化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1341-1352.DOI: 10.16085/j.issn.1000-6613.2022-0952
甲醇供氢体系铜锌双金属催化糠醛加氢转化
萧垚鑫1,2( ), 张军2,3,4(
), 张军2,3,4( ), 胡升5, 单锐2,3,4, 袁浩然2,3,4(
), 胡升5, 单锐2,3,4, 袁浩然2,3,4( ), 陈勇1,2,3,4
), 陈勇1,2,3,4
- 1.华南农业大学生物质工程研究院,广东 广州 510642
2.中国科学院广州能源研究所,广东 广州 510640
3.中国科学院可再生能源重点实验室,广东 广州 510640
4.广东省新能源和可再生能源研究开发与应用重点 实验室,广东 广州 510640
5.中国科学技术大学工程科学学院,安徽 合肥 230026
-
收稿日期:2022-05-23修回日期:2022-11-22出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:袁浩然 -
作者简介:萧垚鑫(1998—),男,硕士研究生,研究方向为生物质高值资源化利用。E-mail:2252164032@qq.com
张军(1987—),男,博士,副研究员,研究方向为有机固废/农林废弃生物质高值资源化利用。E-mail:zhagnjun@ms.giec.ac.cn。 -
基金资助:国家自然科学基金面上项目(51976222);能源清洁利用国家重点实验室开放基金课题(ZJU-CEU2020023)
Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor
XIAO Yaoxin1,2( ), ZHANG Jun2,3,4(
), ZHANG Jun2,3,4( ), HU Sheng5, SHAN Rui2,3,4, YUAN Haoran2,3,4(
), HU Sheng5, SHAN Rui2,3,4, YUAN Haoran2,3,4( ), CHEN Yong1,2,3,4
), CHEN Yong1,2,3,4
- 1.Institute of Biomass Engineering, South China Agricultural University, Guangzhou 510642, Guangdong, China
2.Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (CAS), Guangzhou 510640, Guangdong, China
3.CAS Key Laboratory of Renewable Energy, Guangzhou 510640, Guangdong, China
4.Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou 510640, Guangdong, China
5.School of Engineering Science, University of Science and Technology of China, Hefei 230026, Anhui, China
-
Received:2022-05-23Revised:2022-11-22Online:2023-03-15Published:2023-04-10 -
Contact:YUAN Haoran
摘要:
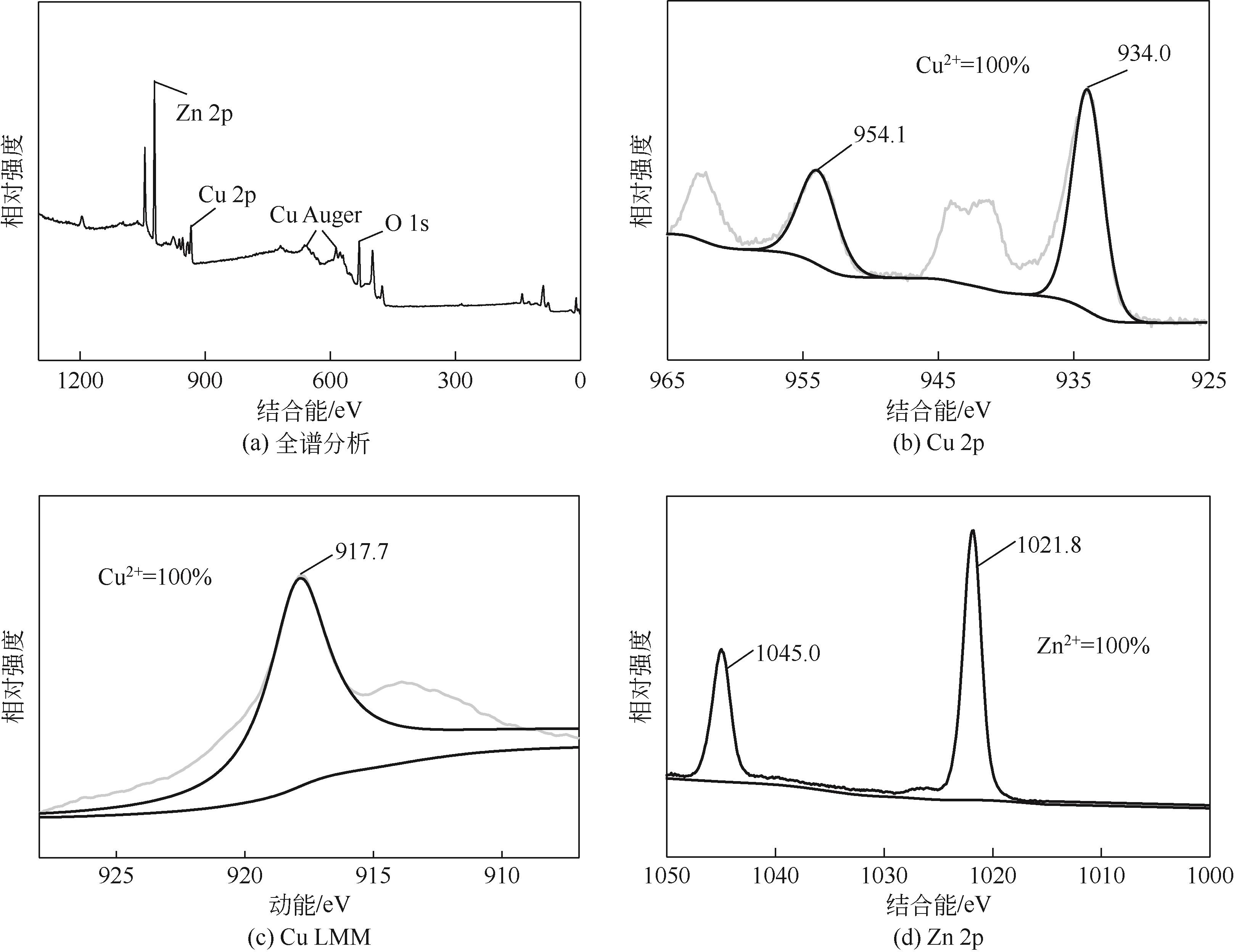
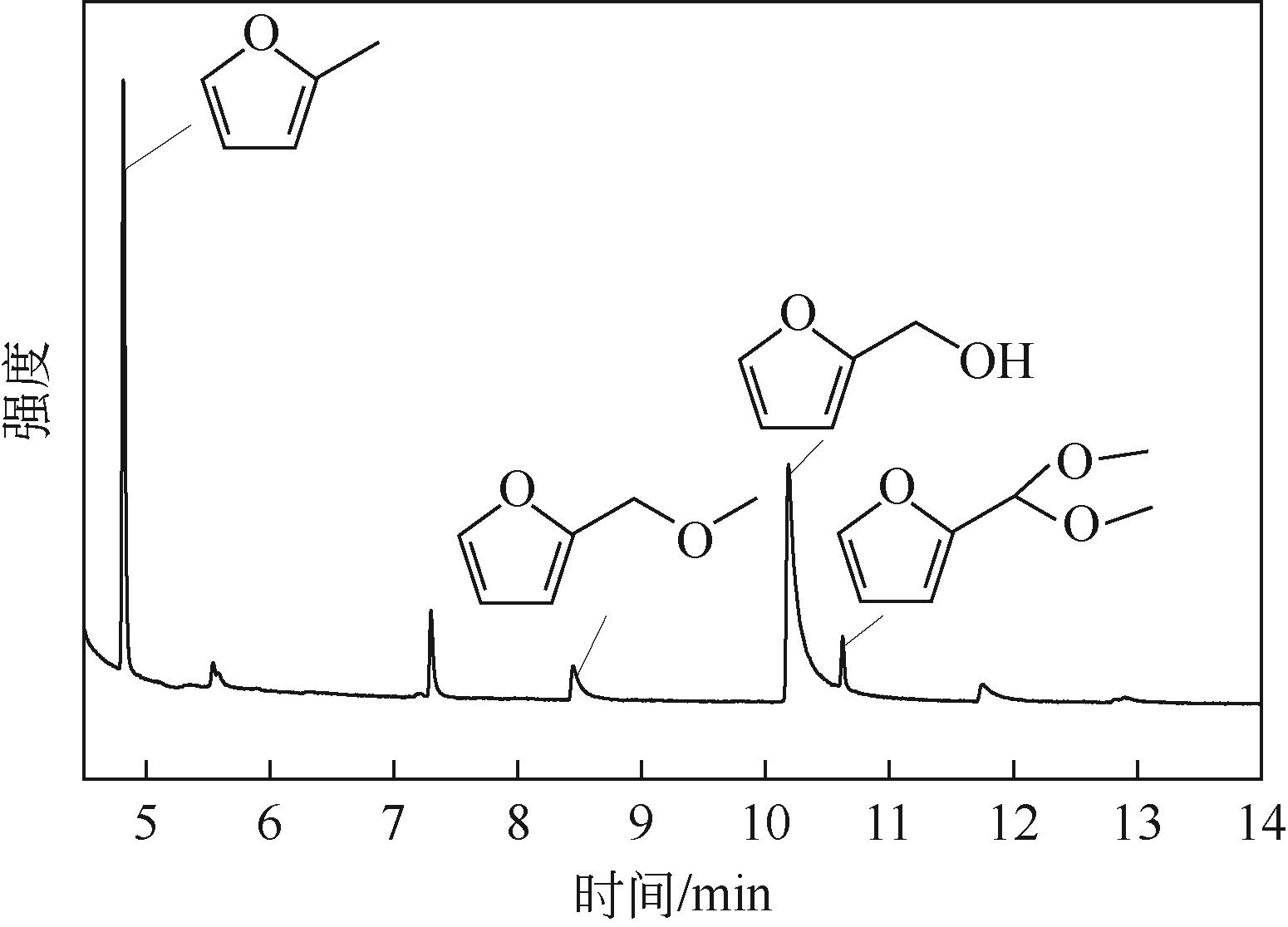
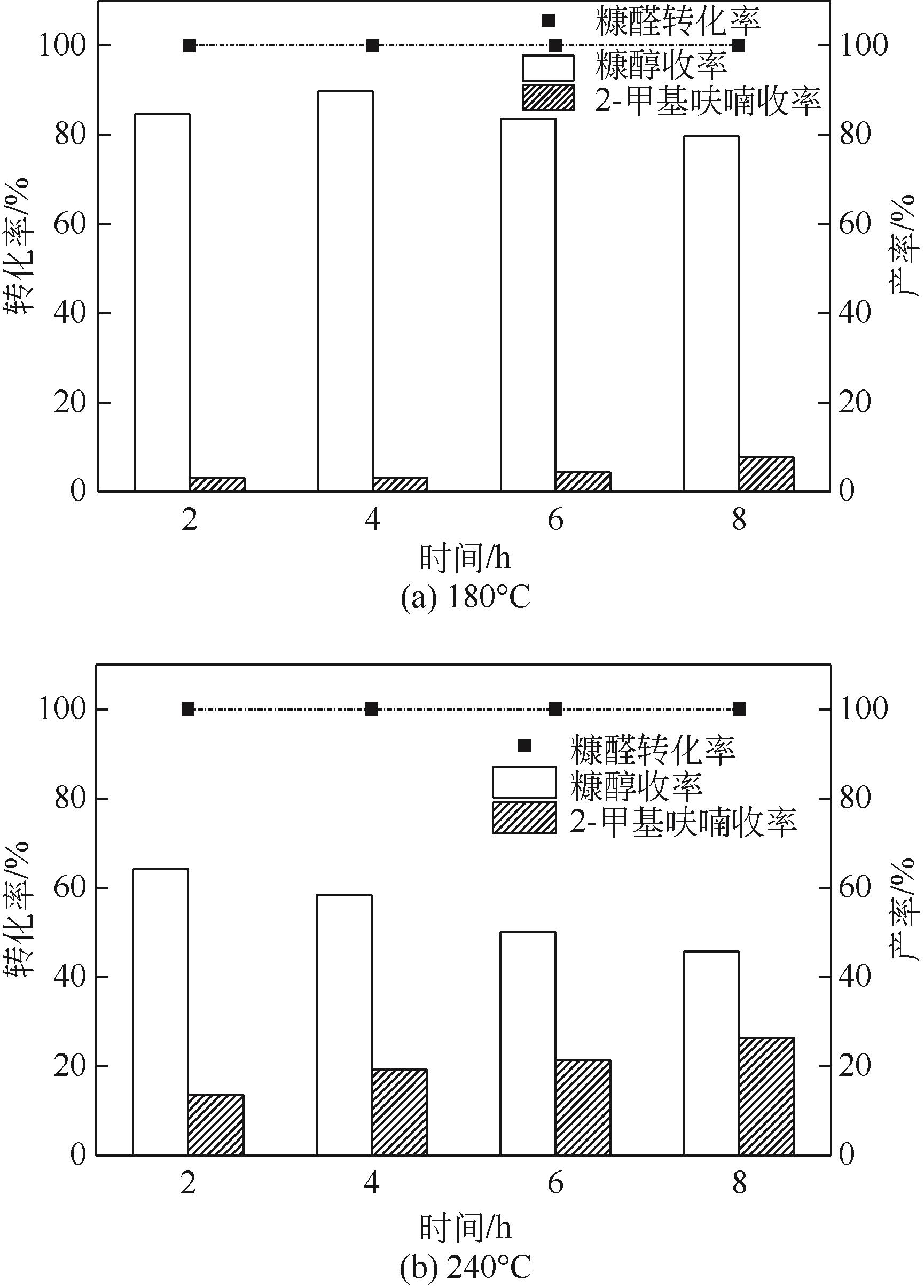
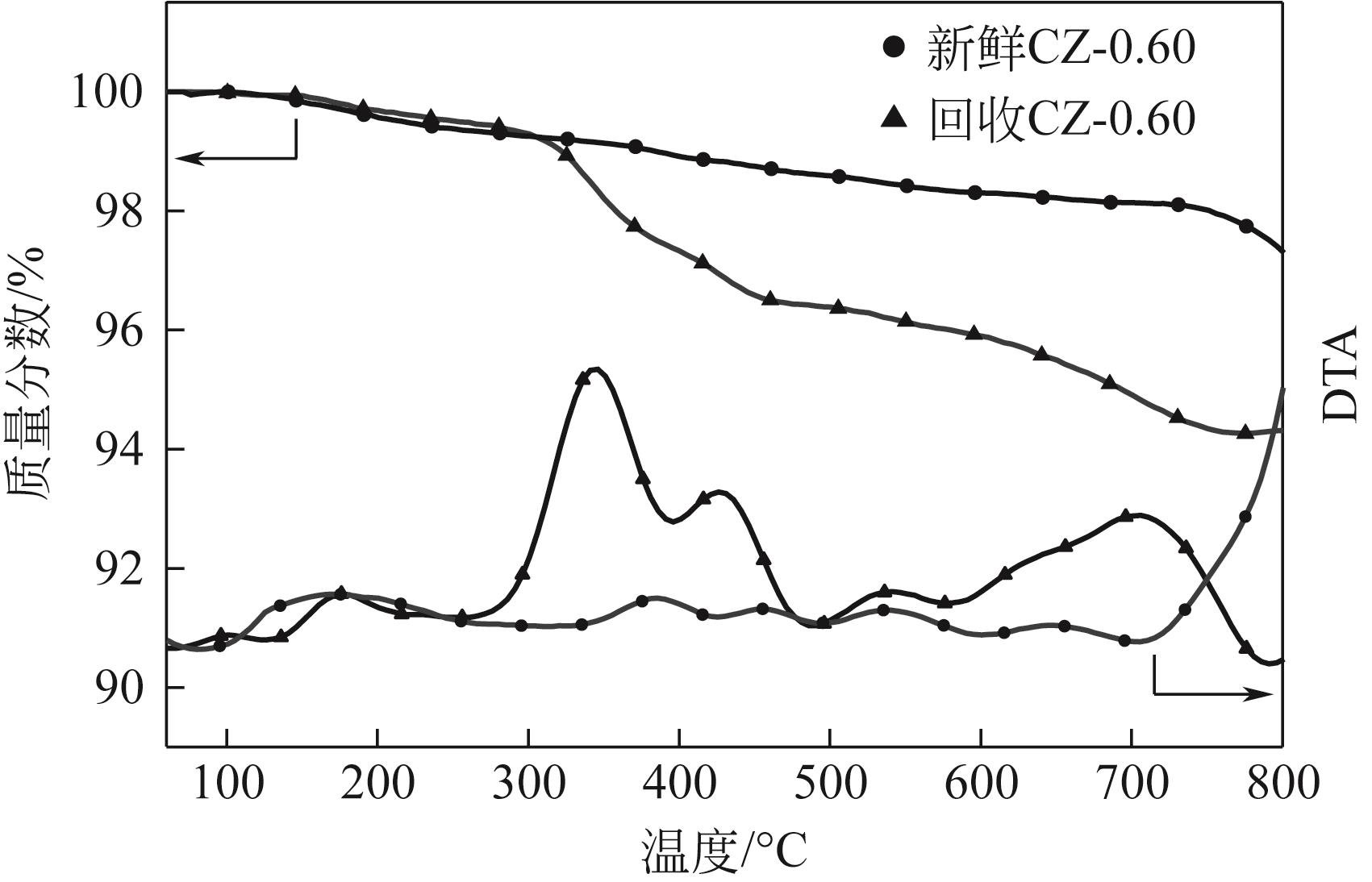
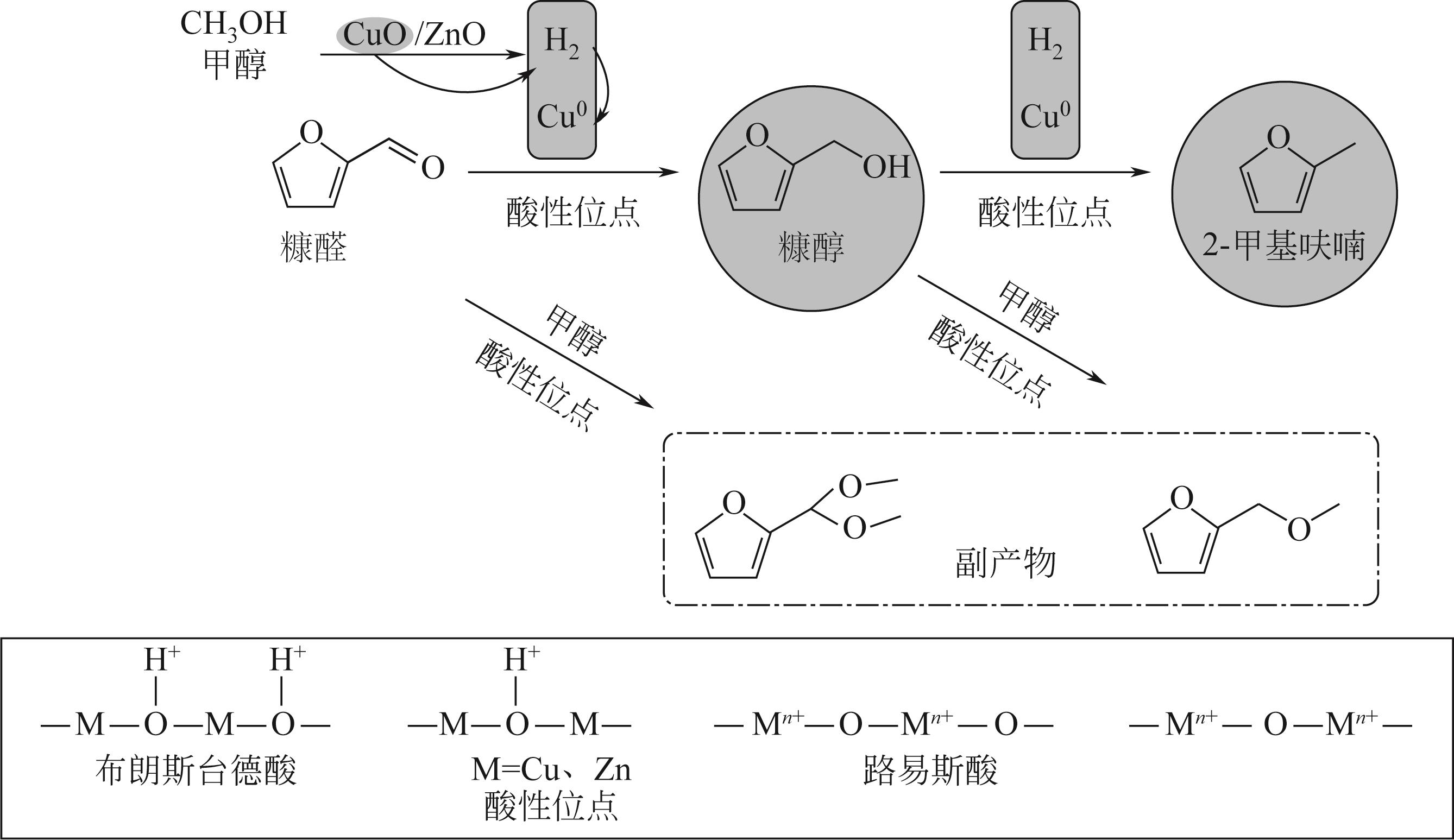
以Cu(NO3)2·3H2O、Zn(NO3)2·6H2O为原料,采用共沉淀法合成了一系列铜锌双金属催化材料。通过电感耦合等离子体发射光谱(ICP-OES)、X射线衍射(XRD)、氮气等温吸附-脱附、扫描电子显微镜(SEM)、氨气程序升温脱附(NH3-TPD)、氢气程序升温还原(H2-TPR)、X射线光电子能谱(XPS)、热重/差热分析(TG/DTA)等手段分析表征了催化剂物理化学特性。催化材料表征结果表明,载体Zn组分的引入显著改善了催化剂结构,形成了丰富的介孔结构和部分酸性位点。甲醇供氢体系糠醛加氢转化实验结果显示,合成的Cu-Zn双金属催化剂在甲醇重整产氢和糠醛加氢反应中表现出优异的活性,其中Cu/Zn摩尔比为0.6的CZ-0.60催化活性最高。当CZ-0.60用量为20mg,在160℃反应4h,糠醛完全转化,糠醇产率达89.7%;而在240℃反应8h,糠醛完全转化,2-甲基呋喃产率达26.3%。CZ-0.60在循环使用过程中仍表现出较好的催化活性,热重分析表明回用的CZ-0.60在750℃下具有良好的热稳定性能。基于上述研究结果,本文提出了甲醇供氢体系铜锌双金属催化糠醛加氢转化可能反应路径。
中图分类号:
引用本文
萧垚鑫, 张军, 胡升, 单锐, 袁浩然, 陈勇. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352.
XIAO Yaoxin, ZHANG Jun, HU Sheng, SHAN Rui, YUAN Haoran, CHEN Yong. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352.
| 组别 | 前体溶液的Cu/Zn比 | Cu浓度 /mol·L-1 | Zn浓度 /mol·L-1 | Cu/Zn | 简记 |
|---|---|---|---|---|---|
| 1 | 0.25 | 0.90 | 4.04 | 0.22 | CZ-0.22 |
| 2 | 0.43 | 1.28 | 3.39 | 0.38 | CZ-0.38 |
| 3 | 0.67 | 1.78 | 2.96 | 0.60 | CZ-0.60 |
| 4 | 1.00 | 2.19 | 2.44 | 0.90 | CZ-0.90 |
| 5 | 1.50 | 2.79 | 2.04 | 1.30 | CZ-1.30 |
表1 样品中实际金属含量及摩尔比
| 组别 | 前体溶液的Cu/Zn比 | Cu浓度 /mol·L-1 | Zn浓度 /mol·L-1 | Cu/Zn | 简记 |
|---|---|---|---|---|---|
| 1 | 0.25 | 0.90 | 4.04 | 0.22 | CZ-0.22 |
| 2 | 0.43 | 1.28 | 3.39 | 0.38 | CZ-0.38 |
| 3 | 0.67 | 1.78 | 2.96 | 0.60 | CZ-0.60 |
| 4 | 1.00 | 2.19 | 2.44 | 0.90 | CZ-0.90 |
| 5 | 1.50 | 2.79 | 2.04 | 1.30 | CZ-1.30 |
| 样品 | 比表面积/m2·g-1 | 孔容/m3·g-1 | 孔径/nm |
|---|---|---|---|
| CZ-0.22 | 113.59 | 6.97 | 3.42 |
| CZ-0.38 | 46.67 | 2.66 | 17.07 |
| CZ-0.60 | 51.96 | 2.62 | 15.35 |
| CZ-0.90 | 38.07 | 1.83 | 15.31 |
| CZ-1.30 | 48.25 | 2.36 | 16.57 |
表2 铜锌双金属催化剂比表面积、孔容及孔径信息
| 样品 | 比表面积/m2·g-1 | 孔容/m3·g-1 | 孔径/nm |
|---|---|---|---|
| CZ-0.22 | 113.59 | 6.97 | 3.42 |
| CZ-0.38 | 46.67 | 2.66 | 17.07 |
| CZ-0.60 | 51.96 | 2.62 | 15.35 |
| CZ-0.90 | 38.07 | 1.83 | 15.31 |
| CZ-1.30 | 48.25 | 2.36 | 16.57 |
| 序号 | 样品 | 糠醛转化率 /% | 产物收率/% | 副产物收率 /% | |
|---|---|---|---|---|---|
| 糠醇 | 2-甲基呋喃 | ||||
| 1 | CZ-0.22 | 100.0 | 70.3 | 6.5 | 23.2 |
| 2 | CZ-0.38 | 100.0 | 71.4 | 6.6 | 22.0 |
| 3 | CZ-0.60 | 100.0 | 69.0 | 12.3 | 18.7 |
| 4 | CZ-0.90 | 100.0 | 72.8 | 4.4 | 22.8 |
| 5 | CZ-1.30 | 100.0 | 70.3 | 8.2 | 21.5 |
表3 Cu-Zn摩尔比对糠醛加氢性能的影响
| 序号 | 样品 | 糠醛转化率 /% | 产物收率/% | 副产物收率 /% | |
|---|---|---|---|---|---|
| 糠醇 | 2-甲基呋喃 | ||||
| 1 | CZ-0.22 | 100.0 | 70.3 | 6.5 | 23.2 |
| 2 | CZ-0.38 | 100.0 | 71.4 | 6.6 | 22.0 |
| 3 | CZ-0.60 | 100.0 | 69.0 | 12.3 | 18.7 |
| 4 | CZ-0.90 | 100.0 | 72.8 | 4.4 | 22.8 |
| 5 | CZ-1.30 | 100.0 | 70.3 | 8.2 | 21.5 |
| 1 | 李全生, 张凯. 我国能源绿色开发利用路径研究[J]. 中国工程科学, 2021, 23(1): 101-111. |
| LI Quansheng, ZHANG Kai. The path for green development and utilization of energy in China[J]. Strategic Study of CAE, 2021, 23(1): 101-111. | |
| 2 | Thomas Bejoy, Midhun C RAJ, Athira K B, et al. Nanocellulose, a versatile green platform: From biosources to materials and their applications[J]. Chemical Reviews, 2018, 118(24): 11575-11625. |
| 3 | MA Jiping, SHI Song, JIA Xiuquan, et al. Advances in catalytic conversion of lignocellulose to chemicals and liquid fuels[J]. Journal of Energy Chemistry, 2019, 36: 74-86. |
| 4 | GALKIN Maxim V, SAMEC Joseph S M. Lignin valorization through catalytic lignocellulose fractionation: A fundamental platform for the future biorefinery[J]. ChemSusChem, 2016, 9(13): 1544-1558. |
| 5 | TESTA Maria Luisa, TUMMINO Maria Laura. Lignocellulose biomass as a multifunctional tool for sustainable catalysis and chemicals: An overview[J]. Catalysts, 2021, 11(1): 125. |
| 6 | YUAN Haoran, LI Chengyu, SHAN Rui, et al. Municipal sludge derived solid acids for levoglucosenone production via cellulose fast pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2022, 167: 105663. |
| 7 | ZHANG Jun, LI Chengyu, YUAN Haoran, et al. Enhancement of aromatics production via cellulose fast pyrolysis over Ru modified hierarchical zeolites[J]. Renewable Energy, 2022, 184: 280-290. |
| 8 | 石宁, 唐文勇, 唐石云, 等. 木质纤维素衍生平台化学品制备液态烷烃的研究进展[J]. 化工进展, 2019, 38(7): 3097-3110. |
| SHI Ning, TANG Wenyong, TANG Shiyun, et al. Advances in the catalytic conversion of lignocellulosic derived platform chemicals into liquid alkanes[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3097-3110. | |
| 9 | 刘志斌, 张学勤. 木质纤维素生物质催化转化为高附加值产品的研究进展[J]. 纤维素科学与技术, 2019, 27(3): 77-82. |
| LIU Zhibin, ZHANG Xueqin. Progress of lignocellulose biomass catalytic conversion to high added-value products[J]. Journal of Cellulose Science and Technology, 2019, 27(3): 77-82. | |
| 10 | 李诗琪, 刘蝈蝈, 张雅静, 等. 糠醛转移加氢制糠醇催化剂的研究进展[J]. 当代化工, 2021, 50(7): 1724-1727. |
| LI Shiqi, LIU Guoguo, ZHANG Yajing, et al. Research progress of catalysts for furfural transfer hydrogenation to furfuryl alcohol[J]. Contemporary Chemical Industry, 2021, 50(7): 1724-1727. | |
| 11 | 陈佳宁, 王慧, 刘慰, 等. 木质纤维素的酸催化精炼研究进展[J]. 中国造纸, 2021, 40(8): 75-82. |
| CHEN Jianing, WANG Hui, LIU Wei, et al. Research progress on acid catalytic refining of lignocellulose[J]. China Pulp & Paper, 2021, 40(8): 75-82. | |
| 12 | 朱晨杰, 张会岩, 肖睿, 等. 木质纤维素高值化利用的研究进展[J]. 中国科学: 化学, 2015, 45(5): 454-478. |
| ZHU Chenjie, ZHANG Huiyan, XIAO Rui, et al. Research progress in catalytic valorization of lignocellulose[J]. Scientia Sinica Chimica, 2015, 45(5): 454-478. | |
| 13 | 熊健, 吕学斌, 任国权, 等. 生物质材料制备糠醛和5-羟甲基糠醛的研究进展[J]. 现代化工, 2022, 42(5): 30-34. |
| XIONG Jian, LV Xuebin, REN Guoquan, et al. Research progress in preparation of furfural and 5-hydroxymethylfurfural from biomass materials[J]. Modern Chemical Industry, 2022, 42(5): 30-34. | |
| 14 | 邓理, 廖兵, 郭庆祥. 纤维素选择性催化转化为重要平台化合物的研究进展[J]. 化工进展, 2013, 32(2): 245-254. |
| DENG Li, LIAO Bing, GUO Qingxiang. Recent progress in selective catalytic conversion of cellulose into key platform molecules[J]. Chemical Industry and Engineering Progress, 2013, 32(2): 245-254. | |
| 15 | 张军, 李丹妮, 袁浩然, 等. 生物质基糠醛和5-羟甲基糠醛加氢转化研究进展[J]. 燃料化学学报, 2021, 49(12): 1752-1767. |
| ZHANG Jun, LI Danni, YUAN Haoran, et al. Advances on the catalytic hydrogenation of biomass-derived furfural and 5-hydroxymethylfurfural[J]. Journal of Fuel Chemistry and Technology, 2021, 49(12): 1752-1767. | |
| 16 | LONG Jingxuan, XU Yufei, ZHAO Wenfeng, et al. Heterogeneous catalytic upgrading of biofuranic aldehydes to alcohols[J]. Frontiers in Chemistry, 2019, 7: 529. |
| 17 | HOANG Anh Tuan, VAN VIET PHAM. 2-Methylfuran (MF) as a potential biofuel: A thorough review on the production pathway from biomass, combustion progress, and application in engines[J]. Renewable and Sustainable Energy Reviews, 2021, 148: 111265. |
| 18 | 聂一凡, 候其东, 李维尊, 等. 糠醛的水解制备和应用研究进展[J]. 化工进展, 2019, 38(5): 2164-2178. |
| NIE Yifan, HOU Qidong, LI Weizun, et al. Advances in production furfural via hydrolysis and application of furfural[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2164-2178. | |
| 19 | 陈伦刚, 张兴华, 张琦, 等. 木质纤维素解聚平台分子催化合成航油技术的进展[J]. 化工进展, 2019, 38(3): 1269-1282. |
| CHEN Lungang, ZHANG Xinghua, ZHANG Qi, et al. Progress in aviation biofuel technology by catalysis synthesis of platform molecules from lignocelluloses depolymerization[J]. Chemical Industry and Engineering Progress, 2019, 38(3): 1269-1282. | |
| 20 | 闫瑞, 郭勇, 李杨, 等. 生物质衍生物催化转化制喷气燃料组分的研究进展[J]. 化工进展, 2016, 35(9): 2735-2745. |
| YAN Rui, GUO Yong, LI Yang, et al. Progress in catalytic production of jet fuel range alkanes from biomass-derivatives[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2735-2745. | |
| 21 | SHIVHARE Atal, KUMAR Abhinav, SRIVASTAVA Rajendra. An account of the catalytic transfer hydrogenation and hydrogenolysis of carbohydrate-derived renewable platform chemicals over non-precious heterogeneous metal catalysts[J]. ChemCatChem, 2021, 13(1): 59-80. |
| 22 | MARISCAL R, MAIRELES-TORRES P, OJEDA M, et al. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy & Environmental Science, 2016, 9(4): 1144-1189. |
| 23 | FANG Wenting, RIISAGER Anders. Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds[J]. Green Chemistry, 2021, 23(2): 670-688. |
| 24 | AN Zhidong, LI Jiang. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts[J]. Green Chemistry, 2022, 24(5): 1780-1808. |
| 25 | 王庄清, 张弨, 赵凤玉. 糠醛及其衍生物催化加氢制备1, 5-戊二醇研究进展[J]. 科学通报, 2019, 64(31): 3165-3172. |
| WANG Zhuangqing, ZHANG Chao, ZHAO Fengyu. Recent advances in the hydrogenolysis of furfural and its derivatives to 1, 5-pentanediol[J]. Chinese Science Bulletin, 2019, 64(31): 3165-3172. | |
| 26 | ZHANG Jun, CHEN Jinzhu. Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5982-5993. |
| 27 | LI Bolong, LI Lulu, SUN Hao, et al. Selective deoxygenation of aqueous furfural to 2-methylfuran over Cu0/Cu2O·SiO2 sites via a copper phyllosilicate precursor without extraneous gas[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12096-12103. |
| 28 | HANSEN Thomas S, BARTA Katalin, ANASTAS Paul T, et al. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol[J]. Green Chemistry, 2012, 14(9): 2457-2461. |
| 29 | NAGARAJA Bhari Mallanna, PADMASRI Aytam Hari, RAJU Burri David, et al. Production of hydrogen through the coupling of dehydrogenation and hydrogenation for the synthesis of cyclohexanone and furfuryl alcohol over different promoters supported on Cu-MgO catalysts[J]. International Journal of Hydrogen Energy, 2011, 36(5): 3417-3425. |
| 30 | 李聪明, 陈阔, 王晓月, 等. 探究Cu/ZnO相互作用对CO2加氢制甲醇反应性能的影响[J]. 物理化学学报, 2021, 37(5): 201-212. |
| LI Congming, CHEN Kuo, WANG Xiaoyue, et al. Understanding the role of Cu/ZnO interaction in CO2 hydrogenation to methanol[J]. Acta Physico-Chimica Sinica, 2021, 37(5): 201-212. | |
| 31 | SEBASTIAN P J, QUINTANA J, AVILA F. Retention of the high optical absorptance in thermally aged black chrome on variably sensitized Cu[J]. Solar Energy Materials and Solar Cells, 1997, 45(1): 65-74. |
| 32 | HU Yubing, ZHANG Yajing, DU Jie, et al. The influence of composition on the functionality of hybrid CuO-ZnO-Al2O3/HZSM-5 for the synthesis of DME from CO2 hydrogenation[J]. RSC Advances, 2018, 8(53): 30387-30395. |
| 33 | EL-SHOBAKY G A, AHMED A S, FAGAL G A, et al. Solid–solid interaction in CuO-ZnO/Al2O3 system under varying conditions[J]. Thermochimica Acta, 1998, 319(1/2): 67-74. |
| 34 | 姚志龙, 闵恩泽. ZnO对CuO-ZnO/Al2O3催化剂催化甘油氢解性能的影响[J]. 精细化工, 2011, 28(9): 866-869, 874. |
| YAO Zhilong, MIN Enze. Effect of ZnO on the hydrogenolysis performance of CuO-ZnO/Al2O3 in catalyzing glycerol[J]. Fine Chemicals, 2011, 28(9): 866-869, 874. | |
| 35 | 郭馨馨, 张希清. BiOCl/Zn-Al LDHs复合材料的制备及光催化性能研究[J]. 石家庄铁道大学学报(自然科学版), 2018, 31(4): 88-95. |
| GUO Xinxin, ZHANG Xiqing. Preparation and photocatalytic activity of BiOCl/Zn-Al layered double hydroxides composites[J]. Journal of Shijiazhuang Tiedao University (Natural Science Edition), 2018, 31(4): 88-95. | |
| 36 | POURMORTAZAVI Seied Mahdi, MARASHIANPOUR Zahra, KARIMI Meisam Sadeghpour, et al. Electrochemical synthesis and characterization of zinc carbonate and zinc oxide nanoparticles[J]. Journal of Molecular Structure, 2015, 1099: 232-238. |
| 37 | EL-SHOBAKY G A, AHMAD A S, AL-NOAIMI A N, et al. Thermal decomposition of basic cobalt and copper carbonates[J]. Journal of Thermal Analysis, 1996, 46(6): 1801-1808. |
| 38 | SONG Hyun-tae, FAZELI Ali, KIM Hyun Dong, et al. Effect of lanthanum group promoters on Cu/(mixture of ZnO and Zn-Al-spinnel-oxides) catalyst for methanol synthesis by hydrogenation of CO and CO2 mixtures[J]. Fuel, 2021, 283: 118987. |
| 39 | DING Wen, LIU Yingwei, WANG Fang, et al. Promoting effect of a Cu-Zn binary precursor on a ternary Cu-Zn-Al catalyst for methanol synthesis from synthesis gas[J]. RSC Advances, 2014, 4(58): 30677-30682. |
| 40 | WANG Jianqiang, WANG Youzhen, XIE Songhai, et al. Partial hydrogenation of benzene to cyclohexene on a Ru-Zn/m-ZrO2 nanocomposite catalyst[J]. Applied Catalysis A: General, 2004, 272(1/2): 29-36. |
| 41 | LIU Hailong, HUANG Zhiwei, KANG Haixiao, et al. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1, 2- and 1, 5-pentanediol over highly dispersed Cu-Al2O3 catalysts[J]. Chinese Journal of Catalysis, 2016, 37(5): 700-710. |
| 42 | MENG Hao, LIU Jiangning, DU Yali, et al. Novel Cu-based oxides catalyst from one-step carbothermal reduction decomposition method for selective catalytic reduction of NO with NH3 [J]. Catalysis Communications, 2019, 119: 101-105. |
| 43 | LU Ping, ZHOU Wei, LI Ying, et al. CuO nanosheets/ZnO nanorods synthesized by a template-free hydrothermal approach and their optical and magnetic characteristics[J]. Ceramics International, 2017, 43(13): 9798-9805. |
| 44 | WANG Yue, LIAO Junyu, ZHANG Jian, et al. Hydrogenation of methyl acetate to ethanol by Cu/ZnO catalyst encapsulated in SBA-15[J]. AIChE Journal, 2017, 63(7): 2839-2849. |
| 45 | WANG Wen, XU Linhua, ZHANG Ruofan, et al. Coexistence of ferromagnetism and paramagnetism in ZnO/CuO nanocomposites[J]. Chemical Physics Letters, 2019, 721: 57-61. |
| 46 | LI Yandong, LIANG Guangfen, WANG Chengrui, et al. Effect of precipitated precursor on the catalytic performance of mesoporous carbon supported CuO-ZnO catalysts[J]. Crystals, 2021, 11(6): 582. |
| 47 | LI Li, MAO Dongsen, YU Jun, et al. Highly selective hydrogenation of CO2 to methanol over CuO-ZnO-ZrO2 catalysts prepared by a surfactant-assisted co-precipitation method[J]. Journal of Power Sources, 2015, 279: 394-404. |
| 48 | MATSON Theodore D, Barta Katalin, IRETSKII Alexei V, et al. One-pot catalytic conversion of cellulose and of woody biomass solids to liquid fuels[J]. Journal of the American Chemical Society, 2011, 133(35): 14090-14097. |
| 49 | GILKEY Matthew J, PANAGIOTOPOULOU Paraskevi, MIRONENKO Alexander V, et al. Mechanistic insights into metal lewis acid-mediated catalytic transfer hydrogenation of furfural to 2-methylfuran[J]. ACS Catalysis, 2015, 5(7): 3988-3994. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [3] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [4] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [5] | 王子健, 柯明, 宋昭峥, 李佳涵, 童燕兵, 孙巾茹. 分子筛催化汽油烷基化降苯技术研究进展[J]. 化工进展, 2023, 42(5): 2371-2389. |
| [6] | 黄起中, 刘冰, 马红鹏, 吕文杰. 基于新型微通道分离技术的甲醇制烯烃废水处理[J]. 化工进展, 2023, 42(2): 669-676. |
| [7] | 郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231. |
| [8] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [9] | 李彬, 潘庆港, 姜爽, 张天永. 高收率氟吡菌酰胺中间体的绿色合成工艺[J]. 化工进展, 2022, 41(S1): 469-476. |
| [10] | 刘鹏龙, 许雄飞, 张玮, 许鑫, 张侃, 王俊文. 甲醇制芳烃K-means-PSO-SVR局部建模及优化[J]. 化工进展, 2022, 41(9): 4691-4700. |
| [11] | 向晟, 王超, 庄钰, 顾偲雯, 张磊, 都健. 变压精馏分离乙酸甲酯-甲醇-乙酸乙酯体系的设计与控制[J]. 化工进展, 2022, 41(8): 4065-4076. |
| [12] | 张嘉琪, 林丽娜, 高文桂, 祝星. CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响[J]. 化工进展, 2022, 41(8): 4213-4223. |
| [13] | 庄雨婷, 王建华, 向智艳, 赵娟, 徐琼, 刘贤响, 尹笃林. 半纤维素及其衍生物转化为γ-戊内酯及其动力学研究进展[J]. 化工进展, 2022, 41(7): 3519-3533. |
| [14] | 李贵贤, 张军强, 杨勇, 范学英, 王东亮. 基于PX选择性强化的短流程甲苯甲醇甲基化PX生产新工艺[J]. 化工进展, 2022, 41(6): 2939-2947. |
| [15] | 王集杰, 韩哲, 陈思宇, 汤驰洲, 沙峰, 唐珊, 姚婷婷, 李灿. 太阳燃料甲醇合成[J]. 化工进展, 2022, 41(3): 1309-1317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||