化工进展 ›› 2021, Vol. 40 ›› Issue (10): 5523-5534.DOI: 10.16085/j.issn.1000-6613.2021-0763
电催化析氢催化剂研究进展
- 中国石油大学(北京)新能源与材料学院,重质油国家重点实验室,北京 102249
-
收稿日期:2021-04-13修回日期:2021-06-15出版日期:2021-10-10发布日期:2021-10-25 -
通讯作者:黄国勇 -
作者简介:王春霞(1984—),女,副教授,硕士生导师,研究方向为纳米材料电催化。E-mail:cxwang@cup.edu.cn 。 -
基金资助:国家自然科学基金青年基金(21804319);国家自然科学基金(52022109);北京市自然科学基金(2202047);中国石油大学(北京)科研基金(2462018YJRC041)
Progress of electrocatalytic hydrogen evolution reaction catalysts
WANG Chunxia( ), SONG Zhaoyi, NI Jiping, PAN Zongwei, HUANG Guoyong(
), SONG Zhaoyi, NI Jiping, PAN Zongwei, HUANG Guoyong( )
)
- College of New Energy and Materials, State Key Laboratory of Heavy Oil Processing, China University of Petroleum-Beijing, Beijing 102249, China
-
Received:2021-04-13Revised:2021-06-15Online:2021-10-10Published:2021-10-25 -
Contact:HUANG Guoyong
摘要:
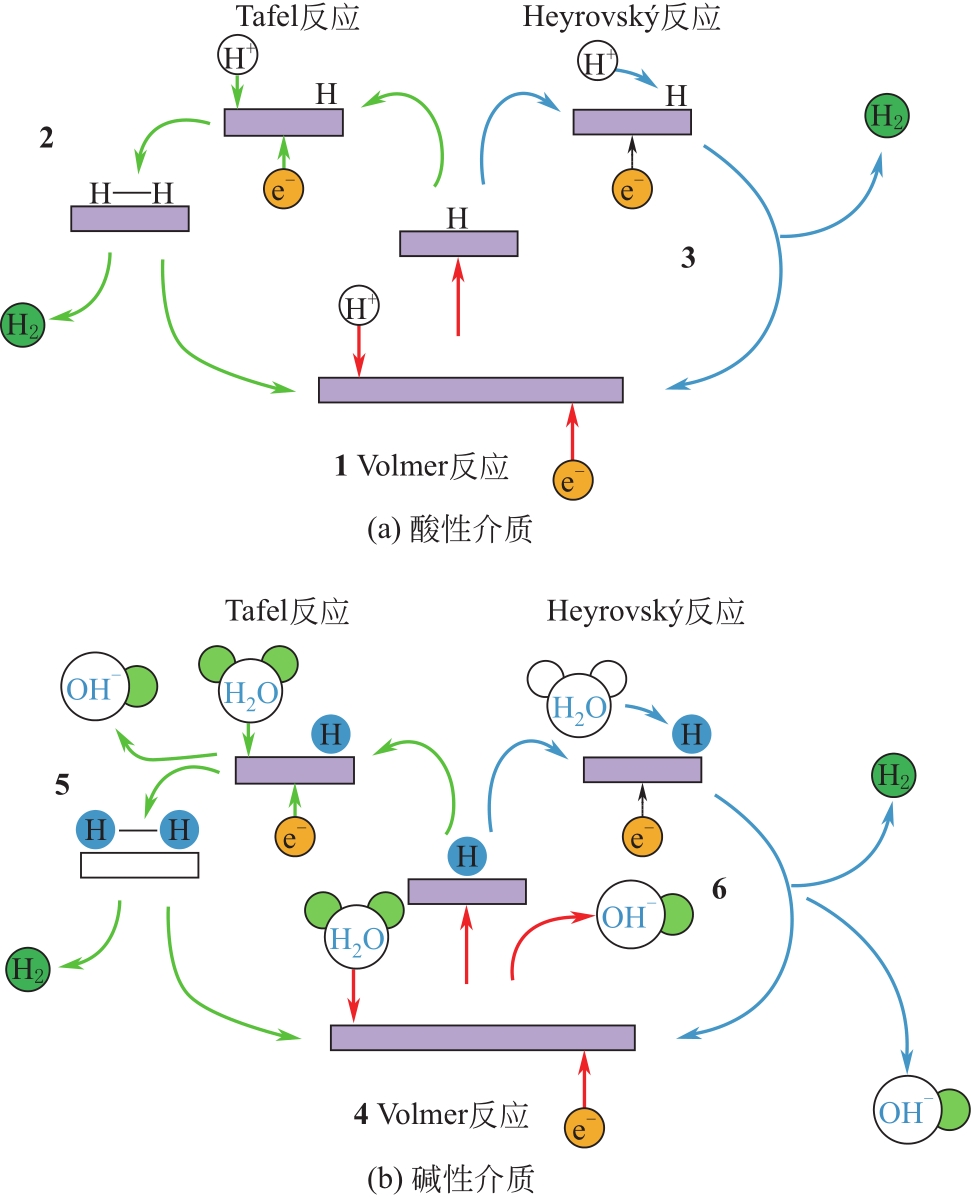
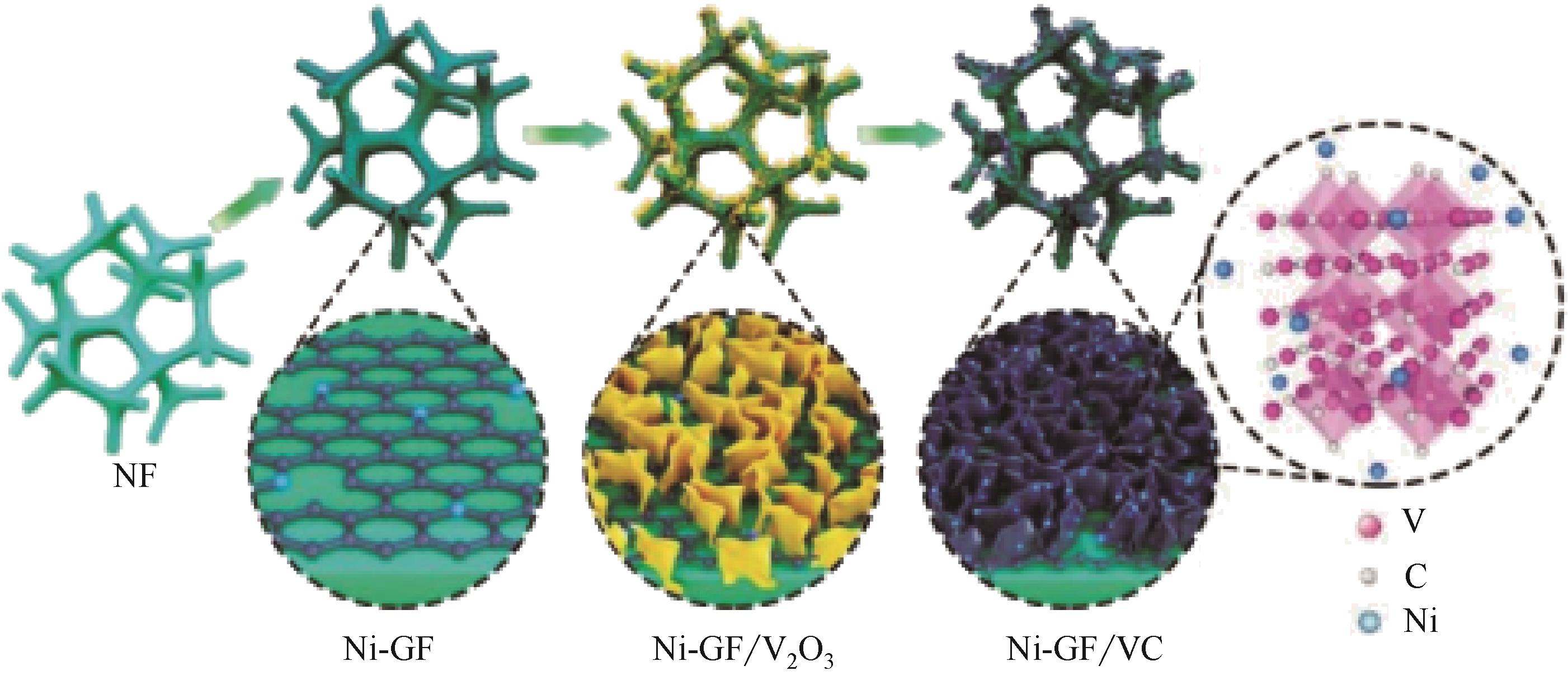
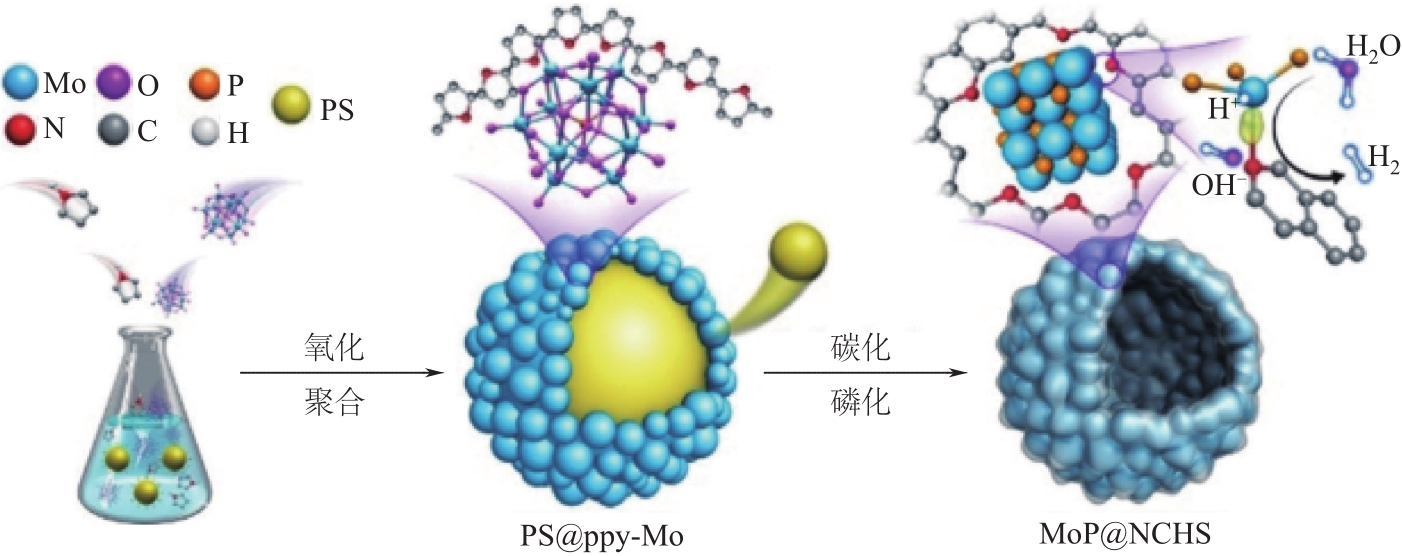
氢能热值高和环境友好性强等特点使其成为未来能源界最具发展潜力的能源之一。电催化析氢反应(hydrogen evolution reaction,HER)作为一种绿色、可持续的产氢方法成为近年来广泛研究的主题。发展高性能、低成本、高活性的析氢催化剂是目前该领域面临的主要挑战。本文总结了近年来高性能催化剂用于HER反应的进展,重点介绍HER反应的基本原理,评估HER催化剂催化性能的典型方法,过渡金属以及化合物、非金属催化剂以及单原子催化剂等电催化析氢催化剂的最新研究进展,系统讨论了催化活性与催化剂形态、结构、组成和合成方法之间的联系,并对催化剂的合成策略、活性位点的固有活性、如何提高活性中心的内在活性和活性位点的数量进行了展望。
中图分类号:
引用本文
王春霞, 宋兆毅, 倪基平, 潘宗卫, 黄国勇. 电催化析氢催化剂研究进展[J]. 化工进展, 2021, 40(10): 5523-5534.
WANG Chunxia, SONG Zhaoyi, NI Jiping, PAN Zongwei, HUANG Guoyong. Progress of electrocatalytic hydrogen evolution reaction catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5523-5534.
| 1 | 李莎莎. 钴基电催化剂的结构设计、合成及其电催化性能[D]. 太原: 太原理工大学, 2019. |
| LI S S. Design, synthesis and electrocatalytic properties of cobalt based electrocatalysts[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 2 | YU P, WANG F M, SHIFA T A, et al. Earth abundant materials beyond transition metal dichalcogenides: a focus on electrocatalyzing hydrogen evolution reaction[J]. Nano Energy, 2019, 58: 244-276. |
| 3 | LAURSEN A B, KEGNAES S, DAHL S, et al. Molybdenum sulfides-efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution[J]. Energy & Environmental Science, 2012, 5(2): 5577-5591. |
| 4 | 吴新勇. 镍基电催化剂的制备及析氢性能研究[D]. 深圳: 深圳大学, 2017. |
| WU X Y. Preparation of nickel-based electrocatalyst and study on its hydrogen evolution performance[D]. Shenzhen: Shenzhen University, 2017. | |
| 5 | PARSONS R. The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen[J]. Transactions of the Faraday Society, 1958, 54:1603-1611. |
| 6 | JARAMILLO T F, JORGENSEN K P, Bonde J, et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts[J]. Science, 2007, 317 (5834): 100-102. |
| 7 | FRYXELL R E, NACHTRIEB N H. Effect of stress on metal electrode potentials[J]. Journal of the Electrochemical Society, 1952, 99(12): 495-503. |
| 8 | FLETCHER S. Tafel slopes from first principles[J]. Journal of Solid State Electrochemistry, 2009, 13(4): 537-549. |
| 9 | WANG E. Electrochemical scanning tunneling microscopy[J]. Analyticalences, 1994, 10(1): 155-156. |
| 10 | 景锋. 镍基硫属自支撑电极的制备及其电解水析氢性能的研究[D]. 武汉: 华中科技大学, 2019. |
| JING F. Research on hydrogen evolution performances of nickel-based chalcogenides self-supported electrodes[D]. Wuhan: Huazhong University of Science and Technology, 2019. | |
| 11 | DE ROOIJ M R. Electrochemical methods: fundamentals and applications[J]. Anti-Corrosion Methods and Materials, 2003, 50(5).DOI: 10.1108/acmm.2003.12850eae.001. |
| 12 | BENCK J D, HELLSTERN T R, KIBSGAARD J, et al. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials[J]. ACS Catalysis, 2016, 4(11): 3957-3971. |
| 13 | ZHU J, HU L S, ZHAO P X, et al. Recent advances in electrocatalytic hydrogen evolution using nanoparticles[J]. Chemical Reviews, 2020, 120(2): 851-918. |
| 14 | HINNEMANN B, MOSES P G, BONDE J, et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution[J]. Journal of the American Chemical Society, 2005, 127(15): 5308-5309. |
| 15 | LI H X, HUANG M, CAO G Y. Markedly different adsorption behaviors of gas molecules on defective monolayer MoS2: a first-principles study[J]. Physical Chemistry Chemical Physics, 2016, 18(22): 15110-15117. |
| 16 | TSAI C, LI H, PARK S, et al. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution[J]. Nature Communications, 2017, 8(1):15113. |
| 17 | ER D Q, YE H, FREY N C, et al. Prediction of enhanced catalytic activity for hydrogen evolution reaction in janus transition metal dichalcogenides[J]. Nano Letters, 2018, 18(6): 3943-3949. |
| 18 | TRAN P D, GOFF A L, HEIDKAMP J, et al. Noncovalent modification of carbon nanotubes with pyrene-functionalized nickel complexes: carbon monoxide tolerant catalysts for hydrogen evolution and uptake[J]. Angewandte Chemie International Edition, 2010, 123(6): 1407-1410 |
| 19 | ZHU C B, MU X K, YU Y, et al. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage[J]. Angewandte Chemie International Edition, 2014, 126: 2184-2188. |
| 20 | GUO Y X, ZHANG X Y, ZHANG X P, et al. Defect-and S-rich ultrathin MoS2 nanosheet embedded N-doped carbon nanofibers for efficient hydrogen evolution[J]. Journal of Materials Chemistry A, 2015, 3 (31): 15927-15934. |
| 21 | LI C G, YAN H G, BRUS L E, et al. Anomalous lattice vibrations of single-and few-layer MoS2[J]. ACS Nano, 2010, 4(5): 2695-2700. |
| 22 | WANG H L, ROBINSON J T, DIANKOV G, et al. Nanocrystal growth on graphene with various degrees of oxidation[J]. Journal of the American Chemical Society, 2010, 132(10): 3270-3271. |
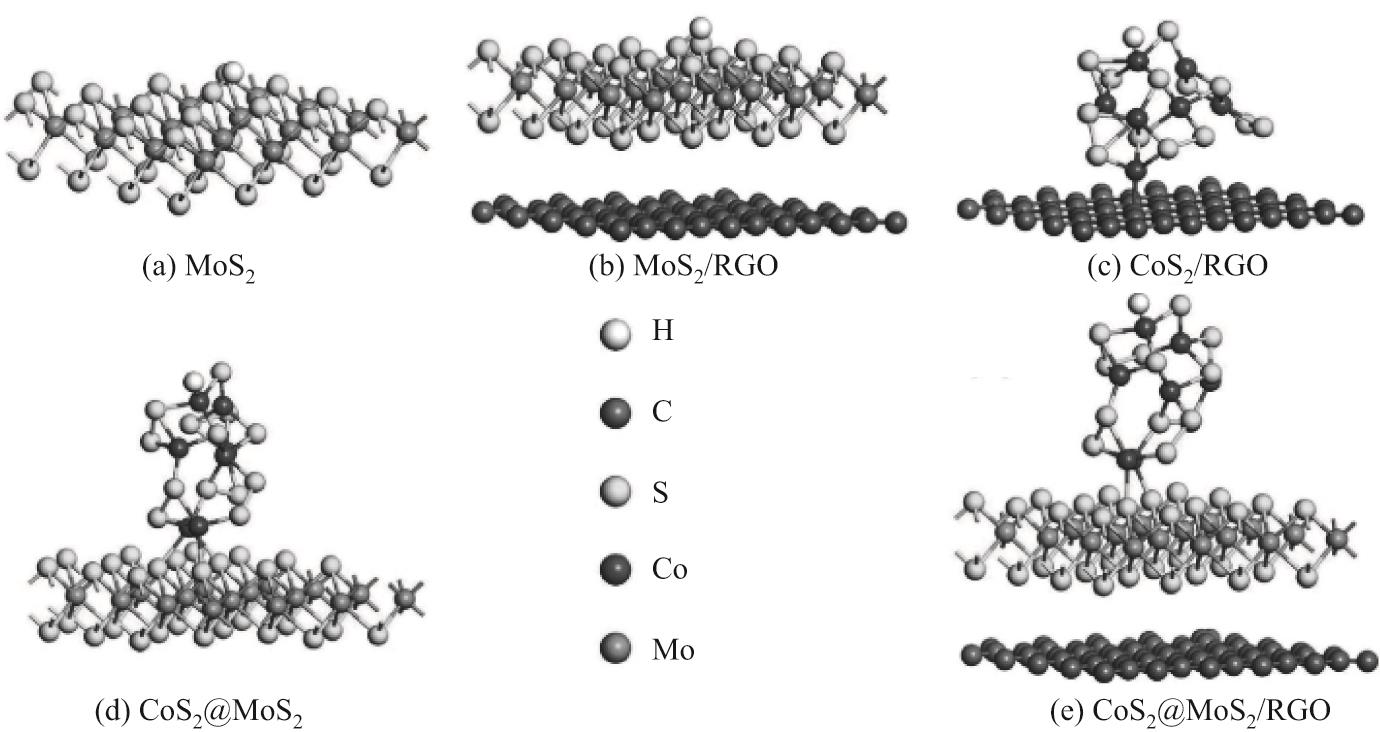
| 23 | GUO Y X, GAN L F, SHANG C S, et al. A cake-style CoS2@MoS2/RGO hybrid catalyst for efficient hydrogen evolution[J]. Advanced Functional Materials, 2017, 27(5): 1-7. |
| 24 | LI L, QIN Z D, RIES L, et al. Role of sulfur vacancies and undercoordinated Mo regions in MoS2 nanosheets towards the evolution of hydrogen[J]. ACS Nano, 2019, 13(6): 6824-6834. |
| 25 | YANG W W, ZHANG S Q, CHEN Q, et al. Conversion of intercalated MoO3 to multi-heteroatoms-doped MoS2 with high hydrogen evolution activity[J]. Advanced Materials, 2020, 32(30): 2001167. |
| 26 | SHI Y, ZHOU Y, YANG D R, et al. Energy level engineering of MoS2 by transition-metal doping for accelerating hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2017, 139(43): 15479-15485. |
| 27 | LIU Y, KELLY T G, CHEN J G, et al. Metal carbides as alternative electrocatalyst supports[J]. ACS Catalysis, 2013, 3(6): 1184-1194. |
| 28 | DONG J H, HAN S, PAK C, et al. High electrochemical performance and stability of co-deposited Pd-Au on phase-pure tungsten carbide for hydrogen oxidation[J]. Topics in Catalysis, 2012, 55: 922-930. |
| 29 | CHEN Z G, GONG W B, CONG S, et al. Eutectoid-structured WC/W2C heterostructures: a new platform for long-term alkaline hydrogen evolution reaction at low overpotentials[J]. Nano Energy, 2019, 68: 104335. |
| 30 | GAO Q S, ZHANG W B, SHI Z P, et al. Structural design and electronic modulation of transition-metal-carbide electrocatalysts toward efficient hydrogen evolution[J]. Advanced Materials, 2018, 31(2): 1802880. |
| 31 | YANG C F, ZHAO R, XIANG H, et al. Ni-activated transition metal carbides for efficient hydrogen evolution in acidic and alkaline solutions[J]. Advanced Energy Materials, 2020, 10(37): 2002260. |
| 32 | LIN Q, SHANG C Q, CHEN Z H, et al. Boron-doped molybdenum carbide as a pH-independent electrocatalyst for the hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45(55): 30659-30665. |
| 33 | ZHAO D, SUN K A, CHEONG W C, et al. Synergistically interactive pyridinic-N-MoP sites: Identified active centers for enhanced hydrogen evolution in alkaline solution[J]. Angewandte Chemie International Edition, 2020, 59(23): 8982-8990. |
| 34 | LI X L, ZHANG J L, ZHANG Y, et al. Copper induced phosphide for enhanced electrochemical hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2020, 45: 21422-21430. |
| 35 | JIAO Y, ZHENG Y, DAVEY K, et al. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped grapheme[J]. Nature Energy, 2016, 1: 16130. |
| 36 | DAI L M, XUE Y H, QU L T, et al. Metal-free catalysts for oxygen reduction reaction[J]. Chemical Reviews, 2015, 115(11): 4823-4892. |
| 37 | 高彩艳. 金属基复合催化剂的制备及电解水性能研究[D]. 郑州: 郑州大学, 2020. |
| GAO C Y. Preparation of metal based composite catalysts and study on electrocatalytic properties[D]. Zhengzhou: Zhengzhou University, 2020. | |
| 38 | ZHANG J T, DAI L M. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(12): 21-28. |
| 39 | VIJAYARAGHAVAN G, STEVENSON K J. Synergistic assembly of dendrimer-templated platinum catalysts on nitrogen-doped carbon nanotube electrodes for oxygen reduction[J]. Langmuir, 2007, 23(10):5279-5282. |
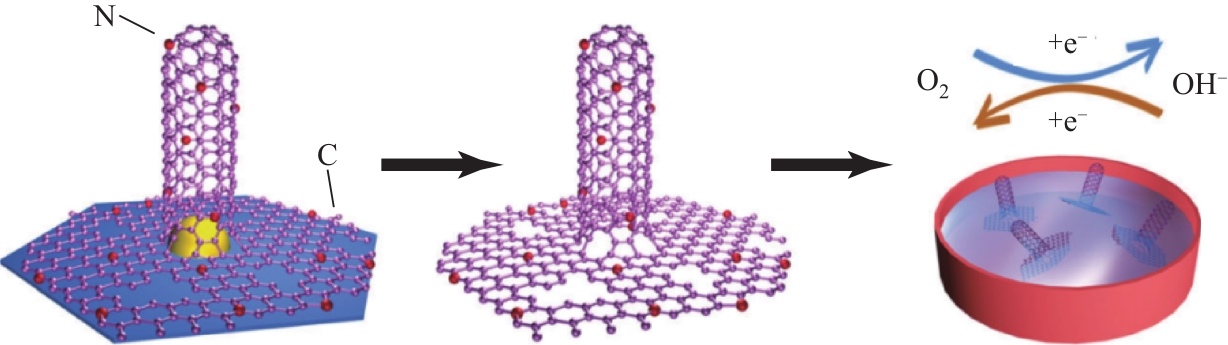
| 40 | LI Y G, DAI H J. Recent advances in zinc-air batteries[J]. Chemical Society Reviews, 2014, 43(15): 5257-5275. |
| 41 | VAZQUEZ-ARENAS J, HIGGINS D, ZHU C, et al. Mechanistic analysis of highly active nitrogen-doped carbon nanotubes for the oxygen reduction reaction[J]. Journal of Power Sources, 2012, 205(1): 215-221. |
| 42 | 周思了. 两种功能化碳材料的电催化析氢性能研究[D]. 芜湖: 安徽师范大学, 2017. |
| ZHOU S L. Study on the electrocatalytic hydrogen evolution performance of two functional carbon materials[D]. Wuhu: Anhui Normal University, 2017. | |
| 43 | ZHENG Y, JIAO Y, ZHU Y H, et al. Hydrogen evolution by a metal-free electrocatalyst[J]. Nature Communications, 2014, 5(4): 3783-3791. |
| 44 | GUO D H, SHIBUYA R, AKIBA C, et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts[J]. Science, 2016, 351(6271):361-365. |
| 45 | LI T H, TANG T M, CUI Z H, et al. Functionalized carbon nanotubes for highly active and metal-free electrocatalysts in hydrogen evolution reaction[J]. Electrocatalysis, 2018, 9: 573-581. |
| 46 | QU K G, ZHENG Y, JIAO Y, et al. Polydopamine-inspired, dual heteroatom-doped carbon nanotubes for highly efficient overall water splitting[J]. Advanced Energy Materials, 2017, 7(9): 160-168. |
| 47 | LI T H, CHEN Y P, HU W H, et al. Ionic liquid in situ functionalized carbon nanotubes as metal-free catalyst for efficient electrocatalytic hydrogen evolution reaction[J]. Nanoscale, 2021, 13: 4444-4450. |
| 48 | ITO D Y, CONG W, FU D T, et al. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction[J]. Angewandte Chemie International Edition, 2015, 51(7): 2131-2136. |
| 49 | ZHAO Z H, XIA Z H. Design principles for dual-element-doped carbon nanomaterials as efficient bifunctional catalysts for oxygen reduction and evolution reactions[J]. ACS Catalysis, 2016, 6(3): 1553-1558. |
| 50 | XING T, ZHENG Y, LI L H, et al. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer grapheme[J]. ACS Nano, 2014, 8(7): 6856-6862. |
| 51 | WANG H, LI X B, GAO L, et al. Three-dimensional graphene networks with abundant sharp edge sites for efficient electrocatalytic hydrogen evolution[J]. Angewandte Chemie International Edition, 2018, 130(1): 198-203. |
| 52 | HOLMBERG N, LAASONEN K. Theoretical insight into the hydrogen evolution activity of open-ended carbon nanotubes[J]. The Journal of Physical Chemistry Letters, 2015, 6(19): 3956-3960. |
| 53 | YUE X, HUANG S L, CAI J J, et al. Heteroatoms dual doped porous graphene nanosheets as efficient bifunctional metal-free electrocatalysts for overall water-splitting[J]. Journal of Materials Chemistry A, 2017, 5(17): 7784-7790. |
| 54 | CHHETRI M, MAITRA S, CHAKRABORTY H, et al. Superior performance of borocarbonitrides, BxCyNz, as stable, low-cost metal-free electrocatalysts for the hydrogen evolution reaction[J]. Energy Environ. Sci., 2016, 9(1): 95-101. |
| 55 | DONG G, ZHANG Y, PAN Q, et al. A fantastic graphitic carbon nitride (g-C3N4) material: electronic structure, photocatalytic and photoelectronic properties[J]. Journal of Photochemistry & Photobiology C: Photochemistry Reviews, 2014, 20: 33-50. |
| 56 | SHALOM M, GIMENEZ S, SCHIPPER F, et al. Controlled carbon nitride growth on surfaces for hydrogen evolution electrodes[J]. Angew. Chem. Int. Ed., 2014, 53(14): 3654-3658. |
| 57 | PEI, Z X, ZHAO J X, HUANG Y, et al. Toward enhanced activity of a graphitic carbon nitride-based electrocatalyst in oxygen reduction and hydrogen evolution reactions via atomic sulfur doping[J]. Journal of Materials Chemistry A, 2016, 4(31): 12205-12211. |
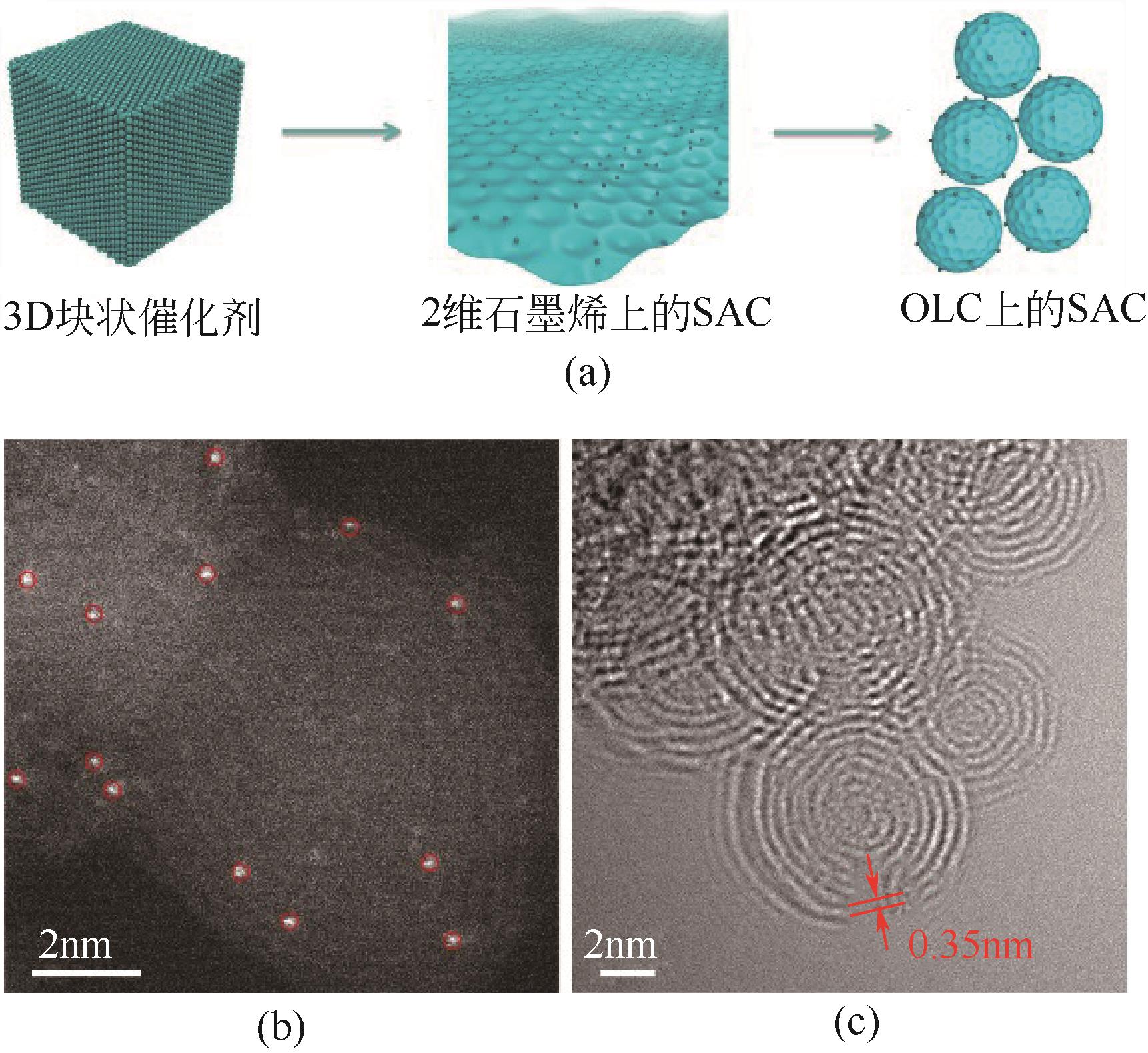
| 58 | QIAO B T, WANG A Q, YANG X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3 (8): 634-641. |
| 59 | WANG A Q, LI J, ZHANG T. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry, 2018, 2 (6): 65-81. |
| 60 | KYRIAKOU G, BOUCHER M B, JEWELL A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335 (6073): 1209-1212. |
| 61 | LIU L C, CORMA A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118 (10): 4981-5079. |
| 62 | LIU J Y, XU J, QIAO B T, et al. Catalysis by supported single metal atoms[J]. ACS Catalysis, 2016, 7 (1): 34-59. |
| 63 | JONES J, XIONG H F, DELARIVA A T, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping[J]. Science, 2016, 353(6295): 150-154. |
| 64 | HUANG Y B, LIANG J, WANG X S, et al. Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions[J]. Chemical Society Reviews, 2017, 46 (1): 126-157. |
| 65 | CHEN Y J, JI S F, WANG Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2017, 56(24): 6937-6941. |
| 66 | ZHENG T T, JIANG K, TA N, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst[J]. Joule, 2019, 3 (1): 265-278. |
| 67 | PENG Y, PAN W Z, WANG N, et al. Ruthenium ion-complexed graphitic carbon nitride nanosheets supported on reduced graphene oxide as high-performance catalysts for electrochemical hydrogen evolution[J]. ChemSusChem, 2018, 11 (1): 130-136. |
| 68 | PENG Y, LU B Z, CHEN L M, et al. Hydrogen evolution reaction catalyzed by ruthenium ion-complexed graphitic carbon nitride nanosheets[J]. Journal of Materials Chemistry A, 2017, 5(34): 18261-18269. |
| 69 | LU B Z, GUO L, WU F, et al. Ruthenium atomically dispersed in carbon outperforms platinum toward hydrogen evolution in alkaline media[J]. Nature Communications, 2019, 10(1): 631-642. |
| 70 | FEI H L, DONG J C, FENG Y X, et al. General synthesis and definitive structural identification of MN4C4 single-atom catalysts with tunable electrocatalytic activities[J]. Nature Catalysis, 2018, 1(1): 63-72. |
| 71 | YIN X P, WANG H J, TANG S F, et al. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution[J]. Angewandte Chemie International Edition, 2018, 57(30): 9382-9386. |
| 72 | ZHOU K L, WANG C H, WANG Z L, et al. Seamlessly conductive Co(OH)2 tailored atomically dispersed Pt electrocatalyst with a hierarchical nanostructure for an efficient hydrogen evolution reaction[J]. Energy & Environmental Science, 2020, 13(9): 3082-3092. |
| 73 | LIU D B, LI X Y, CHEN S M, et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution[J]. Nature Energy, 2019, 4 (6): 512-518. |
| 74 | SHI Y, MA Z R, XIAO Y Y, et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction[J]. Nature Communications, 2021, 12: 3021-3032. |
| 75 | SONG H Q, WU M, TANG Z Y, et al. Single atom Ru doped CoP/CDs nanosheets via splicing of carbon dots for robust hydrogen production[J]. Angewandte Chemie International Edition, 2021, 60(13): 7234-7244. |
| 76 | ZHANG J M, XU X P, YANG L, et al. Single-atom Ru doping induced phase transition of MoS2 and S vacancy for hydrogen evolution reaction[J]. Small Methods, 2019, 3 (12): 1900653. |
| 77 | YANG J, CHEN B X, LIU W, et al. Efficient and robust hydrogen evolution: phosphorus nitride imide nanotubes as supports for anchoring single ruthenium sites[J]. Angewandte Chemie International Edition, 2018, 57 (30): 9495-9500. |
| 78 | FEI H L, DONG J C, ARELLANO-JIMENEZ M J, et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation[J]. Nature Communications, 2015, 6: 8668-8676. |
| 79 | SULTAN S, TIWARI J N, SINGH A N, et al. Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting[J]. Advanced Energy Materials, 2019, 9(22): 1900624.1-1900624.48. |
| 80 | ZHANG L Z, JIA Y, GAO G P, et al. Graphene defects trap atomic Ni species for hydrogen and oxygen evolution reactions[J]. Chem., 2018, 4(2): 285-297. |
| 81 | LIANG L H, JIN H H, ZHOU H, et al. Cobalt single atom site isolated Pt nanoparticles for efficient ORR and HER in acid media[J]. Nano Energy, 2021, 88: 106221. |
| [1] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [2] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [3] | 殷成阳, 侯铭, 杨爽, 毛迪, 刘俊言. 过渡金属改性Cu-SSZ-13分子筛脱硝催化剂研究进展[J]. 化工进展, 2023, 42(6): 2963-2974. |
| [4] | 张国春, 周志辉, 吴红丹. 基于α-Al2O3载体管的新型MXene膜异丙醇脱水性能[J]. 化工进展, 2023, 42(10): 5381-5389. |
| [5] | 李亚男, 年佩, 徐楠, 罗海玉, 魏逸彬. 面向精密流体分离的MXene基膜材料研究进展[J]. 化工进展, 2023, 42(10): 5249-5258. |
| [6] | 段毅, 邹烨, 周书葵, 杨柳. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158. |
| [7] | 王亚楠, 孟秀霞, 张维民, 杨乃涛. 表/界面调控策略在氢/氧反应电极中的研究进展[J]. 化工进展, 2022, 41(5): 2416-2428. |
| [8] | 毛梦雷, 孙丹阳, 孟子晖, 刘文芳. 氧化石墨烯和过渡金属碳/氮化合物固定化酶[J]. 化工进展, 2022, 41(4): 1941-1955. |
| [9] | 贾艳萍, 薛东奇, 刘启帆, 张海丰, 李正, 张兰河. 亚硫酸盐活化技术及其在废水处理中的应用[J]. 化工进展, 2022, 41(1): 418-426. |
| [10] | 田婷婷, 李朝阳, 王召东, 陆慧, 李新冬, 毛艳丽, 宋忠贤, 朱新锋. 过渡金属活化过硫酸盐降解有机废水技术研究进展[J]. 化工进展, 2021, 40(6): 3480-3488. |
| [11] | 黄国勇, 李毅, 屈辰玮, 孙晓华, 李勃天, 戈磊, 叶海木, 张红梅. 热电池用过渡金属二硫化物及其复合材料的研究进展[J]. 化工进展, 2021, 40(4): 2161-2174. |
| [12] | 鲍玉香, 马宏飞, 脱永笑, 祁艳颖, 冯翔, 杨朝合, 陈德. 氯乙烯单体合成催化剂研究进展[J]. 化工进展, 2021, 40(4): 2034-2047. |
| [13] | 吴建国, 吴登峰, 程道建. 丙烷脱氢制丙烯用单原子催化剂研究进展[J]. 化工进展, 2021, 40(12): 6688-6695. |
| [14] | 张婷, 孙晓红, 于宏兵, 董恒. 电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展[J]. 化工进展, 2021, 40(12): 6670-6687. |
| [15] | 石彩, 史峻铭, 滕敏, 王维聪, 额其马林, 黄占华. 过渡金属磷化物在光催化机理方面的研究进展[J]. 化工进展, 2021, 40(11): 6079-6093. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||