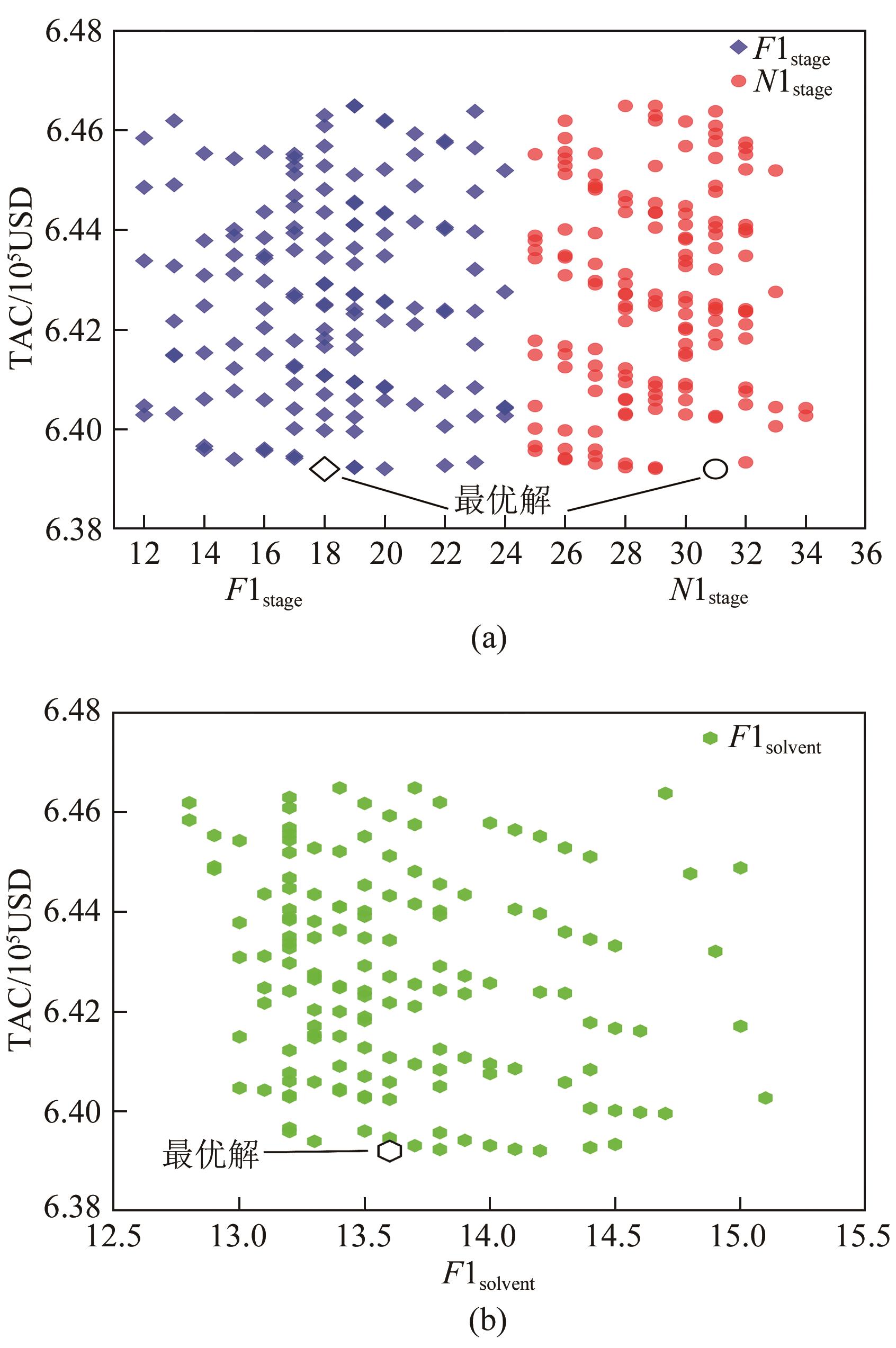
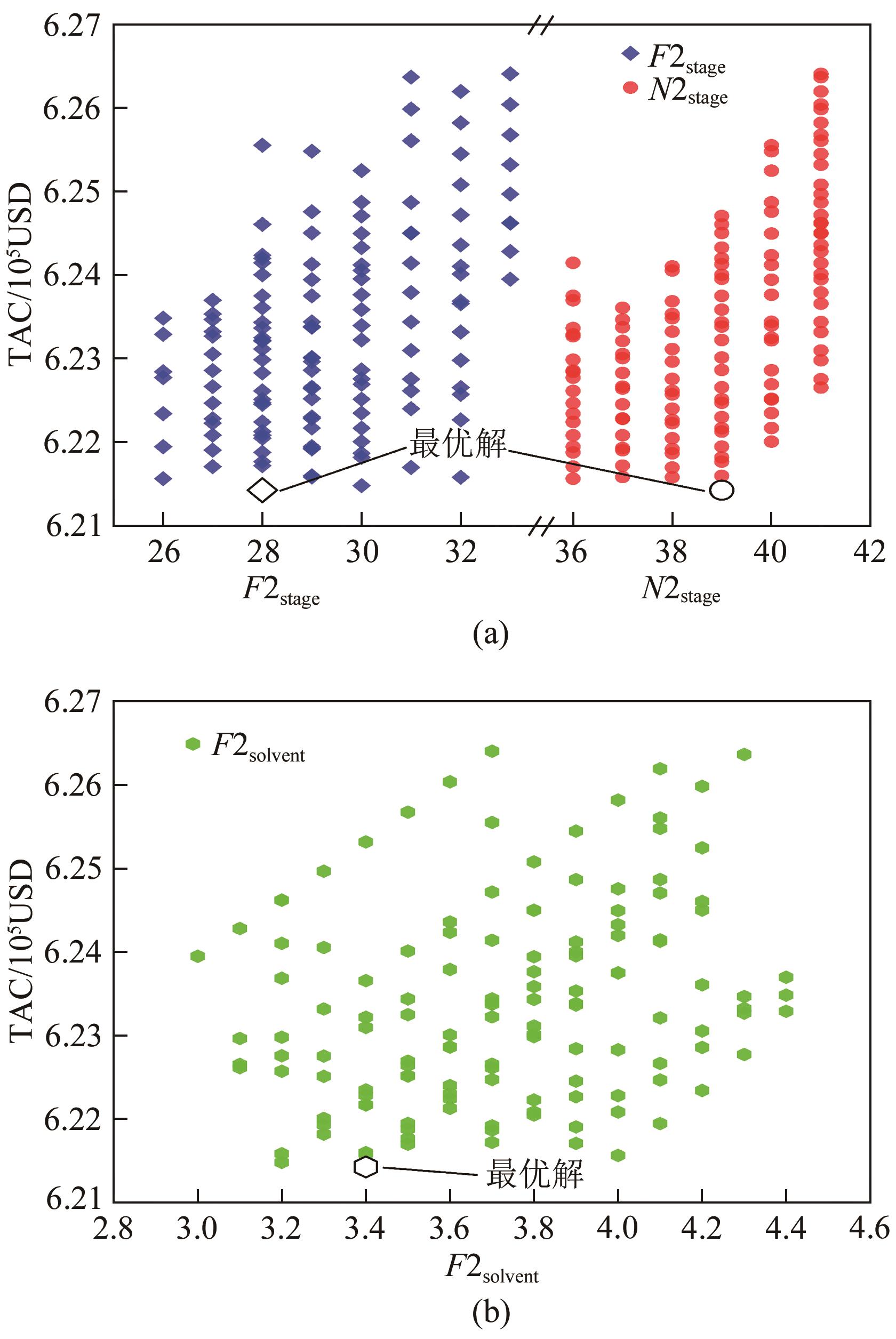
化工进展 ›› 2025, Vol. 44 ›› Issue (1): 75-85.DOI: 10.16085/j.issn.1000-6613.2023-2272
分离乙酸乙酯+乙醇+水体系:离子液体筛选、汽液相平衡和过程模拟
- 大连理工大学化工学院,辽宁 大连 116024
-
收稿日期:2023-12-27修回日期:2024-02-27出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:王维 -
作者简介:李鑫(1998—),男,硕士研究生,研究方向为分离过程强化。E-mail:lxdsg@mail.dlut.edu.cn。
Separation of ethyl acetate+ethanol+water system: Ionic liquids screening, vapor liquid equilibrium and process simulation
LI Xin( ), WANG Wei(
), WANG Wei( ), ZHANG Yu, XIE Qiuyu, YUAN Hao
), ZHANG Yu, XIE Qiuyu, YUAN Hao
- School of Chemical Engineering, Dalian University of Technology, Dalian 116024, Liaoning, China
-
Received:2023-12-27Revised:2024-02-27Online:2025-01-15Published:2025-02-13 -
Contact:WANG Wei
摘要:
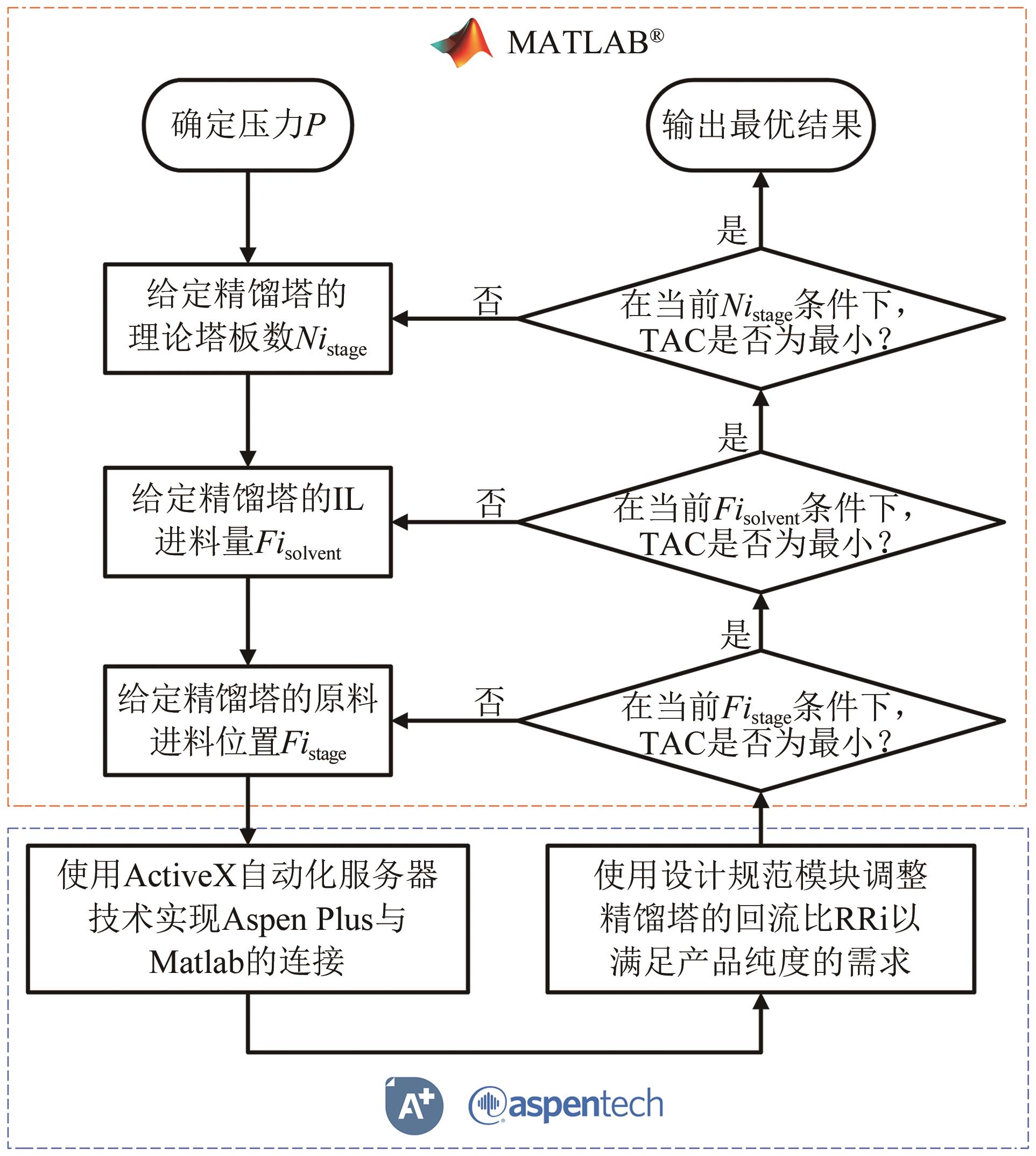
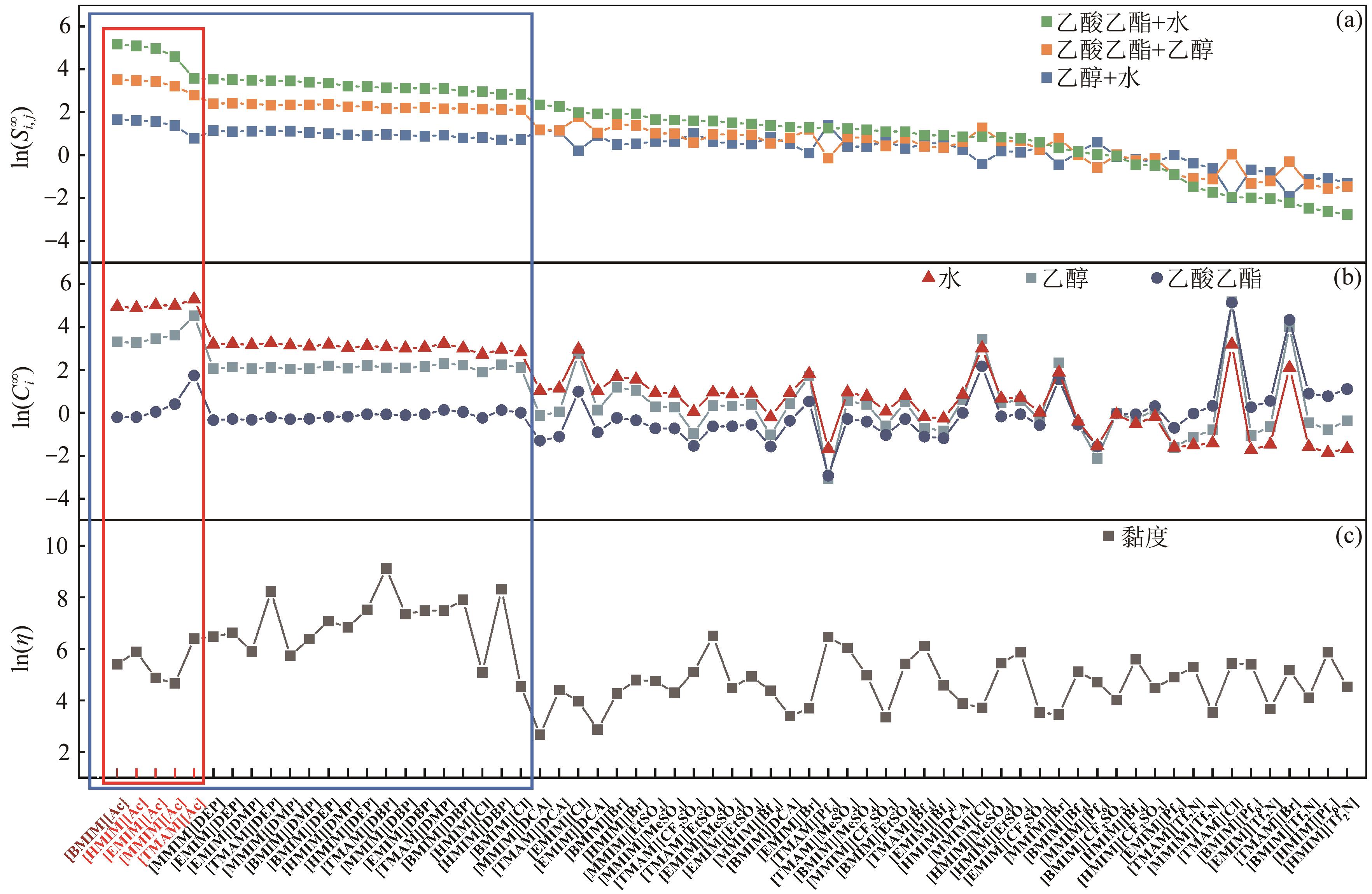
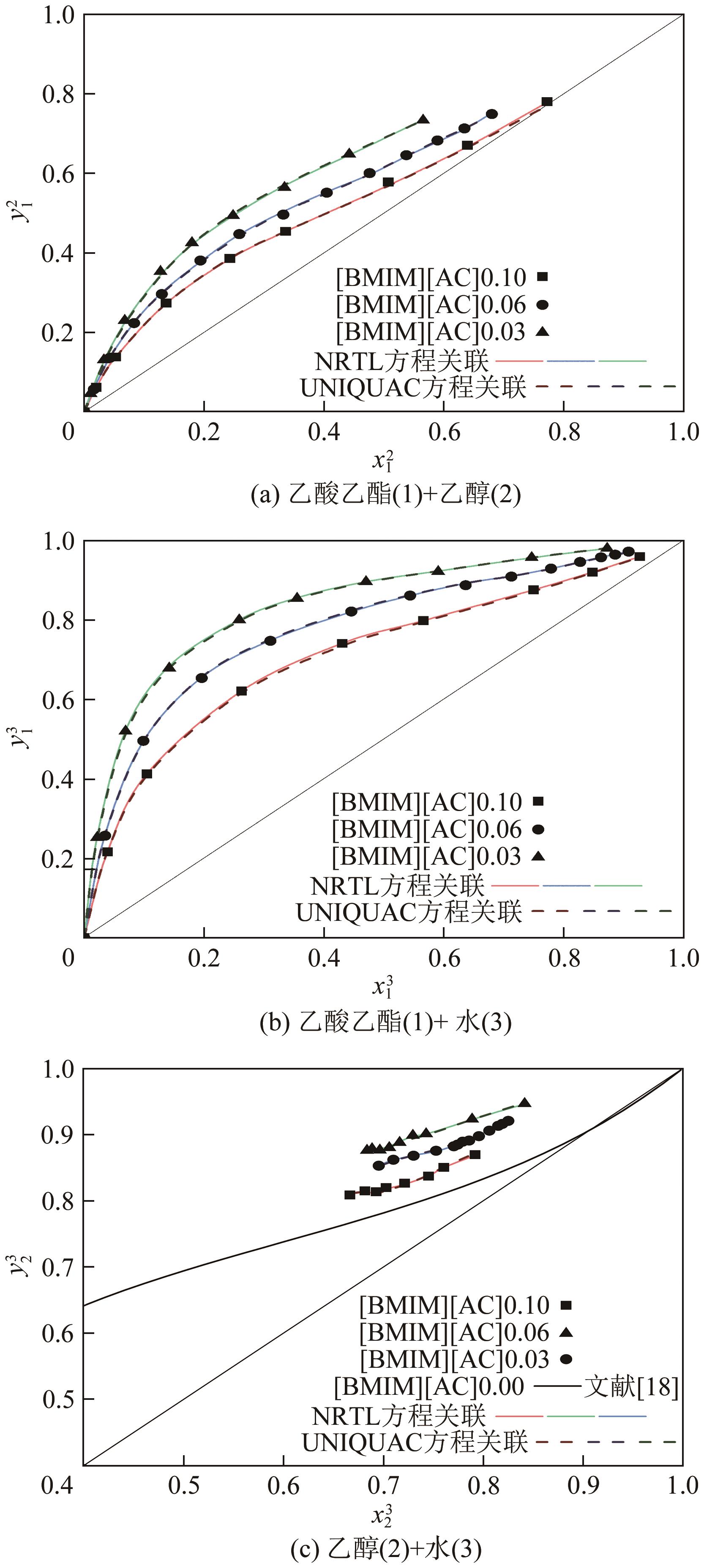
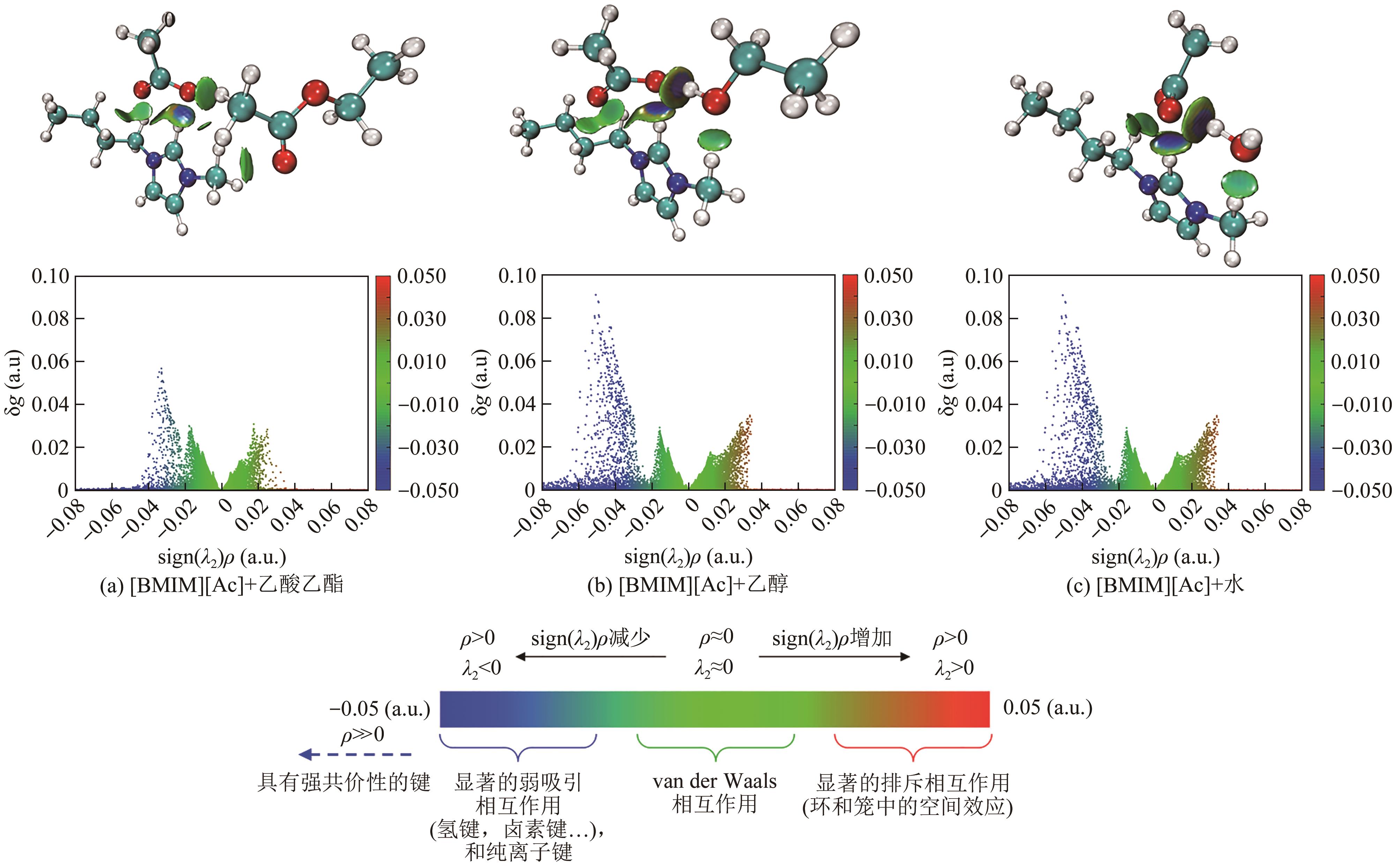
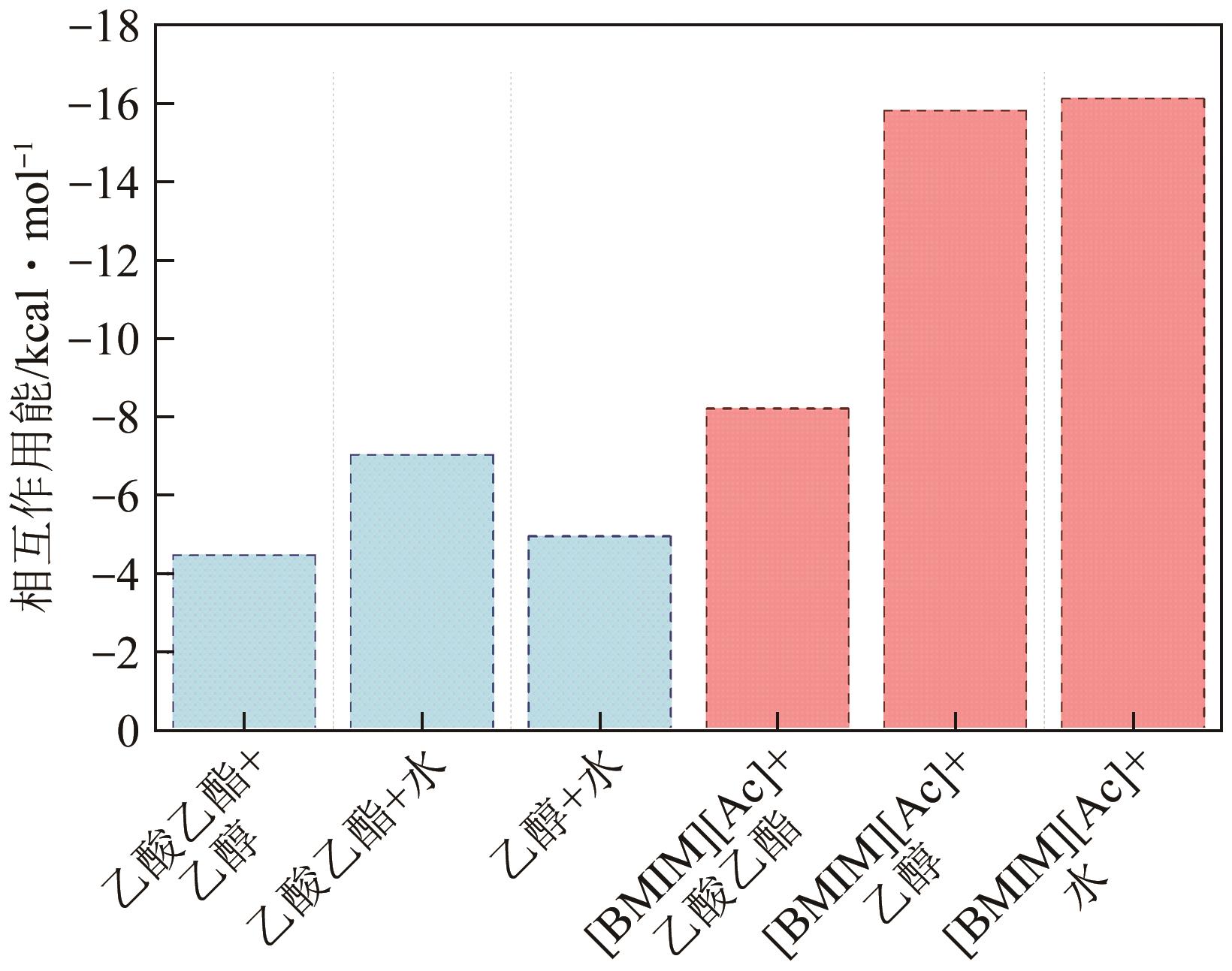
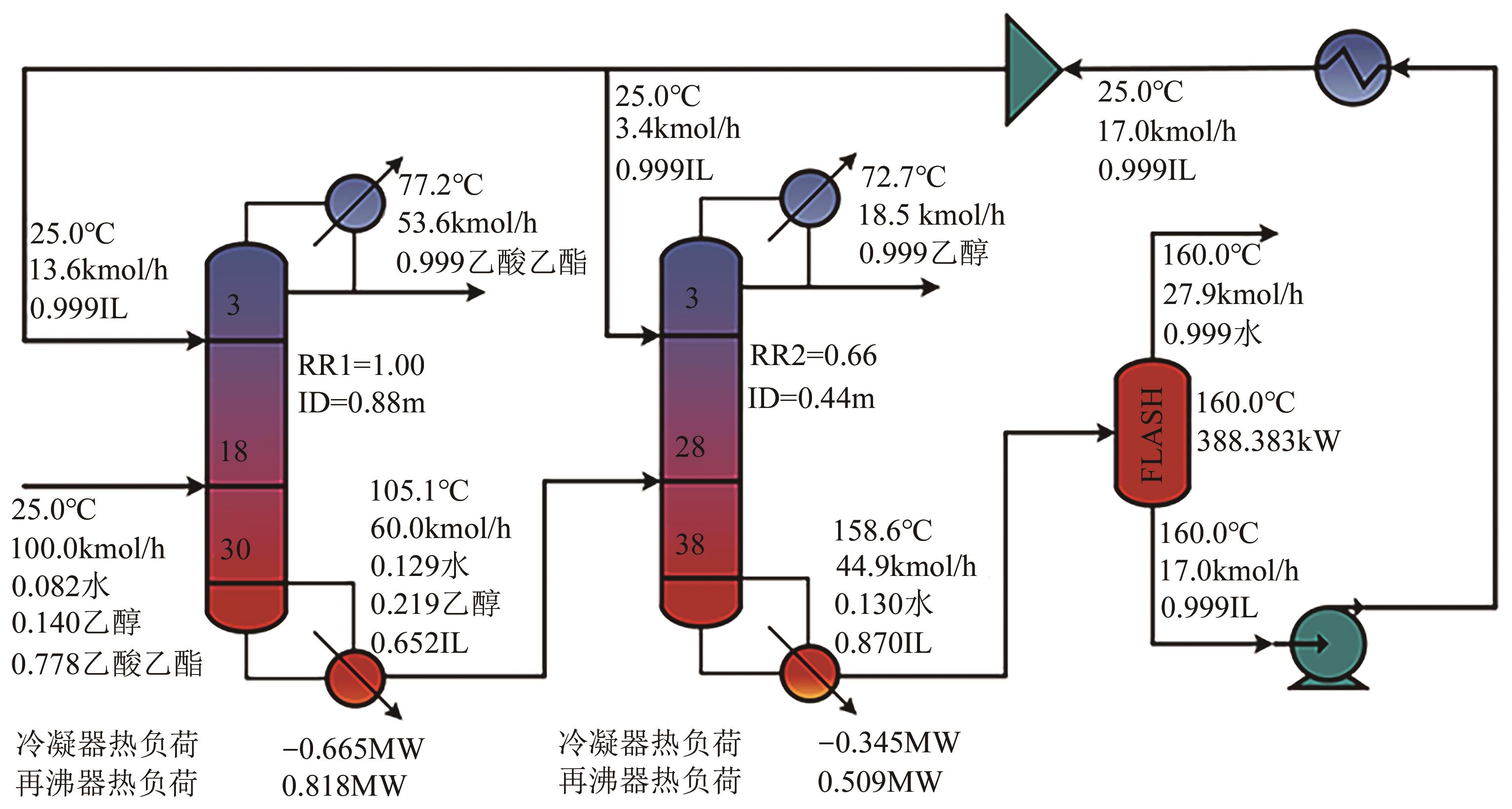
乙酸乙酯、乙醇和水体系能够形成三个二元和一个三元的共沸体系。本文旨在以离子液体(IL)为萃取剂打破这些共沸,实现该三元体系的有效分离。基于COSMO-RS模型和黏度预测模型,计算了65种ILs的选择性、溶解度和黏度,发现1-丁基-3-甲基咪唑醋酸盐([BMIM][Ac])是65种ILs中最符合要求的萃取剂。进行了乙酸乙酯(1)+乙醇(2)+水(3)+IL(4)的四元汽液相平衡(VLE)实验,证实[BMIM][Ac]能够打破所有共沸体系,验证了筛选结果。NRTL和UNIQUAC方程关联四元VLE数据的均方根误差分别为1.69%和2.20%,关联结果可靠。通过量化计算定性和定量分析了IL分离该三元体系的机理,表明[BMIM][Ac]与乙酸乙酯发生弱氢键作用(-8.22kcal/mol,1kcal≈4.186kJ),与乙醇和水分别发生强氢键作用(-15.83kcal/mol和-16.14kcal/mol)。基于方程关联所得参数,模拟并优化了以IL为萃取剂分离乙酸乙酯+乙醇+水体系的萃取精馏过程,显示乙酸乙酯、乙醇和水的产品纯度均可达到0.999。研究结果验证了[BMIM][Ac]作为萃取剂分离该三元共沸体系工业化可行性。
中图分类号:
引用本文
李鑫, 王维, 张羽, 谢湫钰, 袁昊. 分离乙酸乙酯+乙醇+水体系:离子液体筛选、汽液相平衡和过程模拟[J]. 化工进展, 2025, 44(1): 75-85.
LI Xin, WANG Wei, ZHANG Yu, XIE Qiuyu, YUAN Hao. Separation of ethyl acetate+ethanol+water system: Ionic liquids screening, vapor liquid equilibrium and process simulation[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 75-85.
| 参数 | 数值 |
|---|---|
| d | -3.682 |
| e | 9.391 |
| f | 1.066 |
| g | -0.012 |
| h | 0.018 |
| i | -14.852 |
表1 黏度预测模型的参数值
| 参数 | 数值 |
|---|---|
| d | -3.682 |
| e | 9.391 |
| f | 1.066 |
| g | -0.012 |
| h | 0.018 |
| i | -14.852 |
| 组分 | ri | qi |
|---|---|---|
| 乙酸乙酯 | 3.4786 | 3.1160 |
| 乙醇 | 2.1055 | 1.9720 |
| 水 | 0.9200 | 1.4000 |
| [BMIM][Ac] | 8.7838 | 5.2463 |
表2 乙酸乙酯、乙醇、水和[BMIM][Ac]的面积和体积参数值
| 组分 | ri | qi |
|---|---|---|
| 乙酸乙酯 | 3.4786 | 3.1160 |
| 乙醇 | 2.1055 | 1.9720 |
| 水 | 0.9200 | 1.4000 |
| [BMIM][Ac] | 8.7838 | 5.2463 |
| T/K | ||||
|---|---|---|---|---|
| 351.0 | 0.007 | 0.017 | 2.521 | 1.011 |
| 350.1 | 0.033 | 0.077 | 2.382 | 1.010 |
| 348.9 | 0.080 | 0.163 | 2.124 | 1.011 |
| 347.2 | 0.159 | 0.267 | 1.864 | 1.037 |
| 346.1 | 0.251 | 0.353 | 1.621 | 1.075 |
| 345.1 | 0.381 | 0.446 | 1.394 | 1.160 |
| 344.8 | 0.489 | 0.512 | 1.258 | 1.254 |
| 344.9 | 0.630 | 0.593 | 1.128 | 1.438 |
| 345.5 | 0.753 | 0.679 | 1.059 | 1.657 |
| 346.6 | 0.858 | 0.780 | 1.029 | 1.889 |
| 347.9 | 0.926 | 0.869 | 1.017 | 2.050 |
| 349.1 | 0.975 | 0.949 | 1.014 | 2.218 |
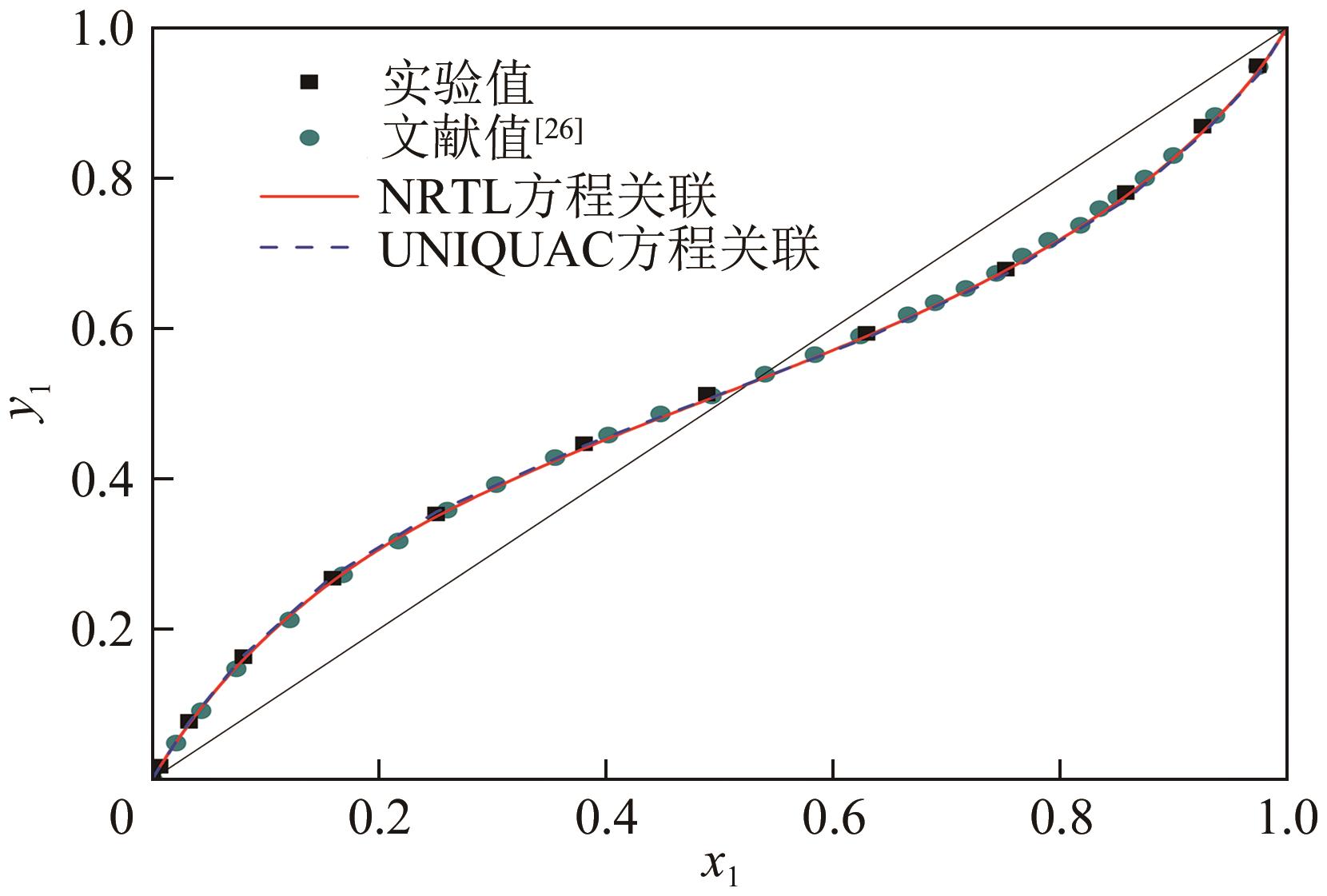
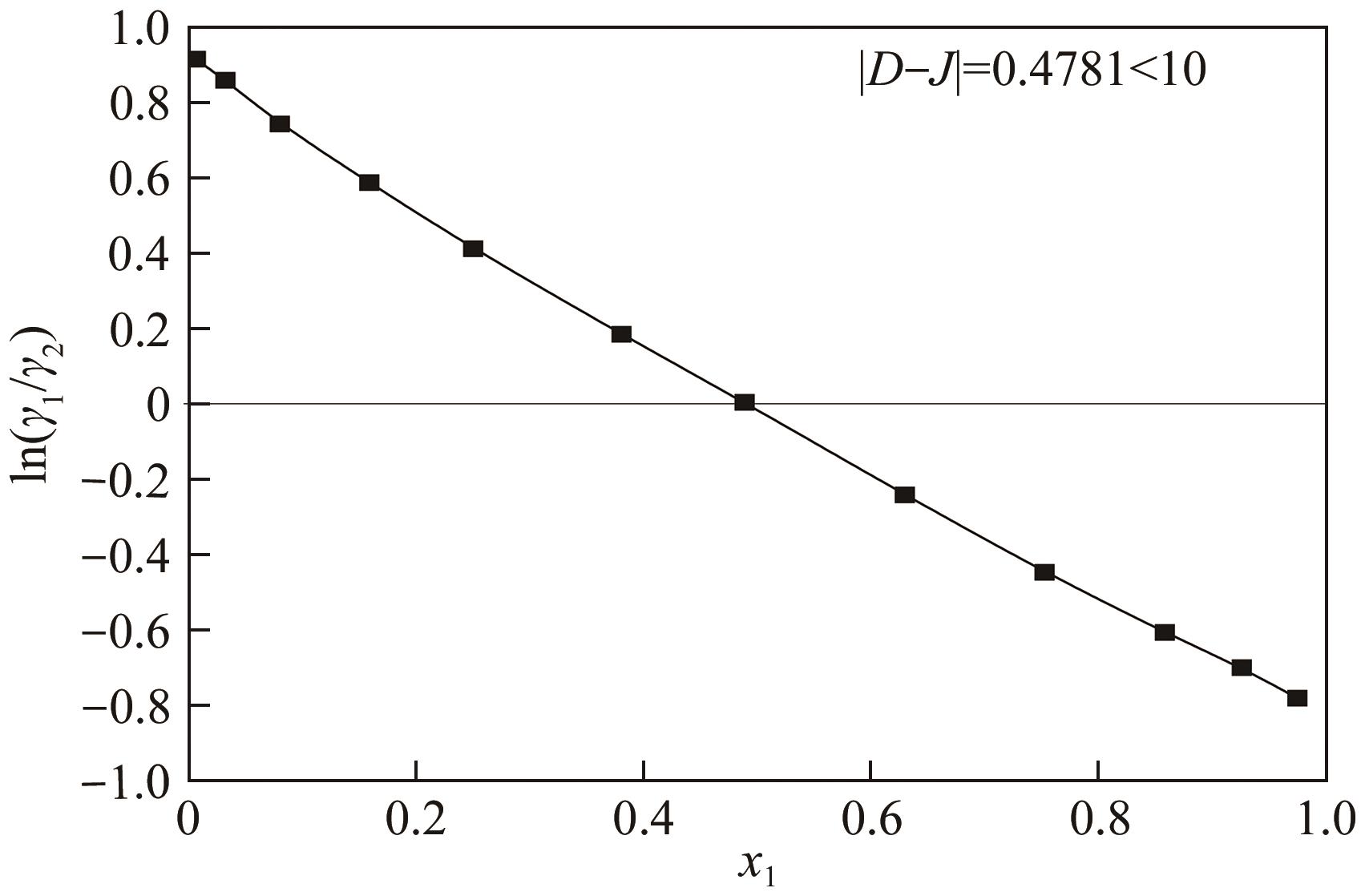
表3 101.3kPa下乙酸乙酯(1)+乙醇(2)的二元VLE数据
| T/K | ||||
|---|---|---|---|---|
| 351.0 | 0.007 | 0.017 | 2.521 | 1.011 |
| 350.1 | 0.033 | 0.077 | 2.382 | 1.010 |
| 348.9 | 0.080 | 0.163 | 2.124 | 1.011 |
| 347.2 | 0.159 | 0.267 | 1.864 | 1.037 |
| 346.1 | 0.251 | 0.353 | 1.621 | 1.075 |
| 345.1 | 0.381 | 0.446 | 1.394 | 1.160 |
| 344.8 | 0.489 | 0.512 | 1.258 | 1.254 |
| 344.9 | 0.630 | 0.593 | 1.128 | 1.438 |
| 345.5 | 0.753 | 0.679 | 1.059 | 1.657 |
| 346.6 | 0.858 | 0.780 | 1.029 | 1.889 |
| 347.9 | 0.926 | 0.869 | 1.017 | 2.050 |
| 349.1 | 0.975 | 0.949 | 1.014 | 2.218 |
| T/K | |||||
|---|---|---|---|---|---|
| 351.8 | 0.031 | 0.013 | 0.637 | 0.050 | 0.768 |
| 350.7 | 0.030 | 0.035 | 0.637 | 0.115 | 0.721 |
| 348.6 | 0.029 | 0.096 | 0.606 | 0.234 | 0.623 |
| 347.6 | 0.032 | 0.178 | 0.555 | 0.340 | 0.541 |
| 347.1 | 0.031 | 0.259 | 0.512 | 0.407 | 0.490 |
| 346.7 | 0.030 | 0.421 | 0.409 | 0.534 | 0.390 |
| 347.3 | 0.031 | 0.556 | 0.314 | 0.633 | 0.312 |
| 348.2 | 0.029 | 0.707 | 0.209 | 0.755 | 0.213 |
| 353.8 | 0.061 | 0.010 | 0.646 | 0.048 | 0.811 |
| 352.4 | 0.062 | 0.029 | 0.645 | 0.119 | 0.759 |
| 350.8 | 0.061 | 0.058 | 0.643 | 0.199 | 0.695 |
| 349.8 | 0.059 | 0.095 | 0.637 | 0.269 | 0.640 |
| 348.7 | 0.059 | 0.147 | 0.612 | 0.351 | 0.572 |
| 348.4 | 0.061 | 0.200 | 0.572 | 0.417 | 0.516 |
| 347.9 | 0.062 | 0.262 | 0.527 | 0.466 | 0.474 |
| 347.8 | 0.059 | 0.327 | 0.482 | 0.522 | 0.426 |
| 347.7 | 0.058 | 0.395 | 0.435 | 0.574 | 0.382 |
| 347.9 | 0.060 | 0.454 | 0.391 | 0.622 | 0.342 |
| 348.0 | 0.061 | 0.506 | 0.353 | 0.662 | 0.308 |
| 348.2 | 0.060 | 0.551 | 0.318 | 0.694 | 0.280 |
| 348.6 | 0.061 | 0.598 | 0.281 | 0.733 | 0.246 |
| 357.9 | 0.102 | 0.006 | 0.609 | 0.040 | 0.843 |
| 356.6 | 0.103 | 0.020 | 0.604 | 0.116 | 0.776 |
| 354.5 | 0.099 | 0.043 | 0.597 | 0.207 | 0.695 |
| 352.1 | 0.098 | 0.084 | 0.577 | 0.324 | 0.595 |
| 351.2 | 0.101 | 0.122 | 0.557 | 0.395 | 0.536 |
| 350.1 | 0.102 | 0.174 | 0.528 | 0.464 | 0.479 |
| 349.1 | 0.100 | 0.244 | 0.487 | 0.538 | 0.416 |
| 348.9 | 0.101 | 0.346 | 0.436 | 0.630 | 0.342 |
| 349.4 | 0.103 | 0.469 | 0.360 | 0.722 | 0.263 |
表4 101.3kPa下乙酸乙酯(1)+乙醇(2)+水(3)+[BMIM][Ac](4)的VLE数据
| T/K | |||||
|---|---|---|---|---|---|
| 351.8 | 0.031 | 0.013 | 0.637 | 0.050 | 0.768 |
| 350.7 | 0.030 | 0.035 | 0.637 | 0.115 | 0.721 |
| 348.6 | 0.029 | 0.096 | 0.606 | 0.234 | 0.623 |
| 347.6 | 0.032 | 0.178 | 0.555 | 0.340 | 0.541 |
| 347.1 | 0.031 | 0.259 | 0.512 | 0.407 | 0.490 |
| 346.7 | 0.030 | 0.421 | 0.409 | 0.534 | 0.390 |
| 347.3 | 0.031 | 0.556 | 0.314 | 0.633 | 0.312 |
| 348.2 | 0.029 | 0.707 | 0.209 | 0.755 | 0.213 |
| 353.8 | 0.061 | 0.010 | 0.646 | 0.048 | 0.811 |
| 352.4 | 0.062 | 0.029 | 0.645 | 0.119 | 0.759 |
| 350.8 | 0.061 | 0.058 | 0.643 | 0.199 | 0.695 |
| 349.8 | 0.059 | 0.095 | 0.637 | 0.269 | 0.640 |
| 348.7 | 0.059 | 0.147 | 0.612 | 0.351 | 0.572 |
| 348.4 | 0.061 | 0.200 | 0.572 | 0.417 | 0.516 |
| 347.9 | 0.062 | 0.262 | 0.527 | 0.466 | 0.474 |
| 347.8 | 0.059 | 0.327 | 0.482 | 0.522 | 0.426 |
| 347.7 | 0.058 | 0.395 | 0.435 | 0.574 | 0.382 |
| 347.9 | 0.060 | 0.454 | 0.391 | 0.622 | 0.342 |
| 348.0 | 0.061 | 0.506 | 0.353 | 0.662 | 0.308 |
| 348.2 | 0.060 | 0.551 | 0.318 | 0.694 | 0.280 |
| 348.6 | 0.061 | 0.598 | 0.281 | 0.733 | 0.246 |
| 357.9 | 0.102 | 0.006 | 0.609 | 0.040 | 0.843 |
| 356.6 | 0.103 | 0.020 | 0.604 | 0.116 | 0.776 |
| 354.5 | 0.099 | 0.043 | 0.597 | 0.207 | 0.695 |
| 352.1 | 0.098 | 0.084 | 0.577 | 0.324 | 0.595 |
| 351.2 | 0.101 | 0.122 | 0.557 | 0.395 | 0.536 |
| 350.1 | 0.102 | 0.174 | 0.528 | 0.464 | 0.479 |
| 349.1 | 0.100 | 0.244 | 0.487 | 0.538 | 0.416 |
| 348.9 | 0.101 | 0.346 | 0.436 | 0.630 | 0.342 |
| 349.4 | 0.103 | 0.469 | 0.360 | 0.722 | 0.263 |
| 组分i | 组分j | NRTL方程 | UNIQUAC方程 | |||
|---|---|---|---|---|---|---|
| δgij /R | δgji /R | αij | δgij /R | δgji /R | ||
| 乙酸乙酯 | 乙醇 | -68.10 | 361.22 | 0.4 | -51.59 | 216.97 |
| 乙醇 | 水 | 201.12 | 222.61 | 0.3 | 549.34 | -229.56 |
| 乙酸乙酯 | 水 | 403.80 | 4968.73 | 0.3 | -146.53 | 6013.95 |
| 乙酸乙酯 | [BMIM][Ac] | 1428.80 | 296.38 | 0.4 | -4944.73 | -117.07 |
| 乙醇 | [BMIM][Ac] | -1207.90 | -869.18 | 0.4 | -5499.16 | -82.69 |
| 水 | [BMIM][Ac] | -1313.00 | -1263.40 | 0.4 | -5800.52 | -331.68 |
| RMSD=1.69% | RMSD=2.20% | |||||
表5 NRTL和UNIQUAC方程对四元VLE实验数据的关联结果及误差
| 组分i | 组分j | NRTL方程 | UNIQUAC方程 | |||
|---|---|---|---|---|---|---|
| δgij /R | δgji /R | αij | δgij /R | δgji /R | ||
| 乙酸乙酯 | 乙醇 | -68.10 | 361.22 | 0.4 | -51.59 | 216.97 |
| 乙醇 | 水 | 201.12 | 222.61 | 0.3 | 549.34 | -229.56 |
| 乙酸乙酯 | 水 | 403.80 | 4968.73 | 0.3 | -146.53 | 6013.95 |
| 乙酸乙酯 | [BMIM][Ac] | 1428.80 | 296.38 | 0.4 | -4944.73 | -117.07 |
| 乙醇 | [BMIM][Ac] | -1207.90 | -869.18 | 0.4 | -5499.16 | -82.69 |
| 水 | [BMIM][Ac] | -1313.00 | -1263.40 | 0.4 | -5800.52 | -331.68 |
| RMSD=1.69% | RMSD=2.20% | |||||
| 1 | MENG Dapeng, DAI Yao, XU Ying, et al. Energy, economic and environmental evaluations for the separation of ethyl acetate/ethanol/water mixture via distillation and pervaporation unit[J]. Process Safety and Environmental Protection, 2020, 140: 14-25. |
| 2 | ERNEST Flick W. Industrial solvents handbook[M]. 5th ed. New Jersey: Noyes Data Corporation, 1998: 818-819. |
| 3 | TOTH Andras Jozsef. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl acetate-ethanol-water highly non-ideal mixture[J]. Separation and Purification Technology, 2019, 224: 490-508. |
| 4 | 李群生, 王亚茹, 文放. 乙醇-水体系分离提纯过程新技术的研究[J]. 化工进展, 2015, 34(12): 4179-4184. |
| LI Qunsheng, WANG Yaru, WEN Fang. Research on the new technology of ethanol-water distillation[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4179-4184. | |
| 5 | 徐东芳, 胡佳静, 王丽丽, 等. 变压精馏分离乙醇-氯仿共沸物的动态特性[J]. 化工进展, 2016, 35(4): 1242-1249. |
| XU Dongfang, HU Jiajing, WANG Lili, et al. Dynamic characteristics of pressure-swing distillation for ethanol-chloroform separation[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1242-1249. | |
| 6 | MA Yixin, CUI Peizhe, WANG Yongkun, et al. A review of extractive distillation from an azeotropic phenomenon for dynamic control[J]. Chinese Journal of Chemical Engineering, 2019, 27(7): 1510-1522. |
| 7 | 张志刚, 张德彪, 张亲亲, 等. 基于COSMO-RS方法筛选离子液体分离乙酸乙酯-乙腈共沸物[J]. 化工学报, 2019, 70(1): 146-153. |
| ZHANG Zhigang, ZHANG Debiao, ZHANG Qinqin, et al. Screening of ionic liquids for separation of ethyl acetate-acetonitrile azeotrope based on COSMO-RS [J]. CIESC Journal, 2019, 70(1): 146-153. | |
| 8 | GERBAUD Vincent, Ivonne RODRIGUEZ-DONIS, HEGELY Laszlo, et al. Review of extractive distillation. Process design, operation, optimization and control[J]. Chemical Engineering Research and Design, 2019, 141: 229-271. |
| 9 | DUAN Cong, LI Chunli. Novel energy-saving methods to improve the three-column extractive distillation process for separating ethyl acetate and ethanol using furfural[J]. Separation and Purification Technology, 2021, 272: 118887. |
| 10 | YANG Ao, ZOU Hechen, I-Lung CHIEN, et al. Optimal design and effective control of triple-column extractive distillation for separating ethyl acetate/ethanol/water with multiazeotrope[J]. Industrial & Engineering Chemistry Research, 2019, 58(17): 7265-7283. |
| 11 | AYUSO Miguel, Andrés CAÑADA-BARCALA, LARRIBA Marcos, et al. Enhanced separation of benzene and cyclohexane by homogeneous extractive distillation using ionic liquids as entrainers[J]. Separation and Purification Technology, 2020, 240: 116583. |
| 12 | 李文秀, 张羽, 曹颖, 等. 离子液体用于四氢呋喃-乙醇-水三元共沸物系分离的研究[J]. 化工学报, 2020, 71(4): 1676-1682. |
| LI Wenxiu, ZHANG Yu, CAO Ying, et al. Study on separation of tetrahydrofuran-ethanol-water ternary azeotropesystem by ionic liquid[J]. CIESC Journal, 2020, 71(4): 1676-1682. | |
| 13 | 李文秀, 张琦, 张亲亲, 等. 含离子液体乙腈-正丙醇体系的等压汽液平衡[J]. 化工学报, 2015, 66(S1): 38-44. |
| LI Wenxiu, ZHANG Qi, ZHANG Qinqin, et al. Isobaric vapor-liquid equilibrium for system of acetonitrile-n-propanol system containing ionic liquids[J]. CIESC Journal, 2015, 66(S1): 38-44. | |
| 14 | ZHANG Lianzheng, WANG Jie, YANG Lin, et al. Separation of isopropyl alcohol+isopropyl acetate azeotropic mixture: Selection of ionic liquids as entrainers and vapor-liquid equilibrium validation[J]. Chinese Journal of Chemical Engineering, 2022, 50: 326-334. |
| 15 | 张清珍, 代成娜, 韩敬莉, 等. 萃取蒸馏脱除油品中硫的过程模拟与优化[J]. 化工进展, 2016, 35(8): 2553-2560. |
| ZHANG Qingzhen, DAI Chengna, HAN Jingli, et al. Desulfurization of oil products by extractive distillation: Simulation and optimization[J]. Chemical Industry and Engineering Progress. 2016, 35(8): 2553-2560. | |
| 16 | 高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选: 实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323. |
| GAO Tengfei, LI Guoxuan, LEI Zhigang. Solvents selection for separation of biphenyl from FCC diesel: Experimental and computational thermodynamics[J]. CIESC Journal, 2022, 73(12): 5314-5323. | |
| 17 | SALLEH M. Zulhaziman M, HADJ-KALI Mohamed K, HASHIM Mohd A,et al. Ionic liquids for the separation of benzene and cyclohexane - COSMO-RS screening and experimental validation[J]. Journal of Molecular Liquids, 2018, 266: 51-61. |
| 18 | MALIK Huzaifa, KHAN Huma Warsi, HASSAN SHAH Mansoor Ul, et al. Screening of ionic liquids as green entrainers for ethanol water separation by extractive distillation: COSMO-RS prediction and aspen plus simulation[J]. Chemosphere, 2022, 311(Pt 2): 136901. |
| 19 | VERMA Vijay Kumar, BANERJEE Tamal. Ionic liquids as entrainers for water+ethanol, water+2-propanol, and water+THF systems: A quantum chemical approach[J]. The Journal of Chemical Thermodynamics, 2010, 42(7): 909-919. |
| 20 | Vicent ORCHILLÉS A, MIGUEL Pablo J, LLOPIS Francisco J, et al. Isobaric vapor-liquid equilibria for the extractive distillation of ethanol+water mixtures using 1-ethyl-3-methylimidazolium dicyanamide[J]. Journal of Chemical & Engineering Data, 2011, 56(12): 4875-4880. |
| 21 | TSANAS Christos, TZANI Andromachi, PAPADOPOULOS Achilleas, et al. Ionic liquids as entrainers for the separation of the ethanol/water system[J]. Fluid Phase Equilibria, 2014, 379: 148-156. |
| 22 | GE Yun, ZHANG Lianzhong, YUAN Xingcai, et al. Selection of ionic liquids as entrainers for separation of (water+ethanol)[J]. The Journal of Chemical Thermodynamics, 2008, 40(8): 1248-1252. |
| 23 | ANDREATTA Alfonsina E, CHARNLEY Matthew P, BRENNECKE Joan F. Using ionic liquids to break the ethanol-ethyl acetate azeotrope[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(12): 3435-3444. |
| 24 | LI Rui, CUI Xianbao, ZHANG Ying, et al. Vapor-liquid equilibrium and liquid-liquid equilibrium of ethyl acetate+ethanol+1-ethyl-3-methylimidazolium acetate[J]. Journal of Chemical & Engineering Data, 2012, 57(3): 911-917. |
| 25 | LI Qunsheng, ZHANG Jiguo, LEI Zhigang, et al. Isobaric vapor-liquid equilibrium for ethyl acetate plus ethanol+1-ethyl-3-methylimidazolium tetrafluoroborate[J]. Journal of Chemical and Engineering Data, 2009, 54(2): 193-197. |
| 26 | Vicent ORCHILLÉS A, MIGUEL Pablo J, VERCHER Ernesto, et al. Isobaric vapor-liquid equilibria for ethyl acetate+ethanol+1-ethyl-3-methylimidazolium trifluoromethanesulfonate at 100 kPa[J]. Journal of Chemical and Engineering Data, 2007, 52(6): 2325-2330. |
| 27 | ZHANG Lianzhong, YUAN Xingcai, QIAO Bingbang, et al. Isobaric vapor-liquid equilibria for water+ethanol+ethyl acetate+1-butyl-3-methylimidazolium acetate at low water mole fractions[J]. Journal of Chemical and Engineering Data, 2008, 53(7): 1595-1601. |
| 28 | MA Shoutao, SHANG Xianyong, LI Lumin, et al. Energy-saving thermally coupled ternary extractive distillation process using ionic liquids as entrainer for separating ethyl acetate-ethanol-water ternary mixture[J]. Separation and Purification Technology, 2019, 226: 337-349. |
| 29 | PAN Qi, SHANG Xianyong, MA Shoutao, et al. Control comparison of extractive distillation configurations for separating ethyl acetate-ethanol-water ternary mixture using ionic liquids as entrainer[J]. Separation and Purification Technology, 2020, 236: 116290. |
| 30 | ZHANG Yu, PAN Yanqiu, ZHANG Tao, et al. A comprehensive method of ionic liquid screening and experimental verification for simultaneous separation of multiple sulfides from oil[J]. Separation and Purification Technology, 2023, 315: 123714. |
| 31 | FREIRE Mara G, TELES Ana Rita R, ROCHA Marisa A A, et al. Thermophysical characterization of ionic liquids able to dissolve biomass[J]. Journal of Chemical and Engineering Data, 2011, 56(12): 4813-4822. |
| 32 | 雷志刚, 王洪有, 许峥, 等. 萃取精馏的研究进展[J]. 化工进展, 2001, 20(9): 6-9. |
| LEI Zhigang, WANG Hongyou, XU Zheng, et al. A review of extractive distillation[J]. Chemical Industry and Engineering Progress, 2001, 20(9): 6-9. | |
| 33 | EIDEN Philipp, BULUT Safak, Tobias KÖCHNER, et al. In silico predictions of the temperature-dependent viscosities and electrical conductivities of functionalized and nonfunctionalized ionic liquids[J]. Journal of Physical Chemistry B, 2011, 115(2): 300-309. |
| 34 | WANG Ping, XU Dongmei, YAN Peisong, et al. Separation of azeotrope (ethanol and ethyl methyl carbonate) by different imidazolium-based ionic liquids: Ionic liquids interaction analysis and phase equilibrium measurements[J]. Journal of Molecular Liquids, 2018, 261: 89-95. |
| 35 | Marek ŁUSZCZYK, MALANOWSKI Stanislaw K. Vapor-liquid equilibrium in α-methylbenzenemethanol + water[J]. Journal of Chemical and Engineering Data, 2006, 51(5): 1735-1739. |
| 36 | HONG Guibing, LEE Ming-Jer, LIN Ho-Mu. Multiphase coexistence for mixtures containing water, 2-propanol, and ethyl acetate[J]. Fluid Phase Equilibria, 2002, 203(1/2): 227-245. |
| 37 | YANG Tzu-Huai, JESSIE LUE Shingjiang. UNIQUAC and UNIQUAC-HB models for the sorption behavior of ethanol/water mixtures in a cross-linked polydimethylsiloxane membrane[J]. Journal of Membrane Science, 2012, 415: 534-545. |
| 38 | SUN Guangming, HUANG Weijia, ZHENG Danxing, et al. Vapor-liquid equilibrium prediction of ammonia-ionic liquid working pairs of absorption cycle using UNIFAC model[J]. Chinese Journal of Chemical Engineering, 2014, 22(1): 72-78. |
| 39 | LU Tian, CHEN Qinxue. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems[J]. Journal of Computational Chemistry, 2022, 43(8): 539-555. |
| 40 | LU Tian, CHEN Feiwu. Multiwfn: A multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 41 | HUMPHREY William, DALKE Andrew, SCHULTEN Klaus. VMD: Visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. |
| 42 | VALDERRAMA José O, SANGA Wilson W, LAZZÚS Juan A. Critical properties, normal boiling temperature, and acentric factor of another 200 ionic liquids[J]. Industrial & Engineering Chemistry Research, 2008, 47(4): 1318-1330. |
| 43 | LUYBEN William L. Distillation design and control using AspenTM simulation[M]. New Jersey: John Wiley & Sons Inc, 2013: 87-89. |
| 44 | LUYBEN William L, YU Cheng-Ching. Reactive distillation design and control[M]. New Jersey: John Wiley & Sons Inc, 2008: 42-43. |
| 45 | WISNIAK Jaime, ORTEGA Juan, Luis FERNÁNDEZ. A fresh look at the thermodynamic consistency of vapour-liquid equilibria data[J]. The Journal of Chemical Thermodynamics, 2017, 105: 385-395. |
| 46 | YUE Kun, ZHOU Guowei. Isobaric vapor-liquid equilibrium for ethyl acetate+ethanol with ionic liquids [MMIM][DMP] and [OMIM][PF6] as entrainers[J]. Journal of Molecular Liquids, 2022, 348: 118404. |
| 47 | CHEN Zhengrun, DAI Yasen, CHI Shuxiu, et al. Analysis and intensification of energy saving process for separation of azeotrope by ionic liquid extractive distillation based on molecular dynamics simulation[J]. Separation and Purification Technology, 2021, 276: 119254. |
| 48 | ZEESHAN Muhammad, NOZARI Vahid, KESKIN Seda, et al. Structural factors determining thermal stability limits of ionic liquid/MOF composites: Imidazolium ionic liquids combined with CuBTC and ZIF-8[J]. Industrial & Engineering Chemistry Research, 2019, 58(31): 14124-14138. |
| [1] | 陈可欣, 李熙, 常福城, 武萧衣, 娄嘉诚, 李会雄. 螺旋管内水-水蒸气两相流压降及流型转变特性[J]. 化工进展, 2025, 44(2): 613-624. |
| [2] | 张迁, 刘鑫, 王冰, 徐晶, 曹晨熙. 复杂风速风向与事件树下储罐区多米诺事故分析[J]. 化工进展, 2025, 44(2): 1170-1182. |
| [3] | 于海, 栾智勇, 姬宜朋, 安申法, 陈家庆, 司政, 任强, 孙丰旭, 宋泽润. 动态水力旋流器内短路流流量的计算方法及影响分析[J]. 化工进展, 2025, 44(1): 135-144. |
| [4] | 乔磊, 张亚新, 魏博, 冉文燊, 马金荣, 王峰. 氧热法气流床电石反应器烧嘴布置参数及操作参数优化[J]. 化工进展, 2025, 44(1): 145-157. |
| [5] | 邢雷, 周晓庆, 蒋明虎, 赵立新, 李新亚, 陈德海. 突缩突扩圆管内离散油滴运动行为及变形特性[J]. 化工进展, 2025, 44(1): 27-37. |
| [6] | 李灏, 孙昱楠, 李健, 陶俊宇, 程占军, 颜蓓蓓, 陈冠益. 陈腐垃圾与原生垃圾共气化特性[J]. 化工进展, 2025, 44(1): 525-537. |
| [7] | 孙建辰, 杨捷, 李军, 孙会东, 牛俊敏, 廖逸飞, 任俊颖, 商辉. 催化剂颗粒排列方式对微波加热效果的影响[J]. 化工进展, 2025, 44(1): 57-65. |
| [8] | 张天昊, 李双喜, 贾祥际, 胡鼎国, 崔瑞焯, 李世聪. 干摩擦釜用机械密封DLC涂层-石墨配副摩擦磨损与温度变形场分析[J]. 化工进展, 2024, 43(S1): 121-133. |
| [9] | 毛宁轩, 万小维, 鞠杰, 胡彦杰, 江浩. 工业气固流化床内流场的CFD-PBM数值模拟和结构优化[J]. 化工进展, 2024, 43(S1): 13-20. |
| [10] | 苏瑶, 陈占秀, 杨历, 邢赫威, 呼和仓, 李源华. 热源温度对非对称纳米通道流动换热的影响[J]. 化工进展, 2024, 43(S1): 144-153. |
| [11] | 张伟业, 朱晓武, 罗永皓, 王志. 复合型叶序微流道混合性能的数值模拟[J]. 化工进展, 2024, 43(S1): 154-165. |
| [12] | 尹少武, 黄若萧, 昝晓君, 童莉葛, 刘传平, 王立. 基于CPCM正六边形砖的蓄热储能系统设计与蓄放热模拟[J]. 化工进展, 2024, 43(S1): 243-254. |
| [13] | 杨会民, 杜加丽, 权亚文, 吴升潇, 靳皎, 吴峰. 侧喷嘴下行床内热量传递特性CFD模拟[J]. 化工进展, 2024, 43(S1): 32-42. |
| [14] | 陈王觅, 席北斗, 李鸣晓, 叶美瀛, 侯佳奇, 于承泽, 魏域芳, 孟繁华. 热解系统碳排放削减技术研究进展[J]. 化工进展, 2024, 43(S1): 479-503. |
| [15] | 李磊, 赵宴民, 田海洋, 李江伟, 周强, 何佳妮, 武琬越. 燃气烟气中低浓度CO2的低能耗高效捕集工艺模拟优化[J]. 化工进展, 2024, 43(S1): 581-589. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||