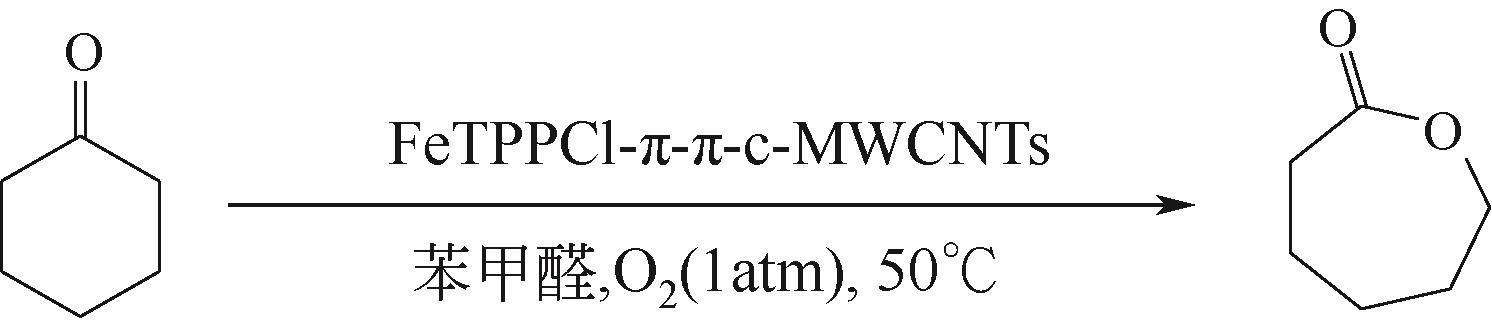化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1332-1340.DOI: 10.16085/j.issn.1000-6613.2022-0818
金属卟啉/碳纳米管仿生催化剂的制备及其在Baeyer-Villiger氧化反应中的催化机理
- 1.江汉大学光电化学材料与器件教育部重点实验室,光电材料与技术学院,湖北 武汉 430056
2.中山大学化学;学院精细化工研究院,广东 广州 510275
-
收稿日期:2022-05-05修回日期:2022-09-16出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:纪红兵 -
作者简介:陈韶云(1984—),女,博士,副教授,研究方向为光电磁功能材料的制备及其性能。E-mail:cescsy@jhun.edu.cn。 -
基金资助:国家自然科学基金(21938001)
Preparation of metalloporphyrin/carbon nanotube biomimetic catalysts and its catalytic mechanism in baeyer-villiger oxidation
CHEN Shaoyun1,2( ), ZHOU Xiantai2, JI Hongbing2(
), ZHOU Xiantai2, JI Hongbing2( )
)
- 1.Key Laboratory of Optoelectronic Chemical Materials and Devices, Ministry of Education, School of Optoelectronic Materials and Technology, Jianghan University, Wuhan 430056, Hubei, China
2.Fine Chemical Industry Research Institute, School of Chemistry, Sun Yat-sen University, Guangzhou 510275, Guangdong, China
-
Received:2022-05-05Revised:2022-09-16Online:2023-03-15Published:2023-04-10 -
Contact:JI Hongbing
摘要:
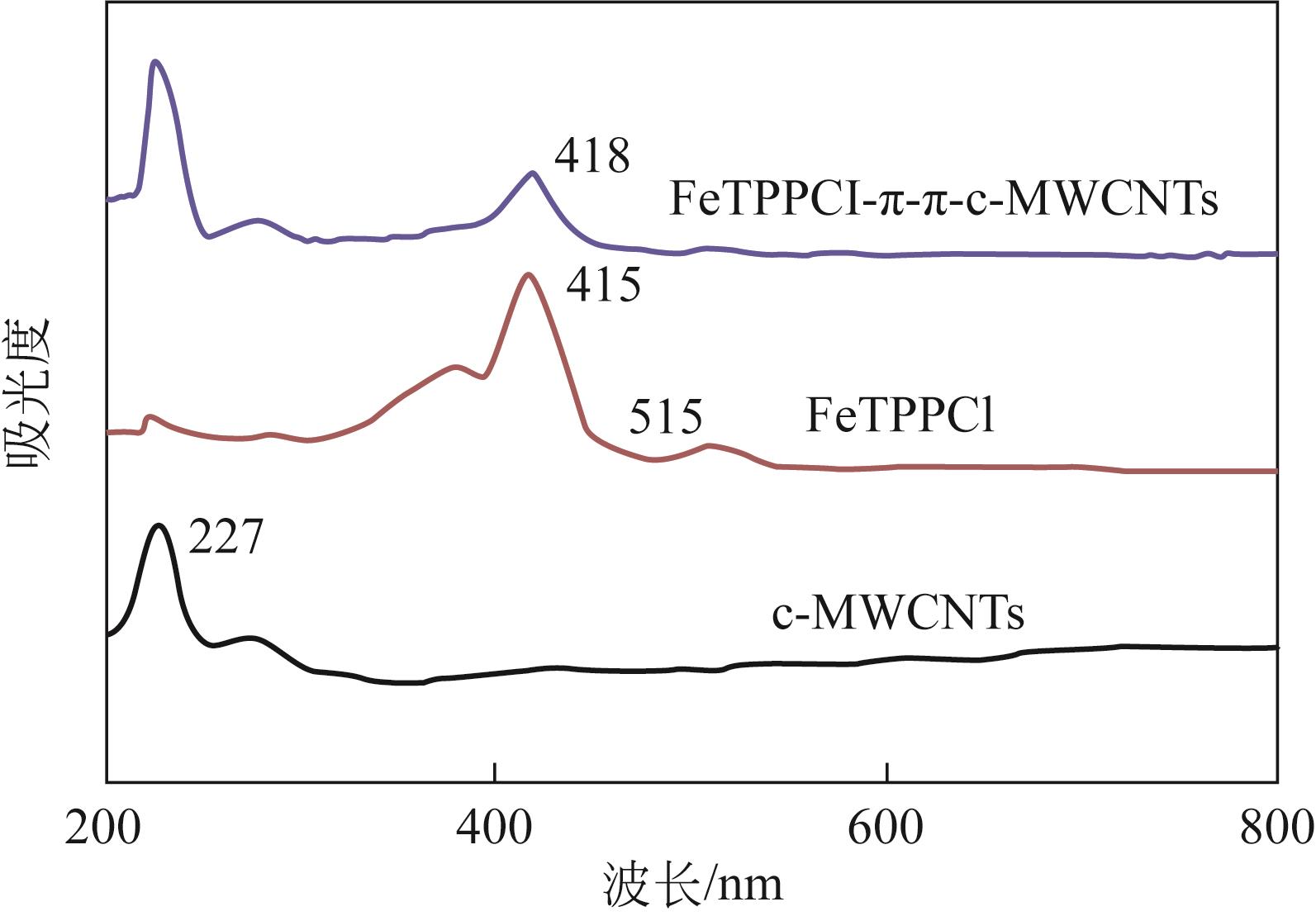
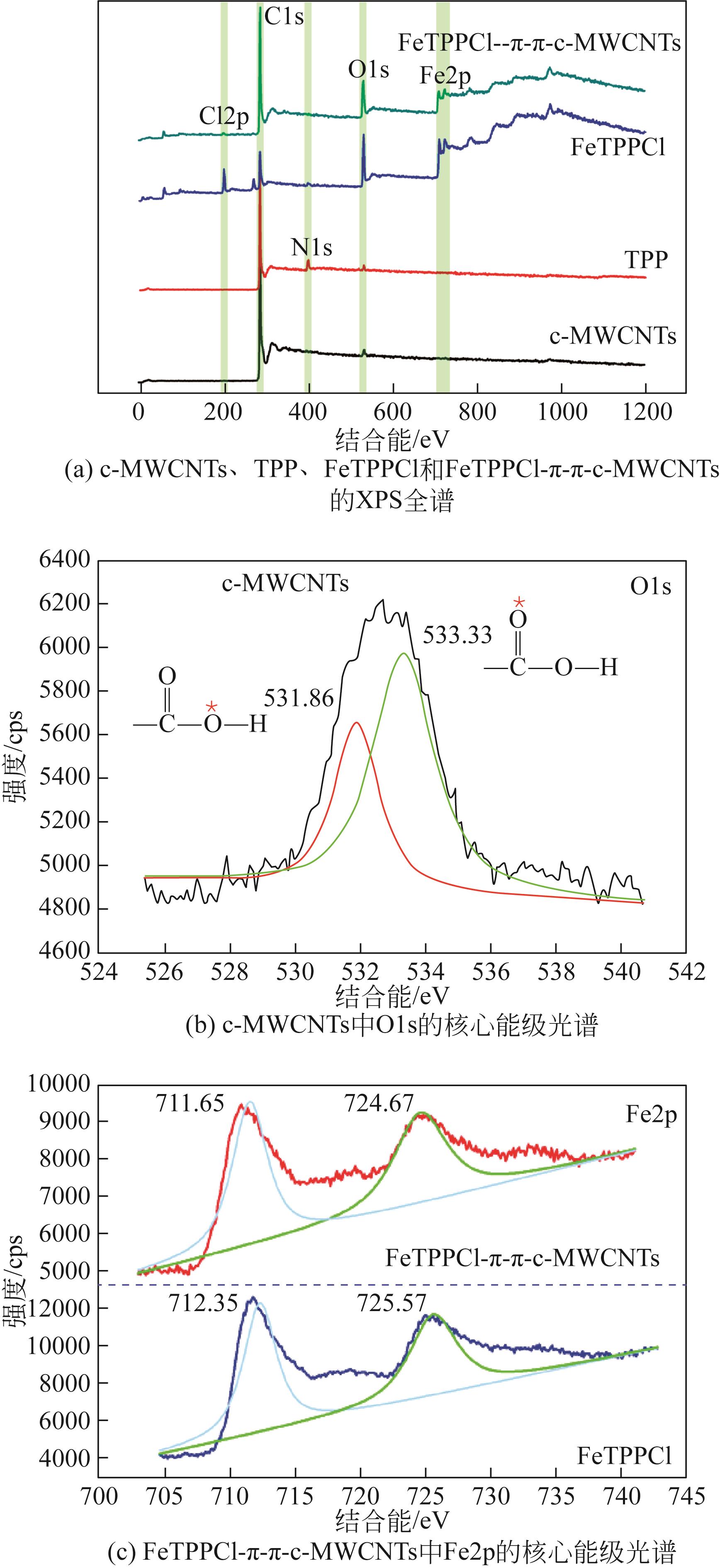
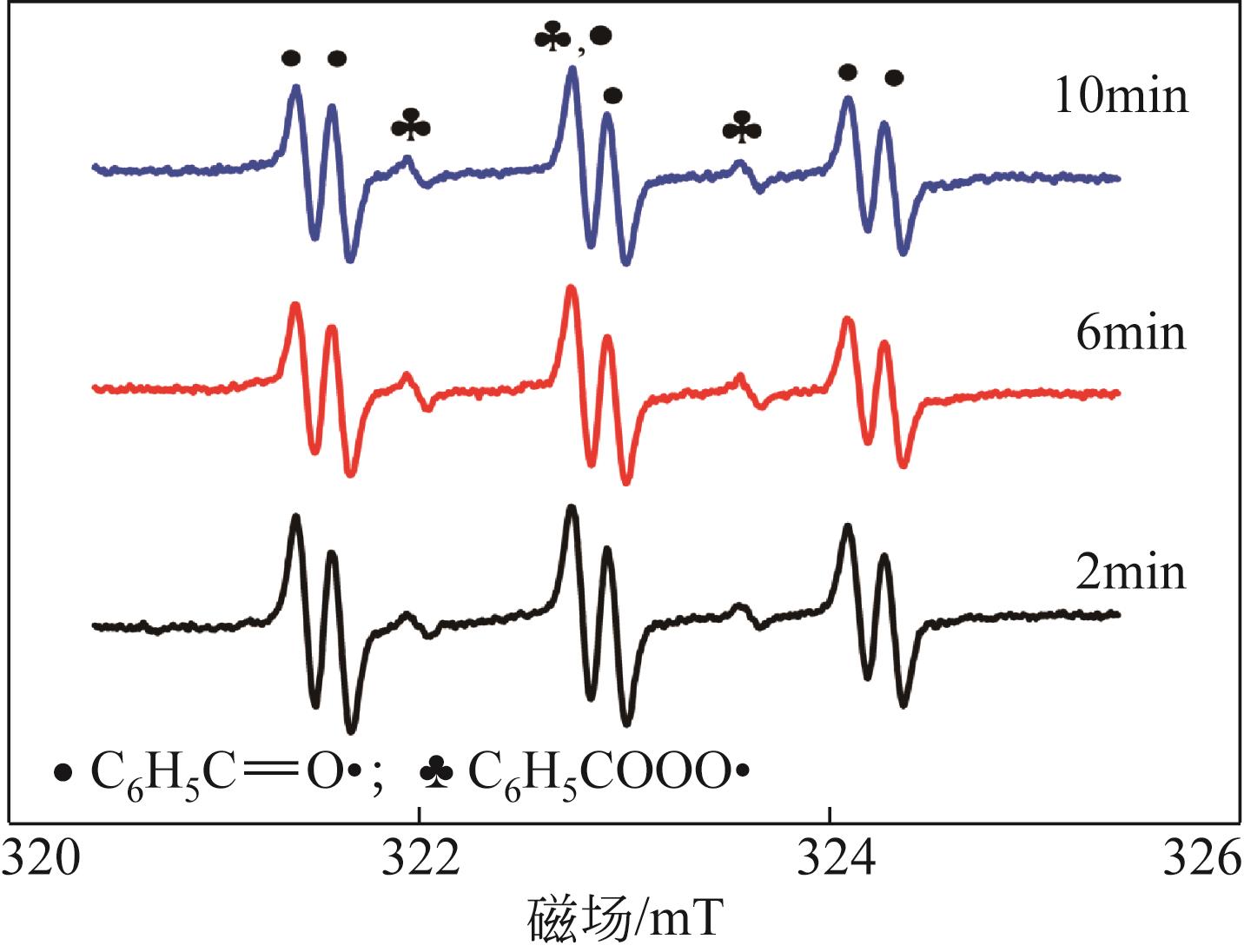
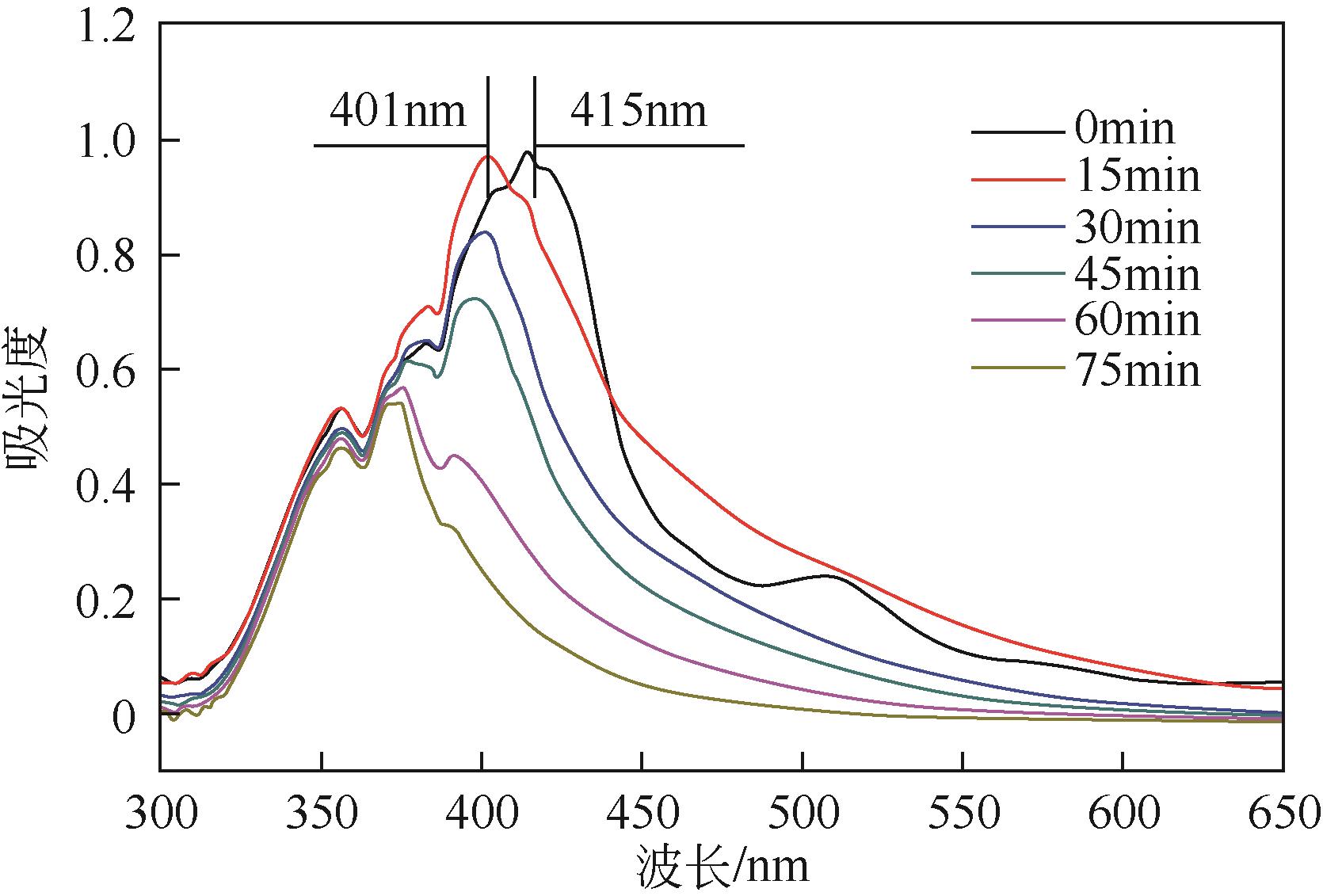
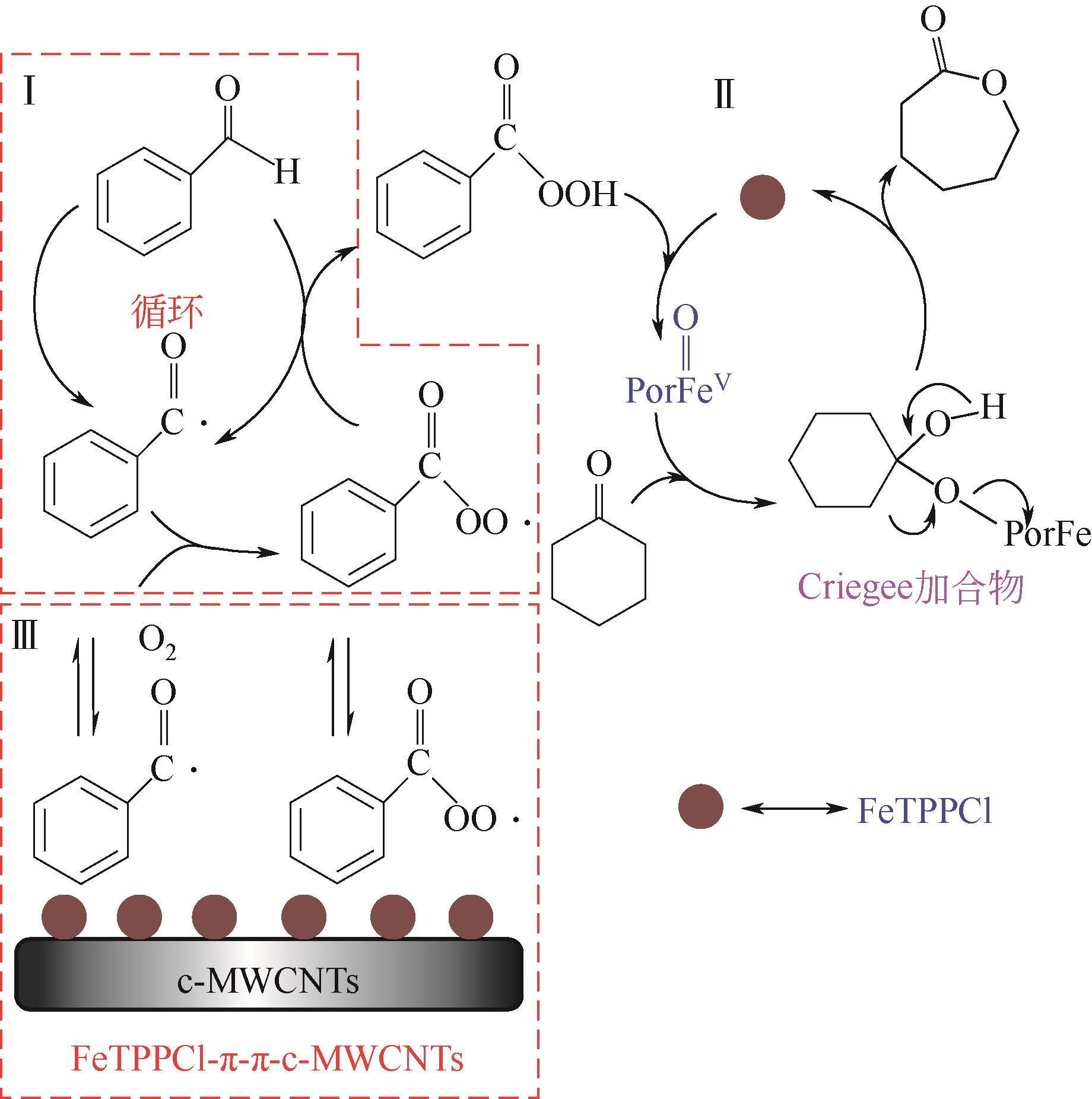
利用金属卟啉环(TPP)上π电子与多壁碳纳米管(MWCNTs)上π电子之间的π-π堆积效应,成功制备了4种金属卟啉/多壁碳纳米管催化剂(FeTPPCl-π-π-c-MWCNTs、FeTPPCl-π-π-MWCNTs、SnTPP-π-π-c-MWCNTs和SnTPP-π-π-MWCNTs)。实验表明,所制备产物可用于酮类化合物Baeyer-Villiger(B-V)氧化反应的仿生催化剂。当反应温度为50℃,1,2-二氯乙烷为溶剂,FeTPPCl-π-π-c-MWCNTs为催化剂,环己酮的转化率达到96%,ε-己内酯的收率为96%。另外,所制备的催化剂对于其他酮类化合物的B-V氧化也表现出良好的催化活性。进一步通过原位电子顺磁共振波谱和原位紫外光谱研究了环己酮的B-V氧化的反应历程,发现金属卟啉/碳纳米管仿生催化剂能同时提高自由基和金属卟啉高价活性物种的稳定性,这有利于增强金属卟啉仿生催化酮类化合物的活性。
中图分类号:
引用本文
陈韶云, 周贤太, 纪红兵. 金属卟啉/碳纳米管仿生催化剂的制备及其在Baeyer-Villiger氧化反应中的催化机理[J]. 化工进展, 2023, 42(3): 1332-1340.
CHEN Shaoyun, ZHOU Xiantai, JI Hongbing. Preparation of metalloporphyrin/carbon nanotube biomimetic catalysts and its catalytic mechanism in baeyer-villiger oxidation[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1332-1340.
| 编号 | 催化剂 | 环己酮的 转化率/% | ε-己内酯的收率/% | ε-己内酯的选择性/% |
|---|---|---|---|---|
| 1 | — | 22 | 22 | >99 |
| 2 | FeTPPCl-π-π-c-MWCNTs | 96 | 96 | >99 |
| 3 | FeTPPCl-π-π-MWCNTs | 80 | 80 | >99 |
| 4 | SnTPP-π-π-c-MWCNTs | 64 | 64 | >99 |
| 5 | SnTPP-π-π-MWCNTs | 60 | 60 | >99 |
| 6 | FeTPPCl-π-π-c-MWCNTs | 0 | 0 | — |
表1 不同催化剂对环己酮B-V氧化的影响
| 编号 | 催化剂 | 环己酮的 转化率/% | ε-己内酯的收率/% | ε-己内酯的选择性/% |
|---|---|---|---|---|
| 1 | — | 22 | 22 | >99 |
| 2 | FeTPPCl-π-π-c-MWCNTs | 96 | 96 | >99 |
| 3 | FeTPPCl-π-π-MWCNTs | 80 | 80 | >99 |
| 4 | SnTPP-π-π-c-MWCNTs | 64 | 64 | >99 |
| 5 | SnTPP-π-π-MWCNTs | 60 | 60 | >99 |
| 6 | FeTPPCl-π-π-c-MWCNTs | 0 | 0 | — |
| 编号 | 溶剂 | 反应温度 /℃ | 环己酮的 转化率/% | ε-己内酯的收率/% | ε-己内酯的选择性/% |
|---|---|---|---|---|---|
| 1 | 1,4-二氧六环 | 50 | 4 | 4 | >99 |
| 2 | 三氟甲苯 | 50 | 85 | 85 | >99 |
| 3 | 乙腈 | 50 | 66 | 66 | >99 |
| 4 | 乙酸乙酯 | 50 | 74 | 74 | >99 |
| 5 | 1,2-二氯乙烷 | 60 | 97 | 97 | >99 |
| 6 | 1,2-二氯乙烷 | 50 | 96 | 96 | >99 |
| 7 | 1,2-二氯乙烷 | 40 | 59 | 59 | >99 |
| 8 | 1,2-二氯乙烷 | 30 | 21 | 21 | >99 |
表2 溶剂和温度对环己酮B-V氧化反应的影响
| 编号 | 溶剂 | 反应温度 /℃ | 环己酮的 转化率/% | ε-己内酯的收率/% | ε-己内酯的选择性/% |
|---|---|---|---|---|---|
| 1 | 1,4-二氧六环 | 50 | 4 | 4 | >99 |
| 2 | 三氟甲苯 | 50 | 85 | 85 | >99 |
| 3 | 乙腈 | 50 | 66 | 66 | >99 |
| 4 | 乙酸乙酯 | 50 | 74 | 74 | >99 |
| 5 | 1,2-二氯乙烷 | 60 | 97 | 97 | >99 |
| 6 | 1,2-二氯乙烷 | 50 | 96 | 96 | >99 |
| 7 | 1,2-二氯乙烷 | 40 | 59 | 59 | >99 |
| 8 | 1,2-二氯乙烷 | 30 | 21 | 21 | >99 |
| 编号 | 催化剂用量 /mg | 环己酮的 转化率/% | ε-己内酯的 收率/% | ε-己内酯的 选择性/% |
|---|---|---|---|---|
| 1 | 0 | 22 | 22 | >99 |
| 2 | 1.2 | 60 | 60 | >99 |
| 3 | 2.5 | 70 | 70 | >99 |
| 4 | 5.0 | 96 | 96 | >99 |
| 5 | 10.0 | 95 | 95 | >99 |
表3 催化剂用量对环己酮B-V氧化反应的影响
| 编号 | 催化剂用量 /mg | 环己酮的 转化率/% | ε-己内酯的 收率/% | ε-己内酯的 选择性/% |
|---|---|---|---|---|
| 1 | 0 | 22 | 22 | >99 |
| 2 | 1.2 | 60 | 60 | >99 |
| 3 | 2.5 | 70 | 70 | >99 |
| 4 | 5.0 | 96 | 96 | >99 |
| 5 | 10.0 | 95 | 95 | >99 |
| 编号 | 苯甲醛的用量 /mmol | 环己酮的 转化率/% | ε-己内酯的 收率/% | ε-己内酯的 选择性/% |
|---|---|---|---|---|
| 1 | 1 | 45 | 45 | >99 |
| 2 | 2 | 55 | 55 | >99 |
| 3 | 4 | 96 | 96 | >99 |
| 4 | 6 | 98 | 97 | >99 |
| 5 | 8 | 96 | 97 | >99 |
表4 苯甲醛用量对环己酮B-V氧化反应的影响
| 编号 | 苯甲醛的用量 /mmol | 环己酮的 转化率/% | ε-己内酯的 收率/% | ε-己内酯的 选择性/% |
|---|---|---|---|---|
| 1 | 1 | 45 | 45 | >99 |
| 2 | 2 | 55 | 55 | >99 |
| 3 | 4 | 96 | 96 | >99 |
| 4 | 6 | 98 | 97 | >99 |
| 5 | 8 | 96 | 97 | >99 |
| 编号 | 酮类反应物 | 产物 | 时间 /h | 转化率 /% | 收率 /% |
|---|---|---|---|---|---|
| 1 |  |  | 8 | 96 | >99 |
| 2 |  |  | 10 | 5 | >99 |
| 3 |  |  | 10 | 81 | >99 |
| 4 |  |  | 10 | 93 | >99 |
| 5 |  |  | 8 | 98 | >99 |
| 6 |  |  | 8 | 98 | >99 |
| 7 |  |  | 8 | 17 | >99 |
表5 FeTPPCl-π-π-c-MWCNTs作为催化剂对不同酮类的B-V氧化反应效
| 编号 | 酮类反应物 | 产物 | 时间 /h | 转化率 /% | 收率 /% |
|---|---|---|---|---|---|
| 1 |  |  | 8 | 96 | >99 |
| 2 |  |  | 10 | 5 | >99 |
| 3 |  |  | 10 | 81 | >99 |
| 4 |  |  | 10 | 93 | >99 |
| 5 |  |  | 8 | 98 | >99 |
| 6 |  |  | 8 | 98 | >99 |
| 7 |  |  | 8 | 17 | >99 |
| 编号 | 苯甲醛 | 催化剂 | BHT | 环己酮的转化率/% |
|---|---|---|---|---|
| 1 | × | × | × | 0 |
| 2 | × | ○ | × | 0 |
| 3 | × | ○ | ○ | 0 |
| 4 | ○ | × | × | 22 |
| 5 | ○ | × | ○ | 0 |
| 6 | ○ | ○ | × | 96 |
| 7 | ○ | ○ | ○ | 0 |
表6 苯甲醛、催化剂和BHT自由基捕集剂对环己酮转化率的影响
| 编号 | 苯甲醛 | 催化剂 | BHT | 环己酮的转化率/% |
|---|---|---|---|---|
| 1 | × | × | × | 0 |
| 2 | × | ○ | × | 0 |
| 3 | × | ○ | ○ | 0 |
| 4 | ○ | × | × | 22 |
| 5 | ○ | × | ○ | 0 |
| 6 | ○ | ○ | × | 96 |
| 7 | ○ | ○ | ○ | 0 |
| 1 | RENZ M, MEUNIER B. 100 years of Baeyer-villiger oxidations[J]. European Journal of Organic Chemistry, 1999, (4): 737-750. |
| 2 | 李子辉, 蒋晶, 金章勇, 等. 生物可降解PCL/PLA开孔发泡材料制备及吸油性能[J]. 化工学报, 2020, 71(12): 5842-5853. |
| LI Zihui, JIANG Jing, JIN Zhangyong, et al. Preparation and oil absorption performance of biodegradable PCL/PLA open-cell foam material[J]. CIESC Journal, 2020, 71(12): 5842-5853. | |
| 3 | BATISTE D C, MEYERSOHN M S, WATTS A, et al. Efficient polymerization of methyl-ε-caprolactone mixtures to access sustainable aliphatic polyesters[J]. Macromolecules, 2020, 53(5): 1795-1808. |
| 4 | ROSA R P, FERREIRA F V, SARAVIA A P K, et al. A combined computational and experimental study on the polymerization of ε-caprolactone[J]. Industrial & Engineering Chemistry Research, 2018, 57(40): 13387-13395. |
| 5 | CHÁVEZ G, HATTI-KAUL R, SHELDON R A, et al. Baeyer-Villiger oxidation with peracid generated in situ by CaLB-CLEA catalyzed perhydrolysis[J]. Journal of Molecular Catalysis B: enzymatic, 2013, 89: 67-72. |
| 6 | XIAO Guansheng, GAO Xi, YAN Weiting, et al. Baeyer-Villiger oxidation of cyclohexanone by hydrogen peroxide with Fe3O4@GO as catalyst under solvent free conditions[J]. Catalysis Letters, 2019, 149(7): 1765-1771. |
| 7 | OLSZÓWKA J E, KARCZ R, NAPRUSZEWSKA B D, et al. Effect of MgAl hydrotalcite crystallinity on catalytic Baeyer-Villiger oxidation of cyclohexanone with H2O2/acetonitrile[J]. Catalysis Communications, 2018, 107: 48-52. |
| 8 | ILOVAISKY A I, MERKULOVA V M, VIL’ V, et al. Regioselective baeyer-villiger oxidation of steroidal ketones to lactones using BF3/H2O2 [J].European Journal of Organic Chemistry, 2020, 3: 402-405. |
| 9 | DE GONZALO G, ALCÁNTARA A. Multienzymatic processes involving baeyer-villiger monooxygenases[J]. Catalysts, 2021, 11: 605. |
| 10 | LI Peilin, MA Yunjian, LI Yongru, et al. Cascade synthesis from cyclohexane to ε-caprolactone by visible-light-driven photocatalysis combined with whole-cell biological oxidation[J]. ChemBioChem, 2020, 21(13): 1852-1855. |
| 11 | SOLÉ J, BRUMMUND J, CAMINAL G, et al. Enzymatic synthesis of trimethyl-ε-caprolactone: process intensification and demonstration on a 100 L scale[J]. Organic Process Research & Development, 2019, 23(11): 2336-2344. |
| 12 | LIU Chunhua, WANG Zhuo, XIAO Liyun, et al. Acid/base-co-catalyzed formal baeyer-villiger oxidation reaction of ketones: using molecular oxygen as the oxidant[J]. Organic Letters, 2018, 20(16): 4862-4866. |
| 13 | FILATOV M, RECKIEN W, PEYERIMHOFF S D, et al. What are the reasons for the kinetic stability of a mixture of H2 and O2? [J]. The Journal of Physical Chemistry A, 2000, 104(51): 12014-12020. |
| 14 | JIANG Qing, SHENG Wenbing, GUO Xiangdong, et al. Metalloporphyrin-catalyzed aerobic oxidation of 2-methoxy-4-methylphenol as a route to vanillin[J]. Journal of Molecular Catalysis A: Chemical, 2013, 373: 121-126. |
| 15 | ADAM F, OOI W T. Selective oxidation of benzyl alcohol to benzaldehyde over Co-metalloporphyrin supported on silica nanoparticles[J]. Applied Catalysis A: General, 2012, 445/446: 252-260. |
| 16 | ZHOU Xiantai, JI Hongbing. Manganese porphyrin immobilized on montmorillonite: a highly efficient and reusable catalyst for the aerobic epoxidation of olefins under ambient conditions[J]. Journal of Porphyrins and Phthalocyanines, 2012, 16(9): 1032-1039. |
| 17 | ZHOU Xiantai, REN Gangli, JI Hongbing. Kinetic and mechanism of the aqueous selective oxidation of sulfides to sulfoxides: insight into the cytochrome P450-like oxidative metabolic process[J]. Journal of Porphyrins and Phthalocyanines, 2013, 17: 1104-1112. |
| 18 | LIU W, GROVES J T. Manganese porphyrins catalyze selective C-H bond halogenations[J]. Journal of the American Chemical Society, 2010, 132(37): 12847-12849. |
| 19 | ZHOU Xiantai, JI Hongbing, YUAN Qiulan. Baeyer-Villiger oxidation of ketones catalyzed by iron(Ⅲ) meso-tetraphenylporphyrin chloride in the presence of molecular oxygen[J]. Journal of Porphyrins and Phthalocyanines, 2008, 12(2): 94-100. |
| 20 | LAN Hongyun, ZHOU Xiantai, JI Hongbing. Remarkable differences between benzaldehyde and isobutyraldehyde as coreductant in the performance toward the iron(Ⅲ) porphyrins-catalyzed aerobic Baeyer-Villiger oxidation of cyclohexanone, kinetic and mechanistic features[J]. Tetrahedron, 2013, 69(21): 4241-4246. |
| 21 | CHEN Shaoyun, ZHOU Xiantai, LI Yang, et al. Biomimetic Baeyer-Villiger oxidation of ketones with SnO2 as cocatalyst, features in activating carbonyl group of substrates[J]. Chemical Engineering Journal, 2014, 241: 138-144. |
| 22 | NABAE Y, ROKUBUICHI H, MIKUNI M, et al. Catalysis by carbon materials for the aerobic baeyer–villiger oxidation in the presence of aldehydes[J]. ACS Catalysis, 2013, 3(2): 230-236. |
| 23 | MARKITON M, BONCEL S, JANAS D, et al. Highly active nanobiocatalyst from lipase noncovalently immobilized on multiwalled carbon nanotubes for baeyer-villiger synthesis of lactones[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1685-1691. |
| 24 | SZELWICKA A, ZAWADZKI P, SITKO M, et al. Continuous flow chemo-enzymatic baeyer-villiger oxidation with superactive and extra-stable enzyme/carbon nanotube catalyst: an efficient upgrade from batch to flow[J]. Organic Process Research & Development, 2019, 23(7): 1386-1395. |
| 25 | CAI Zhihui, LIU Duo, HUANG Jiangnan, et al. Solvent-free production of ε-caprolactone from oxidation of cyclohexanone catalyzed by nitrogen-doped carbon nanotubes[J]. Industrial & Engineering Chemistry Research, 2022, 61(5): 2037-2044. |
| 26 | MAITY S, RAM F, DHAR B B. Phosphorous-doped graphitic material as a solid acid catalyst for microwave-assisted synthesis of β-ketoenamines and baeyer-villiger oxidation[J]. ACS Omega, 2020, 5(26): 15962-15972. |
| 27 | XING Chen, TAN Rong, HAO Pengbo, et al. Graphene oxide supported chlorostannate (Ⅳ) ionic liquid: Brønsted-Lewis acidic combined catalyst for highly efficient Baeyer-Villiger oxidation in water[J]. Molecular Catalysis, 2017, 433: 37-47. |
| 28 | CHEN Shaoyun, ZHOU Xiantai, WANG Jiexiang, et al. Promoting the aerobic Baeyer-Villiger oxidation of ketones over carboxylic multi-walled carbon nanotubes[J]. Molecular Catalysis, 2017, 438: 152-158. |
| 29 | PEREIRA M M, DIAS L D, CALVETE M J. Metalloporphyrins: bioinspired oxidation catalysts[J].ACS Catalysis, 2018, 8(11): 10784-10808. |
| 30 | DAS S K, SUBBAIYAN N K, D’SOUZA F, et al. Formation and photoinduced properties of zinc porphyrin-SWCNT and zinc phthalocyanine-SWCNT nanohybrids using diameter sorted nanotubes assembled via metal-ligand coordination and π-π stacking[J]. Journal of Porphyrins and Phthalocyanines, 2011, 15(9/10): 1033-1043. |
| 31 | WANG Cun, YUAN Ruo, CHAI Yaqin, et al. Non-covalent iron(Ⅲ)-porphyrin functionalized multi-walled carbon nanotubes for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite[J]. Electrochimica Acta, 2012, 62: 109-115. |
| 32 | CHITTA R, SANDANAYAKA A, SCHUMACHER A, et al. Donor-acceptor nanohybrids of zinc naphthalocyanine or zinc porphyrin noncovalently linked to single-wall carbon nanotubes for photoinduced electron transfer[J]. Journal of Physical Chemistry C, 2007, 111: 6947-6955. |
| 33 | BANERJEE I, MONDAL D, MARTIN J, et al. Photoactivated antimicrobial activity of carbon nanotube-porphyrin conjugates[J]. Langmuir, 2010, 26(22): 17369-17374. |
| 34 | LI Yang, ZHOU Xiantai, JI Hongbing. Cocatalytic effect of cobalt acetate on aerobic cyclohexene oxidation catalyzed by manganese porphyrin[J]. Catalysis Communications, 2012, 27: 169-173. |
| 35 | LAN Hongyun, ZHOU Xiantai, JI Hongbing. Remarkable differences between benzaldehyde and isobutyraldehyde as coreductant in the performance toward the iron(Ⅲ) porphyrins-catalyzed aerobic Baeyer-Villiger oxidation of cyclohexanone, kinetic and mechanistic features[J]. Tetrahedron, 2013, 69(21): 4241-4246. |
| 36 | JANZEN E G, BLACKBURN B J. Detection and identification of short-lived free radicals by an electron spin resonance trapping technique[J]. Journal of the American Chemical Society, 1968, 90(21): 5909-5910. |
| 37 | BERLINER L J, KHRAMTSOV V, FUJII H, et al. Unique in vivo applications of spin traps[J]. Free Radical Biology and Medicine, 2001, 30(5): 489-499. |
| 38 | JANZEN E G, LIN C R, HINTON R D. Spontaneous free-radical formation in reactions of m-chloroperbenzoic acid with C-phenyl-N-tert-butylnitrone (PBN) and 3- or 4-substituted PBN’s[J]. The Journal of Organic Chemistry, 1992, 57(6): 1633-1635. |
| 39 | LEISCH H, MORLEY K, LAU P C K. Baeyer-Villiger monooxygenases: more than just green chemistry[J]. Chemical Reviews, 2011, 111(7): 4165-4222. |
| 40 | YACHNIN B J, SPRULES T, MCEVOY M B, et al. The substrate-bound crystal structure of a Baeyer-Villiger monooxygenase exhibits a Criegee-like conformation[J]. Journal of the American Chemical Society, 2012, 134(18): 7788-7795. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||