化工进展 ›› 2021, Vol. 40 ›› Issue (7): 3957-3975.DOI: 10.16085/j.issn.1000-6613.2020-1612
氨气深度脱除材料与技术研究进展
- 浙江大学化学工程与生物工程学院,浙江 杭州 310029
-
收稿日期:2020-08-13修回日期:2020-10-26出版日期:2021-07-06发布日期:2021-07-19 -
通讯作者:邢华斌 -
作者简介:陈沛坤(1995—),男,硕士研究生,研究方向为分离工程与高纯化学品制造。E-mail:9651214@qq.com 。 -
基金资助:国家自然科学基金(21725603);浙江大学衢州研究院科技计划(IZQ2019-KJ-003)
Progress in materials and technologies for deep removal of ammonia gas
CHEN Peikun( ), ZHANG Yuanbin, CUI Xili, XING Huabin(
), ZHANG Yuanbin, CUI Xili, XING Huabin( )
)
- College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310029, Zhejiang, China
-
Received:2020-08-13Revised:2020-10-26Online:2021-07-06Published:2021-07-19 -
Contact:XING Huabin
摘要:
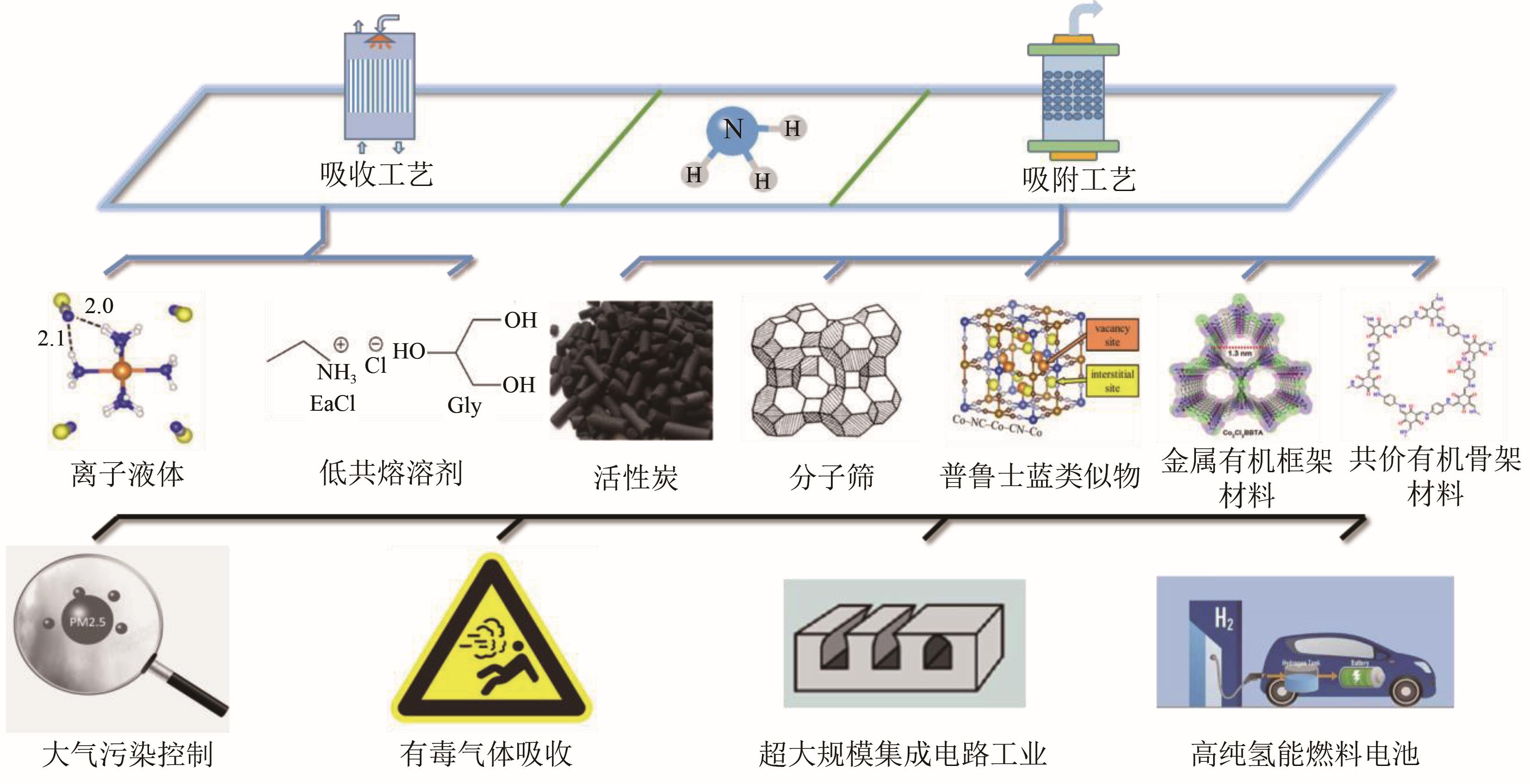
氨气(NH3)作为一种危害性碱性有毒气体,不仅危害环境,而且会对人体造成不可逆伤害。在电子信息、能源等行业,极微量的NH3即可影响产品品质、降低过程性能。因此,NH3的深度脱除在工业上具有重要的意义。本文综述了近年来NH3深度脱除的工艺现状,分析了NH3脱除材料如离子液体、低共熔溶剂、改性活性炭、分子筛、改性氧化铝、金属盐类、金属有机框架材料、多孔有机聚合物、共价有机骨架材料、氧化石墨烯、普鲁士蓝类似物对NH3的分离性能。总结了深度脱除NH3的工艺特点和脱氨材料的性能,浅析了该领域发展面临的问题,并对未来的发展方向提出了建议。
中图分类号:
引用本文
陈沛坤, 张袁斌, 崔希利, 邢华斌. 氨气深度脱除材料与技术研究进展[J]. 化工进展, 2021, 40(7): 3957-3975.
CHEN Peikun, ZHANG Yuanbin, CUI Xili, XING Huabin. Progress in materials and technologies for deep removal of ammonia gas[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3957-3975.
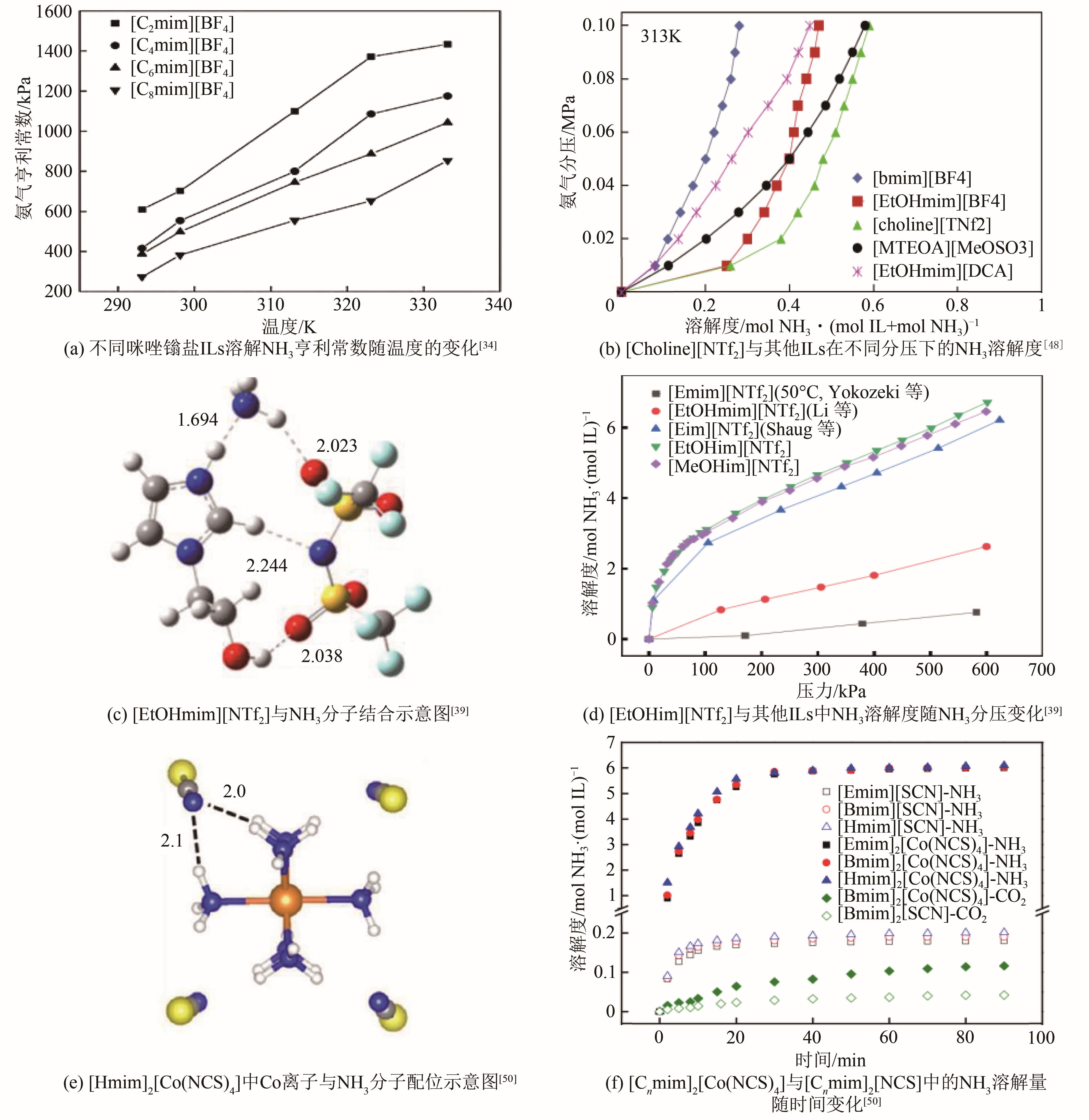
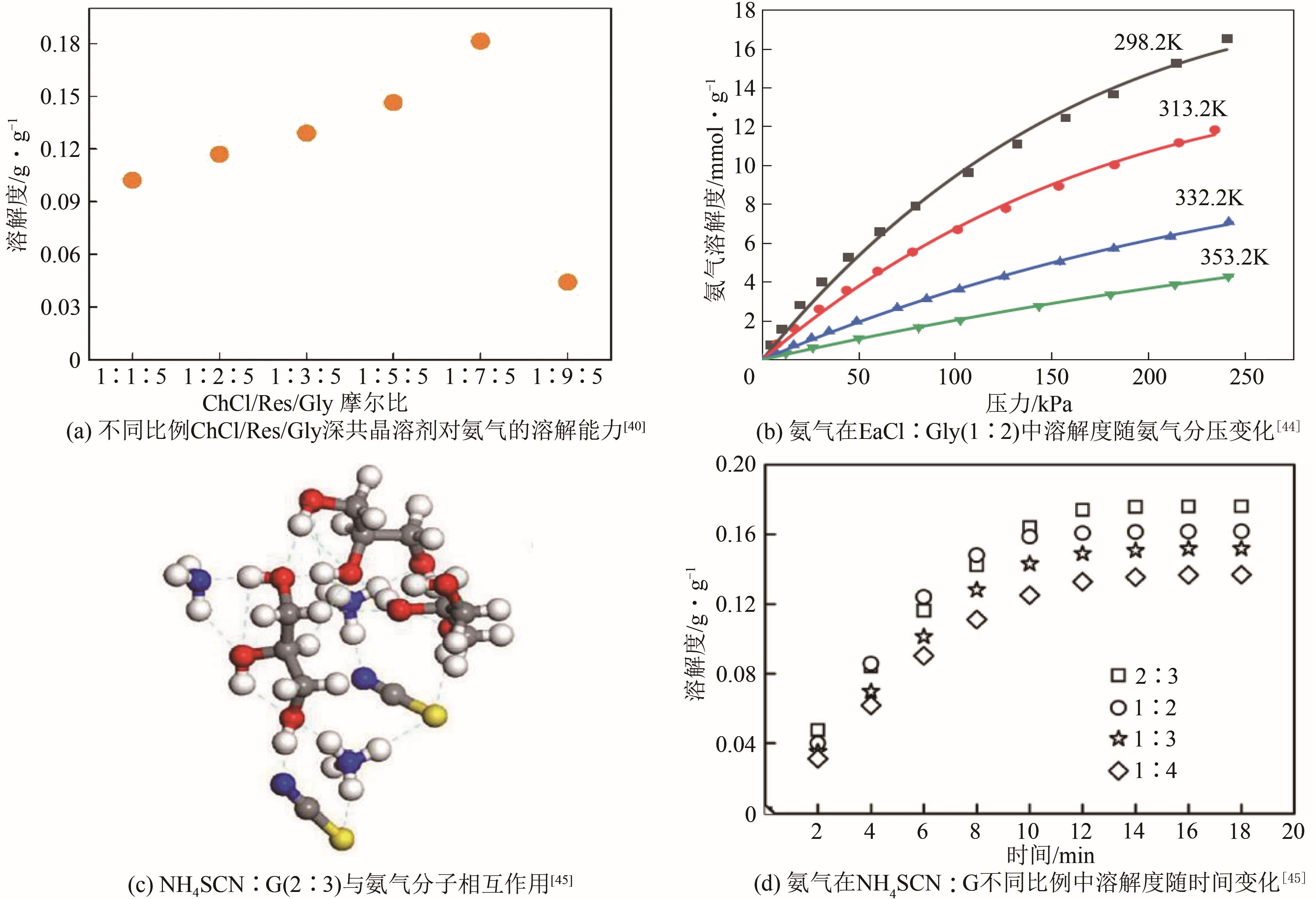
| 吸收剂种类 | 吸收条件 | 吸收量/mmol·g-1 | 文献 | 吸收剂种类 | 吸收条件 | 吸收量/mmol·g-1 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| 温度/K | 压力/kPa | 温度/K | 压力/kPa | ||||||
| [Emim][Ac] | 298.3 | 101.3 | 1.88 | [ | [Choline][NTf2] | 293.15 | 100 | 4.82 | [ |
| [Emim][SCN] | 298.1 | 101.3 | 2.64 | [ | [EtOHmim[NTf2] | 313 | 127 | 2.05 | [ |
| [Emim][EtOSO3] | 298.15 | 418 | 2.35 | [ | [EtOHim][NTf2] | 313 | 100 | 7.88 | [ |
| [DMEA][Ac] | 298.1 | 163 | 5.50 | [ | [Bim][NTf2] | 313 | 100 | 6.65 | [ |
| [Bmim][PF6] | 298.0 | 174 | 1.84 | [ | [Cnmim]2[Co(NCS)4] | 303 | 100 | 10.59 | [ |
| [Hmim][Cl] | 297.8 | 133 | 1.45 | [ | ChCl∶Res∶Gly(1∶3∶5) | 313 | 101 | 7.65 | [ |
| [Emim][NTf2] | 323.15 | 171 | 0.24 | [ | ChCl∶D-f∶Gly(1∶3∶5) | 313 | 101 | 6.47 | [ |
| [Bmim][BF4] | 313.15 | 101.3 | 1.71 | [ | Bmim∶MeSO3(1∶1) | 313.2 | 172.6 | 1.05 | [ |
| [C2MIM][BF4] | 298.15 | 110 | 0.94 | [ | ChCl∶PhOH∶EG(1∶5∶4) | 313.2 | 101.3 | 6.99 | [ |
| [C4MIM][BF4] | 298.15 | 220 | 2.01 | [ | NH4SCN:Gly(2∶3) | 303 | 100 | 10.35 | [ |
| [C6MIM][BF4] | 298.15 | 220 | 2.24 | [ | ChCl∶ImZ∶EG(3∶7∶14) | 313.2 | 101.6 | 4.91 | [ |
| [C8MIM][BF4] | 298.15 | 120 | 1.41 | [ | EaCl∶Gly(1∶2) | 298.2 | 106.7 | 9.63 | [ |
表1 不同吸收剂的NH3吸收性能
| 吸收剂种类 | 吸收条件 | 吸收量/mmol·g-1 | 文献 | 吸收剂种类 | 吸收条件 | 吸收量/mmol·g-1 | 文献 | ||
|---|---|---|---|---|---|---|---|---|---|
| 温度/K | 压力/kPa | 温度/K | 压力/kPa | ||||||
| [Emim][Ac] | 298.3 | 101.3 | 1.88 | [ | [Choline][NTf2] | 293.15 | 100 | 4.82 | [ |
| [Emim][SCN] | 298.1 | 101.3 | 2.64 | [ | [EtOHmim[NTf2] | 313 | 127 | 2.05 | [ |
| [Emim][EtOSO3] | 298.15 | 418 | 2.35 | [ | [EtOHim][NTf2] | 313 | 100 | 7.88 | [ |
| [DMEA][Ac] | 298.1 | 163 | 5.50 | [ | [Bim][NTf2] | 313 | 100 | 6.65 | [ |
| [Bmim][PF6] | 298.0 | 174 | 1.84 | [ | [Cnmim]2[Co(NCS)4] | 303 | 100 | 10.59 | [ |
| [Hmim][Cl] | 297.8 | 133 | 1.45 | [ | ChCl∶Res∶Gly(1∶3∶5) | 313 | 101 | 7.65 | [ |
| [Emim][NTf2] | 323.15 | 171 | 0.24 | [ | ChCl∶D-f∶Gly(1∶3∶5) | 313 | 101 | 6.47 | [ |
| [Bmim][BF4] | 313.15 | 101.3 | 1.71 | [ | Bmim∶MeSO3(1∶1) | 313.2 | 172.6 | 1.05 | [ |
| [C2MIM][BF4] | 298.15 | 110 | 0.94 | [ | ChCl∶PhOH∶EG(1∶5∶4) | 313.2 | 101.3 | 6.99 | [ |
| [C4MIM][BF4] | 298.15 | 220 | 2.01 | [ | NH4SCN:Gly(2∶3) | 303 | 100 | 10.35 | [ |
| [C6MIM][BF4] | 298.15 | 220 | 2.24 | [ | ChCl∶ImZ∶EG(3∶7∶14) | 313.2 | 101.6 | 4.91 | [ |
| [C8MIM][BF4] | 298.15 | 120 | 1.41 | [ | EaCl∶Gly(1∶2) | 298.2 | 106.7 | 9.63 | [ |
| 分子筛 | 温度/K | 低压 | 常压 | ||
|---|---|---|---|---|---|
| NH3分压/kPa | 平衡吸附量/mmol·g-1 | NH3分压/kPa | 平衡吸附量/mmol·g-1 | ||
| 4A zeolite TG242 | 298.15 | 0.0171 | 2.039 | 97.8 | 8.717 |
| 5A zeolite KE154 | 298.15 | 0.0058 | 1.864 | 97.6 | 7.674 |
| 13X zeolite WE894 | 298.15 | 0.0041 | 1.742 | 93.8 | 9.326 |
| 5A zeolite Lancaster | 298.15 | 0.0126 | 3.206 | 97.7 | 7.815 |
| 13X zeolite Lancaster | 298.15 | 0.058 | 3.786 | 97.4 | 9.326 |
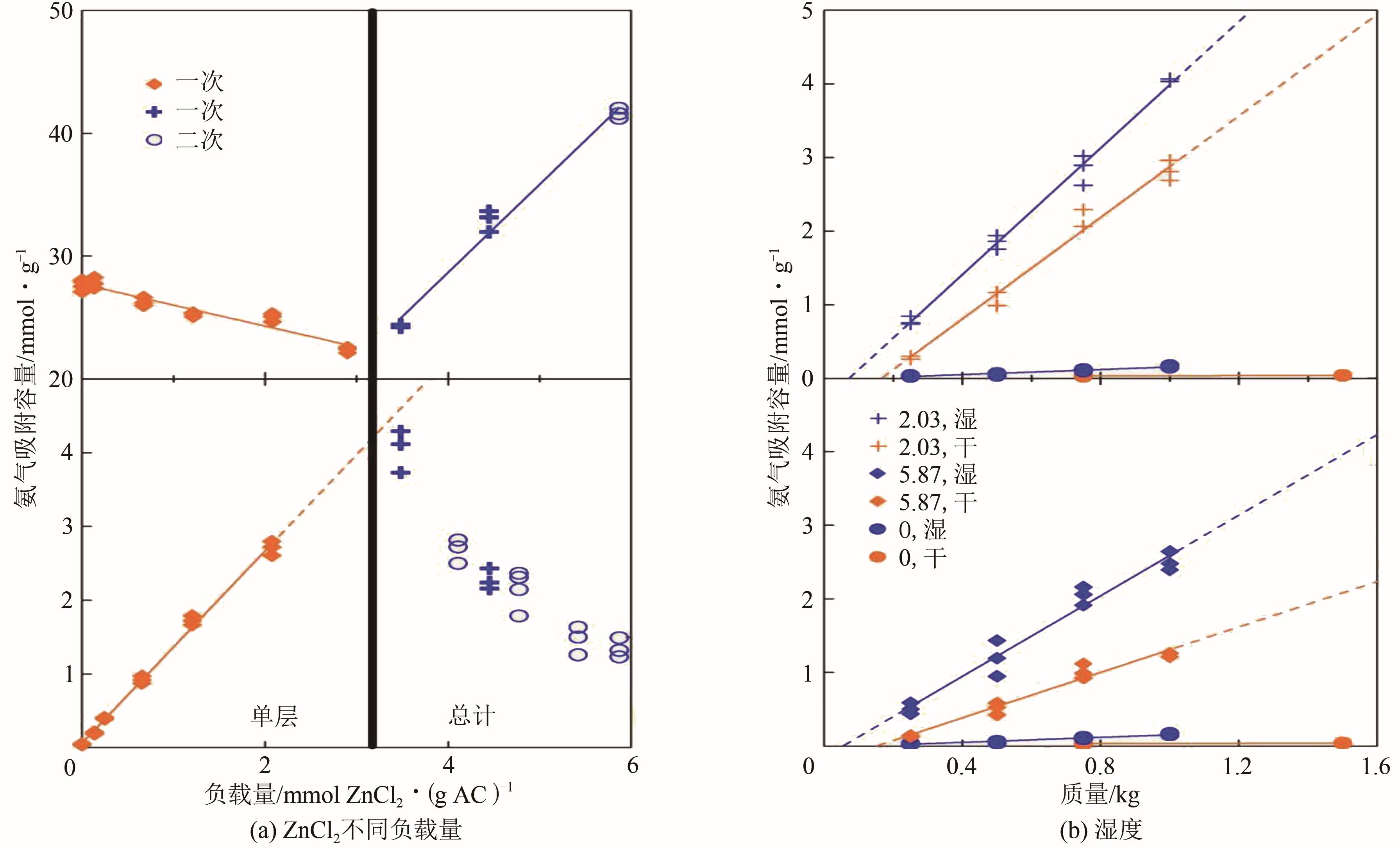
| 5A zeolite Sigma | 298.15 | 0.0085 | 2.460 | 98.7 | 7.430 |
| 13X zeolite Sigma | 298.15 | 0.0115 | 2.489 | 96.7 | 9.030 |
表2 不同分子筛的氨气静态吸附性能[3,74]
| 分子筛 | 温度/K | 低压 | 常压 | ||
|---|---|---|---|---|---|
| NH3分压/kPa | 平衡吸附量/mmol·g-1 | NH3分压/kPa | 平衡吸附量/mmol·g-1 | ||
| 4A zeolite TG242 | 298.15 | 0.0171 | 2.039 | 97.8 | 8.717 |
| 5A zeolite KE154 | 298.15 | 0.0058 | 1.864 | 97.6 | 7.674 |
| 13X zeolite WE894 | 298.15 | 0.0041 | 1.742 | 93.8 | 9.326 |
| 5A zeolite Lancaster | 298.15 | 0.0126 | 3.206 | 97.7 | 7.815 |
| 13X zeolite Lancaster | 298.15 | 0.058 | 3.786 | 97.4 | 9.326 |
| 5A zeolite Sigma | 298.15 | 0.0085 | 2.460 | 98.7 | 7.430 |
| 13X zeolite Sigma | 298.15 | 0.0115 | 2.489 | 96.7 | 9.030 |
| 材料 | 组成 | 298K、1bar下动态吸附容量/mmol·g-1 |
|---|---|---|
| PB | Na0.05Fe[Fe(CN)6]0.70·5.3H2O | 3.1 |
| CoPBA | K0.05Co[Co(CN)6]0.66·4.4H2O | 1.9 |
| FePBA(CoⅢ) | Fe[Co(CN)6]2·3.4H2O(estimated) | 2.5 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.66 | K0.64Cu[Fe(CN)6]0.66·3.2H2O | 2.6 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.59 | K0.33Cu[Fe(CN)6]0.59·4.1H2O | 2.1 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.57 | K0.19Cu[Fe(CN)6]0.57·4.4H2O | 2.7 |
| CuPBA | K0.05Cu[Fe(CN)6]0.46·5.0H2O | 2.1 |
| 离子交换树脂(IE) | 0.38 | |
| 13X 分子筛(ZL) | 0.28 | |
| AC 分子筛 | 0.02 |
表3 不同普鲁士蓝类似物在10μL/L的氨气浓度下穿透吸附性能[84]
| 材料 | 组成 | 298K、1bar下动态吸附容量/mmol·g-1 |
|---|---|---|
| PB | Na0.05Fe[Fe(CN)6]0.70·5.3H2O | 3.1 |
| CoPBA | K0.05Co[Co(CN)6]0.66·4.4H2O | 1.9 |
| FePBA(CoⅢ) | Fe[Co(CN)6]2·3.4H2O(estimated) | 2.5 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.66 | K0.64Cu[Fe(CN)6]0.66·3.2H2O | 2.6 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.59 | K0.33Cu[Fe(CN)6]0.59·4.1H2O | 2.1 |
| CuPBA(FeⅡ),[Fe(CN)6]·Cu=0.57 | K0.19Cu[Fe(CN)6]0.57·4.4H2O | 2.7 |
| CuPBA | K0.05Cu[Fe(CN)6]0.46·5.0H2O | 2.1 |
| 离子交换树脂(IE) | 0.38 | |
| 13X 分子筛(ZL) | 0.28 | |
| AC 分子筛 | 0.02 |
| 材料 | 298K、1bar下静态吸附容量 /mmol·g-1 | 298K、1bar下动态吸附容量/mmol·g-1 | 文献 | ||
|---|---|---|---|---|---|
| NH3浓度/μL·L-1 | 干燥 | 潮湿(80% RH) | |||
| MOF-74 | — | 9900 | 5.47 | — | [ |
| MOF-5 | — | 9900 | 0.35 | — | [ |
| MOF-177 | — | 9900 | 2.47 | — | [ |
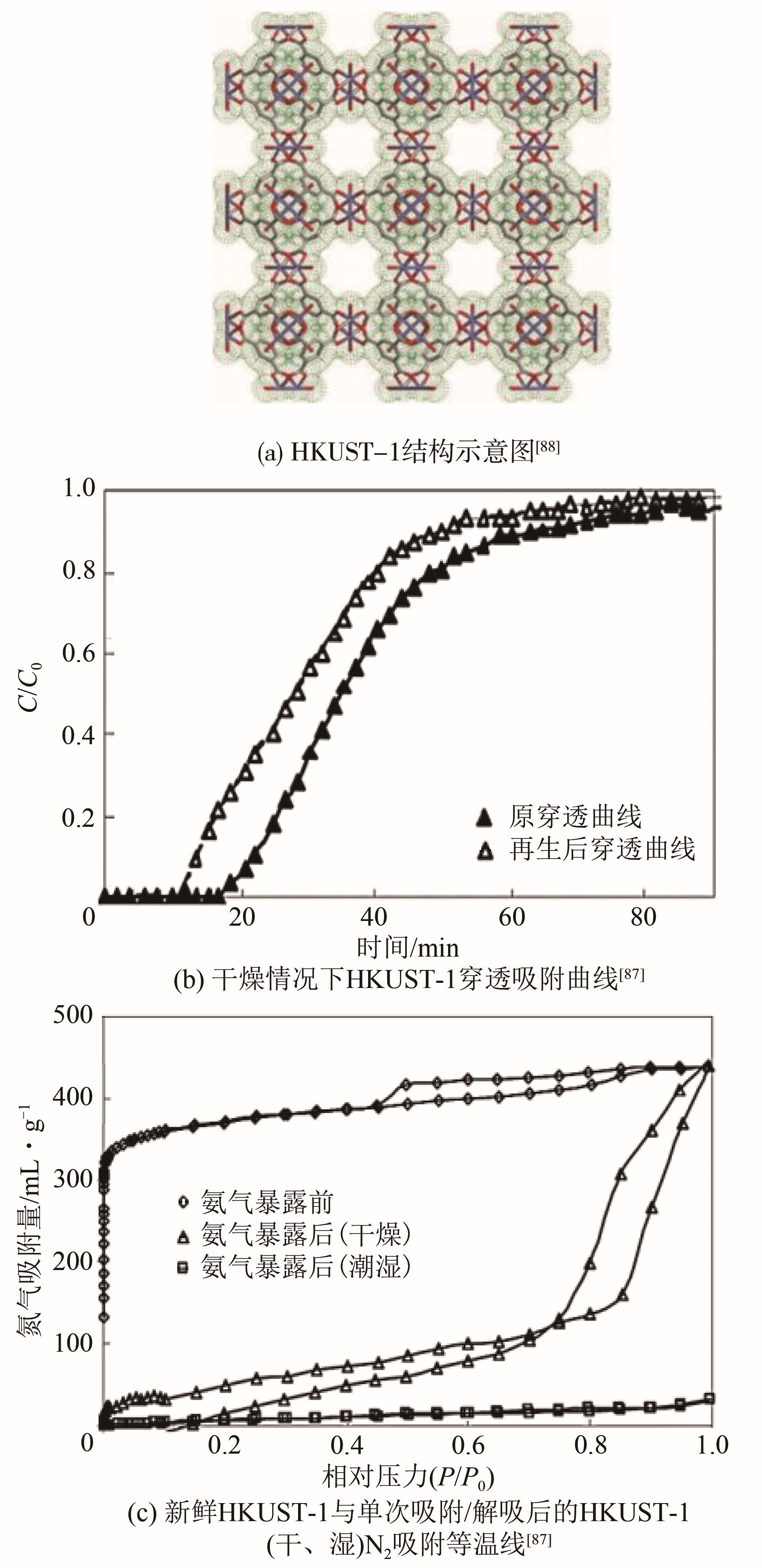
| HKUST-1 | 12.1 | 1000 | 6.6 | 8.9 | [ |
| Co-MOF-74 | — | 1000 | 6.7 | 4.3 | [ |
| Mg-MOF-74 | — | 1000 | 7.6 | 1.7 | [ |
| DUT-6 | 12 | — | — | — | [ |
| DUT-6-(OH)2 | 16.4 | — | — | — | [ |
| UiO-66 | — | 1438 | 1.79 | 2.75 | [ |
| UiO-66-NH2 | 9.84 | 1438 | 3.56 | 3.01 | [ |
| UiO-66-OH | — | 2876 | 5.69 | 2.77 | [ |
| Mn2Cl2BTDD | 15.47 | — | — | — | [ |
| Cu2Cl2BBTA | 19.79 | 1000 | 7.52 | 5.73 | [ |
| Co2Cl2 BBTA | 17.95 | 1000 | 8.56 | 4.36 | [ |
| MFM-300(Al) | 13.9(293K) | — | — | — | [ |
| NU-1401 | 8.7 | 2976 | 8.41 | 5.7 | [ |
| Cu(BDC) | 17.2 | 1000 | — | — | [ |
| Zn(BDC) | 14.1 | 1000 | — | — | [ |
表4 不同金属有机框架材料的氨气吸附性能
| 材料 | 298K、1bar下静态吸附容量 /mmol·g-1 | 298K、1bar下动态吸附容量/mmol·g-1 | 文献 | ||
|---|---|---|---|---|---|
| NH3浓度/μL·L-1 | 干燥 | 潮湿(80% RH) | |||
| MOF-74 | — | 9900 | 5.47 | — | [ |
| MOF-5 | — | 9900 | 0.35 | — | [ |
| MOF-177 | — | 9900 | 2.47 | — | [ |
| HKUST-1 | 12.1 | 1000 | 6.6 | 8.9 | [ |
| Co-MOF-74 | — | 1000 | 6.7 | 4.3 | [ |
| Mg-MOF-74 | — | 1000 | 7.6 | 1.7 | [ |
| DUT-6 | 12 | — | — | — | [ |
| DUT-6-(OH)2 | 16.4 | — | — | — | [ |
| UiO-66 | — | 1438 | 1.79 | 2.75 | [ |
| UiO-66-NH2 | 9.84 | 1438 | 3.56 | 3.01 | [ |
| UiO-66-OH | — | 2876 | 5.69 | 2.77 | [ |
| Mn2Cl2BTDD | 15.47 | — | — | — | [ |
| Cu2Cl2BBTA | 19.79 | 1000 | 7.52 | 5.73 | [ |
| Co2Cl2 BBTA | 17.95 | 1000 | 8.56 | 4.36 | [ |
| MFM-300(Al) | 13.9(293K) | — | — | — | [ |
| NU-1401 | 8.7 | 2976 | 8.41 | 5.7 | [ |
| Cu(BDC) | 17.2 | 1000 | — | — | [ |
| Zn(BDC) | 14.1 | 1000 | — | — | [ |
| 1 | KLERKE A, CHRISTENSEN C H, NФRSKOV J K, et al. Ammonia for hydrogen storage: challenges and opportunities[J]. Journal of Materials Chemistry, 2008, 18(20): 2304-2310. |
| 2 | KHAN N A, HASAN Z, JHUNG S H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): a review[J]. Journal of Hazardous Materials, 2013, 244/245: 444-456. |
| 3 | HELMINEN J, HELENIUS J, PAATERO E, et al. Comparison of sorbents and isotherm models for NH3-gas separation by adsorption[J]. AIChE Journal, 2000, 46(8): 1541-1555. |
| 4 | ZHOU Ying, CHENG Shuiyuan, LANG Jianlei, et al. A comprehensive ammonia emission inventory with high-resolution and its evaluation in the Beijing-Tianjin-Hebei (BTH) region, China[J]. Atmospheric Environment, 2015, 106: 305-317. |
| 5 | PAULOT Fabien, JACOB Daniel J. Hidden cost of U.S. agricultural exports: particulate matter from ammonia emissions[J]. Environmental Science & Technology, 2014, 48(2): 903-908. |
| 6 | KATZ Michael J, HOWARTH Ashlee J, MOGHADAM Peyman Z, et al. High volumetric uptake of ammonia using Cu-MOF-74/Cu-CPO-27[J]. Dalton Transactions, 2016, 45(10): 4150-4153. |
| 7 | KATZ Michael J, RAMNIAL Taramatee, YU Huazhong, et al. Polymorphism of Zn[Au(CN)2]2 and its luminescent sensory response to NH3 vapor[J]. Journal of the American Chemical Society, 2008, 130(32): 10662-10673. |
| 8 | BARIN Gokhan, PETERSON Gregory W, CROCELLA Valentina, et al. Highly effective ammonia removal in a series of Bronsted acidic porous polymers: investigation of chemical and structural variations[J]. Chemical Science, 2017, 8(6): 4399-4409. |
| 9 | 蒋载阳, 江书忠, 曹霞, 等. 论猪舍中氨气的危害[J]. 中国猪业, 2018, 13(8): 58-60, 62. |
| JIANG Zaiyang, JIANG Shuzhong, CAO Xia, et al. On the harm of ammonia gas in pigsty [J]. China Pig Industry, 2018, 13(8): 58-60, 62. | |
| 10 | NUERNBERG G B, MOREIRA M A, ERNANI P R, et al. Efficiency of basalt zeolite and Cuban zeolite to adsorb ammonia released from poultry litter[J]. Journal of Environmental Management, 2016, 183: 667-672. |
| 11 | HUANG R J, ZHANG Y L, BOZZETTI C, et al. High secondary aerosol contribution to particulate pollution during haze events in China[J]. Nature, 2014, 514(7521): 218-222. |
| 12 | MACDONALD S A, HINSBERG W D, WENDT H R, et al. Airborne contamination of a chemically amplified resist. 1. Identification of problem[J]. Chemistry of Materials, 1993, 5(3): 348-356. |
| 13 | LIN I K, BAI H L, WU B J. Analysis of relationship between inorganic gases and fine particles in cleanroom environment[J]. Aerosol and Air Quality Research, 2010, 10(3): 245-254. |
| 14 | KITAJIMA H, SHIRAMIZU Y. Requirements for contamination control in the gigabit era[J]. IEEE Transactions on Semiconductor Manufacturing, 1997, 10(2): 267-272. |
| 15 | YEH C F, HSIAO C W, LIN S J, et al. The removal of airborne molecular contamination in cleanroom using PTFE and chemical filters[J]. IEEE Transactions on Semiconductor Manufacturing, 2004, 17(2): 214-220. |
| 16 | CHIEN C L, TSAI C J, KU K W, et al. Ventilation control of air pollutant during preventive maintenance of a metal etcher in semiconductor industry[J]. Aerosol Air Quality Research, 2007, 7(4): 469-488. |
| 17 | HASSEL B A VAN, KARRA J R, SANTANA J, et al. Ammonia sorbent development for on-board H2 purification[J]. Separation and Purification Technology, 2015, 142: 215-226. |
| 18 | YUAN X Z, LI H, YU Y, et al. Diagnosis of contamination introduced by ammonia at the cathode in a polymer electrolyte membrane fuel cell[J]. International Journal of Hydrogen Energy, 2012, 37(17): 12464-12473. |
| 19 | DECOSTE Jared B, DENNY Michael S, PETERSON Gregory W JR, et al. Enhanced aging properties of HKUST-1 in hydrophobic mixed-matrix membranes for ammonia adsorption[J]. Chemical Science, 2016, 7(4): 2711-2716. |
| 20 | MAIA G D N, DAY G B, GATES R S, et al. Ammonia biofiltration and nitrous oxide generation during the start-up of gas-phase compost biofilters[J]. Atmospheric Environment, 2012, 46: 659-664. |
| 21 | 王佩琳, 王宗爽, 问立宁, 等. 《无机化学工业污染物排放标准》(GB 31573—2015)解读[J]. 环境影响评价, 2016, 38(5): 30-34. |
| WANG Peilin, WANG Zongshuang, WEN Lining, et al. An interpretation of emission standards of pollutants for inorganic chemical industry (GB 31573—2015) [J]. Environmental Impact Assessment,2016,38(5):30-34. | |
| 22 | BHOWN A, CUSSLER E L. Mechanism for selective ammonia transport through poly(vinylammonium thiocyanate) membranes[J]. Journal of the American Chemical Society, 1991, 113(3): 742-749. |
| 23 | TABE-MOHAMMADI A. A review of the applications of membrane separation technology in natural gas treatment[J]. Separation Science and Technology, 1999, 34(10): 2095-2111. |
| 24 | SOPYAN I. Kinetic analysis on photocatalytic degradation of gaseous acetaldehyde, ammonia and hydrogen sulfide on nanosized porous TiO2 films[J]. Science and Technology of Advanced Materials, 2007, 8(1/2): 33-39. |
| 25 | TAKATA Tomoka, TSUNOJI Nao, TAKAMITSU Yasuyuki, et al. Incorporation of various heterometal atoms in CHA zeolites by hydrothermal conversion of FAU zeolite and their performance for selective catalytic reduction of NOx with ammonia[J]. Microporous and Mesoporous Materials, 2017, 246: 89-101. |
| 26 | CHEN C Y, TSAI T H, CHANG C H, et al. Airlift bioreactor system for simultaneous removal of hydrogen sulfide and ammonia from synthetic and actual waste gases[J]. Journal of Environmental Science and Health Part A:Toxic/Hazardous Substances & Environmental Engineering, 2018, 53(8): 694-701. |
| 27 | PAGANS E, FONT X, SANCHEZ A. Biofiltration for ammonia removal from composting exhaust gases[J]. Chemical Engineering Journal, 2005, 113(2/3): 105-110. |
| 28 | ASHTARI Ahmad Kalbasi, MAJD Amir M Samani, RISKOWSKI Gerald L, et al. Removing ammonia from air with a constant pH, slightly acidic water spray wet scrubber using recycled scrubbing solution[J]. Frontiers of Environmental Science & Engineering, 2016, 10(6): 1-10. |
| 29 | GUO Jing, JIAO Weizhou, QI Guisheng, et al. Applications of high-gravity technologies in gas purifications: a review[J]. Chinese Journal of Chemical Engineering, 2019, 27(6): 1361-1373. |
| 30 | VIKRANT K, KUMAR V, KIM K H, et al. Metal-organic frameworks (MOFs): potential and challenges for capture and abatement of ammonia[J]. Journal of Materials Chemistry A, 2017, 5(44): 22877-22896. |
| 31 | 樊腾. 超重力法胶清脱氨及氨气回收技术基础研究[D].太原: 中北大学, 2019. |
| FAN Teng. Basic research on super-gravity gel deamination and ammonia gas recovery technology[D]. Taiyuan: North University of China, 2019. | |
| 32 | YOKOZEKI A, SHIFLETT M B. Vapor-liquid equilibria of ammonia plus ionic liquid mixtures[J]. Applied Energy, 2007, 84(12): 1258-1273. |
| 33 | YOKOZEKI A, SHIFLETT M B. Ammonia solubilities in room-temperature ionic liquids[J]. Industrial & Engineering Chemistry Research, 2007, 46(5): 1605-1610. |
| 34 | LI Guihua, ZHOU Qing, ZHANG Xiangping, et al. Solubilities of ammonia in basic imidazolium ionic liquids[J]. Fluid Phase Equilibria, 2010, 297(1): 34-39. |
| 35 | YANG Lifeng, QIAN Siheng, WANG Xiaobing, et al. Energy-efficient separation alternatives: metal-organic frameworks and membranes for hydrocarbon separation[J]. Chemical Society Reviews, 2020, 49(15): 5359-5406. |
| 36 | LI Zhijie, ZHANG Xiangping, DONG Haifeng, et al. Efficient absorption of ammonia with hydroxyl-functionalized ionic liquids[J]. RSC Advances, 2015, 5(99): 81362-81370. |
| 37 | SHANG D W, BAI L, ZENG S J, et al. Enhanced NH3 capture by imidazolium-based protic ionic liquids with different anions and cation substituents[J]. Journal of Chemical Technology and Biotechnology, 2018, 93(5): 1228-1236. |
| 38 | SHANG D W, ZHANG X P, ZENG S J, et al. Protic ionic liquid [Bim][NTf2] with strong hydrogen bond donating ability for highly efficient ammonia absorption[J]. Green Chemistry, 2017, 19(4): 937-945. |
| 39 | YUAN Lei, ZHANG Xiangping, REN Baozeng, et al. Dual-functionalized protic ionic liquids for efficient absorption of NH3 through synergistically physicochemical interaction[J]. Journal of Chemical Technology and Biotechnology, 2020, 95(6): 1815-1824. |
| 40 | LI Y H, ALI M C, YANG Q W, et al. Hybrid deep eutectic solvents with flexible hydrogen-bonded supramolecular networks for highly efficient uptake of NH3[J]. ChemSusChem, 2017, 10(17): 3368-3377. |
| 41 | ZHONG F Y, PENG H L, TAO D J, et al. Phenol-based ternary deep eutectic solvents for highly efficient and reversible absorption of NH3[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3258-3266. |
| 42 | AKHMETSHINA A I, PETUKHOV A N, MECHERGUI A, et al. Evaluation of methanesulfonate-based deep eutectic solvent for ammonia sorption[J]. Journal of Chemical and Engineering Data, 2018, 63(6): 1896-1904. |
| 43 | ZHONG F Yu, ZHOU Linsen, SHEN Jian, et al. Rational design of azole-based deep eutectic solvents for highly efficient and reversible capture of ammonia[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(16): 14170-14179. |
| 44 | JIANG W J, ZHONG F Y, LIU Y, et al. Effective and reversible capture of NH3 by ethylamine hydrochloride plus glycerol deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10552-10560. |
| 45 | DENG Dongshun, GAO Bao, ZHANG Chao, et al. Investigation of protic NH4SCN-based deep eutectic solvents as highly efficient and reversible NH3 absorbents[J]. Chemical Engineering Journal, 2019, 358: 936-943. |
| 46 | AKI S, MELLEIN B R, SAURER E M, et al. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids[J]. Journal of Physical Chemistry B, 2004, 108(52): 20355-20365. |
| 47 | SHI W, MAGINN E J. Molecular simulation of ammonia absorption in the ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (emim Tf2N)[J]. AIChE Journal, 2009, 55(9): 2414-2421. |
| 48 | BEDIA Jorge, PALOMAR Jose, Maria GONZALEZ-MIQUEL, et al. Screening ionic liquids as suitable ammonia absorbents on the basis of thermodynamic and kinetic analysis[J]. Separation and Purification Technology, 2012, 95: 188-195. |
| 49 | PALOMAR Jose, Maria GONZALEZ-MIQUEL, BEDIA Jorge, et al. Task-specific ionic liquids for efficient ammonia absorption[J]. Separation and Purification Technology, 2011, 82: 43-52. |
| 50 | ZENG S J, LIU L, SHANG D W, et al. Efficient and reversible absorption of ammonia by cobalt ionic liquids through Lewis acid-base and cooperative hydrogen bond interactions[J]. Green Chemistry, 2018, 20(9): 2075-2083. |
| 51 | PAIVA Alexandre, CRAVEIRO Rita, AROSO Ivo, et al. Natural deep eutectic solvents-solvents for the 21st century[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(5): 1063-1071. |
| 52 | CHEN K J, SCOTT H S, MADDEN D G, et al. Benchmark C2H2/CO2 and CO2/C2H2 separation by two closely related hybrid ultramicroporous materials[J]. Chem., 2016, 1(5): 753-765. |
| 53 | OU J Z, GE Wanyin y, CAREY B, et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing[J]. ACS Nano, 2015, 9(10): 10313-10323. |
| 54 | WANG Xiaoxing, MA Xiaoliang, ZHAO Shuqi, et al. Nanoporous molecular basket sorbent for NO2 and SO2 capture based on a polyethylene glycol-loaded mesoporous molecular sieve[J]. Energy & Environmental Science, 2009, 2(8): 878-882. |
| 55 | YANG Cuiting, MIAO Guang, PI Yunhong, et al. Abatement of various types of VOCs by adsorption/catalytic oxidation: a review[J]. Chemical Engineering Journal, 2019, 370: 1128-1153. |
| 56 | YANG Ralpht. Gas separation by adsorption processes[J]. Chemical Engineering Science, 1988, 43(4): 985. |
| 57 | VOHRA M. Treatment of gaseous ammonia emissions using date palm pits based granular activated carbon[J]. International Journal of Environmental Research and Public Health, 2020, 17(5): 14. |
| 58 | HUANG C C, LI H S, CHEN C H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia[J]. Journal of Hazardous Materials, 2008, 159(2-3): 523-527. |
| 59 | TENG H S, YEH T S, HSU L Y. Preparation of activated carbon from bituminous coal with phosphoric acid activation[J]. Carbon, 1998, 36(9): 1387-1395. |
| 60 | MOCHIZUKI Takuya, KUBOTA Mitsuhiro, MATSUDA Hitoki, et al. Adsorption behaviors of ammonia and hydrogen sulfide on activated carbon prepared from petroleum coke by KOH chemical activation[J]. Fuel Processing Technology, 2016, 144: 164-169. |
| 61 | FORTIER H, WESTREICH P, SELIG S, et al. Ammonia, cyclohexane, nitrogen and water adsorption capacities of an activated carbon impregnated with increasing amounts of ZnCl2, and designed to chemisorb gaseous NH3 from an air stream[J]. Journal of Colloid and Interface Science, 2008, 320(2): 423-435. |
| 62 | WANG J T, JIANG W Y, ZHANG Z X, et al. Mesoporous carbon beads impregnated with transition metal chlorides for regenerative removal of ammonia in the atmosphere[J]. Industrial & Engineering Chemistry Research, 2017, 56(12): 3283-3290. |
| 63 | PETIT Camille, KARWACKI Christopher, PETERSON Greg, et al. Interactions of ammonia with the surface of microporous carbon impregnated with transition metal chlorides[J]. Journal of Physical Chemistry C, 2007, 111(34): 12705-12714. |
| 64 | PARK S J, JIN S Y. Effect of ozone treatment on ammonia removal of activated carbons[J]. Journal of Colloid and Interface Science, 2005, 286(1): 417-419. |
| 65 | KIM B J, PARK S J. Effects of carbonyl group formation on ammonia adsorption of porous carbon surfaces[J]. Journal of Colloid and Interface Science, 2007, 311(1): 311-314. |
| 66 | LEPINASSE E, SPINNER B. Production de froid par couplage de réacteurs solide-gaz I: Analyse des performances de tels systèmes[J]. International Journal of Refrigeration, 1994, 17(5): 309-322. |
| 67 | MOCHIDA I, KORAI Y, SHIRAHAMA M, et al. Removal of SOx and NOx over activated carbon fibers[J]. Carbon, 2000, 38(2): 227-239. |
| 68 | PARK S J, KIM B J. Ammonia removal of activated carbon fibers produced by oxyfluorination[J]. Journal of Colloid and Interface Science, 2005, 291(2): 597-599. |
| 69 | ZHENG W H, HU J T, RAPPEPORT S, et al. Activated carbon fiber composites for gas phase ammonia adsorption[J]. Microporous and Mesoporous Materials, 2016, 234: 146-154. |
| 70 | SAMADDAR P, SON Y S, TSANG D C W, et al. Progress in graphene-based materials as superior media for sensing, sorption, and separation of gaseous pollutants[J]. Coordination Chemistry Reviews, 2018, 368: 93-114. |
| 71 | SEREDYCH M, BANDOSZ T J. Adsorption of ammonia on graphite oxide/aluminium polycation and graphite oxide/zirconium-aluminium polyoxycation composites[J]. Journal of Colloid and Interface Science, 2008, 324(1-2): 25-35. |
| 72 | LI Yunguo, PATHAK Biswarup, NISAR Jawad, et al. Metal-decorated graphene oxide for ammonia adsorption[J]. EPL, 2013, 103(2): 28007. |
| 73 | PETIT C, BANDOSZ T J. Graphite oxide/polyoxometalate nanocomposites as adsorbents of ammonia[J]. Journal of Physical Chemistry C, 2009, 113(9): 3800-3809. |
| 74 | HELMINEN J, HELENIUS J, PAATERO E, et al. Adsorption equilibria of ammonia gas on inorganic and organic sorbents at 298.15K[J]. Journal of Chemical and Engineering Data, 2001, 46(2): 391-399. |
| 75 | MATITO-MARTOS I, MARTIN-CALVO A, ANIA C O, et al. Role of hydrogen bonding in the capture and storage of ammonia in zeolites[J]. Chemical Engineering Journal, 2020, 387:124062. |
| 76 | SAHA Dipendu, DENG Shuguang. Characteristics of ammonia adsorption on activated alumina[J]. Journal of Chemical and Engineering Data, 2010, 55(12): 5587-5593. |
| 77 | KIM J, LEE H, VO H T, et al. Bead-shaped mesoporous alumina adsorbents for adsorption of ammonia[J]. Materials, 2020, 13(6): 13. |
| 78 | LIU C Y, AIKA K. Absorption and desorption behavior of ammonia with alkali earth halide and mixed halide[J]. Chemistry Letters, 2002, (8): 798-799. |
| 79 | HUBERTY Mark S, WAGNER Andrew L, MCCORMICK Alon, et al. Ammonia absorption at haber process conditions[J]. AIChE Journal, 2012, 58(11): 3526-3532. |
| 80 | MALMALI Mandi, LE Giang, HENDRICKSON Jennifer, et al. Better absorbents for ammonia separation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(5): 6536-6546. |
| 81 | WESTMAN S, WERNER P E, SCHULER T, et al. X-ray investigations of ammines of alkaline earth metal halides. I. the structures of CaCl2(NH3)8, CaCl2(NH3)2 and the decomposition product CaClOH[J]. Acta Chemica Scandinavica Series A:Physical and Inorganic Chemistry, 1981, 35(7): 467-472. |
| 82 | JIANG Yong, TAKAHASHI Akira, KAWAMOTO Tohru, et al. High performance sorption and desorption behaviours at high working temperatures of ammonia gas in a cobalt-substituted prussian blue analogue[J]. Chemical Communications, 2018, 54(84): 11961-11964. |
| 83 | JIANG Yong, TAKAHASHI Akira, KAWAMOTO Tohru, et al. Unique adsorption and desorption behaviour of ammonia gas at heating temperature using the Prussian blue analogue Zn3[Co(CN)6]2[J]. Inorganica Chimica Acta, 2020, 501: 119273. |
| 84 | TAKAHASHI Akira, MINAMI Kimitaka, NODA Keiko, et al. Trace ammonia removal from air by selective adsorbents reusable with water[J]. ACS Applied Materials & Interfaces, 2020, 12(13): 15115-15119. |
| 85 | REZAEI E, SCHLAGETER B, NEMATI M, et al. Evaluation of metal oxide nanoparticles for adsorption of gas phase ammonia[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 422-431. |
| 86 | 崔希利, 邢华斌. 金属有机框架材料分离低碳烃的研究进展[J]. 化工学报, 2018, 69(6): 2339-2352. |
| CUI Xili, XING Huabin. Separation of light hydrocarbons with metal-organic frameworks [J]. Journal of Chemical Industry and Engineering, 2018, 69(6): 2339-2352. | |
| 87 | PETERSON G W, WAGNER G W, BALBOA A, et al. Ammonia vapor removal by Cu3(BTC)2 and its characterization by MAS NMR[J]. Journal of Physical Chemistry C, 2009, 113(31): 13906-13917. |
| 88 | CHUI S S Y, LO S M F, CHARMANT J P H, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n[J]. Science, 1999, 283(5405): 1148-1150. |
| 89 | Grant GLOVER T, PETERSON Gregory W, SCHINDLER Bryan J, et al. MOF-74 building unit has a direct impact on toxic gas adsorption[J]. Chemical Engineering Science, 2011, 66(2): 163-170. |
| 90 | SPANOPOULOS Ioannis, XYDIAS Pantelis, MALLIAKAS Christos D, et al. A straight forward route for the development of metal-organic frameworks functionalized with aromatic -OH groups: synthesis, characterization, and gas (N2, Ar, H2, CO2, CH4, NH3) sorption properties[J]. Inorganic Chemistry, 2013, 52(2): 855-862. |
| 91 | JASUJA Himanshu, PETERSON Gregory W, DECOSTE Jared B, et al. Evaluation of MOFs for air purification and air quality control applications: ammonia removal from air[J]. Chemical Engineering Science, 2015, 124: 118-124. |
| 92 | RIETH Adam J, TULCHINSKY Yuri, DINCA Mircea. High and reversible ammonia uptake in mesoporous azolate metal organic frameworks with open Mn, Co, and Ni sites[J]. Journal of the American Chemical Society, 2016, 138(30): 9401-9404. |
| 93 | RIETH Adam J, DINCA Mircea. Controlled gas uptake in metal-organic frameworks with record ammonia sorption[J]. Journal of the American Chemical Society, 2018, 140(9): 3461-3466. |
| 94 | GODFREY Harry G W, SILVA Ivan DA, BRIGGS Lydia, et al. Ammonia storage by reversible host-guest site exchange in a robust metal-organic framework[J]. Angewandte Chemie International Edition, 2018, 57(45): 14778-14781. |
| 95 | ZHANG Yuanyuan, ZHANG Xuan, CHEN Zhijie, et al. A flexible interpenetrated zirconium-based metal-organic framework with high affinity toward ammonia[J]. ChemSusChem, 2020, 13(7): 1710-1714. |
| 96 | CHEN Yang, DU Yadan, LIU Puxu, et al. Removal of ammonia emissions via reversible structural transformation in M(BDC) (M=Cu, Zn, Cd) metal-organic frameworks[J]. Environmental Science & Technology, 2020, 54(6): 3636-3642. |
| 97 | BRITT David, TRANCHEMONTAGNE David, YAGHI Omar M. Metal-organic frameworks with high capacity and selectivity for harmful gases[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(33): 11623-11627. |
| 98 | PETIT Camille, HUANG Liangliang, JAGIELLO Jacek, et al. Toward understanding reactive adsorption of ammonia on Cu-MOF/graphite oxide nanocomposites[J]. Langmuir, 2011, 27(21): 13043-13051. |
| 99 | DECOSTE J B, PETERSON G W, SCHINDLER B J, et al. The effect of water adsorption on the structure of the carboxylate containing metal-organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66[J]. Journal of Materials Chemistry A, 2013, 1(38): 11922-11932. |
| 100 | GHOSH P, KIM K C, SNURR R Q. Modeling water and ammonia adsorption in hydrophobic metal-organic frameworks: single components and mixtures[J]. Journal of Physical Chemistry C, 2014, 118(2): 1102-1110. |
| 101 | CHEN C C, ZHU H, LI B G, et al. Structuring metal-organic framework materials into hierarchically porous composites through one-pot fabrication strategy[J]. Chemistry: A European Journal, 2020, 26(15): 3358-3363. |
| 102 | DING M L, CAI X C, JIANG H L. Improving MOF stability: approaches and applications[J]. Chemical Science, 2019, 10(44): 10209-10230. |
| 103 | LIN Y Q, WU H C, YASUI T, et al. Development of an HKUST-1 nanofiller-templated poly(ether sulfone) mixed matrix membrane for a highly efficient ultrafiltration process[J]. ACS Applied Materials & Interfaces, 2019, 11(20): 18782-18796. |
| 104 | PETIT Camille, MENDOZA Barbara, BANDOSZ Teresa J. Reactive adsorption of ammonia on Cu-based MOF/graphene composites[J]. Langmuir, 2010, 26(19): 15302-15309. |
| 105 | TRAN THANH Tung, MANH TRUNG Tran, FELLER Jean-Francois, et al. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers[J]. Carbon, 2020, 159: 333-344. |
| 106 | ZHANG Y Y, ZHANG X, CHEN Z J, et al. A flexible interpenetrated zirconium-based metal-organic framework with high affinity toward ammonia[J]. Chemsuschem, 2020, 13(7): 1710-1714. |
| 107 | YANG S H, SUN J L, RAMIREZ-CUESTA A J, et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host[J]. Nature Chemistry, 2012, 4(11): 887-894. |
| 108 | AGUILA Briana, SUN Qi, PERMAN Jason A, et al. Efficient mercury capture using functionalized porous organic polymer[J]. Advanced Materials, 2017, 29(31): 1700665. |
| 109 | SHAO Lishu, SANG Yafei, HUANG Jianhan, et al. Triazine-based hyper-cross-linked polymers with inorganic-organic hybrid framework derived porous carbons for CO2 capture[J]. Chemical Engineering Journal, 2018, 353: 1-14. |
| 110 | BHANJA Piyali, MODAK Arindam, BHAUMIK Asim. Porous organic polymers for CO2 storage and conversion reactions[J]. ChemCatChem, 2019, 11(1): 244-257. |
| 111 | LIANG J, HUANG Y B, CAO R. Metal-organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates[J]. Coordination Chemistry Reviews, 2019, 378: 32-65. |
| 112 | GE Yuxin, ZHOU Hao, JI Yujin, et al. Understanding water adsorption and the impact on CO2 capture in chemically stable covalent organic frameworks[J]. Journal of Physical Chemistry C, 2018, 122(48): 27495-27506. |
| 113 | SHINDE Digambar B, OSTWAL Mayur, WANG Xinbo, et al. Chlorine-functionalized keto-enamine-based covalent organic frameworks for CO2 separation and capture[J]. CrystEngComm, 2018, 20(47): 7621-7625. |
| 114 | WANG Mengting, ZHOU Xin, ZANG Xiaohuan, et al. Determination of pesticides residues in vegetable and fruit samples by solid-phase microextraction with a covalent organic framework as the fiber coating coupled with gas chromatography and electron capture detection[J]. Journal of Separation Science, 2018, 41(21): 4038-4046. |
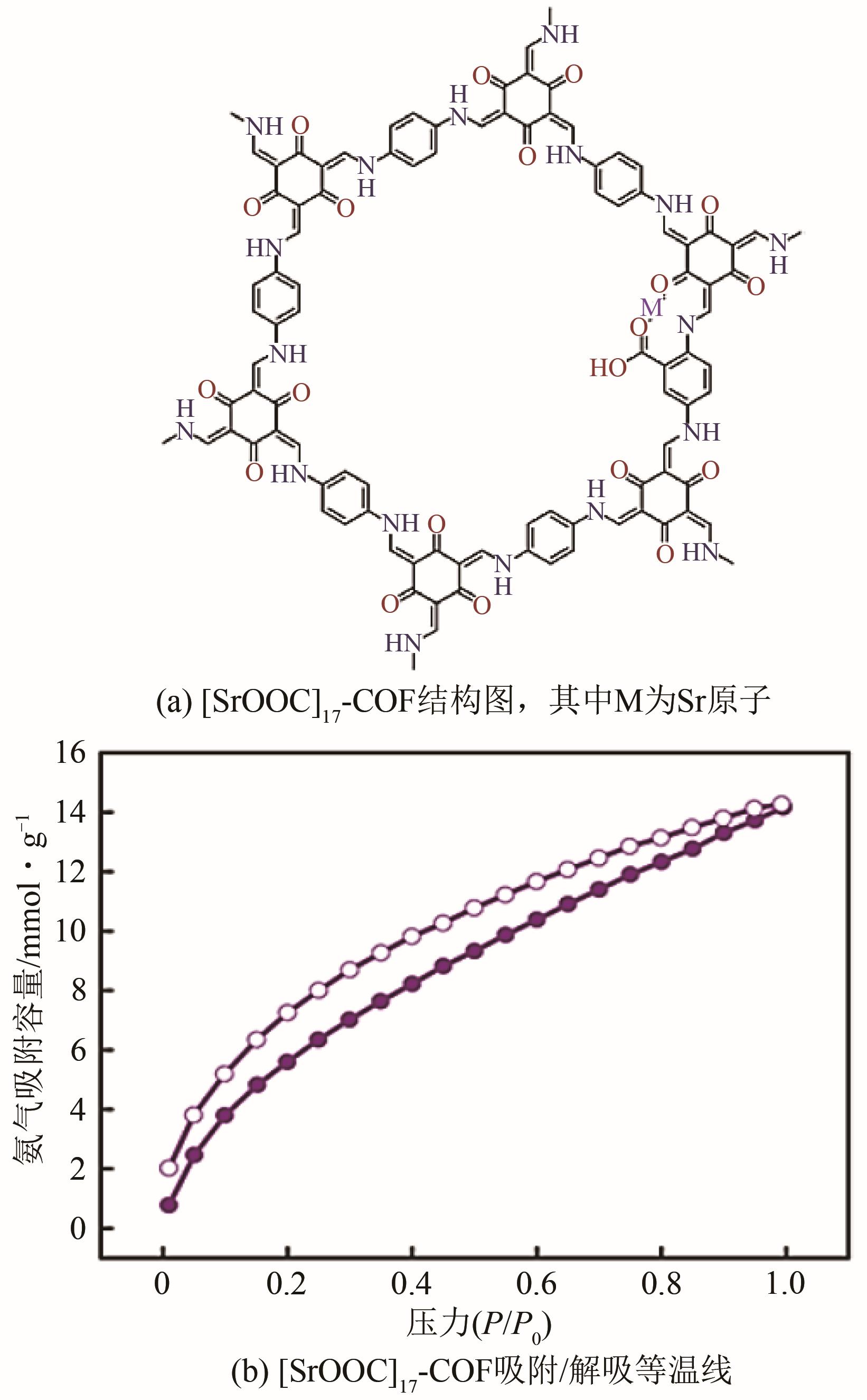
| 115 | YANG Yajie, FAHEEM Muhammad, WANG Lili, et al. Surface pore engineering of covalent organic frameworks for ammonia capture through synergistic multivariate and open metal site approaches[J]. ACS Central Science, 2018, 4(6): 748-754. |
| 116 | CHENG Youdong, ZHAI Linzhi, YING Yunpan, et al. Highly efficient CO2 capture by mixed matrix membranes containing three-dimensional covalent organic framework fillers[J]. Journal of Materials Chemistry A, 2019, 7(9): 4549-4560. |
| 117 | LI Peng, HE Yabing, GUANG Jie, et al. A homochiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohols[J]. Journal of the American Chemical Society, 2014, 136(2): 547-549. |
| 118 | LI Peng, HE Yabing, ZHAO Yunfeng, et al. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature[J]. Angewandte Chemie International Edition, 2015, 54(2): 574-577. |
| 119 | WANG Hailong, LI Bin, WU Hui, et al. A flexible microporous hydrogen-bonded organic framework for gas sorption and separation[J]. Journal of the American Chemical Society, 2015, 137(31): 9963-9970. |
| 120 | HISAKI Ichiro, SUZUKI Yuto, GOMEZ Eduardo, et al. Acid responsive hydrogen-bonded organic frameworks[J]. Journal of the American Chemical Society, 2019, 141(5): 2111-2121. |
| 121 | LIN R B, HE Y B, LI P, et al. Multifunctional porous hydrogen-bonded organic framework materials[J]. Chemical Society Reviews, 2019, 48(5): 1362-1389. |
| 122 | HUMBECK Jeffrey F VAN, MCDONALD Thomas M, JING Xiaofei, et al. Ammonia capture in porous organic polymers densely functionalized with bronsted acid groups[J]. Journal of the American Chemical Society, 2014, 136(6): 2432-2440. |
| 123 | DOONAN Christian J, TRANCHEMONTAGNE David J, Grant GLOVER T, et al. Exceptional ammonia uptake by a covalent organic framework[J]. Nature Chemistry, 2010, 2(3): 235-238. |
| 124 | KANG D W, KANG M J, KIM H J, et al. A hydrogen-bonded organic framework (HOF) with type Ⅳ NH3 adsorption behavior[J]. Angewandte Chemie International Edition, 2019, 58(45): 16152-16155. |
| [1] | 李季桐, 王刚, 熊亚选, 徐钱. 不同工质单效吸收式制冷系统的能量和㶲分析[J]. 化工进展, 2023, 42(S1): 104-112. |
| [2] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [3] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [4] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [5] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [6] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [7] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [8] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [11] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [12] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [13] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [14] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [15] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||