化工进展 ›› 2025, Vol. 44 ›› Issue (5): 2429-2440.DOI: 10.16085/j.issn.1000-6613.2024-2057
• 合成生物制造 • 上一篇
化学-生物级联转化CO2合成单细胞蛋白研究进展
吴孟勤1,2,3( ), 王佳瑶2,3,4, 徐友强1(
), 王佳瑶2,3,4, 徐友强1( ), 王钰2,3(
), 王钰2,3( )
)
- 1.北京工商大学食品与健康学院,北京 100048
2.中国科学院天津工业生物技术研究所低碳合成 工程生物学重点实验室,天津 300308
3.国家合成生物技术创新中心,天津 300308
4.天津科技大学生物工程学院,天津 300222
-
收稿日期:2024-12-18修回日期:2025-01-25出版日期:2025-05-25发布日期:2025-05-20 -
通讯作者:徐友强,王钰 -
作者简介:吴孟勤(1998—),女,博士研究生,研究方向为微生物进化技术与单细胞蛋白合成。E-mail:znwmqin@163.com.cn。 -
基金资助:国家重点研发计划(2024YFA0918100);天津市自然科学基金(24JCJQJC00010)
Progress in cascade conversion of CO2 to single cell protein through chemical and biological catalysis
WU Mengqin1,2,3( ), WANG Jiayao2,3,4, XU Youqiang1(
), WANG Jiayao2,3,4, XU Youqiang1( ), WANG Yu2,3(
), WANG Yu2,3( )
)
- 1.School of Food and Health, Beijing Technology and Business University, Beijing 100048, China
2.Key Laboratory of Engineering Biology for Low-Carbon Manufacturing, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China
3.National Center of Technology Innovation for Synthetic Biology, Tianjin 300308, China
4.College of Biotechnology, Tianjin University of Science and Technology, Tianjin 300222, China
-
Received:2024-12-18Revised:2025-01-25Online:2025-05-25Published:2025-05-20 -
Contact:XU Youqiang, WANG Yu
摘要:
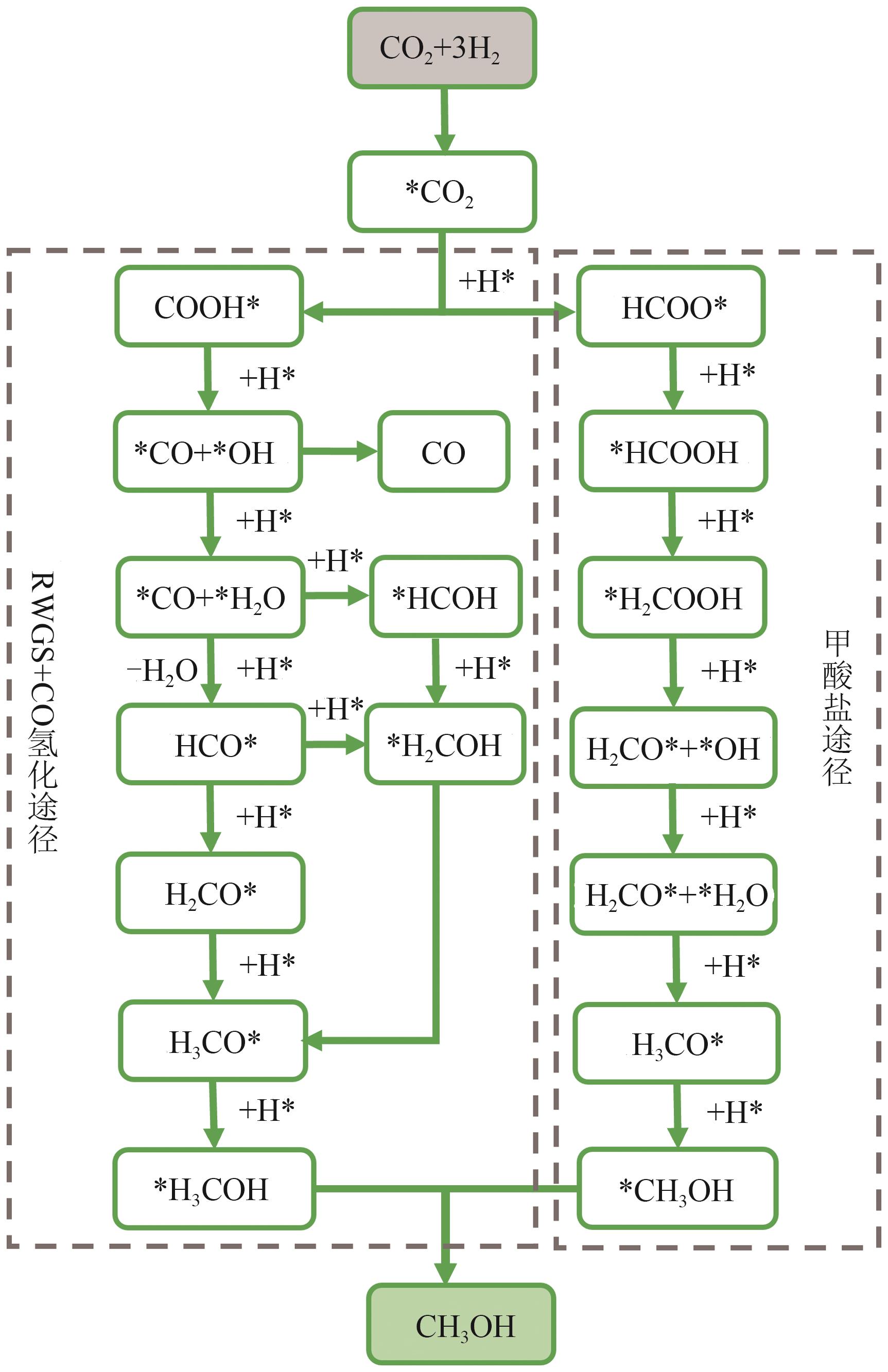
工业发展导致CO2大量排放,加剧了全球温室效应和环境污染。此外,全球人口的不断增长将会导致蛋白质供应不足。通过化学催化CO2还原合成甲醇等有机一碳化合物,进一步通过微生物将甲醇转化为多碳产物,是一条高效的CO2固定和转化利用路线。因此,从原料和产品层面考虑,本文提出了利用化学-生物级联转化CO2生产单细胞蛋白(single cell protein, SCP)的策略,即将CO2通过化学转化生产甲醇,再进一步利用微生物细胞工厂代谢甲醇和无机铵生产SCP,SCP产品有望应用于饲料和食品工业。本文首先介绍了CO2加氢可持续生产甲醇的反应过程及反应机制,总结了相关催化剂的研究进展。其次,介绍了自然界中发现的可利用甲醇的微生物及甲醇代谢途径,以及利用甲醇生产SCP的研究进展。最后,对CO2化学-生物级联转化工业化制造SCP的瓶颈和解决方案进行了展望。
中图分类号:
引用本文
吴孟勤, 王佳瑶, 徐友强, 王钰. 化学-生物级联转化CO2合成单细胞蛋白研究进展[J]. 化工进展, 2025, 44(5): 2429-2440.
WU Mengqin, WANG Jiayao, XU Youqiang, WANG Yu. Progress in cascade conversion of CO2 to single cell protein through chemical and biological catalysis[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2429-2440.
| 催化剂 | 温度 /K | 压力 /MPa | H2∶CO2 | 气体时空速度 /mL∙h-1∙g-1 | CO2转化率 /% | 甲醇选择性 /% | 时空产率 /gMeOH∙gcat-1∙h-1 | 催化剂稳定性/h | 反应 途径 | 参考文献 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu基催化剂 | |||||||||||
| CuZnAl | 533 | 1.5 | 3 | — | 2 | 约54 | 0.73 | — | 甲酸盐 | [ | |
| Cu-20%MgO/ZnO | 473 | 3 | H2∶CO2∶N2= 3∶1∶1 | 7200 | 8.7 | 99 | 0.202 | >120 | — | [ | |
| Cu-ZrO2 | 493 | 3 | H2∶CO2∶Ar= 72∶24∶4 | 15000 | 7.2 | 约77.8 | 2.617 | 约16 | CO加氢 | [ | |
| 30%CuO/49.65%ZnO/20.35%Al2O3 | 575 | 8.5 | 4 | 19000 | 13 | 50 | 0.22 | — | — | [ | |
| 60%CuO-30%ZnO10%Al2O3 | 575 | 8.5 | 4 | 19000 | 21 | 49.5 | 0.425 | — | — | ||
| 60%CuO-27%ZnO/3%La2O3/10%Al2O3 | 575 | 8.5 | 4 | 55000 | 10 | 65 | 0.79 | — | — | ||
| 贵金属基催化剂 | |||||||||||
| Pt(3)/MoO x (30)/TiO2 | 423 | — | 5 | — | — | 约85 | — | — | 甲酸盐 | [ | |
| PdZn/CeO2 | 473 | 2 | 3 | 3600 | 14.1 | 97.2 | 0.17 | >100 | 甲酸盐 | [ | |
| PdZn/ZnO/SiO2 | 533 | 5 | 3 | 60000 | 3.3 | 65.3 | 0.443 | — | — | [ | |
| 金属氧化物催化剂 | |||||||||||
| In2O3/ZrO2 | 573 | 5 | 4∶1 | 16000 | 5.2 | 99.8 | 0.295 | 1000 | — | [ | |
| hexagonalIn2O3 | 543 | 5 | 4∶1 | 20000 | 6.7 | 99.5 | 0.365 | 136 | 甲酸盐 | [ | |
| 固溶体催化剂 | |||||||||||
| ZnO-ZrO2 | 588-593 | 5 | (3∶1)~(4∶1) | 24000 | >10%(单程) | 86~91 | — | >500 | 甲酸盐 | [ | |
| 有序介孔结构ZnO-ZrO2 | 593 | 5.5 | H2∶CO2∶Ar= 72∶24∶4 | 24000 | 约10 | 约81 | 0.708 | 40 | — | [ | |
| 金属有机框架催化剂 | |||||||||||
| Cu@FAU | 513 | 3 | 3 | 12000 | 11.5 | 89.5 | 0.41 | 200 | 甲酸盐 | [ | |
| 20%-Cu@ZrO2-U | 533 | 3 | 3 | 2400 | 12.1 | 70.5 | 0.073 | 100 | 甲酸盐 | [ | |
| In2O3@ZrO2-MIL-68@UiO-66 | 563 | 3 | — | — | 10.4 | 84.6 | 0.29 | — | 甲酸盐 | [ | |
| CuO/s-UiO-66 (4.29%) | 513 | 3 | — | 18000 | 2.43 | 76.8 | 2.649 | 130 | 甲酸盐 | [ | |
| 混合催化剂 | |||||||||||
| PdCu/Ce0.3Zr0.7O2 (PdCu/CZ-3) | 523 | 5 | 3 | 3600 | 25.5 | 30~40 | 0.07 | >100 | 甲酸盐 | [ | |
| Ag/In2O3 | 573 | 5 | H2∶CO2∶N2= 76∶19∶5 | 21000 | 13.6 | 58.2 | 0.453 | 10 | CO加氢 | [ | |
| 0.8%Pd-ZnZrO x | 593 | 4 | H2∶CO2∶N2= 76∶19∶5 | 24000 | 约18 | 约60 | 约0.6 | 100 | 甲酸盐 | [ | |
表1 CO2加氢生产甲醇催化剂的反应条件、催化性能及反应机理
| 催化剂 | 温度 /K | 压力 /MPa | H2∶CO2 | 气体时空速度 /mL∙h-1∙g-1 | CO2转化率 /% | 甲醇选择性 /% | 时空产率 /gMeOH∙gcat-1∙h-1 | 催化剂稳定性/h | 反应 途径 | 参考文献 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu基催化剂 | |||||||||||
| CuZnAl | 533 | 1.5 | 3 | — | 2 | 约54 | 0.73 | — | 甲酸盐 | [ | |
| Cu-20%MgO/ZnO | 473 | 3 | H2∶CO2∶N2= 3∶1∶1 | 7200 | 8.7 | 99 | 0.202 | >120 | — | [ | |
| Cu-ZrO2 | 493 | 3 | H2∶CO2∶Ar= 72∶24∶4 | 15000 | 7.2 | 约77.8 | 2.617 | 约16 | CO加氢 | [ | |
| 30%CuO/49.65%ZnO/20.35%Al2O3 | 575 | 8.5 | 4 | 19000 | 13 | 50 | 0.22 | — | — | [ | |
| 60%CuO-30%ZnO10%Al2O3 | 575 | 8.5 | 4 | 19000 | 21 | 49.5 | 0.425 | — | — | ||
| 60%CuO-27%ZnO/3%La2O3/10%Al2O3 | 575 | 8.5 | 4 | 55000 | 10 | 65 | 0.79 | — | — | ||
| 贵金属基催化剂 | |||||||||||
| Pt(3)/MoO x (30)/TiO2 | 423 | — | 5 | — | — | 约85 | — | — | 甲酸盐 | [ | |
| PdZn/CeO2 | 473 | 2 | 3 | 3600 | 14.1 | 97.2 | 0.17 | >100 | 甲酸盐 | [ | |
| PdZn/ZnO/SiO2 | 533 | 5 | 3 | 60000 | 3.3 | 65.3 | 0.443 | — | — | [ | |
| 金属氧化物催化剂 | |||||||||||
| In2O3/ZrO2 | 573 | 5 | 4∶1 | 16000 | 5.2 | 99.8 | 0.295 | 1000 | — | [ | |
| hexagonalIn2O3 | 543 | 5 | 4∶1 | 20000 | 6.7 | 99.5 | 0.365 | 136 | 甲酸盐 | [ | |
| 固溶体催化剂 | |||||||||||
| ZnO-ZrO2 | 588-593 | 5 | (3∶1)~(4∶1) | 24000 | >10%(单程) | 86~91 | — | >500 | 甲酸盐 | [ | |
| 有序介孔结构ZnO-ZrO2 | 593 | 5.5 | H2∶CO2∶Ar= 72∶24∶4 | 24000 | 约10 | 约81 | 0.708 | 40 | — | [ | |
| 金属有机框架催化剂 | |||||||||||
| Cu@FAU | 513 | 3 | 3 | 12000 | 11.5 | 89.5 | 0.41 | 200 | 甲酸盐 | [ | |
| 20%-Cu@ZrO2-U | 533 | 3 | 3 | 2400 | 12.1 | 70.5 | 0.073 | 100 | 甲酸盐 | [ | |
| In2O3@ZrO2-MIL-68@UiO-66 | 563 | 3 | — | — | 10.4 | 84.6 | 0.29 | — | 甲酸盐 | [ | |
| CuO/s-UiO-66 (4.29%) | 513 | 3 | — | 18000 | 2.43 | 76.8 | 2.649 | 130 | 甲酸盐 | [ | |
| 混合催化剂 | |||||||||||
| PdCu/Ce0.3Zr0.7O2 (PdCu/CZ-3) | 523 | 5 | 3 | 3600 | 25.5 | 30~40 | 0.07 | >100 | 甲酸盐 | [ | |
| Ag/In2O3 | 573 | 5 | H2∶CO2∶N2= 76∶19∶5 | 21000 | 13.6 | 58.2 | 0.453 | 10 | CO加氢 | [ | |
| 0.8%Pd-ZnZrO x | 593 | 4 | H2∶CO2∶N2= 76∶19∶5 | 24000 | 约18 | 约60 | 约0.6 | 100 | 甲酸盐 | [ | |
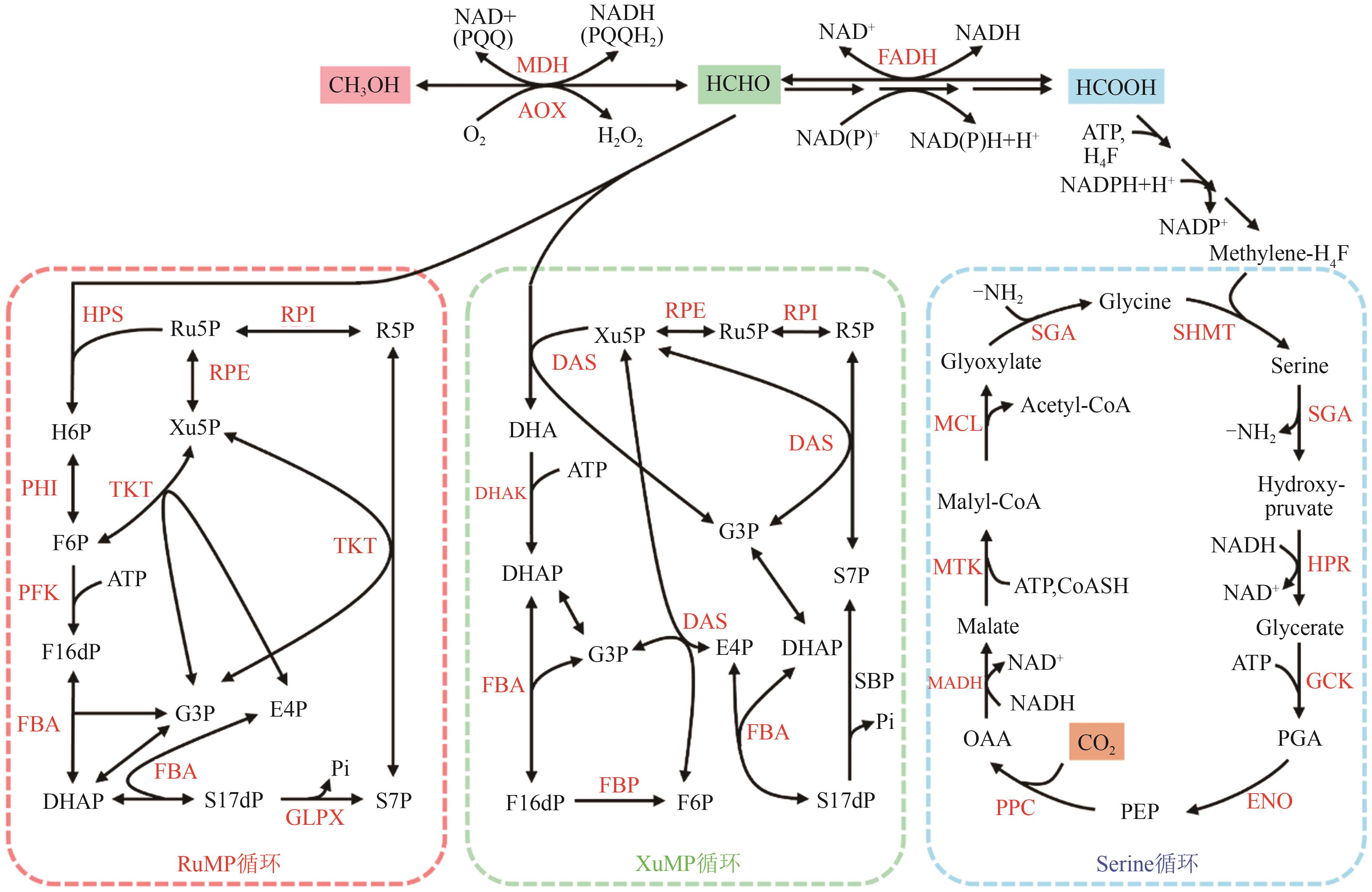
图2 天然甲基营养菌的甲醇代谢途径MDH—甲醇脱氢酶(methanol dehydrogenase);AOX—甲醇氧化酶(alcohol oxidase);FADH—甲醛脱氢酶;HPS—己糖-6-磷酸合酶(3-hexulose-6-phosphate synthase);PHI—6-磷酸-3-己酮糖异构酶(6-phospho-3-hexuloisomerase);PFK—6-磷酸果糖激酶(6-phosphofructokinase);FBA—果糖二磷酸醛缩酶/景天庚酮糖-1,7-二磷酸醛缩酶(fructose-bisphosphate aldolase/sedoheptulose-bisphosphate aldolase);TKT—转酮醇酶(transketolase);RPE—磷酸核酮糖差向异构酶(ribulose-phosphate 3-epimerase);RPI—核糖5-磷酸异构酶(ribose-5-phosphate isomerase);GLPX—双功能果糖二磷酸酶/景天庚酮糖双磷酸酯酶(bifunctional fructose bisphosphatase/sedoheptulose bisphosphatase);DAS—二羟基丙酮合酶(dihydroxyacetone synthase);DHAK—二羟基丙酮激酶(dihydroxyacetone kinase);FBP—果糖二磷酸酶(fructose bisphosphatase);SBP—景天庚酮糖双磷酸酯酶(sedoheptulose bisphosphatase);SHMT—丝氨酸羟甲基转移酶(serine hydroxymethyltransferase);SGA—丝氨酸乙醛酸氨基转移酶(serine glyoxylate aminotransferase);HPR—羟基丙酮酸还原酶(hydroxypyruvate reductase);GCK—甘油酸激酶(glycerate kinase);ENO—烯醇化酶(enolase);PPC—磷酸烯醇丙酮酸羧化酶(phosphoenolpyruvate carboxylase);MADH—苹果酸脱氢酶(malate dehydrogenase);MTK—苹果酸硫激酶 (malate thiokinase);MCL—苹果酰辅酶A裂解酶(malyl-CoA lyase);Ru5P—核酮糖-5-磷酸(ribulose-5-phosphate);Ru15dP—核酮糖-1,5-二磷酸(ribulose-1,5-bisphosphate);R5P—核糖-5磷酸(ribose-5-phosphate);Xu5P—木酮糖-5-磷酸(xylulose-5-phosphate);H6P—3-己酮糖-6-磷酸(hexulose-6-phosphate);F6P—果糖-6磷酸(fructose-6-phosphate);F16dP—果糖-1,6-二磷酸(fructose-1,6-bisphosphate);DHAP—磷酸二羟基丙酮(dihydroxyacetone phosphate);DHA—二羟基丙酮(dihydroxyacetone);G3P—甘油醛-3-磷酸(glyceraldehyde-3-phosphate);E4P—赤藓糖-4-磷酸(erythrose-4-phosphate);S7P—景天庚酮糖-7-磷酸(sedoheptulose-7-phosphate);S17dP—1, 7-二磷酸景天庚酮糖(sedoheptulose-1,7-bisphosphate);PGA—磷酸甘油酸酯(2-phosphate-glycerate);PEP—磷酸烯醇式丙酮酸(phosphoenolpyruvate);OAA—草酰乙酸(oxaloacetate)
| 1 | DUSENGE Mirindi Eric, DUARTE André Galvao, Danielle A WAY. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration[J]. New Phytologist, 2019, 221(1): 32-49. |
| 2 | TANG Chizhou, TANG Shan, SHA Feng, et al. Insights into the selectivity determinant and rate-determining step of CO2 hydrogenation to methanol[J]. The Journal of Physical Chemistry C, 2022, 126(25): 10399-10407. |
| 3 | BOLAND Mike J, Allan N RAE, VEREIJKEN Johan M, et al. The future supply of animal-derived protein for human consumption[J]. Trends in Food Science & Technology, 2013, 29(1): 62-73. |
| 4 | PEREIRA Antia G, Maria FRAGA-CORRAL, Paula GARCIA-OLIVEIRA, et al. Single-cell proteins obtained by circular economy intended as a feed ingredient in aquaculture[J]. Foods, 2022, 11(18): 2831. |
| 5 | GAO Yurong, LI Dapeng, LIU Yang. Production of single cell protein from soy molasses using Candida tropicalis [J]. Annals of Microbiology, 2012, 62(3): 1165-1172. |
| 6 | SCHRADER Jens, SCHILLING Martin, HOLTMANN Dirk, et al. Methanol-based industrial biotechnology: Current status and future perspectives of methylotrophic bacteria[J]. Trends in Biotechnology, 2009, 27(2): 107-115. |
| 7 | BERTAU Martin, OFFERMANNS Heribert, PLASS Ludolf, et al. Methanol: the basic chemical and energy feedstock of the future: Asinger’s vision today[M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. |
| 8 | DU Xianlong, JIANG Zheng, SU Dangsheng, et al. Research progress on the indirect hydrogenation of carbon dioxide to methanol[J]. ChemSusChem, 2016, 9(4): 322-332. |
| 9 | Kerstin SCHULTENKÄMPER, BRITO Luciana F, Marina Gil LÓPEZ, et al. Establishment and application of CRISPR interference to affect sporulation, hydrogen peroxide detoxification, and mannitol catabolism in the methylotrophic thermophile Bacillus methanolicus [J]. Applied Microbiology and Biotechnology, 2019, 103(14): 5879-5889. |
| 10 | MO Xuhua, ZHANG Hui, WANG Tianmin, et al. Establishment of CRISPR interference in Methylorubrum extorquens and application of rapidly mining a new phytoene desaturase involved in carotenoid biosynthesis[J]. Applied Microbiology and Biotechnology, 2020, 104(10): 4515-4532. |
| 11 | CAI Peng, DUAN Xingpeng, WU Xiaoyan, et al. Recombination machinery engineering facilitates metabolic engineering of the industrial yeast Pichia pastoris [J]. Nucleic Acids Research, 2021, 49(13): 7791-7805. |
| 12 | SOBHI Mostafa, ZAKARIA Eman, ZHU Feifei, et al. Advanced microbial protein technologies are promising for supporting global food-feed supply chains with positive environmental impacts[J]. Science of the Total Environment, 2023, 894: 165044. |
| 13 | RITALA Anneli, HÄKKINEN Suvi T, TOIVARI Mervi, et al. Single cell protein-state-of-the-art, industrial landscape and patents 2001-2016[J]. Frontiers in Microbiology, 2017, 8: 2009. |
| 14 | REN Menghao, ZHANG Yanmin, WANG Xuan, et al. Catalytic hydrogenation of CO2 to methanol: A review[J]. Catalysts, 2022, 12(4): 403. |
| 15 | AHMAD Kaisar, UPADHYAYULA Sreedevi. Greenhouse gas CO2 hydrogenation to fuels: A thermodynamic analysis[J]. Environmental Progress & Sustainable Energy, 2019, 38(1): 98-111. |
| 16 | MARTIN Oliver, MARTÍN Dr Antonio J, MONDELLI Dr Cecilia, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 17 | WANG Guo, MAO Dongsen, GUO Xiaoming, et al. Methanol synthesis from CO2 hydrogenation over CuO-ZnO-ZrO2-M x O y catalysts (M=Cr, Mo and W)[J]. International Journal of Hydrogen Energy, 2019, 44(8): 4197-4207. |
| 18 | WANG Jijie, LI Guanna, LI Zelong, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. |
| 19 | ZABILSKIY Maxim, SUSHKEVICH Vitaly L, PALAGIN Dennis, et al. The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol[J]. Nature Communications, 2020, 11(1): 2409. |
| 20 | SHARMA Sachin Kumar, KHAN Tuhin Suvra, SINGHA Rajib Kumar, et al. Design of highly stable MgO promoted Cu/ZnO catalyst for clean methanol production through selective hydrogenation of CO2 [J]. Applied Catalysis A: General, 2021, 623: 118239. |
| 21 | YU Jiahui, LIU Shuai, MU Xueliang, et al. Cu-ZrO2 catalysts with highly dispersed Cu nanoclusters derived from ZrO2@HKUST-1 composites for the enhanced CO2 hydrogenation to methanol[J]. Chemical Engineering Journal, 2021, 419: 129656. |
| 22 | Sardar ALI, KUMAR Dharmesh, KHADER Mahmoud M, et al. Synthesis and evaluation of lanthana modified Cu-based catalysts for CO2 hydrogenation to value added products[J]. Molecular Catalysis, 2023, 543: 113146. |
| 23 | TOYAO Takashi, KAYAMORI Shingo, MAENO Zen, et al. Heterogeneous Pt and MoO x co-loaded TiO2 catalysts for low-temperature CO2 hydrogenation to form CH3OH[J]. ACS Catalysis, 2019, 9(9): 8187-8196. |
| 24 | OJELADE Opeyemi A, ZAMAN Sharif F, DAOUS Muhammad A, et al. Optimizing Pd: Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2019, 584: 117185. |
| 25 | ZABILSKIY Dr Maxim, SUSHKEVICH Dr Vitaly L, NEWTON Dr Mark A, et al. Mechanistic study of carbon dioxide hydrogenation over Pd/ZnO-based catalysts: The role of palladium–zinc alloy in selective methanol synthesis[J]. Angewandte Chemie International Edition, 2021, 60(31): 17053-17059. |
| 26 | DANG Shanshan, QIN Bin, YANG Yong, et al. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Science Advances, 2020, 6(25): eaaz2060. |
| 27 | HAN Zhe, TANG Chizhou, SHA Feng, et al. CO2 hydrogenation to methanol on ZnO-ZrO2 solid solution catalysts with ordered mesoporous structure[J]. Journal of Catalysis, 2021, 396: 242-250. |
| 28 | CHAI Yuchao, QIN Bin, LI Bonan, et al. Zeolite-encaged mononuclear copper centers catalyze CO2 selective hydrogenation to methanol[J]. National Science Review, 2023, 10(7): nwad043. |
| 29 | CHEN Guoqing, YU Jun, LI Gonghui, et al. Cu+-ZrO2 interfacial sites with highly dispersed copper nanoparticles derived from Cu@UiO-67 hybrid for efficient CO2 hydrogenation to methanol[J]. International Journal of Hydrogen Energy, 2023, 48(7): 2605-2616. |
| 30 | CUI Wengang, ZHANG Qiang, ZHOU Lei, et al. Hybrid MOF template-directed construction of hollow-structured In2O3@ZrO2 heterostructure for enhancing hydrogenation of CO2 to methanol[J]. Small, 2023, 19(1): 2204914. |
| 31 | WANG Chao, KOSARI Mohammadreza, XI Shibo, et al. Uniform Si-infused UiO-66 as a robust catalyst host for efficient CO2 hydrogenation to methanol[J]. Advanced Functional Materials, 2023, 33(13): 2212478. |
| 32 | WANG Xilong, ALABSI Mohnnad H, ZHENG Peng, et al. PdCu supported on dendritic mesoporous Ce x Zr1- x O2 as superior catalysts to boost CO2 hydrogenation to methanol[J]. Journal of Colloid and Interface Science, 2022, 611: 739-751. |
| 33 | SUN Kaihang, ZHANG Zhitao, SHEN Chenyang, et al. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol[J]. Green Energy & Environment, 2022, 7(4): 807-817. |
| 34 | LEE Kyungho, ANJUM Uzma, ARAÚJO Thaylan Pinheiro, et al. Atomic Pd-promoted ZnZrO x solid solution catalyst for CO2 hydrogenation to methanol[J]. Applied Catalysis B: Environmental, 2022, 304: 120994. |
| 35 | ARAÚJO Thaylan Pinheiro, MITCHELL Sharon, Javier PÉREZ-RAMÍREZ. Design principles of catalytic materials for CO2 hydrogenation to methanol [J]. Advanced Materials, 2024, 36(48): 2470385. |
| 36 | CHEN Kun, FANG Huihuang, WU Simson, et al. CO2 hydrogenation to methanol over Cu catalysts supported on La-modified SBA-15: The crucial role of Cu-LaO x interfaces[J]. Applied Catalysis B: Environmental, 2019, 251: 119-129. |
| 37 | TADA Shohei, SATOKAWA Shigeo. Effect of Ag loading on CO2-to-methanol hydrogenation over Ag/CuO/ZrO2 [J]. Catalysis Communications, 2018, 113: 41-45. |
| 38 | WANG Guo, MAO Dongsen, GUO Xiaoming, et al. Methanol synthesis from CO2 hydrogenation over CuO-ZnO-ZrO2-M x O y catalysts (M=Cr, Mo and W)[J]. International Journal of Hydrogen Energy, 2019, 44(8): 4197-4207. |
| 39 | TWIGG Martyn V, SPENCER Michael S. Deactivation of copper metal catalysts for methanol decomposition, methanol steam reforming and methanol synthesis[J]. Topics in Catalysis, 2003, 22(3): 191-203. |
| 40 | FICHTL Matthias B, SCHLERETH David, JACOBSEN Nikolas, et al. Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts[J]. Applied Catalysis A: General, 2015, 502: 262-270. |
| 41 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. Probing into the multifunctional role of copper species and reaction pathway on copper-cerium-zirconium catalysts for CO2 hydrogenation to methanol using high pressure in situ DRIFTS[J]. Journal of Catalysis, 2020, 382: 129-140. |
| 42 | DASIREDDY Venkata D B C, LIKOZAR Blaž. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity[J]. Renewable Energy, 2019, 140: 452-460. |
| 43 | LEI Hong, HOU Zhaoyin, XIE Jianwei. Hydrogenation of CO2 to CH3OH over CuO/ZnO/Al2O3 catalysts prepared via a solvent-free routine[J]. Fuel, 2016, 164: 191-198. |
| 44 | DOU Maobin, ZHANG Minhua, CHEN Yifei, et al. Theoretical study of methanol synthesis from CO2 and CO hydrogenation on the surface of ZrO2 supported In2O3 catalyst[J]. Surface Science, 2018, 672: 7-12. |
| 45 | WANG Jing, SUN Kaihang, JIA Xinyu, et al. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catalysis Today, 2021, 365: 341-347. |
| 46 | YE Jingyun, LIU Changjun, MEI Donghai, et al. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3(110): A DFT study[J]. ACS Catalysis, 2013, 3(6): 1296-1306. |
| 47 | CHEN Tianyuan, CAO Chenxi, CHEN Tianbao, et al. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In-Zr oxide catalysts[J]. ACS Catalysis, 2019, 9(9): 8785-8797. |
| 48 | CHOU Chenyu, LOBO Raul F. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts[J]. Applied Catalysis A: General, 2019, 583: 117144. |
| 49 | WANG Jijie, TANG Chizhou, LI Guanna, et al. High-performance M a ZrO x (Ma = Cd, Ga) solid-solution catalysts for CO2 hydrogenation to methanol[J]. ACS Catalysis, 2019, 9(11): 10253-10259. |
| 50 | PATIL Tushar, NAJI Arkan, MONDAL Ujjal, et al. Sustainable methanol production from carbon dioxide: Advances, challenges, and future prospects[J]. Environmental Science and Pollution Research International, 2024, 31(32): 44608-44648. |
| 51 | SHULENBERGER Arthur M, JONSSON Fridrik Ragnar, INGOLFSSON Oddur, et al. Process for producing liquid fuel from carbon dioxide and water: US2007244208[P]. 2007-10-18. |
| 52 | ZHOU Zixuan, GAO Peng. Direct carbon dioxide hydrogenation to produce bulk chemicals and liquid fuels via heterogeneous catalysis[J]. Chinese Journal of Catalysis, 2022, 43(8): 2045-2056. |
| 53 | ZHEN Xudong, WANG Yang. An overview of methanol as an internal combustion engine fuel[J]. Renewable and Sustainable Energy Reviews, 2015, 52: 477-493. |
| 54 | CUI Lanyu, WANG Shanshan, GUAN Changge, et al. Breeding of methanol-tolerant methylobacterium extorquens AM1 by atmospheric and room temperature plasma mutagenesis combined with adaptive laboratory evolution[J]. Biotechnology Journal, 2018, 13(6): 1700679. |
| 55 | WESTLAKE Richard. Large-scale continuous production of single cell protein[J]. Chemie Ingenieur Technik, 1986, 58(12): 934-937. |
| 56 | YANG Xueting, ZHENG Zhaojuan, WANG Yu. Bacillus methanolicus: An emerging chassis for low-carbon biomanufacturing[J]. Trends in Biotechnology, 2025, 43(2):274-277. |
| 57 | JAKOBSEN Øyvind M, BENICHOU Aline, FLICKINGER Michael C, et al. Upregulated transcription of plasmid and chromosomal ribulose monophosphate pathway genes is critical for methanol assimilation rate and methanol tolerance in the methylotrophic bacterium Bacillus methanolicus [J]. Journal of Bacteriology, 2006, 188(8): 3063-3072. |
| 58 | CAI Peng, WU Xiaoyan, DENG Jun, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29): e2201711119. |
| 59 | CHEN Frederic Y-H, JUNG Hsin-Wei, TSUEI Chao-Yin, et al. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol[J]. Cell, 2020, 182(4): 933-946.e14. |
| 60 | REITER Michael A, BRADLEY Timothy, BÜCHEL Lars A, et al. A synthetic methylotrophic Escherichia coli as a chassis for bioproduction from methanol[J]. Nature Catalysis, 2024, 7(5): 560-573. |
| 61 | ZHAN Chunjun, LI Xiaowei, LAN Guangxu, et al. Reprogramming methanol utilization pathways to convert Saccharomyces cerevisiae to a synthetic methylotroph[J]. Nature Catalysis, 2023, 6: 435-450. |
| 62 | WITTHOFF Sabrina, SCHMITZ Katja, Sebastian NIEDENFÜHR, et al. Metabolic engineering of Corynebacterium glutamicum for methanol metabolism[J]. Applied and Environmental Microbiology, 2015, 81(6): 2215-2225. |
| 63 | ANTHONY C. Assimilation of carbon by methylotrophs[J]. Biotechnology, 1991, 18: 79-109. |
| 64 | ANTHONY Christopher. How half a century of research was required to understand bacterial growth on C1 and C2 compounds; the story of the serine cycle and the ethylmalonyl-CoA pathway[J]. Science Progress, 2011, 94(Pt 2): 109-137. |
| 65 | KRÜSEMANN Jan L, RAINALDI Vittorio, COTTON Charles AR, et al. The cofactor challenge in synthetic methylotrophy: Bioengineering and industrial applications[J]. Current Opinion in Biotechnology, 2023, 82: 102953. |
| 66 | NAYAK Dipti D, MARX Christopher J. Genetic and phenotypic comparison of facultative methylotrophy between Methylobacterium extorquens strains PA1 and AM1[J]. PLoS One, 2014, 9(9): e107887. |
| 67 | BOZDAG Ahmet, KOMIVES Claire, FLICKINGER Michael C. Growth of Bacillus methanolicus in 2 M methanol at 50 ℃: The effect of high methanol concentration on gene regulation of enzymes involved in formaldehyde detoxification by the ribulose monophosphate pathway[J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(7): 1027-1038. |
| 68 | GUO Yuanke, LIAO Yang, WANG Jing, et al. Methylotrophy of Pichia pastoris: Current advances, applications, and future perspectives for methanol-based biomanufacturing[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(5): 1741-1752. |
| 69 | WANG Yu, FAN Liwen, TUYISHIME Philibert, et al. Synthetic methylotrophy: A practical solution for methanol-based biomanufacturing[J]. Trends in Biotechnology, 2020, 38(6): 650-666. |
| 70 | COTTON Charles AR, CLAASSENS Nico J, Sara BENITO-VAQUERIZO, et al. Renewable methanol and formate as microbial feedstocks[J]. Current Opinion in Biotechnology, 2020, 62: 168-180. |
| 71 | BELKHELFA Sophia, ROCHE David, DUBOIS Ivan, et al. Continuous culture adaptation of Methylobacterium extorquens AM1 and TK 0001 to very high methanol concentrations[J]. Frontiers in Microbiology, 2019, 10: 1313. |
| 72 | MOSER Josef W, PRIELHOFER Roland, GERNER Samuel M, et al. Implications of evolutionary engineering for growth and recombinant protein production in methanol-based growth media in the yeast Pichia pastoris [J]. Microbial Cell Factories, 2017, 16(1): 49. |
| 73 | JIAN Xingjin, GUO Xiaojie, WANG Jia, et al. Microbial microdroplet culture system (MMC): An integrated platform for automated, high-throughput microbial cultivation and adaptive evolution[J]. Biotechnology and Bioengineering, 2020, 117(6): 1724-1737. |
| 74 | FELDMAN M Y. Reactions of nucleic acids and nucleoproteins with formaldehyde[J]. Progress in Nucleic Acid Research and Molecular Biology, 1973, 13: 1-49. |
| 75 | WOOLSTON Benjamin M, KING Jason R, REITER Michael, et al. Improving formaldehyde consumption drives methanol assimilation in engineered E. coli [J]. Nature Communications, 2018, 9(1): 2387. |
| 76 | GAO Jiaoqi, LI Yunxia, YU Wei, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol[J]. Nature Metabolism, 2022, 4(7): 932-943. |
| 77 | Bojana BAJIĆ, Damjan VUČUROVIĆ, Đurđina VASIĆ, et al. Biotechnological production of sustainable microbial proteins from agro-industrial residues and by-products[J]. Foods, 2023, 12(1): 107. |
| 78 | MISHRA Akanksha, NTIHUGA Jean Nepomuscene, MOLITOR Bastian, et al. Power-to-protein: Carbon fixation with renewable electric power to feed the world[J]. Joule, 2020, 4(6): 1142-1147. |
| 79 | MENG Jiao, LIU Shufan, GAO Le, et al. Economical production of Pichia pastoris single cell protein from methanol at industrial pilot scale[J]. Microbial Cell Factories, 2023, 22(1): 198. |
| 80 | GAO Le, MENG Jiao, DAI Wuling, et al. Deciphering cell wall sensors enabling the construction of robust P. pastoris for single-cell protein production[J]. Biotechnology for Biofuels and Bioproducts, 2023, 16(1): 178. |
| 81 | SIMÕES Ana Cristina Pantoja, FERNANDES Rodrigo Pimentel, BARRETO Maysa Silva, et al. Growth of Methylobacterium organophilum in methanol for the simultaneous production of single-cell protein and metabolites of interest[J]. Food Technology and Biotechnology, 2022, 60(3): 338-349. |
| 82 | CARDOSO ALVES Samara, Erick DÍAZ-RUIZ, LISBOA Bruna, et al. Microbial meat: A sustainable vegan protein source produced from agri-waste to feed the world[J]. Food Research International, 2023, 166: 112596. |
| 83 | ALVAREZ-LARIO B, MACARRON-VICENTE J. Uric acid and evolution[J]. Rheumatology, 2010, 49(11): 2010-2015. |
| 84 | MORENO J M, SANCHEZ-MONTERO J M, BALLESTEROS A, et al. Hydrolysis of nucleic acids in single-cell protein concentrates using immobilized benzonase[J]. Applied Biochemistry and Biotechnology, 1991, 31(1): 43-51. |
| 85 | ABOU-ZEID Abou-Zeid A, KHAN Jalaluddin A, ABULNAJA Khalid O. On methods for reduction of nucleic acids content in a single-cell protein from gas oil[J]. Bioresource Technology, 1995, 52(1): 21-24. |
| 86 | LI YU pin, AHMADI Fatemeh, KARIMAN Khalil, et al. Recent advances and challenges in single cell protein (SCP) technologies for food and feed production[J]. NPJ Science of Food, 2024, 8(1): 66. |
| 87 | HARDY Ronald W, PATRO Biswamitra, Catherine PUJOL-BAXLEY, et al. Partial replacement of soybean meal with Methylobacterium extorquens single-cell protein in feeds for rainbow trout (Oncorhynchus mykiss Walbaum)[J]. Aquaculture Research, 2018, 49(6): 2218-2224. |
| 88 | TLUSTY Michael, RHYNE Andrew, SZCZEBAK Joseph T, et al. A transdisciplinary approach to the initial validation of a single cell protein as an alternative protein source for use in aquafeeds[J]. PeerJ, 2017, 5: e3170. |
| 89 | REKDAL Vayu Maini, VAN DER LUIJT Casper R B, CHEN Yan, et al. Edible mycelium bioengineered for enhanced nutritional value and sensory appeal using a modular synthetic biology toolkit[J]. Nature Communications, 2024, 15(1): 2099. |
| [1] | 高建刚, 姜亚鹏, 包宝青, 王书琦, 崔书明. 绿氢转化制绿色甲醇与绿氨[J]. 化工进展, 2025, 44(4): 1987-1997. |
| [2] | 朱国瑜, 葛棋, 付名利. 甲醇重整制氢催化剂耐久性评价和寿命预测方法[J]. 化工进展, 2025, 44(3): 1338-1346. |
| [3] | 左骥, 罗莉, 谢永锴, 陈文尧, 钱刚, 周兴贵, 段学志. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| [4] | 孙悦鹏, 孙延吉, 潘艳秋, 王成宇. 基于BO-LSTM的低温甲醇洗净化气CO2含量预测[J]. 化工进展, 2025, 44(2): 688-697. |
| [5] | 韩英娜, 李丽, 张林子, 安金泽, 李文秀, 张弢. 离子液体萃取精馏分离甲醇-乙腈共沸物[J]. 化工进展, 2025, 44(2): 660-668. |
| [6] | 胡洋, 韩传军, 胡强, 李汶颖, 安全成, 苏洋, 武洪松, 袁果. 固体氧化物燃料电池用甲醇水蒸气重整反应器研究进展[J]. 化工进展, 2025, 44(1): 169-183. |
| [7] | 周渝, 唐甜, 熊子悠, 韦奇. 基于两级微通道分离工艺的甲醇制烯烃废水深度处理[J]. 化工进展, 2025, 44(1): 100-108. |
| [8] | 周渝, 夏太阳, 韦奇, 唐甜, 田磊. 微通道耦合反渗透膜串联处理甲醇制烯烃废水工艺优化[J]. 化工进展, 2024, 43(S1): 43-51. |
| [9] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [10] | 郭鹏, 李红伟, 李贵贤, 季东, 王东亮, 赵新红. 直接甲醇燃料电池阳极催化剂的失活机制及应对策略[J]. 化工进展, 2024, 43(7): 3812-3823. |
| [11] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [12] | 周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738. |
| [13] | 周秋明, 牛丛丛, 吕帅帅, 李红伟, 文富利, 徐润, 李明丰. 通过产物转化分离推动CO2加氢制甲醇过程的研究进展[J]. 化工进展, 2024, 43(5): 2776-2785. |
| [14] | 李海鹏, 吴桐, 王琪, 郜时旺, 王晓龙, 李旭, 高新华, 年佩, 魏逸彬. 透水NaA分子筛膜强化的CO2加氢高效制甲醇[J]. 化工进展, 2024, 43(5): 2834-2842. |
| [15] | 王东亮, 李婧玮, 孟文亮, 杨勇, 周怀荣, 范宗良. 二氧化碳加氢制甲醇过程碳氢利用率的影响因素与工艺优化分析[J]. 化工进展, 2024, 43(5): 2843-2850. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||