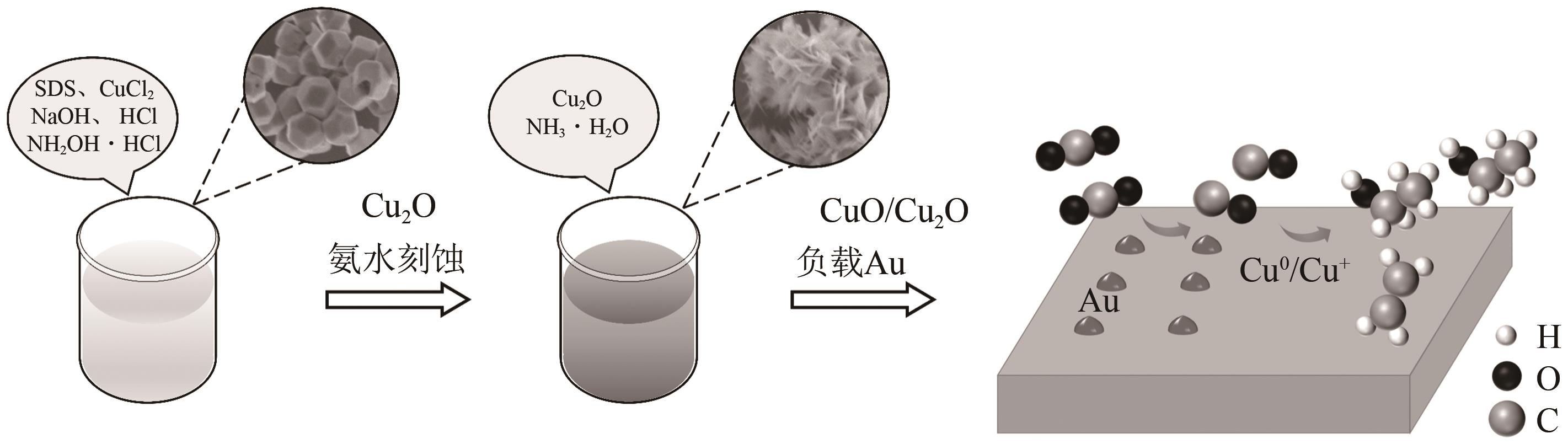
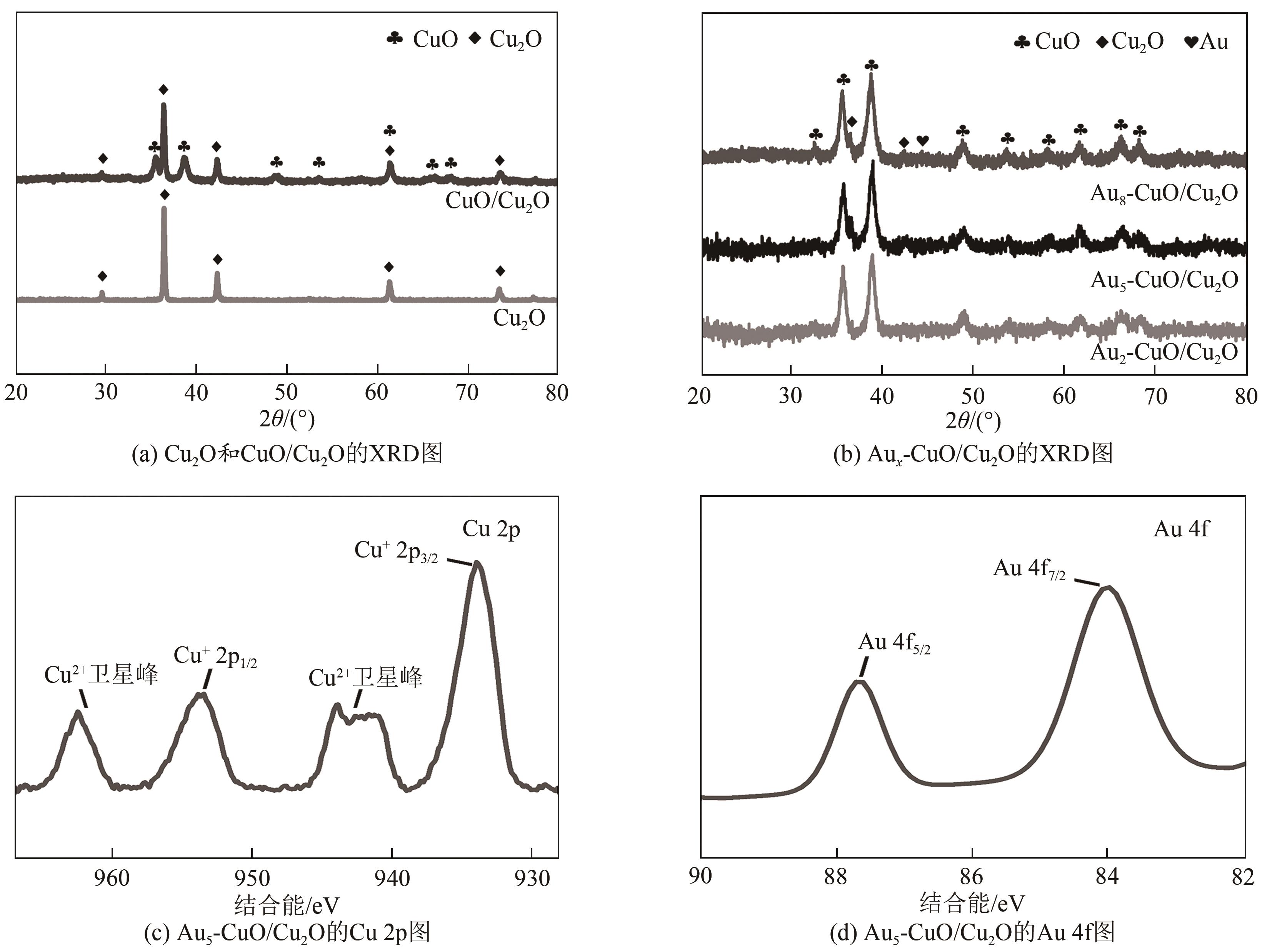
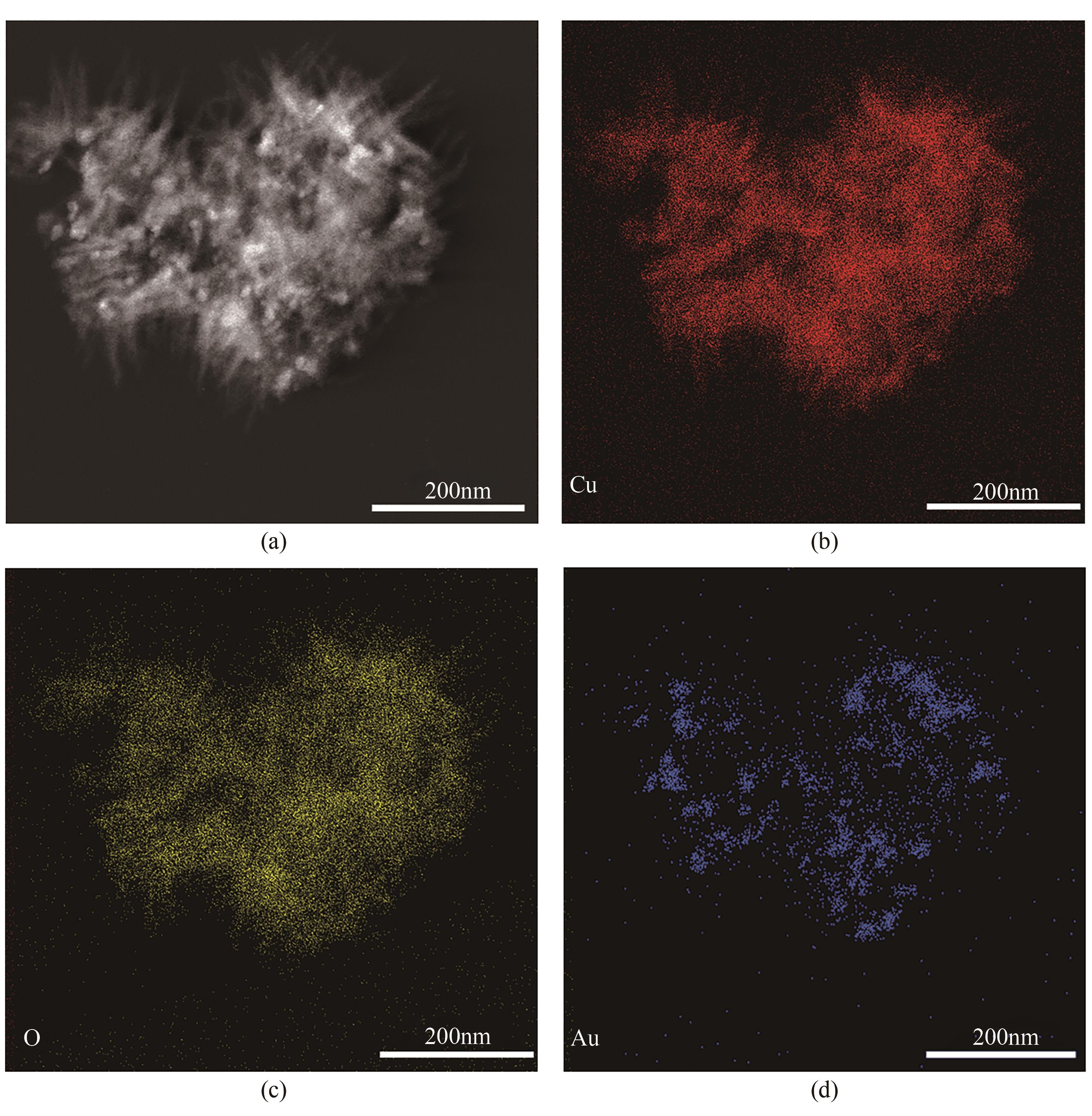
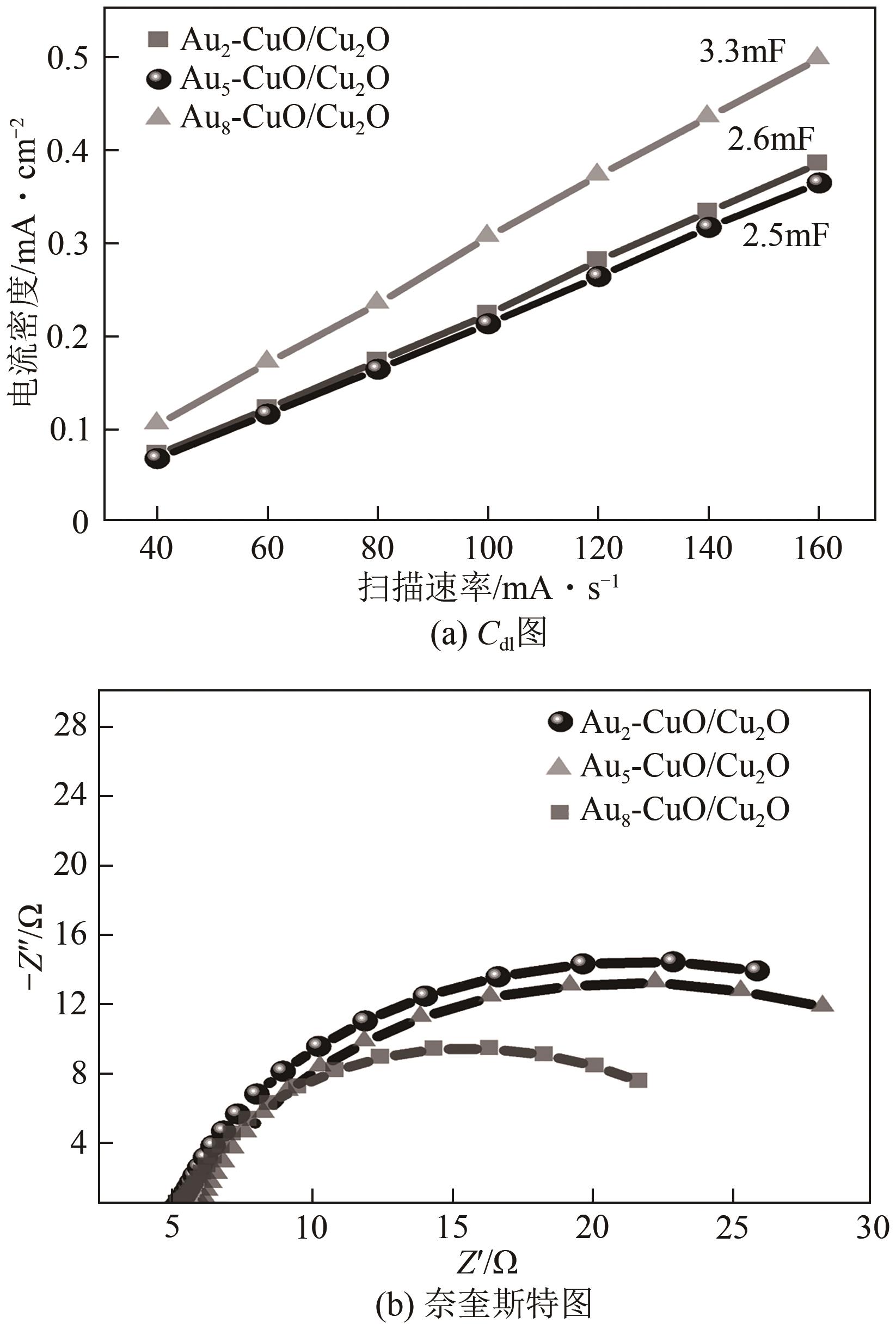
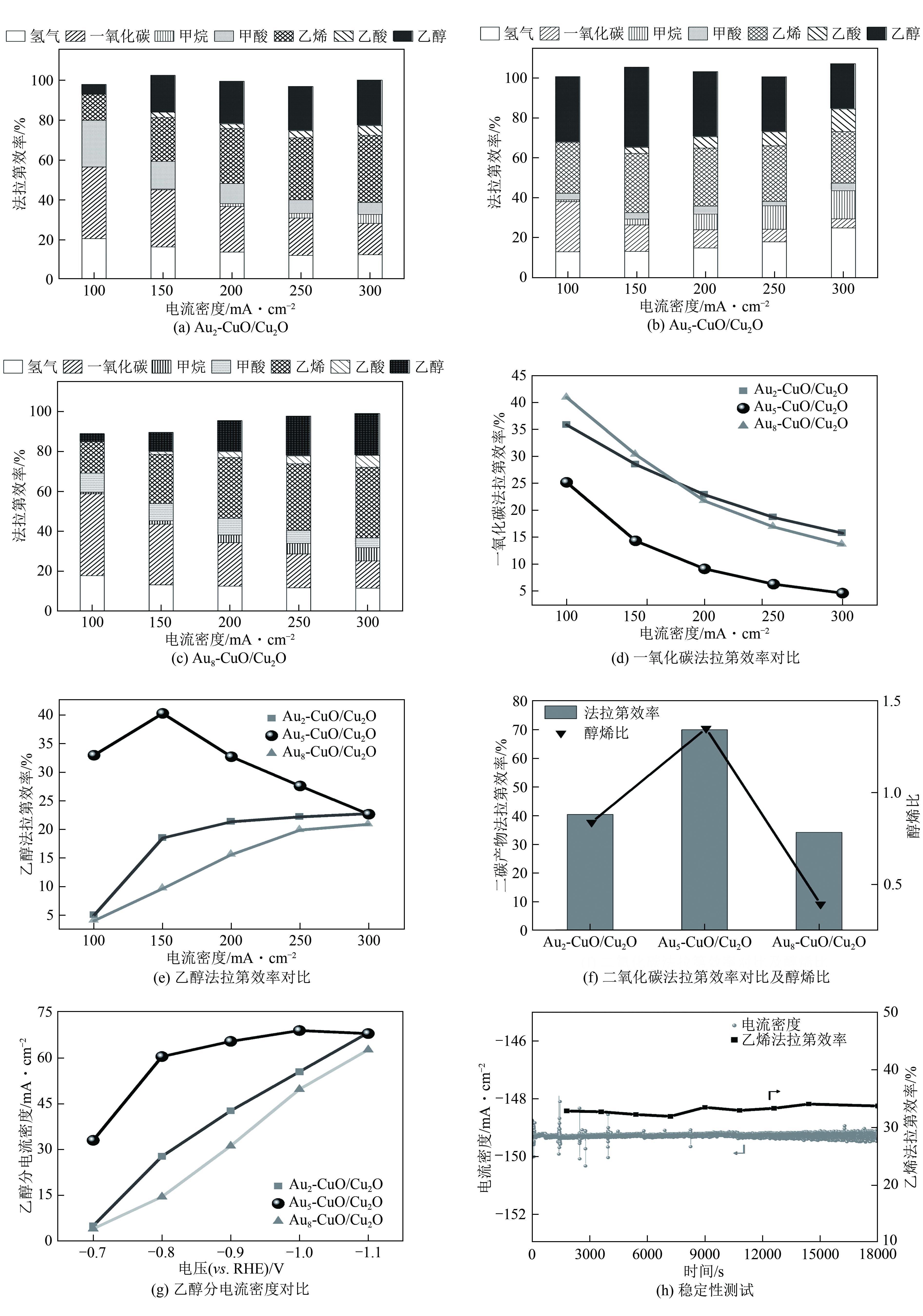
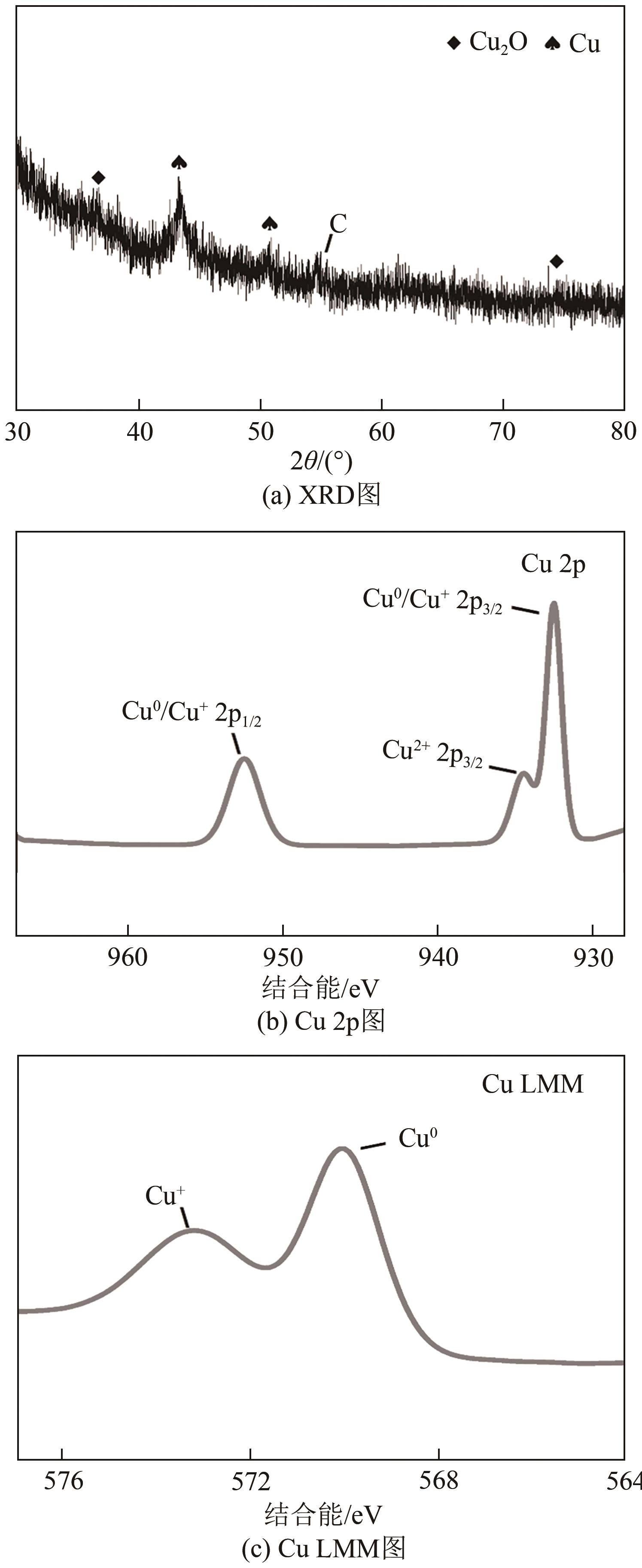
| 1 |
QIAO Jinli, LIU Yuyu, HONG Feng, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675.
|
| 2 |
张育新, 王灿, 舒文祥. 二氧化碳的还原及其利用研究进展[J]. 化工进展, 2023, 42(2): 944-956.
|
|
ZHANG Yuxin, WANG Can, SHU Wenxiang. Research progress of carbon dioxide reduction and utilization[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 944-956.
|
| 3 |
FAN Lei, XIA Chuan, YANG Fangqi, et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products[J]. Science Advances, 2020, 6(8): eaay3111.
|
| 4 |
LI Zhao, WU Rui, ZHAO Lei, et al. Metal-support interactions in designing noble metal-based catalysts for electrochemical CO2 reduction: Recent advances and future perspectives[J]. Nano Research, 2021, 14(11): 3795-3809.
|
| 5 |
WANG Wei, SHANG Lu, CHANG Guojing, et al. Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide[J]. Advanced Materials, 2019, 31(19): e1808276.
|
| 6 |
HOANG Thao T H, VERMA Sumit, MA Sichao, et al. Nanoporous copper-silver alloys by additive-controlled electrodeposition for the selective electroreduction of CO2 to ethylene and ethanol[J]. Journal of the American Chemical Society, 2018, 140(17): 5791-5797.
|
| 7 |
GAO Dunfeng, SINEV Ilya, SCHOLTEN Fabian, et al. Selective CO2 electroreduction to ethylene and multicarbon alcohols via electrolyte-driven nanostructuring[J]. Angewandte Chemie International Edition, 2019, 58(47): 17047-17053.
|
| 8 |
GAO Dunfeng, SCHOLTEN Fabian, ROLDAN CUENYA Beatriz. Improved CO2 electroreduction performance on plasma-activated Cu catalysts via electrolyte design: Halide effect[J]. ACS Catalysis, 2017, 7(8): 5112-5120.
|
| 9 |
LI Haobo, JIANG Yunling, LI Xinyu, et al. C2+ selectivity for CO2 electroreduction on oxidized Cu-based catalysts[J]. Journal of the American Chemical Society, 2023, 145(26): 14335-14344.
|
| 10 |
LI Hefei, LIU Tianfu, WEI Pengfei, et al. High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst[J]. Angewandte Chemie International Edition, 2021, 60(26): 14329-14333.
|
| 11 |
SUN Rongbo, WEI Cong, HUANG Zixiang, et al. Cu2+1O/CuO x heterostructures promote the electrosynthesis of C2+ products from CO2 [J]. Nano Research, 2023, 16(4): 4698-4705.
|
| 12 |
FAN Qikui, ZHANG Xue, GE Xiaohu, et al. Manipulating Cu nanoparticle surface oxidation states tunes catalytic selectivity toward CH4 or C2+ products in CO2 electroreduction[J]. Advanced Energy Materials, 2021, 11(36): 2101424.
|
| 13 |
CLARK Ezra L, HAHN Christopher, JARAMILLO Thomas F, et al. Electrochemical CO2 reduction over compressively strained CuAg surface alloys with enhanced multi-carbon oxygenate selectivity[J]. Journal of the American Chemical Society, 2017, 139(44): 15848-15857.
|
| 14 |
张轩, 黄耀桢, 邵秀丽, 等. 结构化铜基催化剂电化学还原CO2为多碳产物研究进展[J]. 化工进展, 2021, 40(7): 3736-3746.
|
|
ZHANG Xuan, HUANG Yaozhen, SHAO Xiuli, et al. Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3736-3746.
|
| 15 |
TOMBOC Gracita M, CHOI Songa, KWON Taehyun, et al. Potential link between Cu surface and selective CO2 electroreduction: Perspective on future electrocatalyst designs[J]. Advanced Materials, 2020, 32(17): e1908398.
|
| 16 |
ASIRI Abdullah M, GAO Jing, KHAN Sher Bahadar, et al. Revisiting the impact of morphology and oxidation state of Cu on CO2 reduction using electrochemical flow cell[J]. The Journal of Physical Chemistry Letters, 2022, 13(1): 345-351.
|
| 17 |
CHANG Fangfang, LIU Yongpeng, WEI Juncai, et al. In situ surface/interface generation on Cu2O nanostructures toward enhanced electrocatalytic CO2 reduction to ethylene using operando spectroscopy[J]. Inorganic Chemistry Frontiers, 2023, 10(1): 240-249.
|
| 18 |
WANG Shengnan, WANG Dan, TIAN Benqiang, et al. Synergistic Cu+/Cu0 on Cu2O-Cu interfaces for efficient and selective C2+ production in electrocatalytic CO2 conversion[J]. Science China Materials, 2023, 66(5): 1801-1809.
|
| 19 |
MA Yangbo, YU Jinli, SUN Mingzi, et al. Confined growth of silver-copper Janus nanostructures with {100} facets for highly selective tandem electrocatalytic carbon dioxide reduction[J]. Advanced Materials, 2022, 34(19): e2110607.
|
| 20 |
LIU Yuting, LIU Hua, WANG Cheng, et al. Reconstructed Cu/Cu2O(Ⅰ) catalyst for selective electroreduction of CO2 to C2+ products[J]. Electrochemistry Communications, 2023, 150: 107474.
|
| 21 |
CAO Bo, LI Fuzhi, GU Jun. Designing Cu-based tandem catalysts for CO2 electroreduction based on mass transport of CO intermediate[J]. ACS Catalysis, 2022, 12(15): 9735-9752.
|
| 22 |
ZHANG Haochen, CHANG Xiaoxia, CHEN Jingguang G, et al. Computational and experimental demonstrations of one-pot tandem catalysis for electrochemical carbon dioxide reduction to methane[J]. Nature Communications, 2019, 10(1): 3340.
|
| 23 |
WAN Xiankai, WANG Jiaqi, WANG Quanming. Ligand-protected Au55 with a novel structure and remarkable CO2 electroreduction performance[J]. Angewandte Chemie International Edition, 2021, 60(38): 20748-20753.
|
| 24 |
RINGE Stefan, MORALES-GUIO Carlos G, CHEN Leanne D, et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on gold[J]. Nature Communications, 2020, 11(1): 33.
|
| 25 |
ZHANG Lijie, LI Mei, ZHANG Shengbo, et al. Promoting carbon dioxide electroreduction toward ethanol through loading Au nanoparticles on hollow Cu2O nanospheres[J]. Catalysis Today, 2021, 365: 348-356.
|
| 26 |
ZHANG Binbin, WANG Yahui, XU Shanmin, et al. Tuning nanocavities of Au@Cu2O yolk-shell nanoparticles for highly selective electroreduction of CO2 to ethanol at low potential[J]. RSC Advances, 2020, 10(33): 19192-19198.
|
| 27 |
MORALES-GUIO Carlos G, CAVE Etosha R, NITOPI Stephanie A, et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst[J]. Nature Catalysis, 2018, 1: 764-771.
|
| 28 |
SHAN Mengqing, LU Dongsheng, DONG Jiatong, et al. Combined effects of sea urchin-like structure and mixed Cu+/Cu0 states on promoting C2 formation in electrocatalytic CO2 reduction[J]. Frontiers of Chemical Science and Engineering, 2024, 18(3): 30.
|
| 29 |
ZHANG Jianfang, WANG Yan, LI Zhengyuan, et al. Grain boundary-derived Cu+/Cu0 interfaces in CuO nanosheets for low overpotential carbon dioxide electroreduction to ethylene[J]. Advanced Science, 2022, 9(21): e2200454.
|
| 30 |
ZHANG Wei, HUANG Chuqiang, XIAO Qin, et al. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction[J]. Journal of the American Chemical Society, 2020, 142(26): 11417-11427.
|
| 31 |
SHANGGUAN Wenchao, LIU Qing, WANG Ying, et al. Molecular-level insight into photocatalytic CO2 reduction with H2O over Au nanoparticles by interband transitions[J]. Nature Communications, 2022, 13(1): 3894.
|
| 32 |
CHANG Fangfang, WEI Juncai, LIU Yongpeng, et al. Surface/interface reconstruction in situ on Cu2O catalysts with high exponential facets toward enhanced electrocatalysis CO2 reduction to C2+ products[J]. Applied Surface Science, 2023, 611: 155773.
|
| 33 |
LIU Bingqian, YAO Xi, ZHANG Zijing, et al. Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 39165-39177.
|
| 34 |
PAN Wenhao, ZHONG Lei, SHI Zhikai, et al. Interfacial engineering of copper(Ⅰ) oxide nanocubes wrapped with functionalized carbon nanotubes toward carbon dioxide electroreduction to ethylene[J]. Surfaces and Interfaces, 2023, 37: 102708.
|
 ), SHAN Mengqing, WANG Hua(
), SHAN Mengqing, WANG Hua( )
)