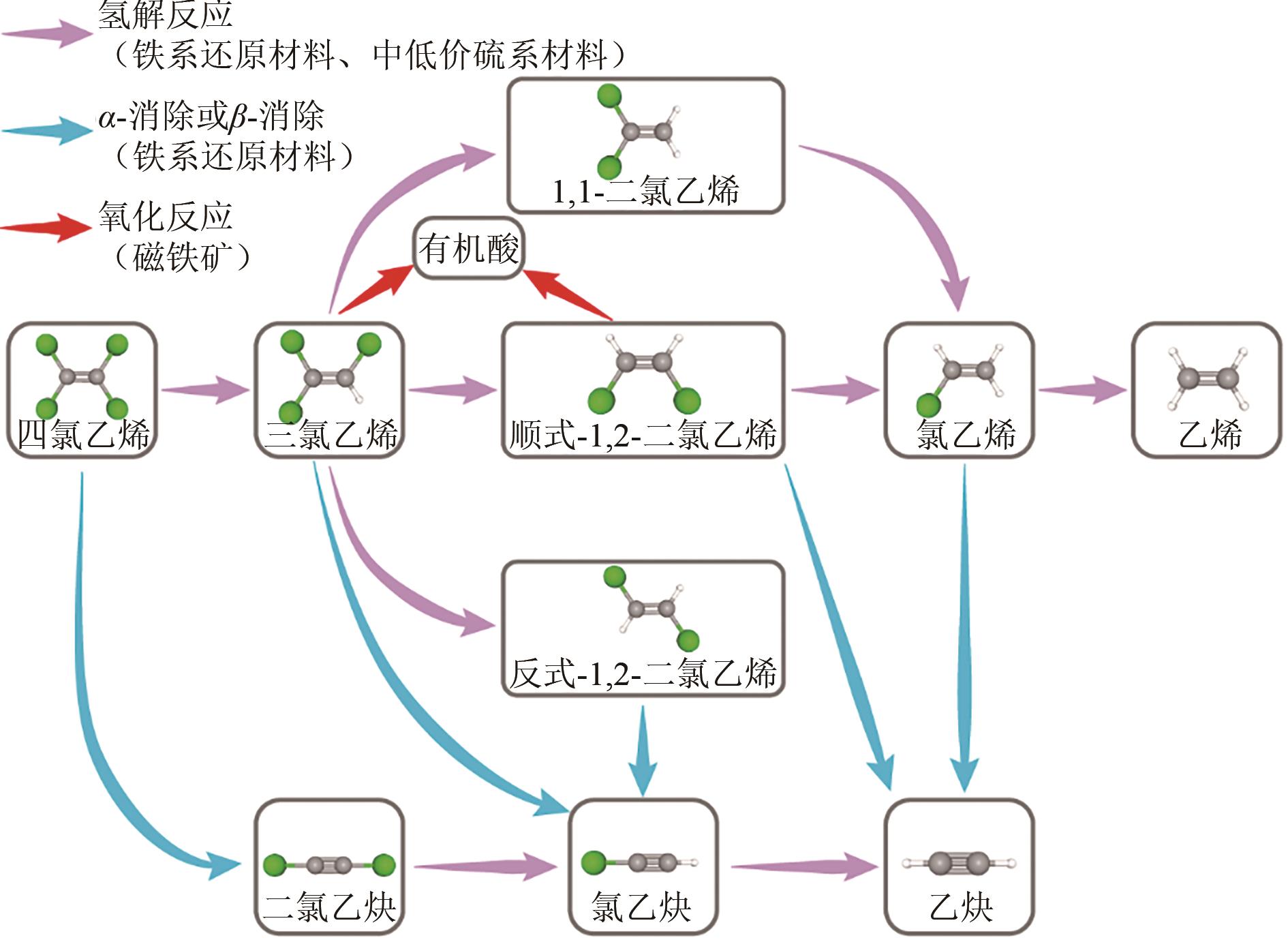
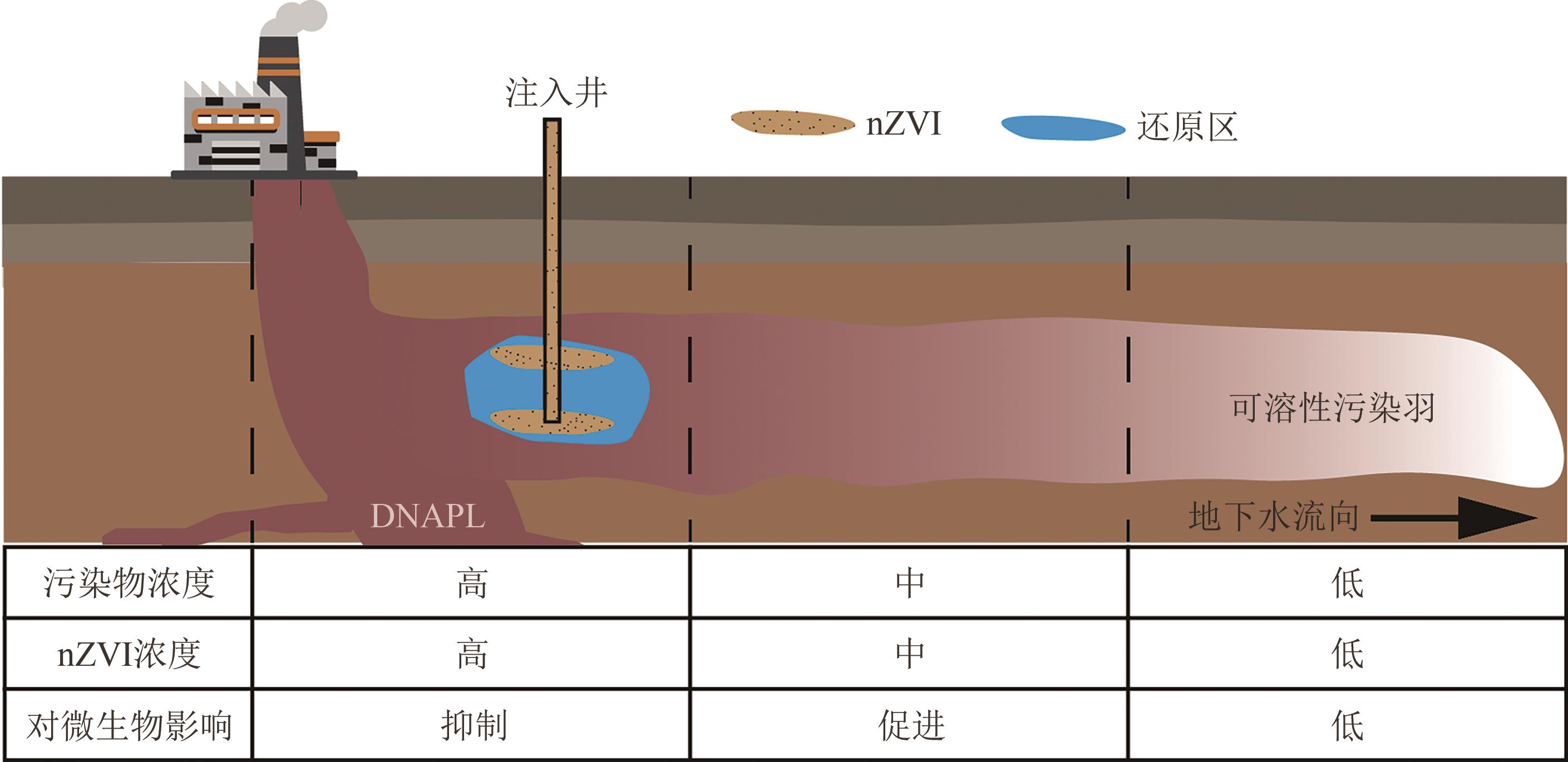
化工进展 ›› 2025, Vol. 44 ›› Issue (1): 500-512.DOI: 10.16085/j.issn.1000-6613.2024-0084
还原材料修复卤代溶剂污染地下水的研究进展
李书鹏1,2,3( ), 独学渊2,3, 李霏2,3, 郭丽莉2,3, 李广贺1,2(
), 独学渊2,3, 李霏2,3, 郭丽莉2,3, 李广贺1,2( )
)
- 1.清华大学环境学院,北京 100084
2.污染场地安全修复技术国家工程实验室,北京 100015
3.北京建工环境修复股份有限公司,北京 100015
-
收稿日期:2024-01-11修回日期:2024-04-13出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:李广贺 -
作者简介:李书鹏(1978—),男,博士研究生,教授级高级工程师,研究方向为土壤和地下水修复技术。E-mail:lishupeng@bceer.com。
Research development of reductive materials for remediation of groundwater contaminated by halogenated solvents
LI Shupeng1,2,3( ), DU Xueyuan2,3, LI Fei2,3, GUO Lili2,3, LI Guanghe1,2(
), DU Xueyuan2,3, LI Fei2,3, GUO Lili2,3, LI Guanghe1,2( )
)
- 1.School of Environment, Tsinghua University, Beijing 100084, China
2.National Engineering Laboratory for Site Remediation Technologies, Beijing 100015, China
3.Beijing Construction Engineering Group Environmental Remediation Co. , Ltd. , Beijing 100015, China
-
Received:2024-01-11Revised:2024-04-13Online:2025-01-15Published:2025-02-13 -
Contact:LI Guanghe
摘要:
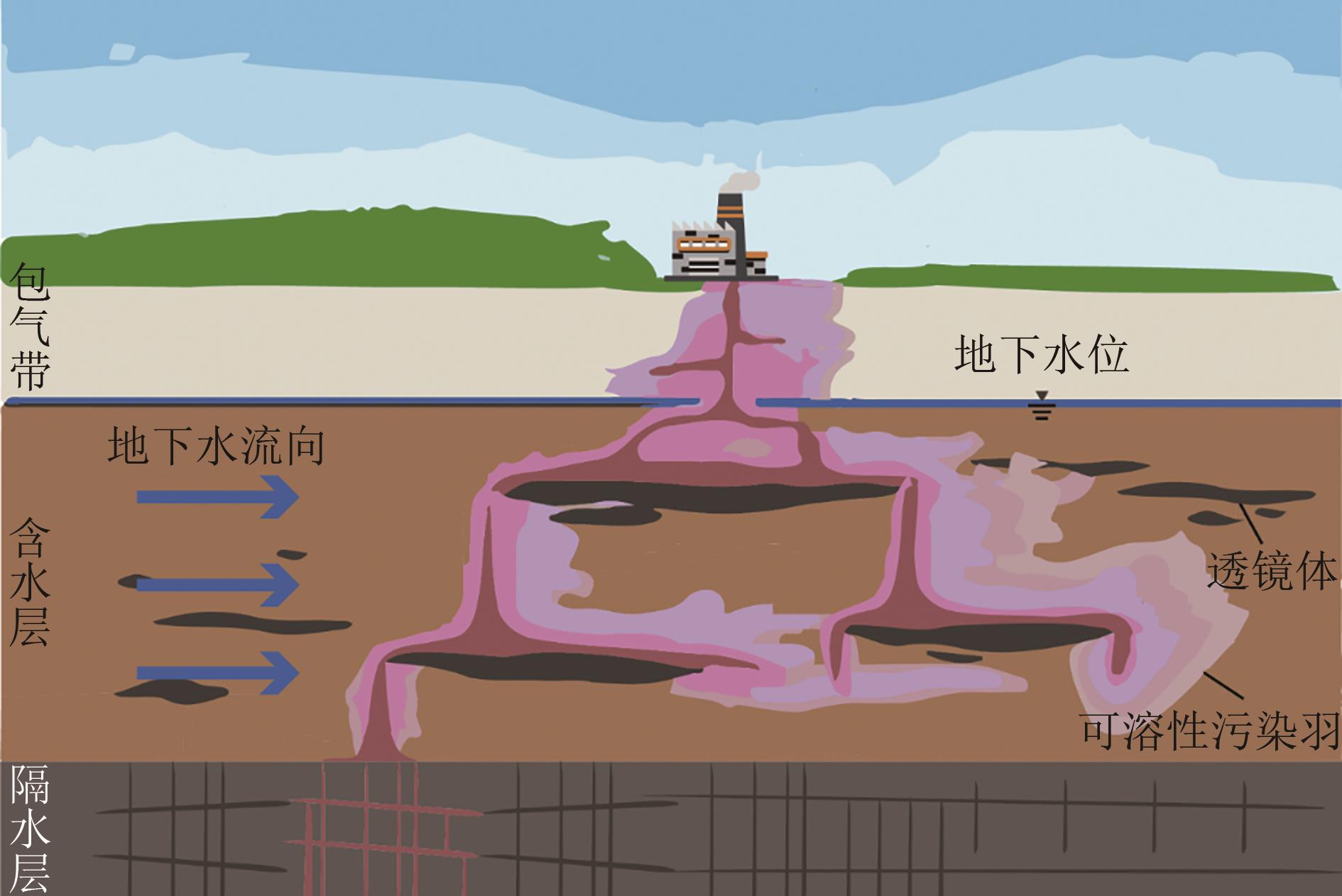
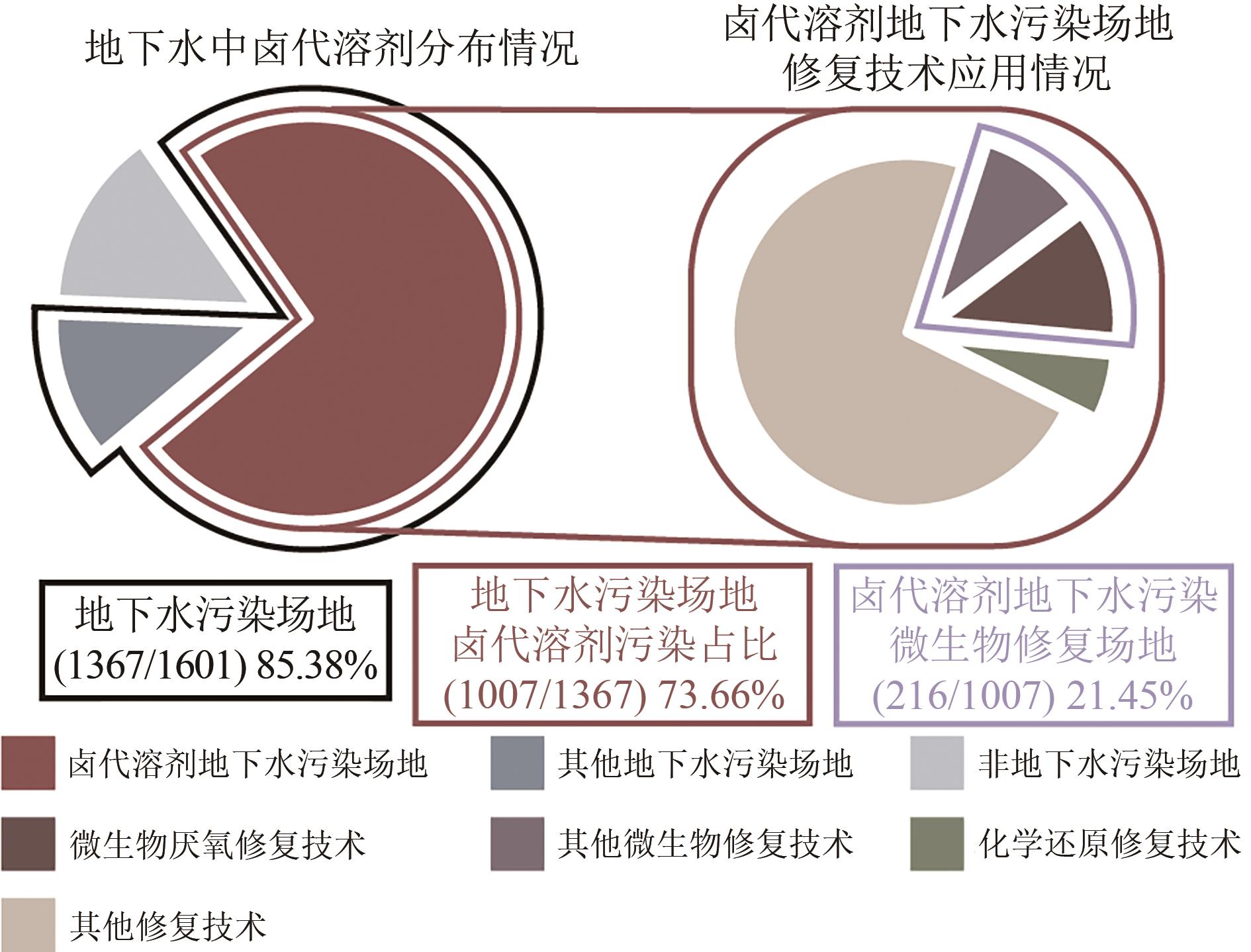
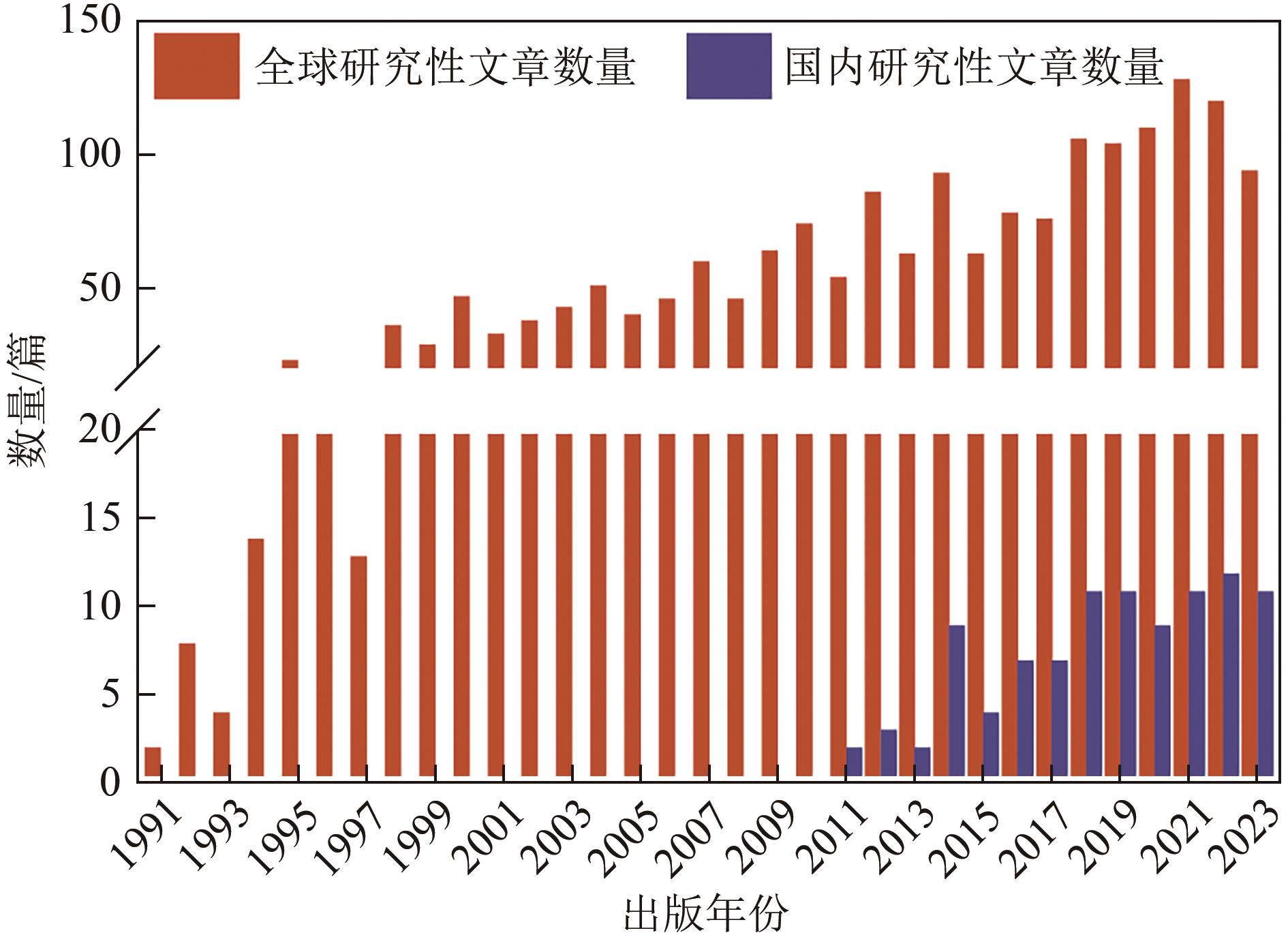
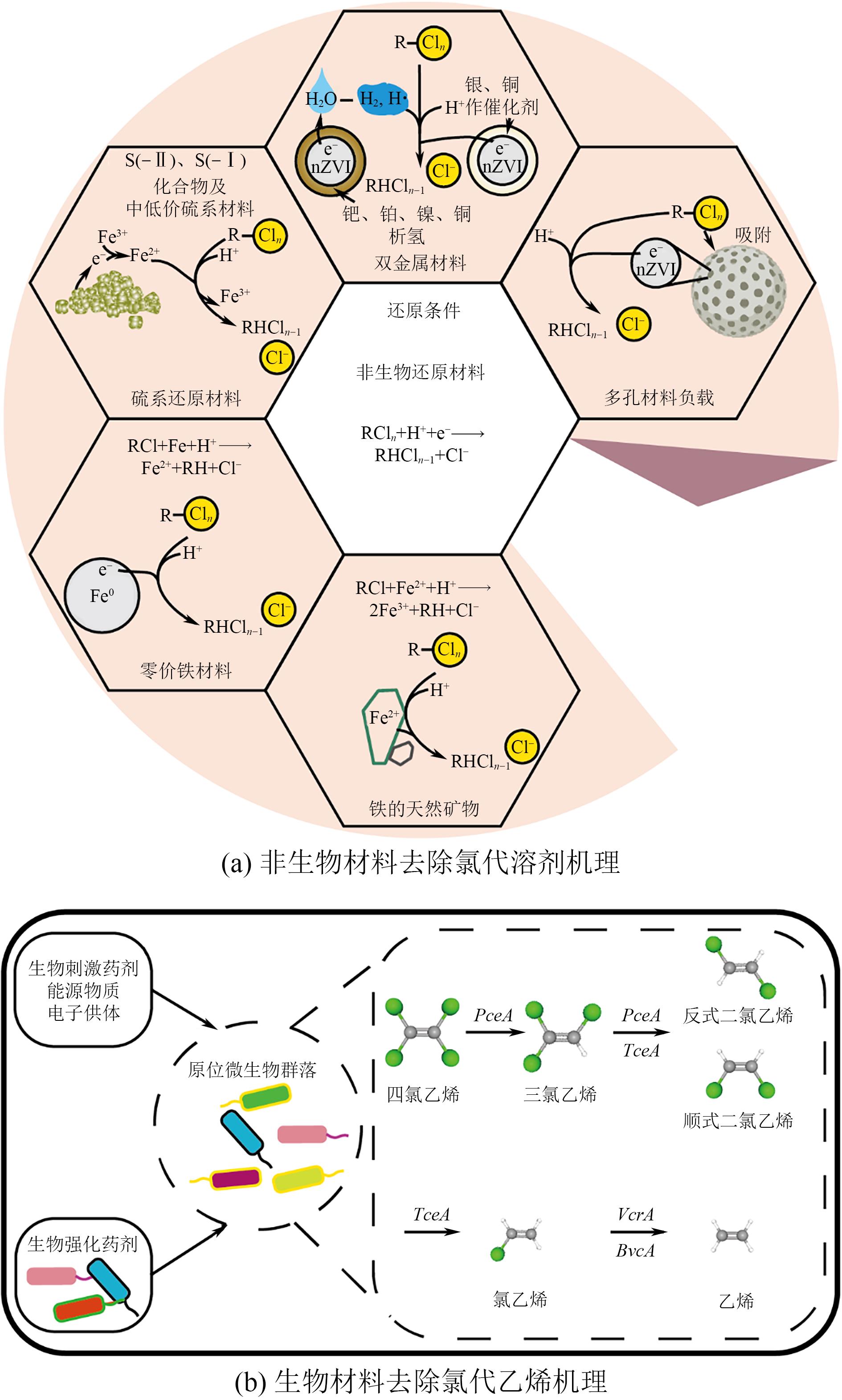
还原材料在卤代溶剂污染地下水修复中具有重要应用价值,但实际应用中仍存在迁移性差、修复效率低及成本高等问题。本文介绍了卤代溶剂污染现状、不同类型还原修复材料的研究进展及其在卤代溶剂污染修复中的应用。简述了这些材料在提升修复效率、降低成本方面的潜力与挑战,旨在分析当前技术的优缺点并探讨未来的改进方向。指出为进一步提升卤代溶剂污染修复效果,应深入研究改性还原药剂以增强其反应活性和迁移能力,同时加强材料技术之间的耦合研究与施工工艺的优化,降低修复成本并提高工程实施的可行性。此外,还应关注修复过程中的环境影响,确保技术的绿色可持续发展。通过这些综合措施,有望推动卤代溶剂污染修复技术迈向更高效、更环保的新阶段。
中图分类号:
引用本文
李书鹏, 独学渊, 李霏, 郭丽莉, 李广贺. 还原材料修复卤代溶剂污染地下水的研究进展[J]. 化工进展, 2025, 44(1): 500-512.
LI Shupeng, DU Xueyuan, LI Fei, GUO Lili, LI Guanghe. Research development of reductive materials for remediation of groundwater contaminated by halogenated solvents[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 500-512.
| 卤代溶剂 | 密度 /g·mL-1 | 水中溶解度 /mg·L-1 | GB 5749—2022 /mg·L-1 |
|---|---|---|---|
| 四氯乙烯 | 1.63 | 200 | 0.04 |
| 三氯乙烯 | 1.46 | 1100 | 0.02 |
| 1,1,1-三氯乙烷 | 1.35 | 1300 | 2 |
| 四氯化碳 | 1.59 | 825 | 0.002 |
| 1,2-二氯乙烷 | 1.25 | 8500 | 0.03 |
| 二氯甲烷 | 1.33 | 20000 | 0.02 |
| 水 | 0.997[ | — | — |
表1 25℃条件下常见卤代溶剂物理性质[11]
| 卤代溶剂 | 密度 /g·mL-1 | 水中溶解度 /mg·L-1 | GB 5749—2022 /mg·L-1 |
|---|---|---|---|
| 四氯乙烯 | 1.63 | 200 | 0.04 |
| 三氯乙烯 | 1.46 | 1100 | 0.02 |
| 1,1,1-三氯乙烷 | 1.35 | 1300 | 2 |
| 四氯化碳 | 1.59 | 825 | 0.002 |
| 1,2-二氯乙烷 | 1.25 | 8500 | 0.03 |
| 二氯甲烷 | 1.33 | 20000 | 0.02 |
| 水 | 0.997[ | — | — |
| 速效成分 | 缓释成分 | |
|---|---|---|
| 能源物质 | 糖浆类(糖蜜、红糖、精炼甘蔗等) | 农产品废弃物(麦麸、秸秆、甲壳素等) |
| 醇类(甲醇、戊醇) | 油类(乳化植物油、豆油等) | |
| 乳清蛋白 | EOS、HRC等 | |
| 电子供体 | 氢气、铁粉 | 乳化油类 |
| 小分子有机酸盐(甲酸盐、乙酸盐、乳酸盐、丙酮酸盐、丁酸盐) | EOS、HRC等 |
表2 生物刺激药剂主要成分
| 速效成分 | 缓释成分 | |
|---|---|---|
| 能源物质 | 糖浆类(糖蜜、红糖、精炼甘蔗等) | 农产品废弃物(麦麸、秸秆、甲壳素等) |
| 醇类(甲醇、戊醇) | 油类(乳化植物油、豆油等) | |
| 乳清蛋白 | EOS、HRC等 | |
| 电子供体 | 氢气、铁粉 | 乳化油类 |
| 小分子有机酸盐(甲酸盐、乙酸盐、乳酸盐、丙酮酸盐、丁酸盐) | EOS、HRC等 |
| 工程应用问题 | 解决策略 |
|---|---|
| 迁移性差 | 乳化(乳化后流动性变好利于迁移) |
| 团聚 | 包覆(CMC可通过静电斥力和空间位阻作用减少nZVI颗粒的聚集)、固相负载(可有效分散nZVI,减少聚集) |
| 生物修复效率低 | 乳化(植物油可以为长期生物降解提供营养)、固相负载(生物炭等材料可以为功能微生物的生长提供营养) |
| 钝化 | 双金属材料(双金属体系下,铁表面的原电池反应将促进形成更多的新鲜表面,增加反应区域)、包覆、固相负载(nZVI进行包覆以及通过固相负载等方式可以有效减少nZVI与空气的接触,从而减少表面钝化)、乳化(疏水植物油溶解氧低,减少表面钝化) |
表3 还原材料工程应用问题及解决策略
| 工程应用问题 | 解决策略 |
|---|---|
| 迁移性差 | 乳化(乳化后流动性变好利于迁移) |
| 团聚 | 包覆(CMC可通过静电斥力和空间位阻作用减少nZVI颗粒的聚集)、固相负载(可有效分散nZVI,减少聚集) |
| 生物修复效率低 | 乳化(植物油可以为长期生物降解提供营养)、固相负载(生物炭等材料可以为功能微生物的生长提供营养) |
| 钝化 | 双金属材料(双金属体系下,铁表面的原电池反应将促进形成更多的新鲜表面,增加反应区域)、包覆、固相负载(nZVI进行包覆以及通过固相负载等方式可以有效减少nZVI与空气的接触,从而减少表面钝化)、乳化(疏水植物油溶解氧低,减少表面钝化) |
| 改性方式 | 材料 | 差异特点 | 成本比较 | 参考文献 |
|---|---|---|---|---|
| 碳载/硅基材料负载 | 生物炭、活性炭、石墨烯、硅酸盐等 | 碳基/硅基材料对卤代溶剂的吸附能力较强,对环境相对友好,可以降低nZVI对地下微生物潜在的生物效应和对整个生态系统的风险 | 农业废弃物或木质材料烧制,成本适中 | [ |
| 矿物负载 | 蒙脱石、累托石、凹凸棒、沸石等 | 矿物材料对金属阳离子有较好的吸附性能,对有机污染物的吸附性能有限,矿物材料对nZVI的分散性改善效果显著 | 自然存在,成本低廉 | [ |
| 聚合物改性 | 羧甲基纤维素、壳聚糖、聚乙二醇等 | 可塑性强,可根据需要进行合成,可作为微生物碳源刺激生物降解作用 | 人工合成材料,成本可控 | [ |
| 硫化 | — | nZVI硫化后疏水性增强,减少nZVI与水的析氢反应,从而提高nZVI对卤代溶剂的降解效率 | 人工合成,成本可控 | [ |
| 乳化 | 食品级表面活性剂、可生物降解植物油、水 | 可生物降解植物油与卤代溶剂疏水性相似,可以增强ZVI与污染物的接触加快反应速度,同时植物油可以为长期生物降解提供营养 | 自然存在,成本低廉 | [ |
表4 改性纳米材料的种类及原理
| 改性方式 | 材料 | 差异特点 | 成本比较 | 参考文献 |
|---|---|---|---|---|
| 碳载/硅基材料负载 | 生物炭、活性炭、石墨烯、硅酸盐等 | 碳基/硅基材料对卤代溶剂的吸附能力较强,对环境相对友好,可以降低nZVI对地下微生物潜在的生物效应和对整个生态系统的风险 | 农业废弃物或木质材料烧制,成本适中 | [ |
| 矿物负载 | 蒙脱石、累托石、凹凸棒、沸石等 | 矿物材料对金属阳离子有较好的吸附性能,对有机污染物的吸附性能有限,矿物材料对nZVI的分散性改善效果显著 | 自然存在,成本低廉 | [ |
| 聚合物改性 | 羧甲基纤维素、壳聚糖、聚乙二醇等 | 可塑性强,可根据需要进行合成,可作为微生物碳源刺激生物降解作用 | 人工合成材料,成本可控 | [ |
| 硫化 | — | nZVI硫化后疏水性增强,减少nZVI与水的析氢反应,从而提高nZVI对卤代溶剂的降解效率 | 人工合成,成本可控 | [ |
| 乳化 | 食品级表面活性剂、可生物降解植物油、水 | 可生物降解植物油与卤代溶剂疏水性相似,可以增强ZVI与污染物的接触加快反应速度,同时植物油可以为长期生物降解提供营养 | 自然存在,成本低廉 | [ |
| 序号 | 地点 | 污染物 | 补救措施 | 修复时间 | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 维拉凡特(巴塞罗那东北约140km处) | 四氯乙烯 | mZVI/乳酸 | 400天 | 83.4~96.3 | [ |
| 2 | 帕斯阔坦克河南岸(北卡罗来纳州伊丽莎白市东南约5km处) | 三氯乙烯、顺式二氯乙烯、氯乙烯 | mZVI-PRB | 长期风险 管控 | 81~99 | [ |
| 3 | 佛罗里达州34号发射中心 | 三氯乙烯 | EZVI(乳化零价铁) | 5月 | 57~100 | [ |
| 4 | 华北平原某污染场地 | 三氯乙烯、三氯甲烷 | 生物炭负载nZVI | 42天 | >90 | [ |
| 5 | 某工业污染场地 | 三氯乙烯 | 生物炭负载nZVI | 2年 | >90 | [ |
| 6 | 某前干洗店 | 四氯乙烯 | 生物炭负载nZVI | 14天 | >90 | [ |
| 7 | 意大利贝加莫附近废弃工业区 | 四氯乙烯、三氯乙烯、二氯丙烷和1,1,2,2-四氯乙烷 | EHC®液体混合物 (一种可溶性有机铁盐) | 6月 | 达成修复 目标 | [ |
表5 国内外传统或新型还原材料在修复卤代溶剂污染地下水的典型修复工程案例
| 序号 | 地点 | 污染物 | 补救措施 | 修复时间 | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 维拉凡特(巴塞罗那东北约140km处) | 四氯乙烯 | mZVI/乳酸 | 400天 | 83.4~96.3 | [ |
| 2 | 帕斯阔坦克河南岸(北卡罗来纳州伊丽莎白市东南约5km处) | 三氯乙烯、顺式二氯乙烯、氯乙烯 | mZVI-PRB | 长期风险 管控 | 81~99 | [ |
| 3 | 佛罗里达州34号发射中心 | 三氯乙烯 | EZVI(乳化零价铁) | 5月 | 57~100 | [ |
| 4 | 华北平原某污染场地 | 三氯乙烯、三氯甲烷 | 生物炭负载nZVI | 42天 | >90 | [ |
| 5 | 某工业污染场地 | 三氯乙烯 | 生物炭负载nZVI | 2年 | >90 | [ |
| 6 | 某前干洗店 | 四氯乙烯 | 生物炭负载nZVI | 14天 | >90 | [ |
| 7 | 意大利贝加莫附近废弃工业区 | 四氯乙烯、三氯乙烯、二氯丙烷和1,1,2,2-四氯乙烷 | EHC®液体混合物 (一种可溶性有机铁盐) | 6月 | 达成修复 目标 | [ |
| 1 | US EPA. Remedy component data for decision documents by media, FYs 1982—2021 (Final NPL, Deleted NPL, and Superfund Alternative Approach Sites)[EB/OL]. (2023-12-18) [2023-12-27]. . |
| 2 | 环境部, 国土资源部, 住房和城乡建设部, 等. 华北平原地下水污染防治工作方案[EB/OL]. (2013-03-08) [2023-12-27]. . |
| 3 | 姜建军. 中国地下水污染现状与防治对策[J]. 环境保护, 2007, 35(19): 16-17. |
| JIANG Jianjun. Present situation of groundwater pollution in China and its prevention and control countermeasures[J]. Environmental Protection, 2007, 35(19): 16-17. | |
| 4 | 汤灵容. 土壤和地下水中挥发性氯代烃污染的快速检测技术[D]. 上海: 上海师范大学, 2011. |
| TANG Lingrong. Rapid detection technology of volatile chlorinated hydrocarbon pollution in soil and groundwater[D]. Shanghai: Shanghai Normal University, 2011. | |
| 5 | CALLAHAN M A. Water-related environmental fate of 129 priority pollutants[M]. Washington DC: US Environmental Protection Agency, 1980: 486. |
| 6 | 周文敏, 傅德黔, 孙宗光. 水中优先控制污染物黑名单[J]. 中国环境监测, 1990, 6(4): 1-3. |
| ZHOU Wenmin, FU Deqian, SUN Zongguang. Blacklist of priority pollutants in water[J]. Environmental Monitoring in China, 1990, 6(4): 1-3. | |
| 7 | GILLHAM Robert W, O’HANNESIN Stephanie F. Enhanced degradation of halogenated aliphatics by zero-valent iron[J]. Groundwater, 1994, 32(6): 958-967. |
| 8 | GLAVEE George N, KLABUNDE Kenneth J, SORENSEN Christopher M, et al. Chemistry of borohydride reduction of iron (Ⅱ) and iron (Ⅲ) ions in aqueous and nonaqueous media. formation of nanoscale Fe, FeB, and Fe2B powders[J]. Inorganic Chemistry, 1995, 34(1): 28-35. |
| 9 | MAYMÓ-GATELL X. Dehalococcoides ethenogenes strain 195: A novel eubacterium that reductively dechlorinates tetrachloroethene (PCE) to ethene[M]. Ithaca: Cornell University, 1997. |
| 10 | LI Fei, DENG Daiyong, ZENG Lingke, et al. Sequential anaerobic and aerobic bioaugmentation for commingled groundwater contamination of trichloroethene and 1,4-dioxane[J]. Science of the Total Environment, 2021, 774: 145118. |
| 11 | PANKOW James F, CHERRY John A. Dense chlorinated solvents and other DNAPLs in groundwater[M]. Portland: Waterloo Press, 1996: 522. |
| 12 | KELL George S. Density, thermal expansivity, and compressibility of liquid water from 0℃ to 150℃: Correlations and tables for atmospheric pressure and saturation reviewed and expressed on 1968 temperature scale[J]. Journal of Chemical and Engineering data, 1975, 20(1): 97-105. |
| 13 | GERHARD Jason I, PANG Tiwee, KUEPER Bernard H. Time scales of DNAPL migration in sandy aquifers examined via numerical simulation[J]. Ground Water, 2007, 45(2): 147-157. |
| 14 | KUEPER Bernard H, ANNABLE M D. Chlorinated solvent source zone remediation[M]. New York: Springer New York, 2014: 6-8. |
| 15 | US EPA. Contaminant of concern data for decision documents by media, FYs 1982—2021 (Final NPL, Deleted NPL, and Superfund Alternative Approach Sites)[EB/OL]. (2023-12-18) [2023-12-27]. . |
| 16 | ELSNER Martin, HOFSTETTER Thomas B. Current perspectives on the mechanisms of chlorohydrocarbon degradation in subsurface environments: Insight from kinetics, product formation, probe molecules, and isotope fractionation[M]. Washington DC: American Chemical Society, 2011: 407-439. |
| 17 | National Center for Biotechnology Information. PubChem Compound Summary for CID 6325, Ethylene[EB/OL]. (2004-09-16) [2024-03-06]. . |
| 18 | National Center for Biotechnology Information. PubChem Compound Summary for CID 31373, Tetrachloroethylene[EB/OL]. (2004-09-16) [2024-03-06]. . |
| 19 | National Center for Biotechnology Information. PubChem Compound Summary for CID 6575, Trichloroethylene [EB/OL]. (2004-09-16) [2024-3-6]. . |
| 20 | National Center for Biotechnology Information. PubChem Compound Summary for CID 638186, trans-1,2-Dichloroethylene[EB/OL]. (2005-03-26) [2024-3-6]. . |
| 21 | National Center for Biotechnology Information. PubChem Compound Summary for CID 643833, cis-1,2-Dichloroethylene[EB/OL]. (2005-03-26) [2024-03-06]. . |
| 22 | National Center for Biotechnology Information. PubChem Compound Summary for CID 6338, Vinyl Chloride[EB/OL]. (2004-09-16) [2024-03-06]. . |
| 23 | GORSKI Christopher A, NURMI James T, TRATNYEK Paul G, et al. Redox behavior of magnetite: Implications for contaminant reduction[J]. Environmental Science & Technology, 2010, 44(1): 55-60. |
| 24 | AYALA-LUIS Karina B, COOPER Nicola G A, KOCH Christian Bender, et al. Efficient dechlorination of carbon tetrachloride by hydrophobic green rust intercalated with dodecanoate anions[J]. Environmental Science & Technology, 2012, 46(6): 3390-3397. |
| 25 | ELSNER Martin, SCHWARZENBACH René P, HADERLEIN Stefan B. Reactivity of Fe (Ⅱ)-bearing minerals toward reductive transformation of organic contaminants[J]. Environmental Science & Technology, 2004, 38(3): 799-807. |
| 26 | FERREY Mark L, WILKIN Richard T, FORD Robert G, et al. Nonbiological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite[J]. Environmental Science & Technology, 2004, 38(6): 1746-1752. |
| 27 | YIN Xin, HUA Han, DYER James, et al. Degradation of chlorinated solvents with reactive iron minerals in subsurface sediments from redox transition zones[J]. Journal of Hazardous Materials, 2023, 445: 130470. |
| 28 | DARLINGTON Ramona, LEHMICKE Leo, ANDRACHEK Richard G, et al. Biotic and abiotic anaerobic transformations of trichloroethene and cis-1,2-dichloroethene in fractured sandstone[J]. Environmental Science & Technology, 2008, 42(12): 4323-4330. |
| 29 | DARLINGTON Ramona, LEHMICKE Leo G, ANDRACHEK Richard G, et al. Anaerobic abiotic transformations of cis-1,2-dichloroethene in fractured sandstone[J]. Chemosphere, 2013, 90(8): 2226-2232. |
| 30 | HE Y, SU C, WILSON J, et al. Identification and characterization methods for reactive minerals responsible for natural attenuation of chlorinated organic compounds in ground water[R]. Washington DC: US Environmental Protection Agency, 2010, R-09/115. |
| 31 | ARNOLD William A, Lynn ROBERTS A. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles[J]. Environmental Science & Technology, 2000, 34(9): 1794-1805. |
| 32 | National Center for Biotechnology Information. PubChem Compound Summary for CID 6366, Vinylidene chloride[EB/OL]. (2005-03-27) [2024-03-06]. . |
| 33 | National Center for Biotechnology Information. PubChem Compound Summary for CID 24227, Dichloroacetylene[EB/OL]. (2005-03-27) [2024-03-06]. . |
| 34 | National Center for Biotechnology Information. PubChem Compound Summary for CID 68975, Chloroethyne[EB/OL]. (2005-03-27)[2024-03-06]. . |
| 35 | National Center for Biotechnology Information. PubChem Compound Summary for CID 6326, Acetylene[EB/OL]. (2004-09-16) [2024-03-06]. . |
| 36 | HE Y T, WILSON J T, SU C, et al. Review of abiotic degradation of chlorinated solvents by reactive iron minerals in aquifers[J]. Groundwater Monitoring & Remediation, 2015, 35(3): 57-75. |
| 37 | TRATNYEK P G, Scherer M M, Johnson T L, et al. Permeable reactive barriers of iron and other zero-valent metals[M]. Boca Raton: CRC Press, 2003: 347-394. |
| 38 | HENDERSON Andrew D, DEMOND Avery H. Long-term performance of zero-valent iron permeable reactive barriers: A critical review[J]. Environmental Engineering Science, 2007, 24(4): 401-423. |
| 39 | GILLHAM R W, VOGAN J, GUI L, et al. In situ remediation of chlorinated solvent plumes[M]. New York: Springer, 2010: 537-571. |
| 40 | O’HANNESIN Stephanie F, GILLHAM Robert W. Long-term performance of an in situ “iron wall” for remediation of VOCs[J]. Groundwater, 1998, 36(1): 164-170. |
| 41 | 王泓泉. 污染地下水可渗透反应墙(PRB)技术研究进展[J]. 环境工程技术学报, 2020, 10(2): 251-259. |
| WANG Hongquan. Study on permeable reactive barrier technology for the remediation of polluted groundwater[J]. Journal of Environmental Engineering Technology, 2020, 10(2): 251-259. | |
| 42 | 李书鹏, 刘鹏, 杜晓明, 等. 采用零价铁-缓释碳修复氯代烃污染地下水的中试研究[J]. 环境工程, 2013, 31(4): 53-58. |
| LI Shupeng, LIU Peng, DU Xiaoming, et al. A field pilot test for restoring chlorohydrocarbon contaminated groundwater using ZVI & controlled releasing carbon material[J]. Environmental Engineering, 2013, 31(4): 53-58. | |
| 43 | 孟梁, 郭琳, 杨洁, 等. 原位修复氯代烃污染场地地下水的现场中试研究[J]. 工业水处理, 2014, 34(8): 18-21. |
| MENG Liang, GUO Lin, YANG Jie, et al. Research on the pilot scale in situ remediation of groundwater polluted by chlorinated hydrocarbons in a contaminated site[J]. Industrial Water Treatment, 2014, 34(8): 18-21. | |
| 44 | OKTAVITRI Nur Indradewi, NAKASHITA Shinya, HIBINO Tadashi, et al. Enhancing pollutant removal and electricity generation in sediment microbial fuel cell with nano zero-valent iron[J]. Environmental Technology & Innovation, 2021, 24: 101968. |
| 45 | 马黎颖, 和明敏, 陈绍华. 异化铁还原菌强化纳米零价铁在环境修复中的应用研究进展[J]. 广州化工, 2020, 48(21): 14-16. |
| MA Liying, HE Mingmin, CHEN Shaohua. Development and application of nanoscale zero-valent iron and dissimilatory iron rreducing bacteria in environmental remediation[J]. Guangzhou Chemical Industry, 2020, 48(21): 14-16. | |
| 46 | CHO Hyun-Hee, LEE Taeyoon, HWANG Sun-Jin, et al. Iron and organo-bentonite for the reduction and sorption of trichloroethylene[J]. Chemosphere, 2005, 58(1): 103-108. |
| 47 | GAO Jie, WANG Wei, RONDINONE Adam J, et al. Degradation of trichloroethene with a novel ball milled Fe-C nanocomposite[J]. Journal of Hazardous Materials, 2015, 300: 443-450. |
| 48 | 王祥, 邓绍坡, 李川, 等. 修复氯代烃污染的生物炭负载纳米零价铁制备条件优化实验[J]. 生态与农村环境学报, 2019, 35(12): 1626-1632. |
| WANG Xiang, DENG Shaopo, LI Chuan, et al. Optimization of preparation conditions for biochar-loaded nano-zero-valent iron with chlorinated hydrocarbons[J]. Journal of Ecology and Rural Environment, 2019, 35(12): 1626-1632. | |
| 49 | 刘嫦娥, 岳敏慧, 谭辉林, 等. 纳米零价铁(nZVI)对蚯蚓-微生物-土壤生态系统的毒性效应研究[J]. 中国生态农业学报, 2021, 29(10): 1722-1732. |
| LIU Chang’e, YUE Minhui, TAN Huilin, et al. Effects of nano-zero-valent iron (nZVI) on earthworm-bacteria-soil systems[J]. Chinese Journal of Eco-Agriculture, 2021, 29(10): 1722-1732. | |
| 50 | Dharmendra S KEN, SINHA Alok. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts[J]. Environmental Nanotechnology, Monitoring & Management, 2020, 14: 100344. |
| 51 | LU Zejia, LI Weiqi, XIN Yiding, et al. The stringent response gene rsh plays multiple roles in Novosphingobium pentaromativorans US6-1’s accommodation to different environmental pollutants: Phenanthrene, copper and nZVI[J]. Environmental Pollution, 2023, 323: 121315. |
| 52 | GARCIA Ariel Nunez, ZHANG Yanyan, GHOSHAL Subhasis, et al. Recent advances in sulfidated zerovalent iron for contaminant transformation[J]. Environmental Science & Technology, 2021, 55(13): 8464-8483. |
| 53 | XU Hao, GAO Mengxi, HU Xi, et al. A novel preparation of S-nZVI and its high efficient removal of Cr(Ⅵ) in aqueous solution[J]. Journal of Hazardous Materials, 2021, 416: 125924. |
| 54 | LIANG Li, LI Xiaoqin, GUO Yiqing, et al. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur[J]. Journal of Hazardous Materials, 2021, 404: 124057. |
| 55 | BOLHARI A. Feasibility of treating chlorinated solvents stored in low permeability zones in sandy aquifers[D]. Fort Collins: Colorado State University, 2012. |
| 56 | GUERRA Peter, BAUER Akemi, REISS Rebecca A, et al. In situ bioremediation of a chlorinated hydrocarbon plume: A superfund site field pilot test[J]. Applied Sciences, 2021, 11(21): 10005. |
| 57 | 孙鸣璐, 董军, 张梦玥, 等. 乳化纳米铁(EZVI)强化地下水氯代烃还原脱氯[J]. 中国环境科学, 2022, 42(6): 2690-2696. |
| SUN Minglu, DONG Jun, ZHANG Mengyue, et al. Enhanced reductive dechlorination of chlorinated hydrocarbons in groundwater by emulsified zero-valentiron[J]. China Environmental Science, 2022, 42(6): 2690-2696. | |
| 58 | MOLENDA Olivia, PUENTES JÁCOME Luz A, CAO Xuan, et al. Insights into origins and function of the unexplored majority of the reductive dehalogenase gene family as a result of genome assembly and ortholog group classification[J]. Environmental Science Processes & Impacts, 2020, 22(3): 663-678. |
| 59 | CHEN Tzu-Wen, CHANG Shuchi. Potential microbial indicators for better bioremediation of an aquifer contaminated with vinyl chloride or 1,1-dichloroethene[J]. Water, Air, & Soil Pollution, 2020, 231: 1-23. |
| 60 | PUENTES JÁCOME Luz A, WANG Po-Hsiang, MOLENDA Olivia, et al. Sustained dechlorination of vinyl chloride to ethene in Dehalococcoides-enriched cultures grown without addition of exogenous vitamins and at low pH[J]. Environmental Science & Technology, 2019, 53(19): 11364-11374. |
| 61 | Jan NĚMEČEK, Jana STEINOVÁ, Roman ŠPÁNEK, et al. Thermally enhanced in situ bioremediation of groundwater contaminated with chlorinated solvents—A field test[J]. Science of the Total Environment, 2018, 622/623: 743-755. |
| 62 | YANG Yi, HIGGINS Steven A, YAN Jun, et al. Grape pomace compost harbors organohalide-respiring Dehalogenimonas species with novel reductive dehalogenase genes[J]. The ISME Journal, 2017, 11(12): 2767-2780. |
| 63 | SCHAEFER Charles E, LIPPINCOTT David R, STEFFAN Robert J. Field-scale evaluation of bioaugmentation dosage for treating chlorinated ethenes[J]. Groundwater Monitoring & Remediation, 2010, 30(3): 113-124. |
| 64 | VAINBERG Simon, CONDEE Charles W, STEFFAN Robert J. Large-scale production of bacterial consortia for remediation of chlorinated solvent-contaminated groundwater[J]. Journal of Industrial Microbiology & Biotechnology, 2009, 36(9): 1189-1197. |
| 65 | MACFARLANE Kim D, CACCIATORE David A, LEIGH Daniel P, et al. Field-scale evaluation of a biobarrier for the treatment of a trichloroethene plume[J]. Remediation Journal, 2011, 22(1): 29-41. |
| 66 | SCHAEFER Charles E, TOWNE Rachael M, VAINBERG Simon, et al. Bioaugmentation for treatment of dense non-aqueous phase liquid in fractured sandstone blocks[J]. Environmental Science & Technology, 2010, 44(13): 4958-4964. |
| 67 | CHRIST John A, Andrew RAMSBURG C, ABRIOLA Linda M, et al. Coupling aggressive mass removal with microbial reductive dechlorination for remediation of DNAPL source zones: A review and assessment[J]. Environmental Health Perspectives, 2005, 113(4): 465-477. |
| 68 | WANG Zhiyuan, HUANG Weilin, PENG Pingan, et al. Rapid dechlorination of 1,2,3,4-TCDD by Ag/Fe bimetallic particles[J]. Chemical Engineering Journal, 2015, 273: 465-471. |
| 69 | RUAN Xia, LIU Hong, WANG Junwen, et al. A new insight into the main mechanism of 2,4-dichlorophenol dechlorination by Fe/Ni nanoparticles[J]. Science of the Total Environment, 2019, 697: 133996. |
| 70 | WANG Rui, TANG Ting, LU Guining, et al. Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems[J]. Science of the Total Environment, 2019, 661: 18-26. |
| 71 | 吴德礼, 王红武, 马鲁铭. Ag/Fe催化还原体系处理水体中氯代烃的研究[J]. 环境科学, 2006, 27(9): 1802-1807. |
| WU Deli, WANG Hongwu, MA Luming. Reductive dechlorination of chlorinated hydrocarbons in water by Ag/Fe catalytic reduction system[J]. Environmental Science, 2006, 27(9): 1802-1807. | |
| 72 | XIE Jituo, LEI Chao, CHEN Wenqian, et al. Catalytic properties of transition metals modified nanoscale zero-valent iron for simultaneous removal of 4-chlorophenol and Cr(Ⅵ): Efficacy, descriptor and reductive mechanisms[J]. Journal of Hazardous Materials, 2021, 403: 123827. |
| 73 | MUELLER Nicole C, Jürgen BRAUN, BRUNS Johannes, et al. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe[J]. Environmental Science and Pollution Research International, 2012, 19(2): 550-558. |
| 74 | WANG Heli, ZHONG Yin, ZHU Xifen, et al. Enhanced tetrabromobisphenol A debromination by nanoscale zero valent iron particles sulfidated with S0 dissolved in ethanol[J]. Environmental Science Processes & Impacts, 2021, 23(1): 86-97. |
| 75 | KRUG T, O’HARA S, WATLING M, et al. Emulsified zero-valent nano-scale iron treatment of chlorinated solvent DNAPL source areas[R]. Washington DC: US Environmental Protection Agency, 2010, ER: 200431. |
| 76 | 田一迪, 王文浩, 戎开雨. 碳负载纳米铁——新型的地下水修复技术及其最新进展[J]. 环境与发展, 2019, 31(11): 62-63. |
| TIAN Yidi, WANG Wenhao, RONG Kaiyu. Carbon-loaded nano-iron—A new type of groundwater remediation technology and its latest development [J]. Environment and Development, 2019, 31(11): 62-63. | |
| 77 | AHMAD Shakeel, LIU Xiaomei, TANG Jingchun, et al. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants[J]. Chemical Engineering Journal, 2022, 431: 133187. |
| 78 | ZHANG Wenying, QIAN Linbo, OUYANG Da, et al. Effective removal of Cr(Ⅵ) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization[J]. Chemosphere, 2019, 221: 683-692. |
| 79 | WU Wenpei, HAN Lu, NIE Xiang, et al. Effects of multiple injections on the transport of CMC-nZVI in saturated sand columns[J]. Science of the Total Environment, 2021, 784: 147160. |
| 80 | ZOU Haowen, HU Erdan, YANG Shangyuan, et al. Chromium(Ⅵ) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant[J]. Science of the Total Environment, 2019, 650: 419-426. |
| 81 | XU Jiang, WANG Yan, WENG Cindy, et al. Reactivity, selectivity, and long-term performance of sulfidized nanoscale zerovalent iron with different properties[J]. Environmental Science & Technology, 2019, 53(10): 5936-5945. |
| 82 | JIAO Weizhou, SONG Yao, ZHANG Dongsheng, et al. Nanoscale zero-valent iron modified with carboxymethyl cellulose in an impinging stream-rotating packed bed for the removal of lead(Ⅱ)[J]. Advanced Powder Technology, 2019, 30(10): 2251-2261. |
| 83 | WANG Shengsen, ZHAO Mingyue, ZHOU Min, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review[J]. Journal of Hazardous Materials, 2019, 373: 820-834. |
| 84 | SUN Peng, WANG Zhiqiang, AN Shengwei, et al. Biochar-supported nZVI for the removal of Cr(Ⅵ) from soil and water: Advances in experimental research and engineering applications[J]. Journal of Environmental Management, 2022, 316: 115211. |
| 85 | DONG Haoran, ZHANG Cong, HOU Kunjie, et al. Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution[J]. Separation and Purification Technology, 2017, 188: 188-196. |
| 86 | BRUTON Thomas A, PYCKE Benny F G, HALDEN Rolf U. Effect of nanoscale zero-valent iron treatment on biological reductive dechlorination: A review of current understanding and research needs[J]. Critical Reviews in Environmental Science and Technology, 2015, 45(11): 1148-1175. |
| 87 | 王振虹, 葛兴彬, 王雅楠, 等. 纳米Fe0粒子对产乙烯脱卤菌群脱氯性能和多样性影响研究[J]. 环境科学学报, 2014, 34(6): 1381-1388. |
| WANG Zhenhong, GE Xingbin, WANG Yanan, et al. Effect of nano-scale zero-valent iron particles on the dechlorination and diversity of Dehalococcoides spp.[J]. Acta Scientiae Circumstantiae, 2014, 34(6): 1381-1388. | |
| 88 | PUIGSERVER Diana, HERRERO Jofre, CARMONA José M. Mobilization pilot test of PCE sources in the transition zone to aquitards by combining mZVI and biostimulation with lactic acid[J]. Science of the Total Environment, 2023, 877: 162751. |
| 89 | WILKIN Richard T, LEE Tony R, SEXTON Molly R, et al. Geochemical and isotope study of trichloroethene degradation in a zero-valent iron permeable reactive barrier: A twenty-two-year performance evaluation[J]. Environmental Science & Technology, 2019, 53(1): 296-306. |
| 90 | HARA Suzanne O’, KRUG Thomas, QUINN Jacqueline, et al. Field and laboratory evaluation of the treatment of DNAPL source zones using emulsified zero-valent iron[J]. Remediation Journal, 2006, 16(2): 35-56. |
| 91 | QIAN Linbo, CHEN Yun, OUYANG Da, et al. Field demonstration of enhanced removal of chlorinated solvents in groundwater using biochar-supported nanoscale zero-valent iron[J]. Science of the Total Environment, 2020, 698: 134215. |
| 92 | HARP Thomas A. Case studies: Closing solvent sites using activated carbon impregnated with iron[C]// Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy. 2010, 14(1): 18. |
| 93 | LEOMBRUNI Alberto, MUELLER Michael, SEECH Alan, et al. Field application of a reagent for in situ chemical reduction and enhanced reductive dichlorination treatment of an aquifer contaminated with tetrachloroethylene (PCE), trichloroethylene, 1,1-dichloroethylene, dichloropropane and 1,1,2,2-tetrachloroethane (r-130)[J]. Environmental Engineering and Management Journal, 2020, 19(10): 1791-1796. |
| [1] | 何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088. |
| [2] | 孙妩娟, 李倩, 李小玲, 石华强, 杨志成, 柯从玉, 王嗣昌, 张群正. 高效石油烃降解菌群的构建及其含油污泥修复性能[J]. 化工进展, 2024, 43(11): 6468-6474. |
| [3] | 白雨虹, 高大文. Trametes versicolor修复石油烃污染土壤及温室气体释放特征[J]. 化工进展, 2024, 43(10): 5922-5931. |
| [4] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [5] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [6] | 房晓宇, 卢滇楠, 刘铮. 污染土壤生物修复技术的进展与工程应用现状[J]. 化工进展, 2023, 42(12): 6498-6506. |
| [7] | 吴中杰, 谢连科, 王晶辉, 黄仁亮. 多级结构羟基硝酸铜纳米酶的制备及其对酚类污染物的降解[J]. 化工进展, 2023, 42(1): 497-505. |
| [8] | 纪冬丽, 叶际亮, 何少林, 苑宏英, 徐龙谭, 李若琳, 王帅, 宋阳, 齐志斌, 葛雁冰. 油页岩原位开采对部分地下水指标影响的分析[J]. 化工进展, 2022, 41(8): 4057-4064. |
| [9] | 吕莹, 胡学武, 陈素素, 刘兴宇, 陈勃伟, 张明江. 多环芳烃污染土壤的微生物修复技术研究进展[J]. 化工进展, 2022, 41(6): 3249-3262. |
| [10] | 李坡, 张珊珊, 施锦秋, 高航, 王明新. 活化过硫酸盐修复苯胺污染地下水及其环境风险[J]. 化工进展, 2022, 41(5): 2753-2760. |
| [11] | 张永祥, 王晋昊, 井琦, 李雅君. 地下水修复中纳米零价铁材料制备及应用综述[J]. 化工进展, 2021, 40(8): 4486-4496. |
| [12] | 唐垂云, 钟娟, 吕莹, 张明江, 孙娟, 刘兴宇. 土壤中铀污染修复技术研究进展[J]. 化工进展, 2021, 40(8): 4587-4599. |
| [13] | 潘云飞, 唐正, 彭欣怡, 高品. 石油烃污染土壤微生物修复技术研究现状及进展[J]. 化工进展, 2021, 40(8): 4562-4572. |
| [14] | 余关龙, 彭海渊, 王世涛, 汪国梁, 陈宏, 杜春艳, 刘媛媛, 孙士权, 禹丽娥, 王建武. 固定化生物吸附剂对Cd(Ⅱ)的去除性能及机理[J]. 化工进展, 2021, 40(5): 2882-2892. |
| [15] | 包清华, 黄立信, 修建龙, 俞理, 崔庆锋, 马原栋, 伊丽娜. 油气田含油污泥生物处理技术研究进展[J]. 化工进展, 2021, 40(5): 2762-2773. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||