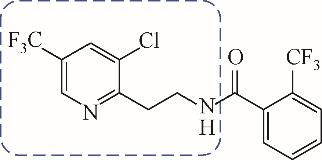
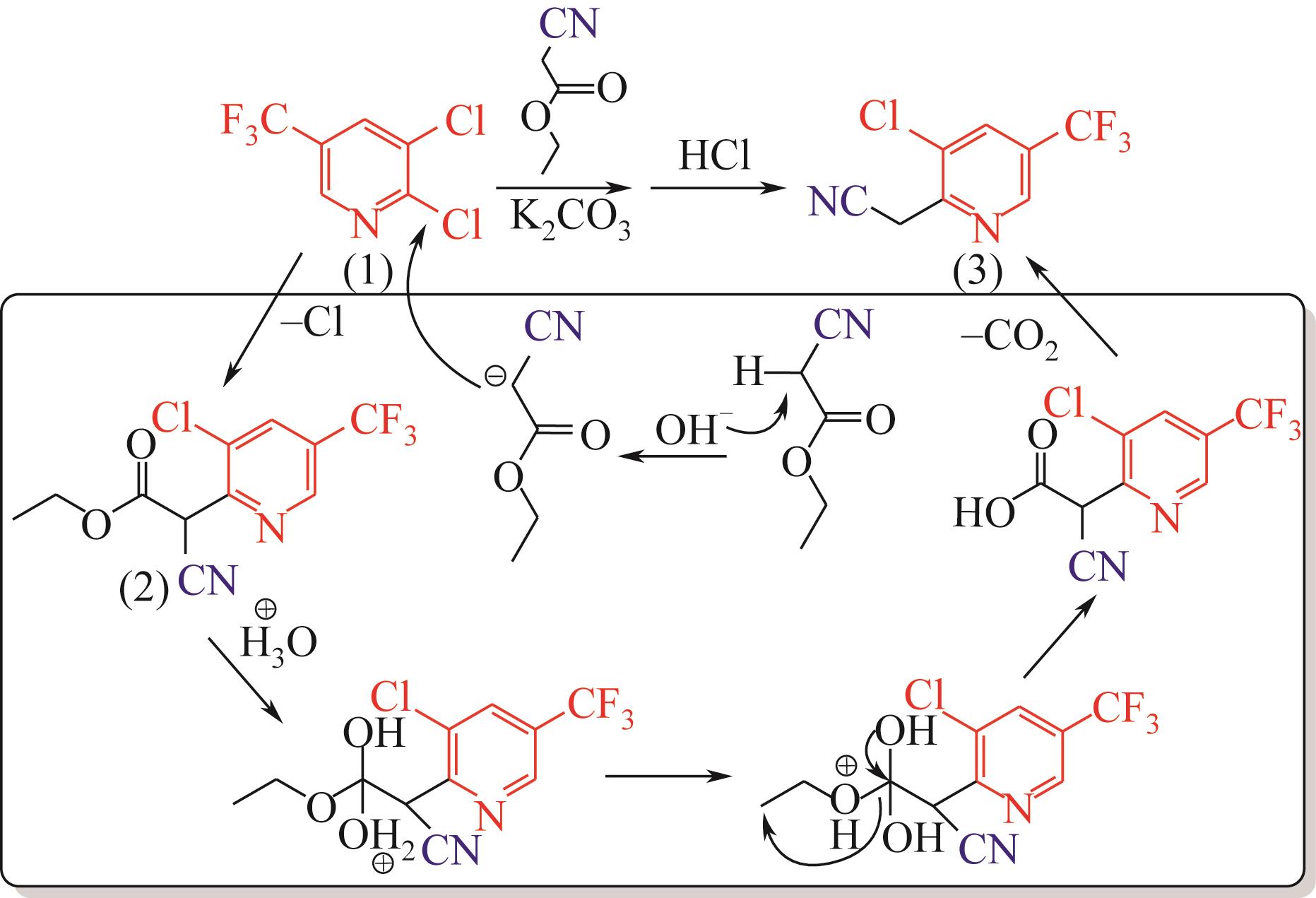
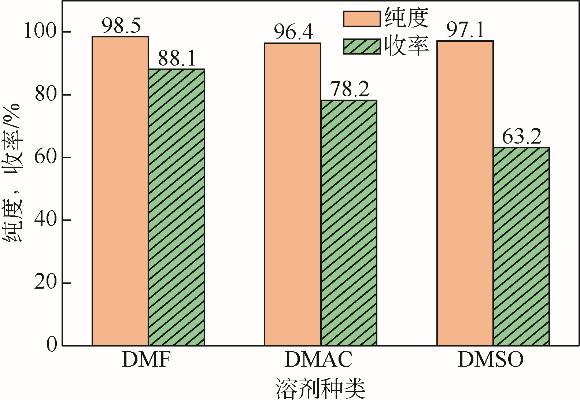
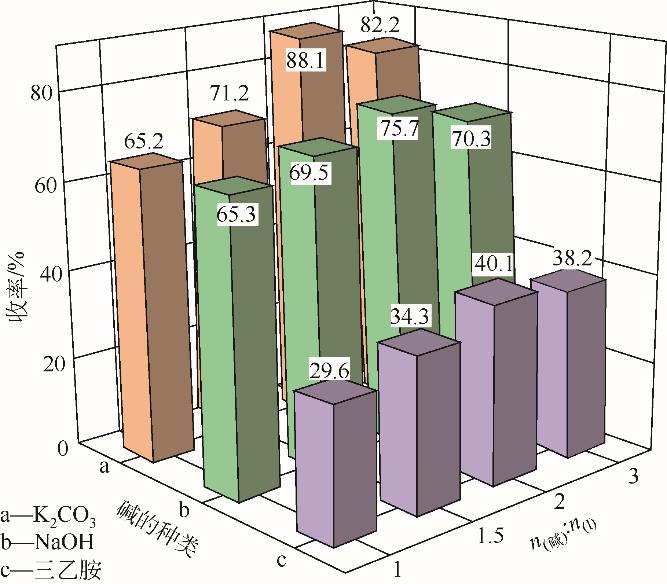
| 1 |
FOUGHT L, MUSSON H G, BLOOMBERG R J,et al. Fluopyram—A new active ingredient from bayer cropScience[J]. Phytopathology, 2009, 99: 36-37.
|
| 2 |
张晓柯, 韩絮, 马薇薇, 等. 江苏省草莓灰霉病菌对氟吡菌酰胺敏感性基线的建立及抗性风险评估[J]. 南京农业大学学报, 2015, 38(5): 810-815.
|
|
ZHANG Xiaoke, HAN Xu, MA Weiwei, et al. Baseline sensitivity of fluopyram and its resistance risk assessment against Botrytis cinerea from strawberry in Jiangsu Province[J]. Journal of Nanjing Agricultural University, 2015, 38(5): 810-815.
|
| 3 |
D·J·曼斯菲尔德, T·库克, P·S·托马斯, 等. 新型2-吡啶基乙基苯甲酰胺衍生物: CN1674784[P]. 2003-08-08.
|
|
MANSFIELD Darren James, COOKE Tracey, THOMAS Peter Stanley, et al. Novel 2-pyridylethylbenzamide derivatives: CN1674784[P]. 2003-08-08.
|
| 4 |
PENG W, LIU Y, LI W, et al. Metabolic and dynamic profiling for risk assessment of fluopyram, a typical phenylamide fungicide widely applied in vegetable ecosystem[J]. Scientific Reports, 2016, 6: 33898.
|
| 5 |
SIEROTZKI H, SCALLIET G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides[J]. Phytopathology, 2013, 103(9): 880-887.
|
| 6 |
AVENOT H F, THOMAS A, GITAITIS R D, et al. Molecular characterization of boscalid- and penthiopyrad-resistant isolates of Didymella bryoniae and assessment of their sensitivity to fluopyram[J]. Pest Management Science, 2012, 68(4): 645-651.
|
| 7 |
周大纲. 新一代优秀杀线虫剂氟吡菌酰胺[J]. 世界农药, 2016, 38(5): 61-63.
|
|
ZHOU Dagang. A new generation of excellent nematicides fluopyram[J]. World Pesticides, 2016, 38(5):61-63.
|
| 8 |
徐英, 徐娜娜, 庄占兴, 等. 氟吡菌酰胺研究开发综述[J]. 世界农药, 2017, 39(6): 37-41.
|
|
XU Ying, XU Nana, ZHUANG Zhanxing, et al. A review of research progress in the development of fluopyram[J]. World Pesticides, 2017, 39(6):37-41.
|
| 9 |
XIA J, XIA Y, NNANNA I A. Structure-function relationship of acyl amino acid surfactants: surface activity and antimicrobial properties[J]. Journal of Agricultural & Food Chemistry, 1995, 43(4): 867-871.
|
| 10 |
刘显. 氧化胺化反应合成2-氨基-6-甲氧基-3-硝基吡啶[J]. 广东化工, 2009, 36(10): 59-60.
|
|
LIU Xian. Synthesis of 2-amino-6-methoxy-3-nitropyridine by oxidative amination[J]. Guangdong Chemical Industry, 2009, 36(10): 59-60.
|
| 11 |
MA Y, STIVALA C E, WRIGHT A M, et al. Enediolate-dilithium amide mixed aggregates in the enantioselective alkylation of arylacetic acids: structural studies and a stereochemical model[J]. Journal of the American Chemical Society, 2013, 135(45): 16853-16864.
|
| 12 |
GILLES B, MICHEL G, EMILIE C, et al. Synthesis of 15N-labeled vicinal diamines through N-activated chiral aziri-dines: tools for the NMR study of platinum-based anti-cancer compounds[J]. Tetrahedron Letters, 2013, 54(6): 545-548.
|
| 13 |
FREDERIC L, PIERRE Y C, PHILIPPE D, et al. Process for the preparation of 2-pyridylethylcarboxamide derivatives: WO2006067103A3[P]. 2006-06-29.
|
| 14 |
COQUERON P Y. Process for the preparation of a 2-ethyl-aminopyridine derivative: EP1674455[P]. 2006-06-18.
|
| 15 |
刘安昌, 冯佳丽, 贺晓璐, 等. 新型杀菌剂氟吡菌酰胺的合成[J]. 农药, 2015, 54(7): 485-486.
|
|
LIU Anchang, FENG Jiali, HE Xiaolu, et al. Synthetic process of novel fungicides fluopyram[J]. Agrochemicals, 2015, 54(7): 485-486.
|
| 16 |
丁成荣, 谢展, 张国富. 3-氯-5-(三氟甲基)-2-乙胺基吡啶的合成[J]. 合成化学, 2015, 23(7): 633-636.
|
|
DING Chengrong, XIE Zhan, ZHANG Guofu. Synthesis of 3-chloro-5-(trifluoromethyl) -2-ethylamino-pyridine[J]. Chinese Journal of Synthetic Chemistry, 2015, 23(7): 633-636.
|
| 17 |
MORADI W A, HIMMLER T, MULLER T N. Catalytic hydrogenation of nitriles: WO2018114810[P]. 2018-06-02.
|
| 18 |
MORADI W A, HIMMLER T, MULLER T N, et al. Process for preparation of 2-aminoethylpyridine derivatives via catalytic hydrogenation of nitriles: WO2015071230[P]. 2015-05-21.
|
| 19 |
孙天孜, 何立, 杜友兴. 3-氯-5-(三氟甲基)-2-乙胺基吡啶的合成[J]. 有机氟工业, 2019 (1): 4-8.
|
|
SUN Tianzi, HE Li, DU Youxing. Synthesis of 3-chloro-5-(trifluoromethyl)-2-ethylamino-pyridine[J]. Organo-Fluorine Industry, 2019 (1): 4-8.
|
| 20 |
陈正伟, 征玉荣, 何彬, 等. 一种 2- (3-氯-5-(三氟甲基)吡啶-2-基)乙胺的合成方法: CN112552231[P]. 2021-03-26.
|
|
CHEN Zhengwei, ZHENG Yurong, HE Bin, et al. A kind of synthetic method of 2-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)ethanamine: CN112552231[P]. 2021-03-26.
|
 ), 潘庆港1, 姜爽1,2(
), 潘庆港1, 姜爽1,2( ), 张天永1,2,3(
), 张天永1,2,3( )
)
 ), PAN Qinggang1, JIANG Shuang1,2(
), PAN Qinggang1, JIANG Shuang1,2( ), ZHANG Tianyong1,2,3(
), ZHANG Tianyong1,2,3( )
)