化工进展 ›› 2020, Vol. 39 ›› Issue (3): 1129-1136.DOI: 10.16085/j.issn.1000-6613.2019-1075
高收率美罗培南侧链中间体的合成
李彬1,2,3( ),史继星1,姜爽1,2(
),史继星1,姜爽1,2( ),张天永1,2,3(
),张天永1,2,3( ),李小康1,周明浩1,刘艺炜1
),李小康1,周明浩1,刘艺炜1
- 1.天津大学化工学院,天津市应用催化科学与工程重点实验室,天津 300354
2.天津化学化工协同创新中心,天津 300072
3.天津市功能精细化学品技术工程中心,天津 300354
Synthesis of meropenem side chain intermediates with high yield
Bin LI1,2,3( ),Jixing SHI1,Shuang JIANG1,2(
),Jixing SHI1,Shuang JIANG1,2( ),Tianyong ZHANG1,2,3(
),Tianyong ZHANG1,2,3( ),Xiaokang LI1,Minghao ZHOU1,Yiwei LIU1
),Xiaokang LI1,Minghao ZHOU1,Yiwei LIU1
- 1.Tianjin Key Laboratory of Applied Catalysis Science and Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300354, China
2.Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
3.Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300354, China
摘要:
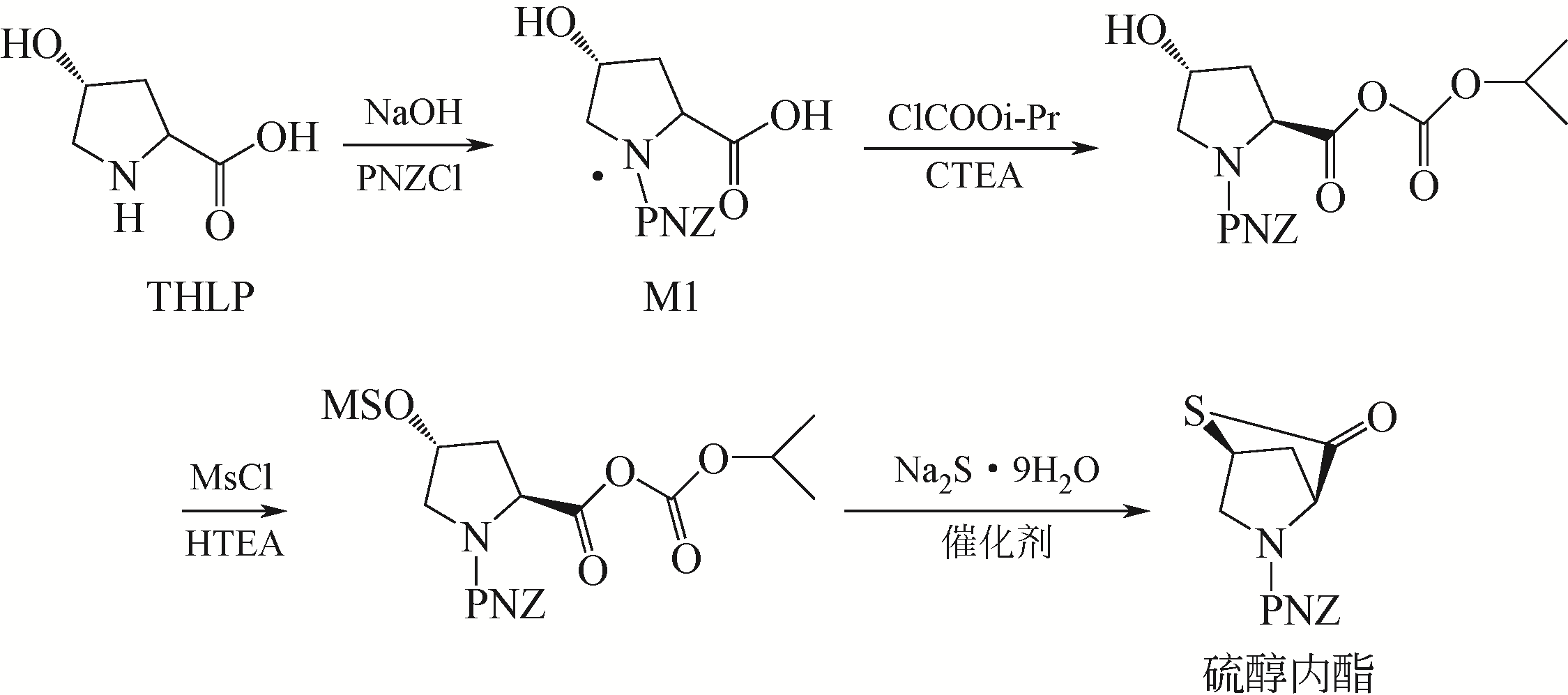
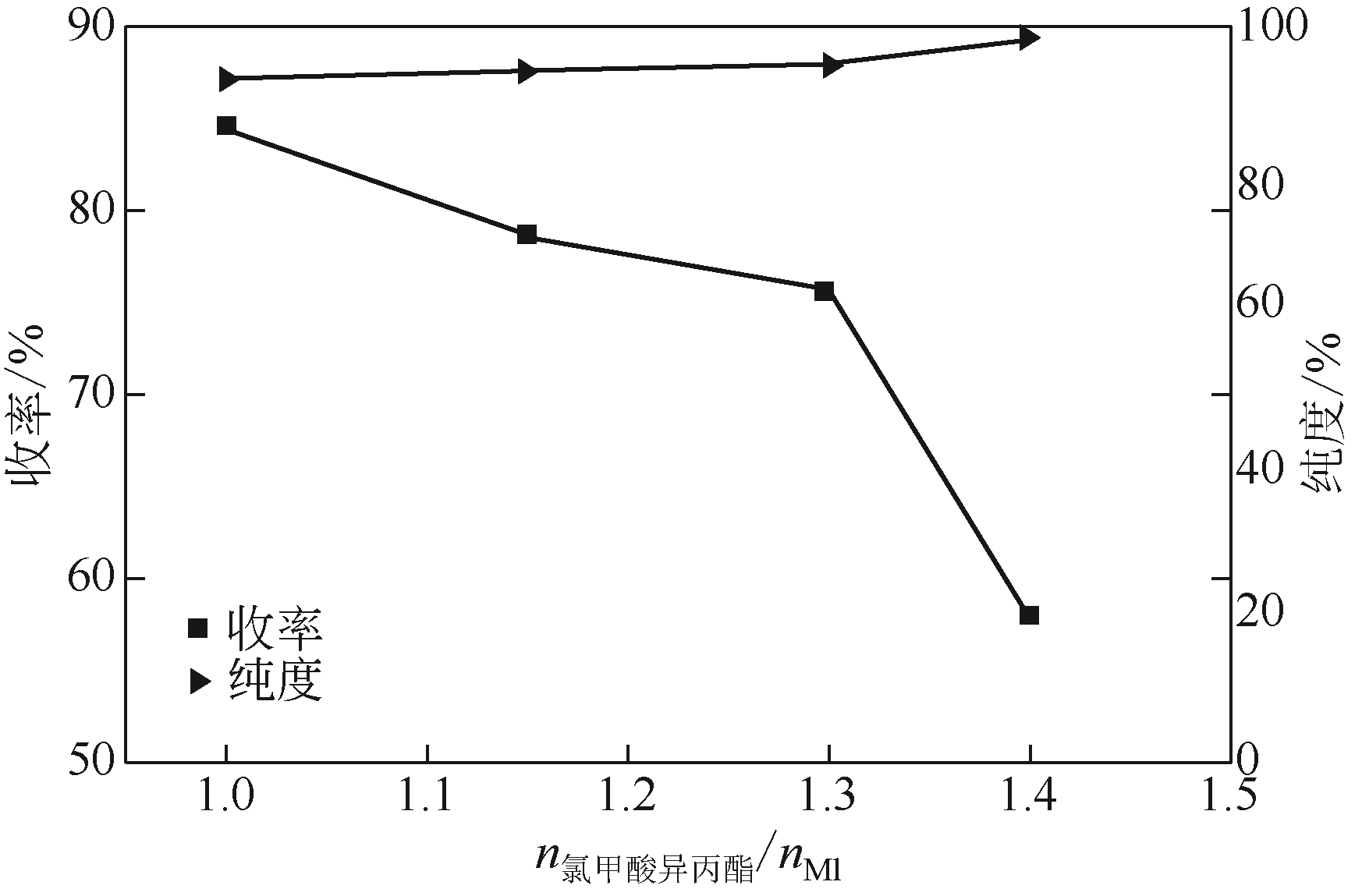
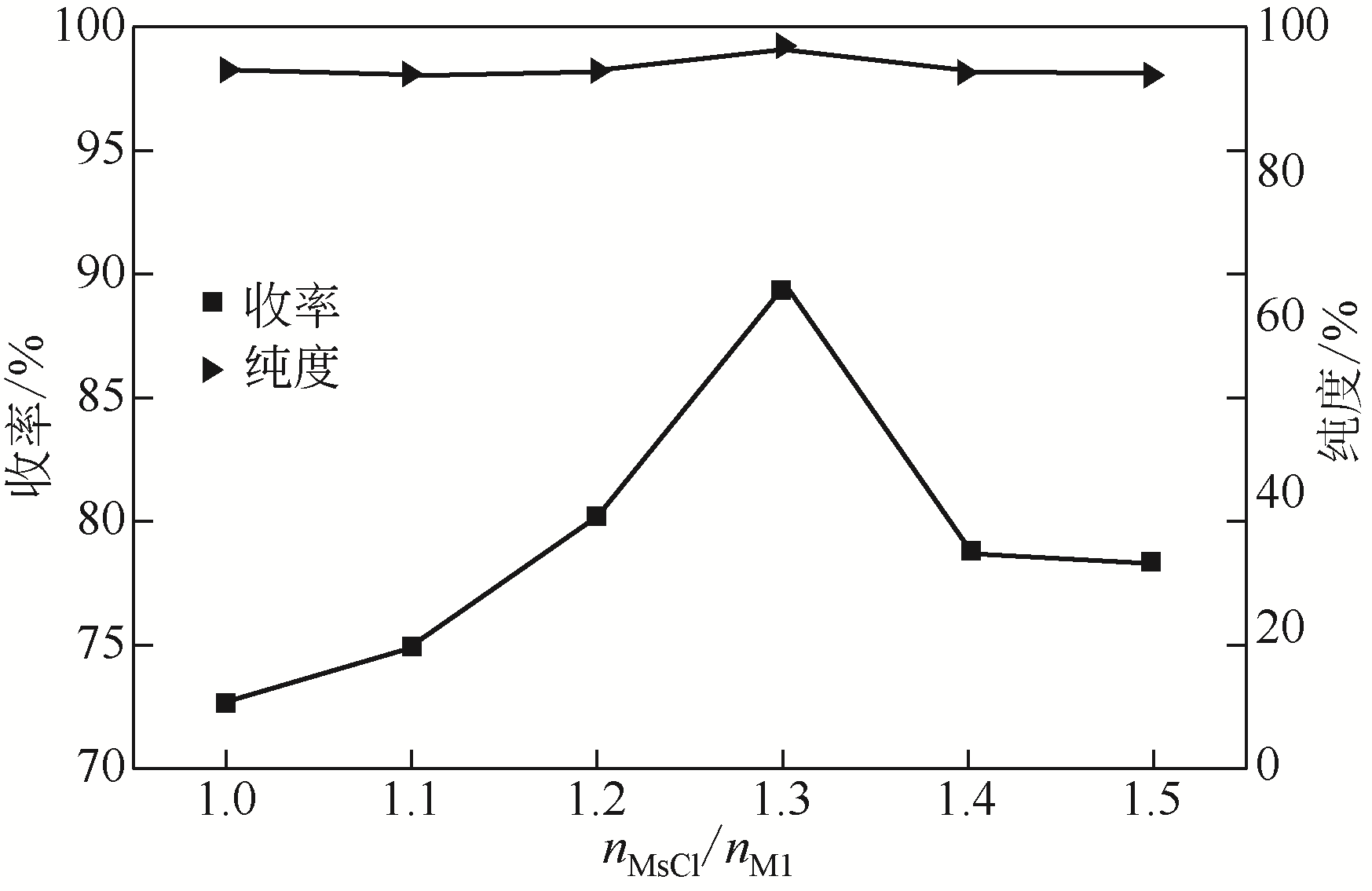
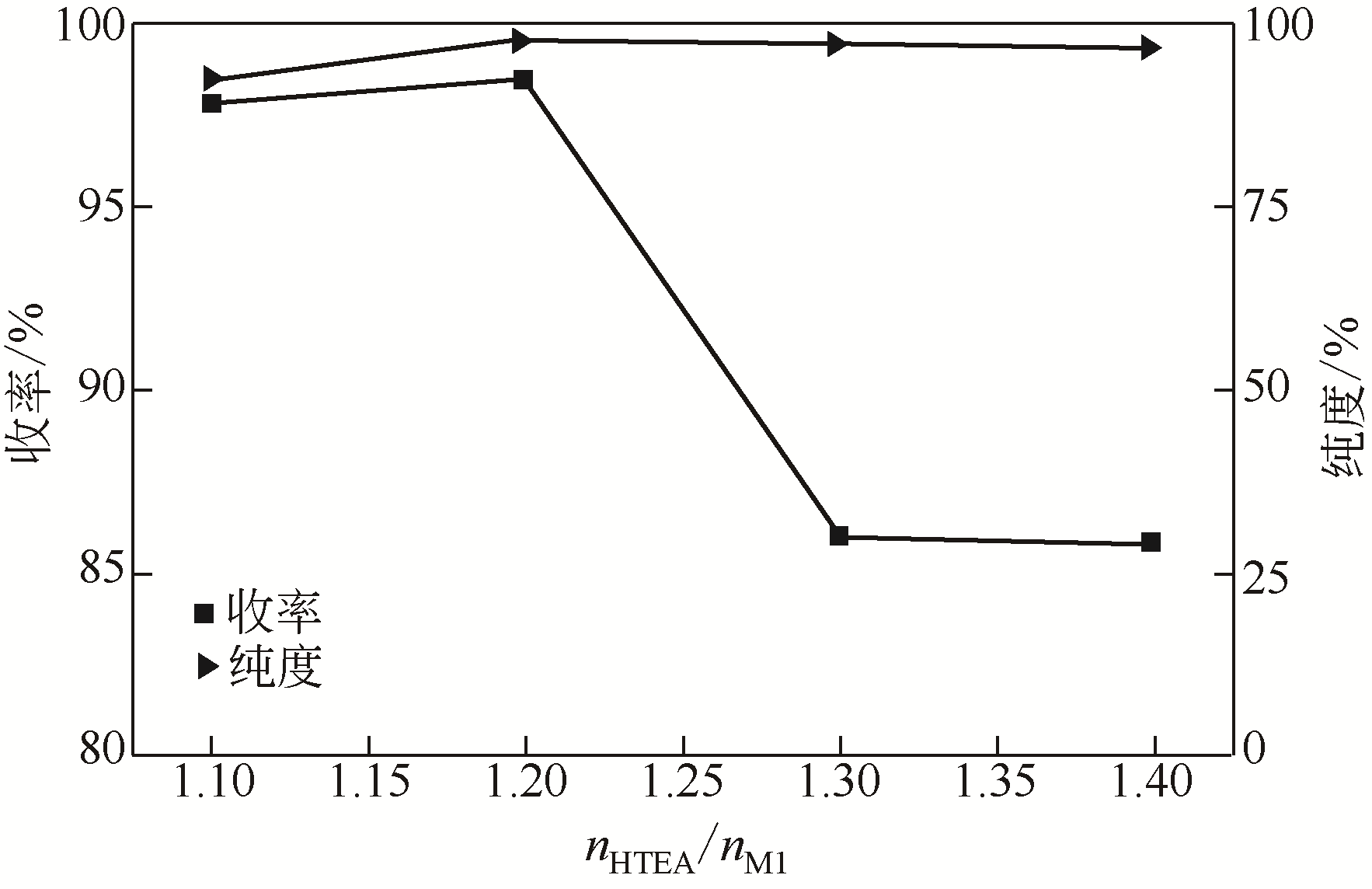
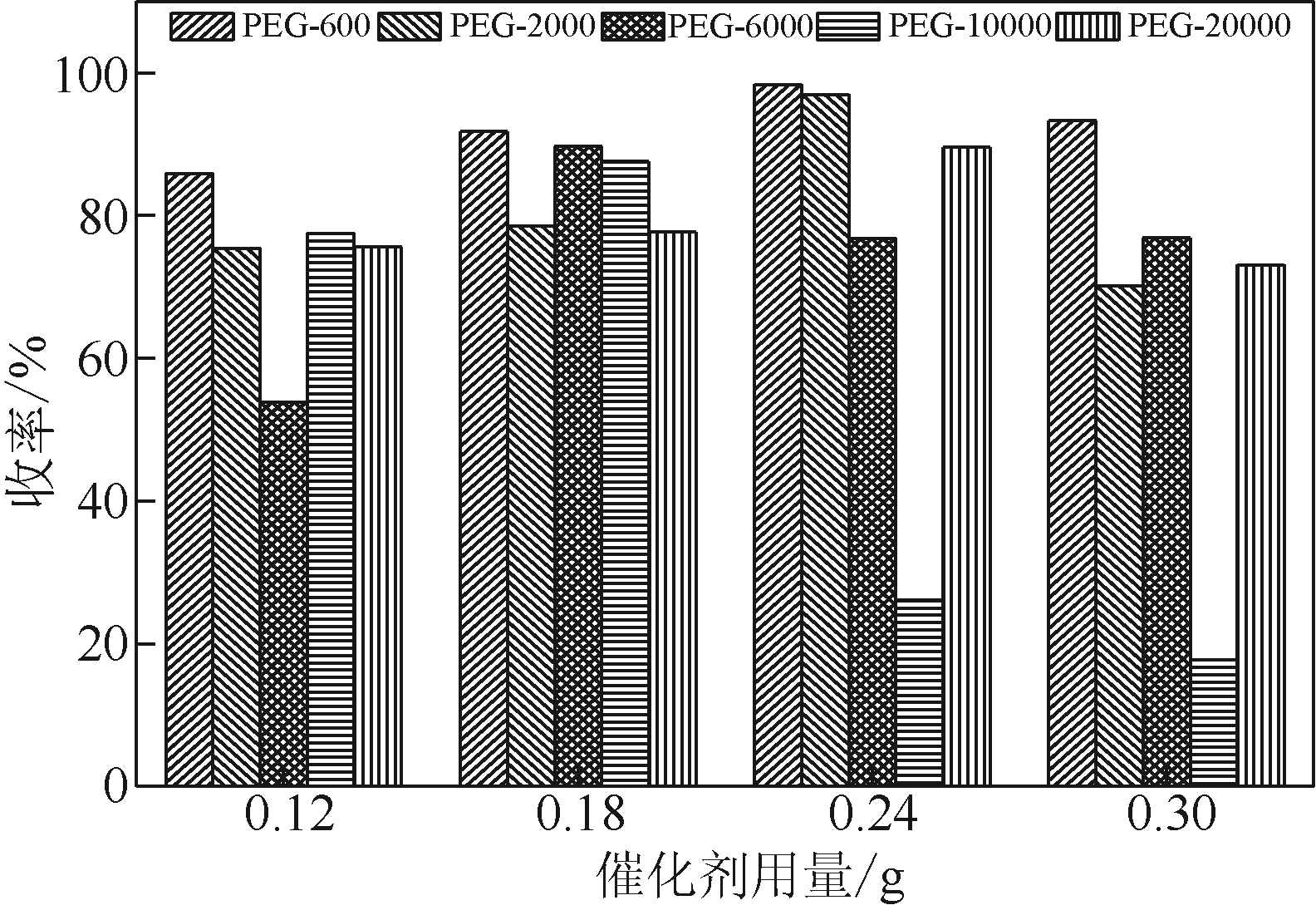
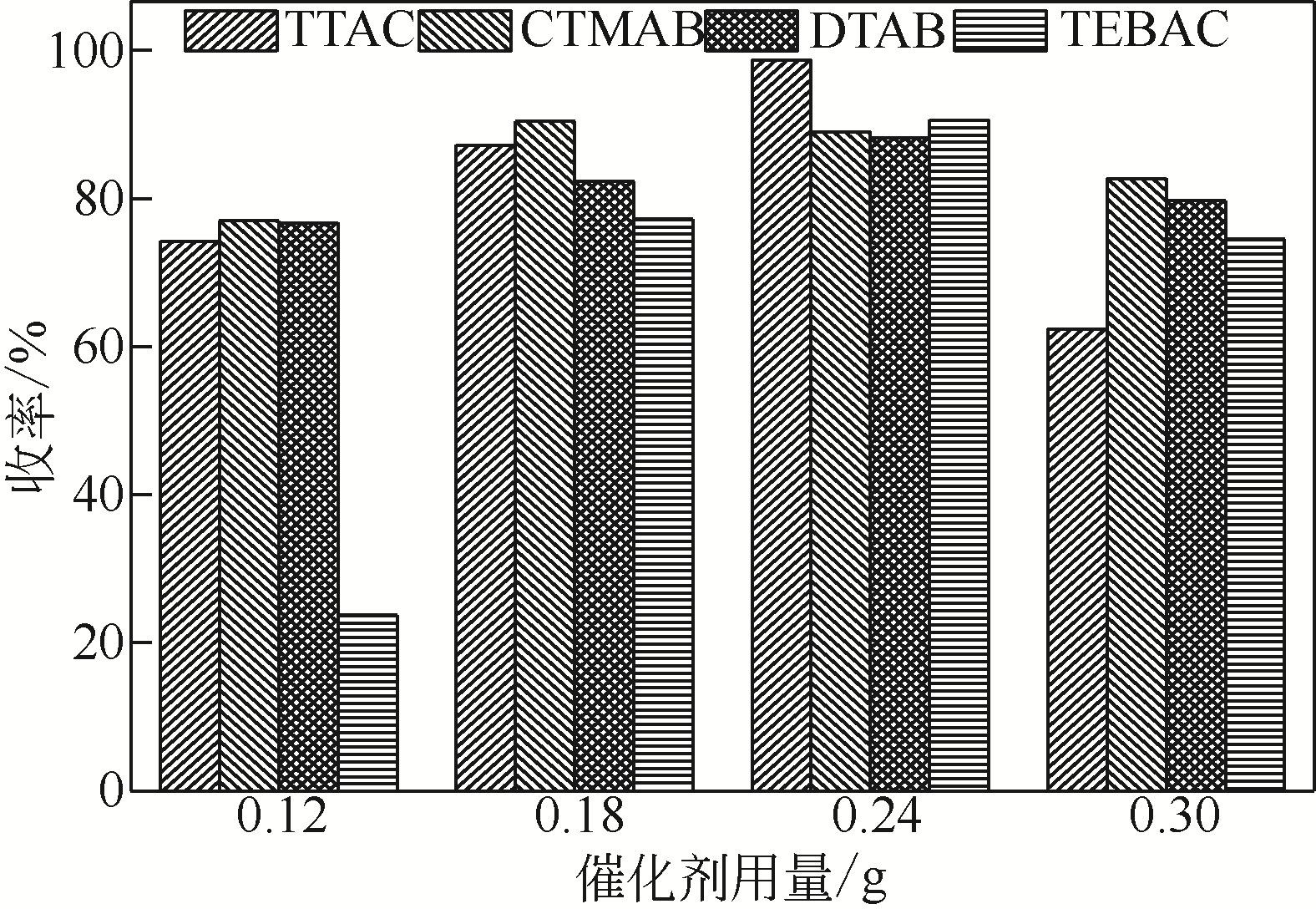
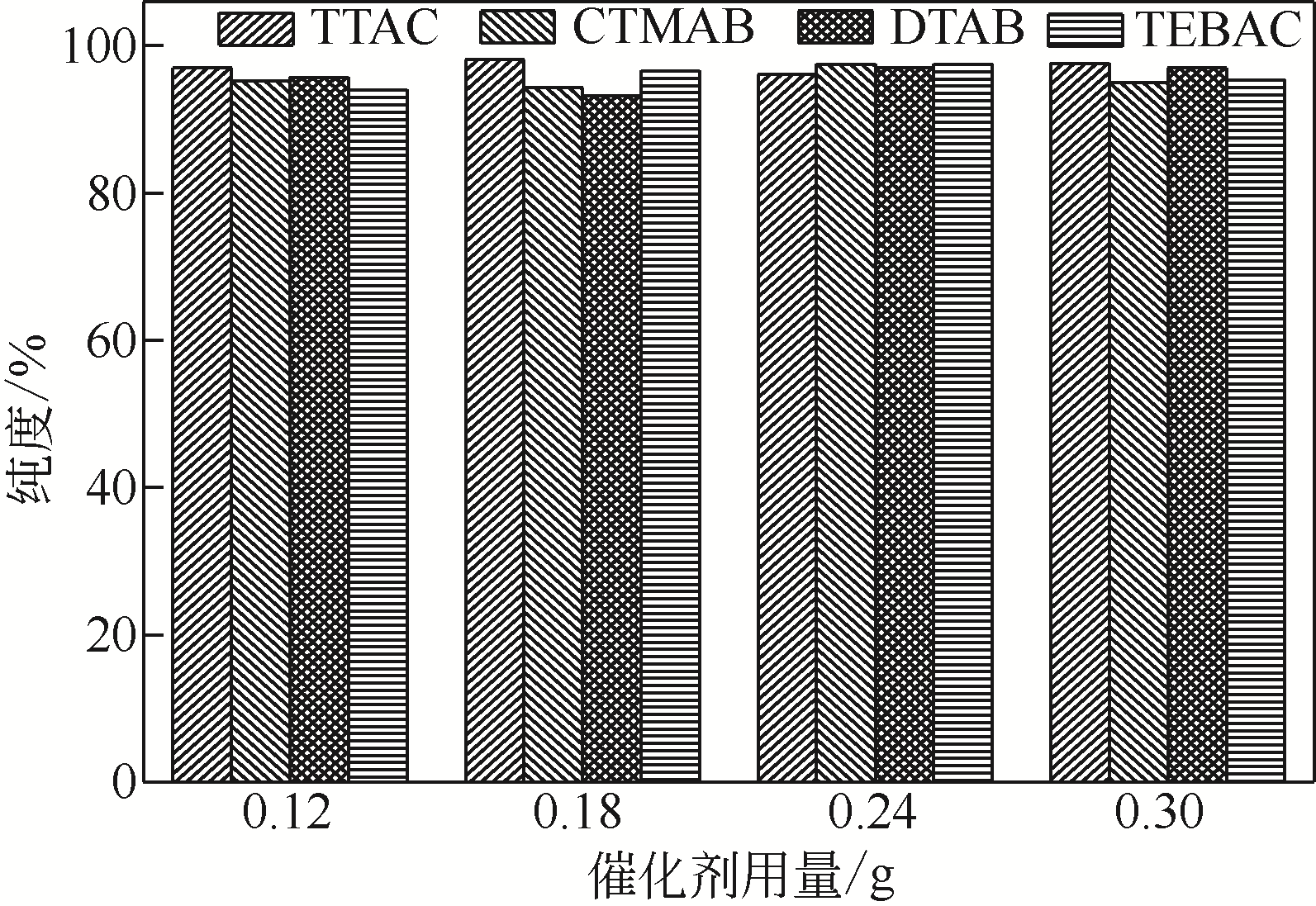
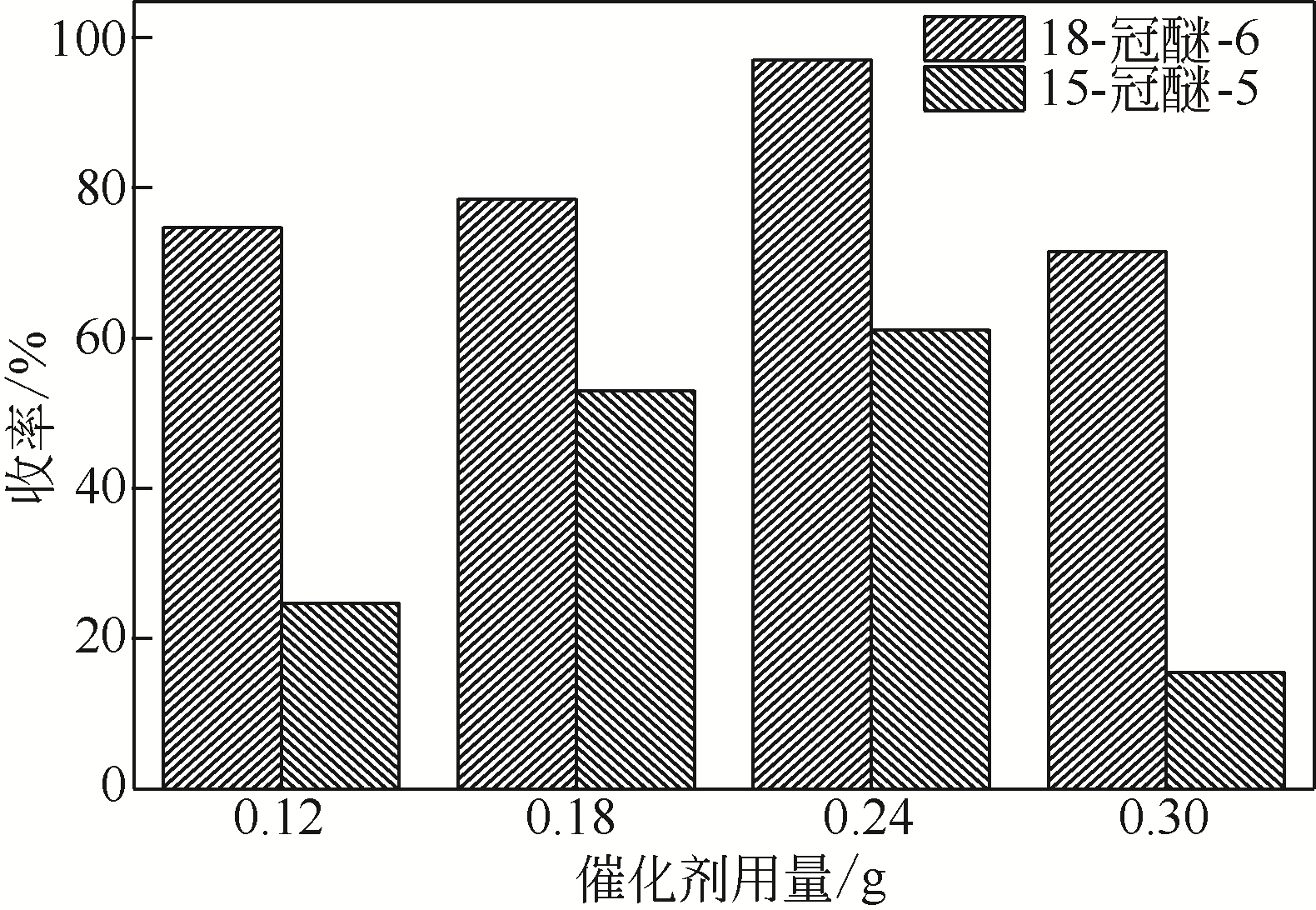
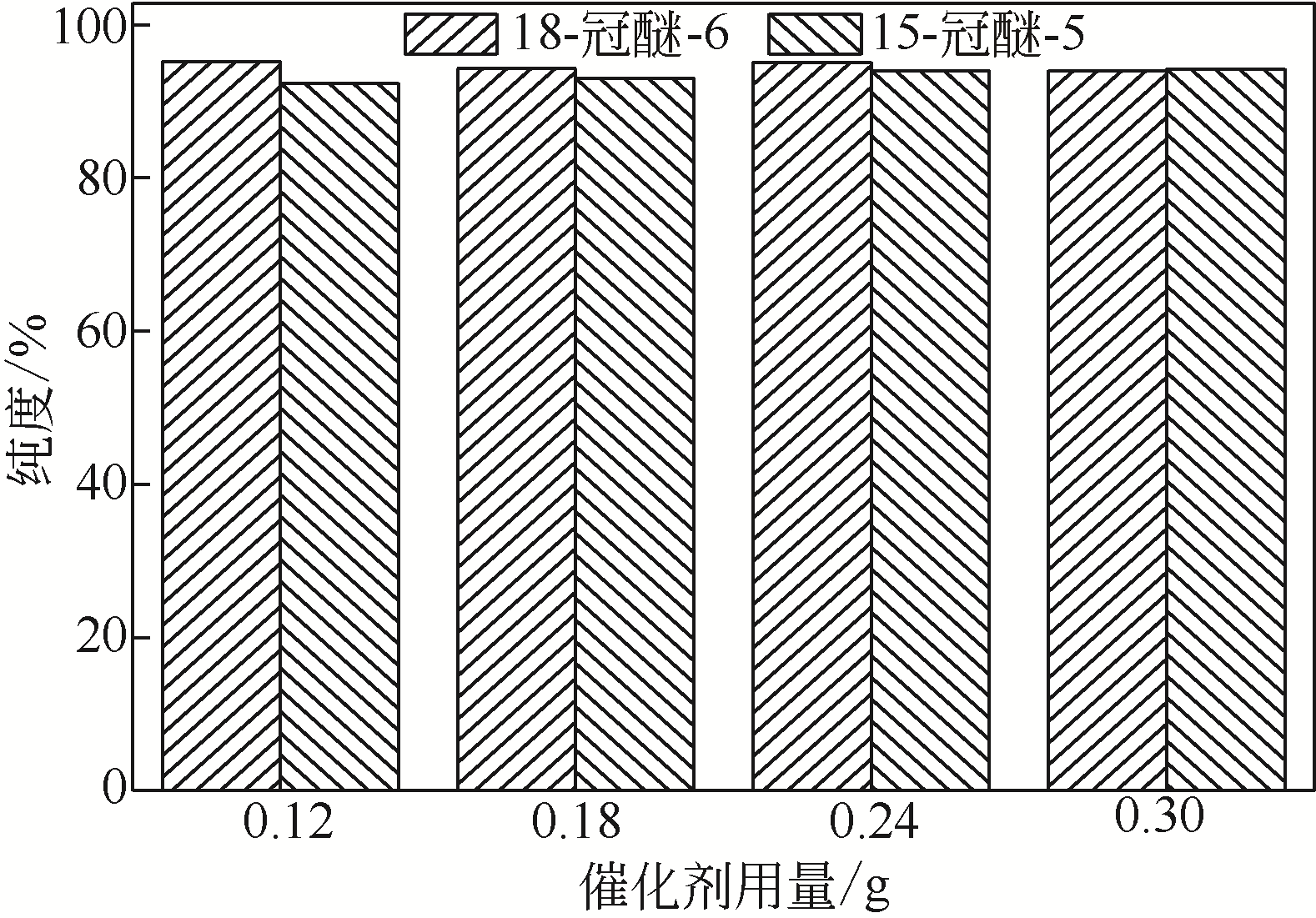
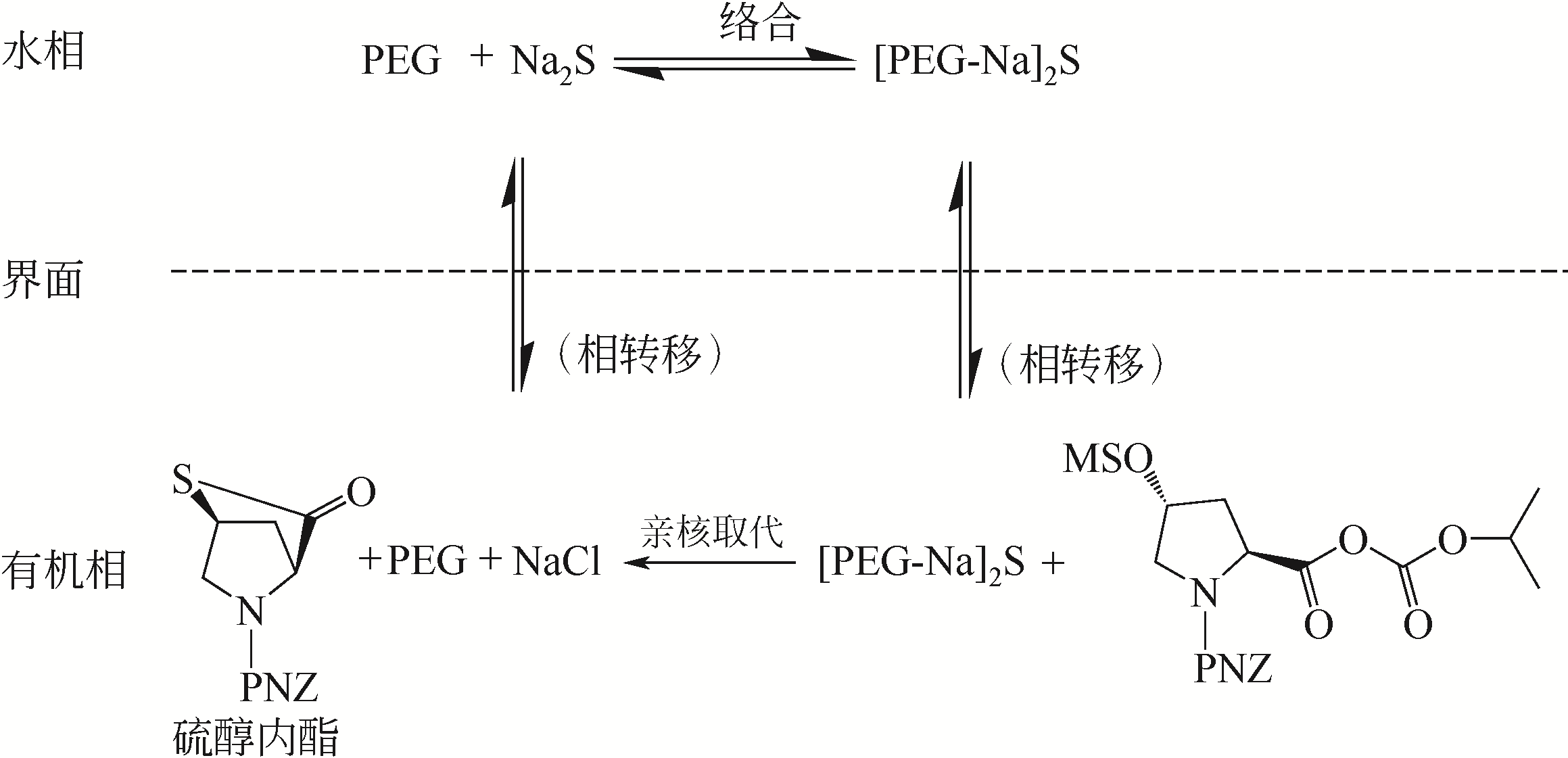
针对现有美罗培南侧链中间体(硫醇内酯)制备工艺成本较高、反应路线复杂、副反应多、收率及粗品纯度较低等缺点,研究了一种简便制备硫醇内酯的方法,通过将M1[(2S,4R)-2-羧基-1-(4-硝基苄氧羰基)吡咯烷)]羧基活化、羟基活化和硫化成环合为一锅法以及添加相转移催化剂法制备硫醇内酯,再进行开环反应得到美罗培南侧链。研究了氯甲酸异丙酯、甲基磺酰氯(MsCl)、三乙胺(TEA)、Na2S·9H2O和三类相转移催化剂[聚乙二醇类(PEG)、季铵盐类和冠醚类]的投料摩尔比对制备硫醇内酯收率和纯度的影响。制备硫醇内酯时加入相转移催化剂既可以加快反应的速率,又可以提高产品纯度及收率。nM1∶n氯甲酸异丙酯∶nMsCl∶
中图分类号: