化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5121-5134.DOI: 10.16085/j.issn.1000-6613.2022-2053
sⅡ型水合物储氢研究进展
岳子瀚1,2,3,4,5( ), 龙臻1,2,3,4(
), 龙臻1,2,3,4( ), 周雪冰1,2,3,4, 臧小亚1,2,3,4, 梁德青1,2,3,4(
), 周雪冰1,2,3,4, 臧小亚1,2,3,4, 梁德青1,2,3,4( )
)
- 1.中国科学院广州能源研究所,广东 广州 510640
2.中国科学院天然气水合物重点实验室,广东 广州 510640
3.广东省新能源和可再生能源研究开发与应用重点实验室,广东 广州 510640
4.天然气水合物国家重点实验室,北京 100028
5.中国科学技术大学纳米科学技术学院,江苏 苏州 215123
-
收稿日期:2022-11-04修回日期:2023-02-03出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:龙臻,梁德青 -
作者简介:岳子瀚(1998—),男,硕士研究生,研究方向为新型固态储氢技术。E-mail:zhyue1@mail.ustc.edu.cn。 -
基金资助:国家自然科学基金(51506202);广东省特支计划(2019BT02L278);广东省重点领域研发计划(2020B111101000403);广东省自然科学基金面上项目(2020A1515010374)
State of the art on hydrogen storage of sⅡ clathrate hydrate
YUE Zihan1,2,3,4,5( ), LONG Zhen1,2,3,4(
), LONG Zhen1,2,3,4( ), ZHOU Xuebing1,2,3,4, ZANG Xiaoya1,2,3,4, LIANG Deqing1,2,3,4(
), ZHOU Xuebing1,2,3,4, ZANG Xiaoya1,2,3,4, LIANG Deqing1,2,3,4( )
)
- 1.Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, Guangdong, China
2.Key Laboratory of Gas Hydrate, Chinese Academy of Sciences, Guangzhou 510640, Guangdong, China
3.Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou 510640, Guangdong, China
4.State Key Laboratory of Natural Gas Hydrate, Beijing 100028, China
5.Nano Science and Technology Institute, University of Science and Technology of China, Suzhou 215123, Jiangsu, China
-
Received:2022-11-04Revised:2023-02-03Online:2023-10-15Published:2023-11-11 -
Contact:LONG Zhen, LIANG Deqing
摘要:
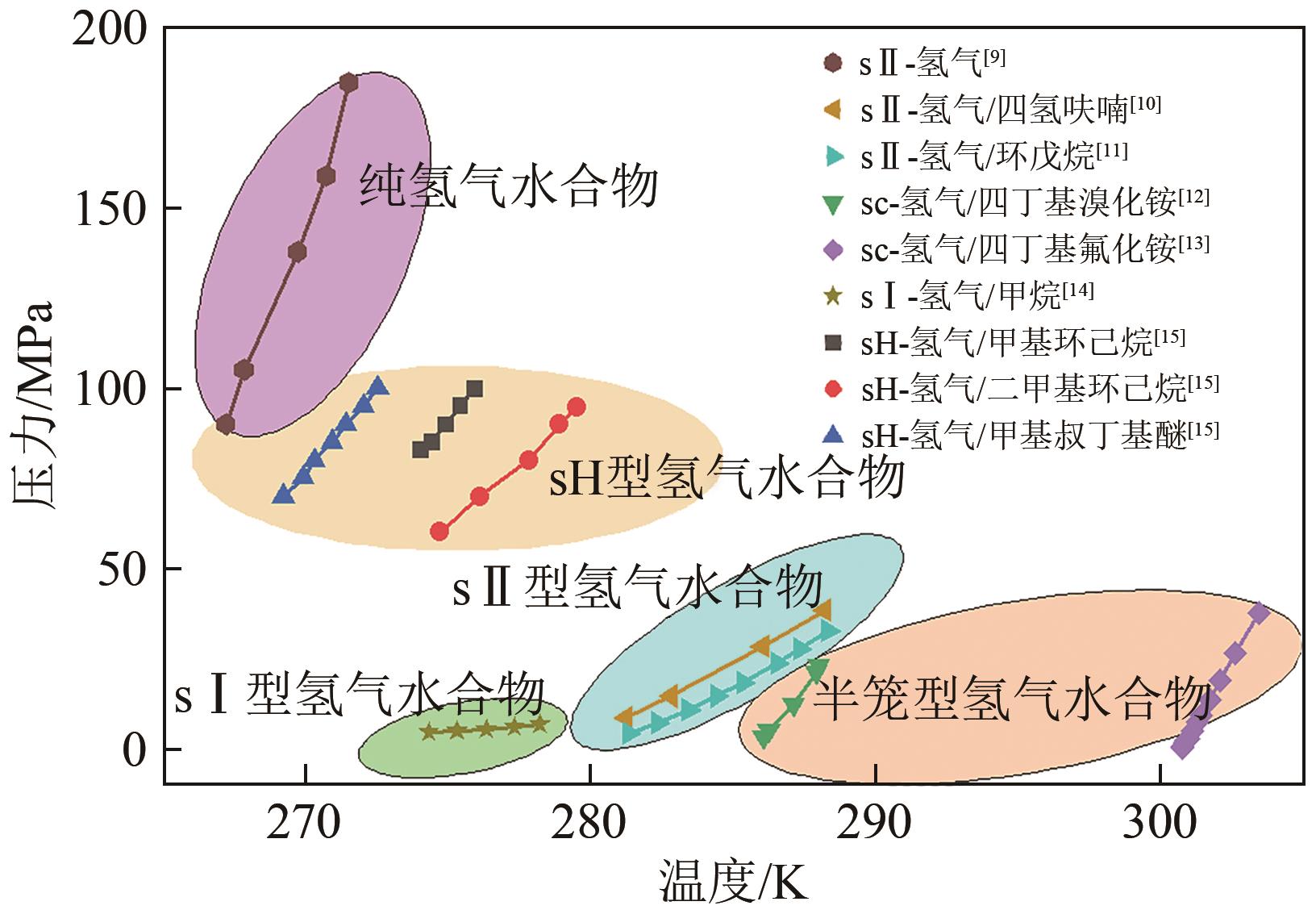
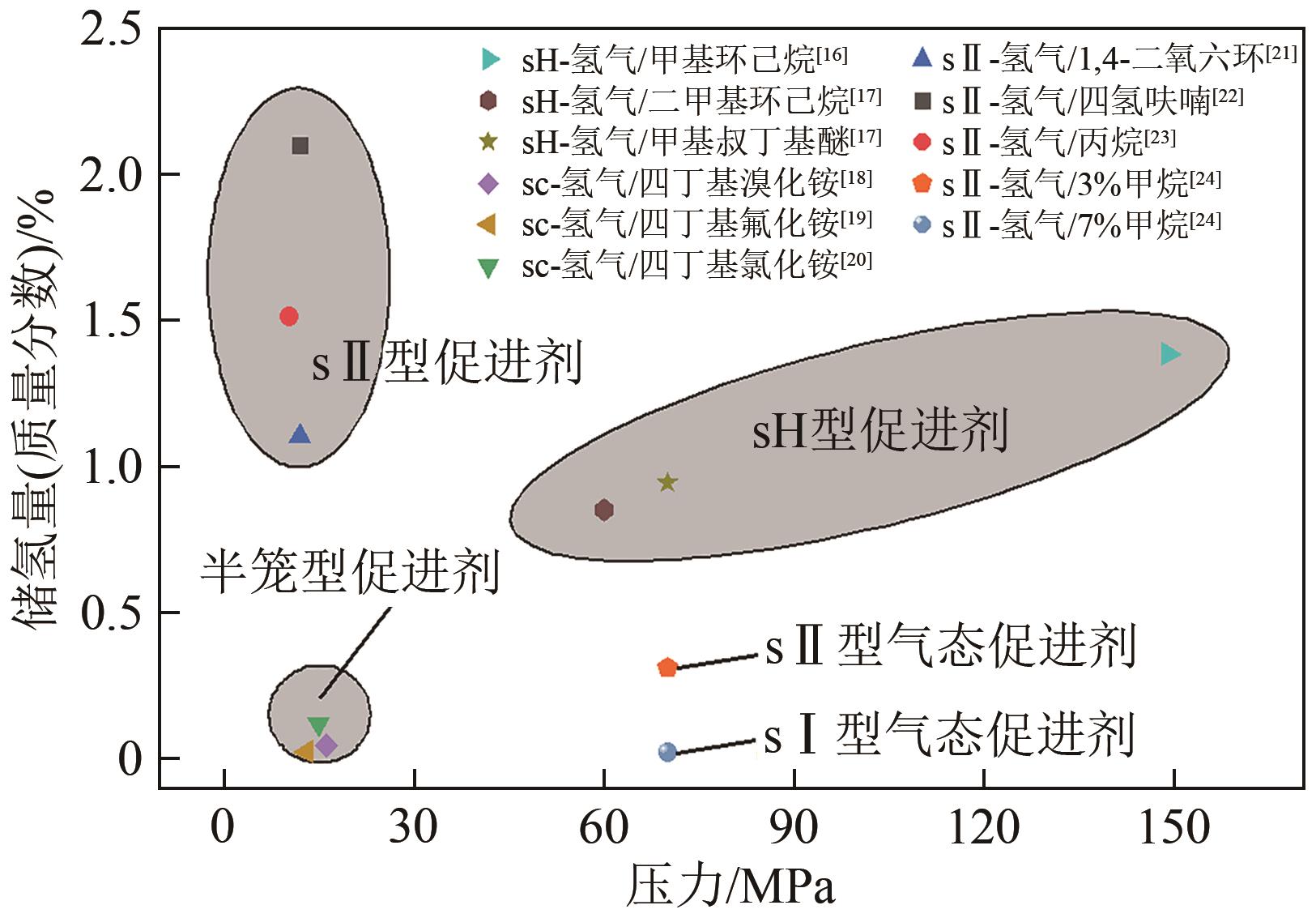
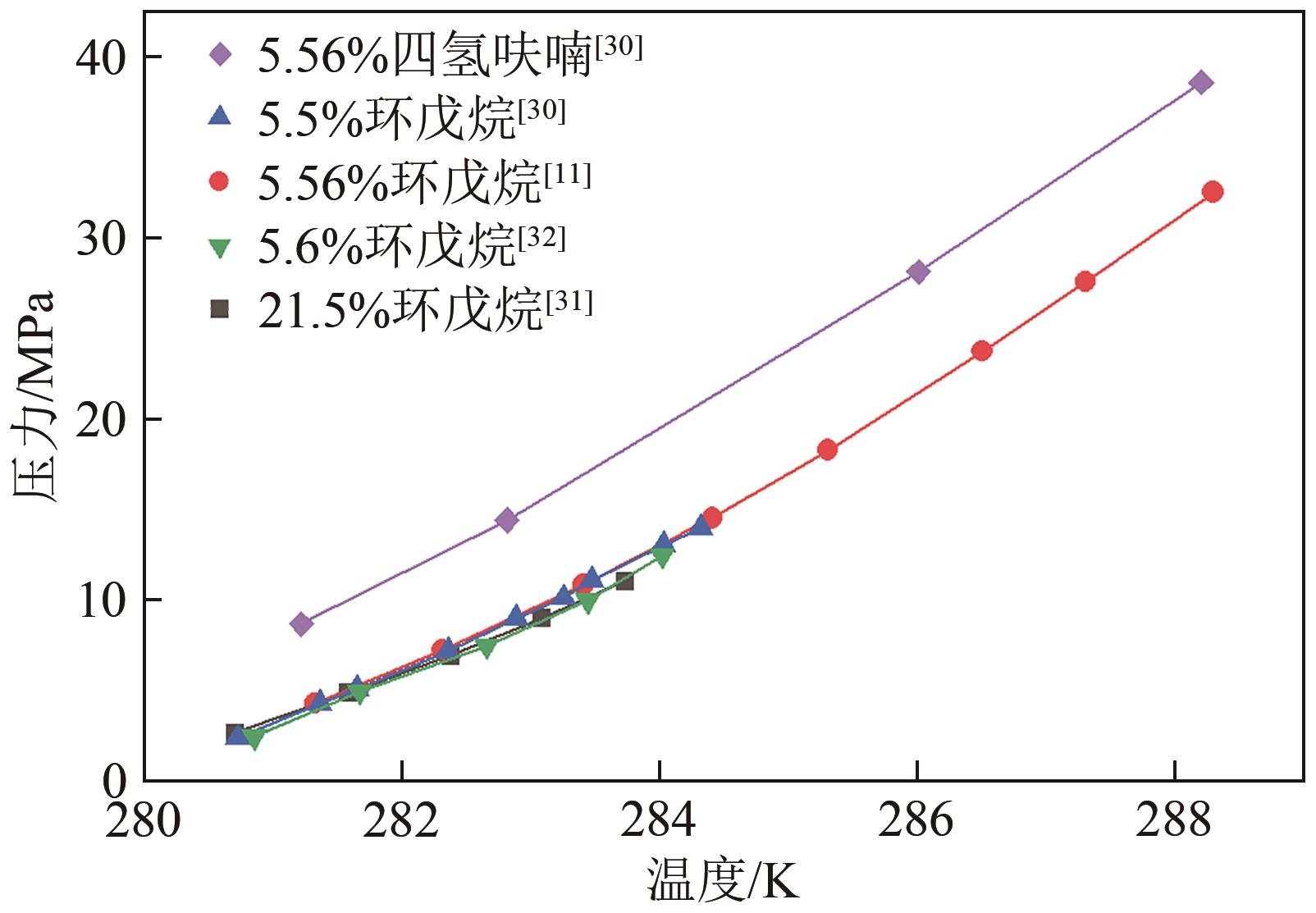
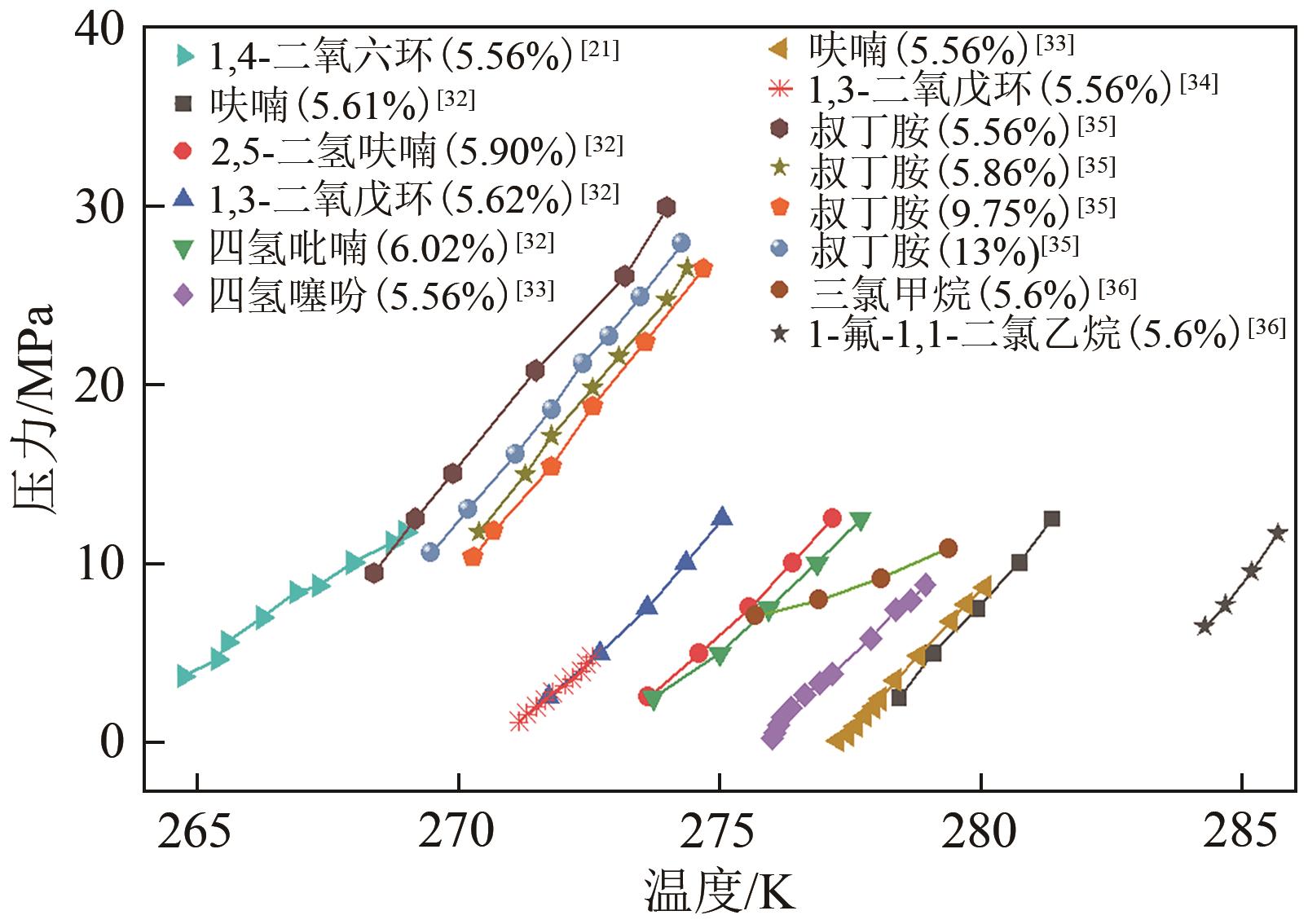
氢能作为一种来源广泛、清洁、零碳的绿色能源载体,是我国实现碳达峰、碳中和愿景的重要抓手。由于氢易燃、易爆、易扩散,如何安全、高效、经济储氢是氢能发展的技术挑战之一。水合物固态储氢则是在不同种类促进剂作用下,通过水分子间氢键作用而形成的三维笼型结构(如Ⅰ型、Ⅱ型、H型以及半笼型)“捕获”氢气分子。综合考量储氢密度和水合物生成稳定性,发现sⅡ型水合物促进剂相对最具有应用潜力。本文首先归纳现有sⅡ型水合物促进剂对氢气水合物生成热力学条件的影响规律,然后比较分析了不同促进剂体系氢气水合物的储氢性能以及晶体微观结构的变化,最后总结国内外目前发展趋势,为水合物储氢的工业化应用提供理论依据和指导。
中图分类号:
引用本文
岳子瀚, 龙臻, 周雪冰, 臧小亚, 梁德青. sⅡ型水合物储氢研究进展[J]. 化工进展, 2023, 42(10): 5121-5134.
YUE Zihan, LONG Zhen, ZHOU Xuebing, ZANG Xiaoya, LIANG Deqing. State of the art on hydrogen storage of sⅡ clathrate hydrate[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5121-5134.
| 1 | 中共中央国务院关于完整准确全面贯彻新发展理念做好碳达峰碳中和工作的意见[N]. 人民日报, 2021-10-25(1). |
| Opinions of the State Council of the CPC Central Committee on completely, accurately and comprehensively implementing the new development concept and doing a good job of carbon peak and carbon neutralization[N]. People’s Daily, 2021-10-25(1). | |
| 2 | 岳来群. 中国确定“十四五”规划及2035年远景目标 油气企业加快改革创新适应转型发展[J]. 国际石油经济, 2021, 29(1): 51-52. |
| YUE Laiqun. China’s 14th Five-Year Plan and long-term goal for 2035 promote oil and gas enterprises to adapt innovation and transformation[J]. International Petroleum Economics, 2021, 29(1): 51-52. | |
| 3 | 高佳佳, 米媛媛, 周洋, 等. 新型储氢材料研究进展[J]. 化工进展, 2021, 40(6): 2962-2971. |
| GAO Jiajia, MI Yuanyuan, ZHOU Yang, et al. Recent developments in new hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 2962-2971. | |
| 4 | 陈晓露, 刘小敏, 王娟, 等. 液氢储运技术及标准化[J]. 化工进展, 2021, 40(9): 4806-4814. |
| CHEN Xiaolu, LIU Xiaomin, WANG Juan, et al. Technology and standardization of liquid hydrogen storage and transportation[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4806-4814. | |
| 5 | SCHLAPBACH L, ZUTTEL A. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414(6861): 353-358. |
| 6 | VOS W L, FINGER L W, HEMLEY R J, et al. Novel H2-H2O clathrates at high pressures[J]. Physical Review Letters, 1993, 71(19): 3150-3153. |
| 7 | MAO W L, MAO H K, GONCHAROV A F, et al. Hydrogen clusters in clathrate hydrate[J]. Science, 2002, 297(5590): 2247-2249. |
| 8 | FLORUSSE L J, PETERS C J, SCHOONMAN J, et al. Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate[J]. Science, 2004, 306(5695): 469-471. |
| 9 | DYADIN Y A, LARIONOV E G, MANAKOV A Y, et al. Clathrate hydrates of hydrogen and neon[J]. Mendeleev Communications, 1999, 9(5): 209-210. |
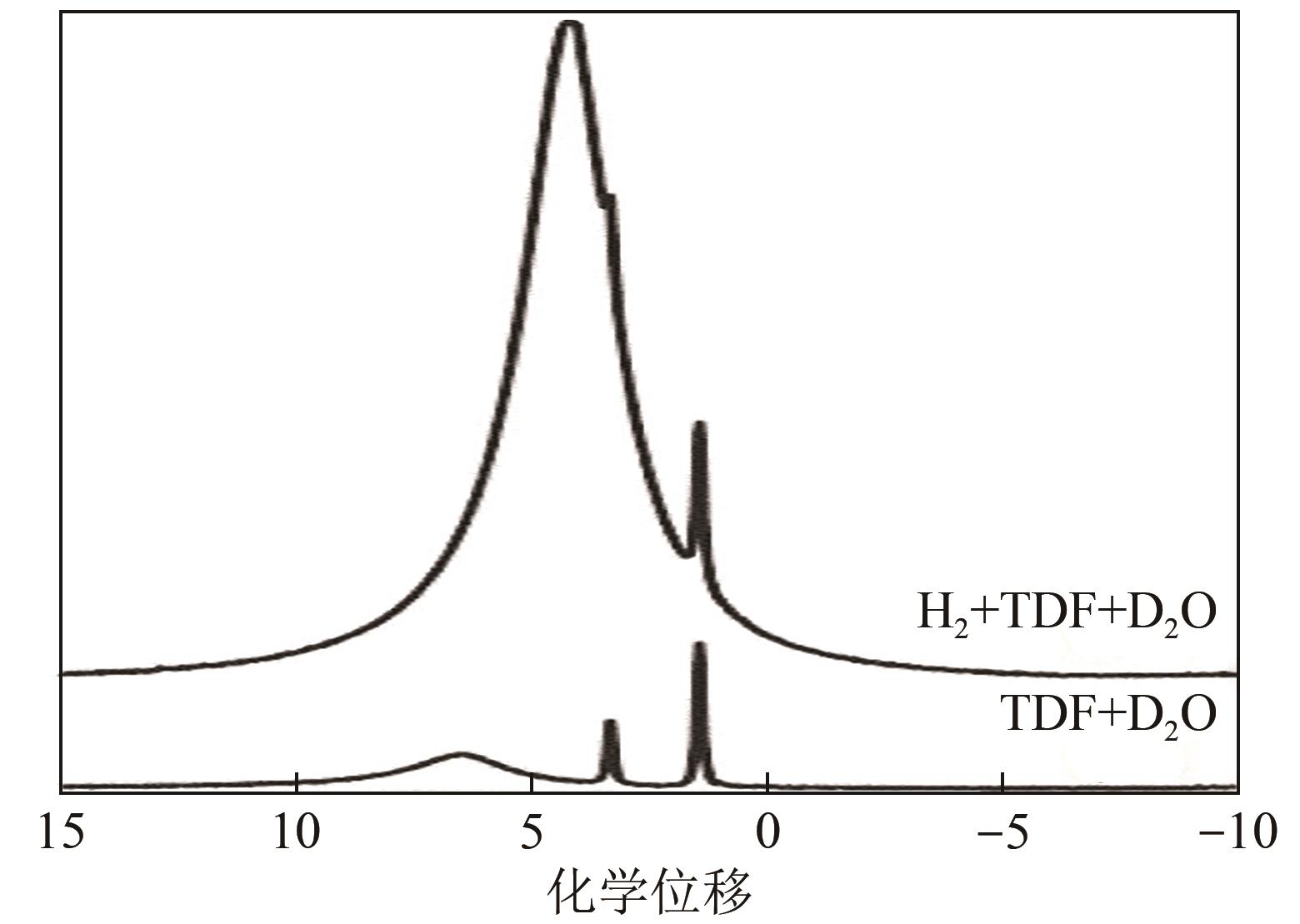
| 10 | HASHIMOTO S, SUGAHARA T, SATO H, et al. Thermodynamic stability of H2 + tetrahydrofuran mixed gas hydrate in nonstoichiometric aqueous solutions[J]. Journal of Chemical & Engineering Data, 2007, 52(2): 517-520. |
| 11 | DU Jianwei, LIANG Deqing, LI Dongliang, et al. Experimental determination of the equilibrium conditions of binary gas hydrates of cyclopentane+oxygen, cyclopentane+nitrogen, and cyclopentane+hydrogen[J]. Industrial & Engineering Chemistry Research, 2010, 49(22): 11797-11800. |
| 12 | ARJMANDI M, CHAPOY A, TOHIDI B. Equilibrium data of hydrogen, methane, nitrogen, carbon dioxide, and natural gas in semi-clathrate hydrates of tetrabutyl ammonium bromide[J]. Journal of Chemical & Engineering Data, 2007, 52(6): 2153-2158. |
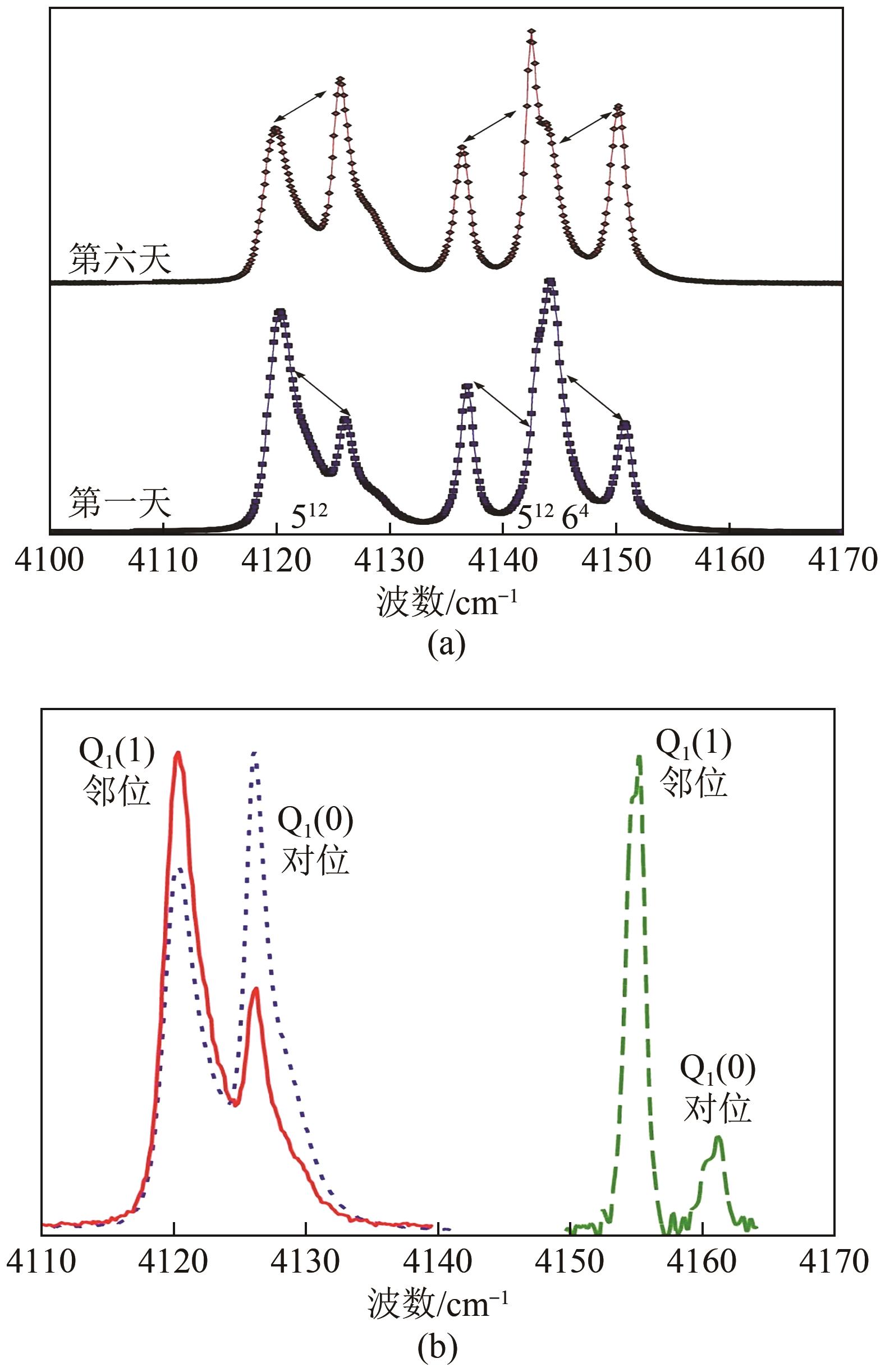
| 13 | SAKAMOTO J, HASHIMOTO S, TSUDA T, et al. Thermodynamic and Raman spectroscopic studies on hydrogen+tetra-n-butyl ammonium fluoride semi-clathrate hydrates[J]. Chemical Engineering Science, 2008, 63(24): 5789-5794. |
| 14 | ZHANG Shixi, CHEN Guangjin, MA Changfeng, et al. Hydrate formation of hydrogen + hydrocarbon gas mixtures[J]. Journal of Chemical & Engineering Data, 2000, 45(5): 908-911. |
| 15 | DUARTE A R C, SHARIATI A, PETERS C J. Phase equilibrium measurements of structure sH hydrogen clathrate hydrates with various promoters[J]. Journal of Chemical & Engineering Data, 2009, 54(5): 1628-1632. |
| 16 | PAPADIMITRIOU N I, TSIMPANOGIANNIS I N, PAPAIOANNOU A T, et al. Monte Carlo study of sⅡ and sH argon hydrates with multiple occupancy of cages[J]. Molecular Simulation, 2008, 34(10/11/12/13/14/15): 1311-1320. |
| 17 | MARTIN A, PETERS C J. Hydrogen storage in sH clathrate hydrates: Thermodynamic model[J]. The Journal of Physical Chemistry B, 2009, 113(21): 7558-7563. |
| 18 | TRUEBA A T, RADOVIC I R, ZEVENBERGEN J F, et al. Kinetics measurements and in situ Raman spectroscopy of formation of hydrogen-tetrabutylammonium bromide semi-hydrates[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5790-5797. |
| 19 | TRUEBA A T, RADOVIC I R, ZEVENBERGEN J F, et al. Kinetic measurements and in situ Raman spectroscopy study of the formation of TBAF semi-hydrates with hydrogen and carbon dioxide[J]. International Journal of Hydrogen Energy, 2013, 38(18): 7326-7334. |
| 20 | DESCHAMPS J, DALMAZZONE D. Hydrogen storage in semiclathrate hydrates of tetrabutyl ammonium chloride and tetrabutyl phosphonium bromide[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3395-3399. |
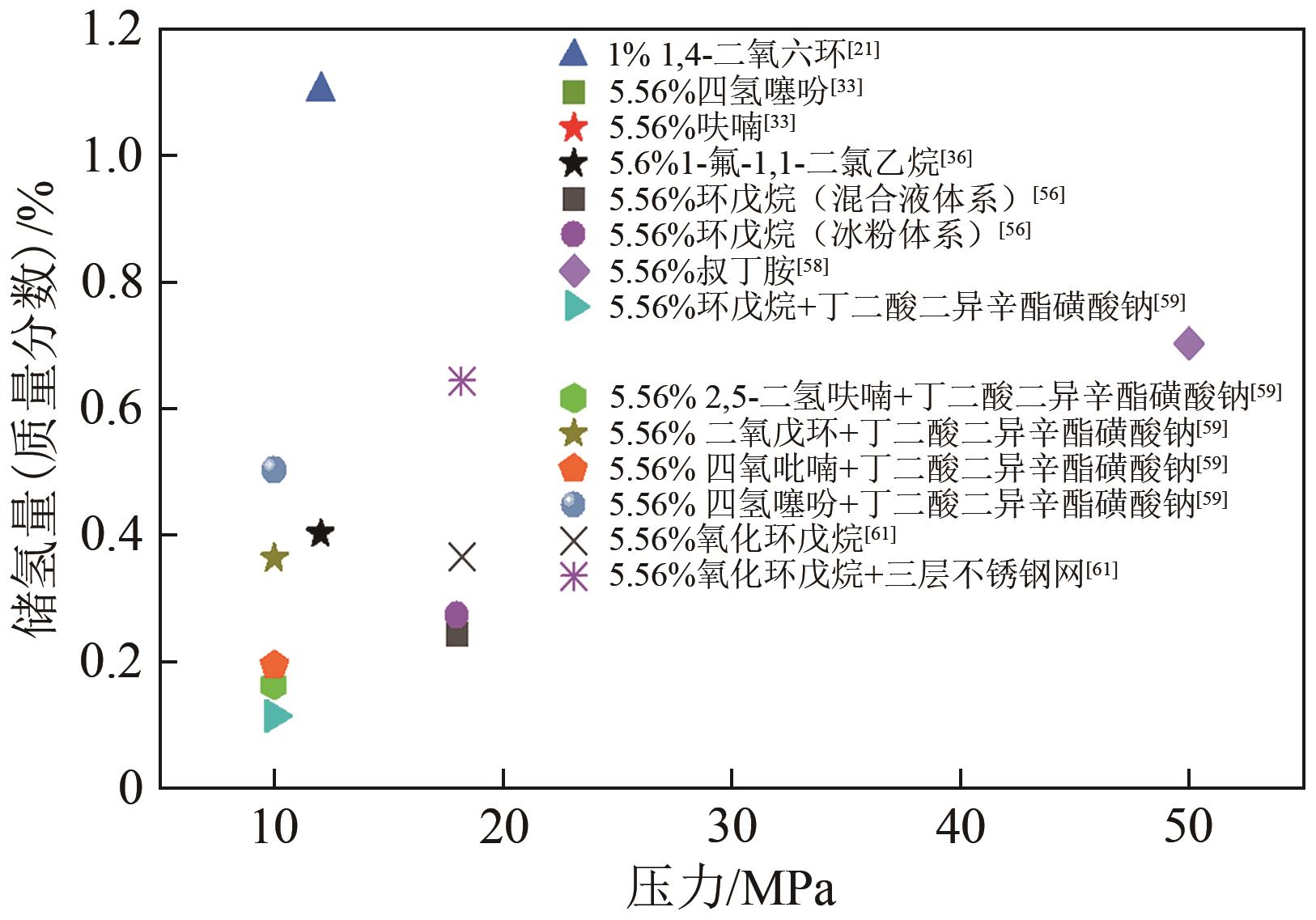
| 21 | YOON J H, HAN J, PARK J, et al. Spectroscopic identification, thermodynamic stability and molecular composition of hydrogen and 1,4-dioxane binary clathrate hydrate[J]. Journal of Physics and Chemistry of Solids, 2008, 69(5/6): 1432-1435. |
| 22 | LEE H, LEE J W, KIM D Y, et al. Tuning clathrate hydrates for hydrogen storage[J]. Nature, 2005, 434(7034): 743-746. |
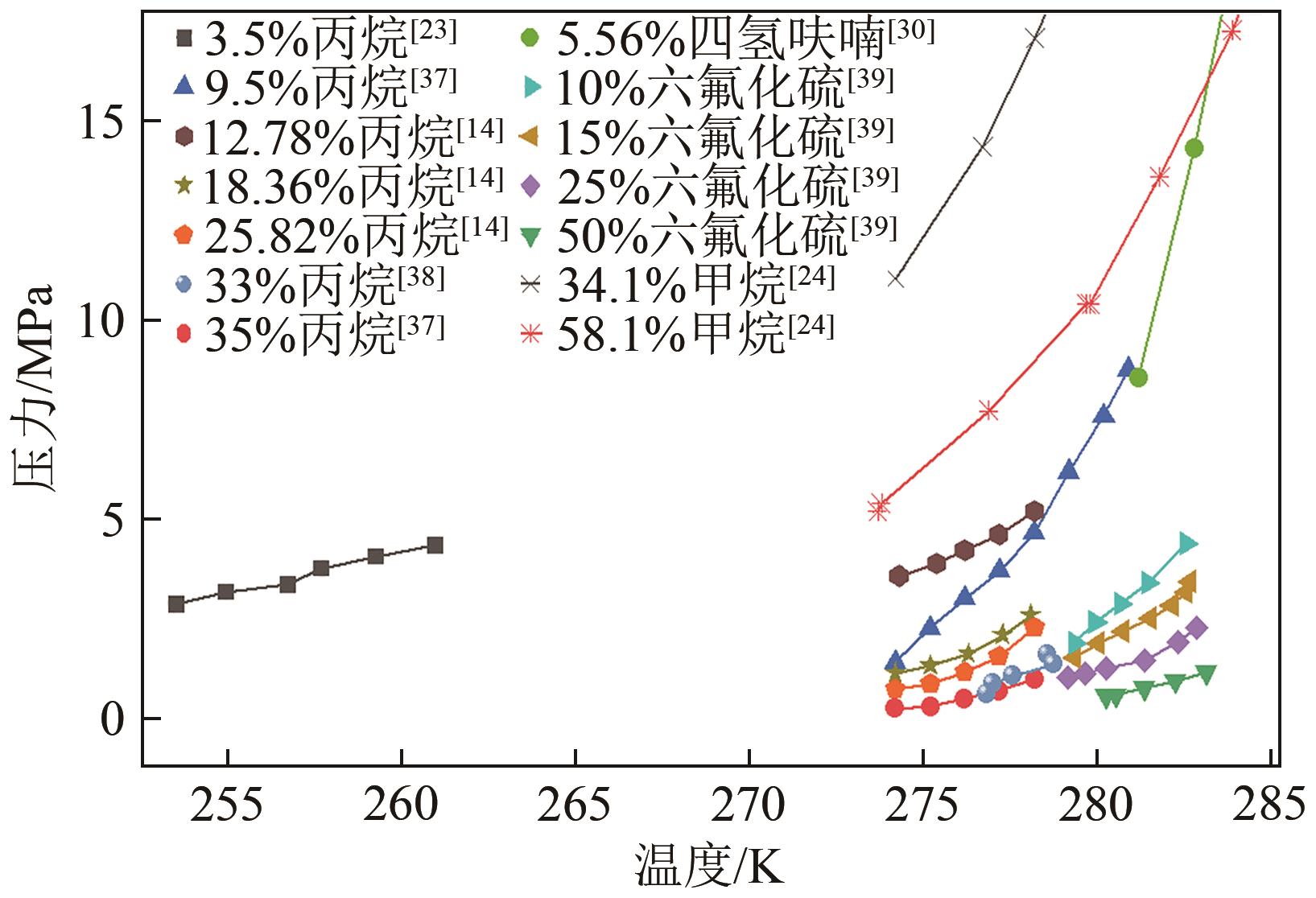
| 23 | 李诵. 丙烷促进水合物储存氢气实验研究[D]. 广州: 华南理工大学, 2018. |
| LI Song. Study of hydrogen storage in hydrate with propane acting as promoter[D]. Guangzhou: South China University of Technology, 2018. | |
| 24 | MATSUMOTO Y, GRIM R G, KHAN N M, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates[J].The Journal of Physical Chemistry C, 2014, 118(7): 3783-3788. |
| 25 | VELUSWAMY H P, KUMAR R, LINGA P. Hydrogen storage in clathrate hydrates: Current state of the art and future directions[J]. Applied Energy, 2014, 122: 112-132. |
| 26 | STROBEL T A, KOH C A, SLOAN E D. Water cavities of sH clathrate hydrate stabilized by molecular hydrogen[J]. The Journal of Physical Chemistry B, 2008, 112(7): 1885-1887. |
| 27 | 李昊阳, 张炜, 李小森, 等. 水合物储氢的研究进展[J]. 化工进展, 2022,41(12): 6285-6294. |
| LI Haoyang, ZHANG Wei, LI Xiaosen, et al. Research process of hydrate-based hydrogen storage[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6285-6294. | |
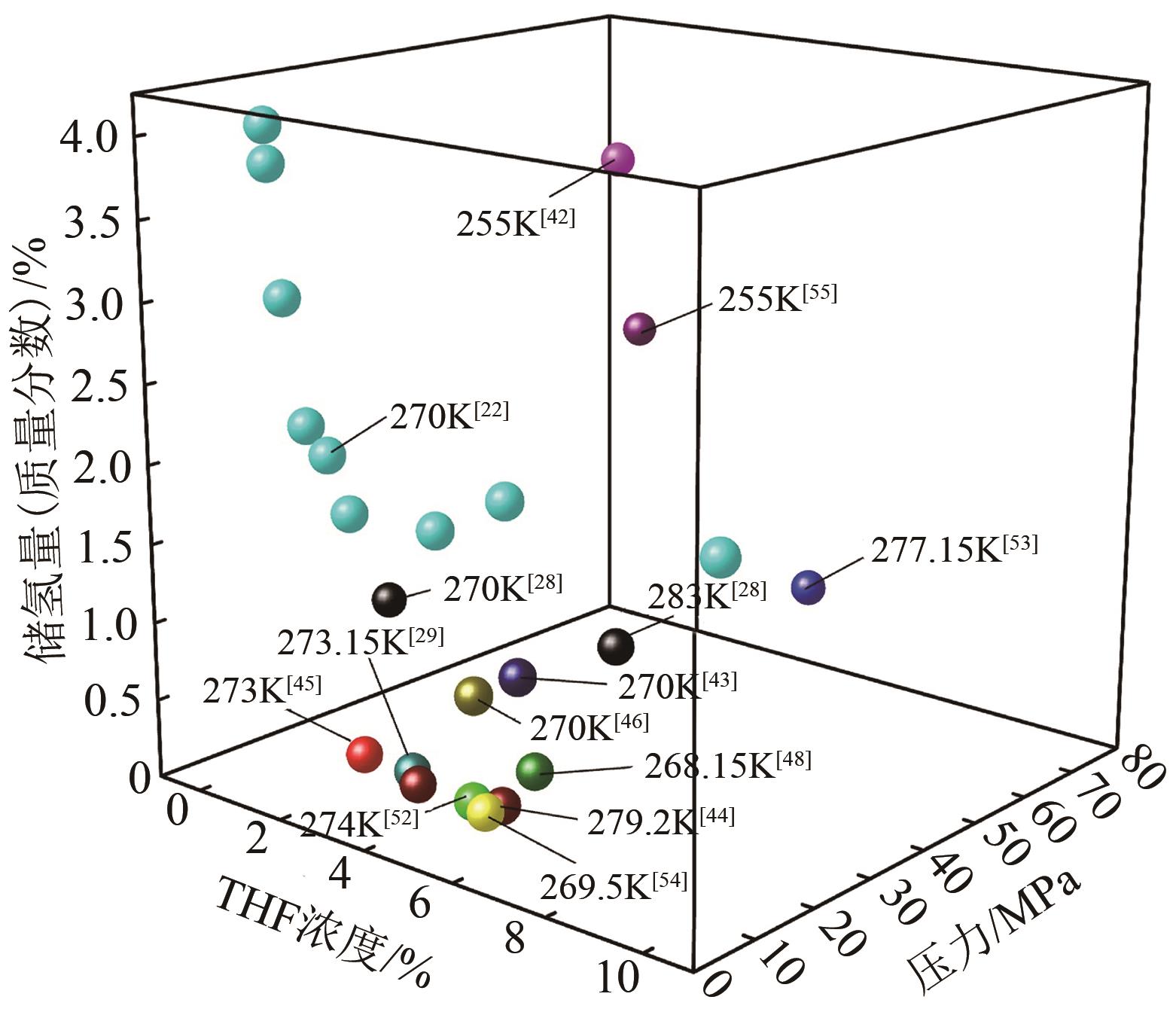
| 28 | ANDERSON R, CHAPOY A, TOHIDI B. Phase relations and binary clathrate hydrate formation in the system H2-THF-H2O[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2007, 23(6): 3440-3444. |
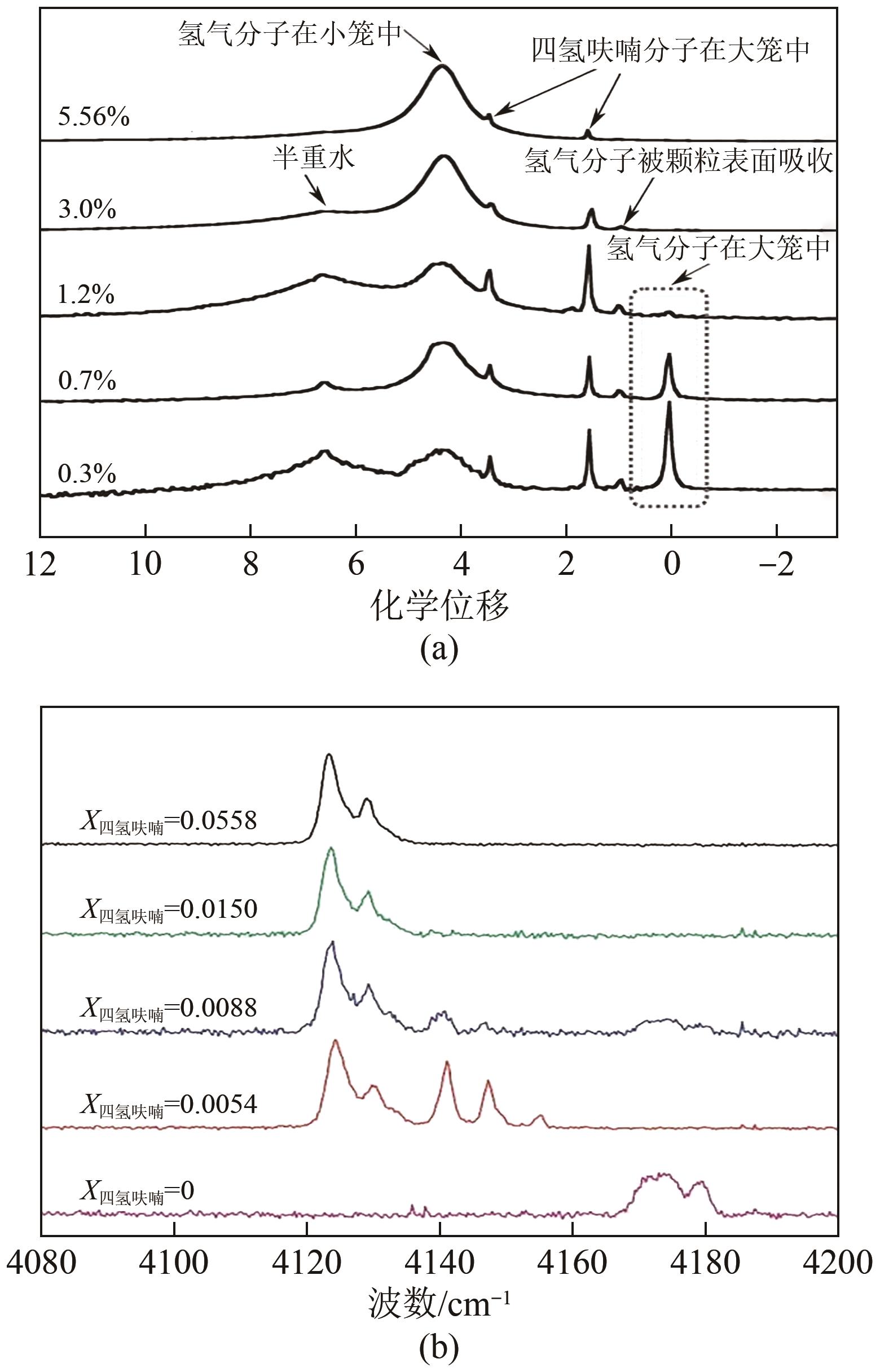
| 29 | CAI Jing, TAO Yuanqing, SOLMS N V, et al. Experimental studies on hydrogen hydrate with tetrahydrofuran by differential scanning calorimeter and in-situ Raman[J]. Applied Energy, 2019, 243: 1-9. |
| 30 | KOMATSU H, YOSHIOKA H, OTA M, et al. Phase equilibrium measurements of hydrogen-tetrahydrofuran and hydrogen-cyclopentane binary clathrate hydrate systems[J]. Journal of Chemical & Engineering Data, 2010, 55(6): 2214-2218. |
| 31 | ZHANG J S, LEE J W. Equilibrium of hydrogen + cyclopentane and carbon dioxide + cyclopentane binary hydrates[J]. Journal of Chemical & Engineering Data, 2009, 54(2): 659-661. |
| 32 | TRUEBA A T, ROVETTO L J, FLORUSSE L J, et al. Phase equilibrium measurements of structure Ⅱ clathrate hydrates of hydrogen with various promoters[J]. Fluid Phase Equilibria, 2011, 307(1): 6-10. |
| 33 | TSUDA T, OGATA K, HASHIMOTO S, et al. Storage capacity of hydrogen in tetrahydrothiophene and furan clathrate hydrates[J]. Chemical Engineering Science, 2009, 64(19): 4150-4154. |
| 34 | ZHANG Y, BHATTACHARJEE G, LINGA P. A robust and highly efficient phase boundary method for determining the thermodynamic equilibrium conditions of bulk gas hydrate systems[J]. Fluid Phase Equilibria, 2021, 540: 113034. |
| 35 | DU Jianwei, LIANG Deqiang, DAI Xingxue, et al. Hydrate phase equilibrium for the (hydrogen + tert-butylamine + water) system[J]. The Journal of Chemical Thermodynamics, 2011, 43(4): 617-621. |
| 36 | YU Chi, FAN Shuanshi, LANG Xuemei, et al. Hydrogen and chemical energy storage in gas hydrate at mild conditions[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14915-14921. |
| 37 | VELUSWAMY H P, YEW J C, LINGA P. New hydrate phase equilibrium data for two binary gas mixtures of hydrogen and propane coupled with a kinetic study[J]. Journal of Chemical & Engineering Data, 2015, 60(2): 228-237. |
| 38 | WANG Pengfei, LI Kehan, YANG Jianyu, et al. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate[J]. Chemical Engineering Journal, 2021, 426: 131279. |
| 39 | PARK D H, LEE B R, SA J H, et al. Gas-hydrate phase equilibrium for mixtures of sulfur hexafluoride and hydrogen[J]. Journal of Chemical & Engineering Data, 2012, 57(5): 1433-1436. |
| 40 | ZHDANOV R K, GETS K V, BELOSLUDOV R V, et al. Theoretical modeling of the thermodynamic properties and the phase diagram of binary gas hydrates of argon and hydrogen[J]. Fluid Phase Equilibria, 2017, 434: 87-92. |
| 41 | BELOSLUDOV R V, ZHDANOV R K, SUBBOTIN O S, et al. Theoretical study of hydrogen storage in binary hydrogen-methane clathrate hydrates[J]. Journal of Renewable and Sustainable Energy, 2014, 6(5): 053132. |
| 42 | SUGAHARA T, HAAG J C, PRASAD P S R, et al. Increasing hydrogen storage capacity using tetrahydrofuran[J]. Journal of the American Chemical Society, 2009, 131(41): 14616-14617. |
| 43 | STROBEL T A, TAYLOR C J, HESTER K C, et al. Molecular hydrogen storage in binary THF-H2 clathrate hydrates[J]. The Journal of Physical Chemistry B, 2006, 110(34): 17121-17125. |
| 44 | VELUSWAMY H P, CHIN W I, LINGA P. Clathrate hydrates for hydrogen storage: The impact of tetrahydrofuran, tetra-n-butylammonium bromide and cyclopentane as promoters on the macroscopic kinetics[J]. International Journal of Hydrogen Energy, 2014, 39(28): 16234-16243. |
| 45 | TALYZIN A. Feasibility of H2-THF-H2O clathrate hydrates for hydrogen storage applications[J]. International Journal of Hydrogen Energy, 2008, 33(1): 111-115. |
| 46 | SAHA D, DENG S G. Accelerated formation of THF-H2 clathrate hydrate in porous media[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2010, 26(11): 8414-8418. |
| 47 | GRIM R G, KERKAR P B, SLOAN E D, et al. Rapid hydrogen hydrate growth from non-stoichiometric tuning mixtures during liquid nitrogen quenching[J]. The Journal of Chemical Physics, 2012, 136(23): 234504. |
| 48 | 臧小亚, 梁德青, 吴能友. 碳纳米管和碳纳米管-四氢呋喃水合物的储氢特性[J]. 高等学校化学学报, 2012, 33(3): 580-585. |
| ZANG Xiaoya, LIANG Deqing, WU Nengyou. H2 adsorption storage capabilities of SWNTs and SWNTs-THF hydrate[J]. Chemical Journal of Chinese Universities, 2012, 33(3): 580-585. | |
| 49 | TIAN Huiquan, ZHANG Zhengcai. Revealing the growth of H2 + THF binary hydrate through molecular simulations[J]. Energy & Fuels, 2020, 34(11): 15004-15010. |
| 50 | NGUYEN T T, PETUYA C, TALAGA D, et al. Promoting the insertion of molecular hydrogen in tetrahydrofuran hydrate with the help of acidic additives[J]. Frontiers in Chemistry, 2020, 8: 550862. |
| 51 | LANG Xuemei, ZHENG Caijuan, FAN Shuanshi, et al. “Similar self-preservation” and decomposition kinetics of tetrahydrofuran-hydrogen hydrate particles[J]. International Journal of Hydrogen Energy, 2022, 47(13): 8457-8466. |
| 52 | MULDER F M, WAGEMAKER M, VAN Eijck L, et al. Hydrogen in porous tetrahydrofuran clathrate hydrate[J]. Chemphyschem, 2008, 9(9): 1331-1337. |
| 53 | OGATA K, HASHIMOTO S, SUGAHARA T, et al. Storage capacity of hydrogen in tetrahydrofuran hydrate[J]. Chemical Engineering Science, 2008, 63(23): 5714-5718. |
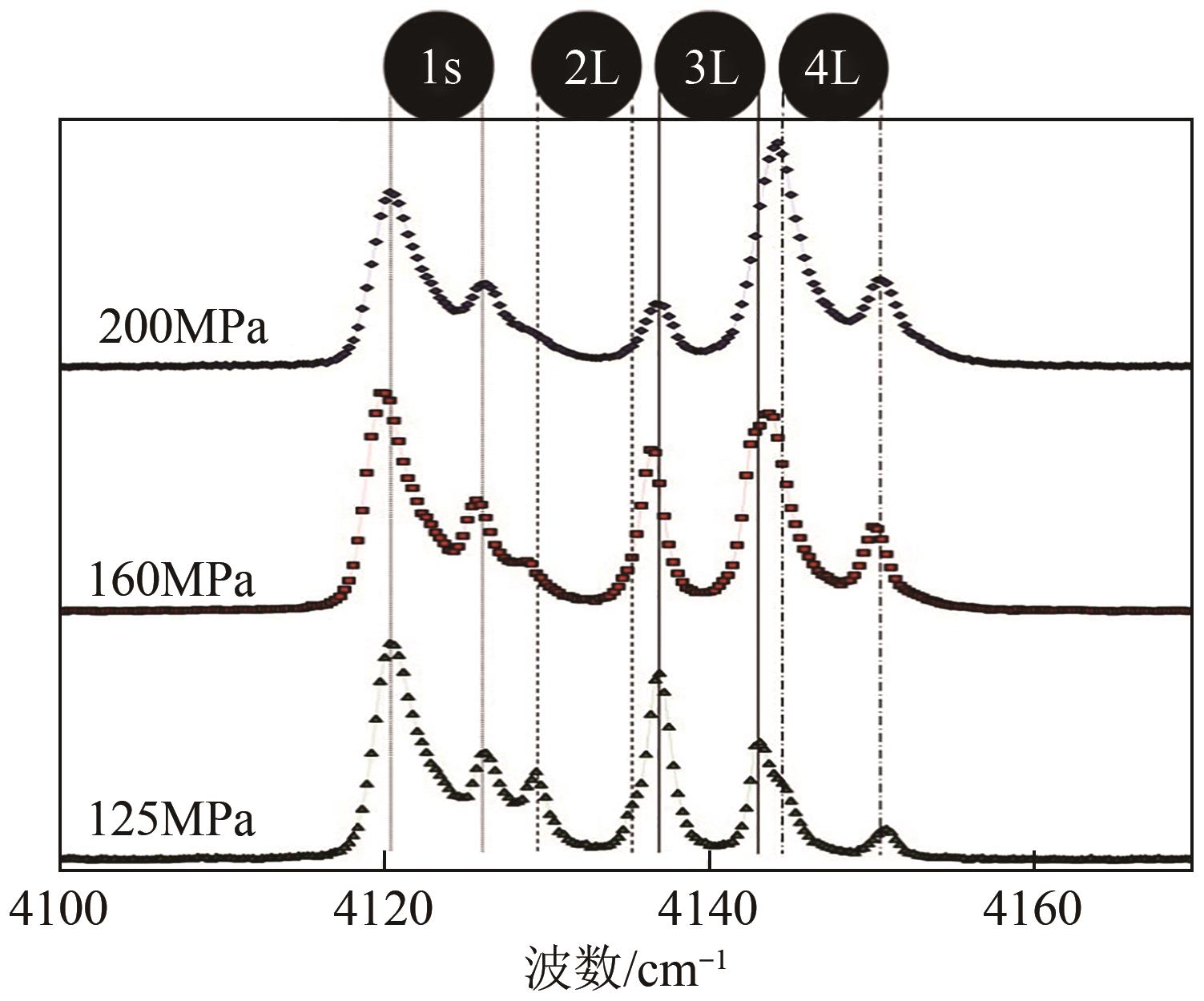
| 54 | NAGAI Y, YOSHIOKA H, OTA M, et al. Binary hydrogen-tetrahydrofuran clathrate hydrate formation kinetics and models[J]. AIChE Journal, 2008, 54(11): 3007-3016. |
| 55 | SUGAHARA T, HAAG J C, WARNTJES A A, et al. Large-cage occupancies of hydrogen in binary clathrate hydrates dependent on pressures and guest concentrations[J]. The Journal of Physical Chemistry C, 2010, 114(35): 15218-15222. |
| 56 | 邓灿, 梁德青, 李栋梁. 环戊烷-氢气水合物形成过程研究[J]. 石油化工, 2009, 38(9): 951-956. |
| DENG Can, LIANG Deqing, LI Dongliang. Formation of cyclopentane-hydrogen clathrate hydrates[J]. Petrochemical Technology, 2009, 38(9): 951-956. | |
| 57 | KAWAMURA T, TAKEYA S, OHTAKE M, et al. Enclathration of hydrogen by organic-compound clathrate hydrates[J]. Chemical Engineering Science, 2011, 66(11): 2417-2420. |
| 58 | PRASAD P S R, SUGAHARA T, SUM A K, et al. Hydrogen storage in double clathrates with tert-butylamine[J]. The Journal of Physical Chemistry A, 2009, 113(24): 6540-6543. |
| 59 | PD P, CANALE V, GERMANI R, et al. Reverse micelles enhance the formation of clathrate hydrates of hydrogen[J]. Journal of Colloid and Interface Science, 2018, 516: 224-231. |
| 60 | ZHANG Y, BHATTACHARJEE G, ZHENG J J, et al. Hydrogen storage as clathrate hydrates in the presence of 1,3-dioxolane as a dual-function promoter[J]. Chemical Engineering Journal, 2022, 427: 131771. |
| 61 | CHEN Siyuan, WANG Yanhong, LANG Xuemei, et al. Rapid and high hydrogen storage in epoxycyclopentane hydrate at moderate pressure[J]. Energy, 2023, 268: 126638. |
| 62 | PARK J, LEE H. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane[J]. Korean Journal of Chemical Engineering, 2007, 24(4): 624-627. |
| 63 | VELUSWAMY H P, CHEN J Y, LINGA P. Surfactant effect on the kinetics of mixed hydrogen/propane hydrate formation for hydrogen storage as clathrates[J]. Chemical Engineering Science, 2015, 126: 488-499. |
| 64 | PANDEY J S, HANSEN J L, VON SOLMS N. Hydrogen-rich natural gas hydrates formation kinetics in the presence of promoters[J]. Chemical Engineering Journal, 2022, 432: 134295. |
| 65 | GHAANI M R, TAKEYA S, ENGLISH N J. Hydrogen storage in propane-hydrate: Theoretical and experimental study[J]. Applied Sciences, 2020, 10(24): 8962. |
| 66 | 尹凯东. 丙烷和甲基环己烷水合物储氢的分子动力学模拟研究[D]. 广州: 华南理工大学, 2020: 24-58. |
| YIN Kaidong. Using propane or methylcyclohexane hydrate as hydrogen storage material: MD simulation[D]. Guangzhou: South China University of Technology, 2020: 24-58. | |
| 67 | WANG Yanhong, YIN Kaidong, FAN Shuanshi, et al. The molecular insight into the “Zeolite-ice” as hydrogen storage material[J]. Energy, 2021, 217: 119406. |
| 68 | FARRANDO-PEREZ J, BALDERAS-XICOHTENCATL R, CHENG Yongqiang, et al. Rapid and efficient hydrogen clathrate hydrate formation in confined nanospace[J]. Nature Communications, 2022, 13: 5953. |
| 69 | LOKSHIN KA, ZHAO Yusheng, HE Duanwei, et al. Structure and dynamics of hydrogen molecules in the novel clathrate hydrate by high pressure neutron diffraction[J]. Physical Review Letters, 2004, 93(12): 125503. |
| 70 | STROBEL T A, KOH C A, SLOAN E D. Hydrogen storage properties of clathrate hydrate materials[J]. Fluid Phase Equilibria, 2007, 261(1/2): 382-389. |
| 71 | STROBEL T A, SLOAN E D, KOH C A. Raman spectroscopic studies of hydrogen clathrate hydrates[J]. The Journal of Chemical Physics, 2009, 130(1): 014506. |
| 72 | GUPTA A, BARON G V, PERREAULT P, et al. Hydrogen clathrates: Next generation hydrogen storage materials[J]. Energy Storage Materials, 2021, 41: 69-107. |
| 73 | STROBEL T A, HESTER K C, SLOAN E D, et al. A hydrogen clathrate hydrate with cyclohexanone: Structure and stability[J]. Journal of the American Chemical Society, 2007, 129(31): 9544-9545. |
| 74 | AHN Y H, MOON S, KOH D Y, et al. One-step formation of hydrogen clusters in clathrate hydrates stabilized via natural gas blending[J]. Energy Storage Materials, 2020, 24: 655-661. |
| 75 | MOON S, LEE Y, SEO D, et al. Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage[J]. Renewable and Sustainable Energy Reviews, 2021, 141: 110789. |
| 76 | LEE W, KANG D W, AHN Y H, et al. Rapid formation of hydrogen-enriched hydrocarbon gas hydrates under static conditions[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(25): 8414-8424. |
| 77 | 郎雪梅, 樊栓狮, 王燕鸿, 等. 笼型水合物为能源化工带来新机遇[J]. 化工进展, 2021, 40(9): 4703-4710. |
| LANG Xuemei, FAN Shuanshi, WANG Yanhong, et al. Opportunities for energy and chemical engineering through clathrate hydrates[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4703-4710. | |
| 78 | 代梦玲, 孙志高, 李娟, 等. 水合物储气促进技术研究进展[J]. 化工进展, 2020, 39(10): 3975-3986. |
| DAI Mengling, SUN Zhigao, LI Juan, et al. Progress on promotion technology for gas storage in hydrates[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 3975-3986. |
| [1] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [5] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [6] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [7] | 史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745. |
| [8] | 刘木子, 史柯柯, 赵强, 李晋平, 刘光. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769. |
| [9] | 李由, 吴越, 钟禹, 林琦璇, 任俊莉. 酸性熔盐水合物预处理麦秆高效制备木糖及其对酶解效率的影响[J]. 化工进展, 2023, 42(9): 4974-4983. |
| [10] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [11] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [12] | 郭晋, 张耕, 陈国华, 朱鸣, 谭粤, 李蔚, 夏莉, 胡昆. 车载液氢气瓶设计技术的研究进展[J]. 化工进展, 2023, 42(8): 4221-4229. |
| [13] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [14] | 王晓晗, 周亚松, 于志庆, 魏强, 孙劲晓, 姜鹏. 不同晶粒尺寸Y分子筛的合成及其加氢裂化反应性能[J]. 化工进展, 2023, 42(8): 4283-4295. |
| [15] | 尹新宇, 皮丕辉, 文秀芳, 钱宇. 特殊浸润性材料在防治油气管道中水合物成核与聚集的应用[J]. 化工进展, 2023, 42(8): 4076-4092. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||