化工进展 ›› 2024, Vol. 43 ›› Issue (S1): 650-661.DOI: 10.16085/j.issn.1000-6613.2024-0813
油页岩灰基分子筛的制备及对亚甲基蓝的吸附
石磊1( ), 王倩1(
), 王倩1( ), 赵晓胜2, 刘宏臣1, 车远军1, 段玉1, 李庆1
), 赵晓胜2, 刘宏臣1, 车远军1, 段玉1, 李庆1
- 1.西安工程大学环境与化学工程学院,陕西 西安 710048
2.沧州师范学院化学与化工系,河北 沧州 061001
-
收稿日期:2024-05-17修回日期:2024-07-14出版日期:2024-11-20发布日期:2024-12-06 -
通讯作者:王倩 -
作者简介:石磊(1997—),女,硕士研究生,研究方向为基于油页岩灰的分子筛制备及其吸附染料分子。E-mail:shileibao2021@163.com。 -
基金资助:国家自然科学基金(22008187)
Synthesis and methyl blue adsorption performance of oil shale ash-based zeolites
SHI Lei1( ), WANG Qian1(
), WANG Qian1( ), ZHAO Xiaosheng2, LIU Hongchen1, CHE Yuanjun1, DUAN Yu1, LI Qing1
), ZHAO Xiaosheng2, LIU Hongchen1, CHE Yuanjun1, DUAN Yu1, LI Qing1
- 1.School of Environmental and Chemical Engineering, Xi'an Polytechnic University, Xi'an 710048, Shaanxi, China
2.College of Chemistry and Chemical Engineering, Cangzhou Normal University, Cangzhou 061001, Hebei, China
-
Received:2024-05-17Revised:2024-07-14Online:2024-11-20Published:2024-12-06 -
Contact:WANG Qian
摘要:
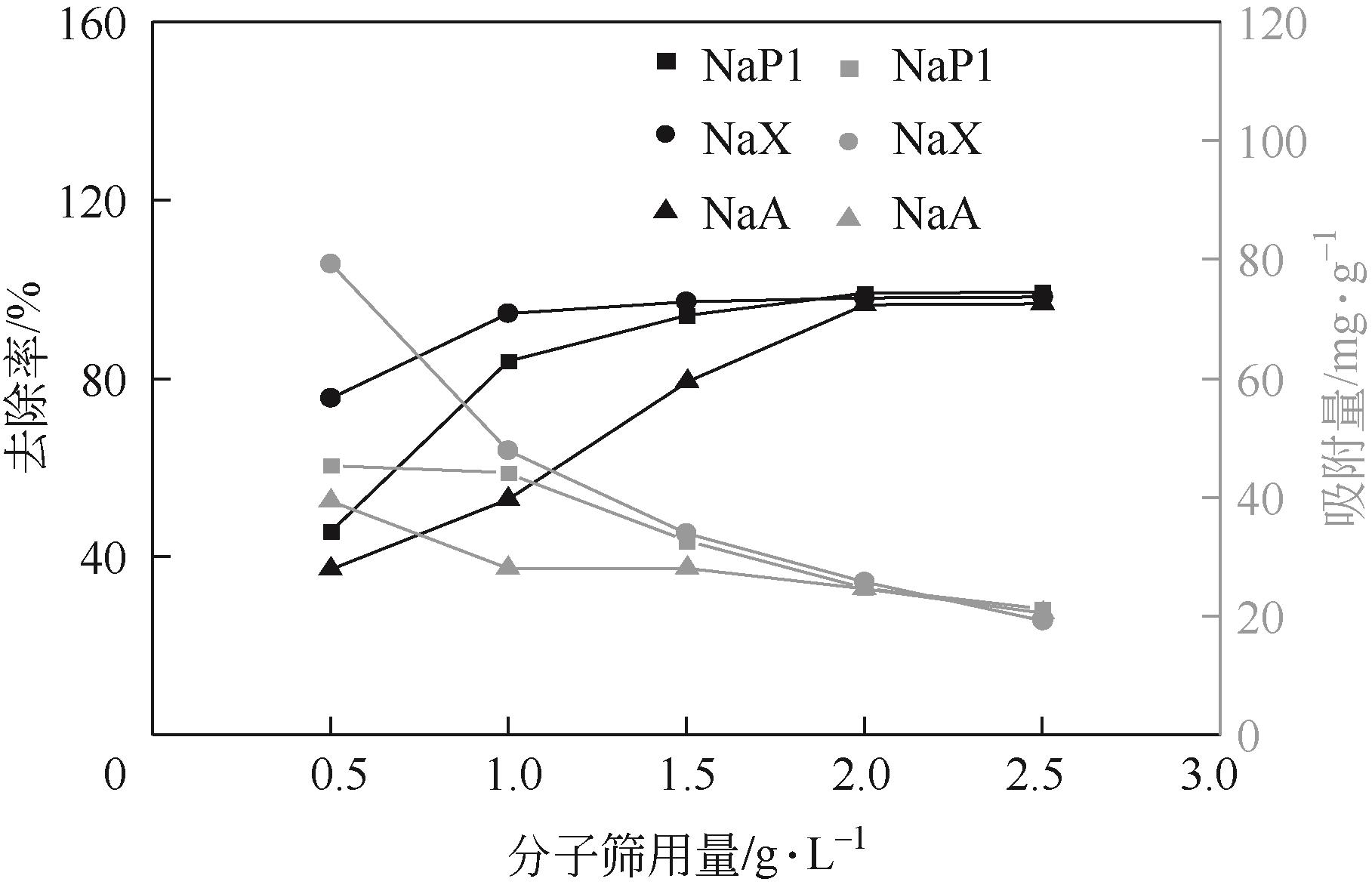
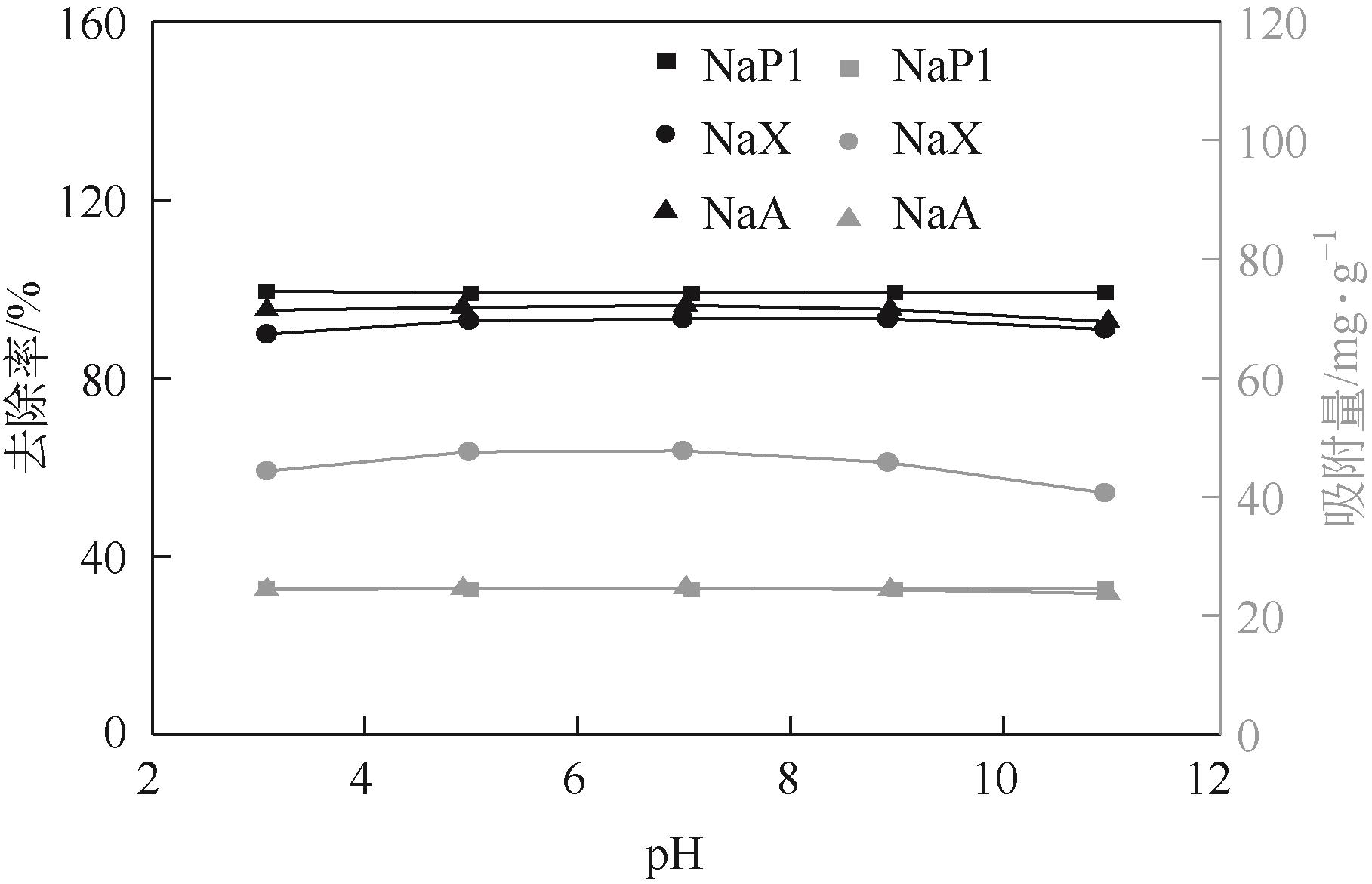
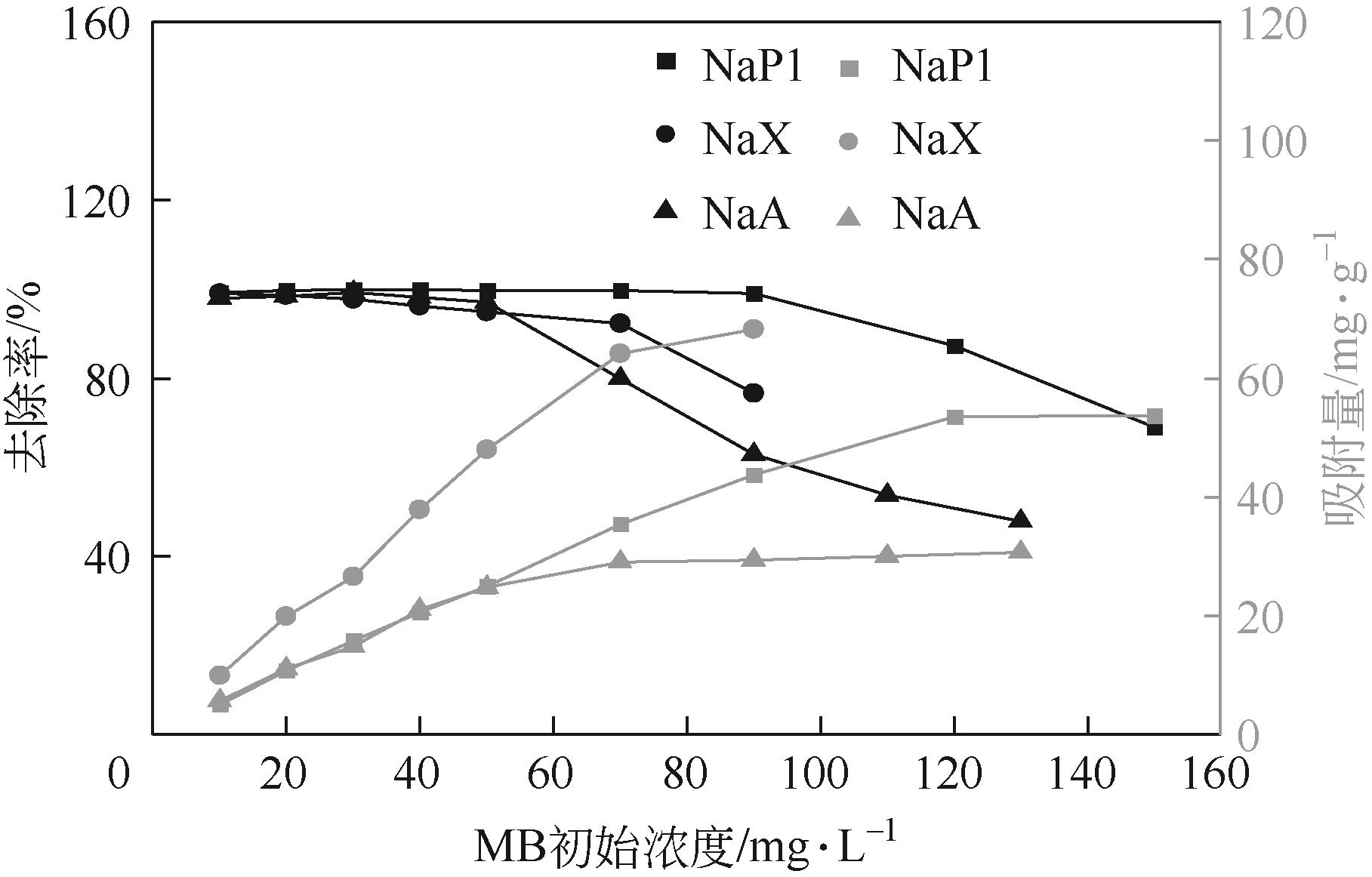
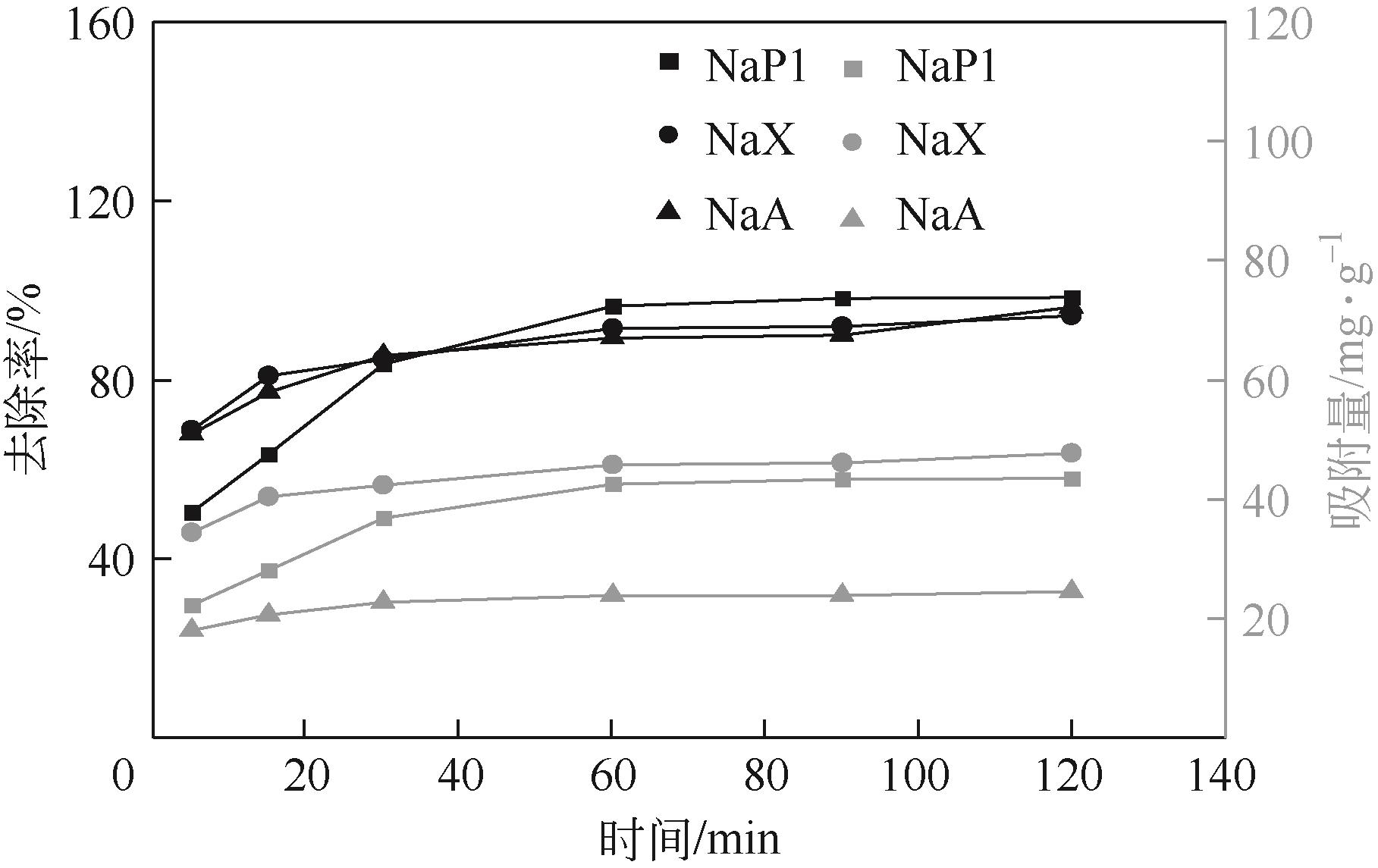
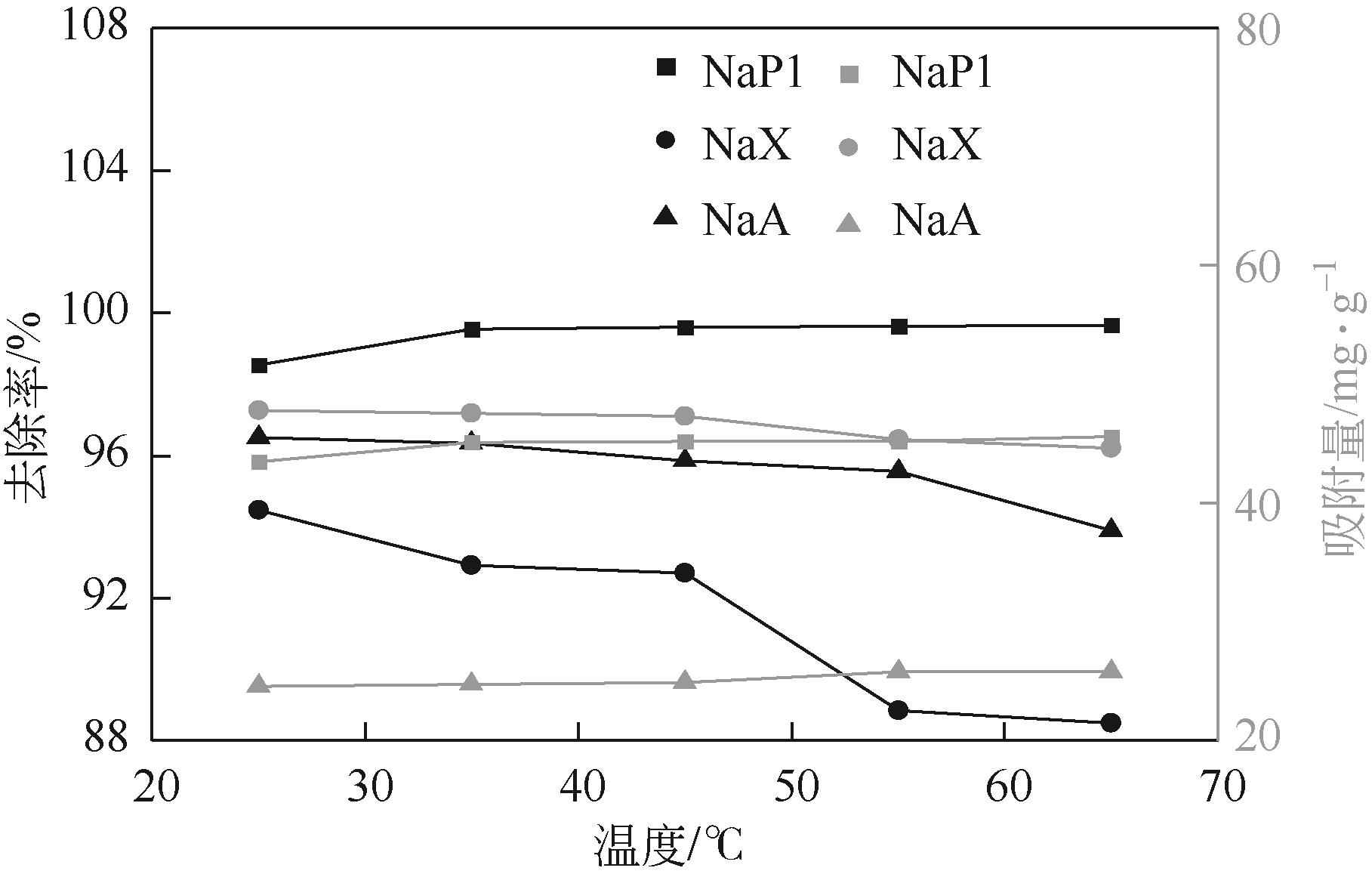
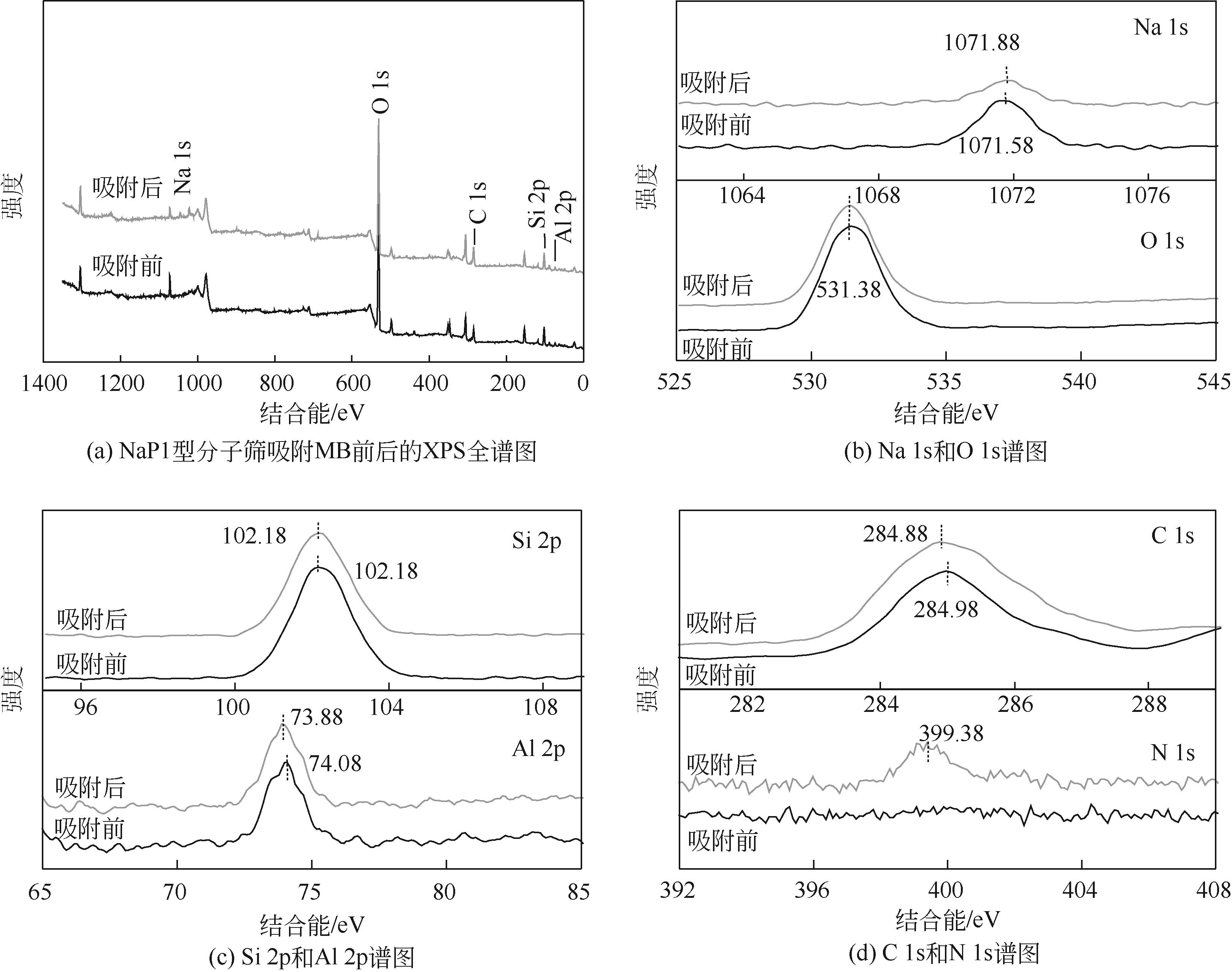
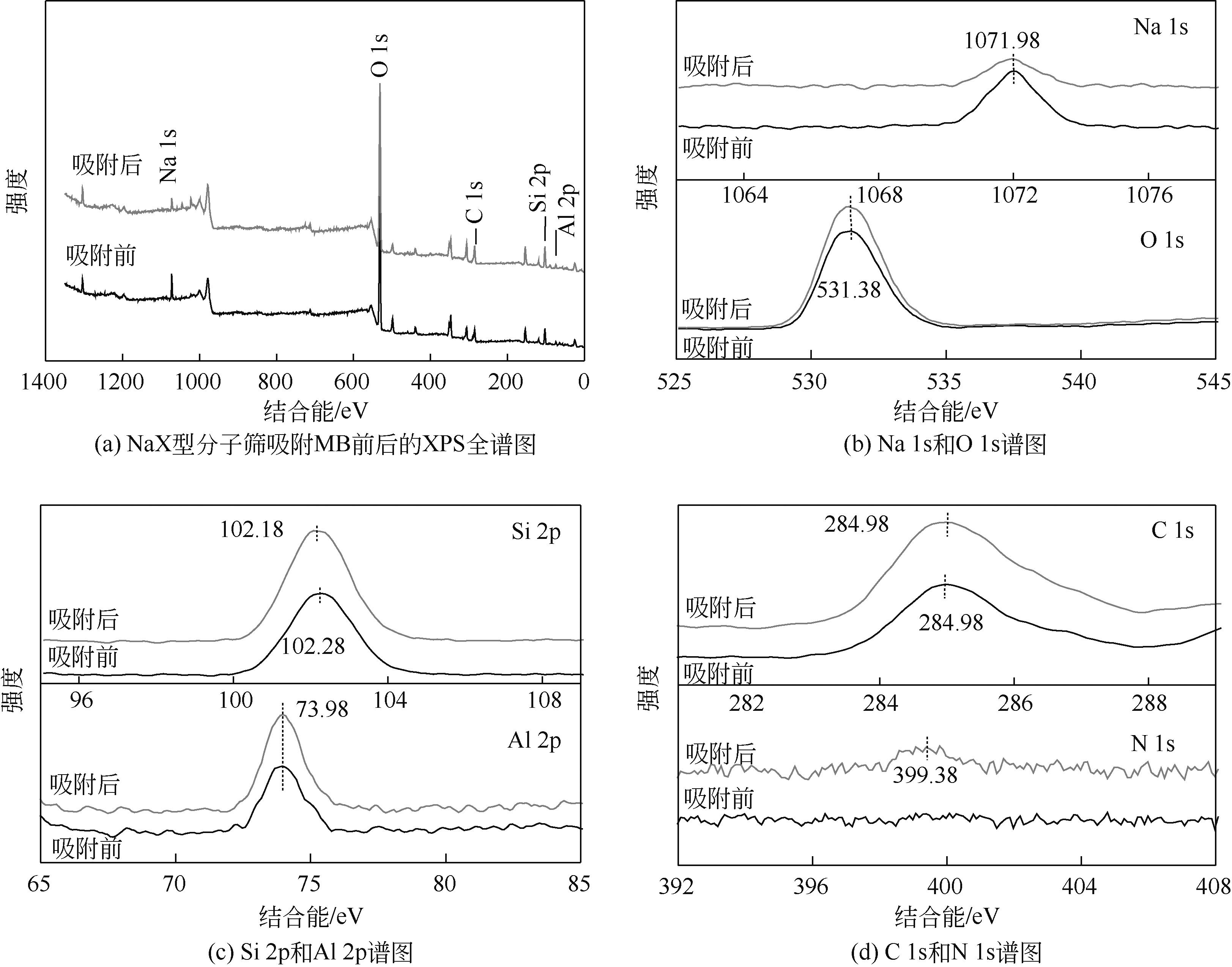
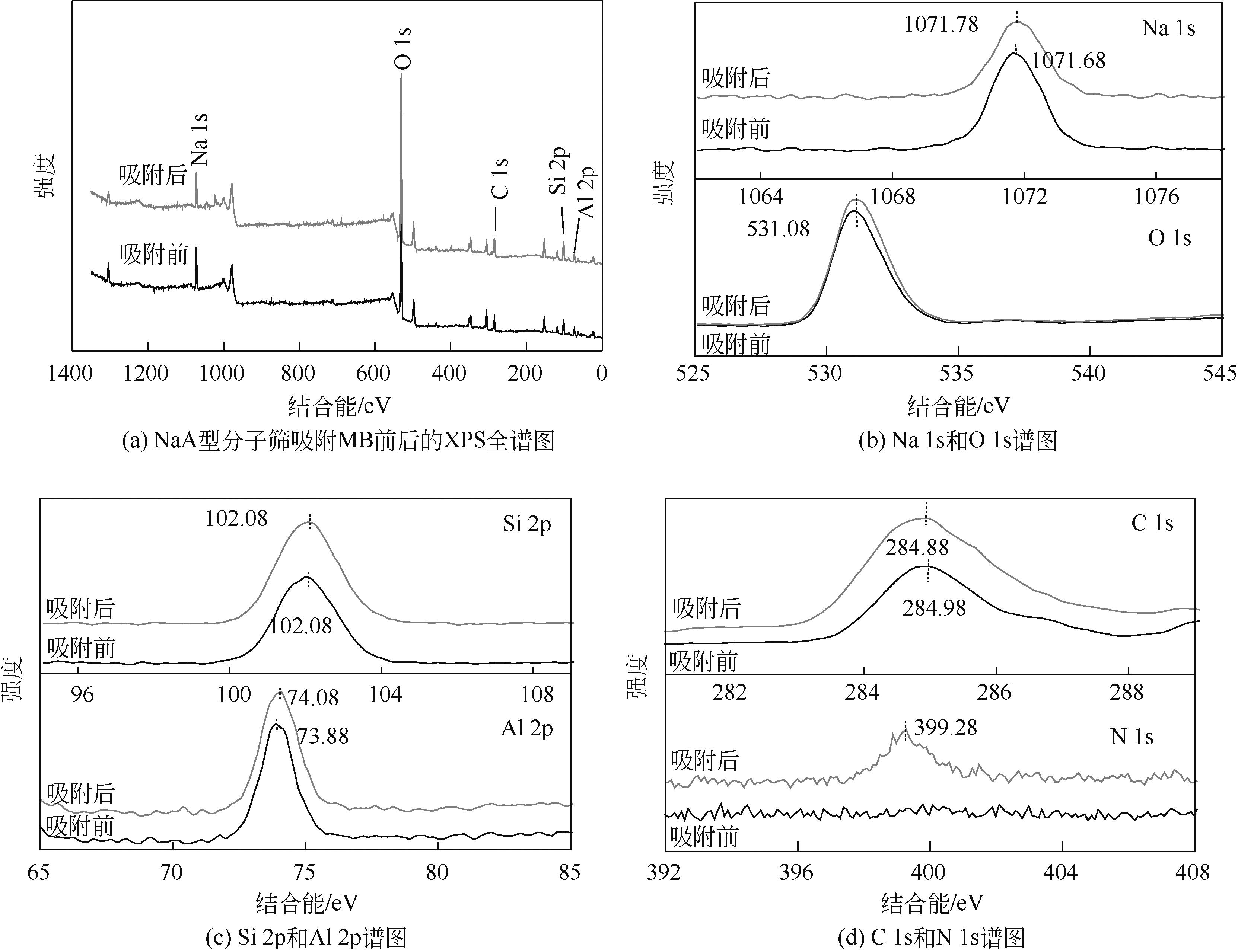
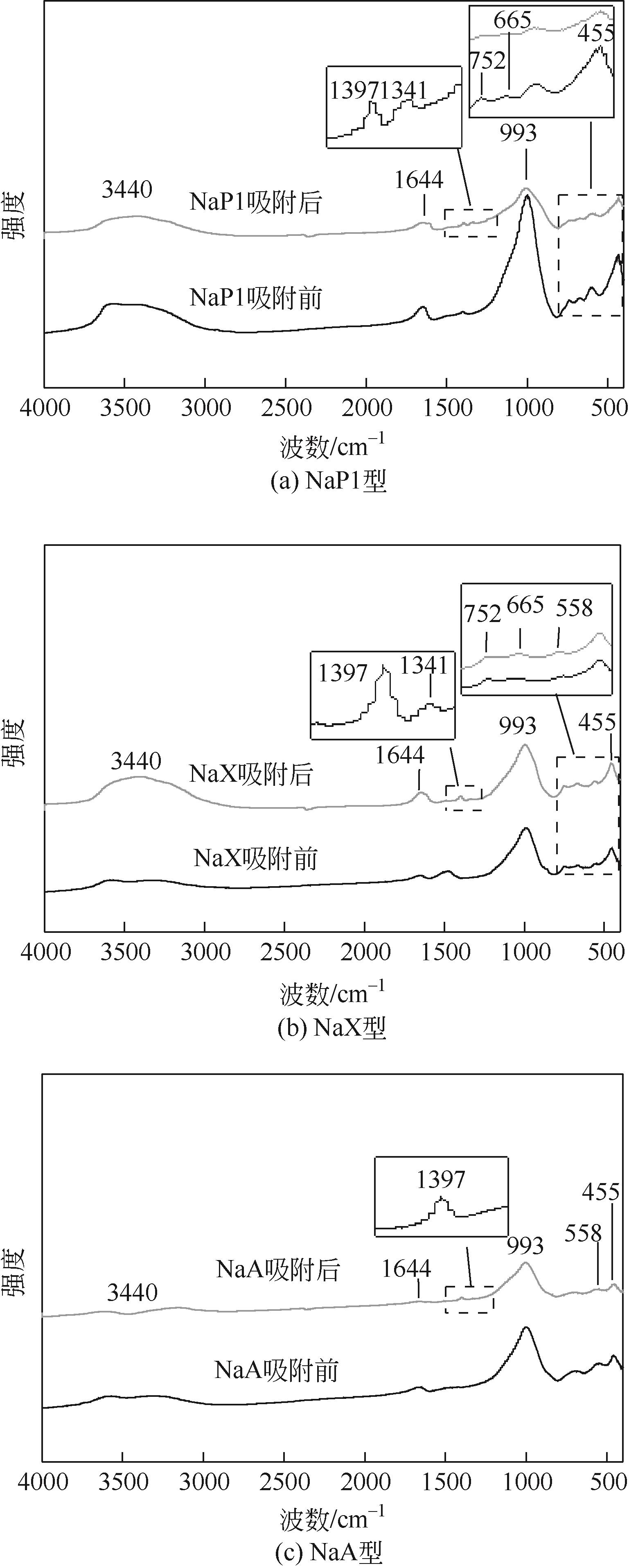
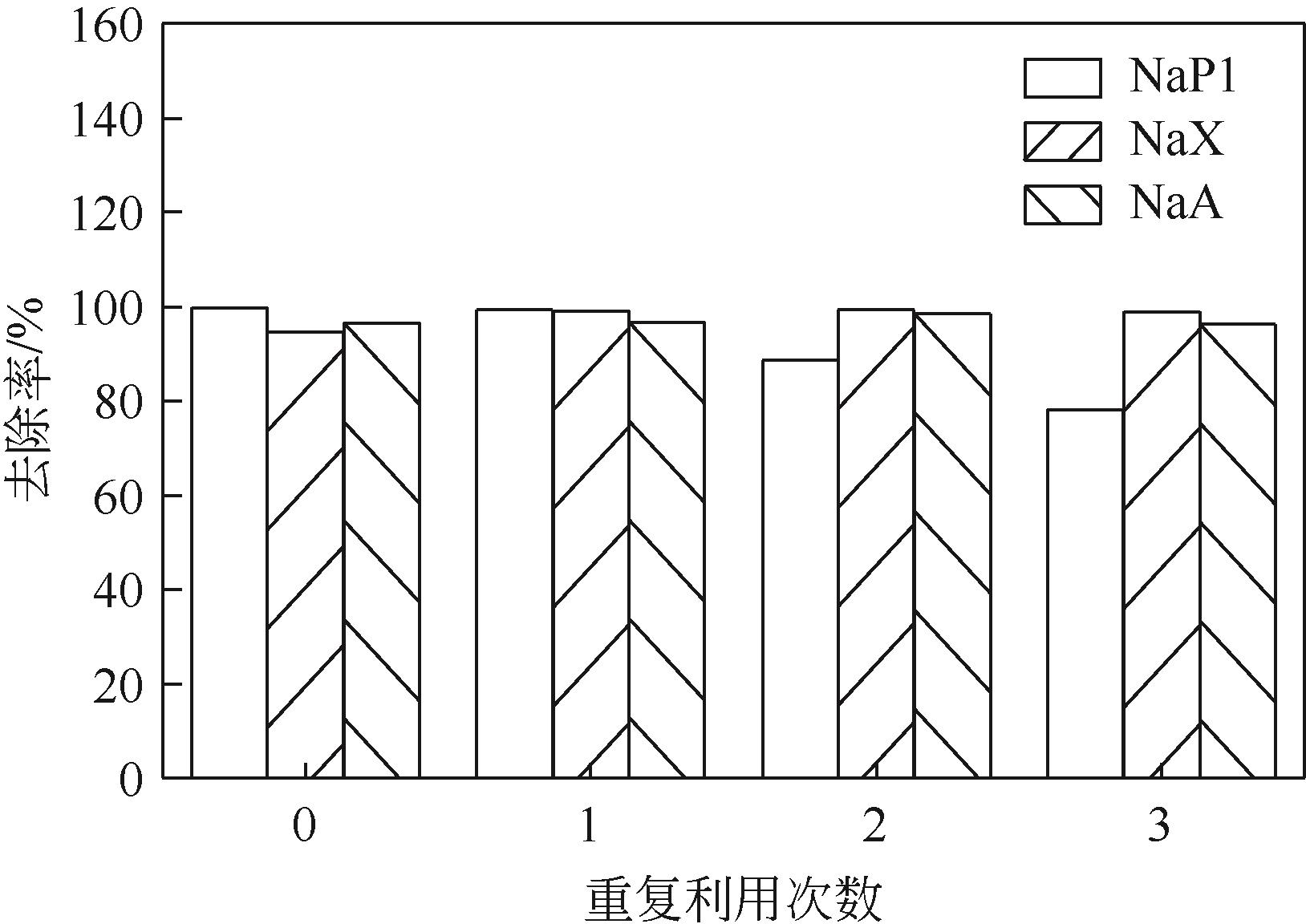
针对油页岩灰基分子筛的类型和结构与其吸附染料分子能力间构效关系不明确的问题,以北票油页岩灰(BPA)为原料制备了NaP1、NaX和NaA三种不同类型的分子筛,并将其用于吸附亚甲基蓝(MB),考察了分子筛用量、MB初始浓度、pH、吸附温度和时间对吸附的影响规律,阐明了分子筛吸附MB过程的吸附机理,明确了不同类型分子筛与其吸附MB能力之间的构效关系。结果表明,分子筛的比表面积和孔结构对其吸附能力影响较大,在最优化吸附条件下,NaP1、NaX和NaA型分子筛对MB的去除率分别为99.7%、94.5%和96.5%,且均具有良好的可再生重复利用性能。吸附过程符合拟二级动力学和Langmuir等温吸附模型。NaP1型分子筛对MB的吸附是自发的、吸热的熵增过程;NaX和NaA型分子筛对MB的吸附是自发的、放热的熵减过程。吸附机理研究表明,静电引力、氢键和孔扩散是分子筛吸附MB过程的主要推动力。可为油页岩灰的资源化利用以及低成本吸附法处理印染废水提供有价值的实践和理论借鉴。
中图分类号:
引用本文
石磊, 王倩, 赵晓胜, 刘宏臣, 车远军, 段玉, 李庆. 油页岩灰基分子筛的制备及对亚甲基蓝的吸附[J]. 化工进展, 2024, 43(S1): 650-661.
SHI Lei, WANG Qian, ZHAO Xiaosheng, LIU Hongchen, CHE Yuanjun, DUAN Yu, LI Qing. Synthesis and methyl blue adsorption performance of oil shale ash-based zeolites[J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 650-661.
| 分子筛 | 灰碱比 | 熔融 | 灰水比 | 老化 | 沉降 | 水热 | 干燥 |
|---|---|---|---|---|---|---|---|
| NaP1 | ABPA∶NaOH=1∶1.5 | 650℃;2h | 1∶10 | 60℃;2h | — | 120℃;10h | 60℃;10h |
| NaX | BPA∶NaOH=1∶2 | 600℃;1h | 1∶6 | 室温;3h | — | 80℃;12h | 110℃;5h |
| NaA | BPA∶NaOH∶Al2O3=1∶0.8∶0.3669 | 700℃;2h | 1∶10 | 室温;3h | 12h | 100℃;24h | 105℃;10h |
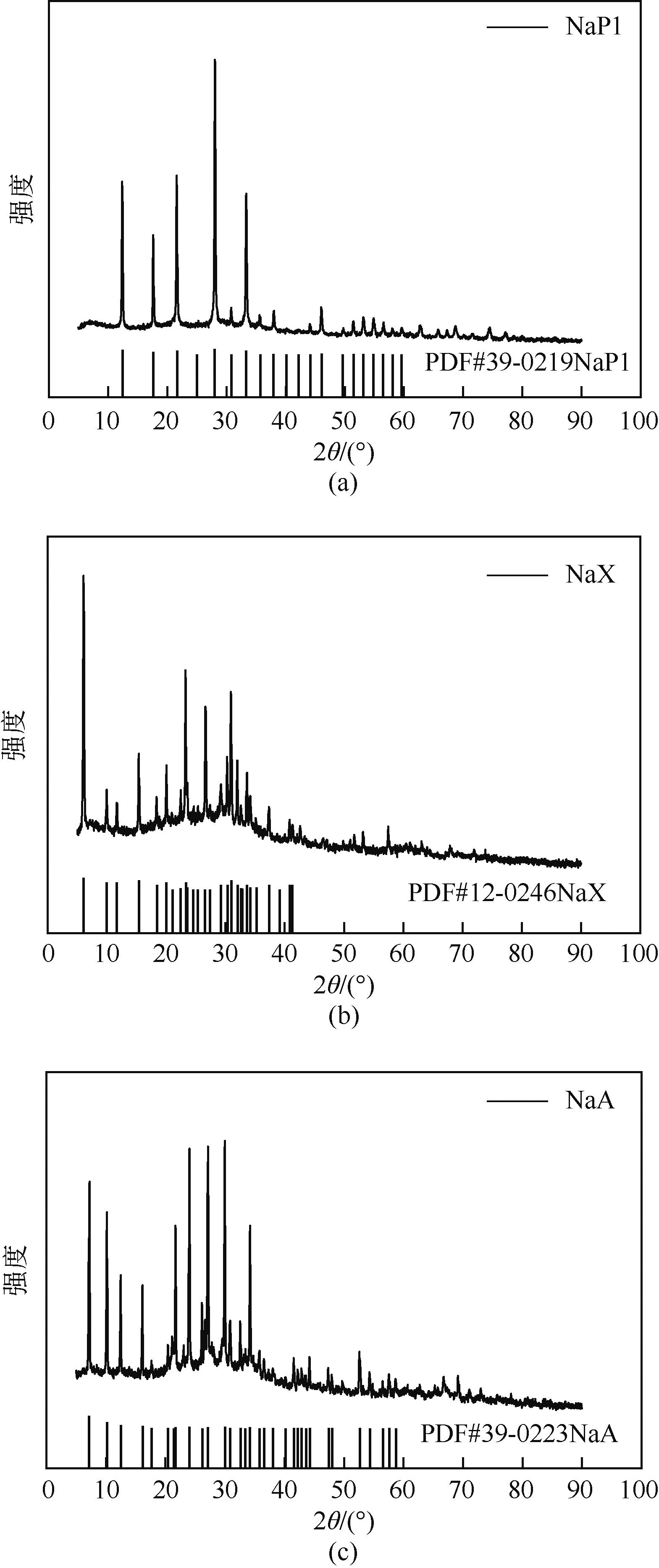
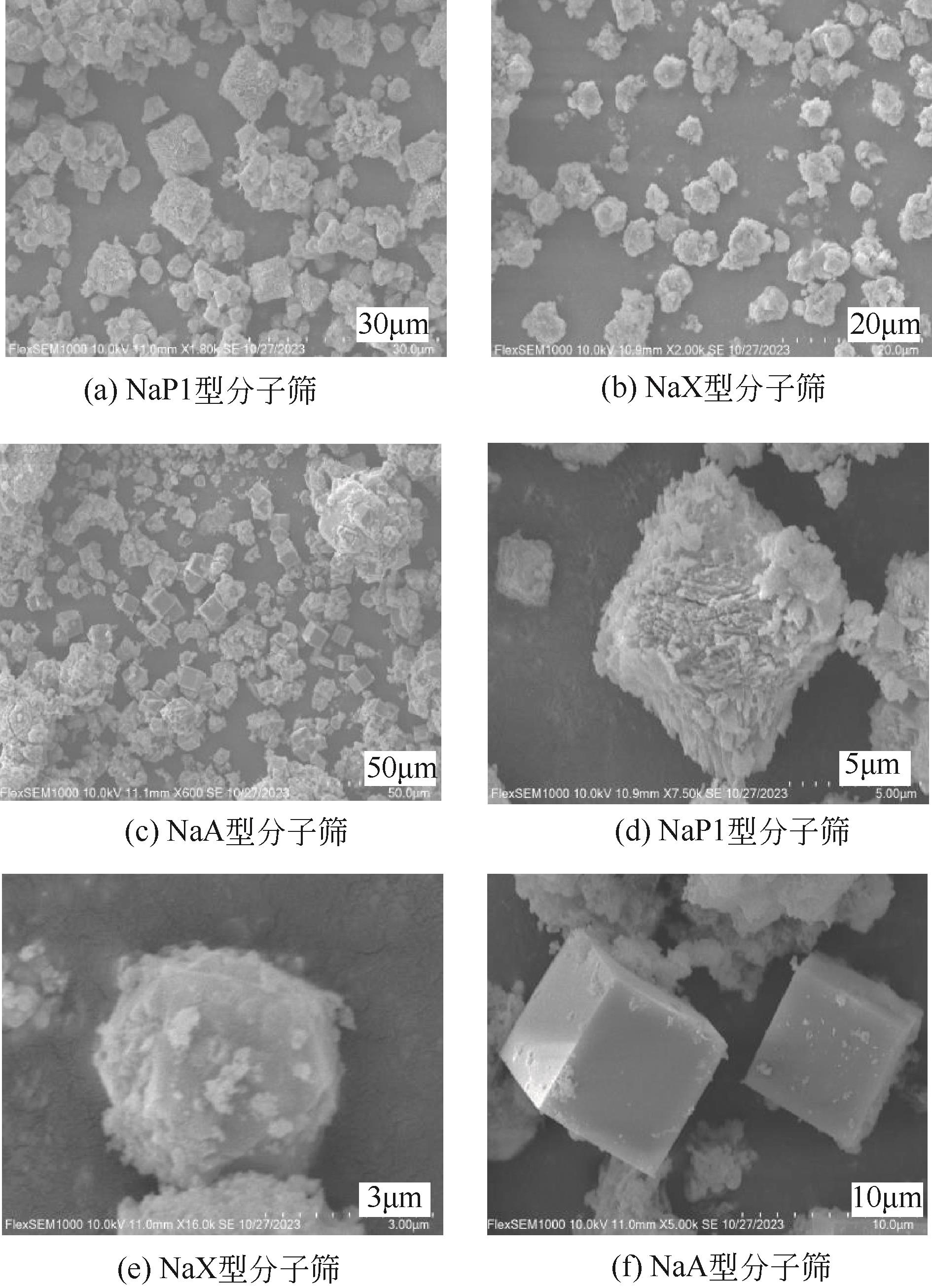
表1 不同类型分子筛的合成条件
| 分子筛 | 灰碱比 | 熔融 | 灰水比 | 老化 | 沉降 | 水热 | 干燥 |
|---|---|---|---|---|---|---|---|
| NaP1 | ABPA∶NaOH=1∶1.5 | 650℃;2h | 1∶10 | 60℃;2h | — | 120℃;10h | 60℃;10h |
| NaX | BPA∶NaOH=1∶2 | 600℃;1h | 1∶6 | 室温;3h | — | 80℃;12h | 110℃;5h |
| NaA | BPA∶NaOH∶Al2O3=1∶0.8∶0.3669 | 700℃;2h | 1∶10 | 室温;3h | 12h | 100℃;24h | 105℃;10h |
| 样品 | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | SO3 | 其他 |
|---|---|---|---|---|---|---|---|---|
| BPA | 60.08 | 14.38 | 9.13 | 5.08 | 3.66 | 3.22 | 2.09 | 2.35 |
| ABPA | 75.76 | 11.39 | 1.94 | 3.11 | 4.40 | 1.79 | 0.10 | 1.49 |
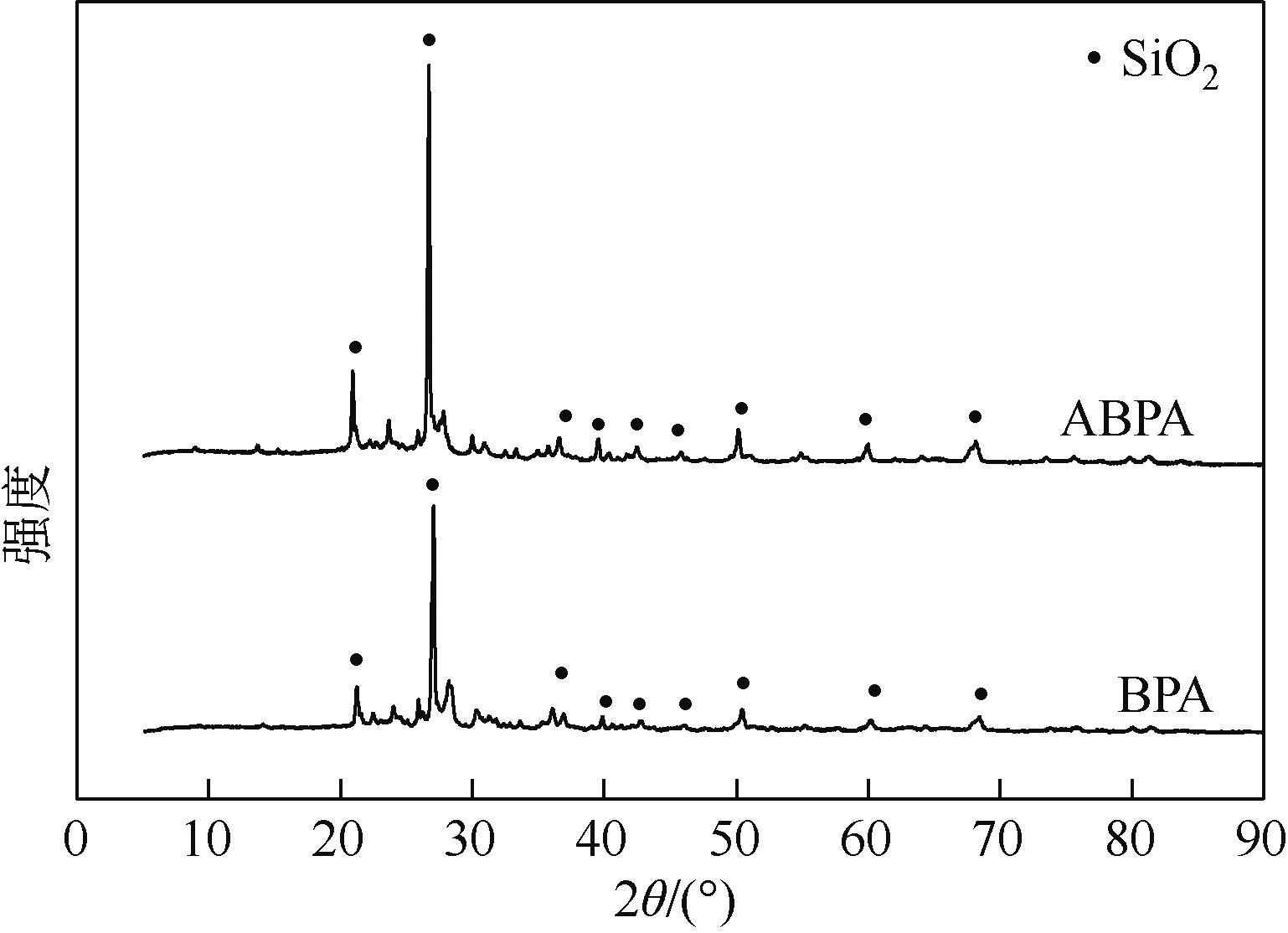
表2 BPA和ABPA的化学组成 (质量分数:%)
| 样品 | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | SO3 | 其他 |
|---|---|---|---|---|---|---|---|---|
| BPA | 60.08 | 14.38 | 9.13 | 5.08 | 3.66 | 3.22 | 2.09 | 2.35 |
| ABPA | 75.76 | 11.39 | 1.94 | 3.11 | 4.40 | 1.79 | 0.10 | 1.49 |
| 分子筛 | 吸附等温线类型 | 回滞环 类型 | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm |
|---|---|---|---|---|---|
| NaP1 | Ⅳ | H3 | 97.90 | 0.097 | 5.73 |
| NaX | Ⅳ | H4 | 351.39 | 0.174 | 5.31 |
| NaA | Ⅳ | H3 | 31.21 | 0.121 | 20.60 |
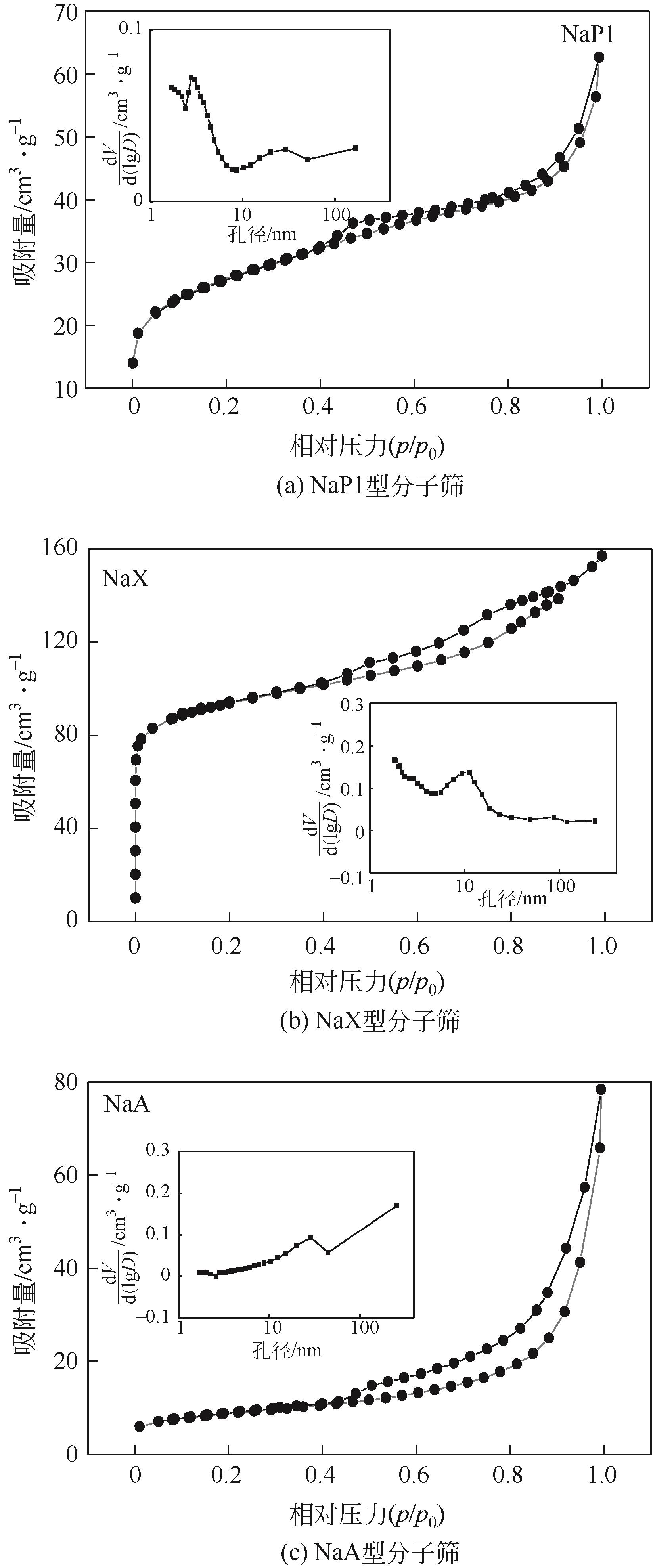
表3 合成分子筛的孔性质参数
| 分子筛 | 吸附等温线类型 | 回滞环 类型 | 比表面积 /m2·g-1 | 孔体积 /cm3·g-1 | 平均孔径 /nm |
|---|---|---|---|---|---|
| NaP1 | Ⅳ | H3 | 97.90 | 0.097 | 5.73 |
| NaX | Ⅳ | H4 | 351.39 | 0.174 | 5.31 |
| NaA | Ⅳ | H3 | 31.21 | 0.121 | 20.60 |
| 分子筛 | 模型 | 参数 | 数值 | ||||
|---|---|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | 55℃ | 65℃ | |||
| NaP1 | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 43.51 | 45.13 | 45.17 | 45.21 | 45.61 |
| qe,cal/mg·g-1 | 42.26 | 23.94 | 10.43 | 3.68 | 1.46 | ||
| k1/min-1 | 0.070 | 0.047 | 0.039 | 0.022 | 0.023 | ||
| R2 | 0.9778 | 0.8938 | 0.7699 | 0.2270 | 0.0463 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 46.51 | 47.62 | 46.21 | 46.02 | 46.00 | |
| k2/g·mg-1·min-1 | 0.003 | 0.003 | 0.008 | 0.019 | 0.049 | ||
| R2 | 0.9981 | 0.9994 | 0.9997 | 0.9996 | 0.9999 | ||
| 内扩散动力学 | C1/mg·g-1 | 19.45 | 21.48 | 29.33 | 35.06 | 40.45 | |
| kp/mg·g-1·min-1/2 | 2.5212 | 2.4930 | 1.7203 | 1.1736 | 0.6013 | ||
| R2 | 0.8503 | 0.7861 | 0.5078 | 0.3960 | 0.4552 | ||
| NaX | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 47.76 | 47.59 | 47.35 | 45.76 | 44.65 |
| qe,cal/mg·g-1 | 11.71 | 9.22 | 9.33 | 4.72 | 8.30 | ||
| k1/min-1 | 0.025 | 0.039 | 0.023 | 0.023 | 0.018 | ||
| R2 | 0.8922 | 0.6879 | 0.6586 | 0.8418 | 0.6734 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 48.47 | 48.47 | 47.82 | 45.93 | 44.82 | |
| k2/g·mg-1·min-1 | 0.006 | 0.009 | 0.008 | 0.020 | 0.008 | ||
| R2 | 0.9992 | 0.9996 | 0.9994 | 0.9999 | 0.9984 | ||
| 内扩散动力学 | C1/mg·g-1 | 33.72 | 34.76 | 33.84 | 38.93 | 33.38 | |
| kp/mg·g-1·min-1/2 | 1.3788 | 1.3598 | 1.3632 | 0.6989 | 1.0673 | ||
| R2 | 0.8682 | 0.6934 | 0.6867 | 0.6749 | 0.8305 | ||
| NaA | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 24.55 | 24.75 | 24.90 | 25.82 | 25.82 |
| qe,cal/mg·g-1 | 5.70 | 6.31 | 10.11 | 5.17 | 6.58 | ||
| k1/min-1 | 0.029 | 0.054 | 0.075 | 0.016 | 0.028 | ||
| R2 | 0.8848 | 0.9250 | 0.8714 | 0.7841 | 0.9527 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 24.94 | 25.18 | 25.29 | 25.89 | 26.32 | |
| k2/g·mg-1·min-1 | 0.015 | 0.021 | 0.022 | 0.012 | 0.012 | ||
| R2 | 0.9997 | 0.9999 | 0.9999 | 0.9971 | 0.9991 | ||
| 内扩散动力学 | C1/mg·g-1 | 17.71 | 19.00 | 20.23 | 19.13 | 18.86 | |
| kp/mg·g-1·min-1/2 | 0.6911 | 0.6112 | 0.4837 | 0.6074 | 0.6797 | ||
| R2 | 0.8292 | 0.7336 | 0.8755 | 0.8296 | 0.9406 | ||
表4 分子筛吸附MB的动力学参数
| 分子筛 | 模型 | 参数 | 数值 | ||||
|---|---|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | 55℃ | 65℃ | |||
| NaP1 | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 43.51 | 45.13 | 45.17 | 45.21 | 45.61 |
| qe,cal/mg·g-1 | 42.26 | 23.94 | 10.43 | 3.68 | 1.46 | ||
| k1/min-1 | 0.070 | 0.047 | 0.039 | 0.022 | 0.023 | ||
| R2 | 0.9778 | 0.8938 | 0.7699 | 0.2270 | 0.0463 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 46.51 | 47.62 | 46.21 | 46.02 | 46.00 | |
| k2/g·mg-1·min-1 | 0.003 | 0.003 | 0.008 | 0.019 | 0.049 | ||
| R2 | 0.9981 | 0.9994 | 0.9997 | 0.9996 | 0.9999 | ||
| 内扩散动力学 | C1/mg·g-1 | 19.45 | 21.48 | 29.33 | 35.06 | 40.45 | |
| kp/mg·g-1·min-1/2 | 2.5212 | 2.4930 | 1.7203 | 1.1736 | 0.6013 | ||
| R2 | 0.8503 | 0.7861 | 0.5078 | 0.3960 | 0.4552 | ||
| NaX | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 47.76 | 47.59 | 47.35 | 45.76 | 44.65 |
| qe,cal/mg·g-1 | 11.71 | 9.22 | 9.33 | 4.72 | 8.30 | ||
| k1/min-1 | 0.025 | 0.039 | 0.023 | 0.023 | 0.018 | ||
| R2 | 0.8922 | 0.6879 | 0.6586 | 0.8418 | 0.6734 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 48.47 | 48.47 | 47.82 | 45.93 | 44.82 | |
| k2/g·mg-1·min-1 | 0.006 | 0.009 | 0.008 | 0.020 | 0.008 | ||
| R2 | 0.9992 | 0.9996 | 0.9994 | 0.9999 | 0.9984 | ||
| 内扩散动力学 | C1/mg·g-1 | 33.72 | 34.76 | 33.84 | 38.93 | 33.38 | |
| kp/mg·g-1·min-1/2 | 1.3788 | 1.3598 | 1.3632 | 0.6989 | 1.0673 | ||
| R2 | 0.8682 | 0.6934 | 0.6867 | 0.6749 | 0.8305 | ||
| NaA | 实验值 拟一级动力学 | qe,exp/mg·g-1 | 24.55 | 24.75 | 24.90 | 25.82 | 25.82 |
| qe,cal/mg·g-1 | 5.70 | 6.31 | 10.11 | 5.17 | 6.58 | ||
| k1/min-1 | 0.029 | 0.054 | 0.075 | 0.016 | 0.028 | ||
| R2 | 0.8848 | 0.9250 | 0.8714 | 0.7841 | 0.9527 | ||
| 拟二级动力学 | qe,cal/mg·g-1 | 24.94 | 25.18 | 25.29 | 25.89 | 26.32 | |
| k2/g·mg-1·min-1 | 0.015 | 0.021 | 0.022 | 0.012 | 0.012 | ||
| R2 | 0.9997 | 0.9999 | 0.9999 | 0.9971 | 0.9991 | ||
| 内扩散动力学 | C1/mg·g-1 | 17.71 | 19.00 | 20.23 | 19.13 | 18.86 | |
| kp/mg·g-1·min-1/2 | 0.6911 | 0.6112 | 0.4837 | 0.6074 | 0.6797 | ||
| R2 | 0.8292 | 0.7336 | 0.8755 | 0.8296 | 0.9406 | ||
| 分子筛 | Langmuir | Freundlich | Dubinin-Radushkevich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL /L·mg-1 | R2 | KF /mg·g-1 | n | R2 | qmax/mg·g-1 | KDR/mol2·J-2 | R2 | |
| NaP1 | 54.1 | 1.881 | 0.9996 | 24.21 | 3.332 | 0.5846 | 53.9 | 7.05×10-8 | 0.9490 |
| NaX | 71.8 | 0.868 | 0.9983 | 27.31 | 2.514 | 0.9032 | 49.6 | 6.90×10-8 | 0.8413 |
| NaA | 30.5 | 1.260 | 0.9996 | 14.44 | 4.721 | 0.6212 | 29.2 | 1.03×10-7 | 0.8712 |
表5 分子筛吸附MB的等温线参数
| 分子筛 | Langmuir | Freundlich | Dubinin-Radushkevich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax/mg·g-1 | KL /L·mg-1 | R2 | KF /mg·g-1 | n | R2 | qmax/mg·g-1 | KDR/mol2·J-2 | R2 | |
| NaP1 | 54.1 | 1.881 | 0.9996 | 24.21 | 3.332 | 0.5846 | 53.9 | 7.05×10-8 | 0.9490 |
| NaX | 71.8 | 0.868 | 0.9983 | 27.31 | 2.514 | 0.9032 | 49.6 | 6.90×10-8 | 0.8413 |
| NaA | 30.5 | 1.260 | 0.9996 | 14.44 | 4.721 | 0.6212 | 29.2 | 1.03×10-7 | 0.8712 |
| 分子筛 | 温度/K | Kc | ΔG /kJ·mol-1 | ΔS /J·mol-1·K-1 | ΔH /kJ·mol-1 |
|---|---|---|---|---|---|
| NaP1 | 298.15 | 67.4695 | -10.440 | 128.5495 | 26.866 |
| 308.15 | 225.7327 | -13.884 | |||
| 318.15 | 246.7785 | -14.571 | |||
| 328.15 | 267.6631 | -15.250 | |||
| 338.15 | 297.3266 | -16.010 | |||
| NaX | 298.15 | 16.9697 | -7.019 | -29.5568 | -15.802 |
| 308.15 | 13.1472 | -6.600 | |||
| 318.15 | 12.6924 | -6.721 | |||
| 328.15 | 7.9724 | -5.664 | |||
| 338.15 | 7.6896 | -5.735 | |||
| NaA | 298.15 | 27.6763 | -8.231 | -9.9983 | -11.374 |
| 308.15 | 26.2860 | -8.375 | |||
| 318.15 | 23.0315 | -8.297 | |||
| 328.15 | 21.5106 | -8.372 | |||
| 338.15 | 15.3951 | -7.686 |
表6 分子筛吸附MB的热力学参数
| 分子筛 | 温度/K | Kc | ΔG /kJ·mol-1 | ΔS /J·mol-1·K-1 | ΔH /kJ·mol-1 |
|---|---|---|---|---|---|
| NaP1 | 298.15 | 67.4695 | -10.440 | 128.5495 | 26.866 |
| 308.15 | 225.7327 | -13.884 | |||
| 318.15 | 246.7785 | -14.571 | |||
| 328.15 | 267.6631 | -15.250 | |||
| 338.15 | 297.3266 | -16.010 | |||
| NaX | 298.15 | 16.9697 | -7.019 | -29.5568 | -15.802 |
| 308.15 | 13.1472 | -6.600 | |||
| 318.15 | 12.6924 | -6.721 | |||
| 328.15 | 7.9724 | -5.664 | |||
| 338.15 | 7.6896 | -5.735 | |||
| NaA | 298.15 | 27.6763 | -8.231 | -9.9983 | -11.374 |
| 308.15 | 26.2860 | -8.375 | |||
| 318.15 | 23.0315 | -8.297 | |||
| 328.15 | 21.5106 | -8.372 | |||
| 338.15 | 15.3951 | -7.686 |
| 1 | 任南琪, 周显娇, 郭婉茜, 等. 染料废水处理技术研究进展[J]. 化工学报, 2013, 64(1): 84-94. |
| REN Nanqi, ZHOU Xianjiao, GUO Wanqian, et al. A review on treatment methods of dye wastewater[J]. CIESC Journal, 2013, 64(1): 84-94. | |
| 2 | DUTA Anca, VISA Maria. Simultaneous removal of two industrial dyes by adsorption and photocatalysis on a fly-ash-TiO2 composite[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2015, 306: 21-30. |
| 3 | HADDAD Mohammadine EL, SLIMANI Rachid, MAMOUNI Rachid, et al. Removal of two textile dyes from aqueous solutions onto calcined bones[J]. Journal of the Association of Arab Universities for Basic and Applied Sciences, 2013, 14(1): 51-59. |
| 4 | CAO Jiashun, LIN Junxiong, FANG Fang, et al. A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies[J]. Bioresource Technology, 2014, 163: 199-205. |
| 5 | DOYLE Aidan M, ALISMAEEL Ziad T, ALBAYATI Talib M, et al. High purity FAU-type zeolite catalysts from shale rock for biodiesel production[J]. Fuel, 2017, 199: 394-402. |
| 6 | LI Hui, ZHENG Feng, WANG Jing, et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance[J]. Chemical Engineering Journal, 2020, 390: 124513. |
| 7 | Piotr ROŻEK, Magdalena KRÓL, Włodzimierz MOZGAWA. Geopolymer-zeolite composites: A review[J]. Journal of Cleaner Production, 2019, 230: 557-579. |
| 8 | WANG Qian, HOU Yucui, WU Weize, et al. A deep insight into the structural characteristics of Yilan oil shale kerogen through selective oxidation[J]. Carbon Resources Conversion, 2019, 2(3): 182-190. |
| 9 | WANG Qian, HOU Yucui, WU Weize, et al. The relationship between the humic degree of oil shale kerogens and their structural characteristics[J]. Fuel, 2017, 209: 35-42. |
| 10 | 张立栋, 刘洪鹏, 贾春霞, 等. 我国油页岩综合利用相关研究进展[J]. 化工进展, 2012, 31(11): 2359-2363. |
| ZHANG Lidong, LIU Hongpeng, JIA Chunxia, et al. Research progress of comprehensive utilization of oil shale in China[J]. Chemical Industry and Engineering Progress, 2012, 31(11): 2359-2363. | |
| 11 | WANG Sha, JIANG Xiumin, HAN Xiangxin, et al. Investigation of Chinese oil shale resources comprehensive utilization performance[J]. Energy, 2012, 42(1): 224-232. |
| 12 | QIAN Yu, YANG Qingchun, ZHANG Jun, et al. Development of an integrated oil shale refinery process with coal gasification for hydrogen production[J]. Industrial & Engineering Chemistry Research, 2014, 53(51): 19970-19978. |
| 13 | SHAWABKEH Reyad, Adnan AL-HARAHSHEH, HAMI Malik, et al. Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater[J]. Fuel, 2004, 83(7/8): 981-985. |
| 14 | Phuong Lan TRAN-NGUYEN, Kim-Phung LY, THANH Luong Huynh Vu, et al. Facile synthesis of zeolite NaX using rice husk ash without pretreatment[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 123: 338-345. |
| 15 | YU Xiaohua, Hailiang LYU, ZHOU Guowei, et al. Absorption of methyl orange by modified fly zeolites[J]. Advanced Materials Research, 2012, 476/477/478: 1365-1369. |
| 16 | 赵晓胜, 周自成, 王倩, 等. 油页岩灰基NaX型沸石的合成及表征[J]. 煤炭与化工, 2022, 45(7): 145-149, 160. |
| ZHAO Xiaosheng, ZHOU Zicheng, WANG Qian, et al. Synthesis and characterization of NaX zeolite based on the oil shale ash[J]. Coal and Chemical Industry, 2022, 45(7): 145-149, 160. | |
| 17 | BAI Shuxia, ZHOU Lingmei, CHANG Zhibing, et al. Synthesis of Na-X zeolite from Longkou oil shale ash by alkaline fusion hydrothermal method[J]. Carbon Resources Conversion, 2018, 1(3): 245-250. |
| 18 | 李燕, 周思奇, 李翔. 油页岩废渣水热合成4A分子筛[J]. 山东化工, 2017, 46(17): 40-41. |
| LI Yan, ZHOU Siqi, LI Xiang. Hydrothermal synthesis of 4A molecular sieve from oil shale waste[J]. Shandong Chemical Industry, 2017, 46(17): 40-41. | |
| 19 | BAO Weiwei, LIU Lu, ZOU Haifeng, et al. Removal of Cu2+ from aqueous solutions using Na-A zeolite from oil shale ash[J]. Chinese Journal of Chemical Engineering, 2013, 21(9): 974-982. |
| 20 | 赵雯, 李函霏, 吴畏. 油页岩灰渣合成P型沸石及对Co2+吸附性研究[J]. 硅酸盐通报, 2021, 40(5): 1720-1727. |
| ZHAO Wen, LI Hanfei, WU Wei. Synthesis of P-type zeolite from oil shale ash and its adsorption to Co2+ [J]. Bulletin of the Chinese Ceramic Society, 2021, 40(5): 1720-1727. | |
| 21 | SHI Lei, WANG Qian, ZHAO Xiaosheng, et al. The methyl blue adsorption performance and mechanism of NaX zeolite synthesized from Huadian oil shale ash[J]. Journal of the Taiwan Institute of Chemical Engineers, 2023, 147: 104904. |
| 22 | SAPAWE N, JALIL AA, TRIWAHYONO S, et al. Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue[J]. Chemical Engineering Journal, 2013, 229: 388-398. |
| 23 | SUPELANO G I, GÓMEZ CUASPUD J A, MORENO-ALDANA L C, et al. Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue[J]. Fuel, 2020, 263: 116800. |
| 24 | CHENG Yong, XU Longjun, LIU Chenglun. NaP1 zeolite synthesized via effective extraction of Si and Al from red mud for methylene blue adsorption[J]. Advanced Powder Technology, 2021, 32(10): 3904-3914. |
| 25 | ZHOU Qi, JIANG Xuguang, QIU Qili, et al. Synthesis of high-quality NaP1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity[J]. Science of the Total Environment, 2023, 855: 158741. |
| 26 | DU Tao, LIU Liying, XIAO Penny, et al. Preparation of zeolite NaA for CO2 capture from nickel laterite residue[J]. International Journal of Minerals, Metallurgy, and Materials, 2014, 21(8): 820-825. |
| 27 | HOR Kar Yan, CHEE Jasmine Mun Cheng, CHONG Mengnan, et al. Evaluation of physicochemical methods in enhancing the adsorption performance of natural zeolite as low-cost adsorbent of methylene blue dye from wastewater[J]. Journal of Cleaner Production, 2016, 118: 197-209. |
| 28 | SIMSEK Gulsah, IMAMOGLU Mustafa. Investigation of equilibrium, kinetic and thermodynamic of methylene blue adsorption onto dehydrated hazelnut husk carbon[J]. Desalination and Water Treatment, 2015, 54(6): 1747-1753. |
| 29 | BAO Weiwei, ZOU Haifeng, GAN Shucai, et al. Adsorption of heavy metal ions from aqueous solutions by zeolite based on oil shale ash: Kinetic and equilibrium studies[J]. Chemical Research in Chinese Universities, 2013, 29(1): 126-131. |
| 30 | MIYAH Youssef, BENJELLOUN Mohammed, LAHRICHI Anissa, et al. Highly-efficient treated oil shale ash adsorbent for toxic dyes removal: Kinetics, isotherms, regeneration, cost analysis and optimization by experimental design[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106694. |
| 31 | 程绿竹, 王宗乾, 王邓峰, 等. 高中空生物质活性碳纤维制备及其对亚甲基蓝的吸附性能[J]. 纺织学报, 2021, 42(2): 129-134. |
| CHENG Lüzhu, WANG Zongqian, WANG Dengfeng, et al. Preparation of highly hollow biomass-based activated carbon fiber and its adsorption property to methylene blue[J]. Journal of Textile Research, 2021, 42(2): 129-134. | |
| 32 | CHENG Yong, XU Longjun, JIANG Zao, et al. Feasible low-cost conversion of red mud into magnetically separated and recycled hybrid SrFe12O19@NaP1 zeolite as a novel wastewater adsorbent[J]. Chemical Engineering Journal, 2021, 417: 128090. |
| 33 | DAI Chengyi, ZHANG Menghan, GUO Xinwen, et al. Mesoporous composite Ni-C-N/SA for selective adsorption of methylene blue from water[J]. Chemical Engineering Journal, 2021, 407: 127181. |
| 34 | LIU Yi, YAN Chunjie, ZHANG Zuhua, et al. A comparative study on fly ash, geopolymer and faujasite block for Pb removal from aqueous solution[J]. Fuel, 2016, 185: 181-189. |
| 35 | CHEN Dan, HU Xin, SHI Lu, et al. Synthesis and characterization of zeolite X from lithium slag[J]. Applied Clay Science, 2012, 59: 148-151. |
| 36 | AHMED Kedir Ebrahim, KUO Dong-Hau, KEBEDE Worku Lakew. In-situ synthesis and characterizations of Bi2(O,S)3/Zn(O,S) composites for visible light hexavalent chromium reduction[J]. Advanced Powder Technology, 2019, 30(8): 1664-1671. |
| 37 | 全姗姗, 朱玉婵, 任占海, 等. 改性MCM-41分子筛对阴离子偶氮染料的吸附作用机理[J]. 化工进展, 2016, 35(1): 320-326. |
| QUAN Shanshan, ZHU Yuchan, REN Zhanhai, et al. Adsorption performance of anionic azo dye by the modified MCM-41 mesoporous molecular sieve[J]. Chemical Industry and Engineering Progress, 2016, 35(1): 320-326. |
| [1] | 张浩, 刘世钰, 沈卫华, 方云进. Ca-ZSM-5催化尿素脱水制备单氰胺[J]. 化工进展, 2024, 43(S1): 365-373. |
| [2] | 何世坤, 张荣花, 李昊阳, 潘晖, 冯君锋. 脱铝分子筛固体酸催化葡萄糖制备5-羟甲基糠醛[J]. 化工进展, 2024, 43(S1): 374-381. |
| [3] | 吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261. |
| [4] | 张叶素, 权燕红, 丁欣欣, 任军. 链状MFI型分子筛的合成与应用[J]. 化工进展, 2024, 43(8): 4382-4392. |
| [5] | 周皞, 王旭瑞, 赵辉爽, 温妮妮, 苏亚欣. CuCe-SAPO-34选择性催化丙烯还原柴油车尾气氮氧化物[J]. 化工进展, 2024, 43(6): 3093-3099. |
| [6] | 曾浩桀, 周媚, 邹镇远, 熊峰, 曾星星, 刘宝玉. 表面钝化型二维ZSM-5分子筛的制备及甲苯与甲醇烷基化性能[J]. 化工进展, 2024, 43(5): 2696-2704. |
| [7] | 张国卿, 宋舒波, 王兴瑞, 巩苗苗, 王旭, 许宇鸿, 冯继越, 张福扬, 陈汇勇. 煤固废基分子筛的制备及其应用进展[J]. 化工进展, 2024, 43(5): 2311-2323. |
| [8] | 汪孟宇, 范鸿霞, 梁长海, 李文英. 分子筛中限制效应对其酸性表征及催化性能的影响[J]. 化工进展, 2024, 43(5): 2600-2610. |
| [9] | 谢小金, 张晓雪, 刘晓玲, 崇明本, 程党国, 陈丰秋. 单晶多级孔ZSM-5分子筛酸性质对正庚烷催化裂解反应传质性能的影响[J]. 化工进展, 2024, 43(5): 2661-2672. |
| [10] | 梁燕燕, 张军亮, 郭云鸦, 张燕挺. 晶种在分子筛合成中的作用研究进展[J]. 化工进展, 2024, 43(3): 1275-1292. |
| [11] | 陈风, 王宣德, 黄伟, 王晓东, 王琰. HZSM-22的粒径调控及Pt/HZSM-22的正十二烷加氢异构催化性能[J]. 化工进展, 2024, 43(3): 1309-1317. |
| [12] | 钱俊明, 郭猛, 任秀秀, 余亮, 钟璟, 徐荣. 芳烃官能化有机硅膜的制备及丙烯/丙烷分离性能[J]. 化工进展, 2024, 43(3): 1428-1435. |
| [13] | 彭程, 徐漪琳, 石钰婧, 张玟, 李宇涛, 王皓冉, 张卫, 占绣萍. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| [14] | 单书月, 罗中秋, 周新涛, 尚波, 田鑫聪, 阎崔蓉. 钢渣构筑Fe-CSH吸附溶液中Pb(Ⅱ)、Cu(Ⅱ)、Zn(Ⅱ)性能及机理[J]. 化工进展, 2024, 43(10): 5867-5880. |
| [15] | 洪学思, 吴省, 宋磊, 缪长喜, 杨为民. 分子筛限域丙烷脱氢催化剂的研究进展[J]. 化工进展, 2024, 43(10): 5517-5526. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||