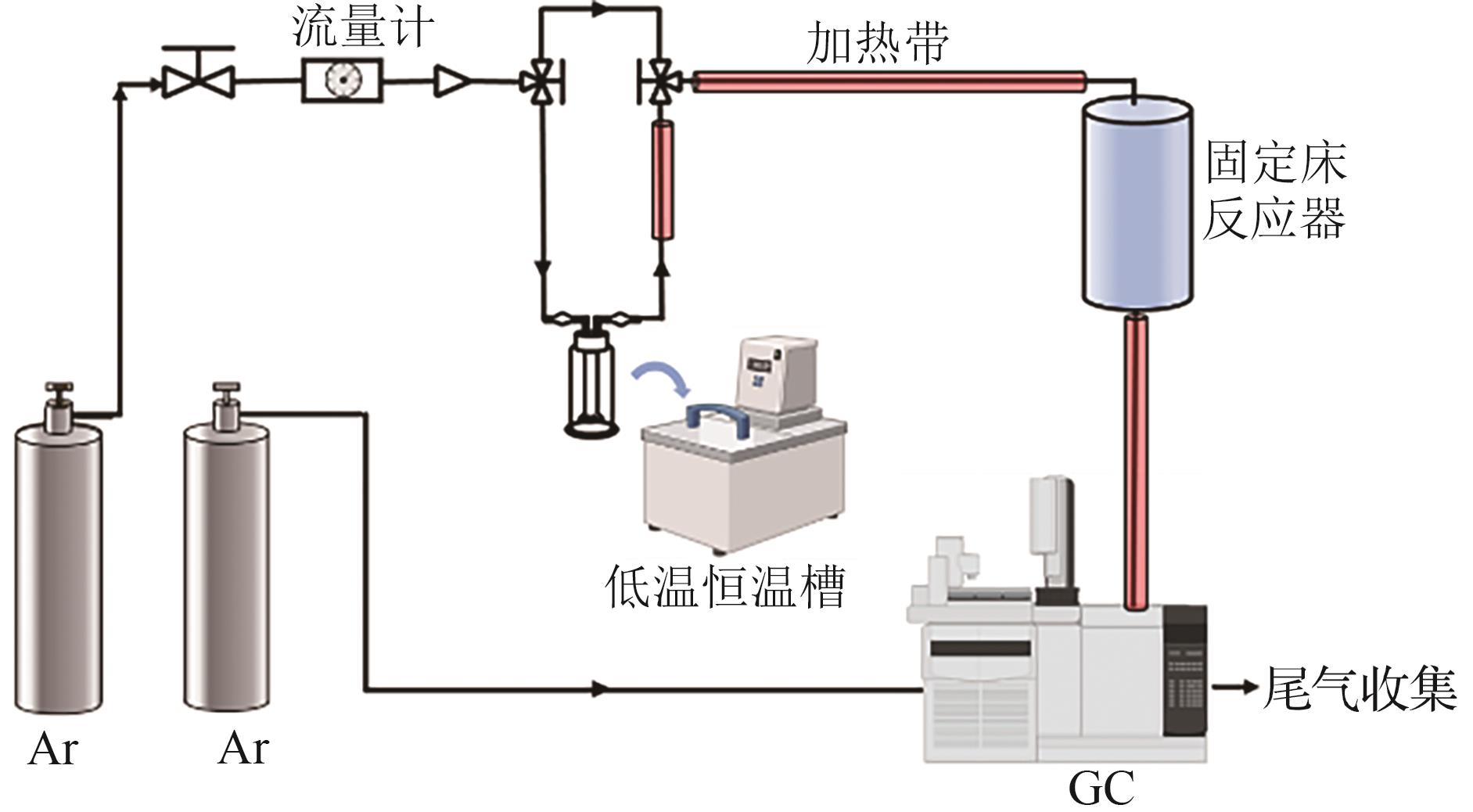
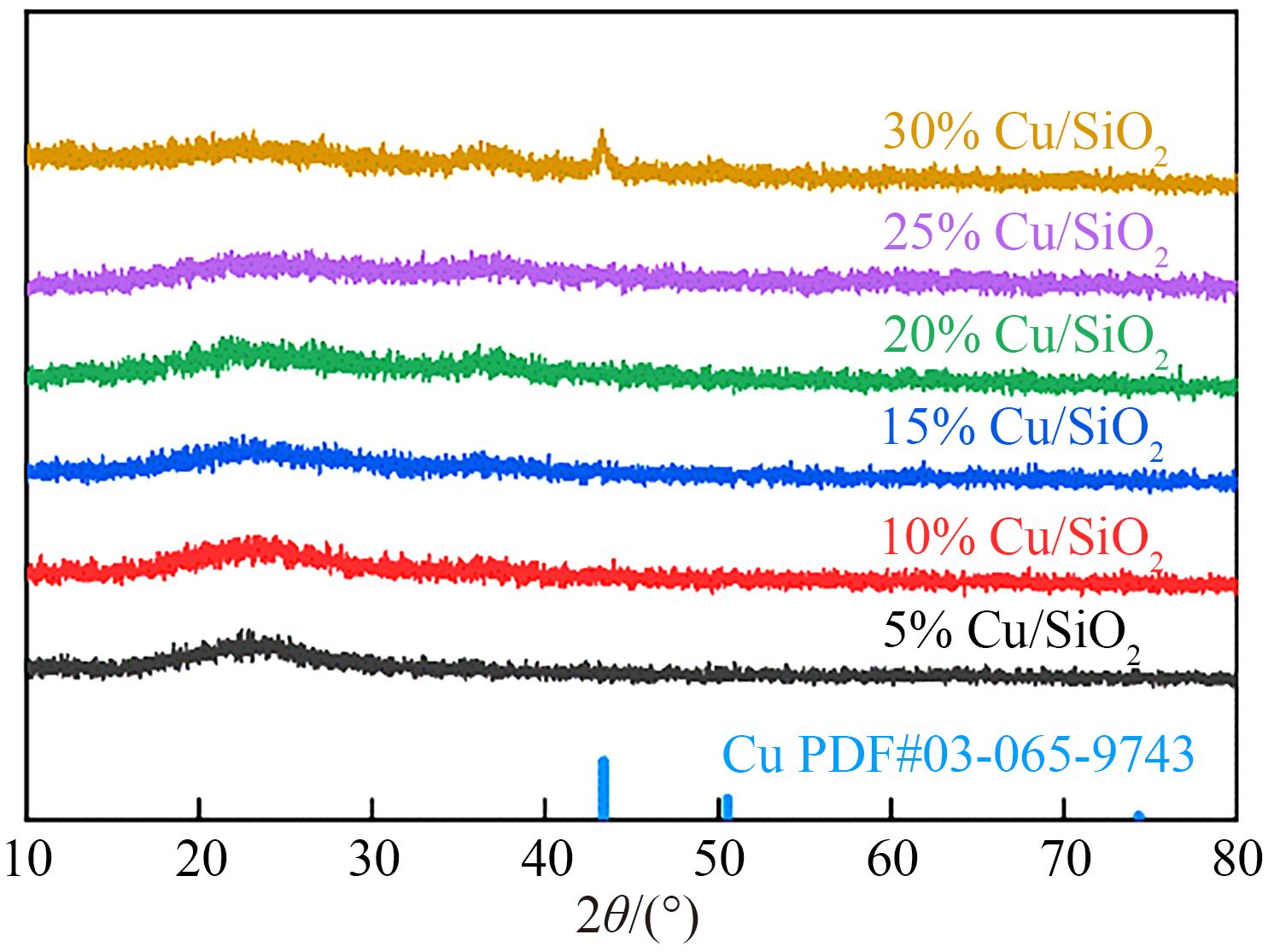
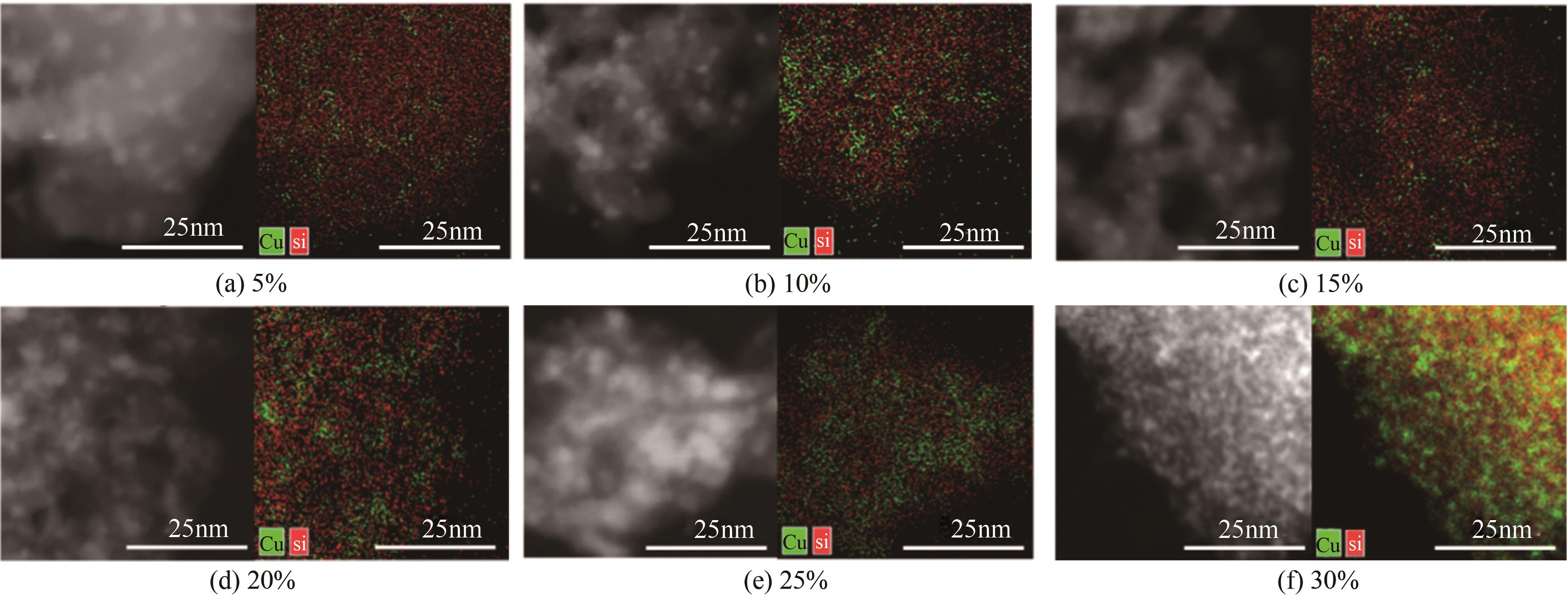
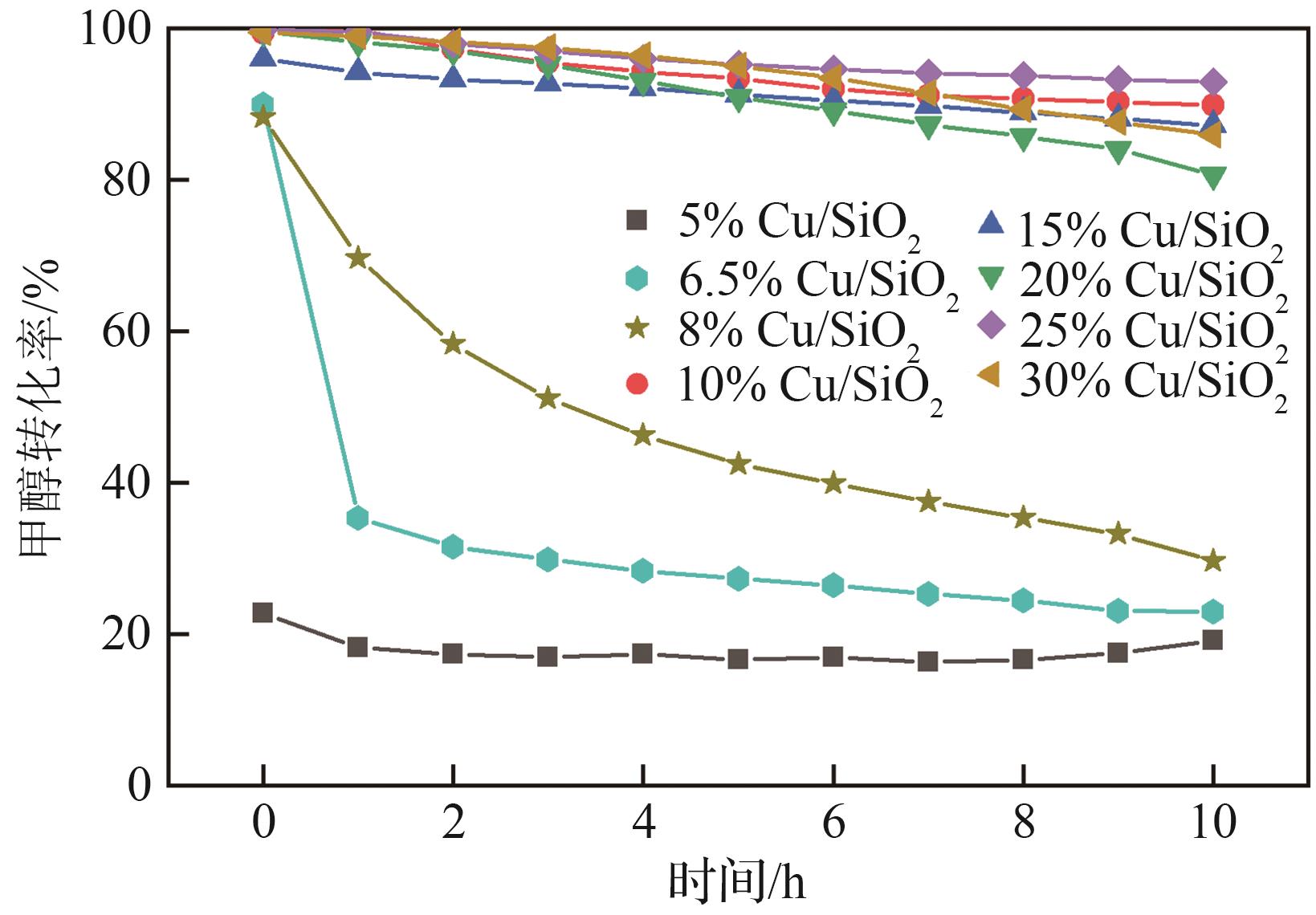
| 1 |
任丽萍, 戴维林, 曹勇, 等. 甲醇脱氢制无水甲醛的高活性Ag-SiO2-Al2O3催化剂[J]. 化学学报, 2003(6): 937-940.
|
|
REN Liping, DAI Weilin, CAO Yong, et al. Highly active Ag-SiO2-Al2O3 catalyst used for the dehydrogenation of methanol to anhydrous formaldehyde[J]. Acta Chimica Sinica, 2003(6): 937-940.
|
| 2 |
SUBARAMANIAN Murugan, PADHY Subarna Sukanya, GOUDA Chandrakanth, et al. Nickel-catalyzed tandem conversion of paraformaldehyde: Methanol to hydrogen and formate/chemo- and stereoselective hydrogenation of alkynes under neutral conditions[J]. Catalysis Science & Technology, 2024, 14(10): 2779-2793.
|
| 3 |
MILLAR Graeme J, MARY Collins. Industrial production of formaldehyde using polycrystalline silver catalyst[J]. Industrial & Engineering Chemistry Research, 2017, 56(33): 9247-9265.
|
| 4 |
THRANE Joachim, MENTZEL Uffe V, THORHAUGE Max, et al. A review and experimental revisit of alternative catalysts for selective oxidation of methanol to formaldehyde[J]. Catalysts, 2021, 11(11): 1329.
|
| 5 |
SU Shoujin, ZAZA Philippe, RENKEN Albert. Catalytic dehydrogenation of methanol to water-free formaldehyde[J]. Chemical Engineering & Technology, 1994, 17(1): 34-40.
|
| 6 |
MERKO Mariia, DELSING Sara, Wilma BUSSER G, et al. Non-oxidative dehydrogenation of methanol to formaldehyde over supported GaO x -based catalysts[J]. Journal of Catalysis, 2023, 427: 115111.
|
| 7 |
LI Han-Jung, LAUSCHE Adam C, PETERSON Andrew A, et al. Using microkinetic analysis to search for novel anhydrous formaldehyde production catalysts[J]. Surface Science, 2015, 641: 105-111.
|
| 8 |
SHAN Junjun, LUCCI Felicia R, LIU Jilei, et al. Water co-catalyzed selective dehydrogenation of methanol to formaldehyde and hydrogen[J]. Surface Science, 2016, 650: 121-129.
|
| 9 |
Ya USACHEV N, KRUKOVSKII I M, KANAEV S A. The nonoxidative methanol dehydrogenation to formaldehyde (A review)[J]. Petroleum Chemistry, 2004, 44(6): 379-394.
|
| 10 |
ZHANG Hongwei, TAN Huiru, JAENICKE Stephan, et al. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen[J]. Journal of Catalysis, 2020, 389: 19-28.
|
| 11 |
KRESSE G, HAFNER J. Ab initio molecular dynamics for liquid metals[J]. Physical Review B, 1993, 47(1): 558-561.
|
| 12 |
KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50.
|
| 13 |
KRESSE G, HAFNER J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium[J]. Physical Review B, 1994, 49(20): 14251-14269.
|
 ), 罗莉, 谢永锴, 陈文尧(
), 罗莉, 谢永锴, 陈文尧( ), 钱刚, 周兴贵, 段学志(
), 钱刚, 周兴贵, 段学志( )
)
 ), LUO Li, XIE Yongkai, CHEN Wenyao(
), LUO Li, XIE Yongkai, CHEN Wenyao( ), QIAN Gang, ZHOU Xinggui, DUAN Xuezhi(
), QIAN Gang, ZHOU Xinggui, DUAN Xuezhi( )
)