉华, 范红梅, 等. SCR脱硝中催化剂表面NH4HSO4生成及分解的原位红外研究[J]. 化工学报, 2015, 66(11): 4460-4468. |
| SHU H, ZHANG Y H, FAN H M, et al. FT-IR study of formation and decomposition of ammonium bisulfates on surface of SCR catalyst for nitrogen removal[J]. CIESC Journal, 2015, 66(11): 4460-4468. | |
| 3 | 唐昊, 李慧, 杨江毅, 等. NH3-SCR工艺中硫酸铵盐的生成与分解机理研究进展[J]. 化工进展, 2018, 37(3): 822-831. |
| TANG H, LI H, YANG J Y, et al. Research progress on the formation and decomposition mechanism of ammonium-sulfate salts in NH3-SCR technology[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 822-831. | |
| 4 | 景有志, 杨丽, 朱淑维, 等. 锰钛系低温选择性催化还原催化剂的抗SO2和抗H2O性能研究进展[J]. 化工进展, 2018, 37(1): 105-111. |
| JING Y Z, YANG L, ZHU S W, et al. Research progress on the SO2 and H2O resistance of Mn-Ti catalysts for low-temperature SCR[J]. Chemical Industry and Engineering Progress, 2018, 37(1): 105-111. | |
| 5 | HUANG R, ZHANG Y, BOZZETTI C, et al. High secondary aerosol contribution to particulate pollution during haze events in China[J]. Nature, 2014, 514: 218-222. |
| 6 | CHENG Y, ZHENG G, WEI C, et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China[J]. Science Advances, 2016, 2(12): e1601530. |
| 7 | 蒋海涛, 蔡兴飞, 付玉玲, 等. 燃煤电厂SO3形成、危害及控制技术[J]. 发电设备, 2013, 27(5): 366-368. |
| JIANG H T, CAI X F, FU Y L, et al. Formation, harms and control technology of SO3 in coal-fired power plants[J]. Power Equipment, 2013, 27(5): 366-368. | |
| 8 | QING M, SU S, WANG L, et al. Effects of H2O and CO2 on the catalytic oxidation property of V/W/Ti catalysts for SO3 generation[J]. Fuel, 2019, 237(1): 545-554. |
| 9 | LI X, WU Z, ZHANG L, et al. An updated acid dew point temperature estimation method for air-firing and oxy-fuel combustion processes[J]. Fuel Processing Technology, 2016, 154: 204-209. |
| 10 | HINDIYARTI L, GLARBORG P, MARSHALL P. Reactions of SO3 with the O/H radical pool under combustion conditions[J]. The Journal of Physical Chemistry A, 2007, 111(19): 3984-3991. |
| 11 | FLEIG D, ANDERSSON K, NORMANN F, et al. SO3 Formation under oxyfuel combustion conditions[J]. Industrial & Engineering Chemistry Research, 2011, 50(14): 8505-8514. |
| 12 | SARBASSOV Y, DUAN L, MANOVIC V, ANTHONY E J. Sulfur trioxide formation/emissions in coal-fired air-and oxy-fuel combustion processes: a review[J]. Greenhouse Gases-Sci Technol., 2018, 8(3): 402-428. |
| 13 | SCHLESINGER R B, ZELIKOFF J T, CHEN L C, et al. Assessment of toxicologic interactions resulting from acute inhalation exposure to sulfuric acid and ozone mixtures[J]. Toxicology and Applied Pharmacology, 1992, 115(2): 183-190. |
| 14 | SARBASSOV Y, DUAN L, JEREMIAS M, et al. SO3 formation and the effect of fly ash in a bubbling fluidised bed under oxy-fuel combustion conditions[J]. Fuel Processing Technology, 2017, 167: 314-321. |
| 15 | GALLOWAY B, PADAK B. Effect of flue gas components on the adsorption of sulfur oxides on CaO[J]. Fuel, 2017, 197: 541-550. |
| 16 | 唐昊, 李文艳, 王琦, 等. 商用选择性催化还原催化剂SO2氧化率控制研究进展[J]. 化工进展, 2017, 36(6): 2143-2149. |
| TANG H, LI W Y, WANG Q, et al. Research progress on the control of SO2 oxidation by commercial SCR catalyst[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2143-2149. | |
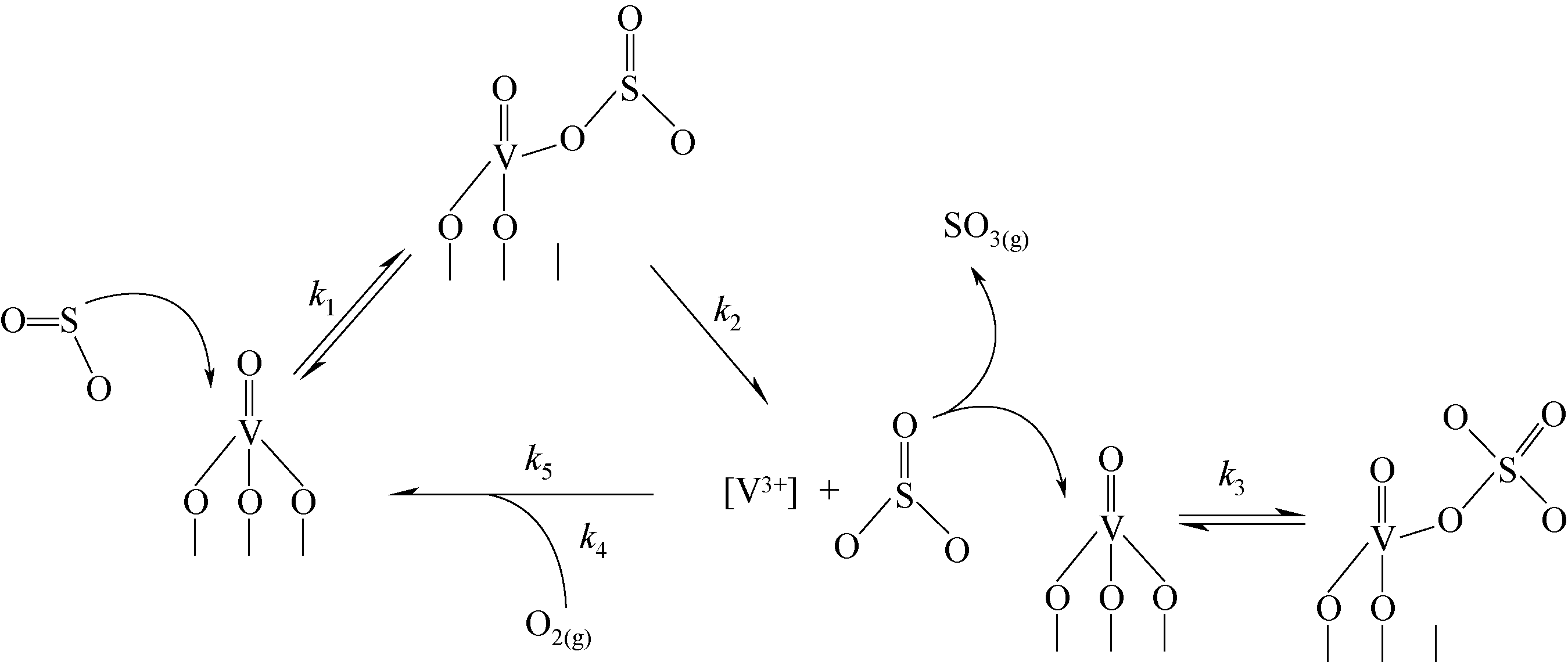
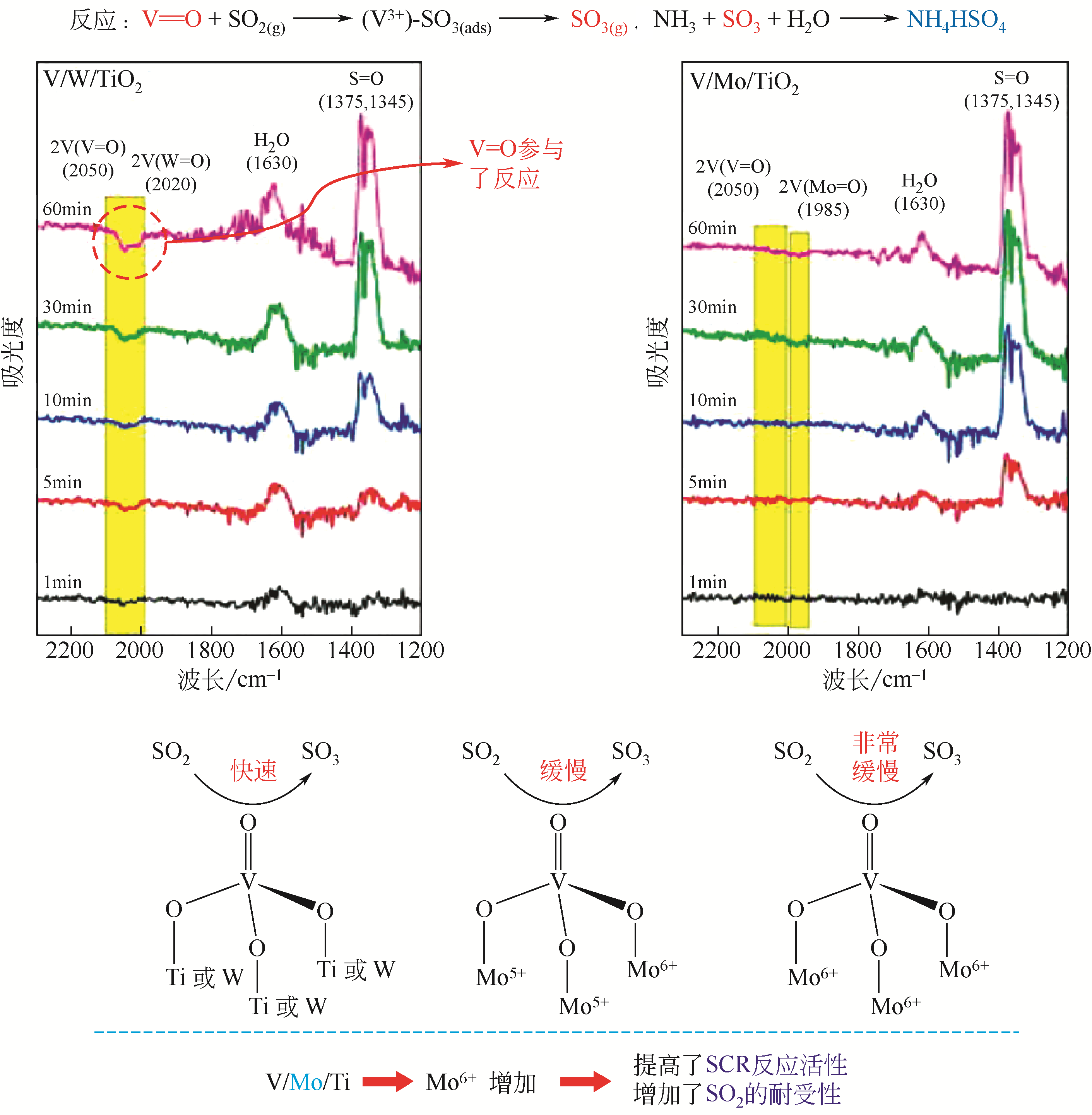
| 17 | QING M, SU S, WANG L, et al. Getting insight into the oxidation of SO2 to SO3 over V2O5-WO3/TiO2 catalysts: reaction mechanism and effects of NO and NH3[J]. Chemical Engineering Journal, 2019, 361: 1215-1224. |
| 18 | KAMATA H, OHARA H, TAKAHASHI K, et al. SO2 oxidation over the V2O5/TiO2 SCR catalyst[J]. Catalysis Letters, 2001, 73(1): 79-83. |
| 19 | JI P, GAO X, DU X, et al. Relationship between the molecular structure of V2O5/TiO2 catalysts and the reactivity of SO2 oxidation[J]. Catalysis Science & Technology, 2016, 6:1187-1194. |
| 20 | DUNN J P, KOPPULA P R, STENGER H G, et al. Oxidation of sulfur dioxide to sulfur trioxide over supported vanadia catalysts[J]. Applied Catalysis B: Environmental, 1998, 19(2): 103-117. |
| 21 | XIONG J, LI Y, LIN Y, et al. Formation of sulfur trioxide during the SCR of NO with NH3 over a V2O5/TiO2 catalyst[J]. RSC Adv., 2019, 9: 38952-38961. |
| 22 | ZHENG C, WANG Y, LIU Y, et al. Formation, transformation, measurement, and control of SO3 in coal-fired power plants[J]. Fuel, 2019, 241: 327-346. |
| 23 | 杨用龙, 苏秋凤, 张杨, 等. 燃煤电站典型超低排放工艺的SO3脱除性能及排放特性[J]. 中国电机工程学报, 2019, 39(10): 2962-2969. |
| YANG Y L, SU Q F, ZHANG Y, et al. Removal performance and emission characteristics of SO3 by typical ultra-low emission technologies in coal-fired power plants[J]. Proceedings of the CSEE, 2019, 39(10): 2962-2969. | |
| 24 | RHUDY R. Sulfuric acid removal process evaluation: short-term results[R]. California: Electric Power Research Institute, 2001. |
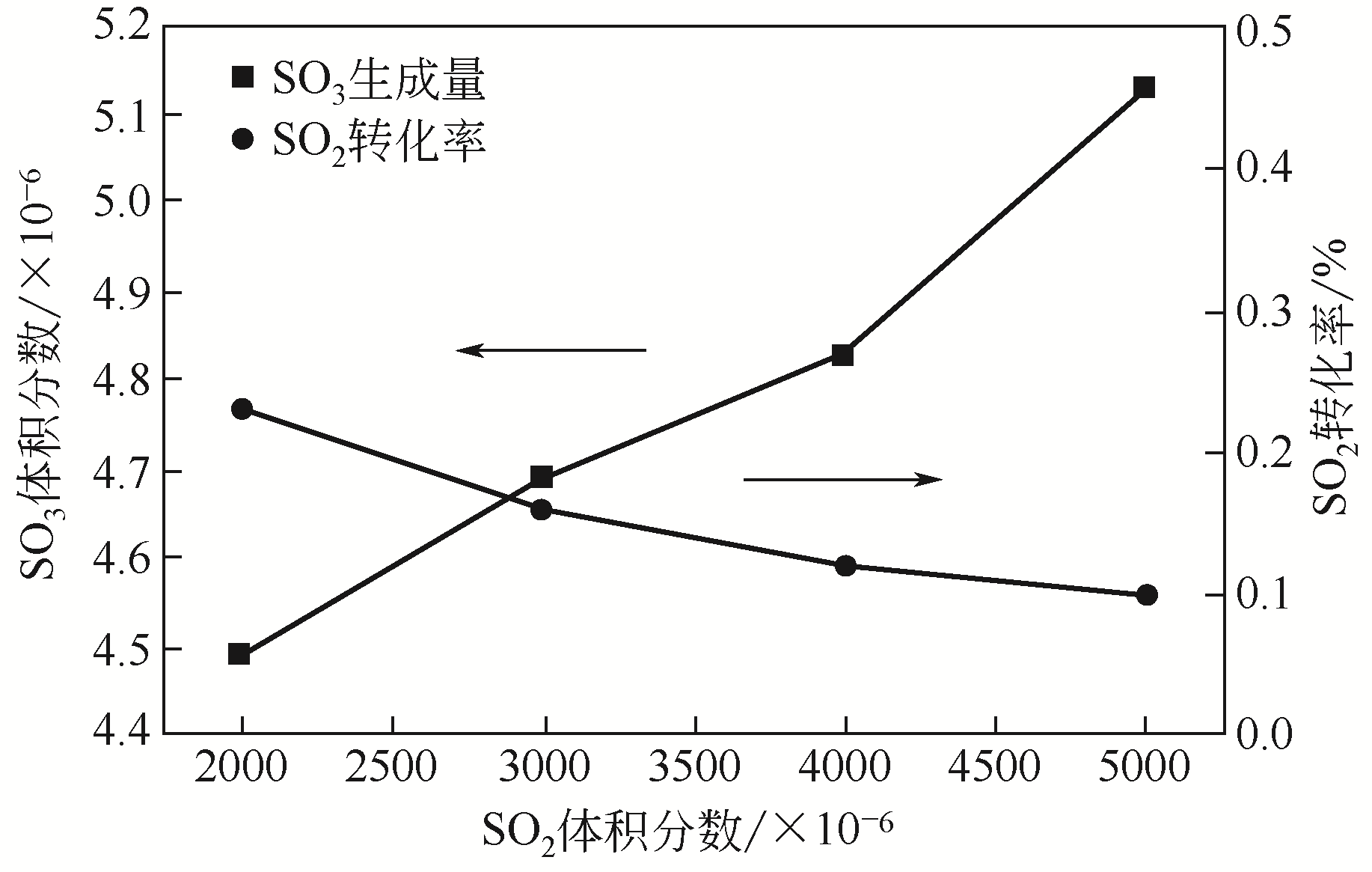
| 25 | 卿梦霞. 燃煤烟气SO3与硫酸氢铵生成机理研究[D]. 武汉: 华中科技大学, 2019. |
| QING M X. Study on generation mechanism of SO3 and ammounium hydrogen sulfate in coal-fired flue gas[D]. Wuhan: Huazhong University of Science and Technology, 2019. | |
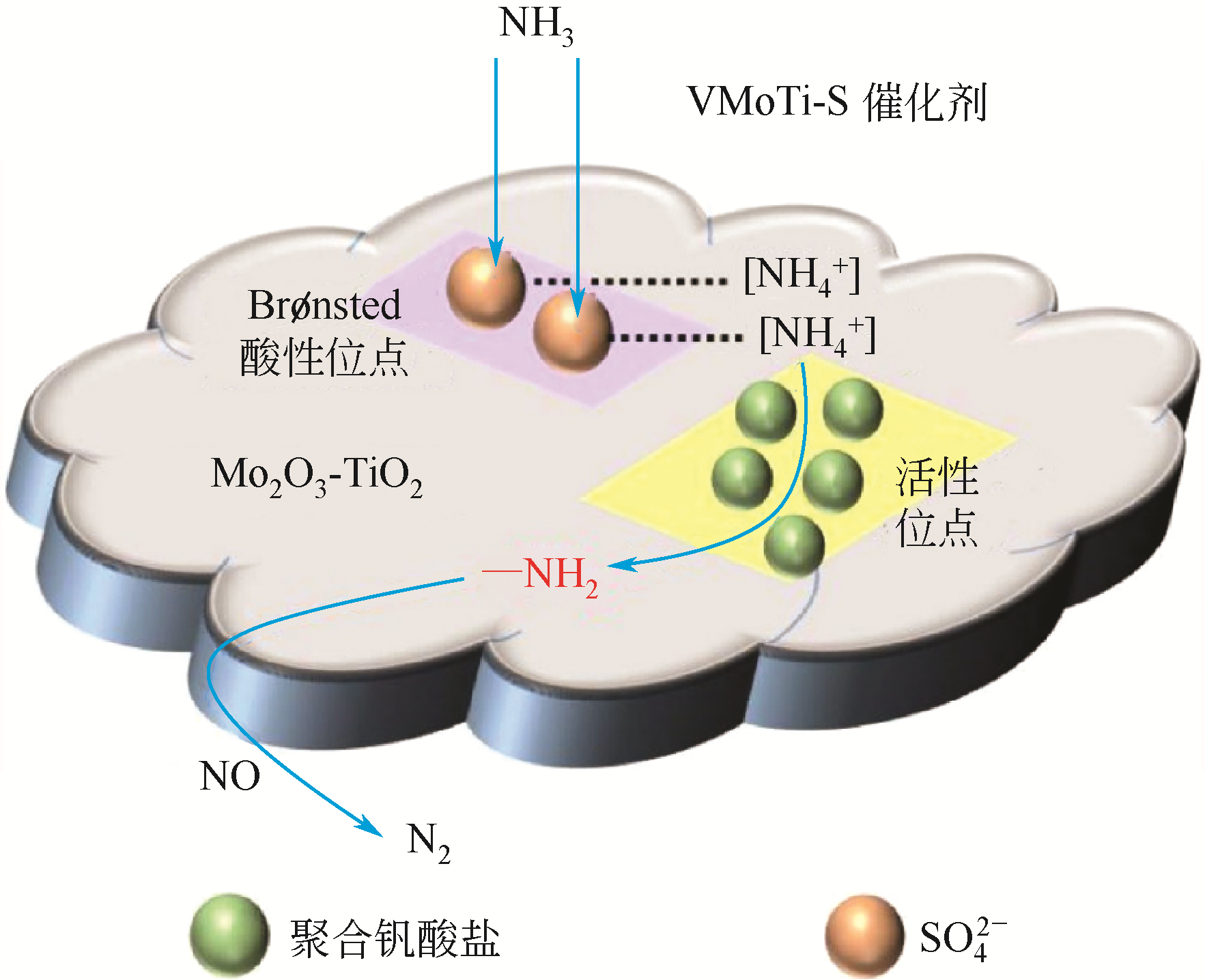
| 26 | XI Y, OTTINGER N A, LIU Z G. New insights into sulfur poisoning on a vanadia SCR catalyst under simulated diesel engine operating conditions[J]. Applied Catalysis B: Environmental, 2014, 160/161: 1-9. |
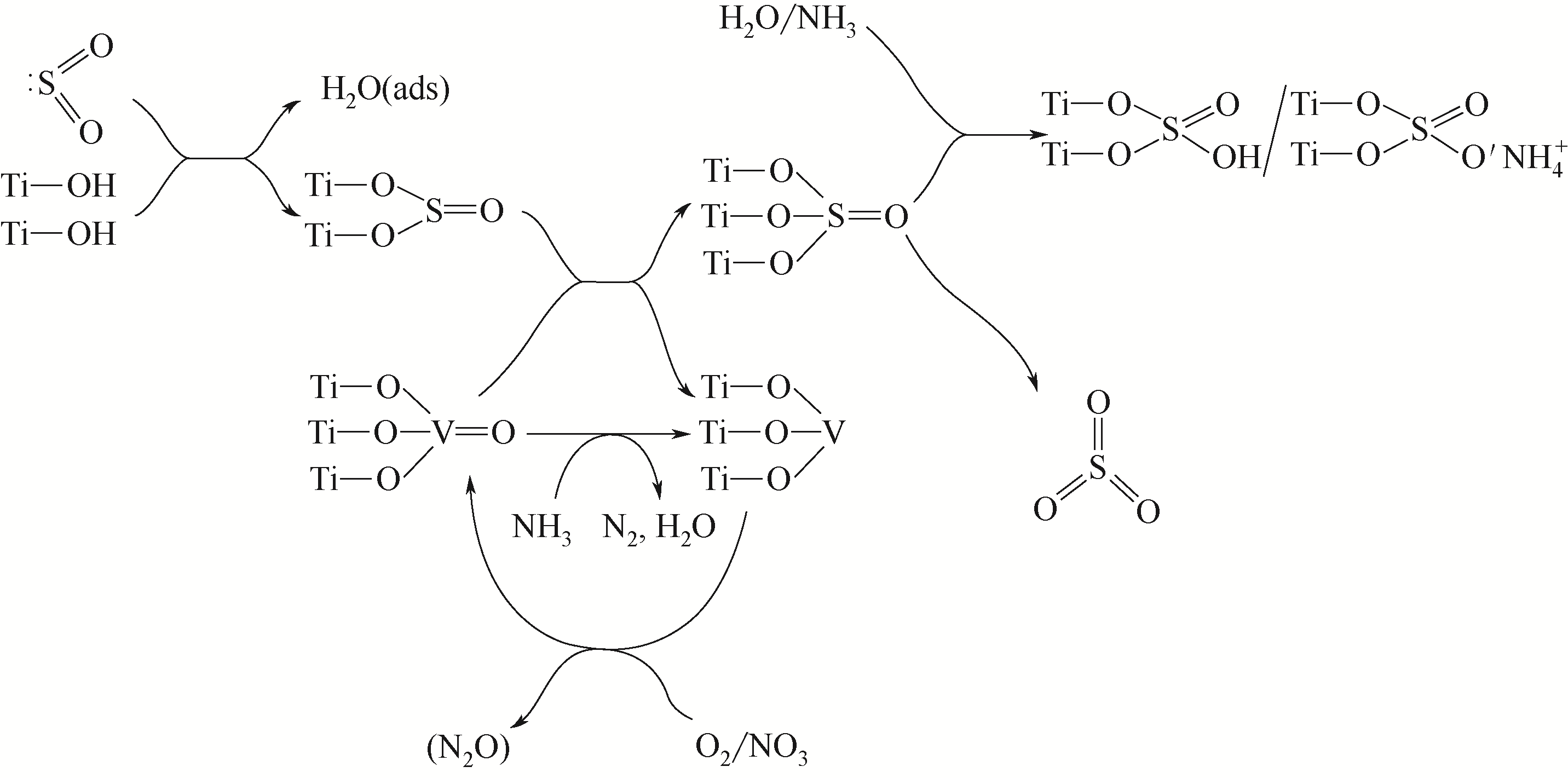
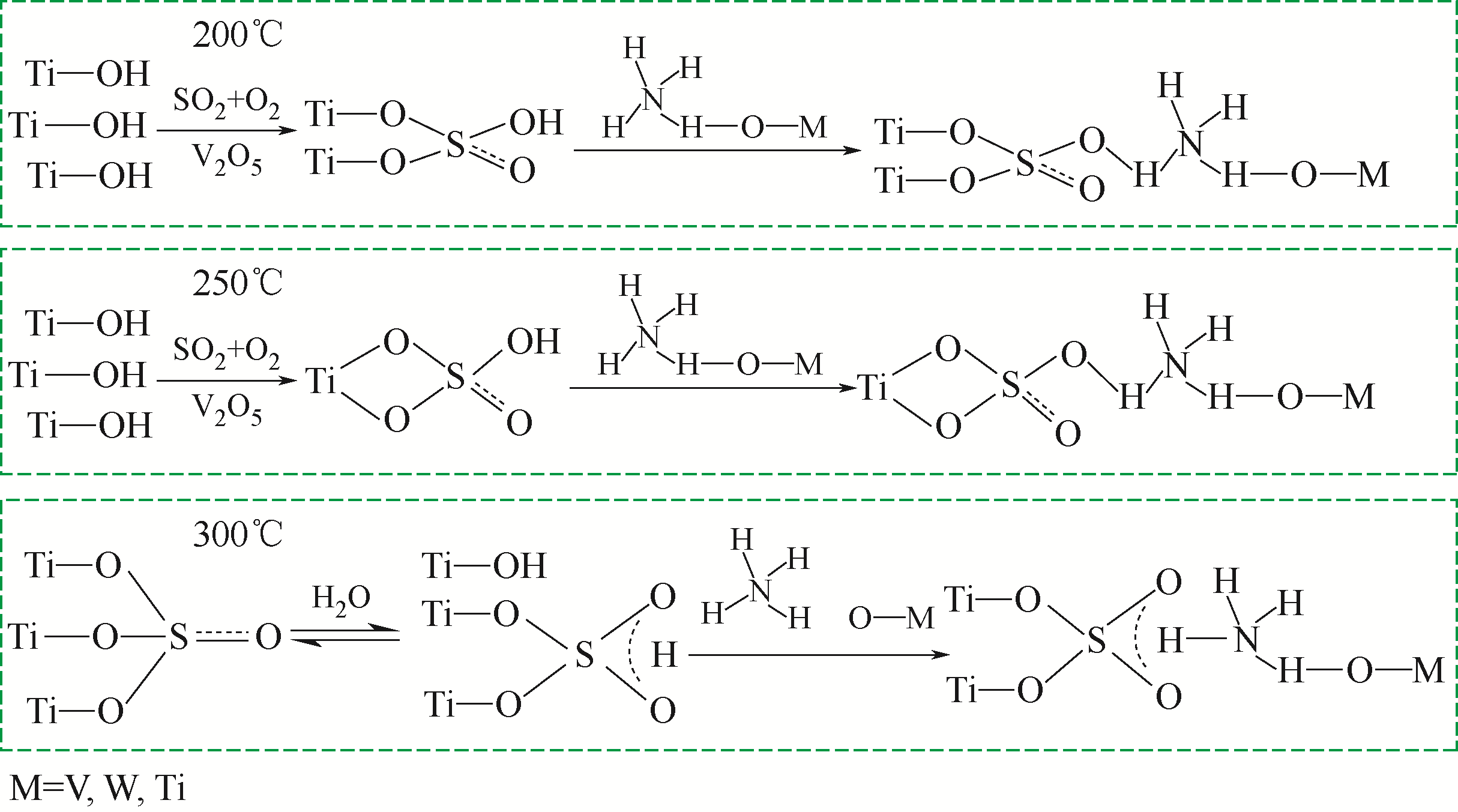
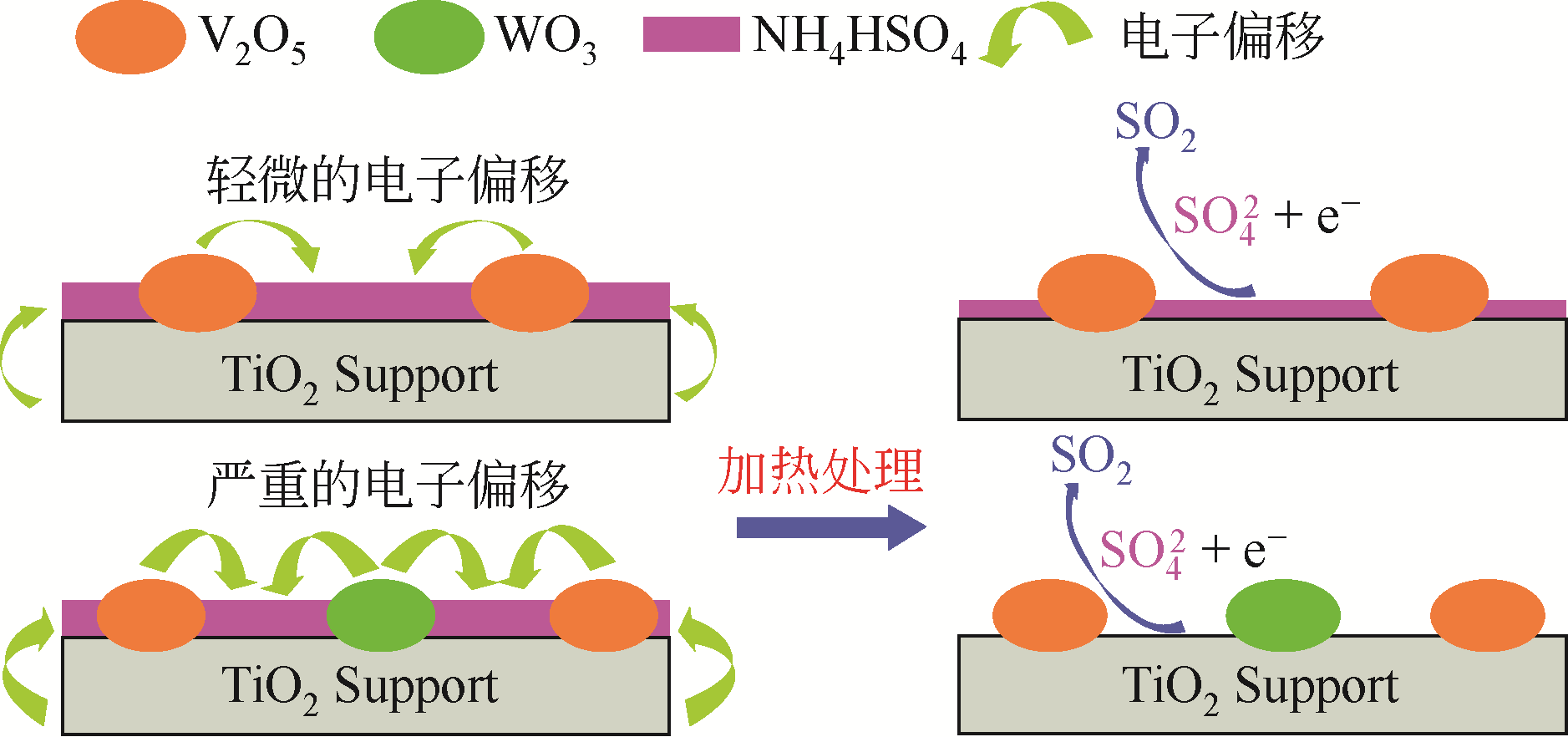
| 27 | SHI Y, SHU H, ZHANG Y, et al. Formation and decomposition of NH4HSO4 during selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts[J]. Fuel Processing Technology, 2016, 150: 141-147. |
| 28 | LI C X, SHEN M Q, YU T, et al. The mechanism of ammonium bisulfate formation and decomposition over V/WTi catalysts for NH3-selective catalytic reduction at various temperatures[J]. Physical Chemistry Chemical Physics, 2017, 19(23): 15194-15206. |
| 29 | WANG X, DU X, LIU S, et al. Understanding the deposition and reaction mechanism of ammonium bisulfate on a vanadia SCR catalyst: a combined DFT and experimental study[J]. Applied Catalysis B: Environmental, 2019, 260: 118168. |
| 30 | CHEN P, WANG Z, CHANG J, et al. Experimental study of the reactivity of Ca-based matters with SO3[C]//2011 Asia-Pacific Power and Energy Engineering Conference, IEEE, 2011. |
| 31 | 陈晓露, 赵钦新, 鲍颖群, 等. SO3脱除技术实验研究[J]. 动力工程学报, 2014, 34(12): 966-971. |
| CHEN X L, ZHAO Q X, BAO Y Q, et al. Experimental research on SO3 removal[J]. Journal of Chinese Society of Power Engineering, 2014, 34(12): 966-971. | |
| 32 | WILHELM J H. SBS injection fights off SO3[J]. Power Eng. Int., 2014, 12(12): 28-30. |
| 33 | MORETTI A L, TRISCORI R J, RITZENTHALER D P. A system approach to SO3 mitigation[C]//Combined Power Plant Air Pollutant Control Mega Symposium, Baltimore, Maryland. USA, 2006. |
| 34 | 高智溥, 胡冬, 张志刚, 等. 碱性吸附剂脱除 SO3 技术在大型燃煤机组中的应用[J]. 中国电力, 2017, 50(7): 102-108. |
| GAO Z B, HU D, ZHANG Z G, et al. Application of alkaline adsorbent removal SO3 technology in large coal-fired units[J]. Electric Power, 2017, 50(7): 102-108. | |
| 35 | BLYTHE G, DOMBROWSKI K. SO3 mitigation guide update[R]. Palo Alto, California, USA: Electric Power Research Institute, 2004: 31-43. |
| 36 | LIU Z, WOO S I. Recent advances in catalytic DeNOx science and technology[J]. Catal. Rev.: Sci. Eng., 2006, 48(1): 43. |
| 37 | BALTIN G, KÖSER H, WENDLANDT K. Reactive desorption of sulfuric acid from ammonium sulfate loaded V2O5-WO3/TiO2 DeNOx‐catalysts[J]. Chemie Ingenieur Technik, 2001, 73(6): 605. |
| 38 | YE D, QU R, SONG H, et al. Investigation of the promotion effect of WO3 on the decomposition and reactivity of NH4HSO4 with NO on V2O5-WO3/TiO2 SCR catalysts[J]. RSC Advances, 2016, 6: 55584-55592. |
| 39 | QIAO J, WANG N, WANG Z, et al. Porous bimetallic Mn2Co1Ox catalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOx with NH3[J]. Catal. Commun., 2015, 72: 111-115. |
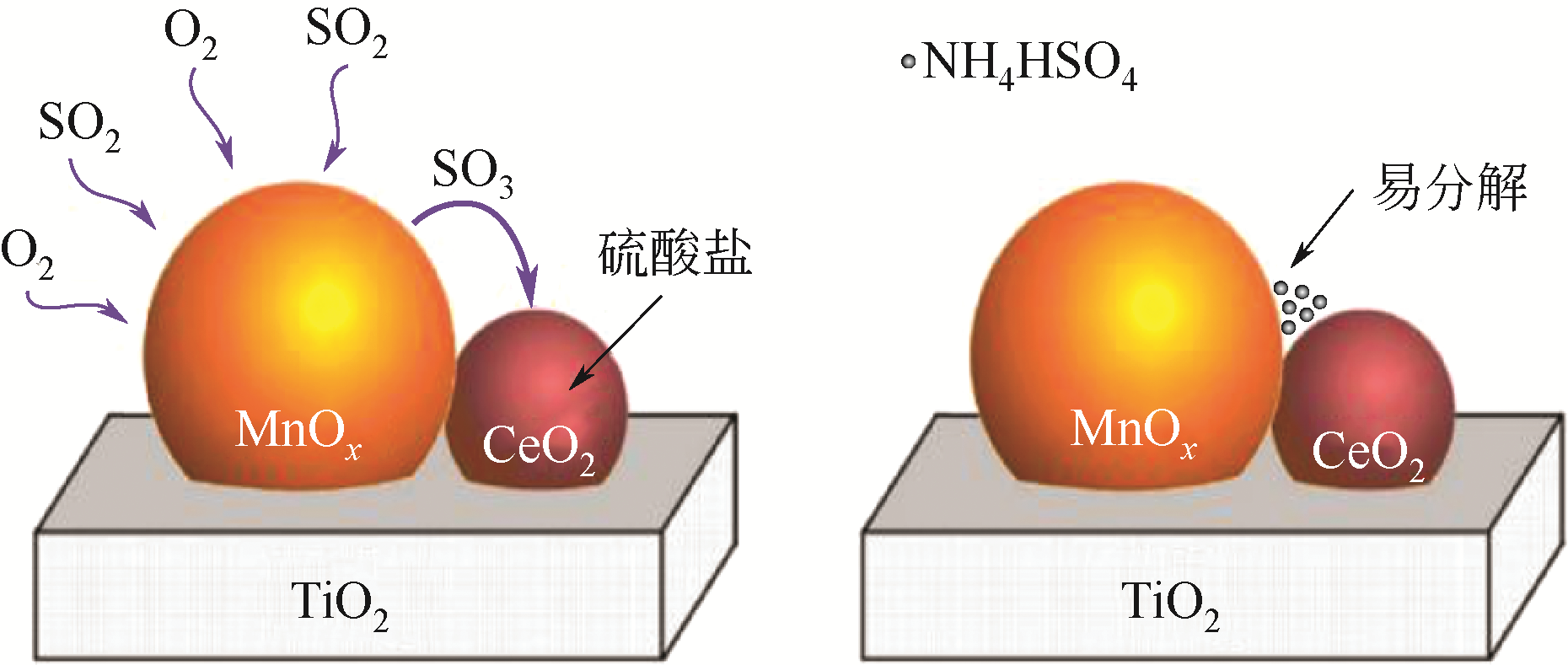
| 40 | WANG F, SHEN B, ZHU S, et al. Promotion of Fe and Co doped Mn-Ce/TiO2 catalysts for low temperature NH3-SCR with SO2 tolerance[J]. Fuel, 2019, 249(1): 54-60. |
| 41 | LIU Z, YI Y, ZHANG S, et al. Selective catalytic reduction of NOx with NH3 over Mn-Ce mixed oxide catalyst at low temperatures[J]. Catalysis Today, 2013, 216: 76-81. |
| 42 | JIN R, LIU Y, WANG Y, et al. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature[J]. Applied Catalysis B: Environmental, 2014, 148/149: 582-588. |
| 43 | KWON D W, PARK K H, HONG S C. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum[J]. Chemical Engineering Journal, 2016, 284: 315-324. |
| 44 | XU Y, WU X, LIN Q, et al. SO2 promoted V2O5-MoO3/TiO2 catalyst for NH3-SCR of NO, at low temperatures[J]. Applied Catalysis A: General, 2019, 570: 42-50. |
| [1] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [2] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [3] | 李佳, 樊星, 陈莉, 李坚. 硝酸生产尾气中NO x 和N2O联合脱除技术研究进展[J]. 化工进展, 2023, 42(7): 3770-3779. |
| [4] | 刘亮, 王朝曦, 李鑫龙, 张高山, 王守阳, 张林林, 陆畅, 卿梦霞. 钒钛系脱硝催化剂抗硫酸氢铵中毒改进措施研究进展[J]. 化工进展, 2023, 42(1): 215-225. |
| [5] | 杨希刚, 陈国庆, 黄林滨, 古世军, 李昌松, 张勇, 金保昇. 尿素法SNCR对大型电站煤粉锅炉运行影响的工业试验[J]. 化工进展, 2022, 41(7): 3573-3581. |
| [6] | 龙红明, 丁龙, 钱立新, 春铁军, 张洪亮, 余正伟. 烧结烟气中NO x 和二 英的减排现状及发展趋势[J]. 化工进展, 2022, 41(7): 3865-3876. 英的减排现状及发展趋势[J]. 化工进展, 2022, 41(7): 3865-3876. |
| [7] | 韩徳琳, 李丹, 王天天, 张海, 张扬, 王随林. 位移钝体稳燃的旋流预混燃烧污染物生成特性[J]. 化工进展, 2022, 41(6): 2915-2923. |
| [8] | 王新宇, 黄亚继, 徐力刚, 李志远, 李偲, 刘晓东. 调节同层二次风以缓解双切圆锅炉高温腐蚀的数值模拟[J]. 化工进展, 2022, 41(5): 2292-2300. |
| [9] | 谭潇, 齐随涛, 周一鸣, 石利斌, 程光旭, 伊春海, 杨伯伦. 非热等离子体协同锰铁双金属催化剂直接催化还原NO[J]. 化工进展, 2022, 41(11): 5850-5857. |
| [10] | 孔祥旭, 张玮, 胡恒, 于嘉朋, 张坤, 徐娜. 工业制SO3气体浓度实时检测及多元非线性回归建模[J]. 化工进展, 2022, 41(11): 5737-5745. |
| [11] | 杨景瑞, 王莹, 陈虎, 吕永康. 氧浓度对调节NO x 氧化度协同CABR法脱硝性能及菌群结构的影响[J]. 化工进展, 2022, 41(11): 6139-6148. |
| [12] | 刘月华, 上官炬, 刘守军, 杨颂, 杜文广. 铁镍复合助剂对煤热解过程中氮迁移规律的影响[J]. 化工进展, 2021, 40(1): 164-172. |
| [13] | 管静, 兰喜龙, 孙红, 柳志刚, 乔彤. 掺杂元素对Mn基催化剂SCR性能及抗硫性能的影响[J]. 化工进展, 2020, 39(6): 2440-2446. |
| [14] | 杨建成,张芹,沈伯雄,袁世磊,王诗宁. 改性柱撑黏土用于烟气脱硝的研究进展[J]. 化工进展, 2020, 39(4): 1493-1499. |
| [15] | 何川, 宋玉宝, 马云龙, 王乐乐, 卞子君. 钙镁氧化物及氢氧化物脱除SO3协同防治催化剂低温失活[J]. 化工进展, 2020, 39(11): 4619-4624. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||