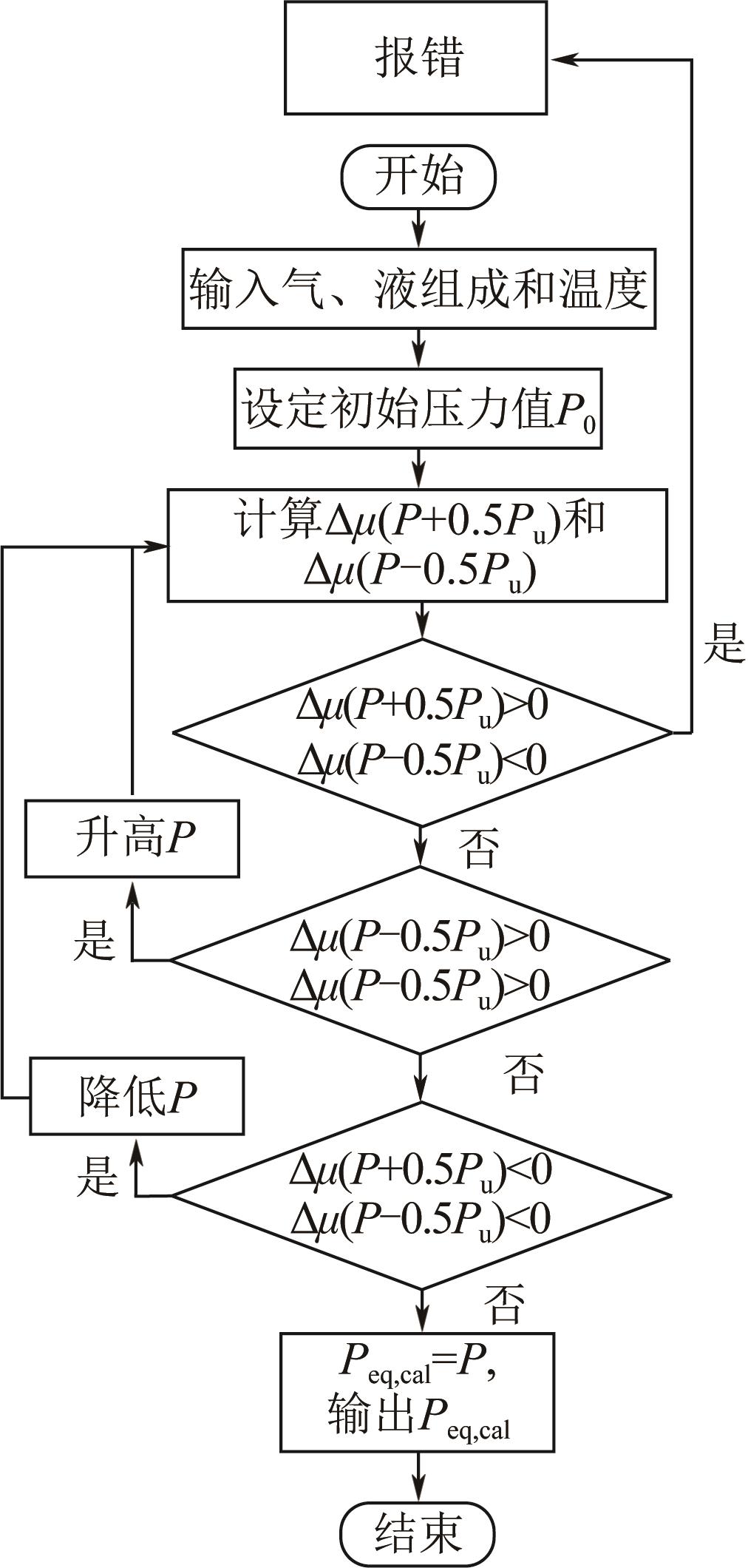
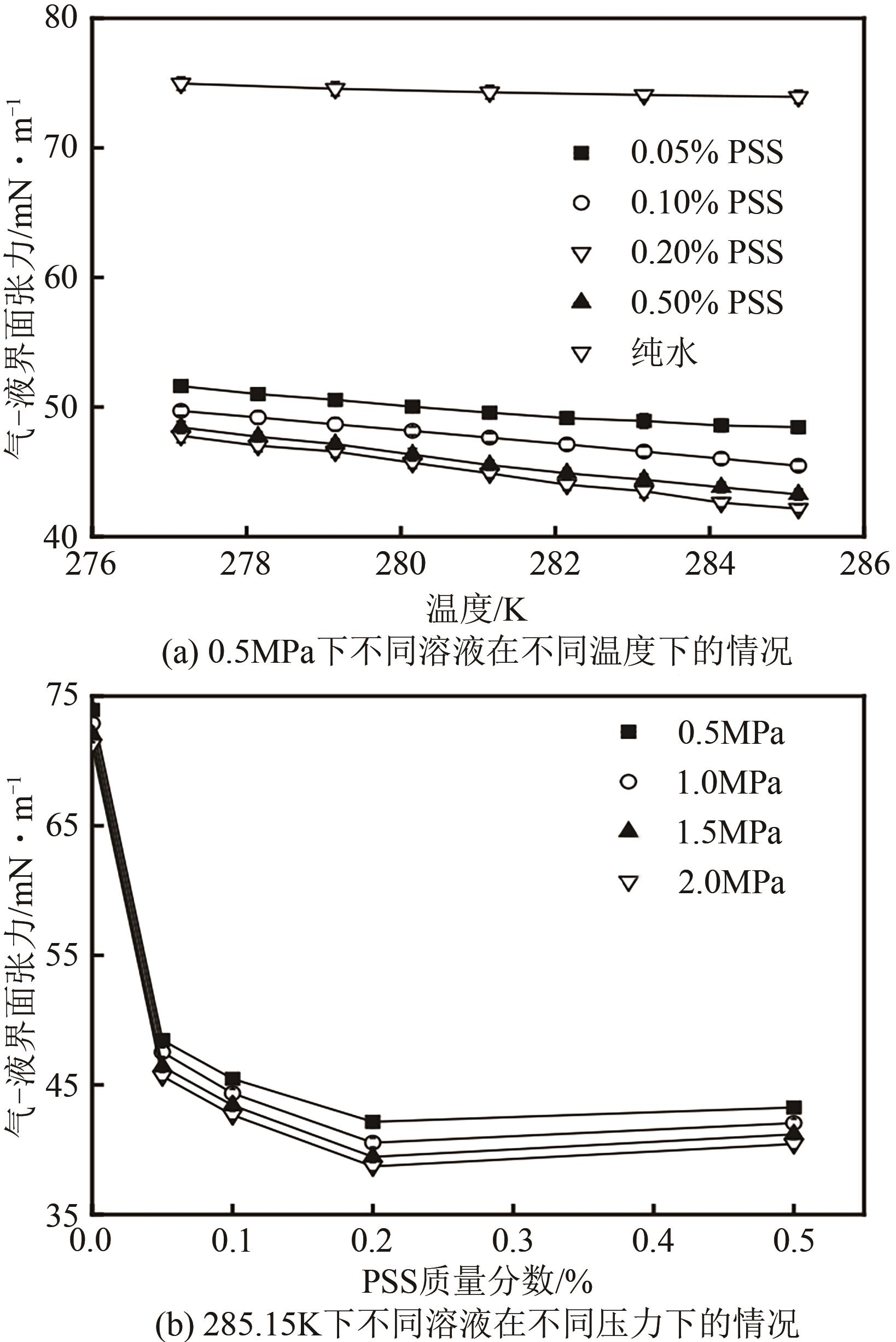
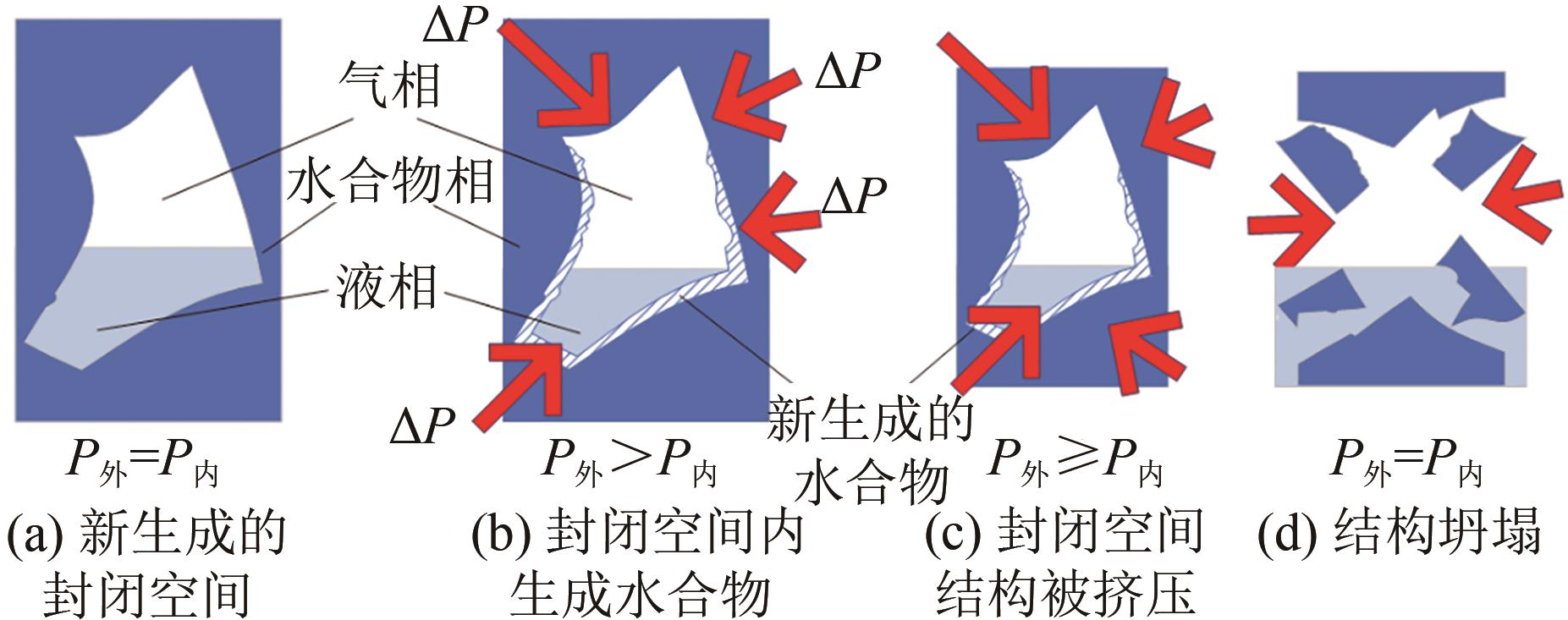
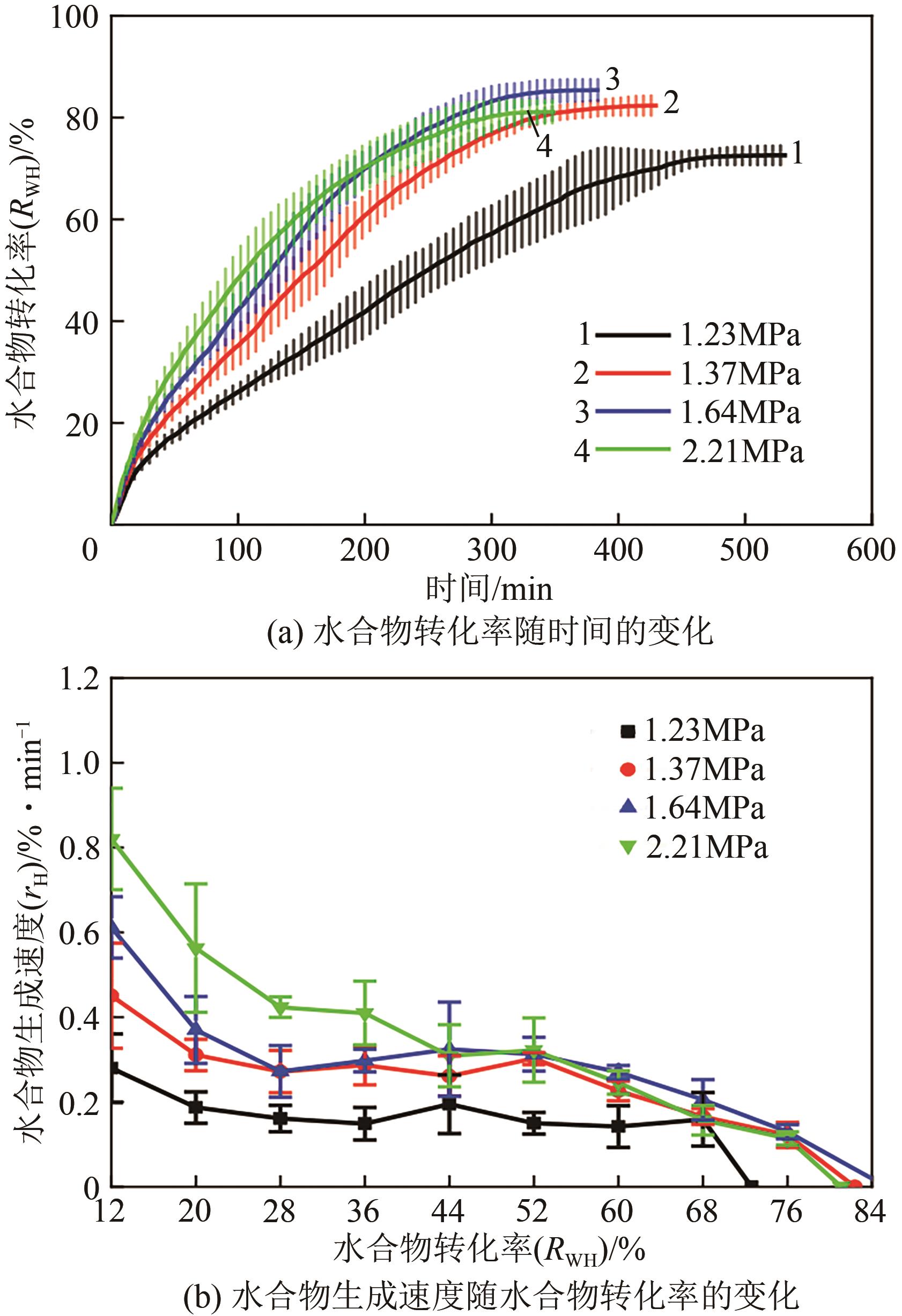
| 1 |
SLOAN E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426: 353-363.
|
| 2 |
代梦玲, 孙志高, 李娟, 等. 水合物储气促进技术研究进展[J]. 化工进展, 2020, 39(10): 3975-3986.
|
|
DAI Mengling, SUN Zhigao, LI Juan, et al. Progress on promotion technology for gas storage in hydrates[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 3975-3986.
|
| 3 |
薛倩, 王晓霖, 李遵照, 等. 水合物利用技术应用进展[J]. 化工进展, 2021, 40(2): 722-735.
|
|
XUE Qian, WANG Xiaolin, LI Zunzhao, et al. Research progresses in hydrate based technologies and processes[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 722-735.
|
| 4 |
樊栓狮, 周静仁, 李璐伶, 等. 水合物法平衡级分离CO2/N2流程模拟分析[J]. 化工进展, 2020, 39(9): 3600-3607.
|
|
FAN Shuanshi, ZHOU Jingren, LI Luling, et al. The simulation and analysis of CO2/N2 separation process by equilibrium stage hydrate-based gas separation method[J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3600-3607.
|
| 5 |
MOLOKITINA N S, NESTEROV A N, PODENKO L S, et al. Carbon dioxide hydrate formation with SDS: further insights into mechanism of gas hydrate growth in the presence of surfactant[J]. Fuel, 2019, 235: 1400-1411.
|
| 6 |
WANG X, ZHANG F, LIPINSKI W. Research progress and challenges in hydrate-based carbon dioxide capture applications[J]. Applied Energy, 2020, 269: 114928.
|
| 7 |
邵伟强, 梁海峰, 张锡彦, 等. 水合物法提纯低浓度煤层气的研究进展[J]. 化工进展, 2021, 40(6): 3143-3150.
|
|
SHAO Weiqiang, LIANG Haifeng, ZHANG Xiyan, et al. Research progress of purification of low-concentration coal-bed methane via hydrate method[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3143-3150.
|
| 8 |
WANG Y, YANG B, LIU Z, et al. The hydrate-based gas separation of hydrogen and ethylene from fluid catalytic cracking dry gas in presence of poly(sodium 4-styrenesulfonate)[J]. Fuel, 2020, 275: 117895.
|
| 9 |
GATET P, DICHARRY C, MARION G, et al. Experimental determination of methane hydrate dissociation curve up to 55MPa by using a small amount of surfactant as hydrate promoter[J]. Chemical Engineering Science, 2005, 60: 5751-5758.
|
| 10 |
PAHLAVANZADEH H, KHANLARKHANI M, REZAEI S, et al. Experimental and modelling studies on the effects of nanofluids (SiO2, Al2O3, and CuO) and surfactants (SDS and CTAB) on CH4 and CO2 clathrate hydrates formation[J]. Fuel 2019, 253: 1392-1405.
|
| 11 |
ZHENG J, CHENG F, LI Y, et al. Progress and trends in hydrate based desalination (HBD) technology: a review[J]. Chinese Journal of Chemical Engineering, 2019, 27: 2037-2043.
|
| 12 |
DICHARRY C, DIAZ J, TORRE J P, et al. Influence of the carbon chain length of a sulfate-based surfactant on the formation of CO2, CH4 and CO2-CH4 gas hydrates[J]. Chemical Engineering Science, 2016, 152: 736-745.
|
| 13 |
ZHONG D L, LI Z, LU Y Y, et al. Evaluation of CO2 removal from a CO2 + CH4 gas mixture using gas hydrate formation in liquid water and THF solutions[J]. Applied Energy, 2015, 158: 133-141.
|
| 14 |
SONG G, LI Y, WANG W, et al. Experimental investigation on the microprocess of hydrate particle agglomeration using a high-speed camera[J]. Fuel, 2019, 237: 475-485.
|
| 15 |
SHI L, DING J, LIANG D. Enhanced CH4 storage in hydrates with the presence of sucrose stearate[J]. Energy, 2019, 180: 978-988.
|
| 16 |
陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 北京: 化学工业出版社, 2020:10-181.
|
|
CHEN G J, SUN C Y, MA Q L. Gas hydrate science and technology[M]. Beijing: Chemical Industry Press, 2020: 10-181.
|
| 17 |
ZANG X, WAN L, HE Y, et al. CO2 removal from synthesized ternary gas mixtures used hydrate formation with sodium dodecyl sulfate (SDS) as additive[J]. Energy, 2020, 190: 116399-116409.
|
| 18 |
ANDO N, KUWABARA Y, MORI Y H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: an experimental study using methane and micelle-forming surfactants[J]. Chemical engineering science, 2012, 73: 79-85.
|
| 19 |
SONG Y M, LIANG R Q, WANG F, et al. Enhanced methane hydrate formation in the highly dispersed carbon nanotubes-based nanofluid[J]. Fuel, 2021, 285: 119234.
|
| 20 |
BAVOH C, LAI B, OSEI H, et, al. A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage[J]. Journal of Natural Gas Science and Engineering, 2019, 64: 52-71.
|
| 21 |
WANG Y, WANG L, HU Z, et, al. The thermodynamic and kinetic effects of sodium lignin sulfonate on ethylene hydrate formation[J]. Energies 2021, 14: 3291.
|
| 22 |
TIAN E, HU C, QIN Y, et al. A study of poly(sodium 4-styrenesulfonate) as draw solute in forward osmosis[J]. Desalination, 2015, 360: 130-37.
|
| 23 |
YU C, CHEN L, SUN B. Experimental characterization of guest molecular occupancy in clathrate hydrate cages: a review[J]. Chinese Journal of Chemical Engineering, 2019, 27: 2189-2206.
|
| 24 |
CHEN G, GUO T. A new approach to gas hydrate modelling[J]. Chemical Engineering Journal, 1998, 71(2): 145-151.
|
| 25 |
CHEN G, GUO T. Thermodynamic modeling of hydrate formation based on new concepts[J]. Fluid Phase Equilibria, 1996, 122(1): 43-65.
|
| 26 |
WANG Y, DENG Y, GUO X, et al. Experimental and modeling investigation on separation of methane from coal seam gas (CSG) using hydrate formation[J]. Energy, 2018, 150: 377-395.
|
| 27 |
XU Z, SUN Q, GAO J, et, al. Experiment and model investigation of D-sorbitol as a thermodynamic hydrate inhibitor for methane and carbon dioxide hydrates[J]. Journal of Natural Gas Science and Engineering, 2021, 90: 103927.
|
| 28 |
PATEL N C, TEJA A S. A new cubic equation of state for fluids and fluid mixtures[J]. Chemical Engineering Science, 1982, 37(3): 463-473.
|
| 29 |
ASADI F, EJTEMAEI M, BIRKETT G, et al. The link between the kinetics of gas hydrate formation and surface ion distribution in the low salt concentration regime[J]. Fuel, 2019, 240: 309-316.
|
| 30 |
HU Y, LEE B, SUM A. Universal correlation for gas hydrates suppression temperature of inhibited systems: Ⅰ. Single salts[J]. AIChE Journal, 2017, 63(11): 5111-5124.
|
| 31 |
JARRAHIANA A, NAKHAEE A. Hydrate-liquid-vapor equilibrium condition of N2 + CO2 + H2O system: measurement and modeling[J]. Fuel, 2019, 237: 769-774.
|
| 32 |
LIAO Z, GUO X, ZHAO Y, et al. Experimental and modeling study on phase equilibria of semiclathrate hydrates of tetra-n-butyl ammonium bromide + CH4, CO2, N2, or gas mixtures[J]. Industrial & Engineering Chemistry Research, 2013, 52(51): 18440-18446.
|
| 33 |
LIAO Z, GUO X, LI Q, et al. Experimental and modeling study on the phase equilibria for hydrates of gas mixtures in TBAB solution[J]. Chemical Engineering Science, 2015, 137: 18440-18446.
|
| 34 |
LI N, ZHANG C, MA Q, et al. Measurements and modeling of interfacial tension for (CO2 + n-alkyl benzene) binary mixtures[J]. The Journal of Supercritical Fluids, 2019, 154: 104625-104633.
|
| 35 |
LUO H, SUN C Y, HUANG Q, et al. Interfacial tension of ethylene and aqueous solution of sodium dodecyl sulfate (SDS) in or near hydrate formation region[J]. Journal of Colloid and Interface Science, 2006, 297: 266-270.
|
| 36 |
MANKO D, ZDZIENNICKA A, JANCZUK B, et al. Surface and volumetric properties of n-octyl-β-d-glucopyranoside and rhamnolipid mixture[J]. Journal of Molecular Liquids, 2016, 219: 801-809.
|
| 37 |
CHENG L, LIAO K, LI Z, et al. The invalidation mechanism of kinetic hydrate inhibitors under high subcooling conditions[J]. Chemical Engineering Science, 2019, 207: 305-316.
|
| 38 |
WANG Y, DU M, GUO X, et al. Experiments and simulations for continuous recovery of methane from coal seam gas (CSG) utilizing hydrate formation[J]. Energy, 2017, 129: 28-41.
|
| 39 |
JIANG L, XU N, LIU Q, et al. Review of morphology studies on gas hydrate formation for hydrate-based technology[J]. Crystal Growth & Design, 2020, 20(12): 8148-8161.
|
 ), 刘智琪2, 孙强2, 刘爱贤1, 杨兰英2, 宫敬3, 郭绪强1(
), 刘智琪2, 孙强2, 刘爱贤1, 杨兰英2, 宫敬3, 郭绪强1( )
)
 ), LIU Zhiqi2, SUN Qiang2, LIU Aixian1, YANG Lanying2, GONG Jing3, GUO Xuqiang1(
), LIU Zhiqi2, SUN Qiang2, LIU Aixian1, YANG Lanying2, GONG Jing3, GUO Xuqiang1( )
)