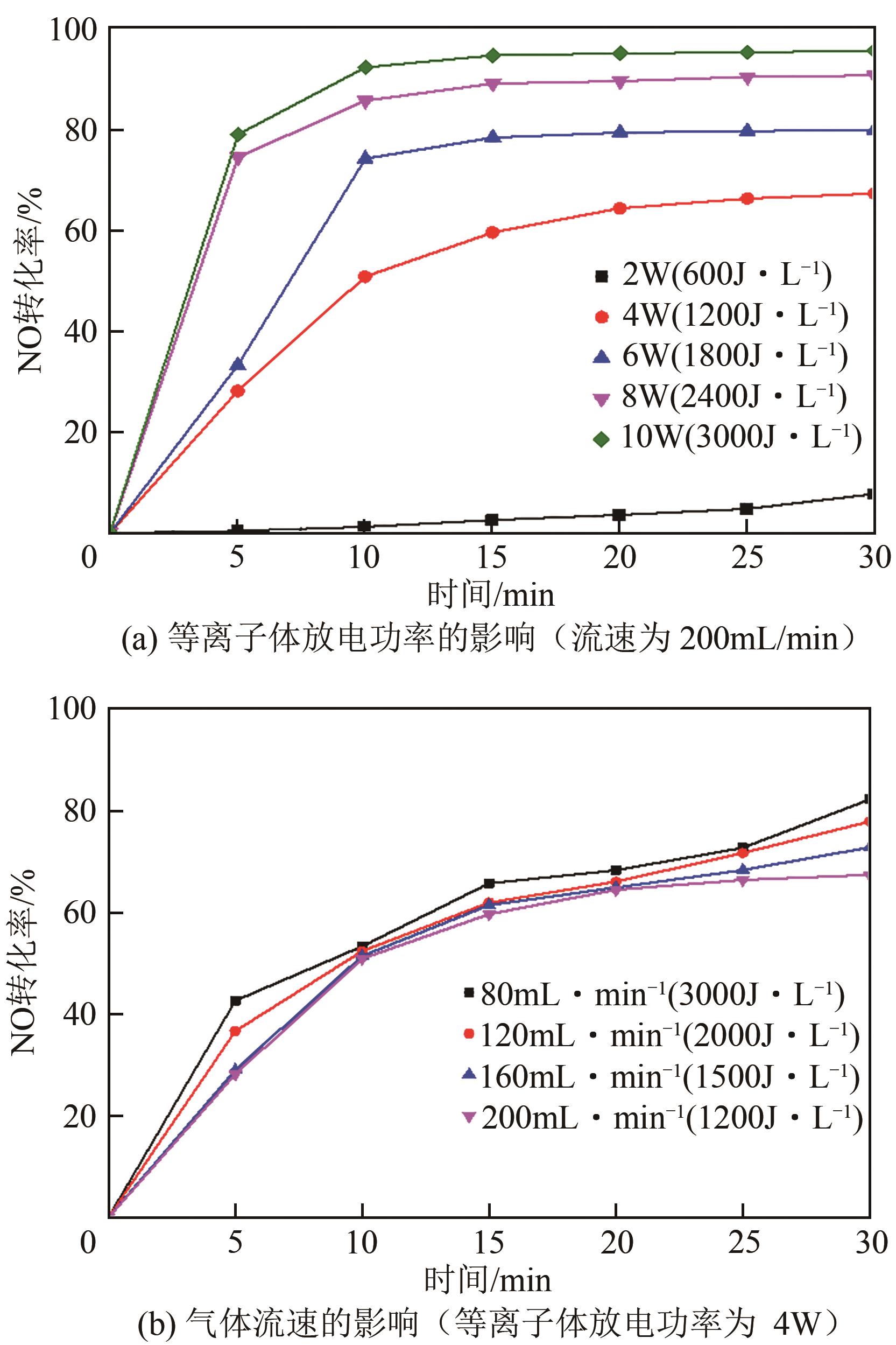
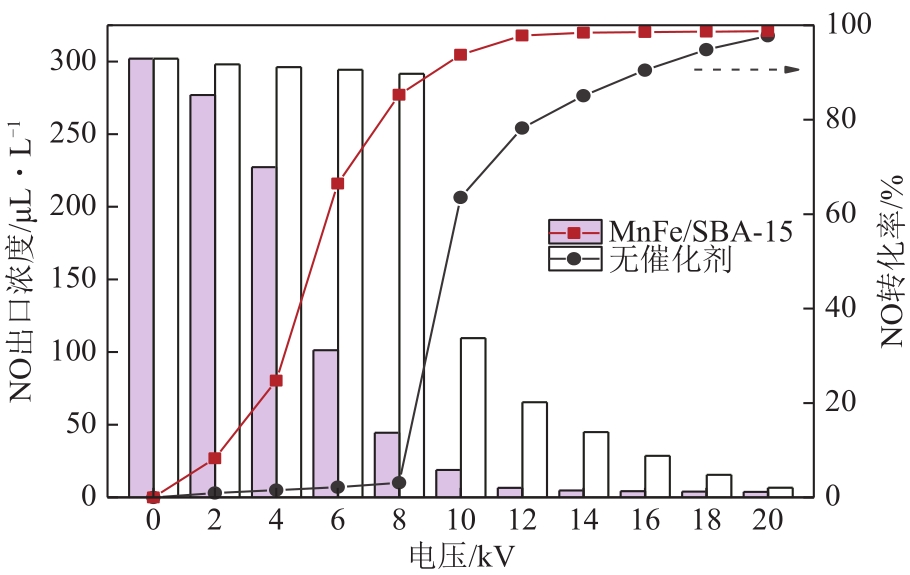
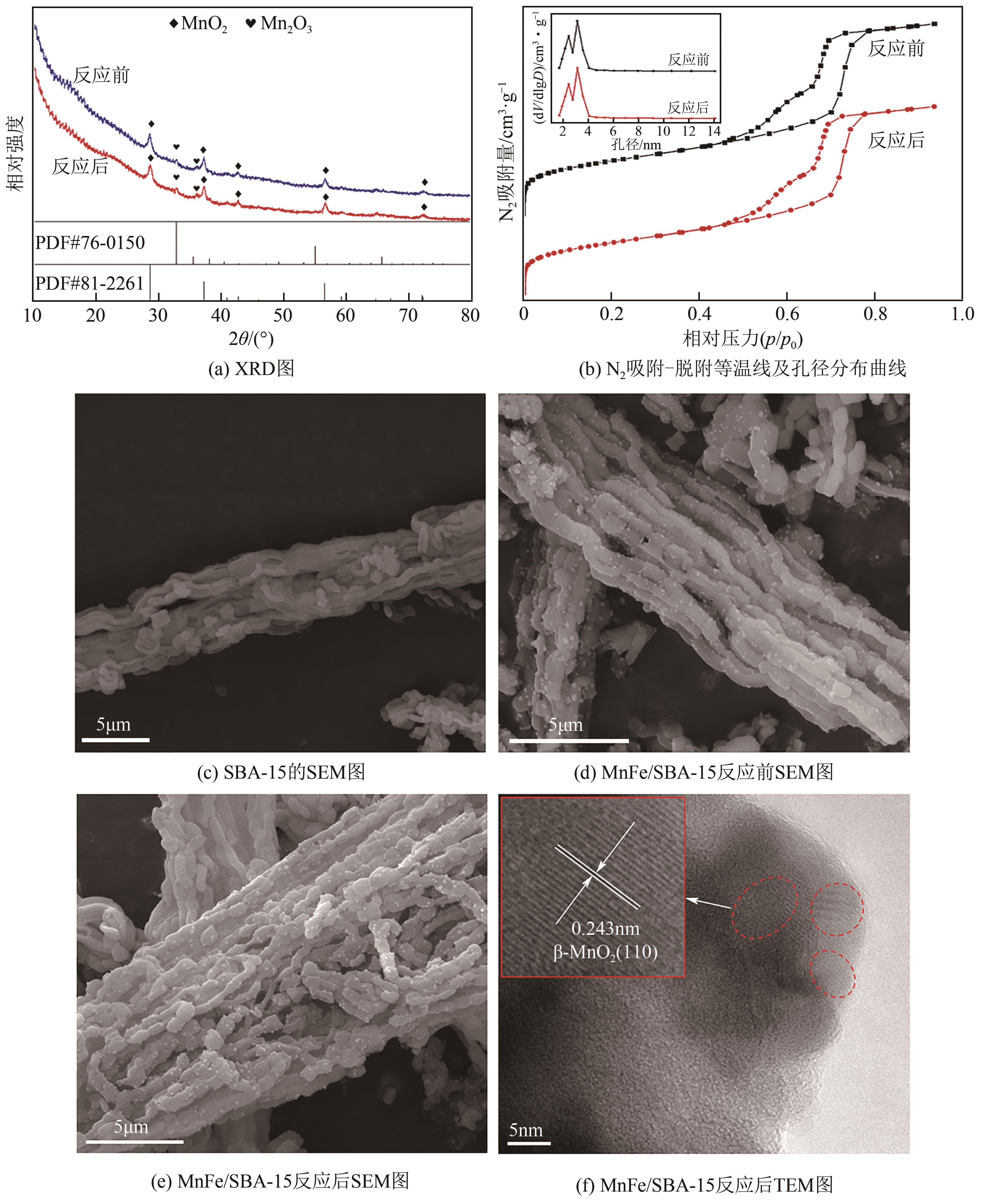
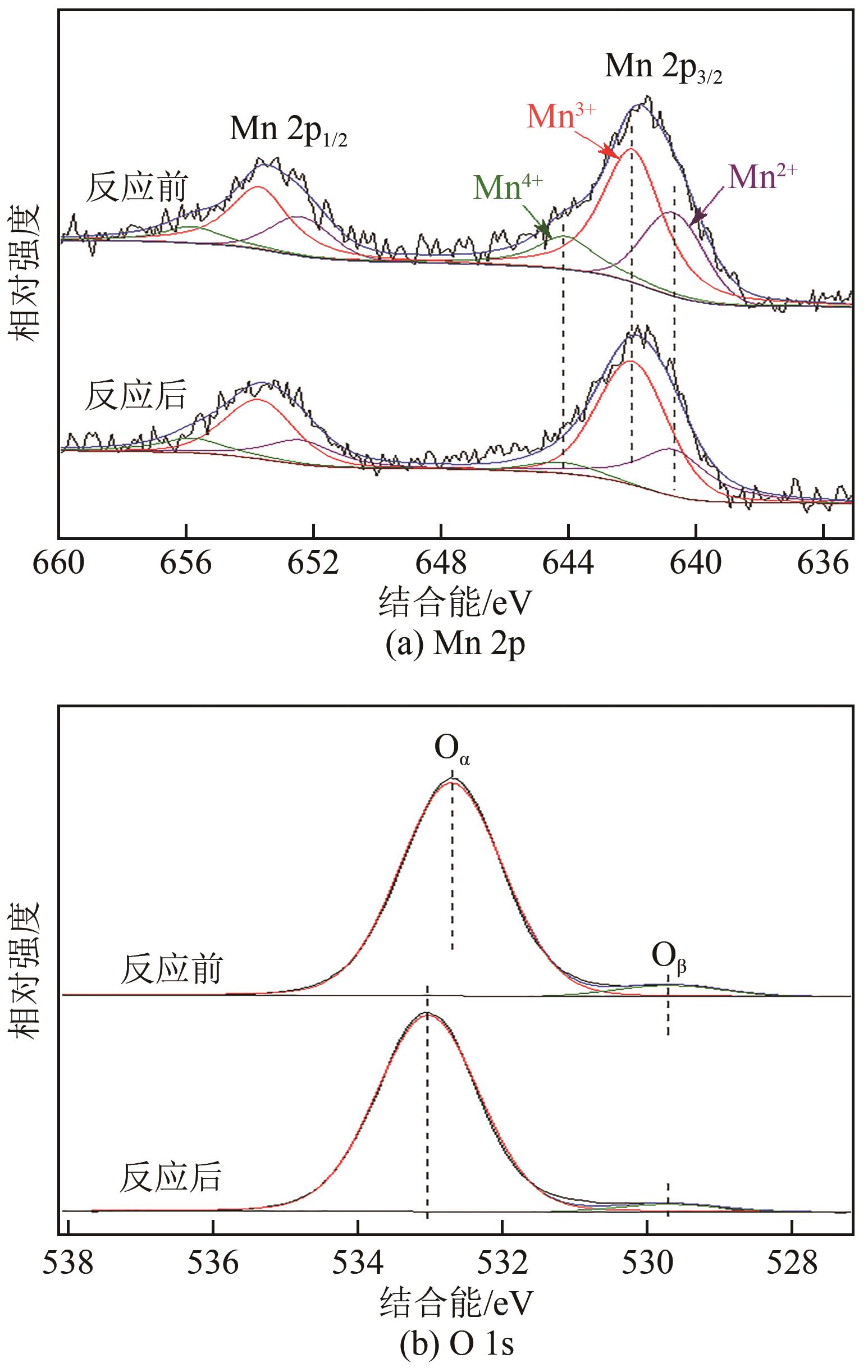
| 1 |
HU Hang, CAI Sixiang, LI Hongrui, et al. Mechanistic aspects of deNO x processing over TiO2 supported Co-Mn oxide catalysts: structure-activity relationships and in situ DRIFTs analysis[J]. ACS Catalysis, 2015, 5(10): 6069-6077.
|
| 2 |
BILKOVA T, FRIDRICHOVA D, PACULTOVA K, et al. Haneda, reaction mechanism of NO direct decomposition over K-promoted Co-Mn-Al mixed oxides-DRIFTS, TPD and transient state studies[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 120: 257-266.
|
| 3 |
GAN Yanling, CUI Suping, MA Xiaoyu, et al. Preparation of Cu-Al/SiO2 porous material and its effect on NO decomposition in a cement kiln[J]. Materials, 2020, 13(1): 1-11.
|
| 4 |
卞俊杰, 王万圆, 满恒孝, 等. BiOX(Cl, Br, I)/Bi2WO6异质结型复合光催化剂用于高浓度氮氧化物的脱除[J]. 化工进展, 2021, 40(11): 6094-6101.
|
|
BIAN Junjie, WANG Wanyuan, MAN Hengxiao, et al. BiOX(Cl, Br, I)/Bi2WO6 heterojunction composites as photocatalysts for high concentration NO removal[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6094-6101.
|
| 5 |
杨永利, 徐东耀, 晁春艳, 等. 负载型Mn基低温NH3-SCR脱硝催化剂研究综述[J]. 化工进展, 2016, 35(4): 1094-1100.
|
|
YANG Yongli, XU Dongyao, CHAO Chunyan, et al. Research advance review on supported Mn-based catalysts at low-temperature selective catalytic reduction of NO x with NH3 [J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1094-1100.
|
| 6 |
ANDANA T, RAPPE K G, GAO Feng, et al. Recent advances in hybrid metal oxide-zeolite catalysts for low-temperature selective catalytic reduction of NO x by ammonia[J]. Applied Catalysis B:Environmental, 2021 291: 1-25.
|
| 7 |
XU Junqiang, CHEN Guorong, GUO Fang, et al. Development of wide-temperature vanadium-based catalysts for selective catalytic reducing of NO x with ammonia: review[J]. Chemical Engineering Journal, 2018, 353: 507-518.
|
| 8 |
LIU Jie, KANG Lin, LI Hongrui, et al. Mn-Fe bi-metal oxides in situ created on metal wire mesh as monolith catalysts for selective catalytic reduction of NO with NH3 [J]. RSC Advances, 2017, 7(64): 40444-40451.
|
| 9 |
YU Rui, ZHAO Zhenchao, SHI Chuan, et al. Insight into the synergic effect of Fe-SSZ-13 zeolite and FeMnTiZrO x catalyst with enhanced reactivity in NH3-SCR of NO x [J]. J. Phys. Chem. C, 2019, 123(4): 2216-2227.
|
| 10 |
戴豪波, 杜凯敏, 郑渭建, 等. NH3-SCR脱硝催化剂研究进展[J]. 现代化工, 2021, 41(5): 40-44, 48.
|
|
DAI Haobo, DU Kaimin, ZHENG Weijian, et al. Research progress on NH3-SCR catalysts[J]. Modern Chemical Industry, 2021, 41(5): 40-44, 48.
|
| 11 |
CHEN Liang, LI Junhua, GE Maofa. The poisoning effect of alkali metals doping over nano V2O5-WO3/TiO2 catalysts on selective catalytic reduction of NO x by NH3 [J]. Chemical Engineering Journal, 2011, 170(2-3): 531-537.
|
| 12 |
高梓寒, 穆杨, 杨润农, 等. 双金属交换的Cu-Mn/SSZ-39催化剂的NH3-SCR脱硝性能[J]. 精细化工, 2021, 38(8): 1621-1627, 1692.
|
|
GAO Zihan, MU Yang, YANG Runnong, et al. NH3-SCR denitrification performance over bimetal exchanged Cu-Mn/SSZ-39 catalyst[J]. Fine Chemicals, 2021, 38(8): 1621-1627, 1692.
|
| 13 |
CAN F, COURTOIS X, ROYER S, et al. An overview of the production and use of ammonia in NSR plus SCR coupled system for NO x reduction from lean exhaust gas[J]. Catalysis Today, 2012, 197(1): 144-154.
|
| 14 |
钟洪玲, 陈鸥, 王洪亮, 等. 超低排放下燃煤电厂氨排放特征[J]. 环境科学研究, 2021, 34(1): 124-131.
|
|
ZHONG Hongling, CHEN Ou, WANG Hongliang, et al. Characteristics of ammonia emission in flue gas from ultra-low emission coal-fired power plants[J]. Research of Environmental Sciences, 2021, 34(1): 124-131.
|
| 15 |
GLICK H S, KLEIN J J, SQUIRE W. Single-pulse shock tube studies of the kinetics of the reaction N2+O2 ⇌ 2NO between 2000—3000K[J]. Journal of Chemical Physics, 1957, 27(4): 850-857.
|
| 16 |
DAMMA D, BONINGARI T, ETTIREDDY P R, et al. Direct decomposition of NO x over TiO2 supported transition metal oxides at low temperatures[J]. Industrial & Engineering Chemistry Research, 2018, 57(49): 16615-16621.
|
| 17 |
李海龙, 肖萍, 王涛, 等. NO分解催化剂的研究进展[J]. 中国科学: 化学, 2014, 44(12): 1951-1965.
|
|
LI Hailong, XIAO Ping, WANG Tao, et al. Recent progress on catalysts used for NO decomposition[J]. Scientia Sinica Chimica, 2014, 44(12): 1951-1965.
|
| 18 |
XU Wentao, ZHOU Jichen, YOU Zhimin, et al. Microwave irradiation coupled with physically mixed MeO x (Me=Mn, Ni) and Cu-ZSM-5 catalysts for the direct decomposition of nitric oxide under excess oxygen[J]. ChemCatChem, 2015, 7(3): 450-458.
|
| 19 |
REDDY G K, PECK T C, ROBERTS C A. CeO2-M x O y (M=Fe, Co, Ni, and Cu)-based oxides for direct NO decomposition[J]. J. Phys. Chem. C, 2019, 123(47): 28695-28706.
|
| 20 |
PECK T C, ROBERTS C A, REDDY G K. Contrasting effects of potassium addition on M3O4(M=Co, Fe, and Mn) oxides during direct NO decomposition catalysis[J]. Catalysts, 2020, 10(5).
|
| 21 |
VANDENBROUCKE Arne M, MORENT Rino, DE GEYTER Nathalie, et al. Non-thermal plasmas for non-catalytic and catalytic VOC abatement[J]. Journal of Hazardous Materials, 2011, 195: 30-54.
|
| 22 |
KIM G T, SEO B H, LEE W J, et al. Effects of applying non-thermal plasma on combustion stability and emissions of NO x and CO in a model gas turbine combustor[J]. Fuel, 2017, 194: 321-328.
|
| 23 |
ZHANG Zhaoshun, CROCKER Mark, CHEN Bingbing, et al. Non-thermal plasma-assisted NO x storage and reduction over cobalt-containing LNT catalysts[J]. Catalysis Today, 2015, 258: 386-395.
|
| 24 |
ZHANG Zhaoshun, SHI Chuan, BAI Zhifeng, et al. Low-temperature H2-plasma-assisted NO x storage and reduction over a combined Pt/Ba/Al and LaMnFe catalyst[J]. Catalysis Science & Technology, 2017, 7(1): 145-158.
|
| 25 |
樊星, 亢思静, 李坚, 等. 非热等离子体强化尿素-SCR脱除NO x [J]. 环境工程, 2019, 37(3): 118-123.
|
|
FAN Xing, KANG Sijing, LI Jian, et al. Removal of NO x by non-thermal plasma enhanced urea-SCR[J]. Environmental Engineering, 2019, 37(3): 118-123.
|
| 26 |
Masaoki OKU, HIROKAWA Kichinosuke, IKEDA Shigero. X-ray photoelectron spectroscopy of manganese-oxygen systems[J]. Journal of Electron Spectroscopy and Related Phenomena, 1975, 7(5): 465-473.
|
| 27 |
ANSELL R O, DICKINSON T, POVEY A F. X-ray photoelectron spectroscopic study of films on colored stainless-steel and colored nilomag alloy-771[J]. Corrosion Science, 1978, 18(3): 245-256.
|
| 28 |
ZHANG Lei, SHI Liyi, HUANG Lei, et al. Rational design of high-performance deNO x catalysts based on Mn x Co3- x O4 nanocages derived from metal-organic frameworks[J]. ACS Catalysis, 2014, 4(6): 1753-1763.
|
| 29 |
焦金珍, 李时卉, 黄碧纯. 石墨烯负载MnO x 催化剂的制备及其低温NH3-SCR活性[J]. 物理化学学报, 2015, 31(07): 1383-1390.
|
|
JIAO Jinzhen, LI Shihui, HUANG Bichun. Preparation of manganese oxides supported on graphene catalysts and their activity in low-temperature NH3-SCR[J]. Acta Physico-Chimica Sinica, 2015, 31(7): 1383-1390.
|
 ), 齐随涛(
), 齐随涛( ), 周一鸣, 石利斌, 程光旭, 伊春海, 杨伯伦
), 周一鸣, 石利斌, 程光旭, 伊春海, 杨伯伦
 ), QI Suitao(
), QI Suitao( ), ZHOU Yiming, SHI Libin, CHENG Guangxu, YI Chunhai, YANG Bolun
), ZHOU Yiming, SHI Libin, CHENG Guangxu, YI Chunhai, YANG Bolun