化工进展 ›› 2023, Vol. 42 ›› Issue (9): 4746-4769.DOI: 10.16085/j.issn.1000-6613.2022-1906
固体储氢材料的研究进展
- 太原理工大学化学工程与技术学院,气体能源高效清洁利用山西省重点实验室,山西 太原 030024
-
收稿日期:2022-10-13修回日期:2023-03-31出版日期:2023-09-15发布日期:2023-09-28 -
通讯作者:刘光 -
作者简介:刘木子(1999—),男,硕士研究生,研究方向为固态储氢材料。E-mail:liumuzi0935@link.tyut.edu.cn。 -
基金资助:国家自然科学基金(21878204);山西省重点研发计划国际合作项目(201903D421073)
Research progress of solid hydrogen storage materials
LIU Muzi( ), SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang(
), SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang( )
)
- Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2022-10-13Revised:2023-03-31Online:2023-09-15Published:2023-09-28 -
Contact:LIU Guang
摘要:
氢的廉价制取、安全储运以及高效应用是目前氢能研究领域的重点,而安全、高效的氢储运是实现氢能规模化应用的技术关键,因此高容量固态储氢材料的研发具有重要的学术意义和应用价值。固体材料储氢因储氢密度大、安全系数高而成为最有前景的储氢技术,得到了研究者们的广泛关注。本文针对目前国内外固体储氢材料研究现状,论述了几种固体储氢材料的研究进展,包括物理吸附类储氢材料、金属基储氢材料、配位氢化物和水合物储氢材料。重点评述了固态储氢材料中最具发展潜力的镁基储氢材料,并阐述了合金化、纳米化、添加催化剂以及复合轻金属配位氢化物等几种改性方法对镁基储氢材料储氢机理、微观结构、热力学性能、动力学性能的影响。制氢-储氢-用氢一体集成化设计应是固态储氢尤其是镁基储氢产业化应用发展道路,而镁基固态储运氢技术的发展,将可能实现氢气安全高效及大规模储运。
中图分类号:
引用本文
刘木子, 史柯柯, 赵强, 李晋平, 刘光. 固体储氢材料的研究进展[J]. 化工进展, 2023, 42(9): 4746-4769.
LIU Muzi, SHI Keke, ZHAO Qiang, LI Jinping, LIU Guang. Research progress of solid hydrogen storage materials[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4746-4769.
| 材料 | Tdes/℃ | 动力学 | 热力学 | 参考 文献 | |
|---|---|---|---|---|---|
| Ea(abs/des)/kJ·mol-1 | ΔH(abs/des)/kJ·mol-1 | ||||
| MgH2@BCNTs | 220 | Abs:5.79%/250℃/5min/80bar Des:5.70%/275℃/约1h/0.002MPa | —/97.94KM | —/68.92 | [ |
| MgH2-10% nano ZrMn2 | 181.9 | Abs:5.3%/100℃/10min/3MPa Des:6.7%/300℃/5min/— | 22.1±2.7AM/82.2±2.7KM | — | [ |
| MgH2-Ni-N-C500 | — | Abs:5.5%/275℃/15s/2MPa Des:5.4%/300℃/5min/0.01MPa | —/87.2±5.4KM | —/ 48.4±2.1 | [ |
| MgH2-9NiMoO4 | — | Abs:6.7%/300℃/60s/3MPa Des:6.7%/300℃/10min/100Pa | —/86.1KM | —/ 73.4 | [ |
| MgH2-5%VTi-5%CNTs | 150 | Abs:5.0%/200℃/30s/2MPa Des:6.0%/300℃/6min/Vacuum | 10.1AM/— | — | [ |
| MgH2-6%MnV2O6 | 183.8 | Abs:3.09%/50℃/10min/3MPa Des:5.57%/250℃/10min/0.09atm | —/81.2±5.6KM | —/ 67.0±0.7 | [ |
| MgH2-0.1TiH2 | — | Abs:1.5%/25℃/4h /2MPa Des:6.0%/300℃/5min/100Pa | 16.4KM/— | — | [ |
| MgH2+fl-TiO2@C | 180.3 | Abs:6.3%/150℃/40min/5MPa Des:6.0%/250℃/7min/1kPa | —/67.10KM | — | [ |
| MgH2-10% N-Nb2O5 | 170 | Abs:6.3%/120℃/1h/50atm Des:5.0%/250℃/3min/— | —/98±3KM | —/76±1 | [ |
| MgH2-10%Co@C | 168 | Abs:— Des:5.8%/300℃/25min/0~0.05MPa | —/84.5KM | — | [ |
| MgH2-7mol%MnFe2O4 | 300 | Abs:— Des:5.05%/300℃/1h/0.1MPa | —/64.55KM | — | [ |
| MgH2-15%VNbO5 | 234 | Abs:5.5%/160℃/10min/2MPa Des:— | —/99±3KM | —/70.65±0.43 | [ |
| 60MgH2/TiO2 | 180 | Abs:2.65%/200℃/11.9min/3MPa Des:2.43%/300℃/10min/0.001MPa | —/106.7KM | — | [ |
| MgH2-10% FeOOH NDs@G | 229.8 | Abs:6.0%/200℃/10min/3.2MPa Des:6.7%/325℃/20min/— | 58.2AM/125.03AM | — | [ |
| MgH2-4% Ni NFs | 143 | Abs:— Des:7.02%/325℃/11min/0.02atm | —/81.5AM | — | [ |
| MgH2-2D TiO2(B) | 200 | Abs:6.01%/100℃/~10s/6MPa Des:6.16%/250℃/10min/0.002MPa | —/75.3±1.0KM | —/76.7 | [ |
| MgH2-10% SrTiO3 | 275 | Abs:6.6%/320℃/1h/27atm Des:5.8%/320℃/60min/1atm | —/109KM | —/76.8 | [ |
| MgH2-TiH2@Gr | 204 | Abs:约6.0%/300℃/约2.5min/15atm Des:6.77%/300℃/30min/1atm | —/88.89±3.86KM | —/74.54 | [ |
| MgH2-10% TiO2@C | 205 | Abs:6.6%/140℃/10min/5MPa Des:6.5%/300℃/7min/ | 38±1AM/106±6KM | —/73.6±0.3 | [ |
| MgH2/Ni@GS | 约130 | Abs:5.4%/250℃/10min/30atm Des:5.4%/250℃/10min/0.01atm | 22.7AM/64.7AM | —/62.1 | [ |
| MgH2-5% K2Ti6O13 | 175 | Abs:6.5%/200℃/0.5min/2.2MPa Des:6.7%/280℃/3min/— | —/105.67KM | — | [ |
| Mg-0.2mol%Nb2O5 | — | Abs:7.0%/300℃/60s/0.84MPa Des:7.0%/300℃/90s/— | —/62 | — | [ |
| MgH2-(Ni-V2O3)@C | 190 | Abs:6.00%/150℃/9min/60atm Des:6.02%/300℃/5min/0.001atm | 42.1AM/84.6AM —/99.4±8.5KM | — | [ |
| MgH2-7.5%(N-Nb2O5@Nb2C) | 178 | Abs:5.0%/90℃/1.95min/3.5MPa Des:6.0%/275℃/7.1min/0.001MPa | 37.7AM/78.1AM | —/78.9±2.3 | [ |
| MgH2-7% TiNb2O7 | 177 | Abs:6.0%/200℃/3min/5MPa Des:5.5%/250℃/10min/— | —/96±4KM | —/74.4 | [ |
| MgH2-Ni@Ti-MX | 175 | Abs:5.4%/125℃/25s/3MPa Des:5.2%/250℃/15min/0.002MPa | 56±4AM/73±3.5KM | 74.4/81.3 | [ |
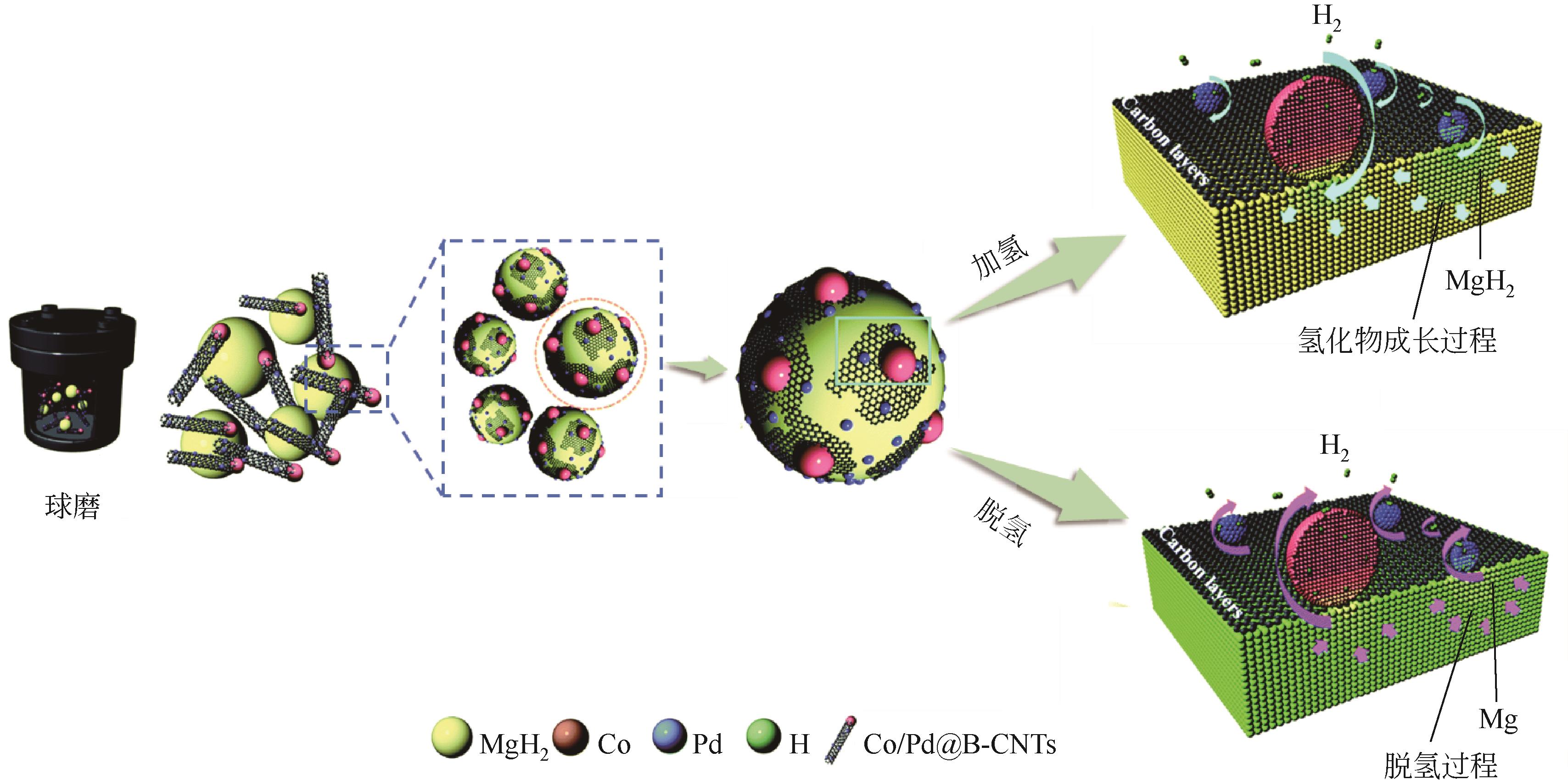
| MgH2-9% NbTiC | 195 | Abs:5.9%/150℃/2.5min/5MPa Des:5.8%/250℃/30min/— | —/ 80±3KM | — | [ |
表1 镁基储氢材料的吸放氢性能对比
| 材料 | Tdes/℃ | 动力学 | 热力学 | 参考 文献 | |
|---|---|---|---|---|---|
| Ea(abs/des)/kJ·mol-1 | ΔH(abs/des)/kJ·mol-1 | ||||
| MgH2@BCNTs | 220 | Abs:5.79%/250℃/5min/80bar Des:5.70%/275℃/约1h/0.002MPa | —/97.94KM | —/68.92 | [ |
| MgH2-10% nano ZrMn2 | 181.9 | Abs:5.3%/100℃/10min/3MPa Des:6.7%/300℃/5min/— | 22.1±2.7AM/82.2±2.7KM | — | [ |
| MgH2-Ni-N-C500 | — | Abs:5.5%/275℃/15s/2MPa Des:5.4%/300℃/5min/0.01MPa | —/87.2±5.4KM | —/ 48.4±2.1 | [ |
| MgH2-9NiMoO4 | — | Abs:6.7%/300℃/60s/3MPa Des:6.7%/300℃/10min/100Pa | —/86.1KM | —/ 73.4 | [ |
| MgH2-5%VTi-5%CNTs | 150 | Abs:5.0%/200℃/30s/2MPa Des:6.0%/300℃/6min/Vacuum | 10.1AM/— | — | [ |
| MgH2-6%MnV2O6 | 183.8 | Abs:3.09%/50℃/10min/3MPa Des:5.57%/250℃/10min/0.09atm | —/81.2±5.6KM | —/ 67.0±0.7 | [ |
| MgH2-0.1TiH2 | — | Abs:1.5%/25℃/4h /2MPa Des:6.0%/300℃/5min/100Pa | 16.4KM/— | — | [ |
| MgH2+fl-TiO2@C | 180.3 | Abs:6.3%/150℃/40min/5MPa Des:6.0%/250℃/7min/1kPa | —/67.10KM | — | [ |
| MgH2-10% N-Nb2O5 | 170 | Abs:6.3%/120℃/1h/50atm Des:5.0%/250℃/3min/— | —/98±3KM | —/76±1 | [ |
| MgH2-10%Co@C | 168 | Abs:— Des:5.8%/300℃/25min/0~0.05MPa | —/84.5KM | — | [ |
| MgH2-7mol%MnFe2O4 | 300 | Abs:— Des:5.05%/300℃/1h/0.1MPa | —/64.55KM | — | [ |
| MgH2-15%VNbO5 | 234 | Abs:5.5%/160℃/10min/2MPa Des:— | —/99±3KM | —/70.65±0.43 | [ |
| 60MgH2/TiO2 | 180 | Abs:2.65%/200℃/11.9min/3MPa Des:2.43%/300℃/10min/0.001MPa | —/106.7KM | — | [ |
| MgH2-10% FeOOH NDs@G | 229.8 | Abs:6.0%/200℃/10min/3.2MPa Des:6.7%/325℃/20min/— | 58.2AM/125.03AM | — | [ |
| MgH2-4% Ni NFs | 143 | Abs:— Des:7.02%/325℃/11min/0.02atm | —/81.5AM | — | [ |
| MgH2-2D TiO2(B) | 200 | Abs:6.01%/100℃/~10s/6MPa Des:6.16%/250℃/10min/0.002MPa | —/75.3±1.0KM | —/76.7 | [ |
| MgH2-10% SrTiO3 | 275 | Abs:6.6%/320℃/1h/27atm Des:5.8%/320℃/60min/1atm | —/109KM | —/76.8 | [ |
| MgH2-TiH2@Gr | 204 | Abs:约6.0%/300℃/约2.5min/15atm Des:6.77%/300℃/30min/1atm | —/88.89±3.86KM | —/74.54 | [ |
| MgH2-10% TiO2@C | 205 | Abs:6.6%/140℃/10min/5MPa Des:6.5%/300℃/7min/ | 38±1AM/106±6KM | —/73.6±0.3 | [ |
| MgH2/Ni@GS | 约130 | Abs:5.4%/250℃/10min/30atm Des:5.4%/250℃/10min/0.01atm | 22.7AM/64.7AM | —/62.1 | [ |
| MgH2-5% K2Ti6O13 | 175 | Abs:6.5%/200℃/0.5min/2.2MPa Des:6.7%/280℃/3min/— | —/105.67KM | — | [ |
| Mg-0.2mol%Nb2O5 | — | Abs:7.0%/300℃/60s/0.84MPa Des:7.0%/300℃/90s/— | —/62 | — | [ |
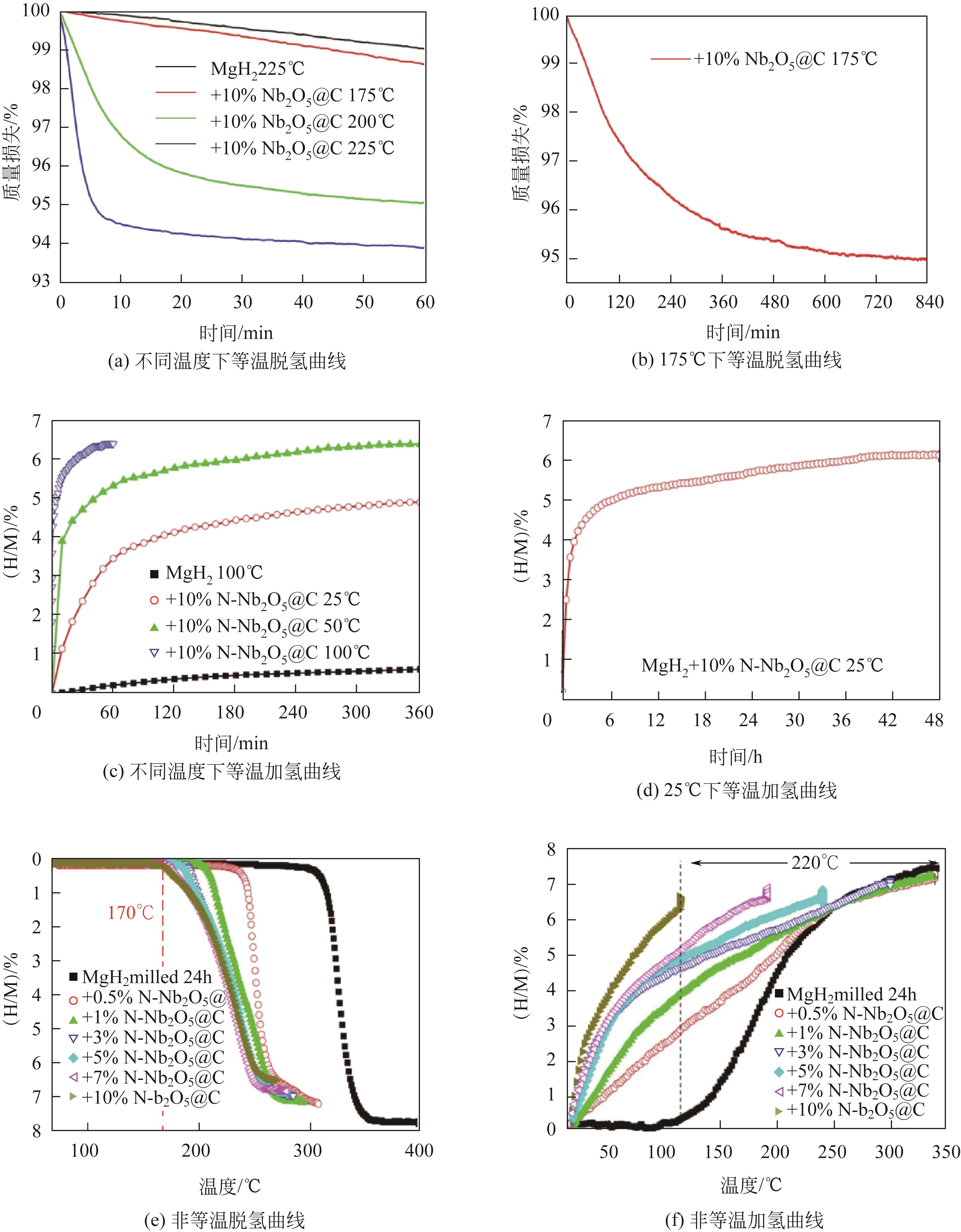
| MgH2-(Ni-V2O3)@C | 190 | Abs:6.00%/150℃/9min/60atm Des:6.02%/300℃/5min/0.001atm | 42.1AM/84.6AM —/99.4±8.5KM | — | [ |
| MgH2-7.5%(N-Nb2O5@Nb2C) | 178 | Abs:5.0%/90℃/1.95min/3.5MPa Des:6.0%/275℃/7.1min/0.001MPa | 37.7AM/78.1AM | —/78.9±2.3 | [ |
| MgH2-7% TiNb2O7 | 177 | Abs:6.0%/200℃/3min/5MPa Des:5.5%/250℃/10min/— | —/96±4KM | —/74.4 | [ |
| MgH2-Ni@Ti-MX | 175 | Abs:5.4%/125℃/25s/3MPa Des:5.2%/250℃/15min/0.002MPa | 56±4AM/73±3.5KM | 74.4/81.3 | [ |
| MgH2-9% NbTiC | 195 | Abs:5.9%/150℃/2.5min/5MPa Des:5.8%/250℃/30min/— | —/ 80±3KM | — | [ |
| 1 | WIEDENHOFER Dominik, LENZEN Manfred, STEINBERGER Julia K. Energy requirements of consumption: Urban form, climatic and socio-economic factors, rebounds and their policy implications[J]. Energy Policy, 2013, 63: 696-707. |
| 2 | GIELEN Dolf, BOSHELL Francisco, SAYGIN Deger, et al. The role of renewable energy in the global energy transformation[J]. Energy Strategy Reviews, 2019, 24: 38-50. |
| 3 | ABE J O, POPOOLA A P I, AJENIFUJA E, et al. Hydrogen energy, economy and storage: Review and recommendation[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15072-15086. |
| 4 | Hervé BARTHÉLÉMY. Hydrogen storage-industrial prospectives[J]. International Journal of Hydrogen Energy, 2012, 37(22): 17364-17372. |
| 5 | SENTHIL KUMAR S, BIBIN C, RAMACHANDRAN M. Design and analysis of hydrogen storage tank with different materials by ansys[J]. IOP Conference Series: Materials Science and Engineering, 2020, 810(1): 012016. |
| 6 | ANWAR Naushad, SHAKEEL Nimra, AHAMED Mohd Imran. Solid-state materials for hydrogen storage[M]//Green Sustainable Process for Chemical and Environmental Engineering and Science. Amsterdam: Elsevier, 2021: 205-223. |
| 7 | YARTYS Volodymyr A, LOTOTSKYY Mykhaylo V. Laves type intermetallic compounds as hydrogen storage materials: A review[J]. Journal of Alloys and Compounds, 2022, 916: 165219. |
| 8 | RUSMAN N A A, DAHARI M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications[J]. International Journal of Hydrogen Energy, 2016, 41(28): 12108-12126. |
| 9 | TARASOV Boris P, ARBUZOV Artem A, VOLODIN Alexey A, et al. Metal hydride-Graphene composites for hydrogen based energy storage[J]. Journal of Alloys and Compounds, 2022, 896: 162881. |
| 10 | SETHIA Govind, SAYARI Abdelhamid. Activated carbon with optimum pore size distribution for hydrogen storage[J]. Carbon, 2016, 99: 289-294. |
| 11 | YUSHIN G, DASH R, JAGIELLO J, et al. Carbide-derived carbons: Effect of pore size on hydrogen uptake and heat of adsorption[J]. Advanced Functional Materials, 2006, 16(17): 2288-2293. |
| 12 | Young-Jung HEO, PARK Soo-Jin. Synthesis of activated carbon derived from rice husks for improving hydrogen storage capacity[J]. Journal of Industrial and Engineering Chemistry, 2015, 31: 330-334. |
| 13 | ZHOU Li, ZHOU Yaping, SUN Yan. Enhanced storage of hydrogen at the temperature of liquid nitrogen[J]. International Journal of Hydrogen Energy, 2004, 29(3): 319-322. |
| 14 | WANG Huanlei, GAO Qiuming, HU Juan. High hydrogen storage capacity of porous carbons prepared by using activated carbon[J]. Journal of the American Chemical Society, 2009, 131(20): 7016-7022. |
| 15 | ELYASSI Mahrokh, RASHIDI Alimorad, HANTEHZADEH Mohammad Reza, et al. Hydrogen storage behaviors by adsorption on multi-walled carbon nanotubes[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2017, 27(1): 285-295. |
| 16 | YE Y, AHN C C, WITHAM C, et al. Hydrogen adsorption and cohesive energy of single-walled carbon nanotubes[J]. Applied Physics Letters, 1999, 74(16): 2307-2309. |
| 17 | LIU Chang, CHEN Yong, WU Chengzhang, et al. Hydrogen storage in carbon nanotubes revisited[J]. Carbon, 2010, 48(2): 452-455. |
| 18 | ZHU Hongwei, LI Xuesong, Lijie CI, et al. Hydrogen storage in heat-treated carbon nanofibers prepared by the vertical floating catalyst method[J]. Materials Chemistry and Physics, 2003, 78(3): 670-675. |
| 19 | Vicente JIMÉNEZ, Ana RAMÍREZ-LUCAS, Paula SÁNCHEZ, et al. Hydrogen storage in different carbon materials: Influence of the porosity development by chemical activation[J]. Applied Surface Science, 2012, 258(7): 2498-2509. |
| 20 | CHEN Xiaohong, XUE Zhiyong, NIU Kai, et al. Li-fluorine codoped electrospun carbon nanofibers for enhanced hydrogen storage[J]. RSC Advances, 2021, 11(7): 4053-4061. |
| 21 | LI Yingwei, YANG Ralph T. Hydrogen storage in low silica type X zeolites[J]. The Journal of Physical Chemistry B, 2006, 110(34): 17175-17181. |
| 22 | ROSI Nathaniel L, ECKERT Juergen, EDDAOUDI Mohamed, et al. Hydrogen storage in microporous metal-organic frameworks[J]. Science, 2003, 300(5622): 1127-1129. |
| 23 | ROWSELL Jesse L C, YAGHI Omar M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal-organic frameworks[J]. Journal of the American Chemical Society, 2006, 128(4): 1304-1315. |
| 24 | SAGARA Tatsuhiko, KLASSEN James, GANZ Eric. Computational study of hydrogen binding by metal-organic framework-5[J]. The Journal of Chemical Physics, 2004, 121(24): 12543-12547. |
| 25 | Gérard FÉREY, LATROCHE Michel, SERRE Christian, et al. Hydrogen adsorption in the nanoporous metal-benzenedicarboxylate M(OH)(O2C-C6H4-CO2) (M=Al3+, Cr3+), MIL-53[J]. Chemical Communications, 2003(24): 2976-2977. |
| 26 | LATROCHE Michel, Suzy SURBLÉ, SERRE Christian, et al. Hydrogen storage in the giant-pore metal-organic frameworks MIL-100 and MIL-101[J]. Angewandte Chemie, 2006, 118(48): 8407-8411. |
| 27 | LI Yingwei, YANG Ralph T. Gas adsorption and storage in metal-organic framework MOF-177[J]. Langmuir, 2007, 23(26): 12937-12944. |
| 28 | SAHA D, WEI Z, DENG S. Equilibrium, kinetics and enthalpy of hydrogen adsorption in MOF-177[J]. International Journal of Hydrogen Energy, 2008, 33(24): 7479-7488. |
| 29 | MUSYOKA Nicholas M, REN Jianwei, LANGMI Henrietta W, et al. Synthesis of rGO/Zr-MOF composite for hydrogen storage application[J]. Journal of Alloys and Compounds, 2017, 724: 450-455. |
| 30 | 龙沛沛, 程绍娟, 赵强, 等. 金属-有机骨架材料的合成及其研究进展[J]. 山西化工, 2008, 28(6): 21-25. |
| LONG Peipei, CHENG Shaojuan, ZHAO Qiang, et al. Development of synthesis of metal organic frameworks[J]. Shanxi Chemical Industry, 2008, 28(6): 21-25. | |
| 31 | QU Jianglan, WANG Yuntao, XIE Lei, et al. Superior hydrogen absorption and desorption behavior of Mg thin films[J]. Journal of Power Sources, 2009, 186(2): 515-520. |
| 32 | ZHANG Liuting, XIAO Xuezhang, XU Chenchen, et al. Remarkably improved hydrogen storage performance of MgH2 catalyzed by multivalence NbH x nanoparticles[J]. The Journal of Physical Chemistry C, 2015, 119(16): 8554-8562. |
| 33 | VARIN R A, CZUJKO T, WRONSKI Z. Particle size, grain size and γ-MgH2 effects on the desorption properties of nanocrystalline commercial magnesium hydride processed by controlled mechanical milling[J]. Nanotechnology, 2006, 17(15): 3856-3865. |
| 34 | LIU Wei, Kondo-Francois AGUEY-ZINSOU. Size effects and hydrogen storage properties of Mg nanoparticles synthesised by an electroless reduction method[J]. Journal of Materials Chemistry A, 2014, 2(25): 9718-9726. |
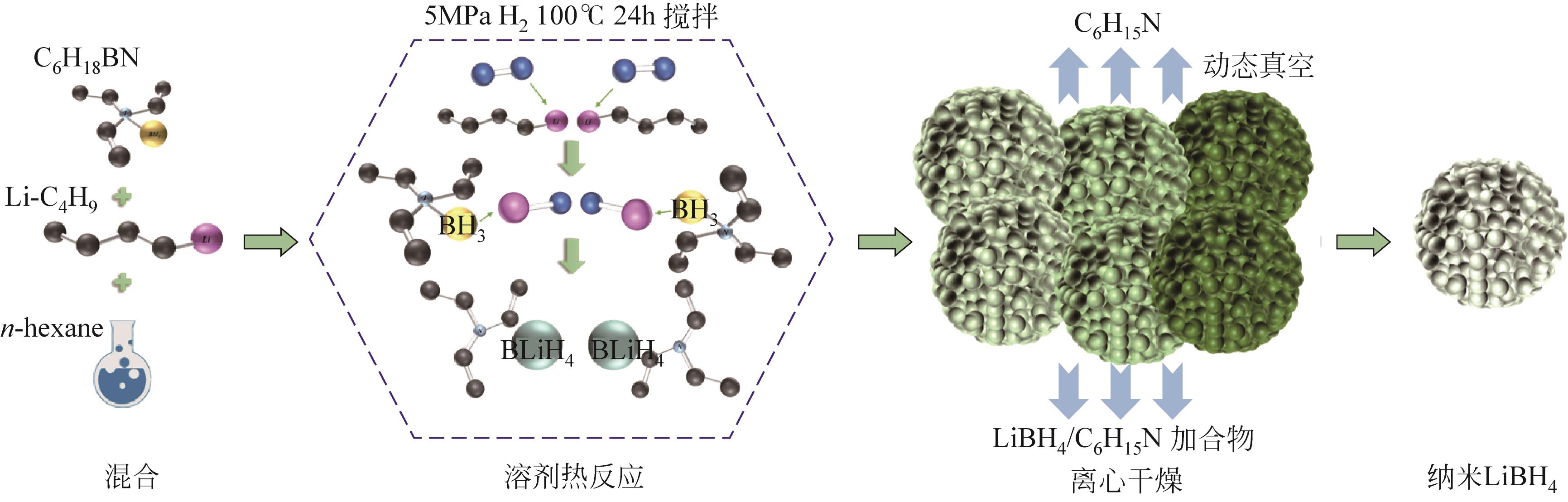
| 35 | ZHANG Xin, LIU Yongfeng, REN Zhuanghe, et al. Realizing 6.7wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides[J]. Energy & Environmental Science, 2021, 14(4): 2302-2313. |
| 36 | SAITA I, TOSHIMA T, TANDA S, et al. Hydrogen storage property of MgH2 synthesized by hydriding chemical vapor deposition[J]. Journal of Alloys and Compounds, 2007, 446/447: 80-83. |
| 37 | CHEN Ming, HU Miaomiao, XIE Xiubo, et al. High loading nanoconfinement of V-decorated Mg with 1 nm carbon shells: Hydrogen storage properties and catalytic mechanism[J]. Nanoscale, 2019, 11(20): 10045-10055. |
| 38 | REILLY James J, WISWALL Richard H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4 [J]. Inorganic Chemistry, 1968, 7(11): 2254-2256. |
| 39 | KHAN Darvaish, ZOU Jianxin, ZENG Xiaoqin, et al. Hydrogen storage properties of nanocrystalline Mg2Ni prepared from compressed 2MgH2Ni powder[J]. International Journal of Hydrogen Energy, 2018, 43(49): 22391-22400. |
| 40 | CAO Wenchao, DING Xin, ZHANG Yong, et al. Enhanced de-/hydrogenation kinetics of a hyper-eutectic Mg85Ni15- x Ag x alloy facilitated by Ag dissolving in Mg2Ni[J]. Journal of Alloys and Compounds, 2022, 917: 165457. |
| 41 | TRAN Xuan Quy, MCDONALD Stuart D, GU Qinfen, et al. Effect of trace Na additions on the hydrogen absorption kinetics of Mg2Ni[J]. Journal of Materials Research, 2016, 31(9): 1316-1327. |
| 42 | ZHANG Xuanzhou, YANG Rong, QU Jianglan, et al. The synthesis and hydrogen storage properties of pure nanostructured Mg2FeH6 [J]. Nanotechnology, 2010, 21(9): 095706. |
| 43 | ZOLLIKER P, YVON K, FISCHER P, et al. Dimagnesium cobalt(Ⅰ) pentahydride, Mg2CoH5, containing square-pyramidal pentahydrocobaltate(4-) (CoH 5 4 - ) anions[J]. Inorganic Chemistry, 1985, 24(24): 4177-4180. |
| 44 | KYOI Daisuke, SATO Toyoto, Ewa RÖNNEBRO, et al. A new ternary magnesium-titanium hydride Mg7TiH x with hydrogen desorption properties better than both binary magnesium and titanium hydrides[J]. Journal of Alloys and Compounds, 2004, 372(1/2): 213-217. |
| 45 | ZHONG H C, WANG H, OUYANG L Z. Improving the hydrogen storage properties of MgH2 by reversibly forming Mg-Al solid solution alloys[J]. International Journal of Hydrogen Energy, 2014, 39(7): 3320-3326. |
| 46 | OUYANG L Z, QIN F X, ZHU M. The hydrogen storage behavior of Mg3La and Mg3LaNi0.1 [J]. Scripta Materialia, 2006, 55(12): 1075-1078. |
| 47 | Pavel RIZO-ACOSTA, CUEVAS Fermin, LATROCHE Michel. Hydrides of early transition metals as catalysts and grain growth inhibitors for enhanced reversible hydrogen storage in nanostructured magnesium[J]. Journal of Materials Chemistry A, 2019, 7(40): 23064-23075. |
| 48 | LU Chong, MA Yanling, LI Fan, et al. Visualization of fast “hydrogen pump” in core-shell nanostructured Mg@Pt through hydrogen-stabilized Mg3Pt[J]. Journal of Materials Chemistry A, 2019, 7(24): 14629-14637. |
| 49 | CUI Jie, LIU Jiangwen, WANG Hui, et al. Mg-TM (TM: Ti, Nb, V, Co, Mo or Ni) core-shell like nanostructures: Synthesis, hydrogen storage performance and catalytic mechanism[J]. Journal of Materials Chemistry A, 2014, 2(25): 9645-9655. |
| 50 | ZHANG J, HE L, YAO Y, et al. Catalytic effect and mechanism of NiCu solid solutions on hydrogen storage properties of MgH2 [J]. Renewable Energy, 2020, 154: 1229-1239. |
| 51 | LIU Meijia, XIAO Xuezhang, ZHAO Shuchun, et al. Facile synthesis of Co/Pd supported by few-walled carbon nanotubes as an efficient bidirectional catalyst for improving the low temperature hydrogen storage properties of magnesium hydride[J]. Journal of Materials Chemistry A, 2019, 7(10): 5277-5287. |
| 52 | ZHANG Xin, LENG Zihan, GAO Mingxia, et al. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2 [J]. Journal of Power Sources, 2018, 398: 183-192. |
| 53 | WANG Zeyi, REN Zhuanghe, JIAN Ni, et al. Vanadium oxide nanoparticles supported on cubic carbon nanoboxes as highly active catalyst precursors for hydrogen storage in MgH2 [J]. Journal of Materials Chemistry A, 2018, 6(33): 16177-16185. |
| 54 | K-f AGUEY-ZINSOU, NICOLAISEN T, ARES FERNANDEZ J R, et al. Effect of nanosized oxides on MgH2 (de)hydriding kinetics[J]. Journal of Alloys and Compounds, 2007, 434/435: 738-742. |
| 55 | WANG Ke, ZHANG Xin, LIU Yongfeng, et al. Graphene-induced growth of N-doped niobium pentaoxide nanorods with high catalytic activity for hydrogen storage in MgH2 [J]. Chemical Engineering Journal, 2021, 406: 126831. |
| 56 | ZOU Jianxin, ZENG Xiaoqin, YING Yanjun, et al. Preparation and hydrogen sorption properties of a nano-structured Mg based Mg-La-O composite[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13067-13073. |
| 57 | LONG Sheng, ZOU Jianxin, LIU Yana, et al. Hydrogen storage properties of a Mg-Ce oxide nano-composite prepared through arc plasma method[J]. Journal of Alloys and Compounds, 2013, 580: S167-S170. |
| 58 | ZOU Jianxin, ZENG Xiaoqin, YING Yanjun, et al. Study on the hydrogen storage properties of core-shell structured Mg-RE (RE=Nd, Gd, Er) nano-composites synthesized through arc plasma method[J]. International Journal of Hydrogen Energy, 2013, 38(5): 2337-2346. |
| 59 | XIAN Kaicheng, WU Meihong, GAO Mingxia, et al. A unique nanoflake-shape bimetallic Ti-Nb oxide of superior catalytic effect for hydrogen storage of MgH2 [J]. Small, 2022, 18(43): 2107013. |
| 60 | SHAN Jiawei, LI Ping, WAN Qi, et al. Significantly improved dehydrogenation of ball-milled MgH2 doped with CoFe2O4 nanoparticles[J]. Journal of Power Sources, 2014, 268: 778-786. |
| 61 | LIN Huai-Jun, MATSUDA Junko, LI Hai-Wen, et al. Enhanced hydrogen desorption property of MgH2 with the addition of cerium fluorides[J]. Journal of Alloys and Compounds, 2015, 645: S392-S396. |
| 62 | FU Yaokun, ZHANG Lu, LI Yuan, et al. Effect of ternary transition metal sulfide FeNi2S4 on hydrogen storage performance of MgH2 [J]. Journal of Magnesium and Alloys, 2022 |
| 63 | ZHU Wen, REN Li, LU Chong, et al. Nanoconfined and in situ catalyzed MgH2 self-assembled on 3D Ti3C2 MXene folded nanosheets with enhanced hydrogen sorption performances[J]. ACS Nano, 2021, 15(11): 18494-18504. |
| 64 | HUANG Tianping, HUANG Xu, HU Chuanzhu, et al. MOF-derived Ni nanoparticles dispersed on monolayer MXene as catalyst for improved hydrogen storage kinetics of MgH2 [J]. Chemical Engineering Journal, 2021, 421: 127851. |
| 65 | WU C Z, WANG P, YAO X, et al. Effect of carbon/noncarbon addition on hydrogen storage behaviors of magnesium hydride[J]. Journal of Alloys and Compounds, 2006, 414(1/2): 259-264. |
| 66 | GU Jian, ZHANG Xiaoping, FU Lei, et al. Study on the hydrogen storage properties of the dual active metals Ni and Al doped graphene composites[J]. International Journal of Hydrogen Energy, 2019, 44(12): 6036-6044. |
| 67 | PINKERTON Frederick E, MEYER Martin S, MEISNER Gregory P, et al. Phase boundaries and reversibility of LiBH4/MgH2 hydrogen storage material[J]. The Journal of Physical Chemistry C, 2007, 111(35): 12881-12885. |
| 68 | LIN Wenping, XIAO Xuezhang, WANG Xuancheng, et al. Extreme high reversible capacity with over 8.0wt% and excellent hydrogen storage properties of MgH2 combined with LiBH4 and Li3AlH6 [J]. Journal of Energy Chemistry, 2020, 50: 296-306. |
| 69 | FAN Xiulin, XIAO Xuezhang, CHEN Lixin, et al. High catalytic efficiency of amorphous TiB2 and NbB2 nanoparticles for hydrogen storage using the 2LiBH4-MgH2 system[J]. Journal of Materials Chemistry A, 2013, 1(37): 11368-11375. |
| 70 | LIU Meijia, ZHAO Shuchun, XIAO Xuezhang, et al. Novel 1D carbon nanotubes uniformly wrapped nanoscale MgH2 for efficient hydrogen storage cycling performances with extreme high gravimetric and volumetric capacities[J]. Nano Energy, 2019, 61: 540-549. |
| 71 | ZHANG Liuting, CAI Zeliang, YAO Zhendong, et al. A striking catalytic effect of facile synthesized ZrMn2 nanoparticles on the de/rehydrogenation properties of MgH2 [J]. Journal of Materials Chemistry A, 2019, 7(10): 5626-5634. |
| 72 | HUANG Yike, AN Cuihua, ZHANG Qiuyu, et al. Cost-effective mechanochemical synthesis of highly dispersed supported transition metal catalysts for hydrogen storage[J]. Nano Energy, 2021, 80: 105535. |
| 73 | CHEN Meng, PU Yanhui, LI Zhenyang, et al. Synergy between metallic components of MoNi alloy for catalyzing highly efficient hydrogen storage of MgH2 [J]. Nano Research, 2020, 13(8): 2063-2071. |
| 74 | YAO Xiangdong, WU Chengzhang, DU Aijun, et al. Metallic and carbon nanotube-catalyzed coupling of hydrogenation in magnesium[J]. Journal of the American Chemical Society, 2007, 129(50): 15650-15654. |
| 75 | FU Huafeng, HU Jia, LU Yangfan, et al. Synergistic effect of a facilely synthesized MnV2O6 catalyst on improving the low-temperature kinetic properties of MgH2 [J]. ACS Applied Materials & Interfaces, 2022, 14(29): 33161-33172. |
| 76 | LU Jun, CHOI Young Joon, FANG Zhigang Zak, et al. Hydrogenation of nanocrystalline Mg at room temperature in the presence of TiH2 [J]. Journal of the American Chemical Society, 2010, 132(19): 6616-6617. |
| 77 | ZHANG Meng, XIAO Xuezhang, LUO Bosang, et al. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures[J]. Journal of Energy Chemistry, 2020, 46: 191-198. |
| 78 | WANG Ke, ZHANG Xin, REN Zhuanghe, et al. Nitrogen-stimulated superior catalytic activity of niobium oxide for fast full hydrogenation of magnesium at ambient temperature[J]. Energy Storage Materials, 2019, 23: 79-87. |
| 79 | WANG Ying, AN Cuihua, WANG Yijing, et al. Core-shell Co@C catalyzed MgH2: Enhanced dehydrogenation properties and its catalytic mechanism[J]. Journal of Materials Chemistry A, 2014, 2(38): 16285-16291. |
| 80 | LI Ping, WAN Qi, LI Ziliang, et al. MgH2 dehydrogenation properties improved by MnFe2O4 nanoparticles[J]. Journal of Power Sources, 2013, 239: 201-206. |
| 81 | VALENTONI Antonio, MULAS Gabriele, ENZO Stefano, et al. Remarkable hydrogen storage properties of MgH2 doped with VNbO5 [J]. Physical Chemistry Chemical Physics, 2018, 20(6): 4100-4108. |
| 82 | REN Li, ZHU Wen, LI Yinghui, et al. Oxygen vacancy-rich 2D TiO2 nanosheets: A bridge toward high stability and rapid hydrogen storage kinetics of nano-confined MgH2 [J]. Nano-Micro Letters, 2022, 14(1): 1-16. |
| 83 | SONG Mengchen, ZHANG Liuting, ZHENG Jiaguang, et al. Constructing graphene nanosheet-supported FeOOH nanodots for hydrogen storage of MgH2 [J]. International Journal of Minerals, Metallurgy and Materials, 2022, 29(7): 1464-1473. |
| 84 | CHEN Jie, XIA Guanglin, GUO Zaiping, et al. Porous Ni nanofibers with enhanced catalytic effect on the hydrogen storage performance of MgH2 [J]. Journal of Materials Chemistry A, 2015, 3(31): 15843-15848. |
| 85 | CHEN Man, XIAO Xuezhang, ZHANG Meng, et al. Insights into 2D graphene-like TiO2 (B) nanosheets as highly efficient catalyst for improved low-temperature hydrogen storage properties of MgH2 [J]. Materials Today Energy, 2020, 16: 100411. |
| 86 | YAHYA M S, ISMAIL M. Catalytic effect of SrTiO3 on the hydrogen storage behaviour of MgH2 [J]. Journal of Energy Chemistry, 2019, 28: 46-53. |
| 87 | VERMA Satish Kumar, BHATNAGAR Ashish, SHUKLA Vivek, et al. Multiple improvements of hydrogen sorption and their mechanism for MgH2 catalyzed through TiH2@Gr[J]. International Journal of Hydrogen Energy, 2020, 45(38): 19516-19530. |
| 88 | XIA Guanglin, TAN Yingbin, CHEN Xiaowei, et al. Monodisperse magnesium hydride nanoparticles uniformly self-assembled on graphene[J]. Advanced Materials, 2015, 27(39): 5981-5988. |
| 89 | KONG Qianqian, ZHANG Huanhuan, YUAN Zhenluo, et al. Hamamelis-like K2Ti6O13 synthesized by alkali treatment of Ti3C2 MXene: Catalysis for hydrogen storage in MgH2 [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(12): 4755-4763. |
| 90 | LAN Zhiqiang, WEN Xiaobin, ZENG Liang, et al. In situ incorporation of highly dispersed nickel and vanadium trioxide nanoparticles in nanoporous carbon for the hydrogen storage performance enhancement of magnesium hydride[J]. Chemical Engineering Journal, 2022, 446: 137261. |
| 91 | LAN Zhiqiang, FU Hong, ZHAO Ruolin, et al. Roles of in situ-formed NbN and Nb2O5 from N-doped Nb2C MXene in regulating the re/hydrogenation and cycling performance of magnesium hydride[J]. Chemical Engineering Journal, 2022, 431: 133985. |
| 92 | ZHANG Lingchao, WANG Ke, LIU Yongfeng, et al. Highly active multivalent multielement catalysts derived from hierarchical porous TiNb2O7 nanospheres for the reversible hydrogen storage of MgH2 [J]. Nano Research, 2021, 14(1): 148-156. |
| 93 | ZHU Wen, PANDA Subrata, LU Chong, et al. Using a self-assembled two-dimensional MXene-based catalyst (2D-Ni@Ti3C2) to enhance hydrogen storage properties of MgH2 [J]. ACS Applied Materials & Interfaces, 2020, 12(45): 50333-50343. |
| 94 | WANG Zeyi, ZHANG Xuelian, REN Zhuanghe, et al. In situ formed ultrafine NbTi nanocrystals from a NbTiC solid-solution MXene for hydrogen storage in MgH2 [J]. Journal of Materials Chemistry A, 2019, 7(23): 14244-14252. |
| 95 | YUAN Zeming, QI Zhen, ZHAI Tingting, et al. Effects of La substitution on microstructure and hydrogen storage properties of Ti-Fe-Mn-based alloy prepared through melt spinning[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(10): 3087-3095. |
| 96 | BALCERZAK M. Structure and hydrogen storage properties of mechanically alloyed Ti-V alloys[J]. International Journal of Hydrogen Energy, 2017, 42(37): 23698-23707. |
| 97 | CHEN X Y, CHEN R R, DING X, et al. Crystal structure and hydrogen storage properties of Ti-V-Mn alloys[J]. International Journal of Hydrogen Energy, 2018, 43(12): 6210-6218. |
| 98 | ZHOU Panpan, CAO Ziming, XIAO Xuezhang, et al. Development of Ti-Zr-Mn-Cr-V based alloys for high-density hydrogen storage[J]. Journal of Alloys and Compounds, 2021, 875: 160035. |
| 99 | ZARYNOW A, GOUDY A J, SCHWEIBENZ R G, et al. The effect of the partial replacement of nickelin LaNi5 hydride with iron, cobalt, and copper on absorption and desorption kinetics[J]. Journal of the Less Common Metals, 1991, 172/173/174: 1009-1017. |
| 100 | LIU Jingjing, LI Kang, CHENG Honghui, et al. New insights into the hydrogen storage performance degradation and Al functioning mechanism of LaNi5- x Al x alloys[J]. International Journal of Hydrogen Energy, 2017, 42(39): 24904-24914. |
| 101 | LIU Jingjing, ZHENG Zhi, CHENG Honghui, et al. Long-term hydrogen storage performance and structural evolution of LaNi4Al alloy[J]. Journal of Alloys and Compounds, 2018, 731: 172-180. |
| 102 | ZHAO Botao, LIU Lifei, YE Yiming, et al. Enhanced hydrogen capacity and absorption rate of LaNi4.25Al0.75 alloy in impure hydrogen by a combined approach of fluorination and palladium deposition[J]. International Journal of Hydrogen Energy, 2016, 41(5): 3465-3469. |
| 103 | ZHU Zhida, ZHU Shuai, LU Haoqi, et al. Stability of LaNi5- x Co x alloys cycled in hydrogen—Part 1. Evolution in gaseous hydrogen storage performance[J]. International Journal of Hydrogen Energy, 2019, 44(29): 15159-15172. |
| 104 | LIU Jingjing, ZHU Shuai, ZHENG Zhi, et al. Long-term hydrogen absorption/desorption properties and structural changes of LaNi4Co alloy with double desorption plateaus[J]. Journal of Alloys and Compounds, 2019, 778: 681-690. |
| 105 | 张怀伟, 郑鑫遥, 刘洋, 等. 稀土元素在储氢材料中的应用进展[J]. 中国稀土学报, 2016, 34(1): 1-10. |
| ZHANG Huaiwei, ZHENG Xinyao, LIU Yang, et al. Application and development of rare earth-based hydrogen storage materials[J]. Journal of the Chinese Society of Rare Earths, 2016, 34(1): 1-10. | |
| 106 | Borislav BOGDANOVIĆ, SCHWICKARDI Manfred. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials[J]. Journal of Alloys and Compounds, 1997, 253/254: 1-9. |
| 107 | ANTON D L. Hydrogen desorption kinetics in transition metal modified NaAlH4 [J]. Journal of Alloys and Compounds, 2003, 356/357: 400-404. |
| 108 | LI Li, WANG Ying, QIU Fangyuan, et al. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst[J]. Journal of Alloys and Compounds, 2013, 566: 137-141. |
| 109 | ZHANG Xin, ZHANG Xuelian, REN Zhuanghe, et al. Amorphous-carbon-supported ultrasmall TiB2 nanoparticles with high catalytic activity for reversible hydrogen storage in NaAlH4 [J]. Frontiers in Chemistry, 2020, 8: 419. |
| 110 | LI Li, QIU Fangyuan, WANG Yijing, et al. Enhanced hydrogen storage properties of TiN-LiAlH4 composite[J]. International Journal of Hydrogen Energy, 2013, 38(9): 3695-3701. |
| 111 | XIA Y, ZHANG H, SUN Y, et al. Dehybridization effect in improved dehydrogenation of LiAlH4 by doping with two-dimensional Ti3C2 [J]. Materials Today Nano, 2019, 8: 100054. |
| 112 | SAZELEE N A, YAHYA M S, ALI N A, et al. Enhancement of dehydrogenation properties in LiAlH4 catalysed by BaFe12O19 [J]. Journal of Alloys and Compounds, 2020, 835: 155183. |
| 113 | WEI Sheng, LIU Jiaxi, XIA Yongpeng, et al. Enhanced hydrogen storage properties of LiAlH4 by excellent catalytic activity of XTiO3@h-BN (X=Co, Ni)[J]. Advanced Functional Materials, 2022, 32(13): 2110180. |
| 114 | XU Juan, MENG Rongrong, CAO Jianyu, et al. Graphene-supported Pd catalysts for reversible hydrogen storage in LiBH4 [J]. Journal of Alloys and Compounds, 2013, 564: 84-90. |
| 115 | LI Zhenglong, GAO Mingxia, WANG Shun, et al. In-situ introduction of highly active TiO for enhancing hydrogen storage performance of LiBH4 [J]. Chemical Engineering Journal, 2022, 433: 134485. |
| 116 | TU Guoping, XIAO Xuezhang, JIANG Yiqun, et al. Composite cooperative enhancement on the hydrogen desorption kinetics of LiBH4 by co-doping with NbCl5 and hexagonal BN[J]. International Journal of Hydrogen Energy, 2015, 40(33): 10527-10535. |
| 117 | YE Jikai, XIA Guanglin, YU Xuebin. In-situ constructed destabilization reaction of LiBH4 wrapped with graphene toward stable hydrogen storage reversibility[J]. Materials Today Energy, 2021, 22: 100885. |
| 118 | ZHANG Xin, ZHANG Wenxuan, ZHANG Lingchao, et al. Single-pot solvothermal strategy toward support-free nanostructured LiBH4 featuring 12wt% reversible hydrogen storage at 400℃[J]. Chemical Engineering Journal, 2022, 428: 132566. |
| 119 | ZHANG Hongming, ZHANG Lu, RODRÍGUEZ-PÉREZ Ismael A, et al. Carbon nanospheres supported bimetallic Pt-Co as an efficient catalyst for NaBH4 hydrolysis[J]. Applied Surface Science, 2021, 540: 148296. |
| 120 | DELMAS J, LAVERSENNE L, ROUGEAUX I, et al. Improved hydrogen storage capacity through hydrolysis of solid NaBH4 catalyzed with cobalt boride[J]. International Journal of Hydrogen Energy, 2011, 36(3): 2145-2153. |
| 121 | NETSKINA O V, OZEROVA A M, KOMOVA O V, et al. Hydrogen storage systems based on solid-state NaBH4/Co x B composite: Influence of catalyst properties on hydrogen generation rate[J]. Catalysis Today, 2015, 245: 86-92. |
| 122 | OUYANG Liuzhang, CHEN Wei, LIU Jiangwen, et al. Enhancing the regeneration process of consumed NaBH4 for hydrogen storage[J]. Advanced Energy Materials, 2017, 7(19): 1700299. |
| 123 | LIU Yongfeng, YANG Yanjing, ZHOU Yifan, et al. Hydrogen storage properties and mechanisms of the Mg(BH4)2-NaAlH4 system[J]. International Journal of Hydrogen Energy, 2012, 37(22): 17137-17145. |
| 124 | PINKERTON Frederick E, MEISNER Gregory P, MEYER Martin S, et al. Hydrogen desorption exceeding ten weight percent from the new quaternary hydride Li3BN2H8 [J]. The Journal of Physical Chemistry B, 2005, 109(1): 6-8. |
| 125 | ICHIKAWA T, HANADA N, ISOBE S, et al. Hydrogen storage properties in Ti catalyzed Li-N-H system[J]. Journal of Alloys and Compounds, 2005, 404/405/406: 435-438. |
| 126 | WEI Jia, LENG Haiyan, LI Qian, et al. Improved hydrogen storage properties of LiBH4 doped Li-N-H system[J]. International Journal of Hydrogen Energy, 2014, 39(25): 13609-13615. |
| 127 | SOMER Mehmet, ACAR Selçuk, Cevriye KOZ, et al. α- and β- Na2[BH4][NH2]: Two modifications of a complex hydride in the system NaNH2-NaBH4; syntheses, crystal structures, thermal analyses, mass and vibrational spectra[J]. Journal of Alloys and Compounds, 2010, 491(1/2): 98-105. |
| 128 | BAI Ying, WU Chuan, WU Feng, et al. Thermal decomposition kinetics of light-weight composite NaNH2-NaBH4 hydrogen storage materials for fuel cells[J]. International Journal of Hydrogen Energy, 2012, 37(17): 12973-12979. |
| 129 | BAI Ying, ZHAO Lulu, WANG Yue, et al. Light-weight NaNH2-NaBH4 hydrogen storage material synthesized via liquid phase ball milling[J]. International Journal of Hydrogen Energy, 2014, 39(25): 13576-13582. |
| 130 | SAHU Parul. Clathrate hydrate technology for water reclamation: Present status and future prospects[J]. Journal of Water Process Engineering, 2021, 41: 102058. |
| 131 | YU Yisong, ZHANG Xianwei, LIU Jianwu, et al. Natural gas hydrate resources and hydrate technologies: A review and analysis of the associated energy and global warming challenges[J]. Energy & Environmental Science, 2021, 14(11): 5611-5668. |
| 132 | VELUSWAMY Hari Prakash, KUMAR Asheesh, SEO Yutaek, et al. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates[J]. Applied Energy, 2018, 216: 262-285. |
| 133 | MATSUMOTO Yuuki, Gary GRIM R, KHAN Naveed M, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3783-3788. |
| 134 | LEE Huen, LEE Jong-won, KIM Do Youn, et al. Tuning clathrate hydrates for hydrogen storage[J]. Nature, 2005, 434(7034): 743-746. |
| 135 | NGUYEN The Thuong, Claire PÉTUYA, TALAGA David, et al. Promoting the insertion of molecular hydrogen in tetrahydrofuran hydrate with the help of acidic additives[J]. Frontiers in Chemistry, 2020, 8: 550862. |
| 136 | LANG Xuemei, ZHENG Caijuan, FAN Shuanshi, et al. “Similar self-preservation” and decomposition kinetics of tetrahydrofuran-hydrogen hydrate particles[J]. International Journal of Hydrogen Energy, 2022, 47(13): 8457-8466. |
| 137 | CHEN Siyuan, WANG Yanhong, LANG Xuemei, et al. Rapid and high hydrogen storage in epoxycyclopentane hydrate at moderate pressure[J]. Energy, 2023, 268: 126638. |
| 138 | GHAANI Mohammad Reza, TAKEYA Satoshi, ENGLISH Niall J. Hydrogen storage in propane-hydrate: Theoretical and experimental study[J]. Applied Sciences, 2020, 10(24): 8962. |
| 139 | 尹凯东. 丙烷和甲基环己烷水合物储氢的分子动力学模拟研究[D]. 广州: 华南理工大学, 2020: 24-58. |
| YIN Kaidong. Using propane or methylcyclohexane hydrate as hydrogen storage material: MD simulation[D]. Guangzhou: South China University of Technology, 2020: 24-58. | |
| 140 | TRUEBA Alondra Torres, RADOVIĆ Ivona R, ZEVENBERGEN John F, et al. Kinetics measurements and in situ Raman spectroscopy of formation of hydrogen-tetrabutylammonium bromide semi-hydrates[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5790-5797. |
| 141 | WANG Yanhong, YIN Kaidong, LANG Xuemei, et al. Hydrogen storage in sH binary hydrate: Insights from molecular dynamics simulation[J]. International Journal of Hydrogen Energy, 2021, 46(29): 15748-15760. |
| 142 | OMRAN Ahmed, NESTERENKO Nikolai, VALTCHEV Valentin. Ab initio mechanistic insights into the stability, diffusion and storage capacity of sⅠ clathrate hydrate containing hydrogen[J]. International Journal of Hydrogen Energy, 2022, 47(13): 8419-8433. |
| 143 | LIU Shengli, ZHANG Wenxiu, WU Huanhua, et al. Molecular hydrogen storage in binary H2-CH4 clathrate hydrates[J]. Journal of Molecular Liquids, 2023, 376: 121496. |
| [1] | 史柯柯, 刘木子, 赵强, 李晋平, 刘光. 镁基储氢材料的性能及研究进展[J]. 化工进展, 2023, 42(9): 4731-4745. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||