化工进展 ›› 2025, Vol. 44 ›› Issue (2): 660-668.DOI: 10.16085/j.issn.1000-6613.2024-0197
离子液体萃取精馏分离甲醇-乙腈共沸物
韩英娜( ), 李丽, 张林子, 安金泽, 李文秀, 张弢(
), 李丽, 张林子, 安金泽, 李文秀, 张弢( )
)
- 沈阳化工大学辽宁省化工分离技术重点实验室,辽宁 沈阳 110142
-
收稿日期:2024-01-26修回日期:2024-05-21出版日期:2025-02-25发布日期:2025-03-10 -
通讯作者:张弢 -
作者简介:韩英娜(1996—),女,硕士研究生,研究方向为化工传质与分离。E-mail:2381573793@qq.com。 -
基金资助:国家自然科学基金(22278272)
Separation of methanol-acetonitrile azeotrope by ionic liquid extractive distillation
HAN Yingna( ), LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao(
), LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao( )
)
- Liaoning Province Key Laboratory of Chemical Separation Technology, Shenyang University of Chemical Technology, Shenyang 110142, Liaoning, China
-
Received:2024-01-26Revised:2024-05-21Online:2025-02-25Published:2025-03-10 -
Contact:ZHANG Tao
摘要:
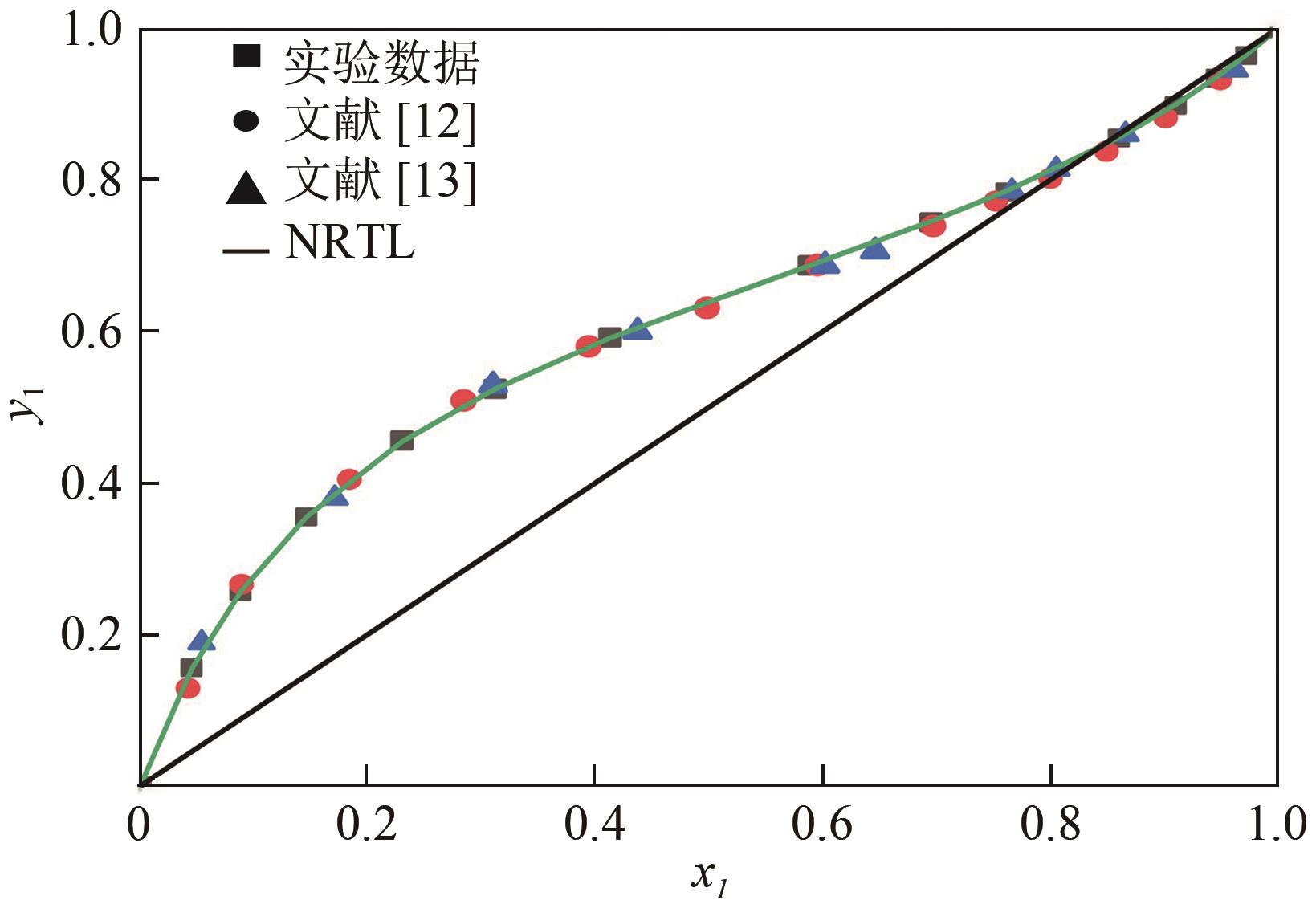
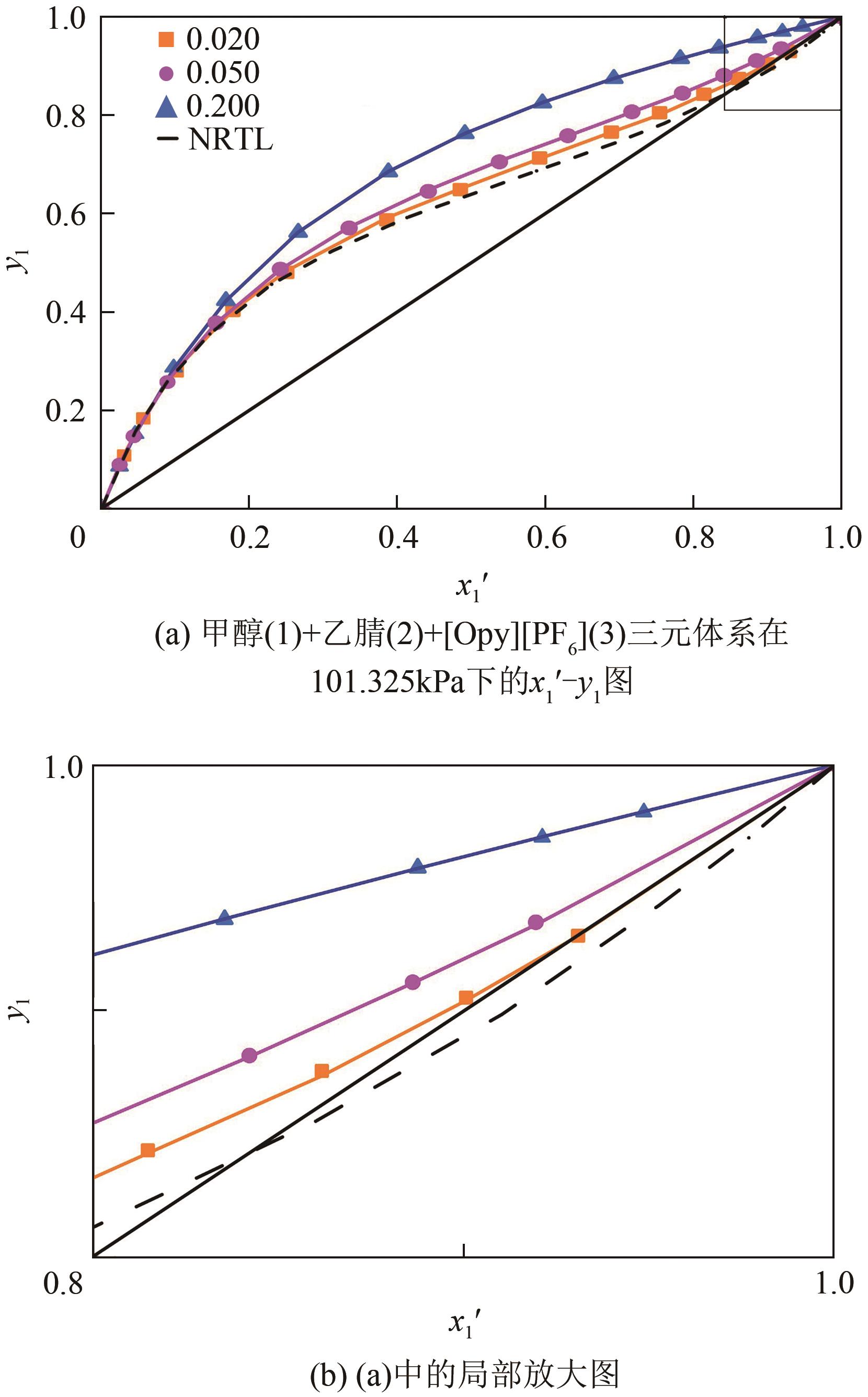
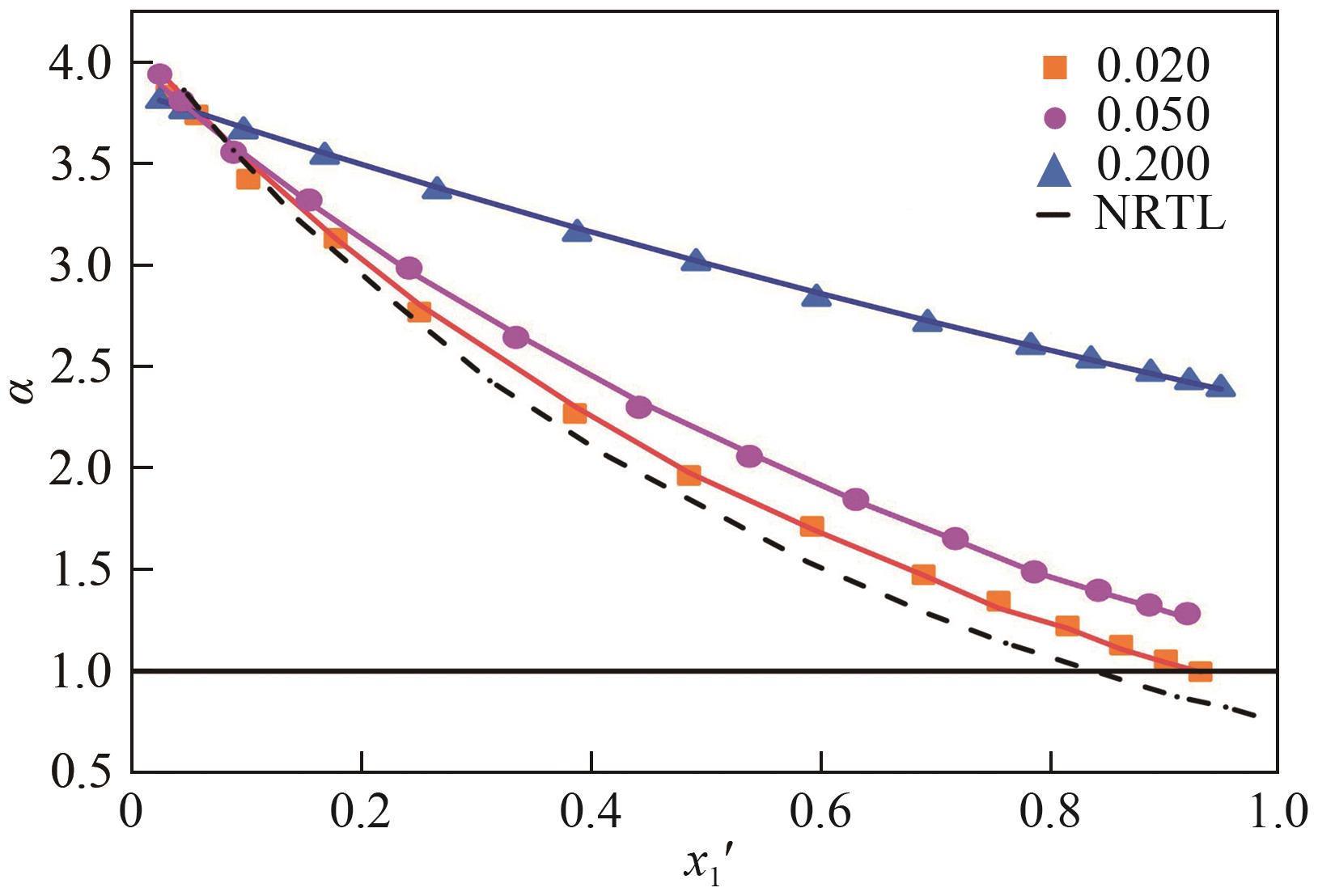
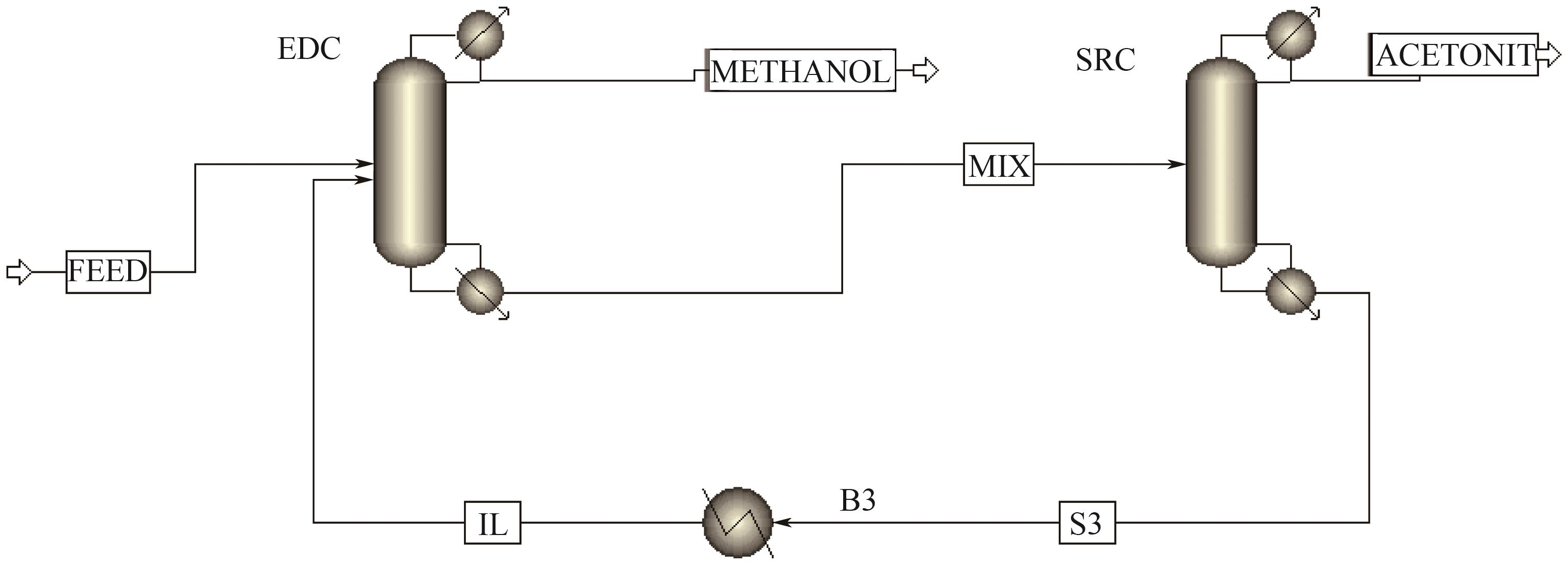
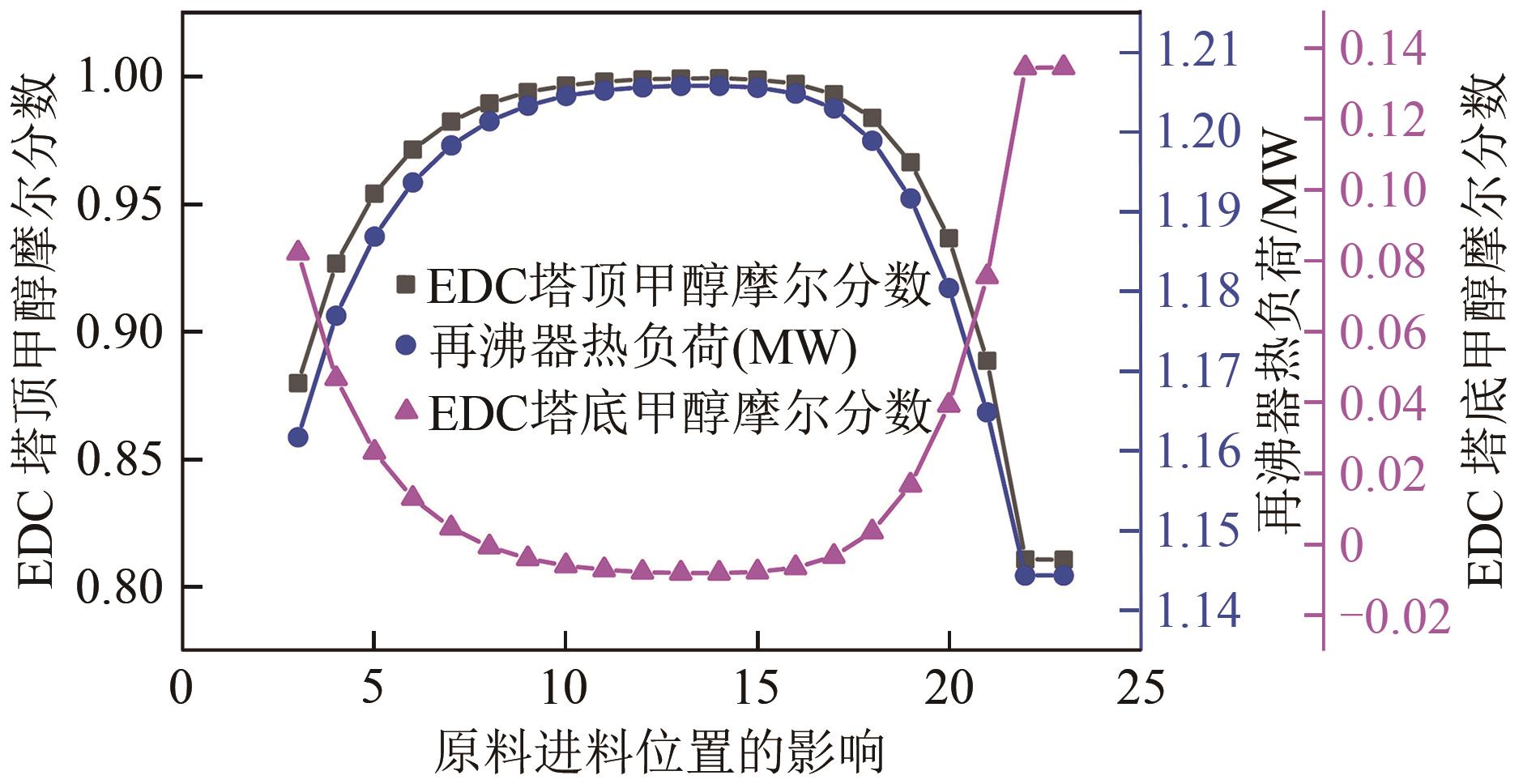
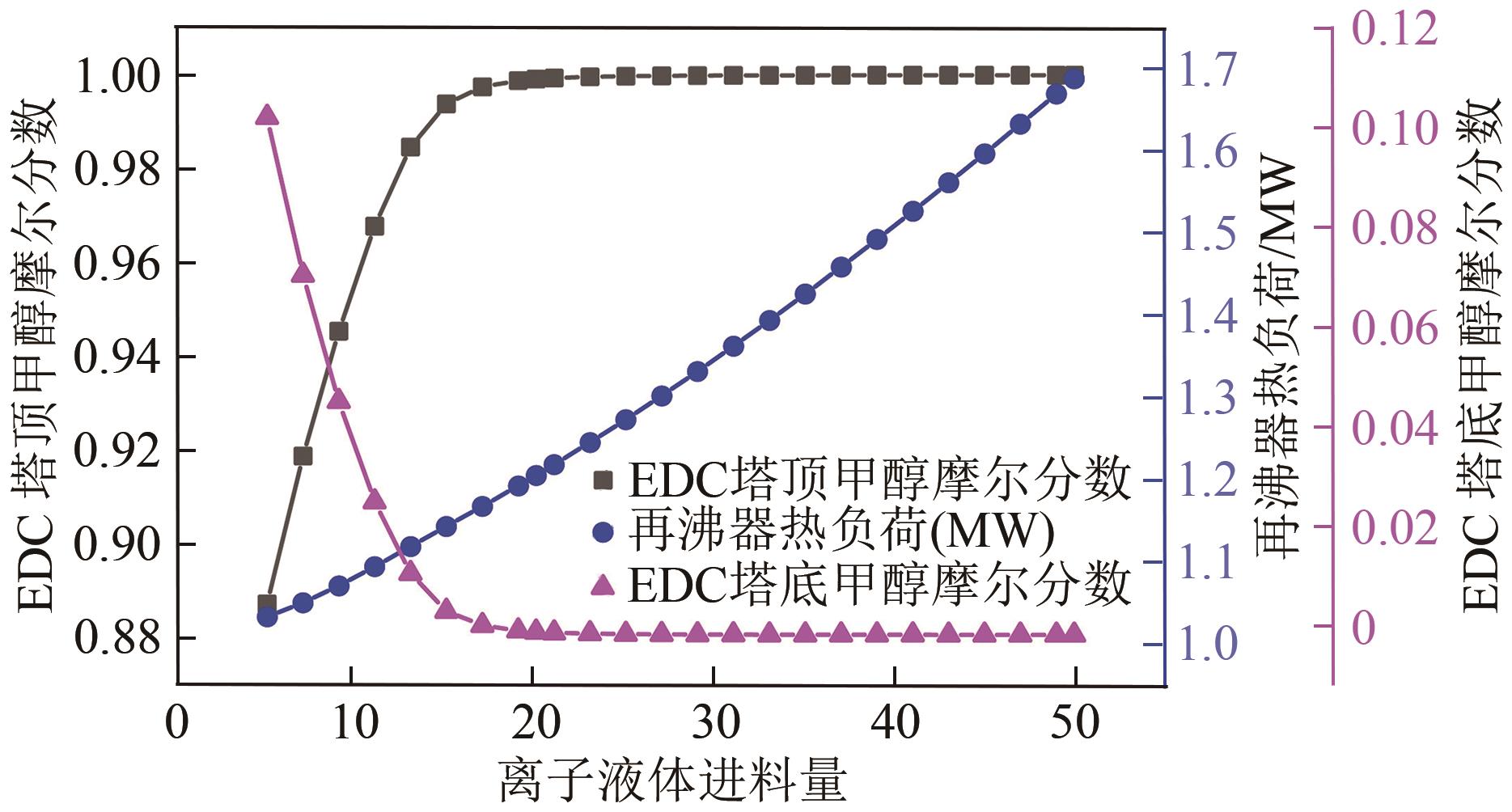
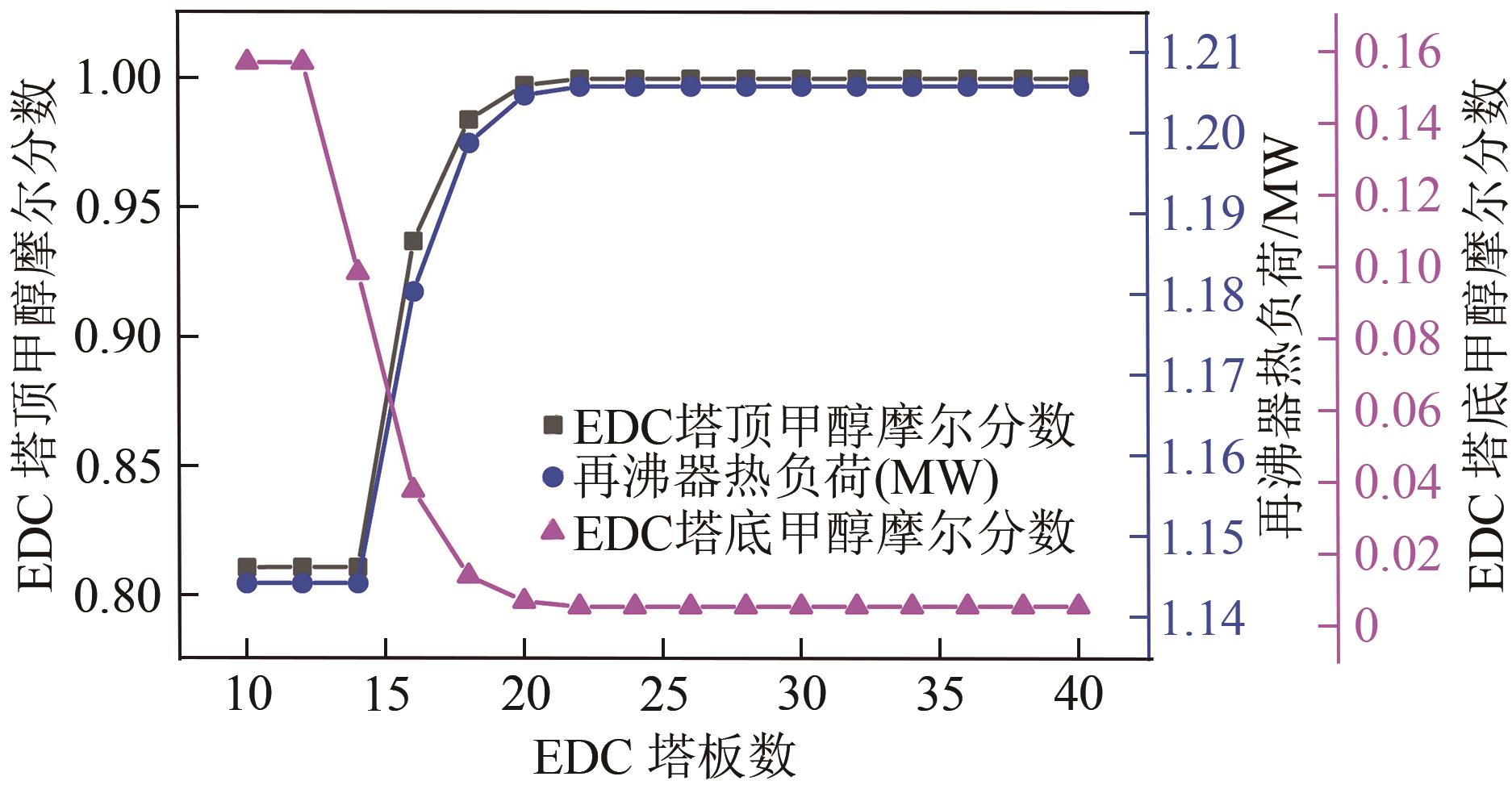
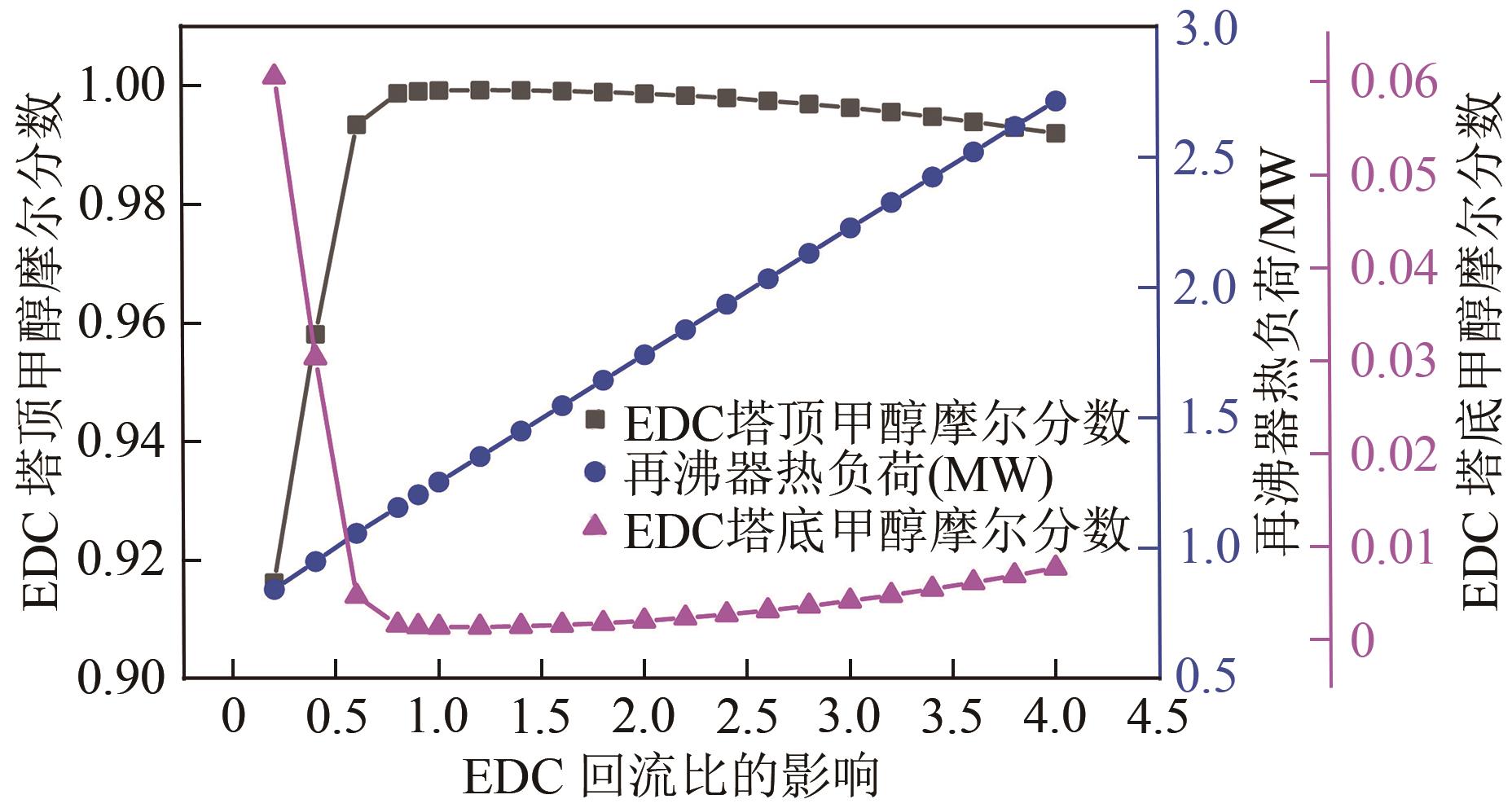
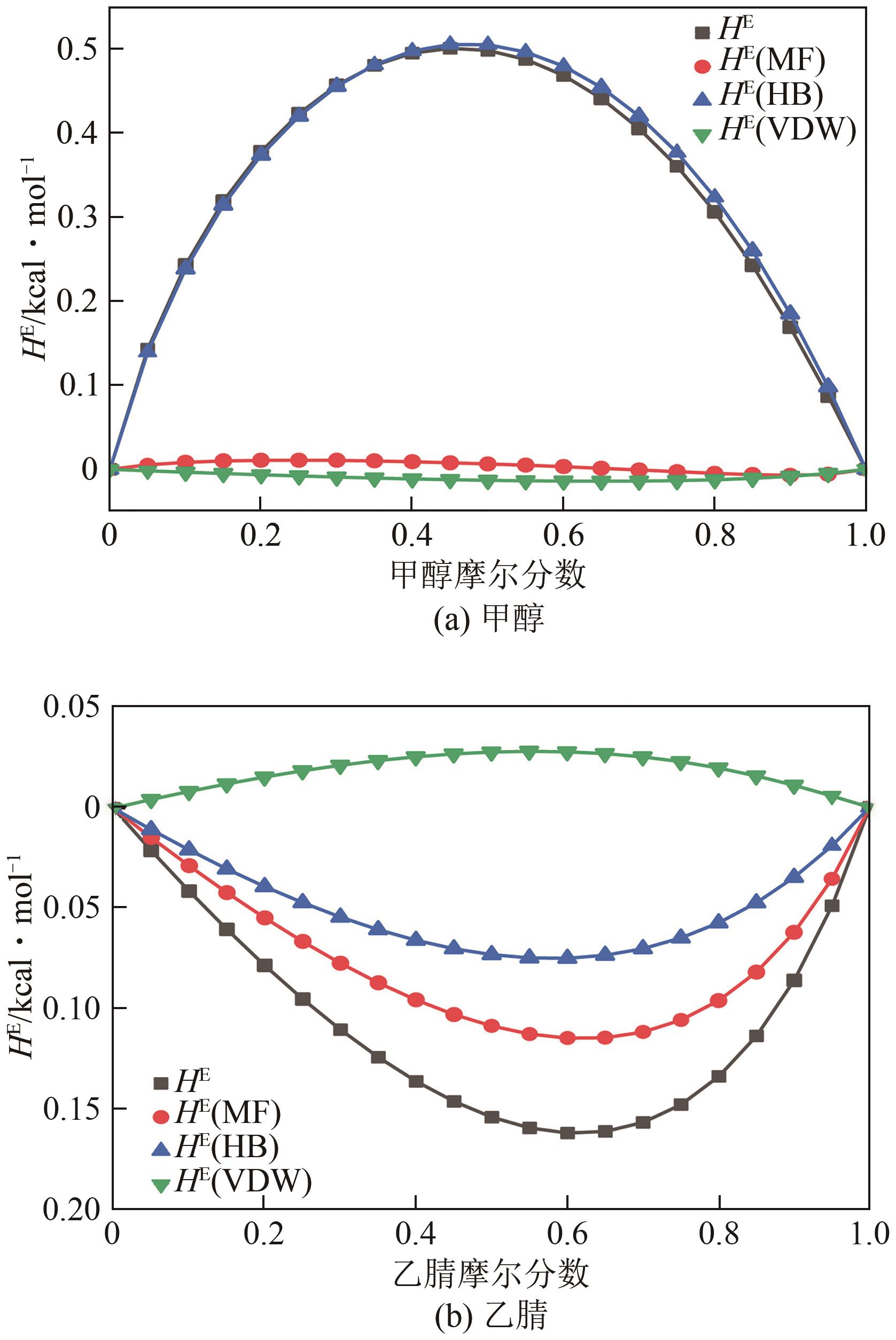
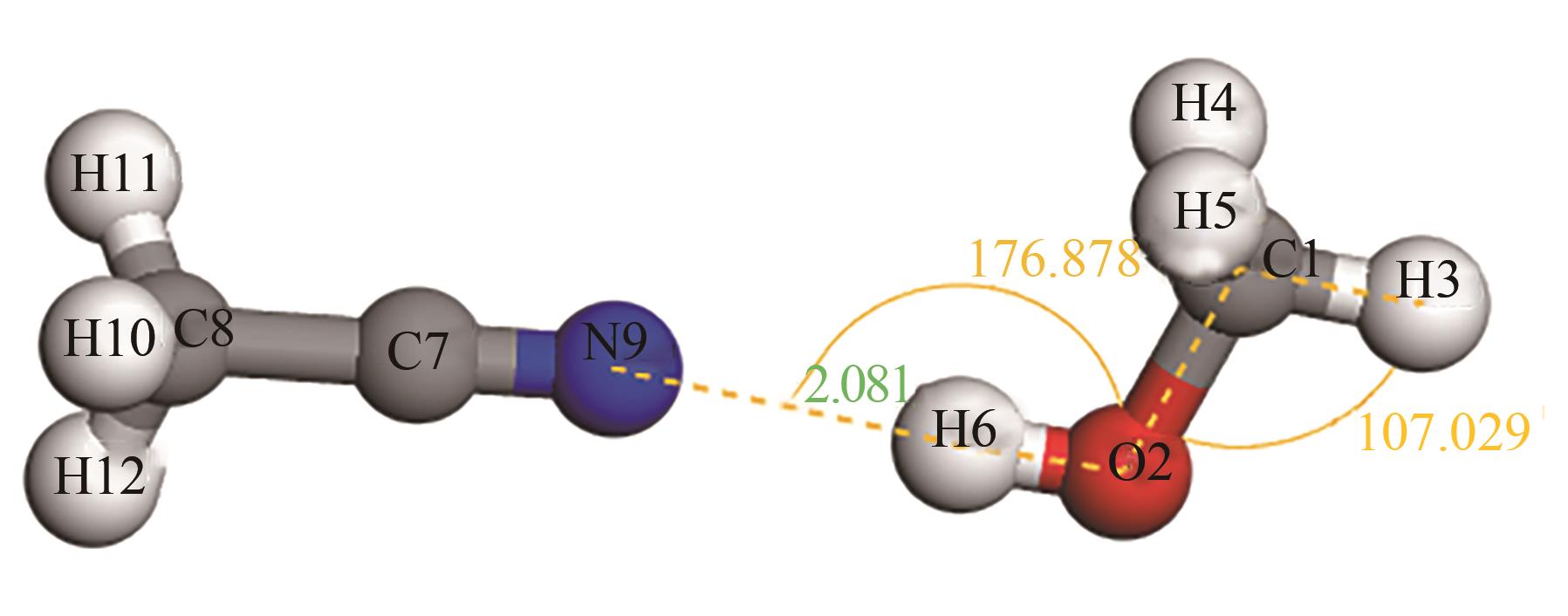
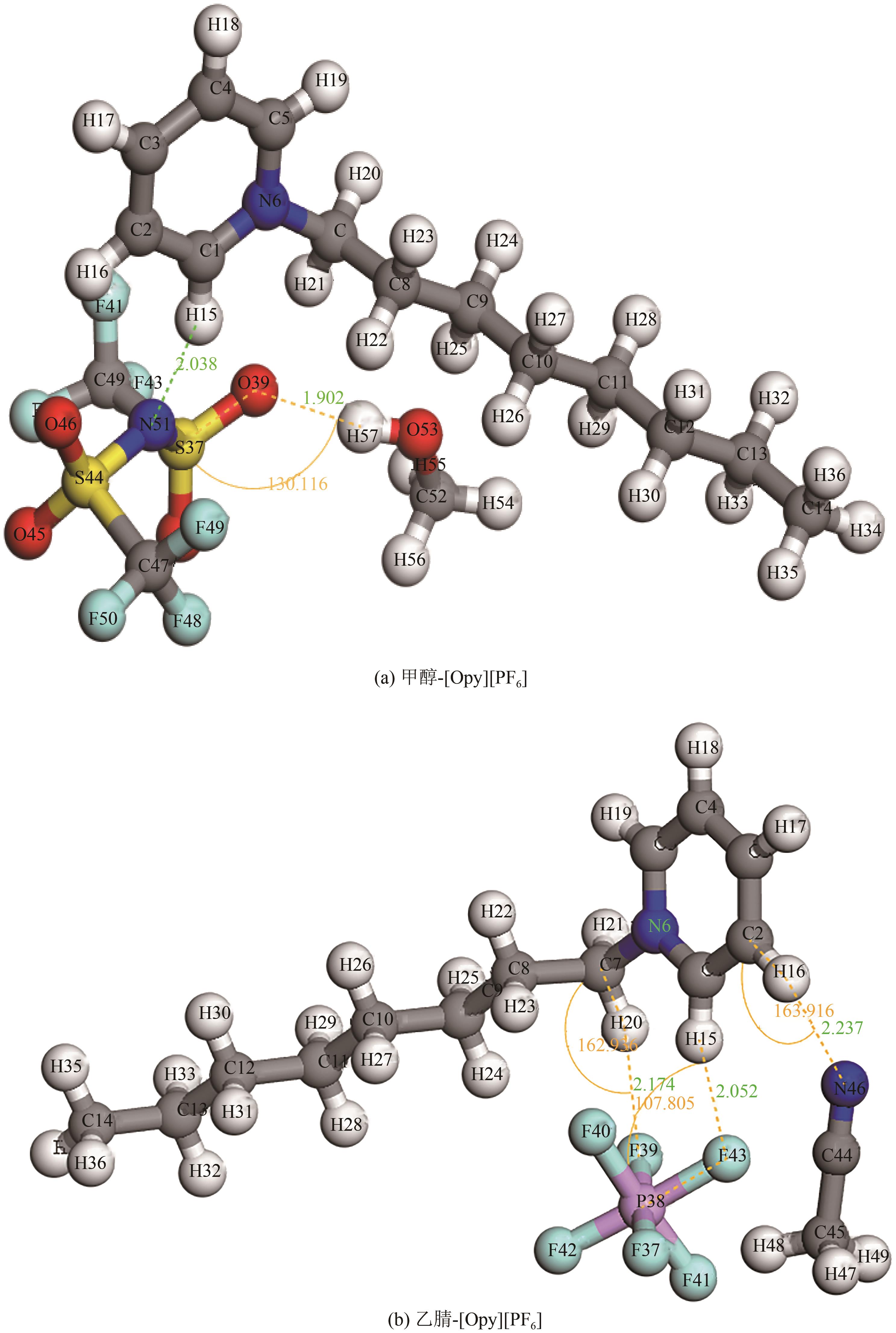
为实现甲醇-乙腈共沸体系的高效分离,本文选取N-辛基吡啶六氟磷酸盐([Opy][PF6])为萃取剂,在101.325kPa下对甲醇-乙腈-[Opy][PF6]三元物系进行等压气液相平衡的测定,获得了三元体系等压气液相平衡(VLE)数据,探究离子液体对甲醇-乙腈气液相平衡的影响,将实验数据与非随机双液(NRTL)模型相关联,得到二元相互作用参数。通过过量焓、高斯分析解释分离机理,结果表明[Opy][PF6]的加入可提高甲醇相对于乙腈的相对挥发度,[Opy][PF6]的含量增加使盐析效果更明显,NRTL模型与实验结果具有良好的一致性,通过Aspen Plus软件中的NRTL模型关联这些数据,发现当[Opy][PF6]的物质的量分数为0.0345时,体系不再共沸,使用Aspen Plus软件模拟萃取精馏流程并优化工艺参数,得到萃取精馏过程的最佳操作条件。模拟结果可作为下一步萃取精馏实验和流程设计的指导。
中图分类号:
引用本文
韩英娜, 李丽, 张林子, 安金泽, 李文秀, 张弢. 离子液体萃取精馏分离甲醇-乙腈共沸物[J]. 化工进展, 2025, 44(2): 660-668.
HAN Yingna, LI Li, ZHANG Linzi, AN Jinze, LI Wenxiu, ZHANG Tao. Separation of methanol-acetonitrile azeotrope by ionic liquid extractive distillation[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 660-668.
| T/K | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 338.00 | 0.972 | 0.964 | 0.980 | 2.229 |
| 337.90 | 0.946 | 0.934 | 0.979 | 2.136 |
| 337.60 | 0.910 | 0.898 | 0.991 | 1.994 |
| 337.30 | 0.860 | 0.855 | 1.010 | 1.846 |
| 337.20 | 0.761 | 0.784 | 1.050 | 1.620 |
| 337.50 | 0.695 | 0.744 | 1.079 | 1.483 |
| 338.00 | 0.588 | 0.687 | 1.154 | 1.322 |
| 339.70 | 0.413 | 0.592 | 1.325 | 1.140 |
| 341.10 | 0.312 | 0.524 | 1.471 | 1.082 |
| 342.90 | 0.230 | 0.456 | 1.621 | 1.039 |
| 345.40 | 0.146 | 0.355 | 1.807 | 1.022 |
| 348.10 | 0.088 | 0.257 | 1.954 | 1.009 |
| 351.00 | 0.045 | 0.156 | 2.067 | 0.998 |
表1 101.325kPa甲醇(1)-乙腈(2)二元体系的等压VLE数据
| T/K | x1 | y1 | γ1 | γ2 |
|---|---|---|---|---|
| 338.00 | 0.972 | 0.964 | 0.980 | 2.229 |
| 337.90 | 0.946 | 0.934 | 0.979 | 2.136 |
| 337.60 | 0.910 | 0.898 | 0.991 | 1.994 |
| 337.30 | 0.860 | 0.855 | 1.010 | 1.846 |
| 337.20 | 0.761 | 0.784 | 1.050 | 1.620 |
| 337.50 | 0.695 | 0.744 | 1.079 | 1.483 |
| 338.00 | 0.588 | 0.687 | 1.154 | 1.322 |
| 339.70 | 0.413 | 0.592 | 1.325 | 1.140 |
| 341.10 | 0.312 | 0.524 | 1.471 | 1.082 |
| 342.90 | 0.230 | 0.456 | 1.621 | 1.039 |
| 345.40 | 0.146 | 0.355 | 1.807 | 1.022 |
| 348.10 | 0.088 | 0.257 | 1.954 | 1.009 |
| 351.00 | 0.045 | 0.156 | 2.067 | 0.998 |
| T/K | x3 | x1 | x1ʹ | y1 | α12 | γ1 | γ2 |
|---|---|---|---|---|---|---|---|
| 338.35 | 0.200 | 0.759 | 0.948 | 0.981 | 2.392 | 1.264 | 0.805 |
| 338.35 | 0.200 | 0.737 | 0.921 | 0.970 | 2.426 | 1.293 | 0.809 |
| 338.15 | 0.200 | 0.710 | 0.887 | 0.958 | 2.468 | 1.330 | 0.809 |
| 338.05 | 0.200 | 0.668 | 0.835 | 0.937 | 2.533 | 1.382 | 0.830 |
| 338.15 | 0.200 | 0.626 | 0.783 | 0.916 | 2.599 | 1.441 | 0.841 |
| 338.45 | 0.200 | 0.555 | 0.693 | 0.876 | 2.713 | 1.544 | 0.875 |
| 338.75 | 0.200 | 0.477 | 0.596 | 0.826 | 2.836 | 1.666 | 0.919 |
| 339.75 | 0.200 | 0.393 | 0.492 | 0.763 | 3.011 | 1.804 | 0.961 |
| 340.95 | 0.200 | 0.311 | 0.388 | 0.685 | 3.156 | 1.951 | 1.014 |
| 342.85 | 0.200 | 0.213 | 0.266 | 0.562 | 3.366 | 2.146 | 1.092 |
| 345.85 | 0.200 | 0.135 | 0.168 | 0.423 | 3.536 | 2.291 | 1.152 |
| 349.65 | 0.200 | 0.078 | 0.098 | 0.287 | 3.661 | 2.343 | 1.167 |
| 352.75 | 0.200 | 0.036 | 0.045 | 0.152 | 3.761 | 2.377 | 1.187 |
| 353.65 | 0.200 | 0.020 | 0.024 | 0.085 | 3.809 | 2.420 | 1.223 |
| 338.35 | 0.050 | 0.873 | 0.919 | 0.936 | 1.283 | 1.044 | 1.436 |
| 338.35 | 0.050 | 0.842 | 0.886 | 0.912 | 1.326 | 1.055 | 1.404 |
| 338.35 | 0.049 | 0.801 | 0.842 | 0.882 | 1.399 | 1.073 | 1.353 |
| 338.35 | 0.048 | 0.748 | 0.786 | 0.845 | 1.489 | 1.101 | 1.305 |
| 338.45 | 0.050 | 0.681 | 0.717 | 0.808 | 1.653 | 1.150 | 1.228 |
| 338.85 | 0.050 | 0.599 | 0.631 | 0.759 | 1.847 | 1.211 | 1.160 |
| 339.45 | 0.051 | 0.511 | 0.538 | 0.706 | 2.059 | 1.290 | 1.111 |
| 340.55 | 0.050 | 0.420 | 0.442 | 0.646 | 2.301 | 1.375 | 1.065 |
| 342.15 | 0.051 | 0.318 | 0.335 | 0.571 | 2.645 | 1.511 | 1.026 |
| 344.45 | 0.049 | 0.230 | 0.242 | 0.488 | 2.986 | 1.635 | 0.993 |
| 347.55 | 0.050 | 0.147 | 0.155 | 0.378 | 3.322 | 1.766 | 0.977 |
| 350.85 | 0.050 | 0.084 | 0.089 | 0.257 | 3.556 | 1.856 | 0.972 |
| 353.95 | 0.049 | 0.041 | 0.043 | 0.147 | 3.809 | 1.944 | 0.963 |
| 355.55 | 0.050 | 0.023 | 0.024 | 0.089 | 3.940 | 1.992 | 0.960 |
| 337.55 | 0.021 | 0.911 | 0.931 | 0.930 | 0.996 | 1.026 | 1.812 |
| 337.35 | 0.021 | 0.882 | 0.901 | 0.905 | 1.056 | 1.040 | 1.731 |
| 337.25 | 0.020 | 0.844 | 0.862 | 0.875 | 1.129 | 1.055 | 1.642 |
| 337.25 | 0.023 | 0.796 | 0.815 | 0.843 | 1.223 | 1.077 | 1.547 |
| 337.35 | 0.020 | 0.740 | 0.755 | 0.806 | 1.345 | 1.104 | 1.442 |
| 337.65 | 0.023 | 0.674 | 0.690 | 0.766 | 1.475 | 1.139 | 1.358 |
| 338.15 | 0.022 | 0.579 | 0.593 | 0.714 | 1.713 | 1.210 | 1.245 |
| 339.05 | 0.020 | 0.476 | 0.485 | 0.649 | 1.963 | 1.294 | 1.167 |
| 340.15 | 0.022 | 0.378 | 0.386 | 0.588 | 2.269 | 1.414 | 1.109 |
| 342.15 | 0.021 | 0.245 | 0.251 | 0.481 | 2.769 | 1.649 | 1.069 |
| 344.65 | 0.022 | 0.174 | 0.178 | 0.403 | 3.132 | 1.777 | 1.030 |
| 348.35 | 0.021 | 0.100 | 0.102 | 0.279 | 3.423 | 1.869 | 1.007 |
| 351.45 | 0.022 | 0.055 | 0.057 | 0.183 | 3.741 | 1.971 | 0.984 |
| 353.15 | 0.021 | 0.030 | 0.031 | 0.108 | 3.842 | 2.022 | 0.990 |
表2 101.325kPa下甲醇(1)+乙腈(2)+[Opy][PF6](3)的VLE数据
| T/K | x3 | x1 | x1ʹ | y1 | α12 | γ1 | γ2 |
|---|---|---|---|---|---|---|---|
| 338.35 | 0.200 | 0.759 | 0.948 | 0.981 | 2.392 | 1.264 | 0.805 |
| 338.35 | 0.200 | 0.737 | 0.921 | 0.970 | 2.426 | 1.293 | 0.809 |
| 338.15 | 0.200 | 0.710 | 0.887 | 0.958 | 2.468 | 1.330 | 0.809 |
| 338.05 | 0.200 | 0.668 | 0.835 | 0.937 | 2.533 | 1.382 | 0.830 |
| 338.15 | 0.200 | 0.626 | 0.783 | 0.916 | 2.599 | 1.441 | 0.841 |
| 338.45 | 0.200 | 0.555 | 0.693 | 0.876 | 2.713 | 1.544 | 0.875 |
| 338.75 | 0.200 | 0.477 | 0.596 | 0.826 | 2.836 | 1.666 | 0.919 |
| 339.75 | 0.200 | 0.393 | 0.492 | 0.763 | 3.011 | 1.804 | 0.961 |
| 340.95 | 0.200 | 0.311 | 0.388 | 0.685 | 3.156 | 1.951 | 1.014 |
| 342.85 | 0.200 | 0.213 | 0.266 | 0.562 | 3.366 | 2.146 | 1.092 |
| 345.85 | 0.200 | 0.135 | 0.168 | 0.423 | 3.536 | 2.291 | 1.152 |
| 349.65 | 0.200 | 0.078 | 0.098 | 0.287 | 3.661 | 2.343 | 1.167 |
| 352.75 | 0.200 | 0.036 | 0.045 | 0.152 | 3.761 | 2.377 | 1.187 |
| 353.65 | 0.200 | 0.020 | 0.024 | 0.085 | 3.809 | 2.420 | 1.223 |
| 338.35 | 0.050 | 0.873 | 0.919 | 0.936 | 1.283 | 1.044 | 1.436 |
| 338.35 | 0.050 | 0.842 | 0.886 | 0.912 | 1.326 | 1.055 | 1.404 |
| 338.35 | 0.049 | 0.801 | 0.842 | 0.882 | 1.399 | 1.073 | 1.353 |
| 338.35 | 0.048 | 0.748 | 0.786 | 0.845 | 1.489 | 1.101 | 1.305 |
| 338.45 | 0.050 | 0.681 | 0.717 | 0.808 | 1.653 | 1.150 | 1.228 |
| 338.85 | 0.050 | 0.599 | 0.631 | 0.759 | 1.847 | 1.211 | 1.160 |
| 339.45 | 0.051 | 0.511 | 0.538 | 0.706 | 2.059 | 1.290 | 1.111 |
| 340.55 | 0.050 | 0.420 | 0.442 | 0.646 | 2.301 | 1.375 | 1.065 |
| 342.15 | 0.051 | 0.318 | 0.335 | 0.571 | 2.645 | 1.511 | 1.026 |
| 344.45 | 0.049 | 0.230 | 0.242 | 0.488 | 2.986 | 1.635 | 0.993 |
| 347.55 | 0.050 | 0.147 | 0.155 | 0.378 | 3.322 | 1.766 | 0.977 |
| 350.85 | 0.050 | 0.084 | 0.089 | 0.257 | 3.556 | 1.856 | 0.972 |
| 353.95 | 0.049 | 0.041 | 0.043 | 0.147 | 3.809 | 1.944 | 0.963 |
| 355.55 | 0.050 | 0.023 | 0.024 | 0.089 | 3.940 | 1.992 | 0.960 |
| 337.55 | 0.021 | 0.911 | 0.931 | 0.930 | 0.996 | 1.026 | 1.812 |
| 337.35 | 0.021 | 0.882 | 0.901 | 0.905 | 1.056 | 1.040 | 1.731 |
| 337.25 | 0.020 | 0.844 | 0.862 | 0.875 | 1.129 | 1.055 | 1.642 |
| 337.25 | 0.023 | 0.796 | 0.815 | 0.843 | 1.223 | 1.077 | 1.547 |
| 337.35 | 0.020 | 0.740 | 0.755 | 0.806 | 1.345 | 1.104 | 1.442 |
| 337.65 | 0.023 | 0.674 | 0.690 | 0.766 | 1.475 | 1.139 | 1.358 |
| 338.15 | 0.022 | 0.579 | 0.593 | 0.714 | 1.713 | 1.210 | 1.245 |
| 339.05 | 0.020 | 0.476 | 0.485 | 0.649 | 1.963 | 1.294 | 1.167 |
| 340.15 | 0.022 | 0.378 | 0.386 | 0.588 | 2.269 | 1.414 | 1.109 |
| 342.15 | 0.021 | 0.245 | 0.251 | 0.481 | 2.769 | 1.649 | 1.069 |
| 344.65 | 0.022 | 0.174 | 0.178 | 0.403 | 3.132 | 1.777 | 1.030 |
| 348.35 | 0.021 | 0.100 | 0.102 | 0.279 | 3.423 | 1.869 | 1.007 |
| 351.45 | 0.022 | 0.055 | 0.057 | 0.183 | 3.741 | 1.971 | 0.984 |
| 353.15 | 0.021 | 0.030 | 0.031 | 0.108 | 3.842 | 2.022 | 0.990 |
| 组分i | 组分j | αij | (Δgij /R) /J·mol-1 | (Δgji /R) /J·mol-1 | ARD/% |
|---|---|---|---|---|---|
| 甲醇 | 乙腈 | 0.4950 | 171.70 | 166.84 | 0.98 |
| 甲醇 | [Opy][PF6] | 0.1000 | -240.33 | -21.77 | |
| 乙腈 | [Opy][PF6] | 0.1926 | 856.77 | -1233.94 |
表3 NRTL模型的参数和相关统计
| 组分i | 组分j | αij | (Δgij /R) /J·mol-1 | (Δgji /R) /J·mol-1 | ARD/% |
|---|---|---|---|---|---|
| 甲醇 | 乙腈 | 0.4950 | 171.70 | 166.84 | 0.98 |
| 甲醇 | [Opy][PF6] | 0.1000 | -240.33 | -21.77 | |
| 乙腈 | [Opy][PF6] | 0.1926 | 856.77 | -1233.94 |
| M | Tb/K | Tc/K | Pc/bar | Vc/mL·mol-1 | ω |
|---|---|---|---|---|---|
| 337.30 | 618.40 | 777.30 | 14.04 | 967.10 | 0.9238 |
表4 [Opy][PF6]的物性参数
| M | Tb/K | Tc/K | Pc/bar | Vc/mL·mol-1 | ω |
|---|---|---|---|---|---|
| 337.30 | 618.40 | 777.30 | 14.04 | 967.10 | 0.9238 |
| 甲醇 | EA/hartree | EB/hartree | EAB/hartree | EBSSE/hartree | ΔE/hartree |
|---|---|---|---|---|---|
| 甲醇(A)-乙腈(B) | -115.765 | -132.796 | -248.568 | 4.676×10-4 | -7.054×10-3 |
| 甲醇(A)-[Opy][PF6] | -2391.049 | -115.764 | -2506.825 | 1.368×10-3 | -9.841×10-3 |
| 乙腈(A)-[Opy][PF6] | -1504.337 | -132.796 | -1637.150 | 1.064×10-3 | -1.634×10-2 |
表5 组分间的相互作用能
| 甲醇 | EA/hartree | EB/hartree | EAB/hartree | EBSSE/hartree | ΔE/hartree |
|---|---|---|---|---|---|
| 甲醇(A)-乙腈(B) | -115.765 | -132.796 | -248.568 | 4.676×10-4 | -7.054×10-3 |
| 甲醇(A)-[Opy][PF6] | -2391.049 | -115.764 | -2506.825 | 1.368×10-3 | -9.841×10-3 |
| 乙腈(A)-[Opy][PF6] | -1504.337 | -132.796 | -1637.150 | 1.064×10-3 | -1.634×10-2 |
| 1 | WANG Yinglong, BU Guangle, GENG Xueli, et al. Design optimization and operating pressure effects in the separation of acetonitrile/methanol/water mixture by ternary extractive distillation[J]. Journal of Cleaner Production, 2019, 218: 212-224. |
| 2 | ZHU Jiujuan, FAN Hanhan, SUN Bing, et al. Effect and mechanism of 1-hexyl-3-methylimidazolium-based ionic liquids on the isobaric vapor-liquid equilibria of methanol+ acetonitrile at 101.3 kPa[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5405-5412. |
| 3 | 赵建军, 刘沐鑫, 丁伯胜, 等. 乙腈与甲醇反应选择性合成丙烯腈催化剂制备及性能研究[J]. 塑料工业, 2018, 46(5): 147-150, 137. |
| ZHAO Jianjun, LIU Muxin, DING Bosheng, et al. Study on preparation and properties of the catalyst for the synthesis of acrylonitrile from acetonitrile and methanol[J]. China Plastics Industry, 2018, 46(5): 147-150, 137. | |
| 4 | ZHANG Yuxin, YU Dan, GUO Fan, et al. Vapor-Liquid Equilibria Measurement of (Methanol + Ethanenitrile + Bis(trifluoromethylsulfonyl) Imide)-Based Ionic Liquids at 101.3 kPa[J]. Journal of Chemical & Engineering Data, 2016, 61(7): 2202-2208. |
| 5 | LI Yanrui, WANG Qiang, LI Xiaoping, et al. Thermodynamic properties of N-octyl pyridinium hexafluorophosphate ionic liquid: Characterization and application in separation of the azeotrope[J]. Journal of Molecular Liquids, 2022, 350: 118434. |
| 6 | 孙小情, 党明岩. 萃取精馏分离丙酮-正庚烷的模拟与优化[J]. 沈阳理工大学学报, 2023, 42(3): 68-74. |
| SUN Xiaoqing, DANG Mingyan. Simulation and optimization of extractive distillation for separation of acetone and N-heptane[J]. Journal of Shenyang Ligong University, 2023, 42(3): 68-74. | |
| 7 | 王丽达. 以离子液体为萃取剂萃取精馏分离四氢呋喃-甲醇共沸体系的研究[D]. 沈阳: 沈阳化工大学, 2022. |
| WANG Lida. Extractive distillation of tetrahydrofuran-methanol azeotropic system using ionic liquid as entrainer [D]. Shenyang: Shenyang University of Chemical Technology, 2022. | |
| 8 | KLAMT Andreas, ECKERT Frank. Prediction of vapor liquid equilibria using COSMOtherm[J]. Fluid Phase Equilibria, 2004, 217(1): 53-57. |
| 9 | BOUDREAUX Andrew, CAMPBELL Craig. Student understanding of liquid-vapor phase equilibrium[J]. Journal of Chemical Education, 2012, 89(6): 707-714. |
| 10 | BESLER Brent H, MERZ Kenneth M Jr, KOLLMAN Peter A. Atomic charges derived from semiempirical methods[J]. Journal of Computational Chemistry, 1990, 11(4): 431-439. |
| 11 | RENON Henri, J-M PRAUSNITZ. Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE Journal, 1968, 14(1): 135-144. |
| 12 | LI Qing, ZHU Wei, LIU Bing, et al. Measurement and correlation of the vapor-liquid equilibrium for methanol+acetonitrile+imidazolium-based ionic liquids at 101.3kPa[J]. The Journal of Chemical Thermodynamics, 2016, 101: 25-30. |
| 13 | LI Yumei, BAI Peng, ZHUANG Qionghong. Isobaric vapor-liquid equilibrium for binary system of methanol and acetonitrile[J]. Fluid Phase Equilibria, 2013, 340: 42-45. |
| 14 | VALDERRAMA José O, SANGA Wilson W, LAZZÚS Juan A. Critical properties, normal boiling temperature, and acentric factor of another 200 ionic liquids[J]. Industrial & Engineering Chemistry Research, 2008, 47(4): 1318-1330. |
| 15 | RUIZ Elia, FERRO Victor R, PALOMAR Jose, et al. Interactions of ionic liquids and acetone: Thermodynamic properties, quantum-chemical calculations, and NMR analysis[J]. The Journal of Physical Chemistry B, 2013, 117(24): 7388-7398. |
| 16 | Gonzalo GARCÍA-MIAJA, TRONCOSO Jacobo, Luis ROMANÍ. Excess properties for binary systems ionic liquid+ethanol: Experimental results and theoretical description using the ERAS model[J]. Fluid Phase Equilibria, 2008, 274(1/2): 59-67. DOI: https://doi.org/10.1016/j.fluid.2008.09.004 . |
| 17 | ORTEGA Juan, VREEKAMP Remko, MARRERO Elena, et al. Thermodynamic properties of 1-butyl-3-methylpyridinium tetrafluoroborate and its mixtures with water and alkanols[J]. Journal of Chemical & Engineering Data, 2007, 52(6): 2269-2276. |
| 18 | S-F BOYS, BERNARDI F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 1970, 19(4): 553-566. |
| [1] | 周渝, 唐甜, 熊子悠, 韦奇. 基于两级微通道分离工艺的甲醇制烯烃废水深度处理[J]. 化工进展, 2025, 44(1): 100-108. |
| [2] | 胡洋, 韩传军, 胡强, 李汶颖, 安全成, 苏洋, 武洪松, 袁果. 固体氧化物燃料电池用甲醇水蒸气重整反应器研究进展[J]. 化工进展, 2025, 44(1): 169-183. |
| [3] | 李鑫, 王维, 张羽, 谢湫钰, 袁昊. 分离乙酸乙酯+乙醇+水体系:离子液体筛选、汽液相平衡和过程模拟[J]. 化工进展, 2025, 44(1): 75-85. |
| [4] | 周渝, 夏太阳, 韦奇, 唐甜, 田磊. 微通道耦合反渗透膜串联处理甲醇制烯烃废水工艺优化[J]. 化工进展, 2024, 43(S1): 43-51. |
| [5] | 陈巨辉, 王振名, 李丹, 王柏森, ZHURAVKOV Michael, SIARHEI Lapatsin, 于广滨. 不同含水率下的生活垃圾气化特性模拟[J]. 化工进展, 2024, 43(9): 4900-4908. |
| [6] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| [7] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [8] | 郭鹏, 李红伟, 李贵贤, 季东, 王东亮, 赵新红. 直接甲醇燃料电池阳极催化剂的失活机制及应对策略[J]. 化工进展, 2024, 43(7): 3812-3823. |
| [9] | 李斯文, 雷敏, 刘玉霜, 董兆琪, 薛丽丽, 赵建社. 离子液体多酸在燃油氧化脱硫中的研究进展[J]. 化工进展, 2024, 43(6): 3322-3335. |
| [10] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [11] | 周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738. |
| [12] | 周秋明, 牛丛丛, 吕帅帅, 李红伟, 文富利, 徐润, 李明丰. 通过产物转化分离推动CO2加氢制甲醇过程的研究进展[J]. 化工进展, 2024, 43(5): 2776-2785. |
| [13] | 李海鹏, 吴桐, 王琪, 郜时旺, 王晓龙, 李旭, 高新华, 年佩, 魏逸彬. 透水NaA分子筛膜强化的CO2加氢高效制甲醇[J]. 化工进展, 2024, 43(5): 2834-2842. |
| [14] | 王东亮, 李婧玮, 孟文亮, 杨勇, 周怀荣, 范宗良. 二氧化碳加氢制甲醇过程碳氢利用率的影响因素与工艺优化分析[J]. 化工进展, 2024, 43(5): 2843-2850. |
| [15] | 庞淑馨, 王昊, 王健宇, 朱卡克, 刘志成. 基于Aspen Plus的甲烷联合重整制合成气过程热力学计算[J]. 化工进展, 2024, 43(5): 2890-2900. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||